Abstract
Radiotherapy causes apoptosis mainly through direct or indirect damage to DNA via ionizing radiation, leading to DNA strand breaks. However, the efficacy of radiotherapy is attenuated in malignant tumor microenvironment (TME), such as hypoxia. Tumor vasculature, due to the imbalance of various angiogenic and anti-angiogenic factors, leads to irregular morphology of tumor neovasculature, disordered arrangement of endothelial cells, and too little peripheral coverage. This ultimately leads to a TME characterized by hypoxia, low pH and high interstitial pressure. This deleterious TME further exacerbates the adverse effects of tumor neovascularization and weakens the efficacy of conventional radiotherapy. Whereas normalization of blood vessels improves TME and thus the efficacy of radiotherapy. In addition to describing the research progress of radiotherapy sensitization and vascular normalization, this review focuses on the strategy and application prospect of modulating vascular normalization to improve the efficacy of radiotherapy sensitization.
Keywords: Vascular normalization, Radiotherapy, Radiation sensitization, Tumor microenvironment, Physical therapy
Highlights
-
•
Vascular normalization can improve tumor hypoxia and help to increase the sensitivity of radiotherapy.
-
•
Vascular normalization inhibitors have limitations in clinical use such as a short therapeutic time window and high toxicity.
-
•
Physical strategies such as laser, electricity, ultrasound, and magnetic field can modulate vascular normalization.
-
•
Physical strategies for modulating vascular normalization have potential for application to radiotherapy sensitization.
1. Introduction
Radiotherapy is one of the longest-used clinical modalities for tumor treatment. It can cause direct damage to DNA through ionizing radiation, leading to DNA single-strand breaks (SSBs) and double-strand breaks (DSBs), as well as indirect damage to DNA through the formation of reactive oxygen species (ROS) and free radicals, which can lead to apoptosis [1]. Clinical studies have shown that more than half (∼70 %) of patients require radiotherapy and in some cases, radiotherapy is the only cancer treatment available [2]. However, the radiation resistance of tumors hinders the application of radiotherapy [3], and people are urgently searching for ways to increase the sensitivity of radiotherapy, First, the innovation of technology to provide more accurate target area [4], which can reduce the radiation dose and improve the radiation effect. The second is the use of radiosensitizers, which can be classified into small molecules, macromolecules and nanomaterials according to their structure [5], among which high atomic number materials are of considerable interest, which can improve the efficacy of radiotherapy by promoting intracellular radiation energy deposition [6]. However, none of them can solve the problem of radiation resistance in radiotherapy. Radiotherapy kills tumor cells through DNA damage, and O2 can prevent the self-repair process of DNA (Fig. 1), which is a necessary element to enhance the DNA damage induced by ionizing radiation in radiotherapy, so hypoxia becomes the main obstacle to radiation resistance in radiotherapy [7]. Studies have shown that the radiation resistance of tumor cells in a low oxygen environment is 2–3 times higher than that in a normal oxygen environment [8]. The efficacy of radiotherapy is severely limited due to the hypoxic state of most solid tumors [9]. Hypoxia not only limits the therapeutic efficacy, but also leads to tumor recurrence and metastasis after radiotherapy. The problem of hypoxia in the tumor microenvironment (TME) needs to be solved urgently, and tumor vascular normalization therapy brings new hope.
Fig. 1.
Mechanisms of radiotherapy and the role of oxygen repair. Radiation induces DNA damage, whereas O2 relieves tumor hypoxia and reacts with DNA free radicals (DNA·), which can repair DNA damage and form more stable DNA peroxides (DNA-OO·, which further prevents cellular DNA repair and thus improves RT treatment outcomes. (RSH, reducing SH-containing compound; SSB, single-strand break; DSB, double-strand break).
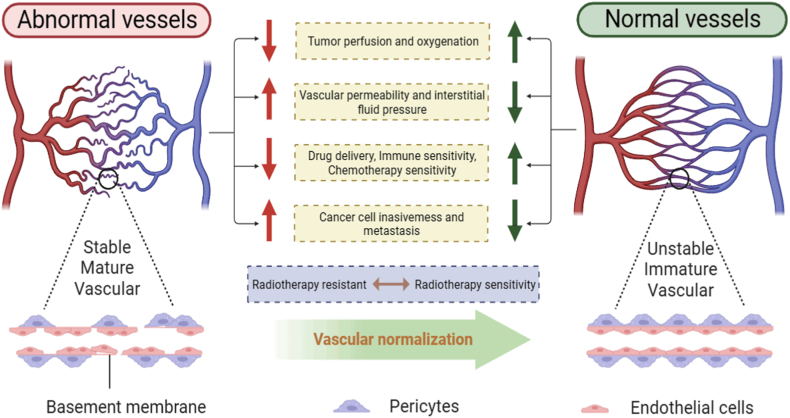
The rapid proliferation of tumor cells necessitates a continuous supply of oxygen and nutrients, thereby accelerating tumor angiogenesis. However, the rapid pace of angiogenesis results in the formation of immature neovascular structures. These newly formed vessels exhibit pronounced curvature, thin vessel walls, and inadequate peripheral coverage, rendering them susceptible to leakage. Additionally, the accumulation of pressure from proliferating cancer cells, stromal cells, and extracellular substances like collagen and hyaluronic acid can compress tumor vessels. In severe cases, this compression can lead to vascular collapse, ultimately resulting in tumor hypoxia [10,11].
As early as 2001, Jain proposed that anti-angiogenic therapy could improve the morphology and function of tumor blood vessels and called it “vascular normalization” [12]. He argued that instead of occluding the tumor vasculature, antiangiogenic therapy restores the structurally and functionally abnormal vasculature to a normal vasculature. This concept is based on the premise that persistent imbalance between pro-angiogenic factors (VEGF, PDGF, TGF-β, etc.) and anti-angiogenic signals leads to structural abnormalities and dysfunction of the tumor vasculature, and suggests that anti-angiogenic therapy can align the endothelial cells, increase pericyte coverage, and restore angiogenic homeostasis to affect the TME, resulting in improvement of tumor hypoxia and perfusion [13]. At the same time, vascular normalization can improve the TME and thus improve the therapeutic efficacy of radiotherapy. In this review, we present current research advances in radiotherapy sensitization and vascular normalization, collate the last 10 years of vascular normalization therapies and, for the first time, describe methods to modulate tumor vascular normalization in terms of drug and physical strategies, with a view to providing a theoretical basis for the combined therapeutic regimen of vascular normalization with radiotherapy, chemotherapy and immunotherapy.
2. Advances in radiotherapy sensitization research
Radiotherapy is an effective modality that utilizes high-energy photon radiation, such as x-rays and γ-rays, to treat cancer cells. It exerts its therapeutic effects by generating ROS through both direct and indirect mechanisms [1]. Clinical studies have demonstrated that radiotherapy is necessary for more than half of cancer patients, and in certain cases, it may be the sole available treatment option [2]. While radiotherapy theoretically holds the potential to eliminate all tumor cells, their varying sensitivities to radiation lead to diverse treatment outcomes [3]. Resistance to radiation poses a significant challenge, despite advancements in radiotherapy techniques and the adoption of new treatments. Metastasis, cancer recurrence, and poor prognosis remain substantial obstacles to achieving better treatment outcomes [14,15]. Consequently, there is a pressing need to develop methods that can alleviate radiation resistance and enhance radiosensitivity.
The progress of science and technology has given rise to innovative approaches in cancer treatment, including radiosensitization techniques. Image-guided radiation therapy (IGRT) employs ultrasound, CT scans, MRI, and other imaging modalities to precisely localize and deliver radiation therapy, thereby improving treatment precision and accuracy [4]. Intensity-modulated radiation therapy (IMRT) is a highly effective method for enhancing the efficacy of radiation treatment. It employs computerized systems and other technologies to measure radiation doses in the tumor target area, enabling precise treatment, reduced radiation dosage, and improved radiosensitization [16]. However, these techniques alone may face challenges in effectively treating tumors. In addition to technological advancements, another avenue for enhancing radiosensitivity and minimizing toxicity in normal tissues is the use of radiosensitizers, which can be categorized into small molecules, macromolecules, and nanomaterials based on their structures [5]. The field of nanotechnology has witnessed the emergence of numerous strategies for radiosensitization. Among them, nanomaterials with high atomic numbers have garnered significant attention. These nanoparticles enhance photoelectric and photon absorption during irradiation, resulting in a dose-enhancement effect. For instance, gold nanoparticles, with an atomic number exceeding 53, exhibit increased absorption of X-rays, thereby promoting intracellular radiation energy deposition and ultimately enhancing the efficacy of radiation therapy [6]. However, it is important to note that while these radiosensitization strategies are promising, they do not address the issue of tumor radiation therapy resistance, which is significantly influenced by hypoxic environments [8]. Moreover, hypoxia can specifically impede the initiation of DNA replication during the early S-phase in tumor cells. Hypoxia induces an upregulation of hemeoxygenases-1, metallothionein II, and c-Jun oncogenes, thereby promoting the aggressiveness of tumor cells. Consequently, hypoxia significantly diminishes the effectiveness of radiotherapy in tumor patients. To enhance the efficacy of radiotherapy in tumor treatment, it is imperative to address tumor hypoxia [17]. In this context, tumor vascular normalization therapy offers a new ray of hope.
3. Advances in vascular normalization research
Abnormal structural and functional tumor vasculature creates a malignant microenvironment, resulting in chemo-resistance, radiotherapy resistance, and immune escape, which can be addressed by vascular normalization [18]. Here we emphasize the role of vascular normalization in radiosensitization (Fig. 2). In 2001, Jain first proposed the vascular normalization theory [12]. In 2011, Goel et al. further elucidated the mechanism of vascular normalization, and by intervening with anti-angiogenic drugs, the dynamic balance between pro-angiogenic and anti-angiogenic factors can be restored in abnormal tumor blood vessels, creating a “time window” for vascular normalization [19]. It is the theory of tumor vascular normalization that raises the clinical status of anti-angiogenic therapy, while with the development of nanotechnology, the application of nanocarriers provides more possibilities for corresponding anti-angiogenic therapy, and provides a theoretical basis for finding more appropriate anti-tumor combination therapy, marking the arrival of the era of induced tumor vascular normalization [18,20]. For example, the vascular normalization “time window” provides an opportunity for tumor chemotherapy, effectively prevents the development of drug resistance, and promotes the development of anti-angiogenic drugs [21]. Nanocarrier delivery of STING agonists also enhances tumor immunotherapy [22]. In addition, anti-angiogenic therapy can repair the structural and functional defects of tumor angiogenesis, which not only facilitates drug delivery but also enhances cancer radiotherapy [23].
Fig. 2.
Structural and functional differences between tumor abnormal vessels and normal vessels. Normal blood vessels have tightly connected endothelial cells, neatly arranged pericytes, intact basement membranes, and smooth blood flow through the vascular lumen. On the contrary, tumor blood vessels have low pericyte coverage, are often separated from endothelial cells, and have incomplete and discontinuous basement membranes, showing structural and functional abnormalities of blood vessels.
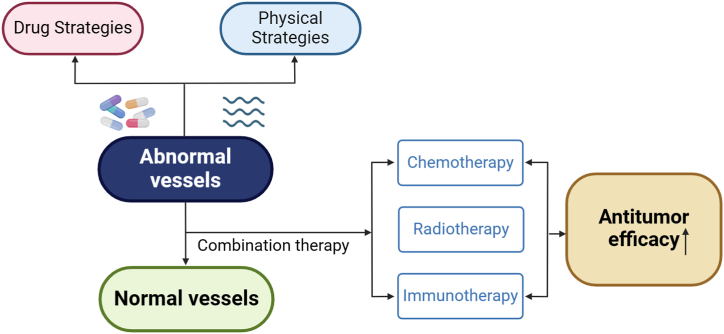
Although pharmacological antiangiogenic therapies can remodel and normalize tumor vasculature, the window of efficacy is limited and these drugs are associated with severe side effects, so we have also concluded that there are some other alternative methods to normalize the vasculature [24]. For example, physical methods such as electricity and ultrasound modulate tumor vascular normalization [25,26], and some nanomaterials such as gold nanoparticles (AuNPs) have also been found to inhibit endothelial Smad2/3 signaling to induce tumor vascular normalization [27]. However, in general, anti-angiogenic drugs targeting vascular normalization are the focus of current research, and the combination of angiogenesis inhibitors with other conventional cancer therapies (including chemotherapy, radiotherapy, immunotherapy, over-the-counter cellular therapy, and cancer vaccines) has been favorably demonstrated in patients with different types of cancer in clinical trials [28] (Fig. 3). The modulatory approach of physical stimulation is also expected to be further developed and utilized.
Fig. 3.
Combined vascular normalization therapy boosts antitumor efficacy. Both drug and physical strategies can modulate vascular normalization, and the combination of conventional treatments (chemotherapy, radiotherapy, immunotherapy) can significantly enhance antitumor efficacy.
4. Strategies to regulate vascular normalization
Based on the application prospect that normalization of blood vessels can sensitize radiotherapy, a variety of methods have been developed, such as inhibiting the production and release of angiogenic factors, promoting the production of anti-angiogenic factors, and normalizing the TME, etc. For these targets of action, we summarize them from the perspectives of drug modulation and physical stimulation (Table 1).
Table 1.
Drug and physical strategies to modulate tumor vascular normalization.
| Strategies | Mechanisms | Representations | |
|---|---|---|---|
| Drug | Targeting HIF-1 | Inhibition of HIF-1α restores normal tumor vasculature | Romidepsin (FK228) and Flavopiridol (alvocidib) [29]; Simvastatin and metformin [30]; Danphenol [31] |
| Targeting VEGF | VEGF-A mediating tumor angiogenesis | Apatinib [32]; lenvatinib and sorafenib [33] | |
| Targeting STAT3 | Sustained activation of STAT3 can be triggered by the expression of HIF-1α | tofacitinib, ruxolitinib and fidaxomicinib [34]; IL-6, JAK2, SOCS1 and PIAS3 [35] | |
| Inhibition of NO | NO is a pro-angiogenic molecule and eNOS plays a dominant role in vascular endothelial growth factor-induced angiogenesis | NO gradient [36] | |
| Targeting CAFs | Inhibits the IL-6/STAT3/AUF1 pathway, normalizes active mammary CAFs and inhibits their paracrine pro-carcinogenic effects, and suppresses tumor angiogenesis | Tocilizumab [37] | |
| Reduces proliferative CAFs to normalize the TME and improve tumor vascular functio | Vismodegib [38] | ||
| Immunization drug | ICIs can mediate vascular normalization by normalizing the TME; recruiting CD8+ T cells and inducing IFN-γ production; and targeting Treg cells to promote TAE aggregation | Nivolumab [39] and Durvalumab [40] | |
| Other drugs | Inhibits bFGF-induced angiogenesis | Salvicine [41] | |
| Inhibits NF-κB transcription factor | Rottlerin [42] and Streptochlorin [43] | ||
| Inhibits Smad2/3 signaling in endothelial cells | Gold nanoparticles [27] | ||
| Physical | Laser | Reduces the number of pro-angiogenic macrophages in tumors | Laser [44] |
| Electricity | Vessel number, VEGF, PD-ECGF, CD31, CD35, and CD105 were reduced, and angiogenesis was inhibited | nsPEFs [45] | |
| Direct killing of tumor vascular endothelial cells and alteration of the microenvironment in tumor tissues indirectly regulate VEGF, HIF-1α and HIF-2α | psPEFs [46] | ||
| Downregulates vascular recruitment of vascular endothelial cells and inhibits eNOS/NO pathway | Wireless electrical stimulation [25] | ||
| Ultrasound | O2-MBs increase strong tumor oxygenation, inhibit HIF-1α/VEGF pathways, and improve vascular structure and function without altering vessel density | Ultrasound -mediated O2-MBs [47] |
|
| Repolarizes macrophages, resulting in the polarization of M2 to tumor-suppressing M1; and remodels tumor vasculature, inducing vascular normalization | UTMD [26] | ||
| Magnetic field | Reduction of tumor blood flow and platelet adhesion in microvessels inhibited tumor angiogenesis | SMF [48] | |
| Inhibitory effect on angiogenesis and tumor development, but the mechanism is not clear | PEMF [[49], [50], [51]] | ||
| Aerobic exercise | Exercise-induced shear stress mediates tumor vascular remodeling through activation of calmodulin phosphatase-NFAT-TSP1 signaling in endothelial cells | Moderate aerobic exercise [24] | |
4.1. Drug strategies
To achieve a balanced regulation between pro-angiogenic and anti-angiogenic factors within the abnormal tumor vasculature and promote vascular normalization, researchers have developed a diverse range of targeted drugs. These drugs aim to regulate specific targets or multiple targets along the signaling pathway involved in tumor angiogenesis. Furthermore, the TME plays a crucial role in fostering the development of abnormal tumor vasculature, with cancer-associated fibroblasts (CAFs) being able to secrete angiogenesis-promoting factors [52]. Consequently, drugs targeting CAFs have been devised to promote blood vessel normalization in response to the interplay within the microenvironment [53]. Additionally, the exploration of immune checkpoint inhibitors (ICIs) not only unleashes T cells to combat tumor cells but also has the potential to regulate tumor vascular normalization [54]. In summary, these various drugs approach the task of tumor vascular normalization from different angles, ultimately improving tumor radiosensitivity.
4.1.1. Targeting HIF-1 (hypoxia-inducible factor-1)
Hypoxia, a characteristic feature of the tumor metabolic microenvironment, plays a crucial role in the development of abnormal angiogenesis [55]. It triggers the activation of hypoxia-inducible factors (HIFs), which regulate signaling pathways associated with hypoxia [56]. HIF serves as a vital regulator in hypoxic conditions, primarily comprising two subunits, HIF-1α and HIF-1β. Under normal oxygen conditions, HIF-1α undergoes ubiquitination and degradation by proteases, leading to decreased stability. However, during cellular hypoxia, HIF-1α is induced and expressed, while HIF-1β is constitutively expressed. HIF-1α forms dimers with HIF-1β, culminating in the activation of downstream genes and binding to the hypoxia response element (HRE) within the promoter region of vascular endothelial growth factor (VEGF), thereby augmenting the transcription and expression of VEGF [57]. Since HIF-1 further stimulates tumor angiogenesis [58], targeted drugs have been developed to sensitize radiotherapy by inhibiting HIF-1 activity and promoting vascular normalization. Studies have demonstrated that increased levels of HIF-1α promote tumor angiogenesis, whereas inhibition of HIF-1α can restore normal vasculature [59]. For instance, Romidepsin (FK228) can reduce the expression of HIF-1α and its downstream gene VEGF, inhibit the DNA binding activity of HIF-1, and thereby hinder angiogenesis [29]. The flavonoid pyrrolopyrimidine derivative Flavopiridol (alvocidib) blocks mRNA transcription, including that of HIF-1α, thereby preventing global transcription [29]. Simvastatin and metformin have also been shown to inhibit the mTOR (mammalian target of rapamycin)/HIF-1α pathway by activating AMPK [30]. Other drugs, such as salvinorin at concentrations of 50 μM and 100 μM, significantly downregulate VEGF, HIF-1α, and PI3K/Akt pathway proteins. Danphenol downregulates VEGF and HIF-1α through significant alterations in the regulation of PI3K/Akt pathway proteins, effectively increasing the sensitivity of ovarian cancer cells to radiation [31]. Additionally, HIF-1 regulates the expression of several other angiogenic factors, including placental growth factor, platelet-derived growth factor β (PDGF-β), and angiopoietin-1 and -2 (Ang-1 and Ang-2) [57]. For example, the angiotensin II receptor antagonist candesartan modulates tumor vascular normalization and improves the sensitivity of radiotherapy during the therapeutic window [60].
4.1.2. Targeting VEGF
As mentioned above, HIF-1 enhances the transcription and expression of VEGF, which is also an important participant in tumor angiogenesis and progression [57]. The VEGF family includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF), with the VEGF-A isoform mediating mainly tumor angiogenesis [61]. Relevant drug therapies include anti-VEGF antibodies (bevacizumab and ramelimumab), receptor tyrosine kinase inhibitors (TKIs, such as sunitinib and sorafenib), VEGF capture molecules (aflibercept, targeting all VEGF molecules and PlGF), and VEGF receptor (VEGFR) inhibitory antibodies (DC101) [19]. Although anti-VEGF monotherapy is not very effective, improvement of tumor hypoxia after treatment improves the sensitivity of radiotherapy [62]. In addition, TKIs have been shown to contribute to vascular normalization and synergize with radiotherapy in situ models of glioblastoma, head and neck cancer, lung cancer, melanoma and colon cancer [8]. It has been shown that the low-dose tyrosine kinase inhibitor Apatinib promotes vascular normalization and hypoxia reduction in lung cancer and sensitizes radiation therapy [32]. The anti-vascular efficacy of different drugs also varies, such as in the treatment of hepatocellular carcinoma (HCC), the anti-angiogenic drugs lenvatinib and sorafenib are multi-targeted TKIs, and experimental comparisons have revealed that lenvatinib induces vascular normalization of hepatocellular carcinoma tumors earlier and more efficiently compared with sorafenib [33]. However, we need to note that the duration of effect of anti-VEGF therapy is relatively short, and combining it with radiotherapy requires careful consideration of the optimal duration of treatment. Long-term treatment with VEGF inhibitors prunes most of the tumor vasculature, including normalized vessels [63]. A corresponding study found that the transforming growth factor (TGF-β) pathway was overexpressed after anti-VEGF-A treatment [64], suggesting that it may play an important role in acquiring treatment resistance.
4.1.3. Targeting STAT3 (signal transducer and activator of transcription 3)
STAT3 is a versatile transcription factor in the cytoplasm that facilitates signal transduction from the plasma membrane to the nucleus across various cellular processes [65]. Upon encountering diverse upstream signals like IL-5, IL-6, IL-10, and EGF, STAT3 undergoes receptor-associated tyrosine kinase-driven phosphorylation, heterodimerizes, and translocates to the nucleus, functioning as a pivotal transcription factor in governing genes responsible for proliferation, differentiation, apoptosis, angiogenesis, and immuno-inflammatory processes [66,67]. Back in the 1990s, it was discovered that impeding STAT3 signaling in B16 tumor cells through transfection of expression vectors containing dominant-negative STAT3 protein variants or STAT3 antisense oligonucleotides effectively repressed VEGF expression [68]. Furthermore, sustained activation of STAT3 stimulates tumor angiogenesis, migration, invasion, and metastasis by triggering the expression of HIF-1α [69]. These findings indicate that targeting STAT3 could potentially induce vascular normalization as a countermeasure against VEGF.
Currently, two primary approaches exist for targeting STAT3 inhibitors or agents: direct inhibition, which directly targets STAT3 activation, and indirect inhibition, which focuses on blocking the upstream pathway of STAT3. Direct inhibitors hinder STAT3 activation by blocking phosphorylation, dimerization, nuclear translocation, and DNA binding through targeting the SH2, DNA-binding, and N-terminal structural domains [70]. Additionally, inhibiting the upstream pathway of STAT3 involves direct inhibitors categorized into three groups: peptides, oligonucleotides, and small molecules [71]. Furthermore, blocking STAT3 upstream signaling frequently entails employing inhibitors of cell surface receptors or phosphokinases, such as tofacitinib, ruxolitinib, fidaxomicinib, and more [34]. Moreover, mounting evidence suggests that non-coding RNAs (ncRNAs) can exert both direct and indirect regulation on STAT3 activity. Among the extensively studied ncRNAs, microRNAs (miRNAs) play a prominent role by directly targeting STAT3 and various components of its signaling pathway (IL-6, JAK2, SOCS1, PIAS3, etc.), thereby modulating STAT3 expression and activation [35]. A study by Yamakuchi et al. revealed that upregulating miR-107 resulted in reduced levels of VEGF and HIF-1β in HCT116 cells, whereas inhibiting endogenous miR-107 led to an increase in VEGF levels [72]. miR-218, by targeting the IL-6 receptor, was found to decrease the levels of VEGF and HIF-1β. Additionally, indirect inhibition of STAT3 activation occurs through JAK3 [73]. miR-375, miR-204, and miR-101 indirectly impede cell growth and angiogenesis by suppressing the translation of JAK2 [[74], [75], [76]]. Furthermore, certain miRNAs can directly target STAT3, suppressing its expression. Zhang et al. discovered that miR-874 significantly curbed angiogenesis in gastric cancer by targeting the 3′ untranslated region (3′-UTR) of STAT3 mRNA, thereby inhibiting the STAT3 pathway and downregulating the angiogenic factor VEGF-A [77]. Likewise, miR-125-5p can restrain STAT3 expression by specifically targeting the 3′-UTR of STAT3 [78]. Through modulation of the STAT3 signaling pathway, miRNAs effectively suppress pathological angiogenesis, offering novel therapeutic opportunities for achieving vascular normalization.
4.1.4. Inhibition of NO (nitric oxide)
NO is a multifunctional signaling molecule that plays a vital role in mediating cancer formation and progression [79]. Nitric oxide synthase (NOS) has three isoenzymes: neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS) [79]. NO interacts with pro-angiogenic and anti-angiogenic molecules, and its dual effect on angiogenesis is dose-dependent [80]. However, in vivo studies support NO as a pro-angiogenic molecule. NO upregulates pro-angiogenic factors such as VEGF, IL-8, and basic fibroblast growth factor, with eNOS playing a dominant role in VEGF-induced angiogenesis [80]. In addition to mediating angiogenesis, eNOS also maintains vascular homeostasis and endothelial function [81]. Furthermore, the elimination of NO production by tumor cells via silencing or inhibition of nNOS creates a NO gradient around blood vessels, the gradient that normalizes the structure and function of abnormal tumor blood vessels [36]. Some studies suggest that inhibiting NO can complement anti-VEGF therapy, and experimental evidence demonstrates that combining NOS inhibition with VEGFR-2 inhibition significantly enhances the anti-tumor effect compared to using either drug alone [82].
4.1.5. Targeting CAFs
CAFs represent a crucial cell population within the TME and exert a significant influence on tumor progression through their interactions within the hypoxic microenvironment [83]. Furthermore, CAFs serve as abundant sources of secreted factors, including cytokines, growth factors, and chemokines, that intricately contribute to tumor cell proliferation, migration, angiogenesis, and metastasis [84]. CAFs play a crucial role in tumor blood vessel development [84]. CAFs secrete epidermal growth factor (EGF), insulin-like growth factor 2 (IGF2), and FGF4, which have been implicated in promoting radioresistance [85], and VEGF can promote tumor angiogenesis [57]. CAFs also secrete chemokines such as CXCL12/SDF-1, which induce angiogenesis [86]. Additionally, CAFs secrete matrix metalloproteinases (MMPs), which degrade the extracellular matrix (ECM) and promote tumor invasion and angiogenesis [87]. Furthermore, CAFs secrete TGF-β, IL-6 and PDGF [88,89]. TGF-β was initially found to be produced by fibroblasts, and subsequently, it was discovered that almost all cell types, including CAFs, can generate TGF-β signaling pathways widely, and TGF-β promotes CAFs generation via either canonical or non-canonical pathways [90,91]. CAF crosstalk enhances tumor vascularization by increasing the association between pericytes and endothelium via a mechanism involving the TGF-β-fibronectin axis [88]. The herbal medicine α-Mangostin (α-M) effectively inactivates CAFs, reduces the production of ECM, and promotes normalization of tumor vasculature [53]. The inhibition of the IL-6/STAT3/AUF1 pathway by Tocilizumab was discovered to restore normalcy to activated breast CAFs and attenuate their paracrine pro-oncogenic effects, thereby suppressing tumor angiogenesis [37]. Vismodegib treatment improves tumor vascular function by reducing proliferative CAFs and contributing to the normalization of the TME [38]. These applications have also demonstrated the clinical value of targeted CAFs as adjuvant therapy to conventional radiotherapy [92].
4.1.6. Immunization drug
ICIs, including antibodies targeting programmed cell death protein 1 (PD-1), programmed death ligand 1, and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), act by suppressing the immune checkpoint pathway and eliciting an anti-tumor response [93]. In addition to their immunomodulatory effects, modulate the tumor vasculature by normalizing the TME [54]. Normalization of blood vessels facilitates lymphocyte infiltration, such as CD4+ T lymphocytes, and active proliferation of CD4+ T lymphocytes in turn further promotes normalization of blood vessels, and such positive feedback regulation not only improves the efficacy of immunotherapy, but also alleviates tumor hypoxia, thus making the tumor sensitive to radiation [54]. Treatment with anti-CTLA-4 or anti-PD-1 drugs normalizes tumor vasculature through the recruitment of CD8+ T cells and the induction of IFN-γ production, which can be further potentiated by ICIs. The normalization of tumor vasculature by ICI treatment depends on the tumor type. A study has found this treatment to be effective in only nine solid tumors [94]. Some studies have demonstrated that targeting Treg cells through ICIs also promotes the aggregation of tumor-associated eosinophils (TAE), which can mediate vascular normalization [95]. The immunotherapeutic agents Nivolumab and Durvalumab have shown vascular normalizing effects and promising clinical benefits when combined with radiotherapy [39,40].
4.1.7. Nanomedicine
With the rapid development of nanotechnology and nanomedicine, high atomic number nanomaterials, such as bismuth-based nanomaterials, magnetic nanomaterials, and selenium nanomaterials, can be used as sensitizers for radiotherapy to overcome radioresistance and increase the efficacy of radiotherapy through the photovoltaic effect of deposition of radiant energy [96]. In addition, nanomaterials can also be used as drug carriers to deliver drugs targeted to the characteristics of the TME, inducing vascular normalization and improving the hypoxic microenvironment, so as to achieve the purpose of sensitization for radiotherapy. For example, acid-responsive mesoporous silica nanoparticles (MSN) intelligently release dopamine, which can significantly inhibit vascular endothelial cell migration and tubule formation, and promote tumor vascular normalization [97]. As well as dextran combined with cuprous oxide (Cu2O@Dex), which releases Cu (Cu+/2+) under acidic conditions, catalyzes the production of NO for vascular normalization [98]. Du et al. used 8-hydroxyquinoline (HQ)-modified AuNPs to obtain AuHQ, which reduces a variety of angiogenic growth factors by inhibiting oxidative stress in tumors and reducing expression by inhibiting oxidative stress in tumors. The results showed that AuHQ treatment induced normalization of tumor vasculature by increasing pericyte coverage and modulating tumor leakage while reducing tumor hypoxia and increasing blood perfusion. In addition, the efficacy of radiotherapy with AuHQ was increased by 38 % compared to the group treated with AuNPs alone [99].
4.1.8. Other drugs
Salvicine [41] specifically inhibited bFGF-induced angiogenesis, whereas it had no effect on VEGF- or EGF-induced angiogenesis. Additionally, certain substances like Rottlerin [42] and Streptochlorin [43] have been identified as inhibitors of the NF-κB transcription factor. These compounds exert inhibitory effects on endothelial cell tube formation, viability, and proliferation by blocking the NF-κB signaling pathway. In addition, recent studies have found that gold nanoparticles, which are usually used as sensitizers for radiotherapy, induce tumor vascular normalization through inhibition of endothelial Smad2/3 signaling, which undoubtedly further expands the development of vascular normalizing drugs [27].
4.2. Physical strategies
Currently, in addition to the above mentioned targets for inhibiting the tumor angiogenic signaling pathway, we can also sensitize radiotherapy by directly improving hypoxic conditions such as transfusion of erythrocytes, administration of erythropoietin, and hyperbaric oxygen (HBO) therapy [8], as well as by using ultrasound waves to disrupt oxygenated microbubbles injected into the tumor [47], or to increase the generation of oxygen within the tumor such as by facilitating the decomposition of H2O2 to produce oxygen [100]. That is, by directly alleviating the hypoxic environment at the tumor site, vascular normalization is promoted (Fig. 2). If oxygen is considered as a drug, these methods basically deliver the drug with the help of physical methods for vascular normalization, and in addition to this, some purely physical strategies for modulating vascular normalization are now being developed. These methods of physical stimulation of vascular normalization are mostly applied to the delivery of chemotherapeutic drugs, from which we can see a broad prospect of application with radiotherapy.
4.2.1. Laser
Traditionally photodynamic therapy can be used to generate cytotoxic ROS that can kill tumor cells by stimulating surface-loaded acoustic sensitizers of nanocarriers under laser irradiation, and can also be designed with H2O2-triggered smart platforms that generate single-linear oxygen (1O2) to activate endothelial TRPV4-eNOS signaling, which mediates tumor vascular normalization [101]. In addition, lasers can directly regulate vascular normalization; for example, researchers have found that lasers reduce the number of pro-angiogenic macrophages in tumors in experiments with mouse tumor models of melanoma and oral cancer. Although laser therapy could not significantly change the total number of F4/80+ cells in perfusion tumors or non-perfusion tumors, the abundance of F4/80+Ly6C+ macrophages decreased significantly after laser therapy, that is, the number of extravascular macrophages promoting angiogenesis was significantly reduced by laser therapy, while the intravascular content was increased, thus increasing tumor perfusion. Effectively promote the structural and functional maturation of tumor blood vessels [44].
4.2.2. Electricity
After 1 week of treatment with nanosecond pulsed electric fields (nsPEFs), the number of blood vessels, VEGF, platelet-derived endothelial cell growth factor (PD-ECGF), CD31, CD35, and CD105 were reduced in a mouse model of melanoma, suggesting that angiogenesis was inhibited [45]. The nsPEFs treatment affects have immediate and delayed vascular effects, most likely through different mechanisms. One of them may be due to the direct electric field-mediated formation of fragile endothelial cell membrane nanopores in capillaries and small vessels. The delayed effect may be due to tumor cell-supported angiogenic death, caspase-associated apoptosis, and adverse effects on small vessels supplying the large blood vessels [45]. In a mouse cervical cancer xenograft model, it was found that picosecond pulsed electric fields (psPEFs) also have an antiangiogenic effect. This study found that with the increase of psPEFs intensity, the mitochondria and endoplasmic reticulum of cancer cells showed varying degrees of swelling and expansion, the connection between cancer cells was reduced, and the structure of cancer cells and vascular endothelial cells was almost unrecognizable, that is, psPEFs showed direct anti-angiogenic effects by acting directly on vascular endothelial cells and cancer cells. In addition, psPEFs can indirectly inhibit the formation of tumor microvessels by changing the microenvironment in the tumor and regulating the expression and transcription levels of angiogenesis related factors. This study confirmed that after psPEFs treatment, the protein expression and mRNA levels of VEGF, HIF-1α and HIF-2α showed a downward trend [46].
Furthermore, recent studies have discovered that wireless electrical stimulation can disrupt intracellular bioelectrical homeostasis and restore normal tumor vascularization [25]. Li et al. developed polarized barium titanate (BTO) nanoparticles exhibiting excellent electromechanical switching capabilities that generate pulsed open-circuit voltages when exposed to low-intensity pulsed ultrasound. They experimentally observed that wireless electrical stimulation suppresses the eNOS/NO pathway by downregulating the recruitment of endothelial cells within the vasculature. In an in vivo hormonal mouse model, it was demonstrated that wireless electrical stimulation can normalize tumor vasculature through the optimization of vascular structure, inducing maturation of the tumor vascular network, reducing vascular leakage, enhancing blood perfusion, and restoring local oxygenation [25]. Studies have also revealed that this method exhibits notable efficacy in enhancing chemotherapy and holds potential as a sensitization strategy for radiotherapy, warranting further validation.
4.2.3. Ultrasound
Antiangiogenic drugs induce normalization of tumor vasculature, but with a short time window, followed by pruning of the vasculature, exacerbating tumor hypoxia. This strategy of ultrasound-modulated vascular normalization can take advantage of ultrasound drug delivery to deliver O2 to the tumor locally and repair the tumor vasculature without altering the tumor vascular density [47]. For example, it has been found that ultrasound combined with microbubbles (MBs) not only mediated drug or gene delivery [102], but also direct loading of O2 with MBs and their local delivery to the tumor site, where it enhances oxygenation and vascular normalization by inhibiting the HIF-1α/VEGF pathway. With the improvement in vascular structure and function, there was no change in vascular density [47]. In addition, in another pancreatic cancer model experiment, the researchers found that ultrasound-targeted microbubble disruption (UTMD) treatment group had increased pericyte coverage of tumor vessels, improved tightness of the endothelial cell layer, increased number of endothelial junctions and prolonged persistence distances, which promoted vascular normalization and consequently improved TME. Additionally, the authors further found that UTMD relies predominantly on the pathway of redifferentiation of tumor-associated macrophages (TAMs) polarization from the M2-type to the M1-type to induce vascular normalization [26].
4.2.4. Magnetic field
In mouse experiments, short-term exposure to magnetic flux densities above approximately 150 mT resulted in significant reductions in intravascular erythrocyte velocity (VRBC) and local blood flow in tumor microvessels. The final experiment demonstrated that a static magnetic field (SMF) of approximately 600 mT inhibited tumor angiogenesis by reducing tumor blood flow and platelet adhesion in the microvasculature [48]. In addition, pulsed electromagnetic field (PEMF) has been found to have an inhibitory effect on angiogenesis and tumor development in several EMF studies [[49], [50], [51]]. It was found that PEMF with the same parameters had different effects on normal and cancer cells, which suggests that the metabolic effects of PEMF are not only related to the parameters [51], but also to the physiological state of the cells. However, there is not enough evidence to explain why these two factors have different effects on angiogenesis, and the mechanism needs to be further investigated.
4.2.5. Aerobic exercise
Although pharmacological anti-angiogenic therapies can modulate vascular normalization, their efficacy is limited and they often come with severe side effects. Therefore, alternative approaches are needed to normalize vasculature. Aerobic exercise has been demonstrated to enhance tumor vascular function in breast and prostate cancer models, but the underlying mechanisms remain unclear [103,104]. In 2016, researchers [24] found that moderate aerobic exercise induced vascular normalization in a mouse model. Shear stress is a mechanical stimulus exerted by blood flow on endothelial cells that regulates vascular integrity. The calmodulin phosphatase-NFAT-TSP1 signaling pathway in endothelial cells is activated after moderate aerobic exercise, and the activation of this signaling pathway is closely related to exercise-induced shear stress. Subsequent experiments demonstrated that increasing vascular shear stress by aerobic exercise leads to alteration and remodeling of tumor vasculature.
Furthermore, the results have shown that moderate aerobic exercise, when compared to chemotherapy alone, induces a significantly greater reduction in tumor growth by improving the delivery of chemotherapy after the normalization of tumor vasculature. Consequently, the normalizing effect of aerobic exercise on blood vessels can serve as an effective adjunct to chemotherapy [24]. Although there is no reference to radiotherapy data yet, we can also see the potential of aerobic exercise to modulate sensitization to radiotherapy after normalization of tumor vasculature.
5. Conclusion
Angiogenesis has long been recognized as a crucial target in cancer therapy. Abnormal proliferation of the tumor vasculature resulting in a hypoxic microenvironment and consequently reduced sensitivity to radiotherapy is a point that should not be overlooked in cancer treatment. The proposal of vascular normalization undoubtedly brings new hope. Drugs or various physical therapies can promote the change of tumor vascular system to a more “mature” or “normal” phenotype, thus reducing tumor hyperpermeability, increasing perivascular cell coverage, normalizing basement membrane, reducing IFP and ameliorating the resulting tumor hypoxia problem [105]. Thus further alleviating the limitation of tumor radiotherapy resistance to achieve the purpose of radiotherapy sensitization. However, initial attempts to target VEGF signaling derived from endothelial cells and tumors have demonstrated limitations due to the intricate mechanisms involved in tumor angiogenesis. We need to take into account the clinical problem that angiogenesis inhibitors have a limited time window to modulate vascular normalization and their efficacy is limited, and these drugs have serious side effects. Currently, we also see the promise of some physical methods, such as light, electricity, ultrasound, magnetic field and other physical methods to regulate tumor vascular normalization, although not yet applied to radiotherapy, we can see the promise of physical modulation strategies in radiotherapy and other combined treatments. Furthermore, in cancer treatment, there are therapeutic limitations in single therapy, and integrated therapy combining multiple treatment options is the current trend of application in cancer treatment, in which the combination of vascular normalization with chemotherapy and immunotherapy has been reported in many cases. For example, in a recent Phase 3 clinical trial, the combination of the vasonormal inhibitor bevacizumab and the checkpoint inhibitor atezolizumab reduced the chance of tumor recurrence after surgery or heat treatment in patients with early diagnosis of HCC by 28 %. The researchers are still following patients to determine if these benefits persist and if the combination of the two improves survival [106]. This indicates that the long-dormant vascular normalization therapy has shown new vitality. We supplement the application potential of vascular normalization in radiotherapy sensitization, aiming to provide new insights and inspiration for the clinical development of new vascular normalization methods, so as to make up for the limitations of traditional therapy in the clinical application of radiotherapy and promote the clinical application of combined therapy. In conclusion, there are several challenges in the clinical application of angiogenesis inhibitors, including the limited “time window” for vascular normalization, suboptimal efficacy, drug toxicity, resistance, and variations in individualized treatment. Further investigation into the internal mechanism of vascular normalization may lead to new breakthroughs or alternatives. Currently, combination therapy offers potential new hope; however, careful attention must be paid to the toxic and side effects of drugs such as the risk of cardiovascular events. Additionally, evaluating the efficacy, safety and prognosis of combination therapy should be a focal point. Simultaneously, exploring strategies for physically regulating vascular normalization is also worthy of more comprehensive research beyond just drug administration; this may include substantive exploration in studying radiation sensitization. We anticipate witnessing new clinical benefits from combination therapy involving vascular normalization.
List of abbreviations
| TME | Tumor microenvironment |
| SSBs | Single-strand breaks |
| DSBs | Double-strand breaks |
| ROS | Reactive oxygen species |
| IGRT | Image-guided radiation therapy |
| IMRT | Intensity-modulated radiation therapy |
| AuNPs | Gold nanoparticles |
| CAFs | Cancer-associated fibroblasts |
| ICIs | Immune checkpoint inhibitors |
| HIF-1 | Hypoxia-inducible factor-1 |
| HIFs | Hypoxia-inducible factors |
| HRE | Hypoxia response element |
| VEGF | Vascular endothelial growth factor |
| FK228 | Romidepsin |
| mTOR | Mammalian target of rapamycin |
| PDGF-β | Platelet-derived growth factor β |
| Ang-1 and Ang-2 | Angiopoietin-1 and -2 |
| PlGF | Placental growth factor |
| TKIs | Tyrosine kinase inhibitors |
| VEGFR | VEGF receptor |
| HCC | Hepatocellular carcinoma |
| TGF-β | Transforming growth factor |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| ncRNAs | Non-coding RNAs |
| miRNAs | MicroRNAs |
| 3′-UTR | 3′ untranslated region |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| nNOS | Neuronal NOS |
| iNOS | Inducible NOS |
| eNOS | Endothelial NOS |
| EGF | Epidermal growth factor |
| IGF2 | Insulin-like growth factor 2 |
| MMPs | Matrix metalloproteinases |
| ECM | Extracellular matrix |
| α-M | α-Mangostin |
| PD-1 | Programmed cell death protein 1 |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| TAE | Tumor-associated eosinophils |
| MSN | Mesoporous silica nanoparticles |
| Cu2O@Dex | dextran combined with cuprous oxide |
| AuHQ | 8-hydroxyquinoline modified AuNPs |
| HBO | Hyperbaric oxygen |
| nsPEFs | Nanosecond pulsed electric fields |
| PD-ECGF | Platelet-derived endothelial cell growth factor |
| psPEFs | Picosecond pulsed electric fields |
| BTO | Barium titanate |
| MBs | Microbubbles |
| UTMD | Ultrasound-targeted microbubble disruption |
| TAMs | Tumor-associated macrophages |
| VRBC | Erythrocyte velocity |
| SMF | Static magnetic field |
| PEMF | Pulsed electromagnetic field |
Funding
This work was supported by the National Natural Science Foundation of China (82272028, 81971621, 82102087), the Key R&D Program of Hunan Province (2021SK2035), the Natural Science Foundation of Hunan Province (2022JJ30039, 2022JJ40392), the Natural Science Foundation of Guangdong Province (2021A1515011177), the Project of Science and Technology Innovation of Hunan Province (2021SK51807).
Data availability statement
There is no additional data available for this study.
Ethics approval and consent to participate
Not applicable.
CRediT authorship contribution statement
Zhili Guo: Writing – review & editing, Writing – original draft, Investigation. Lingling Lei: Writing – review & editing, Writing – original draft, Investigation. Zenan Zhang: Writing – original draft, Investigation. Meng Du: Writing – review & editing, Validation, Conceptualization. Zhiyi Chen: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
References
- 1.Hubenak J.R., Zhang Q., Branch C.D., Kronowitz S.J. Mechanisms of injury to normal tissue after radiotherapy: a review. Plast. Reconstr. Surg. 2014;133(1):49e–56e. doi: 10.1097/01.prs.0000440818.23647.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin O.A., Martin R.F. Cancer radiotherapy: understanding the price of tumor eradication. Front. Cell Dev. Biol. 2020;8:261. doi: 10.3389/fcell.2020.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumari N., Raghavan S.C. G-quadruplex DNA structures and their relevance in radioprotection. Biochim. Biophys. Acta Gen. Subj. 2021;1865(5) doi: 10.1016/j.bbagen.2021.129857. [DOI] [PubMed] [Google Scholar]
- 4.Franzone P., Fiorentino A., Barra S., Cante D., Masini L., Cazzulo E., et al. Image-guided radiation therapy (IGRT): practical recommendations of Italian Association of Radiation Oncology (AIRO) Radiol. Med. 2016;121(12):958–965. doi: 10.1007/s11547-016-0674-x. [DOI] [PubMed] [Google Scholar]
- 5.Gong L., Zhang Y., Liu C., Zhang M., Han S. Application of radiosensitizers in cancer radiotherapy. Int. J. Nanomed. 2021;16:1083–1102. doi: 10.2147/IJN.S290438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Her S., Jaffray D.A., Allen C. Gold nanoparticles for applications in cancer radiotherapy: mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017;109:84–101. doi: 10.1016/j.addr.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Zai W., Kang L., Dong T., Wang H., Yin L., Gan S., et al. coli E. Membrane vesicles as a catalase carrier for long-term tumor hypoxia relief to enhance radiotherapy. ACS Nano. 2021;15(9):15381–15394. doi: 10.1021/acsnano.1c07621. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimura M., Itasaka S., Harada H., Hiraoka M. Microenvironment and radiation therapy. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/685308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moding E.J., Kastan M.B., Kirsch D.G. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat. Rev. Drug Discov. 2013;12(7):526–542. doi: 10.1038/nrd4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain R.K. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol. 2013;31(17):2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmeliet P., Jain R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011;10(6):417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 12.Jain R.K. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 2001;7(9):987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandra R.A., Keane F.K., Voncken F.E.M., Thomas C.R., Jr. Contemporary radiotherapy: present and future. Lancet. 2021;398(10295):171–184. doi: 10.1016/S0140-6736(21)00233-6. [DOI] [PubMed] [Google Scholar]
- 15.Balmukhanov S.B., Yefimov M.L., Kleinbock T.S. Acquired radioresistance of tumour cells. Nature. 1967;216(5116):709–711. doi: 10.1038/216709a0. [DOI] [PubMed] [Google Scholar]
- 16.Ge Y., Wu Q.J. Knowledge-based planning for intensity-modulated radiation therapy: a review of data-driven approaches. Med. Phys. 2019;46(6):2760–2775. doi: 10.1002/mp.13526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z., Zhao Q., Zheng Z., Liu S., Meng L., Dong L., et al. Vascular normalization in immunotherapy: a promising mechanisms combined with radiotherapy. Biomed. Pharmacother. 2021;139 doi: 10.1016/j.biopha.2021.111607. [DOI] [PubMed] [Google Scholar]
- 18.Wang K., Chen Q., Liu N., Zhang J., Pan X. Recent advances in, and challenges of, anti-angiogenesis agents for tumor chemotherapy based on vascular normalization. Drug Discov. Today. 2021;26(11):2743–2753. doi: 10.1016/j.drudis.2021.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Goel S., Duda D.G., Xu L., Munn L.L., Boucher Y., Fukumura D., et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011;91(3):1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen R., Peng L., Zhou W., Wang D., Jiang Q., Ji J., et al. Anti-angiogenic nano-delivery system promotes tumor vascular normalizing and micro-environment reprogramming in solid tumor. J. Contr. Release. 2022;349:550–564. doi: 10.1016/j.jconrel.2022.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Zeng Y., Zhang S., Li S., Song G., Meng T., Yuan H., et al. Normalizing tumor blood vessels to improve chemotherapy and inhibit breast cancer metastasis by multifunctional nanoparticles. Mol. Pharm. 2023;20(10):5078–5089. doi: 10.1021/acs.molpharmaceut.3c00381. [DOI] [PubMed] [Google Scholar]
- 22.Wang-Bishop L., Kimmel B.R., Ngwa V.M., Madden M.Z., Baljon J.J., Florian D.C., et al. STING-activating nanoparticles normalize the vascular-immune interface to potentiate cancer immunotherapy. Sci Immunol. 2023;8(83) doi: 10.1126/sciimmunol.add1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Y.Y., Chen Y.H., Jin J., Yuan Y., Li J.M., Cai X.J., et al. Modulating tumour vascular normalisation using triptolide-loaded NGR-functionalized liposomes for enhanced cancer radiotherapy. J. Liposome Res. 2023;33(3):251–257. doi: 10.1080/08982104.2022.2161095. [DOI] [PubMed] [Google Scholar]
- 24.Schadler K.L., Thomas N.J., Galie P.A., Bhang D.H., Roby K.C., Addai P., et al. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget. 2016;7(40):65429–65440. doi: 10.18632/oncotarget.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C., Xiao C., Zhan L., Zhang Z., Xing J., Zhai J., et al. Wireless electrical stimulation at the nanoscale interface induces tumor vascular normalization. Bioact. Mater. 2022;18:399–408. doi: 10.1016/j.bioactmat.2022.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin L., Du Y., Hao J., Wu R., Du L. UTMD inhibits pancreatic cancer growth and metastasis by inducing macrophage polarization and vessel normalization. Biomed. Pharmacother. 2023;160 doi: 10.1016/j.biopha.2023.114322. [DOI] [PubMed] [Google Scholar]
- 27.Huang N., Liu Y., Fang Y., Zheng S., Wu J., Wang M., et al. Gold nanoparticles induce tumor vessel normalization and impair metastasis by inhibiting endothelial Smad2/3 signaling. ACS Nano. 2020;14(7):7940–7958. doi: 10.1021/acsnano.9b08460. [DOI] [PubMed] [Google Scholar]
- 28.Ansari M.J., Bokov D., Markov A., Jalil A.T., Shalaby M.N., Suksatan W., et al. Cancer combination therapies by angiogenesis inhibitors; a comprehensive review. Cell Commun. Signal. 2022;20(1):49. doi: 10.1186/s12964-022-00838-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirai Y., Chow C.C.T., Kambe G., Suwa T., Kobayashi M., Takahashi I., et al. An overview of the recent development of anticancer agents targeting the HIF-1 transcription factor. Cancers. 2021;13(11) doi: 10.3390/cancers13112813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J.C., Li X.X., Sun X., Li G.Y., Sun J.L., Ye Y.P., et al. Activation of AMPK by simvastatin inhibited breast tumor angiogenesis via impeding HIF-1α-induced pro-angiogenic factor. Cancer Sci. 2018;109(5):1627–1637. doi: 10.1111/cas.13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou H.M., Sun Q.X., Cheng Y. Paeonol enhances the sensitivity of human ovarian cancer cells to radiotherapy-induced apoptosis due to downregulation of the phosphatidylinositol-3-kinase/Akt/phosphatase and tensin homolog pathway and inhibition of vascular endothelial growth factor. Exp. Ther. Med. 2017;14(4):3213–3220. doi: 10.3892/etm.2017.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang S., Zhou Y., Zou L., Chu L., Chu X., Ni J., et al. Low- dose Apatinib promotes vascular normalization and hypoxia reduction and sensitizes radiotherapy in lung cancer. Cancer Med. 2023;12(4):4434–4445. doi: 10.1002/cam4.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Une N., Takano-Kasuya M., Kitamura N., Ohta M., Inose T., Kato C., et al. The anti-angiogenic agent lenvatinib induces tumor vessel normalization and enhances radiosensitivity in hepatocellular tumors. Med. Oncol. 2021;38(6):60. doi: 10.1007/s12032-021-01503-z. [DOI] [PubMed] [Google Scholar]
- 34.McLornan D.P., Pope J.E., Gotlib J., Harrison C.N. Current and future status of JAK inhibitors. Lancet. 2021;398(10302):803–816. doi: 10.1016/S0140-6736(21)00438-4. [DOI] [PubMed] [Google Scholar]
- 35.Zou S., Tong Q., Liu B., Huang W., Tian Y., Fu X. Targeting STAT3 in cancer immunotherapy. Mol. Cancer. 2020;19(1):145. doi: 10.1186/s12943-020-01258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kashiwagi S., Tsukada K., Xu L., Miyazaki J., Kozin S.V., Tyrrell J.A., et al. Perivascular nitric oxide gradients normalize tumor vasculature. Nat. Med. 2008;14(3):255–257. doi: 10.1038/nm1730. [DOI] [PubMed] [Google Scholar]
- 37.Al-Jomah N., Al-Mohanna F.H., Aboussekhra A. Tocilizumab suppresses the pro-carcinogenic effects of breast cancer-associated fibroblasts through inhibition of the STAT3/AUF1 pathway. Carcinogenesis. 2021;42(12):1439–1448. doi: 10.1093/carcin/bgab102. [DOI] [PubMed] [Google Scholar]
- 38.Mpekris F., Papageorgis P., Polydorou C., Voutouri C., Kalli M., Pirentis A.P., et al. Sonic-hedgehog pathway inhibition normalizes desmoplastic tumor microenvironment to improve chemo- and nanotherapy. J. Contr. Release. 2017;261:105–112. doi: 10.1016/j.jconrel.2017.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bozorgmehr F., Hommertgen A., Krisam J., Lasitschka F., Kuon J., Maenz M., et al. Fostering efficacy of anti-PD-1-treatment: Nivolumab plus radiotherapy in advanced non-small cell lung cancer - study protocol of the FORCE trial. BMC Cancer. 2019;19(1):1074. doi: 10.1186/s12885-019-6205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quintela-Fandino M., Holgado E., Manso L., Morales S., Bermejo B., Colomer R., et al. Immuno-priming durvalumab with bevacizumab in HER2-negative advanced breast cancer: a pilot clinical trial. Breast Cancer Res. 2020;22(1):124. doi: 10.1186/s13058-020-01362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y., Wang L., Chen Y., Qing C. Anti-angiogenic activity of salvicine. Pharm. Biol. 2013;51(8):1061–1065. doi: 10.3109/13880209.2013.776612. [DOI] [PubMed] [Google Scholar]
- 42.Maioli E., Valacchi G. Rottlerin: bases for a possible usage in psoriasis. Curr. Drug Metabol. 2010;11(5):425–430. doi: 10.2174/138920010791526097. [DOI] [PubMed] [Google Scholar]
- 43.Choi I.K., Shin H.J., Lee H.S., Kwon H.J. Streptochlorin, a marine natural product, inhibits NF-kappaB activation and suppresses angiogenesis in vitro. J. Microbiol. Biotechnol. 2007;17(8):1338–1343. [PubMed] [Google Scholar]
- 44.Ottaviani G., Martinelli V., Rupel K., Caronni N., Naseem A., Zandonà L., et al. Laser therapy inhibits tumor growth in mice by promoting immune surveillance and vessel normalization. EBioMedicine. 2016;11:165–172. doi: 10.1016/j.ebiom.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X., Kolb J.F., Swanson R.J., Schoenbach K.H., Beebe S.J. Apoptosis initiation and angiogenesis inhibition: melanoma targets for nanosecond pulsed electric fields. Pigment Cell Melanoma Res. 2010;23(4):554–563. doi: 10.1111/j.1755-148X.2010.00704.x. [DOI] [PubMed] [Google Scholar]
- 46.Wu L., Yao C., Xiong Z., Zhang R., Wang Z., Wu Y., et al. The effects of a picosecond pulsed electric field on angiogenesis in the cervical cancer xenograft models. Gynecol. Oncol. 2016;141(1):175–181. doi: 10.1016/j.ygyno.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Ho Y.J., Chu S.W., Liao E.C., Fan C.H., Chan H.L., Wei K.C., et al. Normalization of tumor vasculature by oxygen microbubbles with ultrasound. Theranostics. 2019;9(24):7370–7383. doi: 10.7150/thno.37750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strieth S., Strelczyk D., Eichhorn M.E., Dellian M., Luedemann S., Griebel J., et al. Static magnetic fields induce blood flow decrease and platelet adherence in tumor microvessels. Cancer Biol. Ther. 2008;7(6):814–819. doi: 10.4161/cbt.7.6.5837. [DOI] [PubMed] [Google Scholar]
- 49.Williams C.D., Markov M.S., Hardman W.E., Cameron I.L. Therapeutic electromagnetic field effects on angiogenesis and tumor growth. Anticancer Res. 2001;21(6a):3887–3891. [PubMed] [Google Scholar]
- 50.Yamaguchi S., Ogiue-Ikeda M., Sekino M., Ueno S. Effects of pulsed magnetic stimulation on tumor development and immune functions in mice. Bioelectromagnetics. 2006;27(1):64–72. doi: 10.1002/bem.20177. [DOI] [PubMed] [Google Scholar]
- 51.Berg H., Günther B., Hilger I., Radeva M., Traitcheva N., Wollweber L. Bioelectromagnetic field effects on cancer cells and mice tumors. Electromagn. Biol. Med. 2010;29(4):132–143. doi: 10.3109/15368371003776725. [DOI] [PubMed] [Google Scholar]
- 52.Öhlund D., Elyada E., Tuveson D. Fibroblast heterogeneity in the cancer wound. J. Exp. Med. 2014;211(8):1503–1523. doi: 10.1084/jem.20140692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng J., Xu M., Wang J., Zhou S., Liu Y., Liu S., et al. Sequential delivery of nanoformulated α-mangostin and triptolide overcomes permeation obstacles and improves therapeutic effects in pancreatic cancer. Biomaterials. 2020;241 doi: 10.1016/j.biomaterials.2020.119907. [DOI] [PubMed] [Google Scholar]
- 54.Huang Y., Kim B.Y.S., Chan C.K., Hahn S.M., Weissman I.L., Jiang W. Improving immune-vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol. 2018;18(3):195–203. doi: 10.1038/nri.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei X., Chen Y., Jiang X., Peng M., Liu Y., Mo Y., et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol. Cancer. 2021;20(1):7. doi: 10.1186/s12943-020-01288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ceranski A.K., Carreño-Gonzalez M.J., Ehlers A.C., Colombo M.V., Cidre-Aranaz F., Grünewald T.G.P. Hypoxia and HIFs in Ewing sarcoma: new perspectives on a multi-facetted relationship. Mol. Cancer. 2023;22(1):49. doi: 10.1186/s12943-023-01750-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Z., Ning F., Wang C., Yu H., Ma Q., Sun Y. Normalization of the tumor microvasculature based on targeting and modulation of the tumor microenvironment. Nanoscale. 2021;13(41):17254–17271. doi: 10.1039/d1nr03387e. [DOI] [PubMed] [Google Scholar]
- 58.Jaśkiewicz M., Moszyńska A., Króliczewski J., Cabaj A., Bartoszewska S., Charzyńska A., et al. The transition from HIF-1 to HIF-2 during prolonged hypoxia results from reactivation of PHDs and HIF1A mRNA instability. Cell. Mol. Biol. Lett. 2022;27(1):109. doi: 10.1186/s11658-022-00408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu S., Guo R., Xu H., Yang J., Luo H., Yeung S.J., et al. 14-3-3σ-NEDD4L axis promotes ubiquitination and degradation of HIF-1α in colorectal cancer. Cell Rep. 2023;42(8) doi: 10.1016/j.celrep.2023.112870. [DOI] [PubMed] [Google Scholar]
- 60.Zhu F., Yao W., Huang Y., Chen Y., Wang Z., Cai X. Candesartan induces tumor vascular normalization to improve the efficacy of radiotherapy in the therapeutic window. Ann. Transl. Med. 2022;10(10):581. doi: 10.21037/atm-22-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caporarello N., Lupo G., Olivieri M., Cristaldi M., Cambria M.T., Salmeri M., Classical VEGF, et al. Notch and Ang signalling in cancer angiogenesis, alternative approaches and future directions. Mol. Med. Rep. 2017;16(4):4393–4402. doi: 10.3892/mmr.2017.7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoon C., Lee H.J., Park D.J., Lee Y.J., Tap W.D., Eisinger-Mathason T.S., et al. Hypoxia-activated chemotherapeutic TH-302 enhances the effects of VEGF-A inhibition and radiation on sarcomas. Br. J. Cancer. 2015;113(1):46–56. doi: 10.1038/bjc.2015.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Bock K., Cauwenberghs S., Carmeliet P. Vessel abnormalization: another hallmark of cancer? Molecular mechanisms and therapeutic implications. Curr. Opin. Genet. Dev. 2011;21(1):73–79. doi: 10.1016/j.gde.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 64.Park S.Y., Piao Y., Jeong K.J., Dong J., de Groot J.F. Periostin (POSTN) regulates tumor resistance to antiangiogenic therapy in glioma models. Mol. Cancer Therapeut. 2016;15(9):2187–2197. doi: 10.1158/1535-7163.MCT-15-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim C.P., Cao X. Structure, function, and regulation of STAT proteins. Mol. Biosyst. 2006;2(11):536–550. doi: 10.1039/b606246f. [DOI] [PubMed] [Google Scholar]
- 66.Dutzmann J., Daniel J.M., Bauersachs J., Hilfiker-Kleiner D., Sedding D.G. Emerging translational approaches to target STAT3 signalling and its impact on vascular disease. Cardiovasc. Res. 2015;106(3):365–374. doi: 10.1093/cvr/cvv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sgrignani J., Garofalo M., Matkovic M., Merulla J., Catapano C.V., Cavalli A. Structural biology of STAT3 and its implications for anticancer therapies development. Int. J. Mol. Sci. 2018;19(6) doi: 10.3390/ijms19061591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niu G., Wright K.L., Huang M., Song L., Haura E., Turkson J., et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21(13):2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 69.Al Zaid Siddiquee K., Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008;18(2):254–267. doi: 10.1038/cr.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee H., Jeong A.J., Ye S.K. Highlighted STAT3 as a potential drug target for cancer therapy. BMB Rep. 2019;52(7):415–423. doi: 10.5483/BMBRep.2019.52.7.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song J., Wang J., Tian S., Li H. Discovery of STAT3 inhibitors: recent advances and future perspectives. Curr. Med. Chem. 2023;30(16):1824–1847. doi: 10.2174/0929867329666220819093117. [DOI] [PubMed] [Google Scholar]
- 72.Yamakuchi M., Lotterman C.D., Bao C., Hruban R.H., Karim B., Mendell J.T., et al. P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 2010;107(14):6334–6339. doi: 10.1073/pnas.0911082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang Y., Ding L., Hu Q., Xia J., Sun J., Wang X., et al. MicroRNA-218 functions as a tumor suppressor in lung cancer by targeting IL-6/STAT3 and negatively correlates with poor prognosis. Mol. Cancer. 2017;16(1):141. doi: 10.1186/s12943-017-0710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gong R., Han R., Zhuang X., Tang W., Xu G., Zhang L., et al. MiR-375 mitigates retinal angiogenesis by depressing the JAK2/STAT3 pathway. Aging (Albany NY) 2022;14(16):6594–6604. doi: 10.18632/aging.204232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu Q., Zhao Y., Wang P. miR-204 inhibits angiogenesis and promotes sensitivity to cetuximab in head and neck squamous cell carcinoma cells by blocking JAK2-STAT3 signaling. Biomed. Pharmacother. 2018;99:278–285. doi: 10.1016/j.biopha.2018.01.055. [DOI] [PubMed] [Google Scholar]
- 76.Wang L., Zhuang L., Rong H., Guo Y., Ling X., Wang R., et al. MicroRNA-101 inhibits proliferation of pulmonary microvascular endothelial cells in a rat model of hepatopulmonary syndrome by targeting the JAK2/STAT3 signaling pathway. Mol. Med. Rep. 2015;12(6):8261–8267. doi: 10.3892/mmr.2015.4471. [DOI] [PubMed] [Google Scholar]
- 77.Zhang X., Tang J., Zhi X., Xie K., Wang W., Li Z., et al. Correction: miR-874 functions as a tumor suppressor by inhibiting angiogenesis through STAT3/VEGF-A pathway in gastric cancer. Oncotarget. 2017;8(17) doi: 10.18632/oncotarget.17402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang Y., Chen Y., Liu J., Zhang B., Yang L., Xue J., et al. MiR-125b-5p/STAT3 Axis regulates drug resistance in osteosarcoma cells by acting on ABC transporters. Stem Cell. Int. 2023;2023 doi: 10.1155/2023/9997676. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Huang Z., Fu J., Zhang Y. Nitric oxide donor-based cancer therapy: advances and prospects. J. Med. Chem. 2017;60(18):7617–7635. doi: 10.1021/acs.jmedchem.6b01672. [DOI] [PubMed] [Google Scholar]
- 80.Van Buren G., 2nd, Camp E.R., Yang A.D., Gray M.J., Fan F., Somcio R., et al. The role of nitric oxide in mediating tumour blood flow. Expert Opin. Ther. Targets. 2006;10(5):689–701. doi: 10.1517/14728222.10.5.689. [DOI] [PubMed] [Google Scholar]
- 81.Sung Y.C., Jin P.R., Chu L.A., Hsu F.F., Wang M.R., Chang C.C., et al. Delivery of nitric oxide with a nanocarrier promotes tumour vessel normalization and potentiates anti-cancer therapies. Nat. Nanotechnol. 2019;14(12):1160–1169. doi: 10.1038/s41565-019-0570-3. [DOI] [PubMed] [Google Scholar]
- 82.Camp E.R., Yang A., Liu W., Fan F., Somcio R., Hicklin D.J., et al. Roles of nitric oxide synthase inhibition and vascular endothelial growth factor receptor-2 inhibition on vascular morphology and function in an in vivo model of pancreatic cancer. Clin. Cancer Res. 2006;12(8):2628–2633. doi: 10.1158/1078-0432.CCR-05-2257. [DOI] [PubMed] [Google Scholar]
- 83.Katagiri T., Kobayashi M., Yoshimura M., Morinibu A., Itasaka S., Hiraoka M., et al. HIF-1 maintains a functional relationship between pancreatic cancer cells and stromal fibroblasts by upregulating expression and secretion of Sonic hedgehog. Oncotarget. 2018;9(12):10525–10535. doi: 10.18632/oncotarget.24156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luo H., Tu G., Liu Z., Liu M. Cancer-associated fibroblasts: a multifaceted driver of breast cancer progression. Cancer Lett. 2015;361(2):155–163. doi: 10.1016/j.canlet.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 85.Chu T.Y., Yang J.T., Huang T.H., Liu H.W. Crosstalk with cancer-associated fibroblasts increases the growth and radiation survival of cervical cancer cells. Radiat. Res. 2014;181(5):540–547. doi: 10.1667/RR13583.1. [DOI] [PubMed] [Google Scholar]
- 86.Portella L., Bello A.M., Scala S. CXCL12 signaling in the tumor microenvironment. Adv. Exp. Med. Biol. 2021;1302:51–70. doi: 10.1007/978-3-030-62658-7_5. [DOI] [PubMed] [Google Scholar]
- 87.Najafi M., Farhood B., Mortezaee K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell. Biochem. 2019;120(3):2782–2790. doi: 10.1002/jcb.27681. [DOI] [PubMed] [Google Scholar]
- 88.Zonneville J., Safina A., Truskinovsky A.M., Arteaga C.L., Bakin A.V. TGF-β signaling promotes tumor vasculature by enhancing the pericyte-endothelium association. BMC Cancer. 2018;18(1):670. doi: 10.1186/s12885-018-4587-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Del Nero M., Colombo A., Garbujo S., Baioni C., Barbieri L., Innocenti M., et al. Advanced cell culture models illuminate the interplay between mammary tumor cells and activated fibroblasts. Cancers. 2023;15(9) doi: 10.3390/cancers15092498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sahai E., Astsaturov I., Cukierman E., DeNardo D.G., Egeblad M., Evans R.M., et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer. 2020;20(3):174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ringuette Goulet C., Bernard G., Tremblay S., Chabaud S., Bolduc S., Pouliot F. Exosomes induce fibroblast differentiation into cancer-associated fibroblasts through TGFβ signaling. Mol. Cancer Res. 2018;16(7):1196–1204. doi: 10.1158/1541-7786.MCR-17-0784. [DOI] [PubMed] [Google Scholar]
- 92.Wang Z., Tang Y., Tan Y., Wei Q., Yu W. Cancer-associated fibroblasts in radiotherapy: challenges and new opportunities. Cell Commun. Signal. 2019;17(1):47. doi: 10.1186/s12964-019-0362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li X., Song W., Shao C., Shi Y., Han W. Emerging predictors of the response to the blockade of immune checkpoints in cancer therapy. Cell. Mol. Immunol. 2019;16(1):28–39. doi: 10.1038/s41423-018-0086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tian L., Goldstein A., Wang H., Ching Lo H., Sun Kim I., Welte T., et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544(7649):250–254. doi: 10.1038/nature21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carretero R., Sektioglu I.M., Garbi N., Salgado O.C., Beckhove P., Hämmerling G.J. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat. Immunol. 2015;16(6):609–617. doi: 10.1038/ni.3159. [DOI] [PubMed] [Google Scholar]
- 96.Zhang A., Gao L. The refined application and evolution of nanotechnology in enhancing radiosensitivity during radiotherapy: transitioning from gold nanoparticles to multifunctional nanomaterials. Int. J. Nanomed. 2023;18:6233–6256. doi: 10.2147/IJN.S436268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taleb M., Ding Y., Wang B., Yang N., Han X., Du C., et al. Dopamine delivery via pH-sensitive nanoparticles for tumor blood vessel normalization and an improved effect of cancer chemotherapeutic drugs. Adv. Healthcare Mater. 2019;8(18) doi: 10.1002/adhm.201900283. [DOI] [PubMed] [Google Scholar]
- 98.Wang M., Yan Z., Sun M., Feng X., Wang W., Yuan Z. Cuprous oxide-based dual catalytic nanostructures for tumor vascular normalization-enhanced chemodynamic therapy. ACS Appl. Nano Mater. 2023;6(8):6911–6919. [Google Scholar]
- 99.Wang X., Niu X., Zhang X., Zhang Z., Gao X., Wang W., et al. Construction of an AuHQ nano-sensitizer for enhanced radiotherapy efficacy through remolding tumor vasculature. J. Mater. Chem. B. 2021;9(21):4365–4379. doi: 10.1039/d1tb00515d. [DOI] [PubMed] [Google Scholar]
- 100.Liang R., Liu L., He H., Chen Z., Han Z., Luo Z., et al. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@manganese dioxide to inhibit tumor growth and metastases. Biomaterials. 2018;177:149–160. doi: 10.1016/j.biomaterials.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 101.Zhang S., Li Y., Li Z., Wang G., Liao A., Wang J., et al. Intelligent nanodelivery system-generated (1) O(2) mediates tumor vessel normalization by activating endothelial TRPV4-eNOS signaling. Small. 2022;18(17) doi: 10.1002/smll.202200038. [DOI] [PubMed] [Google Scholar]
- 102.Li Y., Chen Z., Ge S. Sonoporation: underlying mechanisms and applications in cellular regulation. %J BIOI. 2021;2(1):29–36. [Google Scholar]
- 103.Jones L.W., Viglianti B.L., Tashjian J.A., Kothadia S.M., Keir S.T., Freedland S.J., et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J. Appl. Physiol. 2010;108(2):343–348. doi: 10.1152/japplphysiol.00424.2009. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McCullough D.J., Stabley J.N., Siemann D.W., Behnke B.J. Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. J. Natl. Cancer Inst. 2014;106(4) doi: 10.1093/jnci/dju036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.El Alaoui-Lasmaili K., Faivre B. Antiangiogenic therapy: markers of response, "normalization" and resistance. Crit. Rev. Oncol. Hematol. 2018;128:118–129. doi: 10.1016/j.critrevonc.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 106.Leslie M. New partners reinvigorate a once-touted cancer treatment. Science. 2023;380(6646):679–680. doi: 10.1126/science.adi7657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no additional data available for this study.