Abstract
Background
Alzheimer's disease (AD) presents as a widespread neurodegenerative condition impacting over 55 million individuals globally, with an annual rise of 10 million new cases. Despite its staggering prevalence, the absence of a definitive cure establishes the need for a revisit.
Methods
We explore the alternative strategies, focusing on the potential therapeutic efficacy of ethanolic extracts derived from the fruit and leaf of Ficus racemosa Linn.
Results
The investigation comprehensively explores pharmacognostic, phytochemical, toxicological, and pharmacological characteristics. In addition to pharmacognostic and physicochemical analyses, toxicological evaluations conducted on experimental animals demonstrated the innocuous nature of the ethanolic extracts (from both fruit and leaf) of F. racemosa, as evidenced by assessments of hemocompatibility, oxidative parameters, and vital organ histology. Phytochemical profiling via GC-MS identified 48 and 80 phytoconstituents in the fruit and leaf extracts, respectively. These constituents were screened for bioactive potential using the “Lipinski Rule of Five,” resulting in the selection of 25 and 33 constituents from fruit and leaf extracts, respectively. Subsequent molecular docking studies against the AChE enzyme revealed promising interactions of the selected phytoconstituents. Furthermore, the top-scoring phytoconstituents were subjected to in silico screening to assess their interactions with β- and γ-secretase enzymes, in addition to the AChE enzyme. The cumulative findings substantiate the therapeutic utility of the plant extracts, particularly in the context of AD.
Conclusion
In conclusion, our investigation highlights the promising therapeutic potential of selected phytoconstituents derived from ethanolic extracts of F. racemosa in mitigating AD pathology by targeting key enzyme sites such as AChE, β-, and γ-secretase.
Keywords: Alzheimer’s disease, Ficus racemosa, phytoconstituents, toxicology, molecular docking
Graphical Abstract
Graphical Abstract.

The diagrammatic presentation of the involvement of target protein in the pathology of Alzheimer’s disease with the extraction, phytochemical, toxicological assessment, and the possible role of Ficus racemosa as a therapeutic agent.
Introduction
Alzheimer’s disease (AD) is a typical form of dementia and may progressively increase with age. Dementia represents one of the most common neurodegenerative disorders, including AD. A recent report revealed that over 55 million individuals are suffering from dementia worldwide, with an additional nearly 10 million cases attributed to accidental brain injuries and associated neuronal damage.1,2 This trend has gradually risen recently, especially in areas with a high sociodemographic index. An epidemiological study from the USA reveals that about 5% of individuals in the age group 65 to 74, 31.1% in the age group 75 to 84, and 33.3% in the age group 85 or older are suffering from Alzheimer’s dementia.3 Furthermore, an estimated 152 million people are at risk of AD and other dementias by 2050.4
The accumulation of the protein fragment β-amyloid into clumps (β-amyloid plaques) on neurons, and the accumulation of tau protein inside neurons are considered the major pathological alterations associated with ADs.5 Although the complete sequence of events is unclear, β-amyloid may begin accumulating before abnormal tau, and increased β-amyloid accumulation is associated with subsequent increases in tau.3 The formation of amyloid plaques condenses neurotransmission, leading to neurodegeneration, a key feature of ADs. Other brain changes linked to Alzheimer’s involve inflammation and atrophy, which are supposed to be triggered by toxic β-amyloid and tau protein activation in the microglia. Although microglia attempt to overcome the accumulated toxic proteins and debris from dead and dying cells, overburden fails the clearing process and leads to chronic inflammation in neuronal cells.6
Despite the towering prevalence and disease burden of ADs in the aged population becoming a major public health concern worldwide, we are still seeking novel drug therapies. Medicinal plants are widely used across the globe as an alternative therapeutic strategy for the prevention or treatment of diverse ailments. Among the countless species of medicinal plants, Ficus racemosa Linn (a member of the Moraceae family) stands out with a rich chemical composition. In addition to previous reports, our recent study has unveiled its multifaceted health benefits against neurodegenerative disorders based on possible molecular mechanisms.7,8 Sumi et al.9 revealed the antioxidant, anti-inflammatory, and anti-apoptotic potential of F. racemosa (FR), which could be a prominent strategy to reverse neurodegeneration.9 However, limited reports are available on the toxicological impact of FR, restricting its therapeutic application. The comprehensive investigation, including pharmacognostic, toxicological, and phytochemical analysis, can significantly estimate the promising therapeutic effects of unexplored herbal extracts targeting specific receptor sites. The study includes the first-time reporting of diverse phytochemicals accessed by GC–MS, in vitro and in vivo toxicological profiles of plant extracts. Furthermore, the therapeutic efficacy of bioactive phytoconstituents (hits or leads) to target AChE, β-secretase, and γ-secretase were examined by an in-silico approach, adding a significant dimension to underline the therapeutic potential of FR.
Materials and methods
Pharmacognostic analysis
Collection and identification of plant
F. racemosa Linn leaves and fruits were collected from a departmental herbal garden and were identified from the National Institute of Science Communication and Information Resources (NISCAIR), New Delhi, where the voucher specimen for leaves and fruit were housed under the authentication number NIScPR/RHMD/Consult/2021/3936-37-1 and NIScPR/RHMD/Consult/2021/3936-37-2 respectively under the supervision of Chief Scientist Dr Sunita Garg (RHMD, CSIR-NISCAIR).
Microscopic study of leaf, fruit, and powder
The microscopical examination includes the detailed observation of transverse sections of the leaf and fruit of F. racemosa Linn under an inverted microscope. Staining techniques were employed to aid in identifying and characterizing various anatomical features. Moreover, the distinctive characteristics of powdered material (F. racemosa’s leaf and fruit) were examined over a glass slide by treating it with 2–3 drops of chloral hydrate. Samples were covered with a glass coverslip for microscopic examination. Additionally, organoleptic properties and fluorescence behaviour were assessed. Further, the powder was exposed to different reagents, including nitric acid, sulfuric acid, and sodium hydroxide, followed by observation under a UV chamber to study its fluorescence.10
Physicochemical analysis
Various physicochemical properties of the leaf and fruit of F. racemosa Linn, such as the ash value, total ash value, acid-soluble ash, water-soluble ash, sulphated ash, crude fiber content, foaming index, and swelling index, were assessed.10
Preparation of fruit and leaf extract
The fresh leaves and fruits of F. racemosa Linn were selected, washed under running tap water, and dried at room temperature. Dried leaves and fruits were chopped into small pieces and grinded to a coarse powder using a grinder. Both powders were extracted separately with a fixed ratio of 90% ethanol using the Soxhlet apparatus for 48 h, maintaining a temperature range of 70–75 °C. The solvent was evaporated at 60 °C using a water bath. The obtained extracts were further dried in a desiccator using anhydrous magnesium sulfate. The lyophilization of semi-solid extracts was done to get solid mass.
Toxicological analysis
In vitro study (Hemocompatibility assay)
The haemolytic activity of ethanolic extract of the leaf and fruit of F. racemosa Linn was used to access the hemocompatibility. A comparative analysis of plant extracts was conducted against a standard toxic reagent (Triton X) and normal saline incubated with a fresh blood sample collected from healthy mice by a retro-orbital puncture. In brief, fresh blood samples (100 μL) were placed in separate sample tubes and incubated with equilibrated volumes of normal saline, Triton X (1%), and either the ethanolic extract of leaf or the ethanolic extract of fruit. Each mixture was prepared in separate tubes to ensure distinct reactions. These mixtures were allowed to react for 1 h under controlled conditions, including a temperature of 37 °C in a 5% CO2 incubator at 95% humidity. The absorbance of the treated samples was measured at 540 nm, which indicates the presence of haemoglobin or lysis.11 Normal saline and Triton X were considered negative and positive controls, respectively. The hemolytic rate was calculated using the following formula:
 |
Moreover, the freshly collected blood (1 mL) was used to measure agglutination and rouleaux formation after exposure to different extracts. Fresh and uncoagulated blood was centrifugated at 2,000 g for 20 min, which formed the pellets. These pellets were resuspended in normal saline at a ratio of 1:9. Subsequently, an aliquot from this resuspended blend was mixed with normal saline (stock solution) in a ratio of 1:6 mL. Finally, the mixture (1 mL) was exposed to different extracts in an equivalent amount and incubated for 1 h under controlled conditions, including 37 °C temperature in a 5% CO2 incubator at 95% humidity. Following the incubation, a smear of the cell suspension from each mixture was prepared and examined under the microscope at 100×.11
In vivo assessment for safety and toxicity profile
Experimental protocol on animals to perform the toxicological study for different ethanolic extracts of leaves and fruit of F. racemosa Linn was duly approved by the Institutional Animal Ethics Committee (Approval number: GDGU/IAEC/2023/24) in accordance with the guidelines established by the CPCSEA New Delhi, India. Wistar albino mice (20–25 g) of either sex were selected for the study. All animals were initially acclimatized for one week before initiating the experimental protocol under the control environmental conditions, including temperature (22 ± 2°) and relative humidity (60%). Additionally, the mice were maintained on standard chow and drinking water ad-libitum under a typical day/night cycle (12/12 h respectively) throughout the study. This study considered eighteen animals, which were randomly divided (using Random Allocation Software 2.0) into three groups (n = 6), which represent normal control (treated with normal saline) and two treatment groups of each extract (Leaf and fruit).
Different functional, biochemical, and histological parameters were assessed to evaluate the toxic impact of plant extracts. Instant behavioral toxic impacts on animals were accessed by Functional Observational Battery (FOB) at fixed time intervals,12 including 0, 10, 30, and 60 min after the single administration of different extracts (100 mg/kg/p.o) of F. racemosa Linn. Furthermore, the assessment of biochemical parameters includes the reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), and thiobarbituric acid reactive substances (TBARS) were evaluated in the serum samples of mice of respective groups.12,13 Animals were pretreated with different plant extracts (100 mg/kg/p.o/day) for 14 days to estimate the impact on oxidative parameters. In addition, to access the oxidative parameters, we have performed histological analyses to investigate the structural alterations in treated mice’s vital organs (brain, liver, heart, and kidney) compared to normal control animals.14 The biochemical (serum) and histological (tissues) samples were collected at the end of the experimental protocol of 14 days.
Phytochemical analysis
Identification of primary and secondary phytochemical metabolites
The ethanolic extract was subjected to investigate the presence of diverse phytoconstituents, including alkaloids, polyphenols, saponins, flavonoids, tannins, and terpenoids in each extract, as reported in the previous report.15 Briefly. The presence of primary metabolites, including resins, Amino acids, carbohydrates, gums and mucilage, fats, and fixed oils, were analyzed as described in the recent report.16 Whereas secondary metabolites, including polyphenols, Flavonoids, alkaloids, tannins, Terpenoid, Anthraquinones, and saponins were investigated as reported in another report.10,15,17
Gas chromatography and mass spectroscopy (GC–MS) analysis
A qualitative analysis of various extracts of F. racemosa Linn was conducted to identify the spectrum of plant phytochemicals using gas chromatography–mass spectrometry (GC–MS) at the Central Instrumentation Laboratory, CUPB Ghuddha, Bathinda. The plant extracts were dissolved in 70% ethanol to obtain a particulate-free solution suitable for GC–MS analysis. This solution was vigorously stirred for 10 s using a vortex stirrer and filtered through a 0.2-micron membrane filter. The resulting clear extract was utilized for GC–MS analysis.
The identification of compounds was performed via GC–MS using a QP-2010 Ultra instrument. Electron ionization with a 70-eV ionizing energy was employed for GC–MS detection. Helium gas (99.99%) was used as the carrier gas at a constant flow rate of 1 mL/min. A 1 μL sample of the plant extract was injected (with a split ratio of 10:1) into the system, with an injector temperature of 250 °C and an ion source temperature of 200 °C. The total operating time for each sample was approximately 63.10 min. The obtained spectra of the extract compounds were compared and matched using the MS Program of the National Institute of Standards and Technology (NIST) Version 14.0 and the Wiley 8.0 library database. The NIST database contains over 6,200 patterns for interpreting GC–MS data. The retention time, molecular formula, and molecular weight of the sample materials were recorded during the analysis.
Molecular docking analysis
Screening of bioactive compounds (Lipinski rule)
Identifying specific bioactive compounds involved a meticulous process that utilized Lipinski’s rule of five. Lipinski’s rules, introduced by Lipinski et al. (1997), provide valuable guidelines for assessing the drug-likeness of chemical compounds based on their physicochemical properties. These rules suggest that compounds with molecular weights below 500 Daltons, logP values less than 5, no more than 5 hydrogen bond donors, and no more than 10 hydrogen bond acceptors are more likely to exhibit favorable oral bioavailability. This rule was systematically applied to scrutinize and filter through the comprehensive list of phytochemical compounds derived from the Gas Chromatography–Mass Spectrometry (GC–MS) analysis of extracts based on molecular weight, log (p) value, and H-bond donor and acceptor.18,19
Molecular interaction of the selected bioactive compound with AChE
Molecular docking is employed to determine the optimal alignment of ligands and proteins. The selected bioactive compounds of F. racemosa Linn were further analyzed for molecular docking to find the interaction score with the targeted protein (Acetylcholinesterase, AChE). The glide module of Schrodinger’s Maestro molecular modeling suite release 2021–2022 was used for molecular docking interaction between ligand (selected bioactive phytoconstituents of ethanolic extract of fruit and leaf) and protein (AChE). The ligand structures were retrieved from the PubChem compound database at NCBI (http://pubchem.ncbi.nlm.nih.gov/) and underwent preparation via the LigPrep module to generate a 3D structure and minimize the confirmational energies.
For this study, a specific protein (AChE) was chosen initially due to its significant role in neural signal transmission and synaptic interactions. AChE is an enzyme responsible for terminating neuronal signals (PDB ID:4EY7).20 The interactions between protein and bioactive compounds extracted from F. racemosa Linn were examined individually. These proteins’ 3D X-ray crystal structures were obtained from the Protein Data Bank (PDB). These proteins are believed to play a pivotal role in the development and progression of neurodegenerative disorders.
Target protein preparation and docking
Schrodinger’s Maestro molecular modeling suite was used to process and refine the protein structures, transforming the initial raw PDB structures into refined protein models using the protein preparation wizard module. These refined models also involved the removal of any water molecules that were originally present in the structures. Following this, Schrodinger’s Maestro software was utilized for an in-depth analysis of the protein structures. This analysis encompassed the study of structural elements, hydrogen bond interactions, non-bond interactions between ligands and active site residues, and the generation of high-quality visual representations of these findings.
The docking process involved the analysis of both the prepared ligands and target proteins using the protein preparation wizard module. In this process, multiple conformations for the ligands were generated, and final energy refinement was conducted to determine the optimal ligand pose. Subsequently, the docking scores for the best ligand poses with the respective target proteins were calculated for all the tested bioactive compounds.
Molecular interaction with β and γ-secretase
In addition to AChE enzyme, β and γ-secretase plays another prominent role in neurotransmission and could be targeted as a promising site for drug interaction. Thus, the selected bioactive phytoconstituents of fruit and leaf (top 5 compounds having the highest docking score with AChE) were further studied for molecular interaction with these proteins. The PDB ID of these proteins includes β-secretase (PDB ID: 1XN2)21 and γ-secretase (PDB ID:6IYC).22 Following the analysis of the interaction between the bioactive compounds (top 5 molecules having the highest docking score with AChE) were further employed for subsequent docking experiments with two distinct proteins, namely β-secretase and γ-secretase by following the same process as mentioned above for the protein AChE.
Statistical analysis
The data obtained from the experiments was presented as mean ± standard. Experimental data of hemocompatibility and oxidative parameters were analyzed using one-way ANOVA following Tukey’s test. The value of “P” ≤ 0.05 was considered statistically significant. Moreover, the data of toxicological analysis, particularly for FOB, were studied based on repeated measures ANOVA followed by post hoc comparisons using the Bonferroni correction.
Results
Pharmacognostic evaluation
F. racemosa Linn leaves and fruits were collected and authenticated by Chief Scientist Dr Sunita Garg (RHMD, CSIR-NISCAIR) under the authentication number NIScPR/RHMD/Consult/2021/3936-37-1 and NIScPR/RHMD/Consult/2021/3936-37-2 respectively. As per the macroscopic characteristics, the leaves were green (7.5–10 cm) and ovate or elliptic in shape, which grew in a cluster from the main trunk. Raw fruits appeared green, whereas they turned orange, dull reddish, or dark crimson when ripened.
Microscopic analysis for leaf and leaf powder
The microscopic image depicts the outer protective layer of the epidermal cell wall alongside the leaf’s epidermal cells, as illustrated in Fig. 1A. The figure represents transverse section (T.S.) views, including the middle, stereo zoom, and side sections of a leaf from F. racemosa Linn. It exhibits dorso-ventral characteristics and single-layered palisade cells in the upper epidermis. The lower and upper epidermis feature numerous, sometimes uniseriate, unicellular, thin-walled trichomes covering the upper epidermal cells. Furthermore, powder microscopy of the leaf reveals the presence of rhomboidal crystals, stomata, and starch grains (Fig. 1B).
Fig. 1.
Microscopic analysis. Microscopy of a transverse section of the leaf (A) including middle, side section, and stereo zoom of leaf of Ficus racemosa Linn. Epi represents the epidermis cell, and epi CW represents the epidermis cell wall (A), powder microscopy of F. racemosa Linn leaf. RC represents the rhomboidal crystal, ST represents the stomata, and Sr represents the starch grains (B), microscopy of transverse section of fruit including TS at 4×, 10×, and stereo zoom microscopy of F. racemosa Linn’s fruit. Tr represents trichome, SC represents stone cell, WL represents wide lumen, epi represents epidermis, Co.C represents collenchymatous cell, Pa.C represents parenchymatous cell, RC represents rossette crystal, Pa.C represents parenchymatous cell, LD represents lactiferous duct, and VT represents vascular traces (C), powder microscopy of F. racemosa Linn fruit. Uni HH represents unicellular hooked hairs, ep C represents epithelial cells, and SC represents stone cells (D).
Microscopic analysis for fruit and fruit powder
The anatomical composition of F. racemosa fruit was studied based on the microscopy of the T.S (Fig. 1C). Epidermal outgrowths represent the trichomes (plant hairs), and lignified cell walls showed the presence of stone cells (sclereids) that contribute to the fruit’s hardness. Collenchymatous cells also provide structural support and flexibility to young plant tissues. Central cavities refer to wide lumens within the fruit tissue and play roles in storage and gas exchange. The parenchymatous cells are characterized by thin cell walls and large central vacuoles. Crystalline structures showed the presence of rosette crystals which serve as storage reserves for calcium oxalate. Fruit lates contain lactiferous ducts that release milky fluid rich in secondary metabolites. Vascular traces refer to the remnants within the fruit which participate in transporting throughout the plant. In addition, powder microscopy of F. racemosa fruit reveals the presence of unicellular hooked hairs (Fig. 1D). As mentioned earlier, Stone cells are also observed in the powdered form, contributing to the fruit’s texture and resilience.
Physicochemical parameters
The inorganic residue yield estimates the potential adulteration in mineral content, which may affect the purity of the plant material and is represented by different ash values. Different ash values, including total ash, acid-insoluble ash, and water-soluble ash, were measured as 40%, 8.6%, and 6.6%, respectively, for fruit, while in leaf, it was 20%, 8.8%, and 6.7%, respectively. The higher value of total ash (40% in fruit) indicates the presence of a mineral source and is a good indicator of the purity. The value of acid-insoluble ash also suggests the substantial presence of silicate impurities. Additionally, the sulphated ash value of fruit (9.2%) and (8.7%) of leaf represents the inorganic content of the sample by weight (1 g) (Table 1).
Table 1.
Physicochemical parameters of fruit and leaf of Ficus racemosa Linn.
| Sr. No. | Parameters | Inference | |
|---|---|---|---|
| Fruit | Leaf | ||
| 1. | Total Ash Value | 40% | 20% |
| 2. | Acid Insoluble Ash | 8.6% | 8.8% |
| 3. | Water Soluble Ash | 6.6% | 6.7% |
| 4. | Sulphated Ash | 9.2% | 8.7% |
| 5. | Foaming Index | Less than 100 mL | Less than 100 mL |
| 6. | Swelling Index | 5 mL | 7 mL |
| 7. | Crude Fibre Content | 15% | 9.5% |
This table presents the comparative analysis of various phytochemical parameters between the fruit and leaf extracts. The parameters include total ash value, acid insoluble ash, water soluble ash, sulphated ash, foaming index, swelling index, and crude fibre content. The values are expressed as percentages (%) or volume (mL) as appropriate.
Similarly, the physical attributes of the plant were measured by foaming index, swelling index, and crude fiber content (Table 1). The foaming index measures the ability to form a stable foam due to the presence of saponin. The fruit and leaf foaming index displayed no significant difference (less than 100 mL). Conversely, the swelling index determines the ability to absorb water, which was found more in the leaf 7 mL than in the fruit 5 mL, which indicates a high quantity of mucilage, pectin, and hemicellulose in the leaf part. The content of crude fiber index was higher in the fruit part (15%) than in the leaf (9.5%), showing the extent of ingestion and content absorption. It further indicates the high proportion of cellulose, hemicellulose, and lignin.
Preparation and assessment of fruit and leaf extracts
Soxhlet apparatus was used to perform the hot percolation method (60–70 °C) for 48 h to extract the phytoconstituents of F. racemosa from leaf and fruit samples. The dried sample of fruit (80 g) and leaf (80 g) were extracted using 90% ethanol, which yielded 18.04 g and 21.32 g, respectively. The percentage yield of the extract for fruit and leaf was calculated as 22.55% and 26.65% w/w, respectively.
Toxicological analysis
In-vitro assessment (Hemocompatibility)
The hemolytic rate was assessed following the incubation of RBCs with Triton X and extracts derived from both fruit and leaf samples. Our findings revealed a significant elevation in hemolysis induced by Triton X, a standard toxic agent, compared to the untreated control. However, no evidence of hemolysis was observed in the treatment groups exposed to either fruit or leaf extracts, indicating a protective effect against RBC damage compared to the untreated control (Fig. 2A). Moreover, microscopic examination revealed no discernible morphological alterations or aggregation of RBCs following exposure to the fruit and leaf extracts, mirroring the observations in the untreated control group. These results suggest that the extracts may not only mitigate hemolysis but also maintain the structural integrity of RBCs, like the untreated control group (Fig. 2B).
Fig. 2.
Toxicological analysis. The figure represents the effect of Ficus racemosa extracts (fruit and leaf) on the hemolytic rate (A), on the RBC agglutination rate (B), on the biochemical oxidative markers including GSH, SOD, CAT, and TBARS (C), and on the histology of vital organs (D).
In vivo assessment of the safety and toxicological profile
Assessment of functional observational battery (FOB)
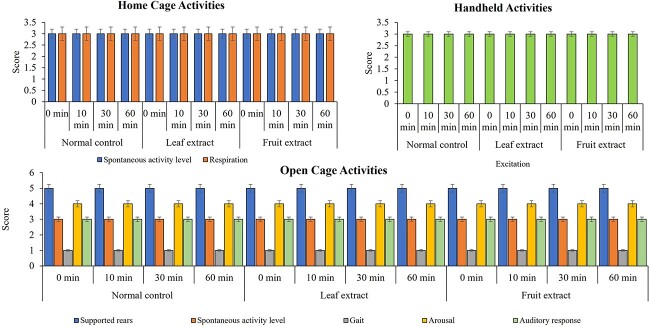
The FOB (functional observational battery) study aimed to observe the instant behavior changes in the animals in response to exposure to different extracts. The results show no measurable change in the animals’ behaviors (spontaneous activity level, respiration, convulsions, tremors, fasiculations, tonus, clonus, vocalization, straubs tail, writhing, retropulsion, and diarrhoea) observed under the home cage compared to normal control animals. Similarly, no major behavioral changes were observed, including lacrimation, salivation, body tone, piloerection, fur appearance, ptosis, and exophthalmia compared to normal control animals during handling. Moreover, a series of open field activities were performed compared to normal control animals, including surface righting, aerial righting, tail pinch and pinna reflexes, visual placing, palpebral closure, and auditory response, which showed no significant alteration upon the exposure of extract. Certain parameters were analyzed based on their activity scores, including spontaneous activity level and respiration in the home cage, and excitation activity during handling. Additionally, supported rearing, spontaneous activity, gait, arousal, and auditory response were evaluated in the open field. The observations indicated no significant changes in these behavioral activities of the animals on the exposure to plant extracts, thereby providing evidence of the absence of immediate toxic effects (Fig. 3). The details of the effect of consequences of fruit and leaf extracts are given in Supplementary Table 1.
Fig. 3.
Behavioural analysis. Figure interprets the toxicological impact of ethanolic extracts of fruit and leaf of Ficus racemosa, based on their activity scores. In comparison to the normal control group, different behavioural parameters, including spontaneous activity level and respiration, were observed in the home cage, excitation activity was observed during handling, and spontaneous activity, supported rearing, gait, arousal, and auditory response were observed in the open field.
Assessment of oxidative marker
The oxidative markers (CAT, TBARS, SOD, and GSH) were investigated in blood serum samples of mice to determine the adverse effect of leaf and fruit extracts. The results demonstrated no significant difference in the leaf and fruit treatment groups compared to the normal control, as shown in Fig. 4A, which suggests the regulation of the free radicals and reactive oxygen species. The conclusion suggests the antioxidant potential which may be attributed to the presence of phytoconstituents within the ethanolic extracts.
Fig. 4.
Toxicity analysis in In vivo model. Evaluation of oxidative parameters and histological analysis of vital organs following treatment with ethanolic extracts of Ficus racemosa fruit and leaf. (A) Oxidative stress markers including reduced glutathione (GSH), thiobarbituric acid reactive substances (TBARS), catalase (CAT), and superoxide dismutase (SOD) levels were measured to assess the toxicological impact compared to normal control groups. (B) Histological examination of the brain, liver, heart, and kidney tissues to observe any morphological changes.
Histological analysis
The histology of vital organs was evaluated to observe any structural alteration caused by acute exposure (14 days) to fruit and leaf ethanolic extracts (100 mg/kg/p.o/day). During the examination, we evaluated several histological sections of different vital organs (Brain, liver, heart, and kidney) compared to a photomicrograph of the respective organs of normal control animals under a microscope (40×), as shown in Fig. 4B. There was no sign of neuronal damage or apoptosis in the brain tissue, and no disruption in the myofibrils, hepatocytes, and glomerulus was observed in the treated groups compared to the normal control. Overall, no significant alteration was observed upon the treatment of ethanolic extracts of fruit and leaf, which endorse the non-toxic profiles of F. racemosa based on the structural assessment of the vital organs of mice.
Phytochemical analysis
Assessment of primary and secondary phytochemical metabolites
The phytochemical analysis revealed the presence of various primary and secondary metabolites in the ethanolic extracts of fruit and leaf study of F. racemosa Linn (Table 2). The identification of primary metabolites was conducted through a series of tests. Carbohydrate analysis involved the application of three methods: Benedict’s test and the Molisch test, both of which yielded positive results in leaf and fruit samples.23,24 Conversely, the biuret and Millon’s tests were performed for starch detection, resulting in negative outcomes.17,25,26 Amino acid and resin analyses were performed using the ninhydrin test, nitric acid test, and precipitation test, respectively.23 Remarkably, amino acids exhibited positive outcomes, whereas resin analysis yielded negative results in both extracts. Furthermore, fixed oils and fats analysis yielded notably substantial positive results (Table 2).
Table 2.
Qualitative analysis of primary and secondary metabolites.
| S.No. | Primary metabolites | Ethanolic extract of Leaf | Ethanolic extract of Fruit |
|---|---|---|---|
| 1. | Carbohydrates | + | + |
| 2. | Starch | − | − |
| 3. | Amino acid | + | + |
| 4. | Resins | − | − |
| 6. | Fatty acids | + | + |
| 7. | Fixed oils and fats | + | + |
| Secondary metabolites | |||
| 8. | Anthraquinones | + | + |
| 9. | Alkaloids | + | + |
| 10. | Polyphenols | + | + |
| 11. | Tannins | + | + |
| 12. | Phlobtannins | − | − |
| 13. | Saponins | + | + |
| 14. | Flavanoids | + | + |
| 15. | Catechins | + | + |
| 16. | Chalcones | + | + |
| 17. | Anthocyanins | + | + |
| 18. | Terpenoids | + | + |
| 19. | Cardiac glycosides | + | + |
| 20. | Flavonols and flavones | + | + |
| 21. | Coumarins | + | + |
| 22. | Emodins | + | + |
This table presents the presence (+) or absence (−) of various primary and secondary metabolites in the ethanolic extracts of leaf and fruit. The primary metabolites analyzed include carbohydrates, starch, amino acids, resins, fatty acids, and fixed oils and fats. The secondary metabolites analyzed include anthraquinones, alkaloids, polyphenols, tannins, phlobatannins, saponins, flavonoids, catechins, chalcones, anthocyanins, terpenoids, cardiac glycosides, flavonols and flavones, coumarins, and emodins.
In addition to primary metabolites, the presence of secondary metabolites was identified based on the degree of precipitation or visible color changes during analysis. The degree of precipitation refers to the visible cloudiness in the reaction mixture, indicating the concentration of phytoconstituents in fruit and leaf samples. Alkaloids were confirmed using Mayer’s and Dragendorff’s tests, while polyphenols were identified by the emergence of a blue-green color upon the addition of ferric cyanide solution, indicating a positive result. Terpenoids and cardiac glycosides were tested using the Salkowski and Keller-Killiani tests, respectively, which yielded positive results. The comprehensive phytochemical study revealed the presence of anthraquinones, alkaloids, polyphenols, tannins, saponins, flavonoids, catechins, chalcones, anthocyanins, terpenoids, cardiac glycosides, coumarins, emodins, flavonols, and flavones in the leaf and fruit of F. racemosa. However, phlobatannins were notably absent in fruit and leaf extracts. Despite this, the presence of these phytoconstituents underscores the remarkable potential of the plant.
GC–MS analysis
The assessment of the chemical structure and composition of the extract reveals its biological potential. Notably, there is a lack of prior studies reporting the characterization of phyto compounds in the ethanolic extract of both fruit and leaf of F. racemosa using GC–MS equipment. Our study fills this gap, presenting results from GC–MS analysis showing 48 chemical constituents in the fruit ethanolic extract (Supplementary Table 2) and 80 chemical constituents in the leaf ethanolic extract (Supplementary Table 3). The chromatogram from the GC–MS analysis of leaf and fruit extracts illustrates the separation and identification of individual chemical constituents. The GC–MS chromatogram for leaf and fruit extracts displays distinct peaks for specific compounds, each with their respective retention times (Fig. 5). These analyses provide detailed information on the active principles, including their molecular formula, molecular weight, retention time, and percentage peak area of phytoconstituents in fruit and leaf extracts. Furthermore, the study of extractive value serves as a valuable source of information and can establish suitable standards for determining the quality of plant material in future investigations. Proximate analysis of the data indicates the composition of the ethanolic extracts from the fruit and leaf of F. racemosa Linn., offering insights into their chemical properties and potential applications.
Fig. 5.
Phytochemical analysis. GC–MS chromatogram of ethanolic extract of fruit (A) and leaf (B) of Ficus racemosa Linn.
Molecular docking analysis
Screening of bioactive compounds
The screening of bioactive phytoconstituents was conducted utilizing Schrödinger’s software suite (specifically Maestro). Among the numerous phytoconstituents identified through GC–MS results, a subset of bioactive compounds was chosen based on adherence to “Lipinski’s Rule of Five.” This extensively established rule in drug discovery aids in evaluating the drug-likeness of chemical compounds, predicting their suitability as potential drug candidates, and assessing oral bioavailability. The criteria of “Lipinski’s Rule of Five” involve molecular weight, lipophilicity (LogP), hydrogen bond donors, and acceptors.18
In accordance with these criteria, we selectively chose 25 bioactive phytoconstituents (from a total of 48) from the ethanolic extract of the fruit and 33 phytoconstituents (from a total of 80) from the ethanolic extract of the leaf. Detailed information regarding the selected bioactive phytoconstituents from both the fruit and leaf, including molecular formula, molecular weight, retention time, percentage peak area, as well as lipophilicity (LogP), hydrogen bond donors, and acceptors values, can be found in Table 3. Subsequently, these compounds were employed as ligands for molecular docking studies.
Table 3.
Detail of selected bioactive phytoconstituents identified by GCMS and screened by “Lipinski’s rule of five”.
| S. N. | Compound names (Fruit extract of Ficus racemosa Linn.) | Molecular formula | Retention index | Retention time | Area % | Molecular weight | HB Donor | HB Accept | Lipophilicity (LogP) |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 9,12-Octadecadienoic acid (Z,Z)- | C18H32O2 | 2183 | 37.037 | 1.60 | 280.45 | 1 | 2.75 | 1 |
| 2 | Heptadecanoic acid, ethyl ester | C19H38O2 | 2077 | 36.189 | 0.11 | 298.508 | 0 | 3 | 1 |
| 3. | Behenic alcohol | C22H46O | 2451 | 31.917 | 0.08 | 326.605 | 2 | 2 | 1 |
| 4. | Tetradecanoic acid | C14H28O2 | 1769 | 29.352 | 0.21 | 228.374 | 1 | 2.75 | 1 |
| 5. | Isopropyl linoleate | C21H38O2 | 2228 | 42.825 | 0.14 | 322.53 | 0 | 2 | 1 |
| 6. | Eicosanoic acid, ethyl ester | C57H104O6 | 6149 | 41.637 | 0.14 | 340.588 | 0 | 3 | 1 |
| 7. | Octadecanoic acid, ethenyl ester | C20H38O2 | 2167 | 39.821 | 0.37 | 310.519 | 0 | 4 | 1 |
| 8. | Octadecanoic acid, ethyl ester | C20H40O2 | 2177 | 38.088 | 0.86 | 312.535 | 0 | 3 | 1 |
| 9. | Ethyl Oleate | C20H38O2 | 2185 | 37.710 | 8.48 | 310.519 | 0 | 3 | 1 |
| 10. | Linoleic acid ethyl ester | C20H36O2 | 2193 | 37.469 | 3.33 | 308.503 | 0 | 3 | 1 |
| 11. | Hexadecanoic acid, ethyl ester | C18H36O2 | 1978 | 34.220 | 5.97 | 284.481 | 0 | 3 | 1 |
| 12. | n-Hexadecanoic acid | C16H32O2 | 1968 | 33.752 | 12.3 | 256.428 | 1 | 2.75 | 1 |
| 13. | Dibutyl phthalate | C16H22O4 | 2037 | 33.459 | 0.10 | 278.347 | 0 | 3.5 | 3 |
| 14. | Pentadecanoic acid, ethyl ester | C17H34O2 | 1878 | 32.144 | 0.16 | 270.454 | 0 | 3 | 1 |
| 15. | Pentadecanoic acid | C15H30O2 | 1869 | 31.523 | 0.23 | 242.401 | 1 | 2.75 | 1 |
| 16. | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | C16H22O4 | 1908 | 31.452 | 0.77 | 278.347 | 0 | 3.5 | 3 |
| 17. | Tetradecanoic acid, ethyl ester | C16H32O2 | 1779 | 29.970 | 0.10 | 256.428 | 0 | 3 | 1 |
| 18. | n-Decanoic acid | C10H20O2 | 1372 | 27.521 | 37.5 | 172.267 | 1 | 2.75 | 3 |
| 19. | Pentanedioic acid, diethyl ester | C9H16O4 | 1250 | 17.050 | 0.08 | 188.223 | 0 | 6 | 3 |
| 20. | Nonanoic acid | C9H18O2 | 1272 | 16.936 | 0.08 | 158.24 | 1 | 2.75 | 3 |
| 21. | Maltol | C6H6O3 | 1063 | 13.431 | 0.13 | 126.112 | 0 | 3.75 | 3 |
| 22. | 1,3-Cyclohexanedione, 2-methyl- | C7H10O2 | 1123 | 13.237 | 0.27 | 126.155 | 0 | 4 | 3 |
| 23. | Phenol, 2-methoxy- | C7H8O2 | 1090 | 13.047 | 0.08 | 124.139 | 1 | 0.75 | 3 |
| 24. | Dimethyl trisulfide | C2H6S3 | 972 | 12.644 | 0.54 | 126.249 | 3.2 | 0 | 3 |
| 25. | Carbaril | C12H11NO2 | 1810 | 14.015 | 0.68 | 201.224 | 2 | 3.5 | 3 |
| Compound names (Leaf extract of F. racemosa Linn.) | |||||||||
| 1. | 1-Hexacosanol | C26H54O | 2848 | 49.543 | 1.55 | 382.712 | 2 | 2 | 1 |
| 2. | Squalene | C30H50 | 2914 | 48.263 | 0.23 | 410.725 | 0 | 0 | 1 |
| 3. | Eicosanoic acid, ethyl ester | C22H44O2 | 2375 | 44.917 | 0.07 | 340.588 | 0 | 3 | 1 |
| 4. | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester | C24H50 | 2407 | 43.668 | 0.76 | 330.507 | 2 | 4 | 1 |
| 5. | Hexadecanoic acid, octyl ester | C24H48O2 | 2574 | 43.412 | 0.26 | 368.642 | 0 | 3 | 1 |
| 6. | Tetracosane | C24H50 | 2407 | 41.777 | 0.10 | 338.659 | 0 | 0 | 1 |
| 7. | Carbamic acid, 2-(dimethylamino)ethyl ester | 978 | 39.722 | 0.06 | 132.162 | 5 | 0.5 | 2 | |
| 8. | Octadecanoic acid, ethyl ester | C20H40O2 | 2177 | 38.086 | 0.59 | 312.535 | 0 | 3 | 1 |
| 9. | Ethyl Oleate | C20H38O2 | 2185 | 41.627 | 2.47 | 310.519 | 0 | 3 | 1 |
| 10. | Linoleic acid ethyl ester | C20H36O2 | 2193 | 37.459 | 0.66 | 308.503 | 0 | 3 | 1 |
| 11. | 9-Octadecenoic acid, (E)- | C18H34O2 | 2175 | 37.243 | 0.93 | 282.465 | 1 | 2.75 | 1 |
| 12. | 9,12-Octadecadienoic acid (Z,Z)- | C18H32O2 | 2183 | 37.006 | 1.02 | 280.45 | 1 | 2.75 | 1 |
| 13. | Heptadecanoic acid, ethyl ester | C19H38O2 | 2077 | 36.193 | 0.07 | 298.508 | 0 | 3 | 1 |
| 14. | Heneicosane | C21H44 | 2109 | 34.375 | 0.15 | 296.579 | 0 | 0 | 1 |
| 15. | Hexadecanoic acid, ethyl ester | C18H36O2 | 1978 | 34.213 | 1.89 | 284.481 | 0 | 3 | 1 |
| 16. | 2H-Pyran-2-one, tetrahydro-6-nonyl- | C14H26O2 | 1801 | 34.056 | 0.25 | 226.358 | 0 | 3 | 3 |
| 17. | n-Hexadecanoic acid | C16H32O2 | 1968 | 33.694 | 11.9 | 256.428 | 1 | 2.75 | 1 |
| 18. | Dibutyl phthalate | C16H22O4 | 2037 | 33.454 | 0.29 | 278.347 | 0 | 3.5 | 3 |
| 19. | 7-Hexadecenal, (Z)- | C16H30O | 1808 | 36.322 | 0.24 | 238.412 | 0 | 2 | 1 |
| 20. | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | C16H22O4 | 1908 | 31.447 | 2.09 | 278.347 | 0 | 3.5 | 3 |
| 21. | Eicosen-1-ol, cis-9- | C20H40O | 2260 | 31.861 | 0.73 | 296.535 | 2 | 2 | 1 |
| 22. | 6,9-Octadecadienoic acid, methyl ester | C19H34O2 | 2093 | 29.632 | 1.05 | 294.476 | 1 | 3 | 1 |
| 23. | Tetradecanoic acid | C14H28O2 | 1769 | 29.325 | 0.49 | 228.374 | 1 | 2.75 | 1 |
| 24. | .alpha.-Guaiene | C15H24 | 1490 | 26.151 | 0.16 | 204.355 | 0 | 0 | 3 |
| 25. | Hexadecane | C16H34 | 1612 | 25.488 | 0.11 | 226.445 | 0 | 0 | 1 |
| 26. | Tetradecane | C14H30 | 1413 | 20.264 | 0.19 | 198.391 | 0 | 0 | 1 |
| 27. | Methyl eicosa-5,8,11,14,17-pentaenoate | C21H32O2 | 2316 | 19.913 | 0.10 | 316.483 | 1 | 3 | 1 |
| 28. | Pentanedioic acid, diethyl ester | C9H16O4 | 1250 | 16.743 | 4.38 | 188.223 | 0 | 6 | 3 |
| 29. | 5-Hydroxymethylfurfural | C6H6O3 | 1163 | 15.399 | 2.92 | 126.112 | 1 | 5 | 2 |
| 30. | Mequinol | C7H8O2 | 1090 | 15.203 | 0.12 | 124.139 | 2 | 1.75 | 3 |
| 31. | Carbaril | C12H11NO2 | 1810 | 12.583 | 1.55 | 201.224 | 2 | 3.5 | 3 |
| 32. | cis-9-Hexadecenal | C16H30O | 1808 | 37.137 | 3.99 | 238.412 | 0 | 2 | 1 |
| 33 | Butanoic acid, 2-methyl-, octyl ester. | C13H26O2 | 1417 | 26.497 | 3.25 | 214.347 | 0 | 3 | 1 |
This table presents the detailed GC–MS analysis of phytoconstituents identified in the ethanolic extracts of fruit and leaf of F. racemosa Linn. The table includes compound names, molecular formula, retention index, retention time, area percentage, molecular weight, number of hydrogen bond donors (HB Donor), number of hydrogen bond acceptors (HB Accept), and lipophilicity (LogP).
Molecular interaction of selected bioactive compounds with AChE, and β/γ-secretase
The bioactive phytoconstituents selected from the fruit (25 compounds) and leaf (33 compounds), complying with Lipinski’s Rule, were subjected to interaction studies with an initial target protein, Acetylcholinesterase (AChE). Docking simulations were performed, and the scores were obtained for the interaction of the bioactive ligands with the initial target protein AChE (4EY7) (Supplementary Table 4). While several bioactive phytoconstituents exhibited interactions with the targeted protein structure (AChE), we narrowed down our focus to the top 5 bioactive phytoconstituents for both fruit and leaf, based on their respective docking scores (Table 4). The binding affinity between these top 5 bioactive ligands and AChE was characterized by intermolecular hydrogen bonds, indicating their potential as Anti-Alzheimer agents. Subsequently, the selected top 5 bioactive phytoconstituents from each ethanolic extract of the fruit and leaf were further examined for their interactions with β-secretase (1XN2) and γ-secretase (6IYC), based on molecular docking scores, revealing diverse binding patterns influenced by ligand nature (Table 4). Phytoconstituents from the fruit and leaf extracts, which demonstrated the highest docking scores against both β-secretase and γ-secretase, were identified from the top five bioactive compounds interacting with AChE (Fig. 6). Among these, linoleic acid and behenic alcohol from the fruit, along with linoleic acid and hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester from the leaf, emerged as promising therapeutic candidates. The detailed interactions of these compounds with key target sites implicated in AD (Fig. 7) demonstrate a substantial influence on pathways associated with neurodegenerative disorders. These compounds exhibited the highest docking scores for interactions with AChE, β-secretase, and γ-secretase, indicating their potential therapeutic efficacy.
Table 4.
Promising bioactive phytochemical compounds obtained from fruit and leaf with AchE, β-secretase, γ-secretase.
| S.N | Particular | Docking score of fruit | Docking score of leaf | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Name | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | Eicosanoic acid, ethyl ester | Carbaril | Behenic alcohol | Linoleic acid ethyl ester | Linoleic acid ethyl ester | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 2H-Pyran-2-one, tetrahydro-6-nonyl- | Carbaril | Carbamic acid, 2-(dimethylamino)ethyl ester |
| 2 | Formula | C16H22O4 | C22H44O2 | C12H11NO2 | C22H46O | C20H36O2 | C20H36O2 | C19H38O4 | C14H26O2 | C12H11NO2 | C5H12N2O2 |
| 3 | AchE (4EY7) | −6.85 | −7.09 | −7.23 | −7.46 | −9.26 | −9.26 | −7.97 | −7.68 | −7.23 | −8.29 |
| 4 | β-secretase (1XN2) | −4.45 | −5.32 | −4.37 | −5.74 | −6.46 | −6.46 | −7.353 | −4.341 | −4.696 | −3.449 |
| 5 | γ-secretase (61YC) | −6.264 | −5.458 | −6.068 | −5.672 | −5.467 | −5.467 | −6.427 | −4.705 | −6.068 | −2.67 |
This table presents the molecular docking scores of various phytoconstituents of fruit and leaf extracts of F. racemosa Linn, which primarily selected (Top 5 phytoconstituents of each extract) based on the highest docking score against AChE and further studied against β and γ-secretase. Furthermore, the highlighted column (including Behenic alcohol and Linoleic acid ethyl ester form fruit phytoconstituents, whereas Linoleic acid ethyl ester and Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester) from leaf phytoconstituents represents the most promising compounds of individual extract that have maximum potential to interact with all three types of targets (AChE, β, and γ-secretase). The table includes the names and formulas of the compounds, along with their docking scores against AchE with PDB ID 4EY7, β-secretase with PDB ID 1XN2, and β-secretase with PDB ID 61YC.
Fig. 6.
In-silico analysis (molecular docking). Figures present the top five bioactive compounds from each extract (fruit and leaf extracts) of Ficus racemosa Linn and the screened promising phytoconstituents (2 from each extract) having the highest docking score with all key enzymes (AchE, β-secretase, and γ-secretase respectively) responsible for the pathology of AD.
Fig. 7.
Molecular interaction of selected Phytoconstituents. The figures represent bioactive compounds from fruit and leaf extracts of Ficus racemosa Linn docked with AchE, β -secretase, and γ-secretase.
Discussion
We precisely examined the safety and pharmacological potential of F. racemosa Linn, specifically focusing on its therapeutic implications for AD. The pharmacognostic analysis provided valuable insights into the unique microscopic features of fruit and leaf, essential for authenticating this plant species across various comparative studies.10 Further, the assessment of inorganic residue yield through diverse ash values underscored the significance of monitoring potential mineral adulteration, which is crucial for ensuring the purity of plant materials.10 The observed variations in ash values, including total ash, acid-insoluble ash, and water-soluble ash, shed light on the mineral composition and potential impurities in the extracts. The comprehensive toxicological evaluations, encompassing both in vivo and in vitro studies, reaffirmed the safety of the selected plant extracts.11 Our findings, supported by parameters such as hemocompatibility and oxidative parameters, provided strong evidence of the absence of toxicity. Additionally, behavioral analyses revealed no signs of abnormal social behavior in experimental animals, further corroborating the safety profile of the extracts.11,12 Phytochemical analysis uncovered a diverse array of metabolites, indicative of significant pharmacological potential, particularly in addressing neurodegenerative diseases.27,28 The identified phytoconstituents exhibited a profound impact on antioxidant potential, augmenting the medicinal properties of the plant extracts.29 These metabolites, including anthraquinones, alkaloids, polyphenols, tannins, saponins, and flavonoids, showcased diverse pharmacological properties (antioxidant, anti-inflammatory, antimicrobial, nociceptive, cholesterol-lowering, and immune-boosting effects), underscoring their therapeutic efficacy.30 Anthraquinones and polyphenols are known to reduce oxidative stress and inhibit amyloid-β aggregation, while alkaloids could inhibit acetylcholinesterase, enhancing cholinergic neurotransmission.31,32 Tannins and saponins could also exhibit strong antioxidant and anti-inflammatory properties, protecting neurons from oxidative damage and neuroinflammation.33,34 Flavonoids have been reported to improve cognitive function by enhancing synaptic plasticity and inhibiting amyloid-β accumulation.35 Collectively, these compounds target multiple AD pathologies, offering a comprehensive therapeutic approach. GC–MS analysis further elucidated the presence of numerous pharmacologically active compounds in the ethanolic extracts of both fruit and leaf. Based on the “Lipinski Rule of Five”, the screening process identified specific bioactive constituents with promising pharmacological potential.18
Thus, exploring the molecular landscape, meticulous docking simulations provided invaluable insights into the interactions of bioactive compounds with key proteins, including AChE, β-secretase, and γ-secretase. These proteins or enzymes play pivotal roles in the pathogenesis of AD.36,37 AChE is pivotal in the degradation of acetylcholine, a neurotransmitter crucial for memory and cognitive function. Insufficient acetylcholine levels may activate the amyloidogenic pathway, responsible for the development of neuronal plaques in AD, serving as a distinctive hallmark.8 Furthermore, the enzymes β-secretase and γ-secretase are integral in the generation of β-amyloid plaques through the amyloidogenic pathway, a characteristic feature in the pathology of AD.8,36,37 Our study systematically investigated the potential of selected bioactive phytoconstituents from fruit (25 constituents) and leaf (33 constituents) extracts, initially targeting the protein AChE (PDB ID: 4EY7). Based on their docking scores, the top 5 phytoconstituents of fruit and leaf extracts individually (against AChE enzyme) were chosen for further investigation of their molecular interactions with β-secretase (PDB ID: 1XN2) and γ-secretase (PDB ID: 6IYC). Subsequently, from these top 5 phytoconstituents of leaf and fruit extract individually, the linoleic acid and behenic alcohol from the fruit, along with linoleic acid and hexadecanoic acid 2-hydroxy-1-(hydroxymethyl) ethyl ester from the leaf extracts were further selected as they exhibited the highest binding affinity with AChE (4EY7), β-secretase (1XN2), and γ-secretase (6IYC) simultaneously. Linoleic acid has been reported for its neuroprotective potential in the literature38; behenic alcohol is studied for its strong anti-inflammatory potential,39 whereas hexadecanoic acid 2-hydroxy-1-(hydroxymethyl) ethyl ester is the first time reported phytoconstituent that may be a promising therapeutic approach for AD and other cognitive functions. These selected phytoconstituents have emerged as promising therapeutic agents for targeting AD owing to their favorable safety profiles and high molecular docking scores with AChE, β- and γ-secretase. Modulating the activity of these enzymes presents a strategic approach to impede the formation of β-amyloid plaques, potentially mitigating the progression of AD and its related cognitive impairments.36,37 The promising findings create opportunities for further in-depth analysis of F. racemosa Linn, and their phytoconstituents for potential clinical applications in AD treatment.
Conclusions
Our study identifies novel therapeutic compounds in F. racemosa Linn fruit (linoleic acid and behenic alcohol) and leaf (linoleic acid, hexadecanoic acid, and 2-hydroxy-1-(hydroxymethyl) ethyl ester) with promising anti-Alzheimer’s potential. These compounds demonstrate significant interactions with key enzymes associated with AD pathology, including AChE, β-secretase, and γ-secretase. Linoleic acid, a polyunsaturated fatty acid found in both the fruit and leaf of F. racemosa, is known for its anti-inflammatory properties, which can mitigate neuroinflammation, a critical factor in AD progression. Behenic alcohol, a long-chain fatty alcohol identified in the fruit, exhibits neuroprotective effects, potentially by modulating lipid metabolism and maintaining cellular membrane integrity. The leaf compounds, including hexadecanoic acid and 2-hydroxy-1-(hydroxymethyl) ethyl ester, further contribute to the anti-Alzheimer’s activity through their antioxidant properties, which combat oxidative stress, another hallmark of AD. Our in silico analyses indicate that these compounds exhibit a high binding affinity to AChE, β-secretase, and γ-secretase, suggesting their potential to inhibit the formation of amyloid plaques and neurofibrillary tangles, thereby interfering with critical neurodegenerative pathways. Importantly, our toxicity assessments reveal that these compounds exhibit no cytotoxic effects, highlighting their safety and therapeutic viability. The integration of these natural compounds into therapeutic strategies could offer a multifaceted approach to AD treatment, targeting multiple pathological pathways with minimal adverse effects. These findings underscore the therapeutic efficacy of F. racemosa Linn and its phytoconstituents, warranting further investigation for potential clinical applications in AD treatment.
Supplementary Material
Contributor Information
Anu Rani, Amity Institute of Pharmacy, Amity University Haryana, Gurugram, Haryana 122413, India.
Pritam Babu Sharma, Drug Discovery and Development Cluster, Amity University Haryana, Gurugram, Haryana 122413, India.
Saurabh Bhatia, Amity Institute of Pharmacy, Amity University Haryana, Gurugram, Haryana 122413, India.
Arun K Sharma, Amity Institute of Pharmacy, Amity University Haryana, Gurugram, Haryana 122413, India.
Author contributions
All authors contributed to the formal analysis Writing—review & editing. The data collection, formal analysis, validation, writing, review, and editing were performed by Anu Rani. Conceptualization, validation, writing, review, and editing were performed by Pritam Babu Sharma and Saurabh Bhatia. Conceptualization, Data curation, Roles/Writing—the original draft was contributed by Arun K. Sharma. All the authors validated and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflict of interest statement
The author declared no conflict of interest.
References
- 1. Shin JH. Dementia epidemiology fact sheet 2022. Ann Rehabil Med. 2022:46(2):53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Navia RO, Constantine LA. Palliative care for patients with advanced dementia. Nursing. 2022:52(3):19–26. [DOI] [PubMed] [Google Scholar]
- 3.2023 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 19(4):1598–1695. [DOI] [PubMed] [Google Scholar]
- 4. Li X, Feng X, Sun X, Hou N, Han F, Liu Y. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2019. Front Aging Neurosci. 2022:14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M. Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomedicine. 2019:14:5541–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodríguez-Gómez JA, Kavanagh E, Engskog-Vlachos P, Engskog MKR, Herrera AJ, Espinosa-Oliva AM, Joseph B, Hajji N, Venero JL, Burguillos MA. Microglia: agents of the CNS pro-inflammatory response. Cells. 2020:9(7):1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmed F, Urooj A. Traditional uses, medicinal properties, and phytopharmacology of Ficus racemosa: a review. Pharm Biol. 2010:48(6):672–681. [DOI] [PubMed] [Google Scholar]
- 8. Rani A, Zia-Ul-Sabah, Tabassum F, Sharma AK. Molecular interplay between phytoconstituents of Ficus Racemosa and neurodegenerative diseases. Eur J Neurosci. 2024:59(7):1833–1847. [DOI] [PubMed] [Google Scholar]
- 9. Sumi SA, Siraj MA, Hossain A, Mia MS, Afrin S, Rahman MM. Investigation of the key pharmacological activities of Ficus racemosa and analysis of its major bioactive polyphenols by HPLC-DAD. Evid Based Complement Alternat Med. 2016:2016:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kumar V, Sharma AK, Rajput SK, Pal M, Dhiman N. Pharmacognostic and pharmacological evaluation of Eulaliopsis binata plant extracts by measuring in vitro/in vivo safety profile and anti-microbial potential. Toxicol Res (Camb). 2018:7(3):454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chigurupati S, Abdul Rahman Alharbi N, Sharma AK, Alhowail A, Vardharajula VR, Vijayabalan S, das S, Kauser F, Amin E. Pharmacological and pharmacognostical valuation of Canna indica leaves extract by quantifying safety profile and neuroprotective potential. Saudi J Biol Sci. 2021:28(10):5579–5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma AK, Mukherjee M, Kumar A, Sharma G, Tabassum F, Akhtar MS, Imam MT, Almalki ZS. Preliminary investigation on impact of intergenerational treatment of resveratrol endorses the development of “super-pups”. Life Sci. 2023:314:121322. [DOI] [PubMed] [Google Scholar]
- 13. Sharma AK, Kumar A, Kumar S, Mukherjee S, Nagpal D, Nagaich U, Rajput SK. Preparation and therapeutic evolution of: Ficus benjamina solid lipid nanoparticles against alcohol abuse/antabuse induced hepatotoxicity and cardio-renal injury. RSC Adv. 2017:7(57):35938–35949. [Google Scholar]
- 14. Shivam K, Selvam A, Sangam S, Majood M, Pahari S, Patra R, Sharma AK, Mukherjee M. Graphene quantum dots-hybrid hydrogel as an avant-Garde biomimetic scaffold for diabetic wound healing. Biomater Adv. 2023:149:213395. [DOI] [PubMed] [Google Scholar]
- 15. Chikara P, Deep A, Bansal N, Kumar S, Bansal S, Sharma AK, Singh I, Marwaha RK. Phytochemical screening and biological potential of Agave angustifolia haw. Leaves extract as antioxidant and anticancer agents. Curr Cancer Ther Rev. 2023:19(1):58–66. [Google Scholar]
- 16. Deshmukh MA, Theng MA. Phytochemical screening, quantitative analysis of primary and secondary metabolites of acacia arabica bark. Int J Curr Pharm Res. 2018:10(2):35–37. [Google Scholar]
- 17. Dubale S, Kebebe D, Zeynudin A, Abdissa N, Suleman S. Phytochemical screening and antimicrobial activity evaluation of selected medicinal plants in Ethiopia. J Exp Pharmacol. 2023:15:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ralte L, Khiangte L, Thangjam NM, Kumar A, Singh YT. GC-MS and molecular docking analyses of phytochemicals from the underutilized plant, Parkia timoriana revealed candidate anti-cancerous and anti-inflammatory agents. Sci Rep. 2022:12(1):3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997:23(1–3):3–25. [DOI] [PubMed] [Google Scholar]
- 20. Sie YY, Chen LC, Li CJ, Yuan YH, Hsiao SH, Lee MH, Wang CC, Hou WC. Inhibition of acetylcholinesterase and amyloid-β aggregation by Piceatannol and Analogs: assessing In vitro and In vivo impact on a murine model of scopolamine-induced memory impairment. Antioxidants. 2023:12(7):1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turner RT, 3rd, Hong L, Koelsch G, Ghosh AK, Tang J. Structural locations and functional roles of new subsites S5, S6, and S7 in memapsin 2 (beta-secretase). Biochemistry. 2005:44(1):105–112. [DOI] [PubMed] [Google Scholar]
- 22. Zhou R, Yang G, Guo X, Zhou Q, Lei J, Shi Y. Recognition of the amyloid precursor protein by human g-secretase. Science (1979). 2019:363(6428):0930. [DOI] [PubMed] [Google Scholar]
- 23. Practical Ph’cogonosy - Vallabh Prakashan. Delhi, India: Vallabh Prakashan. https://www.vallabhprakashan.com/prpcogckk.aspx. Accessed 2024 May 26.
- 24. Kancherla N, Dhakshinamoothi A, Chitra K, Komaram RB. Preliminary analysis of Phytoconstituents and evaluation of anthelminthic property of Cayratia auriculata (In vitro). Maedica (Bucur). 2019:14(4):350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sai K, Thapa R, Devkota HP, Joshi KR. Phytochemical screening, free radical scavenging and α-amylase inhibitory activities of selected medicinal plants from western Nepal. Medicines. 2019:6(2):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ali S, Khan MR, Irfanullah, Sajid M, Zahra Z. Phytochemical investigation and antimicrobial appraisal of Parrotiopsis jacquemontiana (Decne) Rehder. BMC Complement Altern Med. 2018:18(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deep A, Narasimhan B, Aggarwal S, Kaushik D, Sharma AK. Thiophene scaffold as prospective central nervous system agent: a review. Cent Nerv Syst Agents Med Chem. 2016:16(2):158–164. [DOI] [PubMed] [Google Scholar]
- 28. Malhotra B, Kulkarni GT, Dhiman N, Joshi DD, Chander S, Kharkwal A, Sharma AK, Kharkwal H. Recent advances on Berberis aristata emphasizing berberine alkaloid including phytochemistry, pharmacology and drug delivery system. J Herb Med. 2021:27:100433. [Google Scholar]
- 29. Sharma AK, Taneja G, Khanna D, Rajput SK. Reactive oxygen species: friend or foe? RSC Adv. 2015:5(71):57267–57276. [Google Scholar]
- 30. Sidhic J, George S, Alfarhan A, Rajagopal R, Olatunji OJ, Narayanankutty A. Phytochemical composition and antioxidant and anti-inflammatory activities of Humboldtia sanjappae Sasidh. & Sujanapal, an endemic medicinal plant to the western Ghats. Molecules. 2023:28(19):6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Albadrani HM, Chauhan P, Ashique S, Babu MA, Iqbal D, Almutary AG, Abomughaid MM, Kamal M, Paiva-Santos AC, Alsaweed M, et al. Mechanistic insights into the potential role of dietary polyphenols and their nanoformulation in the management of Alzheimer’s disease. Biomed Pharmacother. 2024:174:116376. [DOI] [PubMed] [Google Scholar]
- 32. Campora M, Francesconi V, Schenone S, Tasso B, Tonelli M. Journey on naphthoquinone and Anthraquinone derivatives: new insights in Alzheimer’s disease. Pharmaceuticals (Basel). 2021:14(1):1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu S, Wang M, Xiao H, Ye J, Cao L, Li W, Sun G. Advancements in research on the effects of panax notoginseng saponin constituents in ameliorating learning and memory disorders. Heliyon. 2024:10(7):e28581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Siddiqui N, Saifi A, Chaudhary A, Tripathi PN, Chaudhary A, Sharma A. Multifaceted neuroprotective role of Punicalagin: a review. Neurochem Res. 2024:49(6):1427–1436. [DOI] [PubMed] [Google Scholar]
- 35. Choi GY, Kim HB, Hwang ES, Park HS, Cho JM, Ham YK, Kim JH, Mun MK, Maeng S, Park JH. Naringin enhances long-term potentiation and recovers learning and memory deficits of amyloid-beta induced Alzheimer’s disease-like behavioral rat model. Neurotoxicology. 2023:95:35–45. [DOI] [PubMed] [Google Scholar]
- 36. Hur JY. γ-Secretase in Alzheimer’s disease. Exp Mol Med. 2022:54(4):433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vassar R, Kovacs DM, Yan R, Wong PC. The beta-secretase enzyme BACE in health and Alzheimer’s disease: regulation, cell biology, function, and therapeutic potential. J Neurosci. 2009:29(41):12787–12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mercola J, D’Adamo CR. Linoleic acid: a narrative review of the effects of increased intake in the standard American diet and associations with chronic disease. Nutrients. 2023:15(14):3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dar KB, Parry RA, Bhat AH, Beigh AH, Ahmed M, Khaja UM, Ganie AH, Mir MA, Reshi BA, Khan IS, et al. Immunomodulatory efficacy of Cousinia thomsonii C.B. Clarke in ameliorating inflammatory cascade expressions. J Ethnopharmacol. 2023:300:115727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.