Abstract
BACKGROUND
Girls with Turner syndrome (TS) lack a partial or complete sex chromosome, which causes an accelerated decline of their ovarian reserve. Girls have to deal with several dilemmas related to their fertility, while only a limited number of them are referred to a fertility specialist and counselled about options of family planning on time.
OBJECTIVE AND RATIONALE
This scoping review provides an update of the literature on fertility in girls with TS throughout their lifespan and aims to propose a clinical practice guideline on fertility in TS.
SEARCH METHODS
Databases of PubMed, Embase, and Web of science were searched using the following key terms: Turner syndrome, fertility, puberty, pregnancy, sex-hormones, karyotype, fertility preservation, assisted reproductive techniques, and counselling, alongside relevant subject headings and synonymous terms. English language articles published since 2007 were critically reviewed. Pregnancies after using donated oocytes and data about girls with TS with Y-chromosomal content were excluded.
OUTCOMES
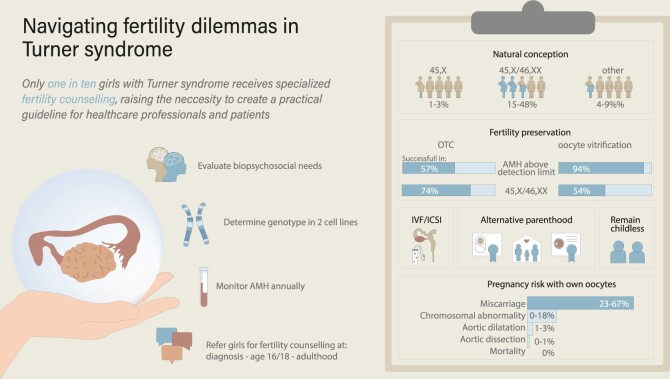
This search identified 1269 studies of which 120 were extracted for the review. The prevalence of natural conception ranged from 15% to 48% in women with 45,X/46,XX, 1% to 3% in women with 45,X, and 4% to 9% in women with other TS karyotypes. When assessing a girl’s fertility potential, it was crucial to determine the karyotype in two cell lines, because hidden mosaicism may exist. In addition to karyotype, assessment of anti-Müllerian hormone (AMH) played a significant role in estimating ovarian function. Girls with AMH above the detection limit were most likely to experience spontaneous thelarche, menarche, and ongoing ovarian function during the reproductive lifespan. Fertility preservation became more routine practice: vitrification of oocytes was reported in 58 girls with TS and a median of five oocytes were preserved per stimulation. Ovarian tissue cryopreservation has demonstrated the presence of follicles in approximately 30% of girls with TS, mostly in girls with mosaic-TS, spontaneous puberty, and AMH above the detection limit. Although girls and their parents appreciated receiving counselling on fertility in TS, only one in ten girls with TS received specialized counselling. Unfamiliarity with fertility preservation techniques or uncertainties regarding the eligibility of a girl for fertility preservation constituted barriers for healthcare professionals when discussing fertility with girls with TS.
WIDER IMPLICATIONS
There currently is a high demand for fertility preservation techniques in girls with TS. A reliable prognostic model to determine which girls with TS might benefit from fertility preservation is lacking. Only a minority of these girls received comprehensive fertility counselling on the full spectrum of fertility, including uncertainties of fertility preservation, pregnancy risks, and alternatives, such as adoption. Fertility preservation could be a viable option for girls with TS. However, the question remains whether enough oocytes can be obtained for a realistic prospect of a live birth. It is important that girls and parents are empowered with the necessary information to make a well-informed decision.
Keywords: Turner syndrome, puberty, fertility, fertility preservation, pregnancy, assisted reproduction, counselling
Graphical Abstract
Graphical Abstract.
To guide girls with Turner syndrome with their fertility dilemmas, we recommended that their fertility potential is assessed and that they receive counselling on every available option of family planning at an early age. AMH, anti-Müllerian hormone; OTC, ovarian tissue cryopreservation; TS, Turner syndrome
Introduction
The majority of girls and women with Turner syndrome (TS) face a challenging journey regarding their fertility, with infertility often becoming a certainty (Sutton et al., 2005). The partial or complete absence of one sex chromosome results in an accelerated loss of ovarian reserve at an early age. Consequently, women with TS have to make difficult decisions about family planning and are confronted with insecurities about their fertility and ability to conceive (Schleedoorn et al., 2020; Falsey et al., 2022; Fearon, 2023a). In addition, women with TS are at higher risk of pregnancy complications (Bernard et al., 2016; Calanchini et al., 2020).
Over the past decades, there has been an increasing request from parents of girls with TS to discuss fertility and fertility preservation options. Accordingly, this led to a growing research interest in fertility preservation in TS, including oocyte vitrification and ovarian tissue cryopreservation (OTC), and the emergence of new data (Brouillet et al., 2023; Nadesapillai et al., 2023b). However, there are still research gaps that remain, since it is extremely difficult to predict which girls will be diagnosed with premature ovarian insufficiency (POI) at a very young age, and which girls will conceive naturally in the future. This poses a significant challenge for medical professionals in providing fertility counselling, especially since there is no consensus on how to address this topic and on how to determine which girls with TS should or should not receive fertility preservation. As a result, only a minority of girls with TS are referred for counselling about options for family planning (Morgan et al., 2019).
This comprehensive scoping review provides an update of recent literature on clinical practice. This review aims to equip healthcare professionals with an overview of original research to enable a well-informed counselling process about fertility in TS. We cover five key topics: understanding fertility potential; chance of natural conception; understanding fertility potential; fertility preservation (vitrification of oocytes and OTC); assisted reproduction with own oocytes; and counselling on fertility.
Methods
This scoping review includes studies regarding women diagnosed with TS and includes topics related to puberty, fertility, fertility preservation, assisted reproduction with own oocytes, pregnancy risks, and counselling. Studies published in English between 1 January 2007 and 1 September 2023 were included.
Protocol
A scoping review was used to establish all types of available evidence including medical, social, cultural and ethical aspects, and to identify any research gaps (Munn et al., 2018). To present the literature in a comprehensive manner, a systematic approach was employed to assess and organize the literature into tables. Quantitative studies, qualitative studies, as well as expert opinions were included. The PRISMA extension for scoping reviews checklist (PRISMA-ScR; Tricco et al., 2018) was used to develop the protocol. The protocol, including eligibility criteria and study selection, was predefined. The protocol can be provided upon request.
Search strategy
The electronic databases of PubMed, Embase, and Web of Science were searched for eligible publications. An experienced librarian supported during the search process. The final search was performed on 1 September 2023. The full electronic search is available in Supplementary Data File S1.
Screening and selection
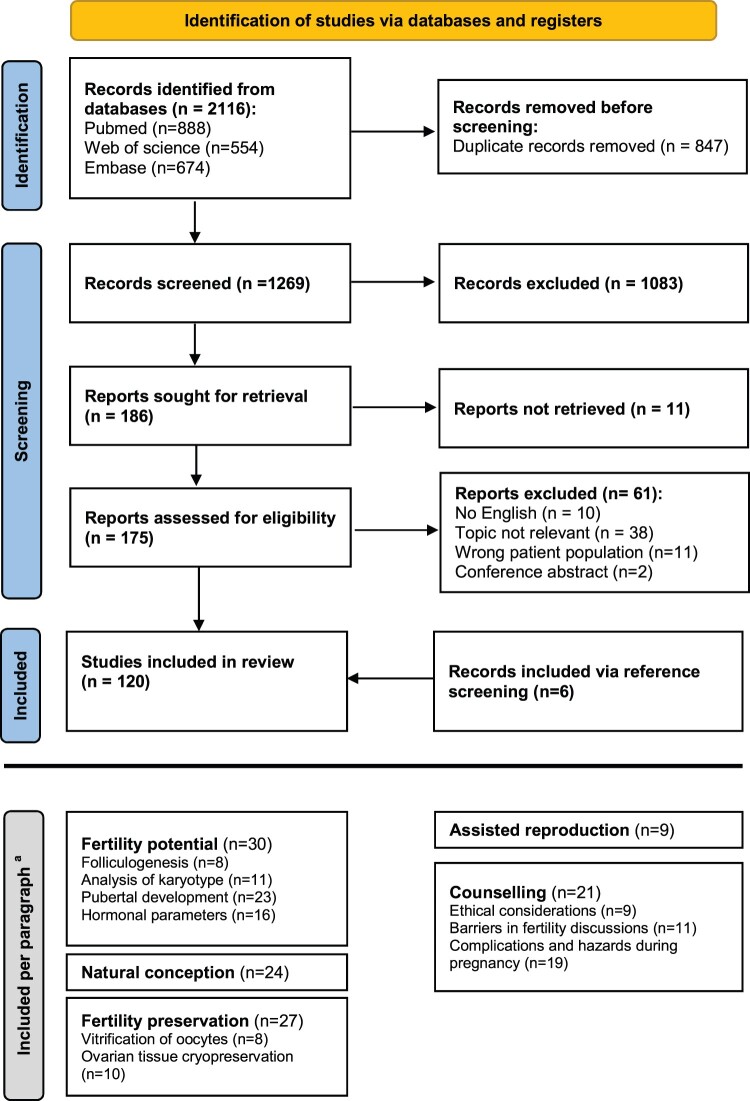
Publications derived from the electronic databases were transferred to EndNote version 20.3, Clarivate, Philadelphia, PA. After the removal of duplicates, publications were exported to Covidence systematic review software, Veritas Health Innovation, Melbourne Australia. All studies were independently screened for title and abstract by two authors (S.v.d.C. and J.v.d.V., K.F. or S.N.). Conflicts were discussed with a third author. The same process was repeated for full-text assessment (S.v.d.C. and K.F.). Reviews and conference abstracts were excluded for data extraction. Data about individuals with Y-chromosomal material or a small deletion (distal to Xq24) were excluded. Reviews were retained for reference screening. The PRISMA-ScR flow chart visualizes the study selection process (Fig. 1).
Figure 1.
PRISMA flowchart for scoping reviews. PRISMA 2020 flow diagram for new systematic reviews, which included searches of databases and registers only. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: n71. doi: 10.1136/bmj.n71. aSome studies are included in multiple paragraphs.
Data analysis
Studies included for data extraction were exported to a predefined data-charting form in Excel. The data-charting form was designed by the first author (S.v.d.C.), with input from the other authors, and tested within the research team. Predetermined themes were assigned by the research team (headings in results section) and divided into subthemes (subheadings in results section) based on the available literature.
After analysis of the literature, women with TS were stratified into three groups according to their genotype: monosomy 45,X, mosaicism 45,X/46,XX or ‘other’ karyotype. The group ‘other’ karyotypes included women with structural aberration of the X-chromosome or women with mosaicism with 47,XXX. Karyotypes were determined in lymphocytes unless otherwise described.
To satisfy reproducibility, the following definitions were used: natural conception—pregnant without the help of assisted reproduction techniques (only studies that give clear statements of natural conception were included); spontaneous thelarche—girls with TS who experienced the onset of breast development of at least Tanner stage B2 (Marshall and Tanner, 1969) without induction with oestrogens; spontaneous menarche—girls with TS who experienced vaginal bleeding without oestrogen induction; and POI—girls or women with hypergonadotropic hypogonadism and/or with symptoms of ovarian insufficiency (e.g. no or stunted puberty, flushes or irregular menstrual cycle) before the age of 40 years.
Results
Figure 1 provides a schematic representation of the review process: 1269 articles were screened, of which 120 were eligible for data extraction.
Understanding fertility potential
The advancements in understanding folliculogenesis in women with TS has led to significant progress in comprehending the reproductive capacities. Likewise, various clinical parameters, including karyotype, pubertal development, and hormone levels, play a crucial role in estimating the fertility potential and ovarian reserve at different ages.
Folliculogenesis
The accelerated decline in the number of oocytes in women with TS becomes evident as early as foetal development, as indicated by an increased number of oocytes showing signs of apoptosis. Furthermore, a significantly reduced number of oogonia in the ovaries of girls with TS was observed (Modi et al., 2003; Reynaud et al., 2004). Research conducted over the past two decades suggested several mechanisms to explain this phenomenon. One hypothesis suggests that the inability of oocytes to undergo meiotic pairing during the prophase stage can be attributed to the absence of a sex chromosome (Burgoyne and Baker, 1985). Another hypothesis suggests that the lack of gap or tight junctions, which are crucial for interaction between oocytes and granulosa cells, impairs the formation of primordial follicles (Rogers et al., 1992). Additionally, it has been hypothesized that a reduced number of oogonial mitoses may contribute to the lower follicle numbers compared to women without TS (Gosden, 1995). Recent research has provided more information on folliculogenesis in women with TS, including follicle density, morphology, and chromosomal content.
Reduced follicle density was observed in the ovaries of foetuses with TS with gestational age of 12–42 weeks. In 22/24 45,X-foetuses and 2/5 45,X/46,XX foetuses, no follicles were found, while numerous follicles were found in all control ovaries (Lundgaard Riis et al., 2021). After birth, the follicle density was also significantly lower in ovaries of prepubertal girls with TS (131–2121 follicles/mm3) and peri- and post-pubertal girls with TS (50–872 follicles/mm3) compared to age-matched controls (respectively, 2946–7401 and 1553–2045 follicles/mm3) (Peek et al., 2023). Other studies confirmed the reduced follicle density in the ovarian cortex of women with TS (Borgström et al., 2009; Peek et al., 2019, 2023; Schleedoorn et al., 2022; Nadesapillai et al., 2023b). In contrast, Mamsen et al. (2019) observed that 7/9 girls with TS with follicles in the ovarian cortex had a follicle density within 95% CI of age-matched controls, although the CI was broad and not specified. To note, when assessing histology sections, it is important to consider that follicles are not evenly distributed in the ovary. Since only a very small ovarian cortex fragment is often used to determine the number of follicles, this might cause misinterpretation of the total ovarian reserve (Lambalk et al., 2004).
In addition to the reduced numbers of follicles, an abnormal morphology is often observed. Abnormal morphology affected 31% of follicles of girls with TS, which was significantly higher compared to individuals without TS with 12% abnormal follicles (Peek et al., 2023). The abnormal morphology of follicles included an incomplete or distorted layer of granulosa cells (Mamsen et al., 2019; Nadesapillai et al., 2023b), partially missing connection to the basal lamina (Mamsen et al., 2019), a less clear structure of follicles, and darker and smaller oocytes (Lundgaard Riis et al., 2021). Abnormal morphology of follicles was observed more frequently in girls with a structural aberration of the X-chromosome compared to girls with TS without a structural aberration (Schleedoorn et al., 2022; Nadesapillai et al., 2023b).
The chromosomal content of primordial follicles of 17 girls with mosaic-TS contained a normal X-chromosomal content in 95% of oocytes (143/150), while in granulosa and stromal cells high levels of aneuploidy were observed, respectively 20–100% and 3–95% (Peek et al. 2019, 2023). Similar observations were described in a case report of a girl with 45,X karyotype and a hidden mosaicism in the ovarian cells (Nadesapillai et al., 2021b). In line with these studies, Balen et al. (2010) published a case report showing that all retrieved oocytes had a normal karyotype. Nevertheless, it is imperative to acknowledge that these oocytes have undergone a process of selection by ovarian hyperstimulation, which might lead to a higher probability of a normal karyotype.
Considering the abnormal morphology and aneuploidy of granulosa and stromal cells, the developmental potential of small follicles could be impaired. The capacity of primordial follicles in girls with TS to complete folliculogenesis was explored by grafting ovarian cortex tissue of girls with mosaic-TS in immunocompromised mice (Peek et al., 2023). This study established that primordial follicles of cryopreserved-thawed ovarian cortex tissue of girls with mosaic-TS were indeed capable of developing into antral follicles. Interestingly, with the expansion of the granulosa cell layer during folliculogenesis, the percentage of aneuploid granulosa cells decreased considerably during folliculogenesis, resulting in a higher proportion of 46,XX granulosa cells in secondary and antral follicles.
Analysis of karyotype
The diagnosis TS is routinely confirmed by karyotyping of 20 lymphocytes. However, Graff et al. (2020) compared the conventional karyotyping in 20 lymphocytes with FISH on 200 buccal cells. The results showed a hidden mosaicism in 8/40 women with 45,X-TS (20%). Similar results were found in other studies where different karyotypes or ratios in karyotype were identified in the analysis of multiple cell lines (Brambila-Tapia et al., 2009; Freriks et al., 2013; Castronovo et al., 2014; Ros et al., 2014; Peek et al., 2019, 2023; Schleedoorn et al., 2022; Nadesapillai et al., 2023b).
These studies indicate that genotyping of two cell lines provides a more accurate risk assessment of POI (Nadesapillai et al., 2021a). However, despite the analysis of the genotype in lymphocytes and buccal cells, the genotype in ovarian cells can still be different (Nadesapillai et al., 2021b).
Pubertal development
Girls with a 45,X/46,XX karyotype had the highest prevalence of spontaneous thelarche (69%), while spontaneous thelarche was reported in only 18% of girls with a monosomy 45,X and in 42% of girls with ‘other’ karyotypes (Table 1). According to a descriptive study by Hankus et al. (2018) involving 110 girls with TS, girls with 45,X were two times less likely to experience spontaneous thelarche compared to girls with a non-45,X karyotype. The median age of onset of spontaneous thelarche varied between 9 and 14 years (Tanaka et al., 2015; Hamza et al., 2018; Hankus et al., 2018; Wu and Li, 2019; Ruszała et al., 2020; Witkowska-Krawczak et al., 2023).
Table 1.
Overview of studies reporting on spontaneous thelarche and menarche in girls with Turner syndrome.
| Study type | Authors | Spontaneous thelarche n(%) |
Spontaneous menarche n(%) |
Karotype analysis a | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 45, X | 45,X/46,XX | Other | Total | 45, X | 45, X/46, XX | Other | ||||
| Retrospective cohort | Lau et al. (2009) | 6/28 (21%) | 1/13 (8%) | 5/9 (56%) | 0/6 (0%) | 4/28 (14%) | 0/13 (0%) | 4/9 (44%) | 0/6 (0%) | NR | |
| Hagen et al. (2010b) | 15/44 (34%) | 1/18 (5%) | 8/9 (89%) | 6/17 (35%) | NR | NR | NR | NR | ≥10 | ||
| Carpini et al. (2012) | 16/32 (50%) | 0/12 (0%) | 10/10 (100%) | 6/10 (60%) | 6/32 (19%) | NR | NR | NR | 50 | ||
| Castronovo et al. (2014) | NR | NR | NR | NR | 6/40 (15%) | 2/31 (6%) | 4/5 (80%) | 0/4 (0%) | ≥30 | ||
| Negreiros et al. (2014) | 52/116 (45%) | 12/54 (22%) | 31/50 (62%) | 9/12 (75%) | 19/52 (37%) | 3/12 (25%) | 16/31 (52%) | 0/9 (0%) | NR | ||
| Lunding et al. (2015) | 35/117 (30%) | 3/48 (6%) | 15/23 (65%) | 17/46 (37%) | 29/117 (32%) | 2/48 (5%) | 15/23(65%) | 12/46 (26%) | ≥10 | ||
| Tanaka et al. (2015) | 77/212 (36%) | 13/64 (20%) | 17/28 (61%) | 47/120 (39%) | 31/212 (15%) | 4/64 (6%) | 13/28 (46%) | 14/120 (12%) | NR | Mosaic included other numerical TS karyotypes. | |
| Bucerzan et al. (2017) | 13/30 (43%) | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Maggio et al. (2018) | 3/19 (16%) | NR/7 | NR/1 | NR/11 | 3/19 (16%) | NR | NR | NR | NR | ||
| Hankus et al. (2018) | 53/110 (48%) | 19/61 (31%) | 9/11 (81%) | 25/38 (66%) | 22/110 (20%) | 6/61 (10%) | NR | NR | ≥10 | ||
| Ruszała et al. (2020) | 16/35 (46%) | 2/12 (16%) | NR | NR | NR | NR | NR | NR | NR | Subgroup non-45, X included 45, X/46, XX, and ‘other’. | |
| Wang et al. (2023) | 16/66 (24%) | 6/28 (21%) | 7/12 (58%) | 3/26 (12%) | NR | NR | NR | NR | ≥10 | ||
| Witkowska-Krawczak et al. (2023) | 19/34 (56%) | 2/11 (18%) | NR | NR | 16/31 (52%) | 2/11 (18%) | NR | NR | ≥20 | Subgroup non-45, X included 45, X/46, XX, and ‘other’. | |
|
| |||||||||||
| Case control | Visser et al. (2013) | 22/41 (54%) | NR | NR | NR | 8/47 (17%) | NR | NR | NR | ≥30 | |
| Hamza et al. (2018) | 7/21 (33%) | NR | NR | NR | 5/21 (23%) | NR | NR | NR | NR | ||
|
| |||||||||||
| Observational | Borgström et al. (2009) | 19/49 (39%) | 3/22 (14%) | 5/6 (83%) | 11/21 (52%) | 13/49 (26%) | 2/22 (9%) | 4/6 (67%) | 7/21 (23%) | 15–105 | |
| Nadesapillai et al. (2023b) | 25/52 (48%) | 1/14 (7%) | 15/20 (75%) | 9/18 (50%) | 8/52 (15%) | 0/14 (0%) | 8/20 (40%) | 0/18 (0%) | 30 | Additional genotyping on 100 buccal cells with FISH analysis. | |
|
| |||||||||||
| Total (%) | 394/1006 (39%) | 63/357 (18%) | 122/178 (69%) | 133/314 (42%) | 170/810 (21%) | 21/276 (8%) | 64/122 (52%) | 33/224 (15%) | |||
Number of lymphocytes used for conventional blood karyotyping.
NR, not reported; TS, Turner syndrome.
Spontaneous menarche occurred in 52% of girls with mosaicism 45,X/46,XX, compared to 8% in girls with monosomy 45,X and 15% in girls with ‘other’ karyotypes (Table 1). Girls with 45,X were three times less likely to experience spontaneous menarche compared to girls with non-45,X karyotypes (Hankus et al., 2018). Despite initial expectations of a correlation between karyotype and the occurrence of menarche, subsequent investigations by Castronovo et al. (2014) and Aso et al. (2010) did not reveal statistically significant results. The median age of spontaneous menarche varied between 11 and 14.6 years (Aso et al., 2010; Homer et al., 2010; Carpini et al., 2012; NegreiRos et al., 2014; Lunding et al., 2015; Tanaka et al., 2015; Bucerzan et al., 2017; Hamza et al., 2018; Hankus et al., 2018; Wu and Li, 2019; Komura et al., 2021; Witkowska-Krawczak et al., 2023).
POI was reported in 7–8% of girls with 45,X/46,XX, in 100% of girls with 45,X and in 47–67% of girls with TS with ‘other’ karyotypes aged younger than 21 years (Lunding et al., 2015; Fitz et al., 2021). The majority of studies reported that 77–100% of girls with monosomy 45,X required induction of puberty (Aso et al., 2010; Hagen et al., 2010b; Carpini et al., 2012; NegreiRos et al., 2014; Lunding et al., 2015; Wu and Li, 2019; Ruszała et al., 2020). A single retrospective study by Fitz et al. (2021) found a considerable variety in the time from the diagnosis of TS until POI (mean 4.9 years±SD 4.5 years). POI emerged at a median age of 15 years in girls with 45,X/46,XX. This was significantly younger in girls with 45,X, in which hypergonadotropic levels were seen at a median age of 10 years. Hence, an inverse correlation was observed between the ratio of aneuploidy and the age at POI. Specifically, there is an observed decrease of 0.09 year in the age of onset of POI for every 1% increase in the presence of 45,X-cells. Moreover, Mercer et al. (2013) described a small cohort of 20 girls with Xq deletion and mild phenotype of TS. They found a higher incidence of POI, at an earlier age, in girls with a larger deletion of the X-chromosome compared to girls with smaller deletions.
Hormonal parameters
Several hormonal parameters have been identified as useful markers of ovarian function in girls with TS. Among these, FSH, LH, and anti-Müllerian hormone (AMH) are the most used. These hormones can provide valuable information during different stages of development, from mini puberty in infancy to puberty and thereafter.
Mini puberty is the activation of the hypothalamic–pituitary–gonadal axis during the neonatal period. This results in a temporary increase of gonadotrophins and sex steroid levels, and mainly occurs in the first 3–6 months of life (Ljubicic et al., 2022). This time frame might provide a fundamental 6-month diagnostic window of the ovarian potential in girls with TS to enable early fertility counselling (Carpini et al., 2018). The study of Johannsen et al. (2018) compared the levels of FSH and LH during mini puberty of four girls with TS (aged 2–5 months) to 1840 girls without TS and found that FSH and LH concentrations of all four girls with TS were above the female reference range. AMH levels of seven girls with TS before the age of 5 years are described (Hagen et al., 2010a; Johannsen et al., 2018). Six girls had AMH below the detection limit (45,X n = 2, miscellaneous karyotype n = 4) and one girl with 45,X/46,XX had AMH within the reference range of girls without TS. Information about their pubertal development is not presented.
During mid-childhood (5–10 years old), the FSH concentration of 70 girls with TS remained within the reference range, even in girls who turned out to need puberty induction at a later stage. In these girls, the elevation of FSH did not become manifest until the expected age of pubertal development (Hagen et al., 2010b). Although FSH seems to have little predictive value during mid-childhood, two studies did measure difference in FSH in a different pubertal development. Aso et al. (2010) performed a descriptive study and demonstrated that if FSH remained below 10 IU/l until the age of 12 years, a menarche could be expected. In addition, according to the findings of Hankus et al. (2018), the probability of experiencing spontaneous menarche when FSH levels exceeded 6.7 IU/l at the age of 6–10 years, was observed to be reduced. In most girls, FSH remained low during childhood, and in these cases AMH appears to be the most reliable indicator for ovarian reserve (Hagen et al., 2010a; Purushothaman et al., 2010; Kallio et al., 2012; Visser et al., 2013; Lunding et al., 2015; Wang et al., 2023). Lunding et al. (2015) reported on pubertal development and AMH levels before pubertal onset in a longitudinal observational cohort study that included 15 girls with TS. In 4/5 girls with spontaneous thelarche, AMH was above the detection limit (>0.28 ng/ml). However, AMH was also detectable in 3/10 girls who needed induction of puberty (range 0.28–0.56 ng/ml). In the study of Hagen et al. (2010a) all girls with AMH <0.56 ng/ml did not develop spontaneous thelarche. Moreover, AMH-levels were correlated with karyotype, as described by Wang et al. (2023), who found significant higher levels of AMH in young girls with 45,X/46,XX aged 4–7 years, compared to girls with 45,X and ‘other’ karyotypes (median values respectively 1.13 ng/ml vs 0.02 and 0.04 ng/ml).
At pubertal age, AMH above the detection limit was associated with spontaneous thelarche (Visser et al., 2013; Lunding et al., 2015; Hamza et al., 2018; Ruszała et al., 2020; Wang et al., 2023). In the cross-sectional study of Hamza et al. (2018), higher AMH levels were found in seven girls with TS ≥12 years of age with spontaneous thelarche compared to 43 girls with TS with induced puberty (2.12 ± 0.76 ng/ml vs 1.01 ± 0.53 ng/ml) and found that the odds of spontaneous thelarche was 18.4 and the odds of spontaneous menarche was 37.5 if AMH was above the detection limit. Similar results were described by Visser et al. (2013), who reported that the odds of spontaneous thelarche was 19.3 if girls with TS ≥12 years had AMH above the detection limit. However, AMH below the detection limit does not necessarily rule out spontaneous thelarche, as described in some cases (Ruszała et al., 2020). This is substantiated by Visser et al. (2013) who described spontaneous thelarche in 38% of girls with TS with AMH below the detection limit, although low AMH (<0.42 ng/ml, Lunding et al., 2015; <0.7 ng/ml, Hagen et al., 2010a) was associated with POI and had a positive predictive value of 95% that POI would occur. The moment when POI might occur could not be predicted using AMH data in the study of Hagen et al. (2010a).
In addition to AMH, inhibin B is also described as a marker to predict ovarian reserve (Hagen et al., 2010b; Messina et al., 2015; Ruszała et al., 2020). Hagen et al. (2010b) reported that spontaneous puberty did not occur in 19/20 girls with TS with repeating inhibin B levels below the detection limit during childhood. In addition, spontaneous thelarche did occur in 9/10 girls with TS (2–16 years) with at least one inhibin B measurement above the detection limit. Levels of inhibin B decreased to below the detection limit in the year before POI, as presented in a case series of four girls with TS (Lunding et al., 2015). The conclusions drawn from these two studies underscore a strong correlation between inhibin B and ovarian function.
Summary
In contrast to FSH, LH or the genotype, which primarily represent probabilities, AMH stands out as a marker for assessing ovarian reserve owing to its direct reflection of the growing pool of follicles. Genotyping of two cell lines can enhance the predictability of ovarian decline.
Research gap: there is a demand for a longitudinal interpretation of ovarian reserve, especially in the prepubertal age group, as the level of AMH has a wide variation between girls and is subject to high inter-test variability.
Natural conception
Studies have reported a low prevalence of natural conception in women with TS (Table 2). Two retrospective cohort studies, Bernard et al. (2016) and Calanchini et al. (2020), found natural conception rates of 5.6% and 13%, respectively, with a mean age of 23–28 years. The prevalence was considerably lower in women with 45,X karyotype (1% and 3.2%) compared to women with 45,X/46,XX karyotype (15% and 48%) and women with other-TS karyotypes (4% and 9%). Although these results indicate the predictive value of karyotype for natural conception, the level of mosaicism appeared not to be predictive for the time to pregnancy, number of pregnancies, live birth or rate of miscarriage (Mortensen et al., 2010; Doğer et al., 2015; Bernard et al., 2016; Calanchini et al., 2020).
Table 2.
Overview of studies reporting on natural conception in women with Turner syndrome.
| Study type | Authors | N | Natural conception n (%) | Age at pregnancy (years)a | Pregnancies per karyotype n(%) | Pregnancies n (%) | Live birth n (%) |
Pregnancy complications (% of pregnancies) |
Karyotype analysis b | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MR | LBW | CA | PE | AD | Mor | |||||||||||
| Retrospective nulli vs primi | Bernard et al. (2016) | 480 | 27 (5.6%) | 27.5 (18–38) | 45, X | 2/181 (1%) | 52 | 30 | 16/52 (31%) | NR | 2/11 (18%) | 2/30 (7%) | 0/52 (0%) | 0/52 (0%) | NR | |
| 45, X/46, XX | 19/130 (15%) | |||||||||||||||
| Other | 6/169 (4%) | |||||||||||||||
| Calanchini et al. (2020) | 141 | 18 (13%) | 23.5 (15–31) | 45, X | 2/62 (3%) | NR | 37 | NR (48%) | 1/14 (7%) | 1/5 (20%) | 2/18 (11%) | 0 | NR | NR | Karyotype was known in 141 women. | |
| 45, X/46, XX | 11/23 (48%) | |||||||||||||||
| Other | 5/56 (9%) | |||||||||||||||
|
| ||||||||||||||||
| Retrospective cohort of pregnancies in TS | Bryman et al. (2011) | 482 | 23 (4.8%) | 27 (16–42) | 45, X | 1 | 82 | 37 (45%) | 37/82 (45%) | NR | 4/37 (11%) | NR | 1/82 (1%) | NR | 10–30 | Results include 4 women with ART. |
| 45, X/46, XX | 25 | |||||||||||||||
| Other | 1 | |||||||||||||||
| Hagman et al. (2011) | 909 | 115 (12.7%) | 30 (18–41) | 45, X | 10 | 124 | 208 | NR | NR | NR | 1/124 (1%) | 1/124 (1%) | 0/124 (0%) | <’94 10–25 ≥’95 ≥ 30 |
||
| 45, X/46, XX | 50 | |||||||||||||||
| Other | 52 | |||||||||||||||
| Unknown | 3 | |||||||||||||||
| Hadnott et al. (2011) | 276 | 5 (1.8%) | 25.6 ± 2.1 | 45, X | 2 | 7 | 7 | NR | 0 | 0 | 0 | 0 | 0 | 50 | ||
| 45, X/46, XX | – | |||||||||||||||
| Other | 3 | |||||||||||||||
| Doğer et al. (2015) | 22 | 11 | 23 (18–32) | 45, X | – | 52 | 17 (33%) | 35/52 (67%) | NR | 1/3 (33%) | NR | NR | NR | 100 | Results include 5 women with ART. | |
| 45, X/46, XX | 17 | |||||||||||||||
| Other | 5 | |||||||||||||||
| Cadoret et al. (2018) | NR | NR | 28.7 ± 6.8 | 45, X | – | 22 | 22 | 5/22 (23%) | NR | 0/22 (0%) | NR | NR | NR | NR | Only ongoing pregnancies are included. | |
| 45, X/46, XX | 16 | |||||||||||||||
| Other | 6 | |||||||||||||||
| Obata et al. (2020) | NR | 5 | NR | 45, X | – | NR | NR | NR | NR | NR | NR | NR | 0 | NR | Patient characteristics were not specified for mode of conception | |
| 45, X/46, XX | 2 | |||||||||||||||
| Other | 3 | |||||||||||||||
| Cauldwell et al. (2022a) | NR | NR | NR | 45, X | 9 | 73 | NR | NR | NR | NR | NR | 0 | NR | NR | Patient characteristics were not specified for mode of conception | |
| 45, X/46, XX | 53 | |||||||||||||||
| Other | 11 | |||||||||||||||
|
| ||||||||||||||||
| Case report | El-Shawarby and Steer (2007) | 1 | 36 | 45, X |
1 | NR | NR | NR | NR | NR | NR | NR | NR | Twin pregnancy. | ||
| Mortensen et al. (2010) | 2 | 28 | 45, X[199]/47, XXX[1] |
2 | 2 | NR | 0 | 0 | 0 | 0 | 0 | NR | Genotyping with FISH on 200 lymphocytes | |||
| 30 | 45, X[200] |
2 | 2 | |||||||||||||
| Alves and Silva (2012) | 1 | 25/28 | 45, X[28]/47, XXX[2] | 2 | 2 | No complications | 30 | |||||||||
| Mavridi et al. (2018) | 1 | 21 | 45, X[52%]/47, XXX[48%] | 1 | 1 | No complications | NR | |||||||||
| Strypstein et al. (2022) | 1 | 29 | 45, X[14%]/46, XX[86%] | 1 | 1 | No complications | NR | Genotyping with FISH on lymphocytes. | ||||||||
Nulli versus primigravida: women with TS who were never pregnant versus women with TS with natural pregnancy.
Mean±SD or Median (range).
Number of lymphocytes used for conventional blood karyotyping.
AD, aortic dissection; CA, congenital abnormalities; LBW, low birthweight; MR, miscarriage rate; Mor, mortality; N, number of cases; NR, not reported; PE, pre-eclampsia; TS, Turner syndrome.
Besides the predictive value of karyotype, a TS diagnosis at an older age or spontaneous menarche could indicate higher chances of natural conception. Bernard et al. (2016) described that women who were diagnosed with TS at an older age (median 20 vs 10 years) were more likely to conceive naturally, probably owing to a milder phenotype. Moreover, 93–100% of women with TS and natural conception experienced spontaneous menarche (Bernard et al., 2016; Calanchini et al., 2020). Pregnancy risks in women with TS are significantly increased compared to women without TS (Hagman et al., 2011; Bernard et al., 2016; Calanchini et al., 2020).
Summary
When counselling young girls on their chance for natural conception, the reported incidence rates of natural conception could be too optimistic. These rates are based on retrospective studies in which women with TS with a mild phenotype were also included and some even were diagnosed after a pregnancy.
Research gap: a prospective study on the incidence of natural conception in the general population of women with TS.
Fertility preservation
Current options for fertility preservation are vitrification of oocytes and OTC. The process of vitrification of oocytes involves ovarian hyperstimulation, frequent ultrasounds, and follicle aspiration, which is typically performed vaginally. The use of vitrified oocytes is widely applied and has a live birth rate of approximately 50% in women without TS aged ≤35 years (Cobo et al., 2016). Vitrification of oocytes is often offered to women with sufficient ovarian reserve (Oktay and Bedoschi, 2014; Talaulikar et al., 2019; Vergier et al., 2019). Girls have to be physically and mentally mature enough to undergo this process. OTC involves a laparoscopic resection of (part of) the ovary. OTC can be conducted at any age and prior to the onset of puberty. This technique has shown a live birth rate of 41% after transplantation of the cryopreserved-thawed ovarian tissue in women without TS (Dolmans et al., 2021).
Vitrification of oocytes
In total, 58 cases of women with TS and vitrification of oocytes are described in 14 retrospective studies and case reports (Table 3). Overall, the first cycle of ovarian hyperstimulation resulted in a median of five vitrified oocytes after follicle aspiration (range 0–21). The majority of women who vitrified at least one oocyte had a 45,X/46,XX karyotype (51%), a median age of 21 years and had experienced a spontaneous onset of thelarche (98%) and menarche (96%). In 94% of cases with at least one vitrified oocyte, an AMH above the detection limit and a FSH below 10 IU/l was observed. With the exception of a single case (Azem et al., 2020), all women with TS undergoing oocyte vitrification exhibited occurrences of spontaneous thelarche and menarche.
Table 3.
Overview of studies reporting on oocyte vitrification in girls with Turner syndrome.
| Study type | Author | N | Procedure | Age (years) | Karyotype in % (number of cells analysed) | Spontaneous thelarche | Spontaneous menarche | AMH (ng/ml) | FSH (IU/l) | AFC | oocytes retrieved | MII-oocytes a vitrified | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retrospective cohort | Oktay and Bedoschi (2014); Oktay et al. (2010) | 3 | Antagonist | 13 | 45, X[90]/47, XXX[10] (30) | Yes | Yes | 1.59 | 5.7 | 6 | 19 | 9 | +1 MII-oocyteb vitrified after IVM |
| 14 | 45, X[45]/46, XX[55] (20) | Yes | Yes | 0.9 | 5.3 | 12 | 11 | 8 | |||||
| 1.7 | 7 | 4 | |||||||||||
| 13 | 45, X[20]/46, XX[80] (20) | Yes | Yes | 0.76 | 5.6 | 6 | 16 | 7 | +5 MII-oocyteb vitrified after IVM | ||||
| Talaulikar et al. (2019) | 7 | Antagonist | 22 | 45, X[100] (NR) | Yes | Yes | 0.49 | 6.9 | 7 | 9 | 6 | ||
| 18 | 45, X[83]/46, XX[17] (NR) | Yes | Yes | 0.43 | 3.2 | 9 | 13 | 8 | |||||
| 18 | 45, X[61]/46, XX[39] (NR) | Yes | Yes | 0.98 | 7.4 | 11 | 9 | 6 | |||||
| 25 | 45, X/46, XX/47, XXX (NR) | Yes | Yes | 1.33 | 2.9 | 12 | 10 | 10 | |||||
| 21 | 45, X[63]/46, XX[37] (NR) | Yes | Yes | 0.49 | 6.2 | 5 | 4 | 4 | |||||
| 22 | 45, X[50]/46, XX[50] (NR) | Yes | Yes | 2.98 | 8.4 | 14 | 6 | 6 | |||||
| 26 | 45, X[88]/46, XX[12] (NR) | Yes | Yes | 1.69 | 7.5 | 11 | 12 | 10 | In two stimulation cycles | ||||
| Vergier et al. (2019) | 3 | Antagonist | 28 | 46, XX del(Xp) (NR) | Yes | Yes | 0.50 | 10 | 6 | 3 | 2 | ||
| 3 | 2 | ||||||||||||
| 7 | 5 | ||||||||||||
| 25 | 45, X0 [100] (100) | Yes | Yes | 8.81 | 6.5 | 18 | 20 | 17 | |||||
| 18 | 46, X[83]/45, X[9]/46, XderX[8] (NR) | Yes | Yes | 13.30 | 5.9 | PCOS | 25 | 20 | |||||
| Martel et al. (2022) | 13 | Antagonist | 13 | Mosaic | NR | Yes | 2.99 | 5.2 | NR | 14 | 12 | ||
| 14 | Turner syndrome | NR | No | <0.16 | 0.4 | NR | 4 | 2 | |||||
| 14 | Mosaic | NR | Yes | 2.08 | 4.5 | NR | 21 | 16 | |||||
| 14 | Turner syndrome | Yes | Yes | 0.03 | 20.6 | NR | 0 | – | Three attempts | ||||
| 15 | Turner syndrome | NR | NR | NR | 3.9 | NR | 0 | – | Two attempts | ||||
| 15 | Turner syndrome | NR | NR | NR | 0.2 | NR | 15 | 15 | |||||
| 15 | Mosaic | NR | Yes | <0.003 | 1.8 | NR | 0 | – | |||||
| 16 | Mosaic | Yes | Yes | 1.63 | 0.5 | NR | 18 | 16 | In two stimulation cycles | ||||
| 17 | Mosaic | NR | Yes | NR | 1.1 | NR | 3 | 0 | Two attempts | ||||
| 18 | Mosaic | NR | NR | NR | <0.1 | NR | 0 | – | Two attempts | ||||
| 21 | Mosaic | NR | Yes | 2.6 | 6.5 | NR | 22 | 12 | |||||
| 21 | Turner syndrome | NR | Yes | <0.03 | 9.3 | NR | 2 | 0 | Two attempts | ||||
| 21 | Mosaic | Yes | Yes | 5.34 | 5.4 | NR | 19 | 14 | |||||
| Brouillet et al. (2023) | 14 | Antagonist | 15 | 45, X[40]/47, XXX[60] (NR) | Yes | Yes | 0.06 | 11 | NR | 0 | – | ||
| 16 | 45, X[5]/46, X, del(X)[95] (NR) | Yes | Yes | 0.4 | 8.9 | 2 |
|
|
|||||
| 16 | 45, X[6]/46, XX[94] (NR) | No | NR | 1.29 | 4.6 | 8 |
|
|
|||||
| 17 | 45, X[18]/46, XX[82] (NR) | Yes | Yes | 1.16 | 3.4 | 6 | 11 | 11 | |||||
| 17 | 45, X[36]/46, XX[64] (NR) | Yes | Yes | 0.85 | 6.4 | 20 | 5 | 5 | |||||
| 18 | 45, X[50]/46, XX[50] (NR) | Yes | Yes | 0.89 | 7.4 | 9 |
|
|
|||||
| 18 | 45, X[89]/47, XXX[11] (NR) | Yes | Yes | 0.54 | 7.6 | 3 | 0 | – | |||||
| 22 | 45, X[83]/47, XXX[17] (NR) | Yes | Yes | 0.6 | 6.3 | 4 |
|
|
|||||
| 22 | 45, X[90]/47, XXX[10] (NR) | Yes | Yes | 0.26 | 20.2 | 9 |
|
|
|||||
| 22 | 45, X[39]/47, XXX[61] (NR) | Yes | Yes | 0.58 | 10.3 | 8 | 2 | 2 | |||||
| 24 | 45, X[95]/46, XX[1]/47, XXX[4] (NR) | Yes | Yes | 2.3 | NR | 12 | 8 | 4 | |||||
| 24 | 45, X[30]/46, XX[70] (NR) | Yes | Yes | NR | NR | 8 |
|
|
|||||
| 30 | 45, X[30]/46, XX[70] (NR) | Yes | Yes | 3.7 | 5.9 | 40 | 9 | 9 | |||||
| 35 | 45, X[32]/46, XX[68] (NR) | Yes | Yes | 1.97 | 7.2 | 15 |
|
|
|||||
| Rodriguez-Wallberg et al. (2023) | 10 | NR | 14–22 | 10/10 | 10/10 | 1.1 ± 1.0 (0.3–3.2) | 5.1 ± 5.4 (0–19) per cycle |
|
|||||
|
| |||||||||||||
| Case report | Kavoussi et al. (2008) | 1 | Antagonist | 28 | Mosaic | NR | NR | NR | 4.3 | 40 | 15 | 13 | |
| Lau et al. (2009) | 1 | Agonist | 16 | 45, X[98]/47, XXX[2] (NR) | Yes | Yes | NR | 6.3 | 6 | 2 | 2 | ||
| Balen et al. (2010) | 1 | Antagonist | 28 | 45, X[93]/46, XX[7] (30) | Yes | Yes | 6.13 | 3.3 | NR | 12 | 9 | Oocytes used for FISH analysis | |
| 10 | 7 | ||||||||||||
| 30 | NA | Embryo preservation, resulting in 13 embryos | |||||||||||
| El-Shawarby et al. (2010) | 1 | Flare-up agonist | 22 | 45, X[86]/47, XXX[11]/46, XX[3] (NR) | Yes | Yes | 1.19 | 4.6 | 7 | 8 | 8 | ||
| Ito et al. (2020) | 1 | Antagonist Duostim | 23 | 45, X [13]/46, XX [87] (30) | Yes | Yes | 3.43 | 10.6 | 3 | 10 | 9 | ||
| 9 | 8 | ||||||||||||
| Azem et al. (2020) | 1 | Antagonist | 7 | 45, X[71]/47, XXX[29] (52) | Prepubertal | Prepubertal | 1.13 | 5.2 | 5 | 0 b | – | ||
| 3 | 6b | 6b | |||||||||||
| Strypstein et al. (2022) | 1 | Antagonist | 24 | 45, X[14]/46, XX[86] (NR) | Yes | Yes | 6.4 | 3.4 | 37 | 11 | 8 | ||
| 27 | 21 | ||||||||||||
| Ulrich et al. (2022) | 1 | Antagonist | 14 | Mosaic | NR | NR | <0.1 | 6 | 14 | 11 | 11 | In four stimulation cycles, of which the first was cancelled due to poor response. | |
Stimulation cycles without oocytes preserved are shown in bold.
Meta-phase II oocytes.
First cycle GnRH-agonist trigger and no oocytes retrieved, second cycle hCG trigger and six oocytes retrieved, which shows the inactivity of the hypothalamus–pituitary–ovarian axis in a prepubertal girl.
AFC, antral follicle count; AMH, anti-Müllerian hormone; N, number of cases; NR, not reported; TS, Turner syndrome.
Many retrospective cohort studies reported to have a small cohort, though all tried to determine predictive parameters for successful vitrification of oocytes. A correlation between AMH level or antral follicle count (AFC) with the number of vitrified oocytes could not be established (Talaulikar et al., 2019; Martel et al., 2022; Brouillet et al., 2023). Brouillet et al. (2023) found a negative correlation with basal FSH levels and oocyte yield (r = −0.6) and a positive correlation with the percentage of 46,XX-cells and oocyte yield (r = 0.77). This could not be confirmed by other studies (Vergier et al., 2019; Martel et al., 2022). However, some studies suggested that exceptions might exist and that individual variations might play a role. This was demonstrated in reports in which oocytes could be vitrified in cases with AMH levels below the detection limit (Martel et al., 2022; Ulrich et al., 2022), or basal FSH level above 20 IU/l (Brouillet et al., 2023).
Since in most cases of women with TS it is difficult to vitrify a sufficient number of mature oocytes, the study of Oktay and Bedoschi (2014) applied 24-h IVM to increase the number of mature oocytes in three cases of girls with TS (aged 13, 13, and 14 years). This resulted in 6/25 immature oocytes being vitrified as mature oocytes.
Literature on the success rates of oocyte vitrification in women with TS is still scarce. There is merely one case report that described a live birth after the use of vitrified oocytes in a woman with a 45,X[14%]/46,XX[86%]-karyotype in lymphocytes. At the age of 24 years, 29 oocytes were vitrified after two stimulation cycles. All vitrified oocytes were fertilized with ICSI, resulting in 11 embryos. She delivered a healthy girl after the second embryo transfer. The case report described a live birth rate of 3% per vitrified oocyte (Strypstein et al., 2022).
Ovarian tissue cryopreservation
Current knowledge of OTC in TS is limited to six cohort studies and five case reports, involving a total of 195 girls with TS aged between 3 and 22 years (Table 4). Borgström et al. (2009) performed a laparoscopy on 57 girls with TS. In 10/57 no ovarian biopsy was obtained because streak ovaries were seen during laparoscopy. In 15/57 (26%) girls with TS, follicles were detected in the ovarian biopsies. Nadesapillai et al. (2023b) performed a unilateral ovariectomy in 93 girls with TS. In 30/93 (32%) girls with TS, follicles were found in an ovarian cortex fragment. Both prospective cohort studies did not exclude girls based on karyotype, hormone levels or age, since no reliable predictive parameters for finding follicles were available at that time. Mamsen et al. (2019) retrospectively evaluated histological sections of girls with TS who underwent OTC. In 9/15 (60%) girls follicles were found. Zajicek et al. (2023) described follicles in 6/9 (67%) girls with TS, however they preselected girls based on at least one positive parameter: detectable AMH, normal FSH or antral follicles seen on transabdominal ultrasound. Rodriguez-Wallberg et al. (2023) performed ovarian biopsies in 10 girls with TS, but the tissue was not analysed for presence of follicles.
Table 4.
Overview of studies reporting on ovarian tissue cryopreservation in girls with Turner syndrome.
| Study type | Author | N | Procedure | Age (years) | Girls with follicles n/cohort (%) | Karyotype | Prepubertal | No spontaneous thelarche | Spontaneous thelarche | Spontaneous menarche b | AMH (ng/ml) | FSH (IU/l) | Follicles/mm3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prospective cohort study | Borgström et al. (2009) | 57a | Laparoscopic ovarian cortex biopsy. 25–50% of one ovary was biopsied. | 8–19 | 15/57 (26%) | 45,X N = 28 |
3 (11%) | 0/6 (0%) | 1/19 (5%) | 2/3 (67%) | 1/2 (50%) | 0.18–0.39 | 5.5–36.3 | 9–290 |
| 25 (89%) | 6/6 (100%) | 18/19 (95%) | 1/3 (33%) | 1/2 (50%) | 0.08–0.40 | 3.3–123 | – | |||||||
| 45,X/46,XX N = 7 |
6 (86%) | 1/1 (100%) | 1/1 (100%) | 4/5 (80%) | 3/4 (75%) | 0.32–1.40 | 1.6–8.9 | 60–1.200 | ||||||
| 1 (14%) | 0/1 (0%) | 0/1 (0%) | 1/5 (20%) | 1/4 (25%) | NR | NR | – | |||||||
| Other N = 22 |
6 (27%) | 0/1 (0%) | 2/11 (18%) | 4/10 (40%) | 4/7 (57%) | 0.11–1.72 | 1–93.4 | 1–22 | ||||||
| 16 (73%) | 1/1 (100%) | 9/11 (82%) | 6/10 (60%) | 3/7 (43%) | 0.09–0.83 | 7.8–112.4 | – | |||||||
| Nadesapillai et al. (2023b) | 93 | Laparoscopic unilateral ovariectomy | 3–19 | 30/93 (32%) | 45,X N = 28 |
1 (4%) | 0/14 | 1/13 (8%) | 0/1 (0%) | 0 | 0.37 | 5.2 | 3 | |
| 27 (96%) | 14/14 (100%) | 12/13 (92%) | 1/1 (100%) | 0 | <0.10 | 5.0–58.8 | ||||||||
| 45,X/46,XX N = 33 |
22 (67%) | 9/13 (%) | 1/5 (20%) | 12/15 (80%) | 7/8 (%) | 0.8–3.0 | 2.2–7.8 | 0.2–247 | ||||||
| 11 (33%) | 4/13 (%) | 4/5 (80%) | 3/15 (20%) | 1/8 (%) | 0–0.3 | 5.6–60.0 | ||||||||
| Other N = 32 |
7 (22%) | 3/14 (%) | 0/9 (0%) | 4/9 (33%) | 0 | 0.1–1.0 | 2.6–8.1 | 0.4–7 | ||||||
| 25 (78%) | 11/14 (%) | 9/9 (100%) | 5/9 (67%) | 0 | <0.10 | 2.9–40.0 | ||||||||
| Rodriguez-Wallberg et al. (2023) | 10 | Laparoscopic ovarian cortex biopsy | 1–17 | Not analysed | NR | 3/10 | NR | NR | NR | NR | NR | NR | ||
|
| ||||||||||||||
| Retrospective cohort study | Jensen et al. (2017) | 6 | Laparoscopic unilateral ovariectomy | 6–17 | NR | Non-Mosaic Mosaic |
4 2 |
NR | NR | NR | NR | NR | NR | NR |
| Mamsen et al. (2019) | 15 | Laparoscopic ovarian cortex biopsy | 5–22 | 9/15 (60%) | 45,X N = 2 |
1 (50%) | 1/2 (50%) | NR | NR | – | 0.73 | NR | 106 | |
| 1 (50%) | 1/2 (50%) | - | NR | – | NR | 13 | – | |||||||
| 45,X/46,XX N = 4 |
4 (100%) | 1/1 (100%) | NR | NR | 3/3 (100%) | 0.06–20.2 | <0.1–5.1 | 3–519 | ||||||
| – | – | - | - | – | – | – | – | |||||||
| Other N = 9 |
4 (44%) | 0/3 | NR | NR | 4/5 (80%) | 0.30–1.62 | 0.4–5.5 | 1–20 | ||||||
| 5 (78%) | 3/3 (100%) | NR | NR | 1/5 (20%) | <0.04–3.2 | 4.2–82.9 | – | |||||||
| Zajicek et al. (2023) | 9 | Laparoscopic unilateral or partial ovariectomy | 2–18 | 7/9 (78%) | 45,X | 1 | ||||||||
| NR | ||||||||||||||
| 45,X/46,XX | 5 | NR | NR | NR | NR | NR | NR | NR | ||||||
| NR | ||||||||||||||
| Other | NR | |||||||||||||
| 1 | ||||||||||||||
|
| ||||||||||||||
| Case report | Huang et al. (2008) | 1 | Laparoscopic ovarian wedge resection | 16 | 1/1 | 45,X[20]/46,XX[80] | yes | yes | NR | 9.8 | NR | |||
| Balen et al. (2010) | 1 | Laparoscopic bilateral ovarian punch biopsies | 17 | 1/1 | 45,X[93]/46,XX[7] | yes | yes | NR | NR | NR | ||||
| Joshi et al. (2021) | 1 | Laparoscopic unilateral ovariectomy | 5 | 0/1 | NR | yes | NR | NR | – | |||||
| Cheng et al. (2022) | 1 | Laparoscopic unilateral ovariectomy | 3 | 1/1 |
|
yes | 1.06 | 4.27 | 440 | |||||
| Dunlop et al. (2023) | 1 | Laparoscopic bilateral ovarian cortex biopsies | 15 | 1/1 | 45,X/46,X,ring(X) | yes | yes | 0.04 | 1.9 | 3 | ||||
Percentages are based on the number of girls who have a certain patient characteristic (e.g. prepubertal in combination with 45,X karyotype) and who have follicles or not. Girls without follicles in the ovarian cortex are shown in bold.
In 10/57 girls no biopsy was performed, because streak ovaria were seen during laparoscopy.
Girls with spontaneous menarche are also included in group with spontaneous thelarche.
AFC, antral follicle count; AMH, anti-Müllerian hormone; N, number of cases; NR, not reported.
The follicle density varied considerably in girls with TS between 1 and 1200 follicles/mm3 (Table 4) and was about 50% higher in prepubertal girls than in pubertal girls with TS (Peek et al., 2023). Moreover, Nadesapillai et al. (2023b) calculated the total number of follicles in an ovary and found a higher number of follicles in girls with TS and a 46,XX cell line (mean 5407 follicles) compared to girls with TS without a 46,XX cell line (mean 2276 follicles).
Three parameters that might predict the presence of follicles in the ovarian cortex of girls with TS who opt for OTC were described: karyotype, spontaneous pubertal development, and AMH above the detection level. Girls with a 45,X/46,XX genotype had the highest prevalence of finding follicles: respectively 86% (Borgström et al., 2009), 67% (Nadesapillai et al., 2023b), and 100% (Mamsen et al., 2019). The variation in reported outcomes regarding finding follicles is most likely a result of a difference in inclusion criteria. Girls with a 45,X had the lowest chance of follicles. Only in 5/58 (8.6%) girls with 45,X were follicles detected (Table 4). In addition to karyotype, in 58–64% of girls with spontaneous thelarche, follicles were seen in the ovarian cortex after OTC. Studies described a positive predictive value of 0.59–0.62 for the presence of follicles if a girl experienced spontaneous thelarche (Borgström et al., 2009; Nadesapillai et al., 2023b). Moreover, for girls with TS who had AMH above the detection limit (respectively >0.28 ng/ml, >0.1 ng/ml or >0.16 ng/ml), the chance of finding follicles was significantly higher. Girls with AMH above the detection limit had a positive predictive value of 0.64–0.88 for the presence of follicles (Borgström et al., 2009; Nadesapillai et al., 2023b; Zajicek et al., 2023). However, follicles were also present in 5/46 (11%) girls with TS and hormone replacement therapy, in 5/50 (10%) girls without spontaneous onset of thelarche and in 1/37 (2.7%) girls with TS and AMH below the detection limit. The number of follicles was often lower in these specific cases (0.7–1000 follicles) (Borgström et al., 2009; Mamsen et al., 2019; Nadesapillai et al., 2023b). So far, no correlation has been found between age and follicle density in the ovarian cortex tissue (Mamsen et al., 2019; Nadesapillai et al., 2023b).
OTC is still considered an experimental procedure in girls with TS, owing to the lack of reported data on live birth after auto transplantation of ovarian tissue (OTT) (Schleedoorn et al., 2020; Mumford et al., 2023). Three cases of OTT in girls with TS have been documented, and in two cases no recovery of ovarian function was observed (Rodriguez-Wallberg et al., 2023) while in a recently published case report a clinical pregnancy was observed (Dunlop et al., 2023). This first pregnancy occurred in a woman with 45,X/46,ring (X) who had preserved ovarian tissue at the age of 15 years while having regular menstrual cycles. At the age of 23 years, she had been trying to get pregnant for 2 years and experienced irregular menstrual cycles. After OTT, she experienced resumption of regular menstrual bleeding. Approximately 8 months after OTT, a viable intrauterine pregnancy with a gestational age of 6 weeks was observed. This resulted in a miscarriage a few days later. A considerable number of girls with TS who underwent OTC are still very young and have not returned for OTT. Therefore, research efforts have been mainly directed towards obtaining more insights into the potential effectiveness of OTC for girls with TS, the quality of follicles in the ovarian cortex tissue and the potential markers to identify girls who could benefit from OTC.
Recently, Peek et al. (2023) performed a study in which ovarian tissue of girls with TS and age-matched controls was xenografted into immunodeficient mice. After retrieval of the grafted ovarian tissue, three significant observations were made. First, the follicle density decreased in tissue from both prepubertal and pubertal girls with TS and age-matched controls after xenografting. However, the follicle density was significantly higher in prepubertal girls compared to pubertal girls, before and after xenografting. Second, the proportion of growing follicles increased from 2–4% to 28–54%, while the proportion of morphologically abnormal follicles in girls with TS decreased from 30–31% to 6–8%, which is comparable to age-matched controls. Third, granulosa cells of unilaminar follicles from girls with TS were mostly aneuploid, but a considerable decrease in the percentage of aneuploid granulosa cells in secondary and antral follicles was observed after retrieval of the grafted ovarian tissue.
Summary
Vitrification of oocytes is a viable option for adolescents with TS. While the correlation between AMH and successful oocytes vitrification has been challenging to establish owing to small sample sizes in the published literature, combining available data suggests a strong correlation. However, sufficient AMH is needed for a realistic prospect of live birth, as multiple stimulation cycles might be needed. Although spontaneous menstrual bleeding and normal basal FSH reflect intact ovarian function, they do not directly indicate a sufficient ovarian reserve for oocyte vitrification.
Similarly, the presence of follicles in cryopreserved ovarian tissue correlates with AMH levels above the detection limit. Yet, for a realistic chance of live birth following OTT, sufficient AMH, as a correlate of sufficient ovarian reserve, is essential for adequate follicle survival. A 46,XX cell line and spontaneous thelarche increase the likelihood of the presence of follicles in the ovarian cortex. Follicle presence could not be related to age, yet prepubertal girls, with a higher follicle count than pubertal girls, are more likely to benefit from OTC although their follicle number remains significantly lower than that of girls without TS. Small follicles are able to develop into antral follicles after OTT; however, no live births have been reported so far in women with TS.
Research gap: the results of live birth after vitrification of oocytes or OTT.
Assisted reproduction techniques with own oocytes
There are few retrospective studies on ART using oocytes from women with TS. The predominant ART methods used were ICSI and IVF. Women with TS who underwent ovarian hyperstimulation followed by follicle aspiration and IVF or ICSI were aged between 25 and 39 years and had a 45,X/46,XX mosaic karyotype in 78% of all cases. Four women had 45,X monosomy (2%). The number of retrieved mature oocytes per stimulation cycle varied between 0 and 10 oocytes. Studies showed a large variation in clinical pregnancy rate (6–73%) and live birth rate (2.4–63%) per embryo transfer (Table 5).
Table 5.
Overview of studies reporting on ART with own oocytes in women with Turner syndrome.
| Study type | Authors | N | Procedure | Age (years)a | Karyotype | Karyotype analysis b | AMH a (ng/ml) | FSH a (IU/l) | AFC a | Stimulation cycles | MII-oocytes a | Embryos | CPR (%) | LBR (%) | MR (%) | CA | Remarks | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retrospective cohort | Doğer et al. (2015) | 10 | ICSI | NR | NR | 100 | NR | NR | NR | 35 | NR | NR | 5/82 (6.1%) | 2/82 (2.4%) | 3/5 (60%) | NR | ||
| Giles et al. (2020) | 56 | IVF-PGT | 38.2 (37.3 –39.0) | Mosaic | 56 | NR | NR | NR | NR | 65 | 9.1 (7.8 –10.4) | 256 | 13/31 (42%) | 7/31 (22.5%) | 4/13 (31%) | 0 | ||
| Gungor et al. (2021) | 98 | ICSI (-PGT) | 33.2 ± 4.2 | 45,X/46,XX | 55 | 100 | NR | 7.3 ± 1.0 | 8.1 ± 3.3 | 98 | NR | NR | 12/NR | 9/NR | 5/12 (29%) | 0 | Additional genotyping with FISH. | |
| Other | 43 | 17/NR | 9/NR | 8/17 (47%) | ||||||||||||||
| Liao et al. (2021) | 67 | ICSI-PGT | 35.4 ± 5.8 | 45,X | 4 | 60–100 | 3.2 ± 3.4 | NR | 13.5 ± 9.5 | 100 | NR | 213 | 22/30 (73%) | 19/30 (63%) | 3/22 (14%) | NR | ||
| 45,X/46,XX | 51 | |||||||||||||||||
| Other | 12 | |||||||||||||||||
| Acet et al. (2022) | 36 | ICSI | 36.2 ± 1.9 | 45,X[≥10%]/46,XX | 10 | NR | NR | 6.7 ± 1.2 | 10.2 ± 1.4 | 36 | 6.8 ± 0.4 | 5.2 ± 0.4 | NR | 11/36 (30%) | NR | NR | Genotyping with FISH on 400 cells. | |
| 35.5 ± 0.9 | 45,X[<10%]/46,XX | 26 | NR | 5.7 ± 0.7 | 10.9 ± 1.0 | 9.4 ± 1.6 | 6.4 ± 1.3 | NR | (31%) | NR | ||||||||
|
| ||||||||||||||||||
| Case report | Moore et al. (2008) | 1 | ICSI/IVF | 28 | 45,X[5]/46,XX[25] | 30 | NR | 7.3 | NR | 4 | 9 (total 36) | 18 (50%) | 0/7 | 0/7 | – | – | TS-diagnosis after 3 cycles | |
| Manno et al. (2009) | 1 | ICSI-PGT | 33 | 45,X[390]/47,XXX[10] | NR | 0.53 | 8–10 | NR | 2 | 2–3 (total 5) | 0–3 | 1/1 | 2/1 | – | NR | Genotyping with FISH on 400 cells. | ||
| Murakami et al. (2014) | 1 | OI | 38 | 45,X[14]/46,XX[16] | 30 | NR | 3.5 | NR | 9 | – | – | 2 | 1 | 1 | 0 | Mole pregnancy | ||
| Brouillet et al. (2023) | 1 | ICSI | 29 | 45,X[87]/46,XX[13] | NR | 1.78 | 7.6 | 11 | 2 | 3–7 (total 10) | 6 | 1/3 | 0/3 | 1/1 | – | |||
Mean±SD or Median (range).
Number of lymphocytes used for conventional blood karyotyping.
AFC, antral follicle count; AMH, anti-Müllerian hormone; CA, congenital abnormality of live birth; CPR, clinical pregnancy rate per embryo transfer; LBR, live birth rate per embryo transfer; MII, metaphase II oocyte; MR, miscarriage rate per clinical pregnancy; N, number of cases; NR, not reported; OI, ovulation induction; PGT, pre-implantation genetic testing; TS, Turner syndrome.
In addition to ICSI or IVF, four out of 10 studies incorporated preimplantation genetic testing (PGT). Numerous investigations have used PGT to enhance reproductive outcomes, by selecting euploid embryos for transfer, with the aim of optimizing live birth rates, while minimizing the incidence of miscarriages and congenital abnormalities (Giles et al., 2020; Gungor et al., 2021; Liao et al., 2021; Acet et al., 2022). PGT was performed at different phases of embryogenesis: biopsy of polar body (Manno et al., 2009), Day 3 (Giles et al., 2020) or Day 5 (Liao et al., 2021). The study by Liao et al. (2021) revealed a higher incidence of sex chromosome aneuploidy in the embryos of women with TS versus women without TS (7% vs 1.6%). Moreover, Acet et al. (2022) studied whether the level of mosaicism of women with TS contributed to the aneuploidy of embryos, and compared embryos of 10 women with high grade mosaicism (≥10% 45,X) with 26 women with low grade mosaicism (<10% 45,X). To note, a TS diagnosis can only be confirmed when a minimum of 10% of cells are aneuploid, or in combination with clinical signs of TS (Gravholt et al., 2017). Acet et al. (2022) reported a significant higher rate of euploid embryos in women with high-grade mosaic-TS compared to women with low-grade mosaic-TS (39% vs 25%). However, pregnancy rates were not different. Furthermore, the study by Gungor et al. (2021) presented a cohort of 53 women with TS and ICSI-PGT versus 46 women with TS and ICSI-non-PGT and found significantly more pregnancies (36% vs 26%) and fewer miscarriages (11% vs 15%) per women after ICSI-PGT, respectively. Similar results after ICSI-PGT were found by Giles et al. (2020) and Liao et al. (2021), who reported clinical pregnancy rates of 42–72% and miscarriage rates of 14–31%. These studies differ from other studies that described clinical pregnancy rates of 6–31% and miscarriage rate of 60% in ICSI procedures without PGT in women with TS (Doğer et al., 2015; Acet et al., 2022). The study of Liao et al. (2021) concluded that when an euploid embryo, following PGT, was transferred to 72 women with TS, the pregnancy and miscarriage rates were comparable to those without TS. Ten newborns, of which a minimum of seven were born after ICSI-PGT, underwent screening for congenital abnormalities, and both studies reported healthy newborns (Giles et al., 2020; Gungor et al., 2021).
Summary
Research on ART is limited in women with TS. The published studies frequently combined IVF and ICSI with PGT and are biased by including women with a very late diagnosis of TS and mild phenotype. PGT could be of added value to reduce the time to pregnancy and number of miscarriages. However, PGT is only an option for women with a large number of embryos, which are needed to select euploid embryos, limiting this option to a small proportion of women.
Research gap: controlled studies on the value of PGT in women with TS undergoing IVF or ICSI with follow-up on pregnancies and the occurrences of chromosomal abnormalities.
Counselling
Infertility is the most frequently cited concern of girls with TS within each age group and their parents (Sutton et al., 2005). Fertility counselling of girls with TS has been an important research topic over the last years (Table 6). Girls with TS and their parents who had fertility counselling expressed a high degree of appreciation, because counselling was a relevant addition to their overall healthcare experience (Joshi et al., 2021; van der Coelen et al., 2022; Fearon, 2023b; Granero-Molina et al., 2023). Only a minority of girls with TS had received fertility counselling.
Table 6.
Overview of studies reporting on fertility counselling in women with Turner syndrome.
| Study type | Authors | Research aim (A) and methods (M) | Main results from women with TS (Wom), parents (Par), and healthcare professionals (HP) | |
|---|---|---|---|---|
| Delphi study | Schleedoorn et al. (2020) |
|
Wom & Par & HP |
|
|
| ||||
| Focus group | van der Coelen et al. (2022) |
|
Wom |
|
| Par |
|
|||
| HP |
|
|||
|
| ||||
| Interview study | Johnson et al. (2017) |
|
Par |
|
| Nadesapillai et al. (2023a) |
|
Wom & Par |
|
|
| Fearon (2023b) |
|
Wom & Par |
|
|
| Granero-Molina et al. (2023) |
|
Wom |
|
|
|
| ||||
| Survey study | King et al. (2016) |
|
Par |
|
| van Hagen et al. (2017) |
|
Wom |
|
|
| Joshi et al. (2021) |
|
Par |
|
|
| Falsey et al. (2022) |
|
Wom |
|
|
| Theroux et al. (2022) |
|
HP |
|
|
| Retrospective cohort | Morgan et al. (2019) |
|
HP |
|
| Obata et al. (2020) |
|
HP |
|
|
| Calanchini et al. (2020) |
|
HP |
|
|
OR, odds ratio; OTC, ovarian tissue cryopreservation; TS, Turner syndrome.
Ethical considerations in fertility counselling in Turner syndrome
The ethical considerations in fertility counselling for TS can be assigned according to the four pillars of medical ethics: beneficence, autonomy, justice, and non-maleficence.
The beneficence for healthcare professionals in discussing (future) fertility involves exploring different options of family planning for girls with TS (van der Coelen et al., 2022). These options are not only limited to fertility preservation, but also include awaiting natural chances of pregnancy, oocyte donation, adoption, surrogacy, foster care, and remaining childless. In addition to healthcare professionals, girls with TS and their parents too are willing to contribute to research into options of fertility preservation in order to gain more knowledge for all women with TS (van der Coelen et al., 2022; Fearon, 2023b; Nadesapillai et al., 2023a).
Autonomy is valued by involving girls with TS as much as possible during counselling. Ideally, girls with TS are involved in counselling regardless of age and counselling is adjusted accordingly (Schleedoorn et al., 2020). However, the decision whether or not to opt for OTC is sometimes offered at a very young age, and consequently the decision is often made by parents. In this regard, anticipated decision regret plays a major role (Fearon, 2023b). An interview study by Nadesapillai et al. (2023a) and focus group interviews by van der Coelen et al. (2022) with a total of 43 parents who had to make the decision about OTC with their daughter with TS, revealed that parents felt that they had to seize all opportunities for a biological child for their daughter. First, to ensure the girl’s autonomy in making future decisions regarding the usage of frozen ovarian tissue. Second, to alleviate the potential burden of blame of the daughter towards her parents for not seizing the option of OTC. However, parents were also concerned that if ovarian tissue was cryopreserved, their daughter might feel compelled to use the ovarian tissue later in life because her parents had made the decision for her earlier (van der Coelen et al., 2022; Fearon, 2023b; Nadesapillai et al., 2023a).
Justice is given by discussing all options of family planning. Fearon (2023b) conducted semi-structured interviews with 19 adult women with TS and 11 mothers of girls with TS. They established that women with TS appreciated the opportunity to choose between several options, such as waiting for natural conception, fertility preservation, oocyte donation, adoption, surrogacy or otherwise, as is the case for women without TS. This element of justice was confirmed in an ethical Delphi study by Schleedoorn et al. (2020) who reached consensus between 25 healthcare professionals, 10 ethicists, seven women with TS and 13 parents, on whether or not to offer OTC to girls with TS.
The non-maleficence principle weighs heavily for many healthcare professionals and parents. Discussing all options of family planning, even those that may not be applicable to a specific case, might result in significant emotional distress for the girl with TS (Theroux et al., 2022; van der Coelen et al., 2022). In addition, some options, such as OTC, oocyte vitrification, oocyte donation, and ART, are invasive procedures with possible complications and could harm girls with TS. Moreover, these procedures could create false hope, because these techniques have low live birth rates or have not resulted in a live birth yet (OTC) (Taggar and Engmann, 2021; van der Coelen et al., 2022; Fearon, 2023b; Nadesapillai et al., 2023a). Another aspect of non-maleficence to take into account is that a pregnancy in a woman with TS often comes with increased pregnancy risks, and there are other options of family planning available that do not come with these risks, such as adoption, surrogacy or foster care (Paulson, 2019; Schleedoorn et al., 2020; Falsey et al., 2022).
Barriers in fertility discussions
According to the clinical guideline for girls with TS, it is crucial to initiate discussions regarding the shortened reproductive lifespan and to consider fertility preservation counselling at an early age (Gravholt et al., 2017). However, a retrospective study by Morgan et al. (2019) found that only 10% of girls with TS were referred to a fertility specialist and that the parents as well as the girl with TS were present during consultation for 27% of the time. Likewise, a survey study among 226 women with TS reported that 36% had discussed fertility with a healthcare professional (Falsey et al., 2022). Women with TS with spontaneous menarche were most likely to be referred to a fertility specialist (referral in 56–59% of cases) (Morgan et al., 2019; Theroux et al., 2022). These results demonstrated that many women with TS did not receive fertility counselling, indicating that barriers to discussing fertility do indeed exist. Paediatricians recognized that both the unfamiliarity with fertility preservation techniques and a lack of interest from families prevented them from discussing fertility with girls with TS and/or their parents (Theroux et al., 2022). In addition, healthcare professionals were less likely to provide fertility counselling if fertility preservation seemed not feasible, had less access to fertility preservation care or did not have appropriate insurance (Morgan et al., 2019; Theroux et al., 2022; van der Coelen et al., 2022). Although awareness regarding this topic has increased, 92% of healthcare professionals still expressed interest in receiving extra training in fertility counselling. Moreover, appropriate tools and resources for facilitating effective fertility counselling services were highly recommended (Lin et al., 2016; Morgan et al., 2019; Theroux et al., 2022).
Similar to healthcare professionals, parents would also like to have tools to discuss fertility with their daughter, because they too encounter difficulties in determining the appropriate timing and approach to discuss infertility (King et al., 2016; Johnson et al., 2017; Fearon, 2023a). A survey study, which included 215 caretakers of girls with TS, reported that around 50% of the parents had discussed fertility with their daughter (King et al., 2016). When they discussed fertility, they mostly weaved it into normal conversations when their daughter was around 6–10 years old. The other half of respondents had not discussed fertility yet and were dealing with barriers, such as fear of negative emotions, feelings of guilt or a lack of proper resources, that allowed them to have this kind of conversation (King et al., 2016; Johnson et al., 2017). Similar concerns while discussing fertility with their daughter were reported by an interview study with 19 women with TS (aged 21–60 years) and 11 mothers of girls with TS (Fearon, 2023b). Psychosocial maturity was the most important prerequisite for discussing fertility, as was parental confidence with the topic (King et al., 2016; Joshi et al., 2021).
Complications and hazards during pregnancy
The elevated risk profile of pregnant women with TS is widely documented (Tables 2 and 5). Hence, one in two women with TS were afraid for the impact of a pregnancy on their health, which was more apparent in women with TS with cardiac abnormalities (van Hagen et al., 2017; Falsey et al., 2022; Granero-Molina et al., 2023). Studies stressed the importance of pre-conceptional counselling. However, only 25–71% of women with TS had pre-conceptional counselling (van Hagen et al., 2017; Morgan et al., 2019; Obata et al., 2020), and 48–65% had had imaging of the aorta (Calanchini et al., 2020; Obata et al., 2020).
During counselling about a future pregnancy, various risks are discussed, including maternal and foetal complications associated with pregnancy in women with TS. Maternal risks include miscarriages, pre-eclampsia, and the potential dilatation of the aortic diameter, which could lead to aortic dissection and mortality. Additionally, foetal risks, such as small for gestational age and congenital abnormalities, are also addressed. Among these risks, miscarriage stands out as the most prevalent, with reported rates ranging between 23% and 67% (Table 2), compared to 10% in women without TS aged 25–29 years (Magnus et al., 2019). Homer et al. (2010) found a correlation between higher number of 45,X-cells in women with mosaic-TS and the increased prevalence of miscarriage (P = 0.026). Pre-eclampsia occurred in 6–11% of the reported natural conceptions (Hagman et al., 2011; Bernard et al., 2016; Calanchini et al., 2020), compared to 4.6% of women without TS (Abalos et al., 2013). Kooijman et al. (2021) compared the aortic diameter increase in 27 pregnant women with TS with age- and karyotype-matched nulligravida women with TS and did not find a significant difference in aortic diameter in the period before, during or after the pregnancy. Aortic dissection was documented in 0.9–1.2% of pregnancies (2/382 pregnancies), and they both survived (Bryman et al., 2011; Hagman et al., 2011). All other studies reported no aortic dissection. The study of Hagman et al. (2013) reported aortic aneurysm in 1.6% of pregnant women with TS. These two cases were known to have a congenital abnormality of the aorta (aortic coarctation/unspecified septal defect). Reassuringly, all studies reported an absence of mortality caused by cardiovascular events during or post-pregnancy (Mortensen et al., 2010; Hagman et al., 2011, 2013; Hadnott et al., 2011; Bernard et al., 2016; Obata et al., 2020; Ramage et al., 2020; Campens et al., 2021; Grewal et al., 2021). Regarding foetal complications, Ramage et al. (2020) compared pregnancies of women with TS to women without TS and found a higher risk for small for gestational age (odds ratio 4.5) and birth before 37 weeks (odds ratio 2.9). In addition, there is an increased likelihood of chromosomal abnormalities in offspring born to women with TS, with prevalence rates between 0% and 18% (Bernard et al., 2016). Chromosomal analysis was performed in 15 newborns, revealing a TS diagnosis in two girls. In addition, one girl was diagnosed with TS in adulthood because of multiple miscarriages. All three girls had a structural abnormality of the X-chromosome, and so did their mother (Bernard et al., 2016; Calanchini et al., 2020).
Summary
In recent years, there has been an increased focus on fertility counselling in girls with TS and their families. Key considerations include granting the girl’s autonomy in family planning by involving her early in conversations and emphasizing the pregnancy risks. On the other hand, fertility counselling could result in unrealistic hope of biological offspring. This research highlighted the barriers for counselling, such as the fear for negative emotions, the unfamiliarity with the options, and how to discuss fertility while adhering to the ethical principles. As a result, only a minority receives adequate fertility counselling.
Research gap: studies focussing on the implementation of fertility counselling in girls with TS and their families is needed.
Discussion
Summary
This review provides a comprehensive appraisal of the existing literature on fertility in women with TS. Throughout life, women with TS face various fertility dilemmas, since only 5–13% are able to conceive naturally. The prevalence of natural conception is highest in women with mosaic 45,X/46,XX (Bernard et al., 2016; Calanchini et al., 2020). Recently, it has been established that the percentage of 45,X-cells in the ovary generally differs from lymphocytes or buccal cells that are normally used for characterization of the genotype (Peek et al., 2019). This could explain pregnancies in women with 45,X in lymphocytes. While AMH levels indicate ovarian reserve and FSH levels in pubertal girls serve as valuable predictors, longitudinal measurements are essential for accurate prediction of the ovarian function. This is crucial, as spontaneous pubertal development and the presence of a small number of follicles are also observed when the AMH level is below the detection limit (Hankus et al., 2018).
The decline of the ovarian reserve over time is difficult to predict, therefore girls with TS and their parents ask for fertility preservation options. Currently, two fertility preservation techniques are available for girls with TS. The first is vitrification of oocytes: an established technique in women with TS. So far, one live birth has been reported using vitrified oocytes in a woman with TS (Strypstein et al., 2022). Second is OTC, which is considered an experimental technique in girls with TS. The few cases describing OTT in women with TS have not resulted in live birth yet (Dunlop et al., 2023; Rodriguez-Wallberg et al., 2023). However, a xenotransplantation study showed that, although a major decline in follicle density after OTT was observed, small follicles from girls with TS (45,X/46,XX) are capable of continuing folliculogenesis (Peek et al., 2023).
A subset of women who retain ovarian functionality and have a desire to conceive opt for ART, such as IVF or ICSI. The ovarian response to ovarian hyperstimulation in ART in these women with ovarian functionality was comparable to women without TS. The clinical pregnancy rate after IVF or ICSI varied between 6% and 73% and live birth rate between 2% and 63% per embryo transfer (Doğer et al., 2015; Liao et al., 2021).
Fertility counselling in TS is complicated and there is a strong need for a practical guideline. This guideline should give guidance on how to provide counselling and how to embed counselling in daily practice, with clear indications of when to refer girls and on how to decide who is responsible for referral.
Practical guideline: fertility in Turner syndrome
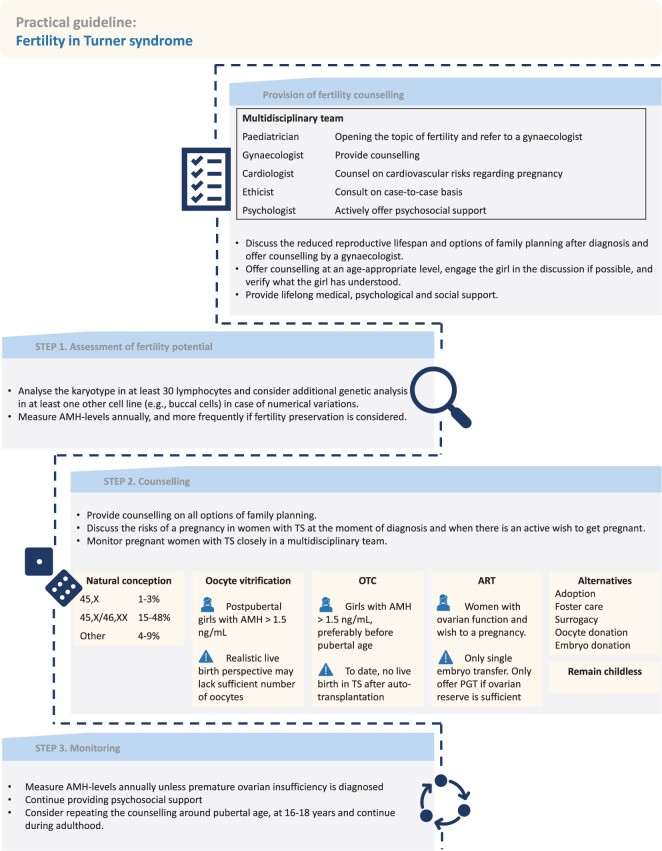
The following practical guideline (Fig. 2) was established based on the results section and clinical opinion from the various experts included in this review.
Figure 2.
Practical guideline for fertility in girls with Turner syndrome. AMH, anti-Müllerian hormone; OTC, ovarian tissue cryopreservation; PGT, pre-implantation genetic testing; TS, Turner syndrome.
Provision of fertility counselling
We advocate a centralized provision of fertility counselling by a dedicated multidisciplinary team, consisting of gynaecologists, paediatricians, (paediatric) cardiologists, psychologists, and medical ethicists. Each expert in this team has a designated role as this would ensure optimized quality and consistency of the counselling process (King et al., 2016; Morgan et al., 2019; Schleedoorn et al., 2019, 2020; Calanchini et al., 2020; Joshi et al., 2021; Granero-Molina et al., 2023). The counselling should include information about options of family planning and cardiac comorbidities and complications associated with pregnancy (Morgan et al., 2019; Schleedoorn et al., 2020). Psychosocial support should be available and actively offered to girls with TS and their parents, especially during the counselling process.
The timing of referral is determined and guided by the preferences and needs of both the girl and her parents. Referral to discuss options of family planning should be considered at time of diagnosis, at pubertal age, at age 16–18 years, and continue during adulthood. It is imperative to address fertility-related information at an age-appropriate level and, if possible, to engage the girl in the discussion. Furthermore, it is crucial to verify if the girl has understood what was explained to her by asking her questions regarding the subject (Mascarenhas et al., 2019; Azem et al., 2020; Schleedoorn et al., 2020; van der Coelen et al., 2022). In case of intellectual disability and/or psychiatric comorbidity, multidisciplinary consultation should be considered before counselling (Gravholt et al., 2017; Björlin Avdic et al., 2021). The key points are to:
discuss the reduced reproductive lifespan and options of family planning after diagnosis and offer counselling by a gynaecologist.
consider repeating the counselling around pubertal age and the age of 16–18 years.
offer counselling at an age-appropriate level, engage the girl in the discussion if possible, and verify what the girl has understood.
provide lifelong medical, psychological, and social support.
Step 1: assessment of fertility potential
Two factors are considered important in establishing the fertility potential of a girl with TS. First, we recommend establishing the karyotype in at least 30 lymphocytes (Gravholt et al., 2017; Berisha et al., 2020). Consider confirming the genotype in a second cell line, preferably buccal cells, in girls with a numerical TS-genotype (e.g. 45,X, 45,X/46,XX or 45,X/47,XXX) to determine the presence of multiple cell lines, and thus estimate the fertility potential more precisely (Graff et al., 2020; Nadesapillai et al., 2023b). Second, we recommend to determine the level of AMH at diagnosis and to evaluate this annually in order to estimate the ovarian reserve (Blakemore et al., 2019). Consider measuring AMH levels more frequently if fertility preservation is considered. With longitudinal parameters, the course of the ovarian function can be monitored more precisely and discussed with the girl and her parents in a timely manner. From the age of 10 years, FSH could be a useful additional marker to AMH in determining a girl’s fertility potential. Increased FSH levels (twice above the upper limit of normal) and/or repeated AMH below the detection limit is indicative of POI (Aso et al., 2010; Hagen et al., 2010b; Hankus et al., 2018; Nadesapillai et al., 2023b). Once POI is diagnosed, repeated measurement of AMH or FSH is no longer useful. The key points are to:
analyse the karyotype in at least 30 lymphocytes and consider additional genetic analysis in at least one other cell line (e.g. buccal cells) in case of numerical variations.
measure AMH levels annually, and more frequently if fertility preservation is considered.
Step 2: counselling
If full assessment of karyotype and hormonal markers is completed, the girl and her parents should be offered counselling on all options of family planning, including the individualized feasibility (Oktay and Bedoschi, 2014; Gravholt et al., 2017; Blakemore et al., 2019; Calanchini et al., 2020; Schleedoorn et al., 2020). Moreover, it should be mentioned that a pregnancy may be discouraged in the future if there are contra-indications for a pregnancy (Mascarenhas et al., 2019; Nawroth et al., 2020; Rodriguez-Wallberg et al., 2020; Schleedoorn et al., 2020). The key point is:
provide counselling on all options of family planning and pregnancy risks.
The following information should be integrated into counselling and discussed on an individual basis.
Natural conception
The prevalence of natural conception is highest (15–48%) in women with mosaic 45,X/46,XX and lowest (1–3%) in women with monosomy 45,X. In women with a structural abnormality of the X-chromosome (‘other’) the prevalence is between 4% and 9% (Bernard et al., 2016; Calanchini et al., 2020). Nevertheless, it should be discussed that fertility declines with age and it is not apparent at prepubertal or pubertal age whether or not women possess the ovarian capacity to conceive naturally. Girls with spontaneous menarche and cyclic bleeding should be informed about the necessity of contraceptives, and in case of considering conception the need for cardiac assessment.
Oocyte vitrification
A viable option for post-pubertal girls with TS who are physically and mentally mature enough to undergo the intensive procedures of ovarian hyperstimulation and subsequent oocyte aspiration (Gravholt et al., 2017). Since 2007, this procedure has been described in 58 cases with TS and has resulted in one live birth in this population. Based on expert opinion, we recommend that oocyte vitrification should only be offered to girls with sufficient levels of AMH in repeated measures, e.g. >1.5 ng/ml, and who experienced spontaneous thelarche and menarche. In cases with lower AMH levels, deviation from standard practices may be justified, subject to informed shared decision making. The question remains how many preserved oocytes will be needed for a realistic prospect of a live birth. In women without TS ≤35 years, the use of 15 vitrified oocytes resulted in a live birth rate of 70% (4.7% per oocyte) and increased to 95% if 25 vitrified oocytes were utilized (3.8% per oocyte) (Cobo et al., 2018). The case report of a live birth in a woman with TS after oocyte vitrification showed a live birth of 3% per vitrified oocyte. So far, no reliable mathematical model for live birth rates after oocyte vitrification in women with TS exists. However, in girls with TS a median of five oocytes (range 0–21) per cycle was vitrified. Given the 23–67% risk of miscarriages for women with TS (Bernard et al., 2016; Borini and Coticchio, 2019; Calanchini et al., 2020), a lower live birth rate than 3% per oocyte could be expected and approximately 35–50 vitrified oocytes might be necessary if a similar likelihood of 95% live birth is being pursued. This high number of retrieved oocytes is not realistic for girls with TS owing to their compromised ovarian reserve. This stresses the importance of realistic counselling to prevent false hope. It should be discussed that several stimulation cycles with oocyte retrieval are necessary to obtain enough oocytes. Therefore, it is crucial to carefully evaluate the medical-ethical justification and the girl’s preferences before proceeding with each subsequent cycle.
The key points are to:
offer oocyte vitrification to post-pubertal girls with spontaneous thelarche and menarche and AMH above 1.5 ng/ml (repeated measures) who are mature enough to undergo the procedure.
mention that for a realistic perspective on live birth a relatively high number of oocytes are needed, with multiple stimulation cycles, which might not be feasible considering the reduced ovarian reserve.
Ovarian tissue cryopreservation
This option for fertility preservation that may also be offered before puberty, since no reproductive maturity is required. Follicles were found in the ovaries of one out of three girls with TS, mostly in girls with 46,XX-cell line, and AMH above the detection limit (Borgström et al., 2009; Peek et al., 2019, 2023; Nadesapillai et al., 2023b). A higher follicle density was found in prepubertal girls compared to pubertal girls (Peek et al., 2023). Nonetheless, in girls with TS, higher rates of morphologically abnormal follicles and a reduced number of follicles were found (Mamsen et al., 2019; Peek et al., 2023). Despite the unfavourable characteristics, the small follicles of girls with 45,X/46,XX were capable of developing to secondary and antral stages after xenotransplantation (Peek et al., 2023). The success of restoration of ovarian function after OTT in girls with TS is limited by a reduced number of follicles, a reduced rate of morphologically normal follicles, and the known loss of 50–90% of oocytes after OTT caused by hypoxia (te Velde and Pearson, 2002; Dolmans et al., 2021). Therefore, based on expert opinion, we suggest discussing OTC as an option for girls with AMH above 1.5 ng/ml (repeated measures), preferably before puberty given their higher number of follicles, and offering it only within a research setting (Peek et al., 2023). Unilateral ovariectomy in women without TS has a low complication risk and shortened the reproductive lifespan by only 1–3 years (Bjelland et al., 2014; Rosendahl et al., 2017; Grynberg et al., 2023). Unfortunately, the impact of unilateral ovariectomy on ovarian function in girls with TS is unknown (Jensen et al., 2017). Moreover, no live births have been reported after OTT in women with TS (Dunlop et al., 2023; Rodriguez-Wallberg et al., 2023). The key points are to:
discuss OTC as an option for girls with AMH above 1.5 ng/ml (repeated measures), preferably before puberty, and offer it only within a research setting. Do not exclude girls from OTC based on karyotype only.
mention that OTC is considered experimental in girls with TS, that a significant loss of follicles is expected after OTT related to the procedure, and that so far no live birth has been reported.
ART
Three items are considered important in ART for women with TS. First, a sufficient oocyte yield is unlikely for most women with TS since the reported cases of women with TS who underwent ART were mostly diagnosed during or after IVF/ICSI and therefore likely to have an oocyte yield comparable to women without TS. Second, we recommend opting for single embryo transfer to prevent complications associated with multiple pregnancy (Söderström-Anttila et al., 2019; Abir et al., 2020; Rodriguez-Wallberg et al., 2020). Third, the added value of PGT in ART may be limited in case of poor ovarian response (Gungor et al., 2021; Liao et al., 2021). High numbers of oocytes are needed to select euploid embryos for transfer. Therefore, we recommend offering PGT only to women with sufficient ovarian reserve, or if the women so desire. The key points are to:
consider ART for women with sufficient ovarian reserve when they express an active wish to become pregnant.
select single embryo transfer.
only offer PGT to women with sufficient ovarian reserve or at the express wish of a woman with TS.
Alternatives
Options of family planning with children of non-genetic origin include adoption, foster care, surrogacy, and donation of oocytes or embryos. A common encountered option is intra-familial donation of oocytes by a sister, niece or mother of the women with TS. This way, the child would be genetically related to the women with TS. However, it is important to note that intra-familial donation could lead to emotional, ethical, and social complexities (Fearon, 2023a). When discussing family planning, it is imperative not to overlook the valid option of leading a childless life. The key point is to:
discuss alternative options of future parenthood and the option to remain childless.
Preconception counselling
Considering the increased health risks of a pregnancy in women with TS, it is strongly recommended to provide preconception counselling (Bondy et al., 2009; Lin et al., 2016; Gravholt et al., 2017; van Hagen et al., 2017; Blakemore et al., 2019; Söderström-Anttila et al., 2019; Abir et al., 2020; Andersen and Gravholt, 2022; Bamba et al., 2022; Brown, 2022). During counselling, several issues should be addressed, including cardiac screening and risks of miscarriage (23–67%), pre-eclampsia (6–7%), small for gestational age (0–7%), and cardiovascular complications such as dissection of the aorta (0–1%) (Doğer et al., 2015; Bernard et al., 2016; Cadoret et al., 2018; Calanchini et al., 2020). None of the studies included in this review described mortality after cardiovascular complications related to a pregnancy. This may be attributed to the enhanced medical care for pregnant women with TS (Cadoret et al., 2018; Andersen and Gravholt, 2022). We recommend close monitoring of pregnant women with TS by a multidisciplinary team consisting of gynaecologists and cardiologists, with frequent perinatal and postnatal controls (Bondy et al., 2009; Gravholt et al., 2017; Mavridi et al., 2018; Abir et al., 2020; Martín et al., 2021; Andersen and Gravholt, 2022; Brown, 2022; Cauldwell et al., 2022a,b). The key points are to:
discuss the risks of a pregnancy in women with TS at the moment of diagnosis and when there is an active wish to get pregnant.
monitor pregnant women with TS closely in a multidisciplinary team.
Step 3: monitoring
We recommend monitoring AMH levels on an annual basis, or at a frequency determined after careful consideration in consultation with the girl or woman with TS (Oktay and Bedoschi, 2019). In cases where AMH consistently is below the detection limit and/or POI is diagnosed, we recommend discontinuing further AMH evaluation. In addition, it is important to maintain psychosocial support and offer information on family planning options if requested.
Society’s impact
Society has a major impact on women with TS when it comes to fertility. Fertility is generally regarded as a goal pursued by all individuals, and the decision to remain childless may necessitate justification within one’s social environment (Fearon, 2023a; Radkowska-Walkowicz and Maciejewska-Mroczek, 2023). This society’s pressure to have children could even be more pronounced in certain ethnic or religious contexts. In addition, women do not always qualify for options such as adoption because they are often wrongly assumed to have a shorter life expectancy or reduced social capabilities (Wasserman and Asch, 2012; Söderström-Anttila et al., 2019; Radkowska-Walkowicz and Maciejewska-Mroczek, 2023). Also, financial capabilities and insurance coverage can play a role in the choice options of family planning (Mascarenhas et al., 2019; Theroux et al., 2022) and this leads to inequity in accessibility to care. However, we advocate offering fertility counselling to all girls with TS to the best of a clinician’s knowledge. A conscious decision about family planning is likely to enhance a woman’s ability to cope with infertility and contribute to her overall satisfaction with her decisions.
Strengths and limitations
In this scoping review, articles were systematically screened and selected using the PRISMA checklist (Tricco et al., 2018) and multiple databases were searched. Whereas other reviews primarily focus on fertility preservation, this review focusses on all facets of fertility. Fertility in TS is examined with a broad scope and a wide time range of more than 15 years, which resulted in a comprehensive presentation of original research. In this way, we have provided tools for a practical guideline for fertility counselling for healthcare professionals. The findings could be limited by the karyotype descriptions of several studies, since the karyotype was either not clear or conducted with a limited number of cells, leading to potential misinterpretation of a girl’s fertility potential. In addition, the karyotype group labelled as ‘other’ consisted of a heterogeneous group. Consequently, formulating precise statements concerning the girls in the ‘other’ group remained challenging. Furthermore, statements and recommendations were sometimes based on a single study because of the limited available literature on several aspects. Moreover, the studies were generally based on small numbers of girls with TS with heterogenous characteristics, thereby impeding the extrapolation of findings.
Future perspectives
Prediction models are necessary to transcend the fertility counselling and identify appropriate candidates for fertility preservation. To achieve this, international collaboration is necessary to pursue large longitudinal cohorts of girls with TS, with and without fertility preservation. These cohorts should have well-defined karyotypes and long-term follow-up of fertility outcome. In the future, IVM of primordial follicles to mature oocytes may provide a solution for cryopreserved ovarian tissue containing low numbers of follicles. However, the development of this technique remains pending. Furthermore, research should be conducted on gene expression in ovarian cells to further understand the pathophysiology of POI in girls with TS. This might provide valuable insights into the potential consequences of aneuploid ovarian cells on folliculogenesis. Finally, comprehensive qualitative research is essential to develop a shared-decision tool for appropriate fertility counselling for girls with TS and their parents.
Conclusion
This review provides insight into the current knowledge of fertility in girls with TS and creates awareness of the importance of repeated discussions about the options of family planning. These options include fertility preservation techniques, as well as alternative options or the conscious decision to remain childless. With this review, we aimed to reduce the barrier for referral to an expert team for fertility counselling and offered a guideline for counselling. The topic of fertility necessitates thorough discussion tailored to the individual needs of girls with TS and their parents. In addition, it requires reconsideration on multiple occasions to enhance decision satisfaction.
Supplementary Material
Contributor Information
Sanne van der Coelen, Department of Obstetrics and Gynaecology, Radboud University Medical Center, Nijmegen, The Netherlands.
Janielle van der Velden, Department of Paediatrics, Amalia Children’s Hospital, Radboud University Medical Center, Nijmegen, The Netherlands.
Sapthami Nadesapillai, Department of Obstetrics and Gynaecology, Radboud University Medical Center, Nijmegen, The Netherlands.
Didi Braat, Department of Obstetrics and Gynaecology, Radboud University Medical Center, Nijmegen, The Netherlands.
Ronald Peek, Department of Obstetrics and Gynaecology, Radboud University Medical Center, Nijmegen, The Netherlands.
Kathrin Fleischer, Department of Reproductive Medicine, Nij Geertgen Center for Fertility, Elsendorp, The Netherlands.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Data availability
Data from screening and full-text review are available from authors upon reasonable request.
Authors’ roles
All authors participated in the design of the review article. Literature search was performed by S.v.d.C. Screening and selection of articles were performed by S.v.d.C., J.v.d.V., K.F., and S.N. The first draft of the manuscript was written by S.v.d.C. with assistance of K.F., R.P., and J.v.d.V. All authors critically revised and confirmed the manuscript. Ten of the included studies in this review originate from our research group. K.F. and J.v.d.V. are involved in the revision group of the international guidelines for management of TS (2023).
Funding
Unconditional funding: Goodlife and Ferring.
Conflict of interest
The authors have no conflicts of interest related to this review.
References
- Abalos E, Cuesta C, Grosso AL, Chou D, Say L.. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol 2013;170:1–7. [DOI] [PubMed] [Google Scholar]
- Abir R, Oron G, Shufaro Y.. Fertility in patients with Turner syndrome. Fertil Steril 2020;114:73–74. [DOI] [PubMed] [Google Scholar]
- Acet F, Sahin G, Ucar AAO, Emirdar V, Karaca E, Durmaz B, Goker ENT, Tavmergen E.. In vitro fertilization and preimplantation genetic diagnosis outcomes in mosaic Turner’s syndrome: a retrospective cohort study from a single referral center experience. J Gynecol Obstet Hum Reprod 2022;51:102405. [DOI] [PubMed] [Google Scholar]
- Alves C, Silva SF.. Spontaneous procreation in Turner syndrome: report of two pregnancies in the same patient. Syst Biol Reprod Med 2012;58:113–115. [DOI] [PubMed] [Google Scholar]
- Andersen NH, Gravholt CH.. Re: pregnancies in women with Turner syndrome: a retrospective multicentre UK study. BJOG 2022;129:1413–1414. [DOI] [PubMed] [Google Scholar]
- Aso K, Koto S, Higuchi A, Ariyasu D, Izawa M, Miyamoto Igaki J, Hasegawa Y.. Serum FSH level below 10 mIU/mL at twelve years old is an index of spontaneous and cyclical menstruation in Turner syndrome. Endocr J 2010;57:909–913. [DOI] [PubMed] [Google Scholar]
- Azem F, Brener A, Malinger G, Reches A, Many A, Yogev Y, Lebenthal Y.. Bypassing physiological puberty, a novel procedure of oocyte cryopreservation at age 7: a case report and review of the literature. Fertil Steril 2020;114:374–378. [DOI] [PubMed] [Google Scholar]
- Balen AH, Harris SE, Chambers EL, Picton HM.. Conservation of fertility and oocyte genetics in a young woman with mosaic Turner syndrome. BJOG 2010;117:238–242. [DOI] [PubMed] [Google Scholar]
- Bamba V, Levitsky LL, Wong AW, Rivera-Cruz G, Scurlock C, Lin AE.. Letter to the editor Re: first live birth after fertility preservation using vitrification of oocytes in a woman with mosaic Turner syndrome. J Assist Reprod Genet 2022;39:777–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berisha SZ, Shetty S, Prior TW, Mitchell AL.. Cytogenetic and molecular diagnostic testing associated with prenatal and postnatal birth defects. Birth Defects Res 2020;112:293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard V, Donadille B, Zenaty D, Courtillot C, Salenave S, Brac de la Perrière A, Albarel F, Fèvre A, Kerlan V, Brue T. et al. ; CMERC Center for Rare Disease. Spontaneous fertility and pregnancy outcomes amongst 480 women with Turner syndrome. Hum Reprod 2016;31:782–788. [DOI] [PubMed] [Google Scholar]
- Bjelland EK, Wilkosz P, Tanbo TG, Eskild A.. Is unilateral oophorectomy associated with age at menopause? A population study (the HUNT2 Survey). Hum Reprod 2014;29:835–841. [DOI] [PubMed] [Google Scholar]
- Björlin Avdic H, Butwicka A, Nordenström A, Almqvist C, Nordenskjöld A, Engberg H, Frisén L.. Neurodevelopmental and psychiatric disorders in females with Turner syndrome: a population-based study. J Neurodev Disord 2021;13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore JK, Wei LS, Quinn GP.. Addressing practical concerns surrounding fertility preservation in patients with Turner syndrome. Fertil Steril 2019;112:651–652. [DOI] [PubMed] [Google Scholar]
- Bondy C, Rosing D, Reindollar R.. Cardiovascular risks of pregnancy in women with Turner syndrome. Fertil Steril 2009;91:e31–e32; author reply e34. [DOI] [PubMed] [Google Scholar]
- Borgström B, Hreinsson J, Rasmussen C, Sheikhi M, Fried G, Keros V, Fridström M, Hovatta O.. Fertility preservation in girls with Turner syndrome: prognostic signs of the presence of ovarian follicles. J Clin Endocrinol Metab 2009;94:74–80. [DOI] [PubMed] [Google Scholar]
- Borini A, Coticchio G.. Oocyte quantity and quality are crucial for a perspective of fertility preservation in women with Turner syndrome. Fertil Steril 2019;111:461–462. [DOI] [PubMed] [Google Scholar]
- Brambila-Tapia AJ, Rivera H, García-Castillo H, Domínguez-Quezada MG, Ip D-R.. 47,XXX/45,X/46,XX mosaicism in a patient with Turner phenotype and spontaneous puberal development. Fertil Steril 2009;92:1747–1747.e5. [DOI] [PubMed] [Google Scholar]
- Brouillet S, Ranisavljevic N, Sonigo C, Haquet E, Bringer-Deutsch S, Loup-Cabaniols V, Hamamah S, Willems M, Anahory T.. Should we perform oocyte accumulation to preserve fertility in women with Turner syndrome? A multicenter study and systematic review of the literature. Hum Reprod 2023;38:1733–1745. [DOI] [PubMed] [Google Scholar]
- Brown RN. An improved understanding of pregnancy in women with Turner syndrome may save lives!. BJOG 2022;129:804. [DOI] [PubMed] [Google Scholar]
- Bryman I, Sylvén L, Berntorp K, Innala E, Bergström I, Hanson C, Oxholm M, Landin-Wilhelmsen K.. Pregnancy rate and outcome in Swedish women with Turner syndrome. Fertil Steril 2011;95:2507–2510. [DOI] [PubMed] [Google Scholar]
- Bucerzan S, Miclea D, Popp R, Alkhzouz C, Lazea C, Pop IV, Grigorescu-Sido P.. Clinical and genetic characteristics in a group of 45 patients with Turner syndrome (monocentric study). Ther Clin Risk Manag 2017;13:613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne PS, Baker TG.. Perinatal oocyte loss in XO mice and its implications for the aetiology of gonadal dysgenesis in XO women. J Reprod Fertil 1985;75:633–645. [DOI] [PubMed] [Google Scholar]
- Cadoret F, Parinaud J, Bettiol C, Pienkowski C, Letur H, Ohl J, Sentilhes L, Papaxanthos A, Winer N, Mathieu d’Argent E. et al. Pregnancy outcome in Turner syndrome: a French multi-center study after the 2009 guidelines. Eur J Obstet Gynecol Reprod Biol 2018;229:20–25. [DOI] [PubMed] [Google Scholar]
- Calanchini M, Aye CYL, Orchard E, Baker K, Child T, Fabbri A, Mackillop L, Turner HE.. Fertility issues and pregnancy outcomes in Turner syndrome. Fertil Steril 2020;114:144–154. [DOI] [PubMed] [Google Scholar]
- Campens L, Baris L, Scott NS, Broberg CS, Bondue A, Jondeau G, Grewal J, Johnson MR, Hall R, De Backer J. et al. ; ROPAC Investigators Group. Pregnancy outcome in thoracic aortic disease data from the Registry of Pregnancy and Cardiac disease. Heart 2021;107:1704–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpini S, Carvalho AB, de Lemos-Marini SHV, Guerra-Junior G, Maciel-Guerra AT.. FSH may be a useful tool to allow early diagnosis of Turner syndrome. BMC Endocr Disord 2018;18:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpini S, Carvalho AB, Guerra-Júnior G, Baptista MT, Lemos-Marini SH, Maciel-Guerra AT.. Spontaneous puberty in girls with early diagnosis of Turner syndrome. Arq Bras Endocrinol Metabol 2012;56:653–657. [DOI] [PubMed] [Google Scholar]
- Castronovo C, Rossetti R, Rusconi D, Recalcati MP, Cacciatore C, Beccaria E, Calcaterra V, Invernizzi P, Larizza D, Finelli P. et al. Gene dosage as a relevant mechanism contributing to the determination of ovarian function in Turner syndrome. Hum Reprod 2014;29:368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauldwell M, Steer PJ, Adamson D, Alexander C, Allen L, Bhagra C, Bolger A, Bonner S, Calanchini M, Carroll A. et al. Pregnancies in women with Turner syndrome: a retrospective multicentre UK study. BJOG 2022a;129:796–803. [DOI] [PubMed] [Google Scholar]
- Cauldwell M, Turner H, Curtis S, Steer P.. Authors’ reply re: pregnancies in women with Turner syndrome: a retrospective multicentre UK study. BJOG 2022b;129:1415–1416. [DOI] [PubMed] [Google Scholar]
- Cheng J, Ruan X, Du J, Jin F, Gu M, Wu Y, Mueck AO.. Ovarian tissue cryopreservation for a 3-year-old girl with Mosaic Turner syndrome in China: first case report and literature review. Front Endocrinol (Lausanne) 2022;13:959912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobo A, García-Velasco J, Domingo J, Pellicer A, Remohí J.. Elective and onco-fertility preservation: factors related to IVF outcomes. Hum Reprod 2018;33:2222–2231. [DOI] [PubMed] [Google Scholar]
- Cobo A, García-Velasco JA, Coello A, Domingo J, Pellicer A, Remohí J.. Oocyte vitrification as an efficient option for elective fertility preservation. Fertil Steril 2016;105:755–764.e8. [DOI] [PubMed] [Google Scholar]
- Doğer E, Çakıroğlu Y, Ceylan Y, Ulak E, Özdamar Ö, Çalışkan E.. Reproductive and obstetric outcomes in mosaic Turner’s syndrome: a cross-sectional study and review of the literature. Reprod Biol Endocrinol 2015;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmans MM, Donnez J, Cacciottola L.. Fertility preservation: the challenge of freezing and transplanting ovarian tissue. Trends Mol Med 2021;27:777–791. [DOI] [PubMed] [Google Scholar]
- Dunlop CE, Jack SA, Telfer EE, Zahra S, Anderson RA.. Clinical pregnancy in Turner syndrome following re-implantation of cryopreserved ovarian cortex. J Assist Reprod Genet 2023;40:2385–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shawarby SA, Sharif F, Conway G, Serhal P, Davies M.. Oocyte cryopreservation after controlled ovarian hyperstimulation in mosaic Turner syndrome: another fertility preservation option in a dedicated UK clinic. BJOG 2010;117:234–237. [DOI] [PubMed] [Google Scholar]
- El-Shawarby SA, Steer CV.. Spontaneous monozygotic monochorionic twin pregnancy in non-mosaic Turner’s syndrome. Int J Gynaecol Obstet 2007;97:155. [DOI] [PubMed] [Google Scholar]
- Falsey E, Cirino AL, Snyder E, Steeves M, Lin AE.. Parenthood among individuals with Turner syndrome: results of an online survey of attitudes towards pregnancy, adoption, and surrogacy. J Community Genet 2022;13:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon K. Infertility, reproductive timing and ‘cure’ in families affected by Turner syndrome. Soc Sci Med 2023a;328:116005. [DOI] [PubMed] [Google Scholar]
- Fearon K. What do families affected by Turner syndrome think of ovarian tissue freezing in childhood? Hum Fertil (Camb) 2023b;26:355–364. [DOI] [PubMed] [Google Scholar]
- Fitz VW, Law JR, Peavey M.. Karyotype is associated with timing of ovarian failure in women with Turner syndrome. J Pediatr Endocrinol Metab 2021;34:319–323. [DOI] [PubMed] [Google Scholar]
- Freriks K, Timmers HJ, Netea-Maier RT, Beerendonk CC, Otten BJ, van Alfen-van der Velden JA, Traas MA, Mieloo H, van de Zande GW, Hoefsloot LH. et al. Buccal cell FISH and blood PCR-Y detect high rates of X chromosomal mosaicism and Y chromosomal derivatives in patients with Turner syndrome. Eur J Med Genet 2013;56:497–501. [DOI] [PubMed] [Google Scholar]
- Giles J, Meseguer M, Mercader A, Rubio C, Alegre L, Vidal C, Trabalon M, Bosch E.. Preimplantation genetic testing for aneuploidy in patients with partial X monosomy using their own oocytes: is this a suitable indication? Fertil Steril 2020;114:346–353. [DOI] [PubMed] [Google Scholar]
- Gosden RG. Ovulation 1: oocyte development throughout life. In: Grudzinskas JG, Yovich JL (eds). Gametes—The Oocyte. United Kingdom: Cambridge University, 1995. [Google Scholar]
- Graff A, Donadille B, Morel H, Villy MC, Bourcigaux N, Vatier C, Borgel A, Khodawardi A, Siffroi JP, Christin-Maitre S.. Added value of buccal cell FISH analysis in the diagnosis and management of Turner syndrome. Hum Reprod 2020;35:2391–2398. [DOI] [PubMed] [Google Scholar]
- Granero-Molina J, Román RA, Del Mar Jiménez-Lasserrotte M, Ruiz-Fernández MD, Ventura-Miranda MI, Granero-Heredia G, Fernández-Medina IM. ‘I’m still a woman’: a qualitative study on sexuality in heterosexual women with Turner syndrome. J Clin Nurs 2023;32:6634–6647. [DOI] [PubMed] [Google Scholar]
- Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, Lin AE, Mauras N, Quigley CA, Rubin K. et al. ; International Turner Syndrome Consensus Group. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol 2017;177:G1–G70. [DOI] [PubMed] [Google Scholar]
- Grewal J, Valente AM, Egbe AC, Wu FM, Krieger EV, Sybert VP, van Hagen IM, Beauchesne LM, Rodriguez FH, Broberg CS. et al. ; AARCC Investigators. Cardiovascular outcomes of pregnancy in Turner syndrome. Heart 2021;107:61–66. [DOI] [PubMed] [Google Scholar]
- Grynberg M, Pytel S, Peigne M, Sonigo C.. The follicular output rate in normo-ovulating women undergoing ovarian stimulation is increased after unilateral oophorectomy. Hum Reprod 2023;38:1162–1167. [DOI] [PubMed] [Google Scholar]
- Gungor N, Güngör K, Kavrut M, Yurci A.. A retrospective series of homologous intracytoplasmic sperm injection cycle results of 99 women with mosaic Turner syndrome. Clin Exp Obstet Gynecol 2021;48:942–948. [Google Scholar]
- Hadnott TN, Gould HN, Gharib AM, Bondy CA.. Outcomes of spontaneous and assisted pregnancies in Turner syndrome: the U.S. National Institutes of Health Experience. Fertil Steril 2011;95:2251–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen CP, Aksglaede L, Sørensen K, Main KM, Boas M, Cleemann L, Holm K, Gravholt CH, Andersson AM, Pedersen AT. et al. Serum levels of anti-Müllerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. J Clin Endocrinol Metab 2010a;95:5003–5010. [DOI] [PubMed] [Google Scholar]
- Hagen CP, Main KM, Kjaergaard S, Juul A.. FSH, LH, inhibin B and estradiol levels in Turner syndrome depend on age and karyotype: longitudinal study of 70 Turner girls with or without spontaneous puberty. Hum Reprod 2010b;25:3134–3141. [DOI] [PubMed] [Google Scholar]
- Hagman A, Källén K, Barrenäs ML, Landin-Wilhelmsen K, Hanson C, Bryman I, Wennerholm UB.. Obstetric outcomes in women with Turner karyotype. J Clin Endocrinol Metab 2011;96:3475–3482. [DOI] [PubMed] [Google Scholar]
- Hagman A, Källén K, Bryman I, Landin-Wilhelmsen K, Barrenäs ML, Wennerholm UB.. Morbidity and mortality after childbirth in women with Turner karyotype. Hum Reprod 2013;28:1961–1973. [DOI] [PubMed] [Google Scholar]
- Hamza RT, Mira MF, Hamed AI, Ezzat T, Sallam MT.. Anti-Müllerian hormone levels in patients with Turner syndrome: relation to karyotype, spontaneous puberty, and replacement therapy. Am J Med Genet A 2018;176:1929–1934. [DOI] [PubMed] [Google Scholar]
- Hankus M, Soltysik K, Szeliga K, Antosz A, Drosdzol-Cop A, Wilk K, Zachurzok A, Malecka-Tendera E, Gawlik AM.. Prediction of spontaneous puberty in Turner syndrome based on mid-childhood gonadotropin concentrations, karyotype, and ovary visualization: a longitudinal study. Horm Res Paediatr 2018;89:90–97. [DOI] [PubMed] [Google Scholar]
- Homer L, Le Martelot MT, Morel F, Amice V, Kerlan V, Collet M, De Braekeleer M.. 45,X/46,XX mosaicism below 30% of aneuploidy: clinical implications in adult women from a reproductive medicine unit. Eur J Endocrinol 2010;162:617–623. [DOI] [PubMed] [Google Scholar]
- Huang JY, Tulandi T, Holzer H, Lau NM, Macdonald S, Tan SL, Chian RC.. Cryopreservation of ovarian tissue and in vitro matured oocytes in a female with mosaic Turner syndrome: case report. Hum Reprod 2008;23:336–339. [DOI] [PubMed] [Google Scholar]
- Ito A, Katagiri Y, Tamaki Y, Fukuda Y, Oji A, Morita M.. DuoStim: a new option for fertility preservation for a woman with Turner syndrome. Gynecol Endocrinol 2020;36:1144–1148. [DOI] [PubMed] [Google Scholar]
- Jensen AK, Rechnitzer C, Macklon KT, Ifversen MR, Birkebæk N, Clausen N, Sørensen K, Fedder J, Ernst E, Andersen CY.. Cryopreservation of ovarian tissue for fertility preservation in a large cohort of young girls: focus on pubertal development. Hum Reprod 2017;32:154–164. [DOI] [PubMed] [Google Scholar]
- Johannsen TH, Main KM, Ljubicic ML, Jensen TK, Andersen HR, Andersen MS, Petersen JH, Andersson AM, Juul A.. Sex differences in reproductive hormones during mini-puberty in infants with normal and disordered sex development. J Clin Endocrinol Metab 2018;103:3028–3037. [DOI] [PubMed] [Google Scholar]
- Johnson EK, Rosoklija I, Shurba A, D’Oro A, Gordon EJ, Chen D, Finlayson C, Holl JL.. Future fertility for individuals with differences of sex development: parent attitudes and perspectives about decision-making. J Pediatr Urol 2017;13:402–413. [DOI] [PubMed] [Google Scholar]
- Joshi VB, Behl S, Pittock ST, Arndt CAS, Zhao Y, Khan Z, Granberg CF, Chattha A.. Establishment of a pediatric ovarian and testicular cryopreservation program for malignant and non-malignant conditions: the Mayo Clinic experience. J Pediatr Adolesc Gynecol 2021;34:673–680. [DOI] [PubMed] [Google Scholar]
- Kallio S, Aittomäki K, Piltonen T, Veijola R, Liakka A, Vaskivuo TE, Dunkel L, Tapanainen JS.. Anti-Mullerian hormone as a predictor of follicular reserve in ovarian insufficiency: special emphasis on FSH-resistant ovaries. Hum Reprod 2012;27:854–860. [DOI] [PubMed] [Google Scholar]
- Kavoussi SK, Fisseha S, Smith YR, Smith GD, Christman GM, Gago LA.. Oocyte cryopreservation in a woman with mosaic Turner syndrome: a case report. J Reprod Med 2008;53:223–226. [PubMed] [Google Scholar]
- King JE, Plamondon J, Counts D, Laney D, Dixon SD.. Barriers in communication and available resources to facilitate conversation about infertility with girls diagnosed with Turner syndrome. J Pediatr Endocrinol Metab 2016;29:185–191. [DOI] [PubMed] [Google Scholar]
- Komura N, Mabuchi S, Sawada K, Nishio Y, Kimura T, Komura H.. Subsequent menstrual disorder after spontaneous menarche in Turner syndrome. Clin Endocrinol (Oxf) 2021;95:163–168. [DOI] [PubMed] [Google Scholar]
- Kooijman SS, Duijnhouwer AL, van Kimmenade RRJ, van Dijk APJ, Hink E, de Boer MJ, Timmermans J, Roos-Hesselink JW.. Influence of pregnancy on aortic diameter in women with the Turner syndrome. Am J Cardiol 2021;140:122–127. [DOI] [PubMed] [Google Scholar]
- Lambalk CB, de Koning CH, Flett A, Van Kasteren Y, Gosden R, Homburg R.. Assessment of ovarian reserve. Ovarian biopsy is not a valid method for the prediction of ovarian reserve. Hum Reprod 2004;32:2485–2495. [DOI] [PubMed] [Google Scholar]
- Lau NM, Huang JY, MacDonald S, Elizur S, Gidoni Y, Holzer H, Chian RC, Tulandi T, Tan SL.. Feasibility of fertility preservation in young females with Turner syndrome. Reprod Biomed Online 2009;18:290–295. [DOI] [PubMed] [Google Scholar]
- Liao J, Luo K, Cheng D, Xie P, Tan Y, Hu L, Lu G, Gong F, Lin G.. Reproductive outcomes after preimplantation genetic testing in mosaic Turner syndrome: a retrospective cohort study of 100 cycles. J Assist Reprod Genet 2021;38:1247–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AE, Karnis MF, Calderwood L, Crenshaw M, Bhatt A, Souter I, Silberbach M, Reindollar RH.. Proposal for a national registry to monitor women with Turner syndrome seeking assisted reproductive technology. Fertil Steril 2016;105:1446–1448. [DOI] [PubMed] [Google Scholar]
- Ljubicic ML, Busch AS, Upners EN, Fischer MB, Petersen JH, Raket LL, Frederiksen H, Johannsen TH, Juul A, Hagen CP.. A biphasic pattern of reproductive hormones in healthy female infants: the COPENHAGEN Minipuberty study. J Clin Endocrinol Metab 2022;107:2598–2605. [DOI] [PubMed] [Google Scholar]
- Lundgaard Riis M, Nielsen JE, Hagen CP, Rajpert-De Meyts E, Græm N, Jørgensen A, Juul A.. Accelerated loss of oogonia and impaired folliculogenesis in females with Turner syndrome start during early fetal development. Hum Reprod 2021;36:2992–3002. [DOI] [PubMed] [Google Scholar]
- Lunding SA, Aksglaede L, Anderson RA, Main KM, Juul A, Hagen CP, Pedersen AT.. AMH as predictor of premature ovarian insufficiency: a longitudinal study of 120 Turner syndrome patients. J Clin Endocrinol Metab 2015;100:E1030–E1038. [DOI] [PubMed] [Google Scholar]
- Maggio MC, De Pietro A, Porcelli P, Serraino F, Angileri T, Di Peri A, Corsello G.. The predictive role of pelvic magnetic resonance in the follow up of spontaneous or induced puberty in Turner syndrome. Ital J Pediatr 2018;44:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus MC, Wilcox AJ, Morken NH, Weinberg CR, Håberg SE.. Role of maternal age and pregnancy history in risk of miscarriage: prospective register based study. BMJ 2019;364:l869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamsen LS, Charkiewicz K, Anderson RA, Telfer EE, McLaughlin M, Kelsey TW, Kristensen SG, Gook DA, Ernst E, Andersen CY.. Characterization of follicles in girls and young women with Turner syndrome who underwent ovarian tissue cryopreservation. Fertil Steril 2019;111:1217–1225.e3. [DOI] [PubMed] [Google Scholar]
- Manno M, Tomei F, Cervi M, Gaspardo G, Antonini-Canterin F, Nicolosi G.. Homologous in vitro fertilization in Turner syndrome: insights from a case report. Fertil Steril 2009;91:1294–1294.e1. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM.. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel RA, Blakemore JK, Fino ME.. The use of oocyte cryopreservation for fertility preservation in patients with sex chromosome disorders: a case series describing outcomes. J Assist Reprod Genet 2022;39:1143–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín M, Adeba A, Hera JM, Martínez J, Cigarrán H, Alvarez-Cabo R.. Turner syndrome, unicuspid aortopathy, and pregnancy: difficult decisions for complex scenarios. Arch Cardiol Mex 2021;91:252–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas M, Oliver J, Bhandari H.. Routes to parenthood for women with Turner syndrome. Obstet Gynaecol 2019;21:43–50. [Google Scholar]
- Mavridi A, Ntali G, Theodora M, Stamatelopoulos K, Michala L.. A spontaneous pregnancy in a patient with Turner syndrome with 45,x/47,xxx mosaicism: a case report and review of the literature. J Pediatr Adolesc Gynecol 2018;31:651–654. [DOI] [PubMed] [Google Scholar]
- Mercer CL, Lachlan K, Karcanias A, Affara N, Huang S, Jacobs PA, Thomas NS.. Detailed clinical and molecular study of 20 females with Xq deletions with special reference to menstruation and fertility. Eur J Med Genet 2013;56:1–6. [DOI] [PubMed] [Google Scholar]
- Messina MF, Aversa T, Salzano G, Costanzo D, Sferlazzas C, Mirabelli S, Zirilli G, Lombardo F.. Inhibin B in adolescents and young adults with Turner syndrome. J Pediatr Endocrinol Metab 2015;28:1209–1214. [DOI] [PubMed] [Google Scholar]
- Modi DN, Sane S, Bhartiya D.. Accelerated germ cell apoptosis in sex chromosome aneuploid fetal human gonads. Mol Hum Reprod 2003;9:219–225. [DOI] [PubMed] [Google Scholar]
- Moore AK, Lynch K, Arny MJ, Dr G.. Turner mosaicism (45,X/46,XX) diagnosed in a young woman subsequent to low oocyte maturity and failed ICSI. Fertil Steril 2008;90:2012–2015.e13. [DOI] [PubMed] [Google Scholar]
- Morgan TL, Kapa HM, Crerand CE, Kremen J, Tishelman A, Davis S, Nahata L.. Fertility counseling and preservation discussions for females with Turner syndrome in pediatric centers: practice patterns and predictors. Fertil Steril 2019;112:740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen KH, Rohde MD, Uldbjerg N, Gravholt CH.. Repeated spontaneous pregnancies in 45,X Turner syndrome. Obstet Gynecol 2010;115:446–449. [DOI] [PubMed] [Google Scholar]
- Mumford BK, Hendriks S, Gomez-Lobo V.. Should ovarian tissue cryopreservation in pediatric patients with Turner syndrome be limited to the research setting? J Pediatr Adolesc Gynecol 2023;36:566–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E.. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 2018;18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Hinokio K, Kiyokawa M, Morine M, Iwasa T.. Successful advanced maternal age pregnancy with mosaic Turner syndrome conceived after ovulation induction with clomiphene citrate: a case report. Case Rep Obstet Gynecol 2014;2014:934740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadesapillai S, van der Coelen S, Goebel L, Peek R, Braat DD, van der Velden JA, Fleischer K, Oerlemans AJ.. Deciding on future fertility: considerations of girls with Turner syndrome and their parents to opt for or against ovarian tissue cryopreservation. Reprod Biomed Online 2023a;46:1017–1025. [DOI] [PubMed] [Google Scholar]
- Nadesapillai S, van der Velden J, Braat D, Peek R, Fleischer K.. The challenge of defining predictive parameters for fertility preservation counseling in young females with Turner syndrome. Acta Obstet Gynecol Scand 2021a;100:1155–1156. [DOI] [PubMed] [Google Scholar]
- Nadesapillai S, van der Velden J, Smeets D, van de Zande G, Braat D, Fleischer K, Peek R.. Why are some patients with 45,X Turner syndrome fertile? A young girl with classical 45,X Turner syndrome and a cryptic mosaicism in the ovary. Fertil Steril 2021b;115:1280–1287. [DOI] [PubMed] [Google Scholar]
- Nadesapillai S, van der Velden J, van der Coelen S, Schleedoorn M, Sedney A, Spath M, Schurink M, Oerlemans A, IntHout J, Beerendonk I. et al. TurnerFertility trial: fertility preservation in young girls with Turner syndrome by freezing ovarian cortex tissue—a prospective intervention study. Fertil Steril 2023b;120:1048–1060. [DOI] [PubMed] [Google Scholar]
- Nawroth F, Schüring AN, von Wolff M.. The indication for fertility preservation in women with Turner syndrome should not only be based on the ovarian reserve but also on the genotype and expected future health status. Acta Obstet Gynecol Scand 2020;99:1579–1583. [DOI] [PubMed] [Google Scholar]
- Negreiros LP, Bolina ER, Guimarães MM.. Pubertal development profile in patients with Turner syndrome. J Pediatr Endocrinol Metab 2014;27:845–849. [DOI] [PubMed] [Google Scholar]
- Obata S, Tsuburai T, Shindo R, Aoki S, Miyagi E, Sakakibara H.. Current situation and outcomes of pregnancy in women with Turner syndrome in Japan. J Obstet Gynaecol Res 2020;46:1728–1734. [DOI] [PubMed] [Google Scholar]
- Oktay K, Bedoschi G.. Oocyte cryopreservation for fertility preservation in postpubertal female children at risk for premature ovarian failure due to accelerated follicle loss in Turner syndrome or cancer treatments. J Pediatr Adolesc Gynecol 2014;27:342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay K, Bedoschi G.. Fertility preservation in girls with Turner syndrome: limitations, current success and future prospects. Fertil Steril 2019;111:1124–1126. [DOI] [PubMed] [Google Scholar]
- Oktay K, Rodriguez-Wallberg KA, Sahin G.. Fertility preservation by ovarian stimulation and oocyte cryopreservation in a 14-year-old adolescent with Turner syndrome mosaicism and impending premature ovarian failure. Fertil Steril 2010;94:753–759.e15. [DOI] [PubMed] [Google Scholar]
- Paulson RJ. Pregnancies in women with Turner syndrome: how do we counsel our patients? Fertil Steril 2019;112:219. [DOI] [PubMed] [Google Scholar]
- Peek R, Nadesapillai S, Thi Nguyen TY, Vassart S, Smeets D, van de Zande G, Camboni A, Braat D, van der Velden J, Donnez J. et al. Assessment of folliculogenesis in ovarian tissue from young patients with Turner syndrome using a murine xenograft model. Fertil Steril 2023;120:371–381. [DOI] [PubMed] [Google Scholar]
- Peek R, Schleedoorn M, Smeets D, van de Zande G, Groenman F, Braat D, van der Velden J, Fleischer K.. Ovarian follicles of young patients with Turner’s syndrome contain normal oocytes but monosomic 45,X granulosa cells. Hum Reprod 2019;34:1686–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman R, Lazareva O, Oktay K, Ten S.. Markers of ovarian reserve in young girls with Turner’s syndrome. Fertil Steril 2010;94:1557–1559. [DOI] [PubMed] [Google Scholar]
- Radkowska-Walkowicz M, Maciejewska-Mroczek E.. ‘Should I Buy Her a Doll’? Motherhood and Turner syndrome in Poland. Med Anthropol 2023;42:177–190. [DOI] [PubMed] [Google Scholar]
- Ramage K, Grabowska K, Silversides C, Quan H, Metcalfe A.. Maternal, pregnancy, and neonatal outcomes for women with Turner syndrome. Birth Defects Res 2020;112:1067–1073. [DOI] [PubMed] [Google Scholar]
- Reynaud K, Cortvrindt R, Verlinde F, De Schepper J, Bourgain C, Smitz J.. Number of ovarian follicles in human fetuses with the 45,X karyotype. Fertil Steril 2004;81:1112–1119. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Wallberg KA, Landin-Wilhelmsen K; Swedish Turner Academy. The complexity of fertility preservation for women with Turner syndrome and the potential risks of pregnancy and cardiovascular complications. Acta Obstet Gynecol Scand 2020;99:1577–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Wallberg KA, Sergouniotis F, Nilsson HP, Lundberg FE.. Trends and outcomes of fertility preservation for girls, adolescents and young adults with Turner syndrome: a prospective cohort study. Front Endocrinol (Lausanne) 2023;14:1135249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers PA, Murphy CR, Leeton J, Hoise MJ, Beaton L.. Turner’s syndrome patients lack tight junctions between uterine epithelial cells. Hum Reprod 1992;7:883–885. [DOI] [PubMed] [Google Scholar]
- Ros C, Serra A, Balasch J, Margarit E, Castelo-Branco C.. Comparative cytogenetic analysis in two tissues with different lineage in Turner’s syndrome patients: correlation with phenotype. Gynecol Endocrinol 2014;30:282–286. [DOI] [PubMed] [Google Scholar]
- Rosendahl M, Simonsen MK, Kjer JJ.. The influence of unilateral oophorectomy on the age of menopause. Climacteric 2017;20:540–544. [DOI] [PubMed] [Google Scholar]
- Ruszała A, Wójcik M, Starzyk J.. Evaluation of the usefulness of antymüllerian hormone and inhibin B as markers of ovarian activity in patients with Turner syndrome—preliminary results. Pediatr Endocrinol Diabetes Metab 2020;26:84–88. [DOI] [PubMed] [Google Scholar]
- Schleedoorn M, van der Velden J, Braat D, Beerendonk I, van Golde R, Peek R, Fleischer K.. TurnerFertility trial: PROTOCOL for an observational cohort study to describe the efficacy of ovarian tissue cryopreservation for fertility preservation in females with Turner syndrome. BMJ Open 2019;9:e030855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleedoorn MJ, Fleischer K, Braat D, Oerlemans A, van der Velden A, Peek R.. Why Turner patients with 45, X monosomy should not be excluded from fertility preservation services. Reprod Biol Endocrinol 2022;20:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleedoorn MJ, Mulder BH, Braat DDM, Beerendonk CCM, Peek R, Nelen W, Van Leeuwen E, Van der Velden A, Fleischer K, Turner Fertility Expert Panel O.. International consensus: ovarian tissue cryopreservation in young Turner syndrome patients: outcomes of an ethical Delphi study including 55 experts from 16 different countries. Hum Reprod 2020;35:1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderström-Anttila V, Pinborg A, Karnis MF, Reindollar RH, Paulson RJ.. Should women with Turner syndrome be allowed to carry their own pregnancies? Fertil Steril 2019;112:220–225. [DOI] [PubMed] [Google Scholar]
- Strypstein L, Van Moer E, Nekkebroeck J, Segers I, Tournaye H, Demeestere I, Dolmans MM, Verpoest W, De Vos M.. First live birth after fertility preservation using vitrification of oocytes in a woman with mosaic Turner syndrome. J Assist Reprod Genet 2022;39:543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton EJ, McInerney-Leo A, Bondy CA, Gollust SE, King D, Biesecker B.. Turner syndrome: four challenges across the lifespan. Am J Med Genet A 2005;139a:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggar AA, Engmann LL.. Should we proceed with more caution when considering additional autologous fertility solutions for some patients with classic Turner syndrome? Fertil Steril 2021;115:1173–1174. [DOI] [PubMed] [Google Scholar]
- Talaulikar VS, Conway GS, Pimblett A, Davies MC.. Outcome of ovarian stimulation for oocyte cryopreservation in women with Turner syndrome. Fertil Steril 2019;111:505–509. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Igarashi Y, Ozono K, Ohyama K, Ogawa M, Osada H, Onigata K, Kanzaki S, Kohno H, Seino Y. et al. Frequencies of spontaneous breast development and spontaneous menarche in Turner syndrome in Japan. Clin Pediatr Endocrinol 2015;24:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velde ER, Pearson PL.. The variability of female reproductive ageing. Hum Reprod Update 2002;8:141–154. [DOI] [PubMed] [Google Scholar]
- Theroux CI, Elliott V, Davis S, Crerand CE, Kremen J, Tishelman A, Hutaff-Lee C, Nahata L.. Fertility counseling practices for patients with Turner syndrome in pediatric endocrine clinics: results of a Pediatric Endocrine Society Survey. Horm Res Paediatr 2022;95:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L. et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–473. [DOI] [PubMed] [Google Scholar]
- Ulrich ND, Raja N, Ellman E, Moravek MB.. Outcomes of fertility preservation consults for women at risk for primary ovarian insufficiency due to history of cancer treatment or mosaic Turner syndrome. J Adolesc Young Adult Oncol 2022;11:427–432. [DOI] [PubMed] [Google Scholar]
- van der Coelen S, van der Velden J, Nadesapillai S, Peek R, Braat D, Schleedoorn M, Fleischer K, Oerlemans A.. The decision-making process regarding ovarian tissue cryopreservation in girls with Turner syndrome by patients, parents, and healthcare providers: a mixed-methods study. Horm Res Paediatr 2022;95:374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hagen IM, Duijnhouwer AL, Ten Kate-Booij MJ, Dykgraaf RH, Duvekot JJ, Utens EM, Roos-Hesselink JW.. Wish to conceive and concerns to develop cardiovascular complications during pregnancy in patients with Turner syndrome. J Psychosom Obstet Gynaecol 2017;38:45–52. [DOI] [PubMed] [Google Scholar]
- Vergier J, Bottin P, Saias J, Reynaud R, Guillemain C, Courbiere B.. Fertility preservation in Turner syndrome: karyotype does not predict ovarian response to stimulation. Clin Endocrinol (Oxf) 2019;91:646–651. [DOI] [PubMed] [Google Scholar]
- Visser JA, Hokken-Koelega AC, Zandwijken GR, Limacher A, Ranke MB, Flück CE.. Anti-Müllerian hormone levels in girls and adolescents with Turner syndrome are related to karyotype, pubertal development and growth hormone treatment. Hum Reprod 2013;28:1899–1907. [DOI] [PubMed] [Google Scholar]
- Wang J, Lan T, Dai X, Yang L, Hu X, Yao H.. The cut-off value of serum anti-Müllerian hormone levels for the diagnosis of Turner syndrome with spontaneous puberty. Int J Endocrinol 2023;2023:6976389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman D, Asch A.. Reproductive medicine and Turner syndrome: ethical issues. Fertil Steril 2012;98:792–796. [DOI] [PubMed] [Google Scholar]
- Witkowska-Krawczak E, Erazmus M, Majcher A, Pyrżak B, Kucharska AM.. Predicted health care profile after transition to adult care in Turner syndrome children-experience of single center. Front Pediatr 2023;11:1173419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HH, Li H.. Karyotype classification, clinical manifestations and outcome in 124 Turner syndrome patients in China. Ann Endocrinol (Paris) 2019;80:10–15. [DOI] [PubMed] [Google Scholar]
- Zajicek M, Volodarsky-Perel A, Shai D, Dick-Necula D, Raanani H, Gruber N, Karplus G, Kassif E, Weisz B, Meirow D.. Evaluation of ovarian reserve in young females with non-iatrogenic ovarian insufficiency to establish criteria for ovarian tissue cryopreservation. Reprod Biomed Online 2023;47:102–109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from screening and full-text review are available from authors upon reasonable request.