Abstract
Human respiratory syncytial virus (RSV) F glycoprotein (RSV-F) can independently interact with immobilized heparin and facilitate both attachment to and infection of cells via an interaction with cellular heparan sulfate. RSV-glycosaminoglycan (GAG) interactions were evaluated using heparin-agarose affinity chromatography. RSV-F from A2- and B1/cp-52 (cp-52)-infected cell lysates, RSV-F derived from a recombinant vaccinia virus, and affinity-purified F protein all bound to and were specifically eluted from heparin columns. In infectivity inhibition studies, soluble GAGs decreased the infectivity of RSV A2 and cp-52, with bovine lung heparin exhibiting the highest specific activity against both A2 (50% effective dose [ED50] = 0.28 ± 0.11 μg/ml) and cp-52 (ED50 = 0.55 ± 0.14 μg/ml). Furthermore, enzymatic digestion of cell surface GAGs by heparin lyase I and heparin lyase III but not chondroitinase ABC resulted in a significant reduction in cp-52 infectivity. Moreover, bovine lung heparin inhibited radiolabeled A2 and cp-52 virus binding up to 90%. Taken together, these data suggest that RSV-F independently interacts with heparin/heparan sulfate and this type of interaction facilitates virus attachment and infectivity.
Human respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract disease in infants and young children worldwide (for a review, see reference 8). The virus contains three surface glycoproteins, designated the attachment (RSV-G), fusion (RSV-F), and small hydrophobic (RSV-SH) proteins. While early studies suggested that RSV-G was necessary for attachment (21), recent evidence indicates that RSV-G is not absolutely required for either RSV binding or infectivity (18).
Previously, a cold-passaged (cp) RSV subgroup B candidate vaccine, designated RSV B1/cp-52 (cp-52), was described that replicated efficiently in vitro (18) and in vivo (9) despite a large deletion that prevents the synthesis of both the RSV-G and -SH proteins. Therefore, the attachment and infectivity of this virus strain could be directly attributed to RSV-F. While it appears that RSV-F alone can mediate both RSV attachment and infectivity, the fusion function of RSV-F is enhanced in the presence of RSV-G and -SH (15).
RSV-F is primarily responsible for penetration of the virus into host cells and subsequent cell-to-cell spread or syncytium formation during RSV infection (28). RSV-F protein is initially translated into the 70-kDa inactive precursor, F0, which is cotranslationally modified by the addition of N-linked carbohydrate (7). F0 is then endoproteolytically cleaved by a cellular trypsin-like protease into two disulfide-linked subunits, F1 and F2, of 48 and 23 kDa, respectively (13, 14). Upon cleavage, the highly conserved hydrophobic N terminus or fusion peptide of the F1 protein is exposed and thought to mediate virus fusion by inserting itself in the target cell membrane (1, 6). In a recent report, RSV-F was shown to interact with RhoA, a small cellular GTPase (25); however, because RhoA inserts itself into the cytoplasmic side of the plasma membrane and is not expressed on the extracellular membrane surface, it seems likely that this is not a receptor for RSV-F.
RSV-G is known to mediate an interaction with immobilized heparin and cellular heparan sulfate (19), and one region responsible for this interaction lies within a linear heparin-binding domain (HBD) of the G protein ectodomain (11). The mechanism by which RSV-F can mediate attachment to cells is not known at this time, and no such interaction with heparin has been described for the F protein. In the present study, we sought to determine if RSV-F could interact with heparin and if such an interaction occurred, whether it was biologically relevant.
Here we report that RSV-F from strains A2 and cp-52 can specifically bind to heparin. Soluble glycosaminoglycans (GAGs) were also able to inhibit both viruses from binding to and infecting Vero cells. In addition, treatment of cells with GAG-specific lyases significantly reduced virus infectivity. Taken together, these data suggest that heparan-like molecules on the surface of cells can be used efficiently by RSV-F to facilitate attachment to and infection of host cells.
MATERIALS AND METHODS
Cells, virus, and purified viral proteins.
Vero cells were grown in Eagle's minimal essential medium (EMEM) containing Earle's salts and 10% fetal bovine serum (FBS; BioWhittaker, Walkersville, Md.). Human RSV strain A2 and measles virus (Edmonston strain) were prepared in Vero cells incubated at 37°C. cp-52 virus was propagated in Vero cells held at 32°C. Recombinant vaccinia viruses expressing the RSV-G (10) or RSV-F (24) gene were also grown on Vero cells. Viral harvests (RSV and measles virus only) were adjusted to 100 mM MgSO4 and 50 mM HEPES (BioWhittaker) prior to clarification at 10,000 × g for 30 min. Virus was concentrated as previously described (22) or pelleted directly from the tissue culture supernatant by overnight centrifugation (9,000 rpm, SS34 rotor) and resuspended in EMEM containing 1% FBS, 100 mM MgSO4, and 50 mM HEPES. Infected cell pellets were resuspended in lysis buffer (20 mM Tris [pH 7.4], 1% NP-40, 0.1 mg of phenylmethylsulfonyl fluoride per ml, 1 μg of aprotinin per ml) for 30 min on ice. Lysates were clarified by centrifugation at 13,000 × g for 5 min, and supernatants (referred to as infected cell lysates) were frozen at −70°C until further use. Protein assays were conducted using the Bio-Rad protein assay according to the method of Bradford (2). Virus was prepared either unlabeled or radiolabeled with Promix 35S-labeled methionine and cysteine (Amersham, Arlington Heights, Ill.; methionine, 12 mCi/ml; cysteine, 11 mCi/ml; specific activities, 9.2 mCi/μg) by adding 50 μCi/ml into methionine- and cysteine-deficient EMEM supplemented with 1% FBS and 2 μg of actinomycin D (Calbiochem, La Jolla, Calif.) per ml 48 to 72 h postinfection. Labeling was allowed to continue for 12 h before harvesting. The titers were 104.2 and 106.2 50% tissue culture infective doses (TCID50) for radiolabeled cp-52 and A2, respectively, and the specific activities for both viruses were 2 × 105 to 3 × 105 cpm/μg of viral protein.
Purified RSV-G and F protein were provided by Wyeth-Lederle Vaccines, and the purity was determined to be 98 and 99%, respectively, based on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis.
HAAC.
For heparin-agarose affinity chromatography (HAAC), chromatography on 1 ml of heparin-agarose or uncoupled cross-linked CL4b agarose (Sigma, St. Louis, Mo.) columns equilibrated with MTN buffer (25 mM morpholineethanesulfonic acid [MES]-NaOH [pH 5.7], 0.1% Triton X-100, 100 mM NaCl) was carried out as previously described with some exceptions (19). Clarified lysates in MTN buffer were applied to the column at a protein concentration of 250 μg of lysate per ml of heparin-agarose and washed twice with 10 column volumes of MTN buffer. Bound protein was eluted with 10 column volumes of MTN buffer containing 2 mg of heparin (porcine intestinal mucosa; Sigma) per ml. The final wash and heparin-eluted fractions were concentrated using Centricon-10 microconcentrators by spinning at 5,000 × g for 1.5 h and analyzed by SDS-PAGE and Western blot. Alternatively, fractions were analyzed for RSV-specific proteins by immunoprecipitation with RSV-monospecific polyclonal antiserum directed against either the RSV-G or RSV-F glycoprotein followed by SDS-PAGE and Western blotting with the same antiserum.
Immunoprecipitation of RSV proteins.
Briefly, 100 μl of protein A-Sepharose (Sigma) was coated with RSV-G or -F monospecific polyclonal rabbit antiserum (1:1,000) before 1 ml of the eluted fraction was added. The sample was reacted overnight at 4°C with rotation, followed by washing three times with immunoprecipitation buffer (0.1 M Tris-HCl [pH 7.5], 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS). Samples were then resuspended in 1× sample buffer containing 5% 2-mercaptoethanol and boiled for 3 min prior to SDS-PAGE and RSV-specific Western blot analysis (20, 27). Blots were probed with either an anti-G, anti-F, or anti-SH monoclonal or polyclonal antiserum diluted 1:1,000, and specific bands were detected using a 1:1,000 dilution of a peroxidase-conjugated goat anti-mouse or goat anti-rabbit immunoglobulin antibody (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) and TMB (3,3′,5,5′-tetramethylbenzidine)-peroxidase substrate (Kirkegaard and Perry Laboratories).
Determination of RSV infectious titers by TCID50 or plaque assay.
Serial dilutions of virus in EMEM containing 1% FBS were inoculated onto confluent Vero cell monolayers in 96-well plates and incubated at 37°C (strain A2) for 6 days. Endpoint titers were determined by the method of Reed and Muench (16). For the titration of cp-52, a plaque assay was performed as described above except after a 1-h virus adsorption, the virus inoculum was removed and cells were overlaid with 100 μl of EMEM containing 1% FBS and 1% methylcellulose. After 6 days, monolayers were fixed and stained with 1% crystal violet, and the plaques were enumerated after visualization under an inverted microscope.
Infectivity inhibition assays.
For infectivity inhibition assays, low-molecular-weight (LMW) heparin (Mr = 6000) from porcine intestinal mucosa, bovine lung (BVL) heparin, de-N-sulfated heparin from bovine intestinal mucosa, and chondroitin sulfate ABC from bovine mucosa were subjected to threefold serial dilution starting at 50 μg of GAG per well in quadruplicate. Diluted GAGs were then incubated with 100 TCID50 of A2, cp-52, or measles virus for 1.5 h at 37°C prior to being transferred to Vero cells in 96-well plates. RSV A2 and measles virus infectivity inhibition assays were terminated at 72 h postinfection, and the monolayers were fixed with 80% methanol, air dried, and then assayed by enzyme-linked immunosorbent assay (ELISA) for virus replication. However, due to limited antigen detectability of cp-52 by ELISA, a plaque assay format was used to quantitate infectivity inhibition of cp-52. Plaques were counted and percent infectivity was determined relative to the mock (phosphate-buffered saline [PBS; pH 7.4])-treated control.
ELISA.
Briefly, after blocking the fixed monolayers with 5% Blotto, viral proteins were detected by adding 50 μl of a polyclonal anti-F antiserum (RSV) or antimatrix antibody (measles virus; Chemicon International, Inc., Temecula, Calif.) diluted 1:1,000 in Blotto containing 0.05% Tween 20 and incubating for 1 h at 37°C. Plates were washed five times with PBS (pH 7.4) containing 0.05% Tween 20, followed by the addition of 50 μl of peroxidase-conjugated goat anti-rabbit or anti-mouse immunoglobulin antibody (1:1,000) for 1 h at 37°C in order to detect RSV- and measles virus-specific antibodies, respectively. The plates were washed, treated with 100 μl of ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)]-peroxidase substrate for 15 min and read on a Vmax kinetic plate reader at 405 nm. After subtracting out background absorbance, values were converted into percent infectivity by dividing the average absorbance of sample wells by the average absorbance from the mock-treated controls and multiplying by 100. In order for assays to be considered valid, the absorbance of the mock-treated virus control had to be at least twice that of the negative (no-virus) control.
Enzyme treatment of cells.
To further delineate the role of GAGs during the RSV infection process, Vero cells were treated with enzymes that cleave specific GAGs from the cell surface. Cells were treated with heparin lyase I (from Flavobacterium heparinum; Sigma), (0.92 IU/mg), heparin lyase III (from F. heparinum; Sigma) (0.37 IU/mg), or chondroitinase ABC (from Proteus vulgaris; Sigma) (0.004 IU/mg), which cleave heparin, heparan sulfate, and chondroitin sulfate, respectively. One hundred microliters of each diluted GAG lyase (see figure legends for enzyme concentrations) was added in quadruplicate wells to a 96-well plate for 1 h at 37°C. GAG lyase was removed, and the cells were washed once with PBS (pH 7.4) prior to inoculation with approximately 100 TCID50 of cp-52. After a 1-h adsorption period, the inoculum was removed and the cells were overlaid with EMEM containing 1% FBS and 1% methylcellulose. After 6 days, the cells were fixed and stained with 1% crystal violet. The number of plaques in the treated versus untreated cells was used to determine the effect of GAG lyase treatment on RSV infectivity. Enzymes purchased from Seikagaku America, Inc., gave identical results. Enzyme activity was assessed as described previously (23). Briefly, 25 μg of heparin, heparan sulfate, or chondroitin sulfate ABC diluted in 0.4 M NaCl was incubated with 2 Sigma units (SU) of heparin lyase I, heparin lyase III, or chondroitinase ABC, respectively, at 37°C for 45 min. GAGs in controls without enzymes were precipitated with 0.5 mg of cetylpyridinium chloride (Sigma) per ml, resulting in a cloudy solution. Enzymatic digestion of the GAGs was indicated if the solutions remained clear (heparin lyase III and chondroitinase ABC) or only slightly cloudy (heparin lyase I) following cetylpyridinium chloride treatment.
RSV binding assays.
35S-labeled RSV A2 or cp-52 was bound to Vero cells in suspension. Cells were prepared by washing cell monolayers with PBS (pH 7.4) followed by the addition of 10 ml of 1 mM EDTA to facilitate removal of cells. Cells were pelleted and washed twice with cold PBS (pH 7.4) before being counted. Labeled virus (50 μl; 5 × 104 cpm/tube) diluted in binding buffer (Tris-buffered saline [TBS] [pH 7.4], 1% bovine serum albumin [BSA; Sigma]) was added to duplicate 1.5-ml microcentrifuge tubes (VWR, West Chester, Pa.) containing the indicated concentration of bovine lung heparin (see figure legends for concentrations) and incubated for 30 min at 37°C. After the virus-GAG incubation, 50 μl of cells (4 × 105 cells) was added to each tube. Virus was allowed to bind for 1 h at 4°C before being washed twice with cold PBS (pH 7.4). The cell pellets were resuspended in 50 μl of PBS (pH 7.4) followed by the addition of 1 ml of EcoLume (ICN, Costa Mesa, Calif.) scintillation cocktail prior to being counted in a Beckman LS-5801 liquid scintillation counter.
Statistical analysis.
Statistical analysis was performed with the Prism version 3.0 software (Graphpad Software, Inc.). The results are expressed as the mean ± 1 standard error of the mean. Where appropriate, results were tested for significance by a one-way analysis of variance followed by the post hoc Tukey test to measure differences among groups. Results having P values of <0.05 were considered significant.
RESULTS
RSV-F binds heparin.
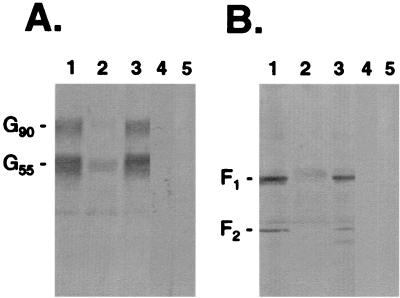
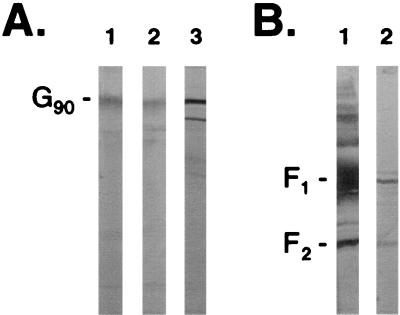
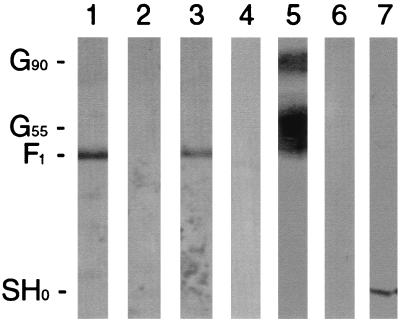
We and others have now shown that RSV-G from infected cell lysates can bind immobilized heparin (19) (Fig. 1A). In addition to G-heparin interactions, in this study RSV-F from infected cell lysates was shown to interact with heparin (Fig. 1B). Neither RSV-G nor -F protein from infected cell lysates bound when uncoupled cross-linked CL-4B agarose was used as a control (Fig. 1A and B, lanes 4). In addition, no antibody reactivity was detected when uninfected cell lysate was subjected to HAAC and screened by Western blot using an anti-G or anti-F antiserum (Fig. 1A and B, lanes 5). Further analysis using lysates from Vero cells infected with vaccinia virus recombinants expressing the genes for either RSV-Ga (subgroup A), RSV-Gb (subgroup B), or RSV-F provided additional evidence that the interactions between either subgroup of RSV-G (Fig. 2A) or RSV-F (Fig. 2B) and heparin were independent interactions. Similarly, affinity-purified RSV-G and -F proteins bound independently in HAAC, confirming that the interactions were not due to the presence of other viral or contaminating cellular proteins. In Fig. 3, a cp-52-infected cell lysate was probed for the presence of RSV surface glycoproteins by Western blot following HAAC. The lysate is positive for RSV-F (Fig. 3, lane 1). Due to a large deletion in its genome, cp-52 was unable to synthesize RSV-G (Fig. 3, lane 4) and SH (Fig. 3, lane 6). To control for the specificity of the antisera used to detect RSV-G and -SH, the anti-G and anti-SH antisera were used to probe an A2-infected Vero cell lysate (Fig. 3, lanes 5 and 7, respectively). The antisera reacted as expected, confirming cp-52's inability to express RSV-G and -SH. Following HAAC of the cp-52-infected cell lysate, RSV-F specifically bound and was eluted by heparin in the absence of the G or SH protein (Fig. 3, lane 3). In each case, specific elution of bound viral protein was significantly increased relative to the minute concentrations detected in the final wash, which was not unexpected due to the inherent leakiness in column chromatography of this type.
FIG. 1.
Heparin-agarose affinity chromatography of RSV (strain A2)-infected Vero cell lysates. HAAC was carried out on infected cell lysates (250 μg of protein per ml of heparin-agarose) followed by immunoprecipitation. Samples were analyzed by SDS-PAGE (4 to 20% gels) followed by Western blot with a polyclonal rabbit anti-G (A) or anti-F (B) antiserum. The infected cell lysate was analyzed for the presence of RSV-specific protein (lane 1) prior to being run over heparin-agarose columns. Columns were washed twice with 10 column volumes of MES buffer, and the final wash was examined (lane 2). Bound proteins were eluted with MES buffer containing heparin (2 mg/ml) (lane 3). As a control to demonstrate that the viral proteins were specifically binding to heparin, identical samples were run over columns containing uncoupled CL-4B agarose, and the heparin-eluted material was examined for the presence of RSV-G and -F proteins (lane 4). In addition, uninfected cell lysate was run over heparin-agarose to control for polyclonal antibody reactivity (lane 5). G90 represents the mature, fully glycosylated form of RSV-G. G55 is a partially glycosylated precursor of G90.
FIG. 2.
Heparin-agarose affinity chromatography of RSV-F proteins. Purified RSV proteins or infected cell lysates from recombinant vaccinia viruses expressing either RSV-G (Ga, subgroup A; Gb, subgroup B) or RSV-F were subject to HAAC followed by Western blot analysis using a polyclonal rabbit anti-G (A) or anti-F (B) antiserum. The assay was carried out as described in Materials and Methods except that purified proteins were used at a concentration of 50 μg/ml of heparin-agarose. (A) Purified RSV-Ga (lane 1), vaccinia virus-expressed RSV-Ga (lane 2), and vaccinia virus-expressed RSV-Gb (lane 3). (B) Purified RSV-Fa (lane 1) and vaccinia virus-expressed RSV-Fa (lane 2). G90 represents the mature, fully glycosylated form of RSV-G.
FIG. 3.
Heparin-agarose affinity chromatography of RSV cp-52-infected Vero cell lysate. Heparin chromatography was performed as described in the legend to Fig. 1, followed by Western blot analysis using an anti-F polyclonal antiserum (lane 1, infected cell lysate; lane 2, final wash; lane 3, heparin-eluted material). To demonstrate that cp-52 is unable to synthesize the G and SH proteins, cp-52 lysate was probed with an anti-G polyclonal antiserum (lane 4) or an anti-SH antiserum (lane 6), and lysate from A2-infected cells was included as a control for anti-G (lane 5) and anti-SH (lane 7) antibody reactivity. G90 represents the mature, fully glycosylated form of RSV-G. G55 is a partially glycosylated precursor of G90. SH0 represents the full-length unglycosylated form of RSV-SH.
Heparin inhibition of RSV A2 and cp-52 infectivity.
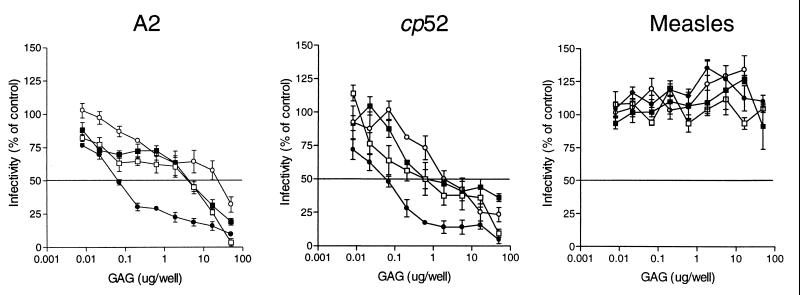
To assess the biological relevance of the RSV-F-heparin interaction, we tested the ability of soluble GAGs, including LMW heparin, BVL heparin, de-N-sulfated heparin, and chondroitin sulfate, to inhibit virus infectivity. Representative dose curves for soluble GAG inhibition of A2, cp-52, and measles virus infectivity are shown in Fig. 4. Based on the data, (50% effective doses) (ED50) were calculated for each of the virus-GAG combinations tested (Table 1). For the A2 and cp-52 viruses, BVL heparin, a heterogeneous population of molecules varying in size and degree of sulfation, was the most effective inhibitor of RSV infectivity, exhibiting ED50 values equivalent to 0.28 ± 0.11 μg/ml and 0.55 ± 0.14 μg/ml, respectively. The ED50 of all other GAGs tested was 10-fold or more higher than that for BVL heparin. In addition, none of the GAGs tested showed any significant inhibition of measles virus infectivity, indicating that the inhibitory effect of the GAGs on RSV infectivity could not be attributed to nonspecific steric or charge-related interactions and likely represents the blockage of a specific interaction between RSV and the cellular GAG heparan sulfate.
FIG. 4.
Inhibition of virus infectivity by various GAGs. RSV A2 or cp-52 or measles virus (100 TCID50) was incubated with BVL heparin (●), LMW heparin (□), de-N-sulfated heparin (■), or chondroitin sulfate (○) for 1.5 h at 37°C before being added to cells. The infection was allowed to progress for 72 h (A2 and measles virus), after which the cells were fixed and subjected to RSV- or measles virus-specific ELISA. Absorbance values at 405 nm were converted to percentages, and infectivity was then plotted as percentage of that of a mock-treated control. Due to poor detection by ELISA, cp-52 infections were stopped at 6 days postinfection and the cells were fixed and stained with 1% crystal violet. Plaques were counted, and percentages were determined versus a mock-treated control. Representative data are from separate experiments and represent the mean ± 1 standard error of the mean. For A2 and cp-52, the data are from single experiments of at least four separate experiments with similar results. Measles virus inhibition was conducted in parallel with A2 and cp-52, and data represent a single experiment from at least two separate experiments with similar results.
TABLE 1.
Soluble GAG competition in paramyxoviruses
| Virus | Mean GAG ED50 (μg/ml)a ± SEM
|
|||
|---|---|---|---|---|
| LMW heparin | BVL heparin | De-N-sulfated heparin | Chondroitin sulfate | |
| A2 | 28.11 ± 0.23 | 0.28 ± 0.11 | 29.96 ± 0.42 | 85 ± 6.8 |
| cp-52 | 3.38 ± 0.17 | 0.55 ± 0.14 | 3.46 ± 0.54 | 11.5 ± 1.94 |
| Measles virusb | >250 | >250 | >250 | >250 |
Results represent the mean ± the standard error of the mean for at least four separate experiments (P < 0.05).
Inhibition of measles virus infectivity did not exceed 50% for any of the GAGs tested, and therefore no ED50 could be calculated.
Effect of GAG lyase treatments on infectivity of RSV cp-52 virus.
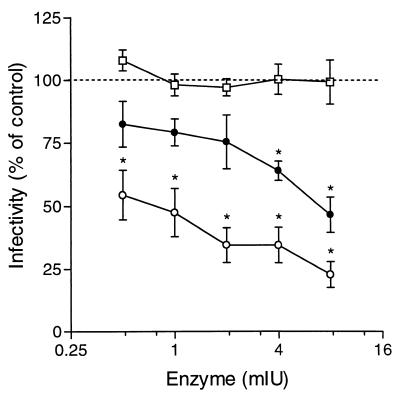
RSV A2 virus infectivity is significantly reduced by treatment of cells with heparin lyase I prior to virus adsorption (19). Therefore, we sought to determine if treatment of cells with a panel of GAG lyases prior to infection with cp-52 had a similar effect. The dependence of cp-52 infectivity on cell surface GAGs was examined by digesting Vero cells with heparin lyase I (cleaves heparin and highly sulfated forms of heparan sulfate), heparin lyase III (degrades only heparan sulfate), or chondroitinase ABC (cleaves chondroitin sulfate types A, B, and C) (5). Treatment of Vero cells with 8 mIU of heparin lyase I or heparin lyase III significantly reduced the infectivity of cp-52, by 53.3% ± 7% and 77.3% ± 5%, respectively, compared to untreated controls. In contrast, the same concentration of chondroitinase ABC did not inhibit the infectivity of cp-52 (Fig. 5).
FIG. 5.
Effect of GAG lyase treatment of Vero cells on RSV cp-52 infectivity. Vero cells were treated for 1 h at 37°C with heparin lyase I (●), heparin lyase III (○), or chondroitinase ABC (□) at the indicated concentrations. Following enzyme treatment, the cells were washed and 100 TCID50 of RSV cp-52 was added for 1 h at 37°C. The inoculum was then removed, and the cells were overlaid with EMEM containing 1% methylcellulose. After 6 days, the cells were fixed and stained with 1% crystal violet. Plaques were counted, and percentages were determined versus a mock-treated control. Data represent the mean ± 1 standard error of the mean of at least three separate experiments. ★, P < 0.05.
Heparin-dependent binding of radiolabeled RSV to cells.
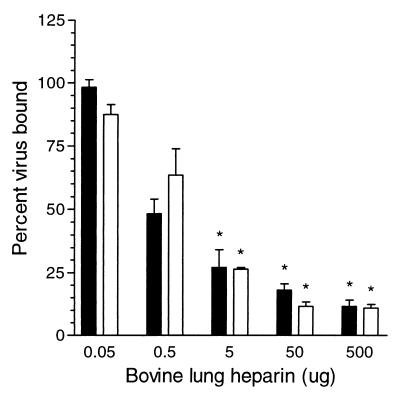
In order to determine if cellular GAGs were required for virus attachment, we looked at the ability of soluble GAGs to inhibit the binding of radiolabeled virus to Vero cells. In the binding competition assay, we choose to use BVL heparin as the competitor, as this GAG demonstrated the highest specific activity against RSV in our infectivity inhibition assays. BVL heparin inhibited A2 and cp-52 binding to Vero cells in a dose-dependent manner (Fig. 6). Five hundred micrograms of BVL heparin decreased A2 and cp-52 binding by 90% compared to the untreated control. LMW heparin yielded similar results (data not shown).
FIG. 6.
Competitive binding of RSV to Vero cells. A2 (solid bars) or cp-52 (open bars) was incubated with the indicated concentration of BVL heparin (50 μl total volume) for 30 min at 37°C. Subsequently, 4 × 105 Vero cells (50 μl total volume) were added to each reaction and incubated at 4°C for 1 h with mixing. Cells were washed twice with cold PBS (pH 7.4). The percentage of bound virus was determined versus untreated controls (PBS only). The maximum amount of virus bound in any given assay ranged from 15 to 20% of the total virus input. Each point represents the mean ± 1 standard error of the mean of at least three separate experiments. ∗, P < 0.05.
DISCUSSION
We report here that RSV-F can interact with heparin and that this interaction is independent of RSV-G. Furthermore, these studies suggest that this interaction is functionally important based on the following evidence: (i) RSV-F from A2- or cp-52-infected cell lysates, from a vaccinia virus recombinant expressing RSV-F (strain A2), and affinity-purified F protein (strain A2) all specifically bound to and eluted from heparin agarose columns; (ii) soluble GAGs were able to inhibit A2 and cp-52 infectivity; (iii) heparin lyase I and heparin lyase III treatment of Vero cells significantly reduced cp-52 infectivity; and (iv) soluble heparin inhibited A2 and cp-52 binding to Vero cells.
cp-52 is a unique tool which allowed characterization of the interactions between RSV-F and cellular membrane constituents in the absence of RSV-G and -SH. The function of RSV-F protein has been primarily associated with fusion of the viral and cellular membranes, allowing release of the viral genome into the cytoplasm, while the attachment function has been attributed to RSV-G protein (for a review, see reference 8). In light of the data presented here and that of others (18), it is evident that the functional properties of the RSV surface glycoproteins may be more complex than previously thought. From studies utilizing cp-52, it appears that RSV-F can facilitate cellular attachment in the absence of RSV-G through a heparan sulfate-like molecule and that this interaction may play a crucial role in the infectivity of the virus. It should be emphasized here that the inability to detect F2 during heparin chromatography of cp-52 does not imply that the F HBD is located within the F1 subunit of the F protein. F2 was eluted and detected following HAAC of A2-infected Vero cell lysate, cell lysate from Vero cells infected with a vaccinia virus recombinant expressing F protein (subgroup A), and purified F protein (subgroup A). Failure to detect cp-52 F2 may merely indicate that the experimental system was not optimized for the detection of the subgroup B F2 subunit. Nevertheless, it would appear then that the other surface glycoproteins, G and SH, act as accessory molecules enhancing virus attachment, fusion, or infectivity, in part via interactions with cellular GAGs (15). Although the exact nature of these interactions is still under investigation, it is possible that both RSV-G and -F interact with cellular heparin-like molecules to enhance the avidity of virus binding, thereby increasing the likelihood of subsequent interactions required for infection.
RSV interactions with cellular GAGs, cp-52 in particular, share similarities with other viruses, including alphaviruses (3), flaviviruses (5), and herpesviruses (23). Therefore, consideration of the model that was proposed for dengue virus binding to cells by Putnak et al. (26) seems appropriate. This model suggests that the initial interaction of the dengue virus E glycoprotein with cell surface GAGs provokes a conformational change allowing E glycoprotein to interact with a putative high-affinity receptor, triggering endocytosis. In a similar scenario, RSV-F interaction with cellular GAGs could result in a conformational change that exposes the fusion peptide or allows RSV-F to interact with a putative high-affinity receptor required for fusion. RhoA is a small GTPase of the Ras superfamily that has been shown to interact with RSV-F both in vitro and in vivo (25). Data show that RhoA facilitates virus-induced syncytium formation, yet it remains unclear whether RhoA is expressed on the cell surface and serves as the receptor for RSV. Further investigation will be required to determine if RhoA is absolutely required for RSV infectivity. Interestingly, preliminary data suggest that an RSV-G deletion mutant may infect CHO pgsA-745 cells, which are reportedly 99% GAG deficient, indicating that RSV-F can infect cells by interacting with cellular membrane components other than GAGs (M. N. Teng, M. E. Peeples, and P. L. Collins, Abstr. 18th Annu. Meet. Am. Soc. Virol., abstr. W17-7, 1999). Nevertheless, our data demonstrate that soluble GAGs block cp-52 attachment and infectivity, suggesting that RSV-F interactions with cellular GAGs facilitate virus entry. In addition, the specificity of the interaction was confirmed through the use of GAG lyases; heparin lyases I and III inhibited virus infectivity, whereas chondroitin sulfate lyase did not. Efforts are currently under way to characterize other interactions of RSV-F that may be required for infection. In contrast to cp-52, the presence of RSV-G and -SH on wild-type virions could render the virus more infectious by increasing the probability of attachment to cellular GAGs and subsequent steps in the fusion process. The lack of RSV-G and -SH may help explain the small-plaque phenotype seen with cp-52 in vitro (18) and attenuation in vivo (9).
How RSV surface glycoproteins interact with cellular GAGs is a growing research area. Currently, two mammalian consensus HBDs, XBBXBX and XBBBXXBX, where B is a basic residue, have been described (for a review, see reference 4). The binding of heparin to HBDs occurs via an electrostatic interaction between the negatively charged sulfate groups on heparin molecules and positively charged basic amino acid residues within the HBD. While many mammalian heparin-binding proteins contain the consensus HBD sequences, it appears as though most viral HBDs do not necessarily conform to the mammalian sequences (11, 12). Sequence analysis of RSV-G revealed a cluster of basic amino acids in the RSV-G ectodomain (180P→K233) (17, 19). Based on our previous work, we identified the presence of a single linear HBD for subgroup A (184AICKRIPNKKPGKKTT198) and subgroup B (183KSICKTIPSNKPKKK197) (11). Interestingly, neither of the RSV-G HBDs conforms to the sequences reported for the consensus mammalian HBDs. A similar sequence analysis of RSV-F revealed that the F2 subunit (strain A2) contains a single mammalian XBBXBX HBD consensus sequence (64IKKIKC69), although this sequence motif is not strictly conserved among other RSV subtypes. However, there are two relatively conserved regions within F2, one reverse-oriented XBBXBX HBD sequence (106NRARRE111) and a cluster of six basic residues (131KKRKRR136) adjacent to the F1-F2 cleavage site, that could function as potential HBD sequences. Analysis of the F1 protein does not reveal the presence of any known mammalian consensus HBD sequence motifs, although we cannot rule out the possibility that conformational determinants play a role in RSV-F-heparin interactions. Efforts are currently under way to experimentally determine the functional HBDs within F1 and F2 that may be involved in interactions with cellular heparin/heparan sulfate.
ACKNOWLEDGMENTS
We thank Lewis Markoff and Prabhakara Atreya for critical reviews of the manuscript and Dan Speelman and Gerald E. Hancock from Wyeth-Lederle Vaccines for the purified G and F glycoproteins and rabbit anti-F and anti-G antisera. We also thank Brian Murphy and Steve Whitehead (NIAID, NIH) for kindly providing RSVB1/cp-52 virus and Peter L. Collins (NIAID, NIH) for kindly providing the anti-SH antiserum and recombinant vaccinia viruses expressing the genes for the surface glycoproteins (G, F, and SH) of RSV strain A2. The vaccinia virus recombinants used in this study were obtained from the World Health Organization Reagent Bank.
REFERENCES
- 1.Anderson K, Stott E J, Wertz G W. Intracellular processing of the human respiratory syncytial virus fusion glycoprotein: amino acid substitutions affecting folding, transport and cleavage. J Gen Virol. 1992;73:1177–1188. doi: 10.1099/0022-1317-73-5-1177. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Byrnes A P, Griffin D E. Binding of Sindbis virus to cell surface heparan sulfate. J Virol. 1998;72:7349–7356. doi: 10.1128/jvi.72.9.7349-7356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardin A D, Weintraub H J R. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Maguire T, Hileman R E, Fromm J R, Esko J D, Linhardt R J, Marks R M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 6.Collins P L, Mottet G. Post-translational processing and oligomerization of the fusion glycoprotein of human respiratory syncytial virus. J Gen Virol. 1991;72:3095–3101. doi: 10.1099/0022-1317-72-12-3095. [DOI] [PubMed] [Google Scholar]
- 7.Collins P L, Huang Y T, Wertz G W. Nucleotide sequence of the gene encoding the fusion (F) glycoprotein of human respiratory syncytial virus. Proc Natl Acad Sci USA. 1984;81:7683–7687. doi: 10.1073/pnas.81.24.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins P L, McIntosh K, Chanock R M. Respiratory syncytial virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1313–1351. [Google Scholar]
- 9.Crowe J E, Jr, Bui P T, Firestone C Y, Connors M, Elkins W R, Chanock R M, Murphy B R. Live subgroup B respiratory syncytial virus vaccines that are attenuated, genetically stable, and immunogenic in rodents and nonhuman primates. J Infect Dis. 1996;173:829–839. doi: 10.1093/infdis/173.4.829. [DOI] [PubMed] [Google Scholar]
- 10.Elango N, Prince G A, Murphy B R, Venkatesan S, Chanock R M, Moss B. Resistance to human respiratory syncytial virus (RSV) infection induced by immunization of cotton rats with a recombinant vaccinia virus expressing the RSV G glycoprotein. Proc Natl Acad Sci USA. 1986;83:1906–1910. doi: 10.1073/pnas.83.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldman S A, Hendry R M, Beeler J A. Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G. J Virol. 1999;73:6610–6617. doi: 10.1128/jvi.73.8.6610-6617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn S J, Ryan P. A heterologous heparin-binding domain can promote functional attachment of a pseudorabies virus gC mutant to cell surfaces. J Virol. 1995;69:834–839. doi: 10.1128/jvi.69.2.834-839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotoh B, Ohnishi Y, Inocencio N M, et al. Mammalian subtilisin-related proteinases in cleavage activation of the paramyxovirus fusion glycoprotein: superiority of furin/PACE to PC2 or PC1/PC3. J Virol. 1992;66:6391–6397. doi: 10.1128/jvi.66.11.6391-6397.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruber C, Levine S. Respiratory syncytial virus polypeptides. III. The envelope associated proteins. J Gen Virol. 1983;64:825–832. doi: 10.1099/0022-1317-64-4-825. [DOI] [PubMed] [Google Scholar]
- 15.Heminway B R, Yu Y, Tanaka Y, Perrine K G, Gustafson E, Bernstein J M, Galinski M S. Analysis of respiratory syncytial virus F, G, and SH proteins in cell fusion. Virology. 1994;200:801–805. doi: 10.1006/viro.1994.1245. [DOI] [PubMed] [Google Scholar]
- 16.Hsiung G D. Virus assay, neutralization test, and antiviral assay. In: Chan V F, editor. Hsiung's diagnostic virology. 4th ed. New Haven, Conn: Yale University Press; 1994. p. 46. [Google Scholar]
- 17.Johnson P R, Spriggs M K, Olmsted R A, Collins P L. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci USA. 1987;84:5625–5629. doi: 10.1073/pnas.84.16.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karron R A, Buonagurio D A, Georgiu A F, Whitehead S S, Adamus J E, Clements-Mann M L, Harris D O, Randolph V B, Udem S A, Murphy B R, Sidhu M S. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc Natl Acad Sci USA. 1997;94:13961–13966. doi: 10.1073/pnas.94.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krusat T, Streckert H-J. Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells. Arch Virol. 1997;142:1247–1254. doi: 10.1007/s007050050156. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Levine S, Klaiber-Franco R, Paradiso P R. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J Gen Virol. 1987;68:2521–2524. doi: 10.1099/0022-1317-68-9-2521. [DOI] [PubMed] [Google Scholar]
- 22.Mbiguino A, Menezes J. Purification of human respiratory syncytial virus: superiority of sucrose gradient over Percoll, renografin and metrizamide gradients. J Virol Methods. 1991;31:161–170. doi: 10.1016/0166-0934(91)90154-r. [DOI] [PubMed] [Google Scholar]
- 23.Neyts J, Snoeck R, Schols D, Balzarini J, Esko J D, Van Schepdael A, De Clercq E. Sulfated polymers inhibit the interaction of human cytomegalovirus with cell surface heparan sulfate. Virology. 1992;189:48–58. doi: 10.1016/0042-6822(92)90680-n. [DOI] [PubMed] [Google Scholar]
- 24.Olmsted R A, Elango N, Prince G A, Murphy B R, Johnson P R, Moss B, Chanock R M, Collins P L. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci USA. 1986;83:7462–7466. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pastey M K, Crowe J E, Jr, Graham B S. RhoA interacts with the fusion glycoprotein of respiratory syncytial virus and facilitates virus-induced syncytium formation. J Virol. 1999;73:7262–7270. doi: 10.1128/jvi.73.9.7262-7270.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putnak J R, Kanesa-Thasan N, Innis B L. A putative cellular receptor for dengue viruses. Nat Med. 1997;3:828–829. doi: 10.1038/nm0897-828. [DOI] [PubMed] [Google Scholar]
- 27.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh E E, Hruska J. Monoclonal antibodies to respiratory syncytial virus proteins: identification of the fusion protein. J Virol. 1983;47:171–177. doi: 10.1128/jvi.47.1.171-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]