In the syncytial Drosophila embryo, nascent actin caps form as local collections of plasma membrane folds and tubules. The centrosome is required for these plasma membrane infoldings and for recruiting Arp2/3 actin networks that reconfigure the membranes for cap growth.
Abstract
Regulated cell shape change requires the induction of cortical cytoskeletal domains. Often, local changes to plasma membrane (PM) topography are involved. Centrosomes organize cortical domains and can affect PM topography by locally pulling the PM inward. Are these centrosome effects coupled? At the syncytial Drosophila embryo cortex, centrosome-induced actin caps grow into dome-like compartments for mitoses. We found the nascent cap to be a collection of PM folds and tubules formed over the astral centrosomal MT array. The localized infoldings require centrosome and dynein activities, and myosin-based surface tension prevents them elsewhere. Centrosome-engaged PM infoldings become specifically enriched with an Arp2/3 induction pathway. Arp2/3 actin network growth between the infoldings counterbalances centrosomal pulling forces and disperses the folds for actin cap expansion. Abnormal domain topography with either centrosome or Arp2/3 disruption correlates with decreased exocytic vesicle association. Together, our data implicate centrosome-organized PM infoldings in coordinating Arp2/3 network growth and exocytosis for cortical domain assembly.
Graphical Abstract
Introduction
Cell shape change involves the deployment of actin networks to specific domains of the cell cortex. The Arp2/3 complex can nucleate actin network growth to expand cortical domains, whereas non-muscle myosin II can pull actin filaments together to contract cortical domains (Bodor et al., 2020; Fernandez-Gonzalez and Harris, 2023; Kelkar et al., 2020; Svitkina, 2020). Signals for inducing local assembly of Arp2/3 and actomyosin networks can come from outside or inside the cell. As an example, outside diffusible factors or anchored ligands act through surface receptors to induce local domains of actin network assembly that direct cell migrations and interactions (Devreotes et al., 2017; Yamada et al., 2019), whereas inside cues from microtubules (MTs) of the mitotic spindle organize an equatorial domain of actomyosin network assembly that drives cell division (Green et al., 2012). Although key signaling molecules are known, e.g., Rho family small G proteins (Lawson and Ridley, 2018), many aspects of inducing a cortical actin domain remain unclear.
Cell-shape change is often associated with folded plasma membrane (PM) domains that can act as membrane reservoirs or signaling platforms. As reservoirs, folded PM domains unfurl to contribute to cortical domain expansion, as evident during cell migration (Goudarzi et al., 2017), asymmetric cell division (LaFoya and Prehoda, 2023), phagocytosis (Masters et al., 2013), and cellularization of syncytial nuclei (Figard et al., 2013). Without folded PM to unfurl, or without exocytic PM addition, cell surface tension can inhibit cortical domain expansion (Raucher and Sheetz, 2000). Intriguingly, patterns of surface topography and tension across the cell have been implicated in localizing actin network induction (Houk et al., 2012; Kessels and Qualmann, 2021; Saha et al., 2018). PM folds also recruit cytoskeletal regulators containing BAR domains that recognize membrane curvature (Kessels and Qualmann, 2021). The SCAR/WAVE complex, a major upstream activator of Arp2/3, forms circular assemblies that preferentially localize to concave regions of PM infoldings (Pipathsouk et al., 2021), and Arp2/3-induced actin networks have been observed to preferentially localize to convex infoldings of giant unilamellar vesicles (Baldauf et al., 2023). In cells migrating on fabricated nanoridges, actin networks preferentially form at resulting PM indentations (Driscoll et al., 2014; Lou et al., 2019; Yang et al., 2023). Nanoridge-induced PM infoldings of activated T cells also accumulate actin networks (Wheatley et al., 2022). PM folds associated with cell-shape changes can form in response to polarized exocytosis (Figard et al., 2016), polarized cortical flows (LaFoya and Prehoda, 2023), and physical interactions with the extracellular environment (Quiroga et al., 2023; Sitarska et al., 2023), whereas PM folding is reduced by surface tension (Kessels and Qualmann, 2021). These examples highlight PM topography as a controllable parameter of cell-shape change, but how generally is PM folding linked to cortical domain growth, especially in vivo?
Another organizer of cortical domains is the centrosome (Elric and Etienne-Manneville, 2014; Tang and Marshall, 2012). Functioning as an internal positional cue, the centrosome has various context-dependent effects on the cell cortex. Centrosomes can locally inhibit cortical actomyosin networks during cell division (Green et al., 2012) and cell polarization (Gan and Motegi, 2021). Reciprocally, centrosomes can induce actin rings around bone reabsorption sites of osteoclasts (Philip et al., 2022) and excess centrosomes heighten Rac-GTP and Arp2/3 activities in endothelial cells (Kushner et al., 2016). Moreover, centrosomes polarize intracellular membrane trafficking to cortical actin domains during cell migration (Elric and Etienne-Manneville, 2014) and immune synapse formation (Douanne and Griffiths, 2021). The centrosome can transmit signals by releasing diffusible molecules, as evident during polarization of the one-cell C. elegans embryo (Zhao et al., 2019). Additionally, motor movements to and from MT minus ends of the centrosome direct intracellular membrane trafficking (Elric and Etienne-Manneville, 2014; Tang and Marshall, 2012) and can redistribute cortical myosin (Chapa-Y-Lazo et al., 2020). The minus-end-directed motor activity of cortically localized dynein can also pull the centrosome to the cell periphery (Elric and Etienne-Manneville, 2014; Tang and Marshall, 2012). Notably, pulling forces between the centrosome and PM can additionally drive PM infoldings, as observed in the C. elegans embryo (Chapa-Y-Lazo et al., 2020; Redemann et al., 2010), in the ascidian embryo (Negishi et al., 2016), and at the immune synapse (Yi et al., 2013). However, a role for centrosome-induced PM infoldings in the formation of a new cortical actin domain has not been reported.
The syncytial Drosophila embryo is an ideal system to study the induction of an expansive cortical domain by the centrosome. The first nuclear divisions occur synchronously, deep within the embryo. Most nuclei then move to the one-cell embryo cortex, where they continue dividing synchronously from nuclear cycles 10–13. At the cortex, transient dome-like compartments form to house individual mitotic spindles and prevent them from colliding. Each dome-like compartment forms from an “actin cap.” A nascent cap is induced above each centrosome of the mitotic spindle at anaphase and telophase, expands laterally as an actin cap, and then bends inward around its perimeter to form a dome-like compartment (Fig. 1 A). Nascent caps for forming two new compartments are thus induced at the apical surface of a single telophase compartment of the preceding cycle. Each new compartment reaches its full depth by the subsequent metaphase and then partially regresses into the next anaphase and telophase (Blake-Hedges and Megraw, 2019; Schmidt and Grosshans, 2018; Tam and Harris, 2024). Significantly, centrosomes are necessary and sufficient for inducing an actin cap (Megraw et al., 1999; Raff and Glover, 1989; Vaizel-Ohayon and Schejter, 1999). In response, the cap grows as a cortical actin domain downstream of Sponge (Spg; a Rac-GEF), Rac-GTP, SCAR, and Arp2/3 (Henry et al., 2022; Postner et al., 1992; Schmidt et al., 2018; Stevenson et al., 2002; Zallen et al., 2002; Zhang et al., 2018), and in combination with membrane exocytosis directed by RalA, Rab8, and the exocyst complex (Holly et al., 2015; Mavor et al., 2016). Cap induction coincides with the cortical assembly of a surrounding actomyosin network (Foe et al., 2000) (Fig. 1 A), which restrains lateral cap growth, guides ingression of the cap perimeter, and generates surface tension over the cap surface (Zhang et al., 2018). The synchronous expansions and ingressions of neighboring caps form pseudocleavage furrows between them, and actomyosin localizes to the base of these furrows until actomyosin disassembly at metaphase (Blake-Hedges and Megraw, 2019; Schmidt and Grosshans, 2018; Tam and Harris, 2024). As a mitotic compartment enters anaphase and telophase, it is unclear how each centrosome initiates a nascent cap for the next round of division. Intriguingly, nascent caps exhibit distinctive PM accumulations (Warn et al., 1984) and lateral cap growth involves Arp2/3-based dispersal of cortical folds (Jiang and Harris, 2019). Thus, we hypothesized that centrosome induction of the nascent cap involves PM infoldings.
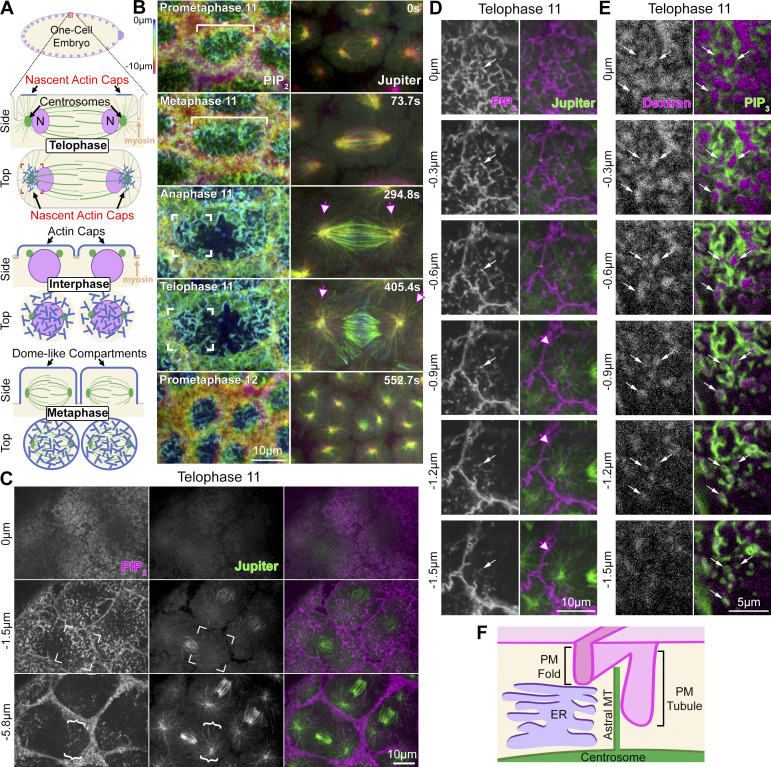
Figure 1.
PM folds and tubules at centrosomal MTs during actin cap formation. (A) Schematics show a nascent actin cap forming above each centrosome of a telophase cortical compartment of the syncytial embryo. Subsequently, each cap expands above each daughter nucleus and grows into a dome-like compartment by metaphase of the next cycle. (B) Coexpressed markers of PM (PH-domain probe for PIP2) and mitotic spindle (Jupiter-GFP) shown from nuclear cycle 11–12 as 3D renderings of maximum intensity projections. Color-coded from apical surface (0 µm) to 10 µm below. White bracket shows PM folds across the full apical surface at prometaphase and metaphase 11 (basal pseudocleavage furrows are red and yellow). Corner brackets show PM folds restricted to apical surface domains above astral centrosomal MTs (pink arrows) at poles of anaphase and telophase 11 compartments. By prometaphase 12, each pole becomes a new compartment. (C) Z-sections show apical PM surface at 0 µm, PM infoldings at −1.5 µm above centrosomal MTs (corner brackets), and cross-sections of PM tubules at −5.8 µm amongst centrosomal MTs (curly brackets). (D) Closely spaced Z sections through one nascent cap show PM folds continuous with PM tubules (white arrows), which extend into centrosomal MTs (pink arrows). Features described in B–D seen in 10/10 embryos. (E) Z sections show the entry of extracellular dye (Dextran) in between folds and into connected tubules (arrows). Seen in 8/8 embryos. (F) Schematic of PM tubule extending from PM fold into astral centrosomal array and between ER membranes (ER imaging in Fig. 2).
Here, we show that the nascent cap is a domain of PM folds and tubules organized by the centrosome and prevented elsewhere by myosin-based cortical tension. Local inductions of Arp2/3-based actin networks appear to push the PM folds apart while maintaining PM tubules and facilitating exocytic vesicle associations as part of cortical domain growth.
Results
Nascent caps are collections of PM folds and tubules integrated with centrosomal MTs
A 1980s non-confocal imaging study of fixed embryos revealed a distinctive morphology of Concanavalin A-stained PM at nascent caps (Warn et al., 1984). To further define nascent cap PM organization, we performed confocal sectioning of live embryos coexpressing the PH domain of PLC-γ fused to mCherry, a probe for the PM lipid phosphatidylinositol (4,5)-bisphosphate (PIP2) (Herszterg et al., 2013), and Jupiter-GFP, an MT-associated protein (Karpova et al., 2006). Color-coded confocal planes allowed tracking of the overall mitotic compartment in 3D through nuclear cycle 11. The prometaphase and metaphase compartment (Fig. 1 B, white brackets) displayed ingressed pseudocleavage furrows around its circumference (Fig. 1 B, red-to-yellow coloring) and folded PM over its entire apical surface (Fig. 1 B, green-to-blue coloring). At anaphase and telophase, PM folds became restricted to compartment poles (Fig. 1 B, corner brackets), astral centrosomal MTs gained abundance and localized below each folded domain (Fig. 1 B, arrows), and surrounding pseudocleavage furrows were shallower. By prometaphase 12, the nascent caps grew into new compartments, each with deep pseudocleavage furrows and PM folds over their apical surfaces (Fig. 1 B). In single confocal sections, telophase nascent cap infoldings were resolved as shallower PM folds (Fig. 1 C, corner brackets at −1.5 µm) and deeper PM puncta (Fig. 1 C, curly brackets at −5.8 µm). These folds and puncta were present in proximity to astral centrosomal MTs, but not to the central spindle between the centrosomes (Fig. 1 C). Serial sectioning and 3D rendering indicated that the PM puncta were cross-sections of PM tubules that extended as continuous structures from PM folds into the embryo (Fig. 1 D, white arrows; Video 1). Astral centrosomal MTs extended up to confocal planes containing PM tubules (Fig. 1 D, pink arrows), but diminished in higher planes dominated by PM folds. Three other distinct PM markers revealed similar organization (a phosphatidylinositol (3,4,5)-trisphosphate (PIP3) probe, Fig. 2 A; Spider-GFP, Fig. 7 F; and Gap43-mCherry, data not shown). Cell-impermeable fluorescent dextran injected into the extracellular space entered wide openings of PM folds and traveled into PM tubules below (Fig. 1 E, arrows). Overall, these data show that the nascent cap forms as a local collection of PM folds which extend deeper PM tubules into the astral centrosomal MT array below (Fig. 1 F schematic).
Video 1.
PM tubules near astral centrosomal MTs at a telophase 11 nascent cap. Surface renderings of deconvolved stacks of co-imaged PIP2 probe (shown first) and Jupiter-GFP (shown second). 0 µm is at apical PM folds (orange) and the movie rotates to show structures 6 µm into the embryo (purple). From the bottom-up, the arc of the surrounding pseudocleavage furrow can be seen to the left with the PIP2 probe. The central spindle can be seen to the right with Jupiter-GFP. The nascent cap is in between the arc of the pseudocleavage furrow and the central spindle. Tips of the PM tubules of the nascent cap are green, and tubules extend down from orange PM folds.
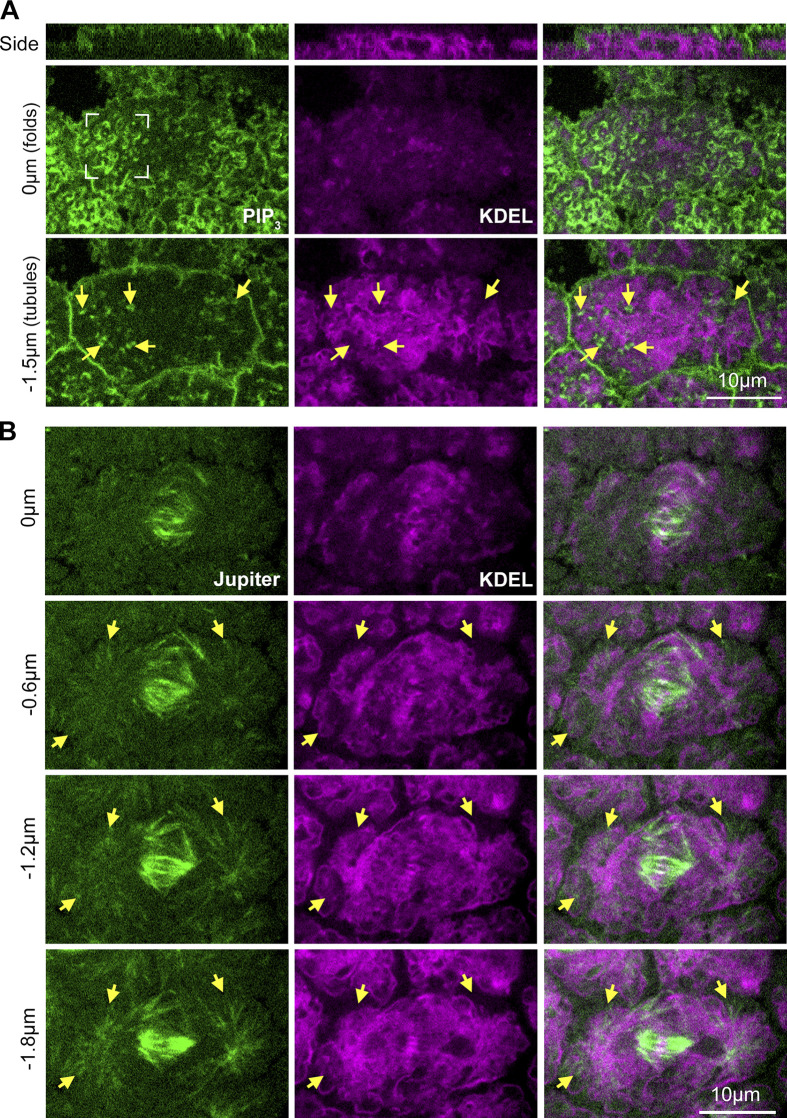
Figure 2.
ER localization relative to nascent cap PM folds and tubules, and to centrosomal MTs. (A) Single sections of co-expressed markers of PM (a PH-domain probe for PIP3) and ER (KDEL-RFP) at telophase 11. PM folds of nascent cap (corner brackets) are mainly above ER. Arrows show PM tubules of nascent cap extending downward between ER regions. Seen in 5/5 embryos. (B) Single sections of co-expressed markers of MTs (Jupiter-GFP) and ER (KDEL-RFP) at telophase 11. Arrows show centrosomal astral MTs emanating upward through ER regions. Seen in 5/5 embryos.
Figure 7.
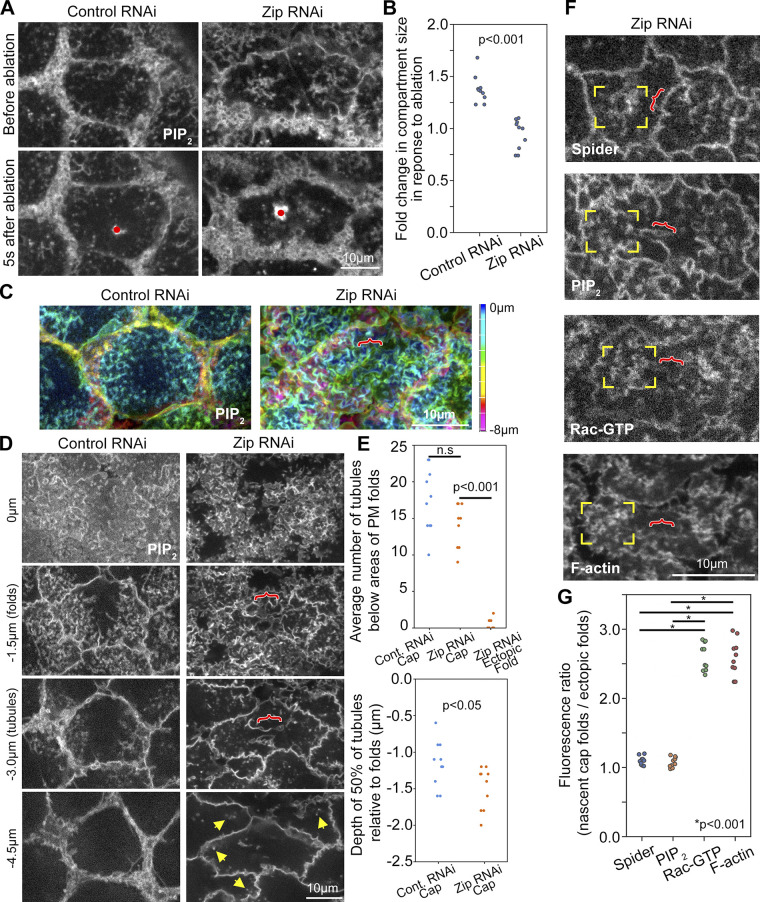
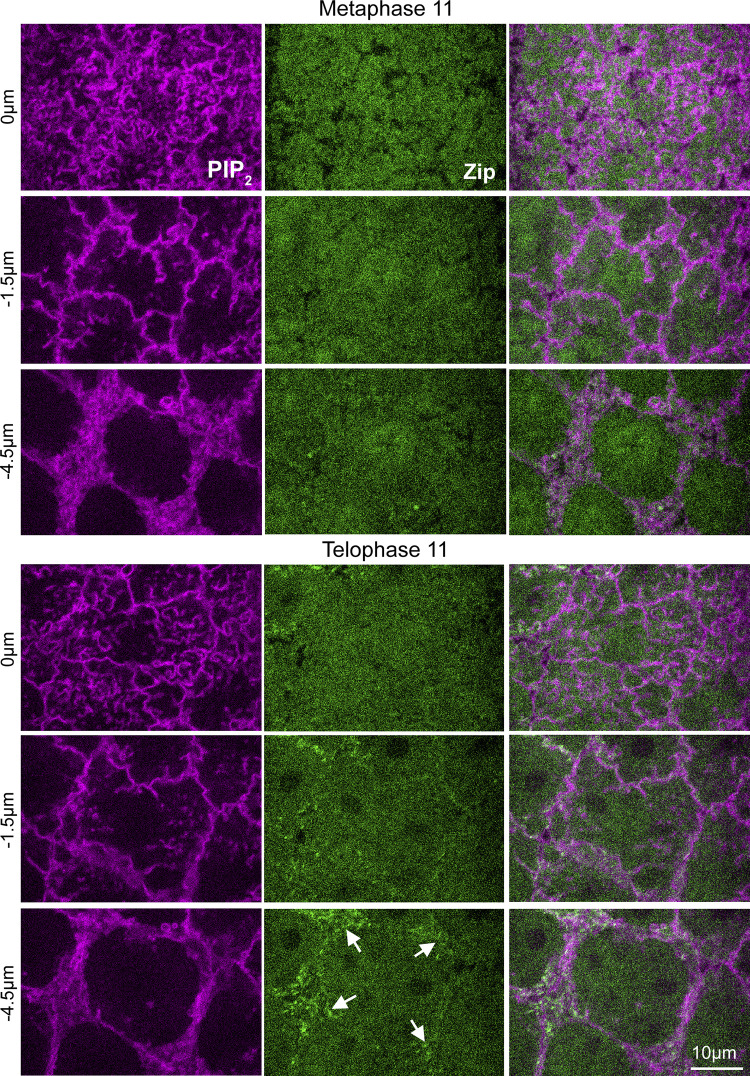
Effect of myosin on telophase compartment surface topography. (A–G) Telophase 11. (A) Single sections show PIP2 probe at pseudocleavage furrows in control and Zip RNAi embryos. Time points before and after ablation are shown. Red dot indicates the ablation site. (B) Compartment areas after ablation compared to pre-ablation areas as ratios in control and Zip RNAi embryos. Embryo values plotted. (C) Maximum intensity projections with color-coded Z-positions (scale) show PM organization detected by PIP2 probe expressed with control and Zip RNAi. The bracket shows ectopic folding between nascent caps in Zip RNAi. (D) Single sections of PIP2 probe in control and Zip RNAi embryos. Red brackets show ectopic folds between nascent caps. Yellow arrows show abnormally deep PM tubules of nascent caps. Minimal tubules were detected below the ectopic folds. Seen in 10/10 Zip RNAi embryos. (E) Quantifications of nascent cap tubule numbers and depths in control and Zip RNAi embryos. Ectopic folds of Zip RNAi embryos were also quantified for tubules. Embryo values plotted. (F) Single sections at level of PM folds show Spider-GFP, PIP2 probe, Rac-GTP probe, and F-actin probe with Zip RNAi. Corner brackets indicate nascent caps. Red brackets show ectopic folds. (G) Quantifications of signal ratios between nascent cap folds and ectopic folds in Zip RNAi embryos. Embryo values plotted.
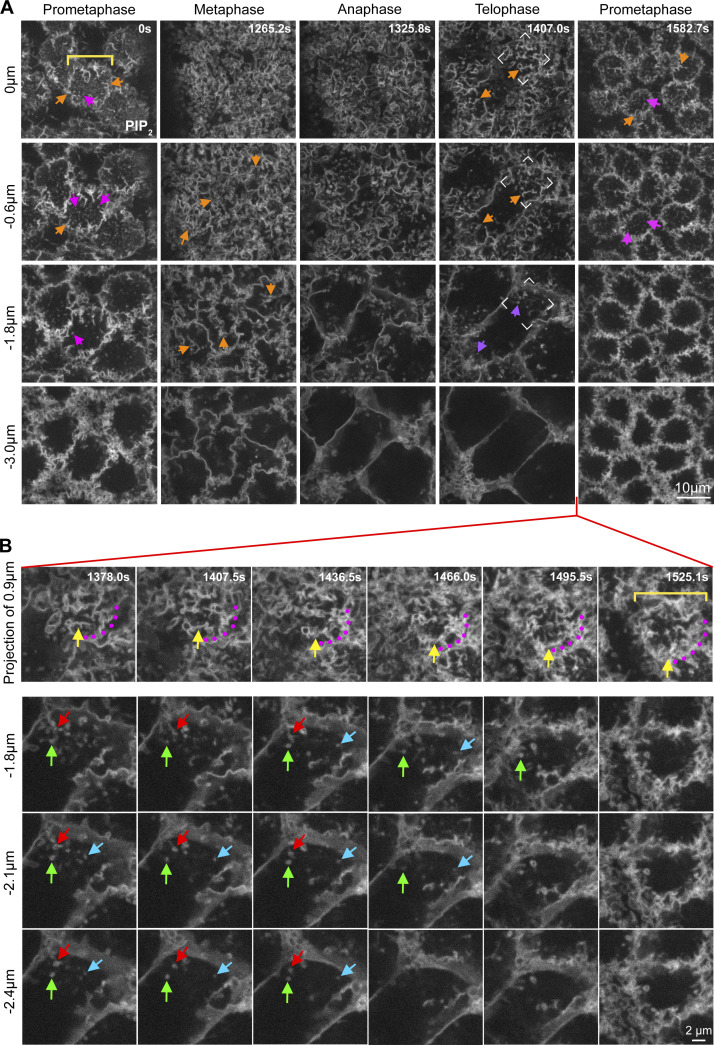
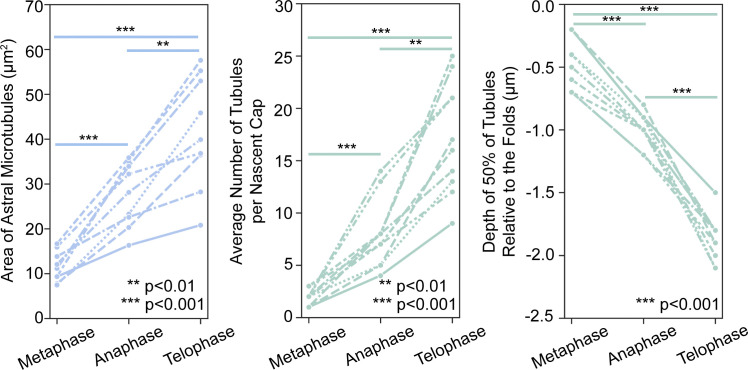
To determine whether the nascent cap PM topography was unique, we examined the apical PM organization of the mitotic compartment through cycle 11 and into cycle 12. At prometaphase 11, the apical PM was relatively flat with an even distribution of PM tubules extending inward directly from the compartment surface (Fig. S1 A, pink arrows). At this stage, PM folds were mainly restricted to the compartment circumference (Fig. S1 A, orange arrows). By metaphase 11, apical PM tubules were replaced by PM folds across the whole apical surface (Fig. S1 A, orange arrows), with few PM tubules below. By telophase, each nascent cap displayed PM folds but now with many tubules below (Fig. S1 A, orange and purple arrows). Quantifications of dual live-imaged PM and MT probes (Fig. 1 B) showed that increasing PM tubule numbers and depths correlated with increasing abundance of astral centrosomal MTs from metaphase to telophase (Fig. S2). From telophase 11 to prometaphase 12, high-time resolution analyses revealed PM fold loss from the cap apical surface (Fig. S1 B, 1525.1 s, bracket), with some folds shifting to the cap perimeter (Fig. S1 B, yellow arrows versus purple dots). Simultaneously, PM tubules retracted from deeper sections (Fig. S1 B, red, green, blue arrows). By prometaphase 12, each new compartment apical surface became repopulated with numerous PM tubules (Fig. S1 A, 1582.7 s, pink arrows), with separate PM folds mainly restricted to the compartment periphery (Fig. S1 A, 1582.7 s, orange arrows). Thus, unique PM topographic domains form where and when nascent caps are induced in association with astral centrosomal MTs.
Figure S1.
Changes to PM morphology over the cell cycle. (A) Z-sections of PIP2–positive PM from nuclear cycle 11–12. The yellow bracket shows a prometaphase compartment. Pink arrows show apical surface tubules at prometaphase. Orange arrows show PM folds at the apical periphery of prometaphase compartments, over the full surface of metaphase compartments, and restricted to the poles of telophase compartments. Purple arrows show PM tubules beneath PM folds at telophase. Corner brackets show a nascent cap at telophase. (B) Higher time resolution imaging of PIP2–positive PM from telophase 11 to prometaphase 12. 1.5 µm projections of the apical surface show movement of a PM fold (yellow arrows) to the periphery of forming compartment (purple dots and yellow bracket). Z-sections show apically directed regression of PM tubules over the same time (red, green, and blue arrows show example tubules). The features described in A and B were seen in 5/5 embryos.
Figure S2.
Comparison of astral centrosomal microtubule abundance, PM tubule numbers, and PM tubule depths from metaphase to telophase of cycle 11. The dataset supporting Fig. 1 B quantified. At metaphase, tubules were assessed across the folded apical domain. At anaphase and telophase, tubules were assessed across the folded nascent caps.
For a fuller understanding of what surrounds the PM folds and tubules at telophase, we investigated the ER, which is organized within each compartment by the MT network (Frescas et al., 2006). Confocal sectioning of live embryos co-expressing a PIP3 probe, a marker of the PM (Britton et al., 2002), and KDEL-RFP, a marker of the ER (Frescas et al., 2006), showed most nascent cap PM folds above the ER (Fig. 2 A, corner brackets), whereas PM tubules extended through gaps of dense ER that otherwise filled the compartment (Fig. 2 A, arrows). Coimaging with Jupiter-GFP showed astral centrosomal MTs extending up through the ER toward the apical surface (Fig. 2 B, arrows). These data indicate that the centrosome and apical surface PM are separated by a dense ER network, but that the ER is infiltrated by centrosomal MTs from below and by PM tubules from above (Fig. 1 F schematic).
An Arp2/3 induction pathway enriches at PM folds and tubules of the nascent cap
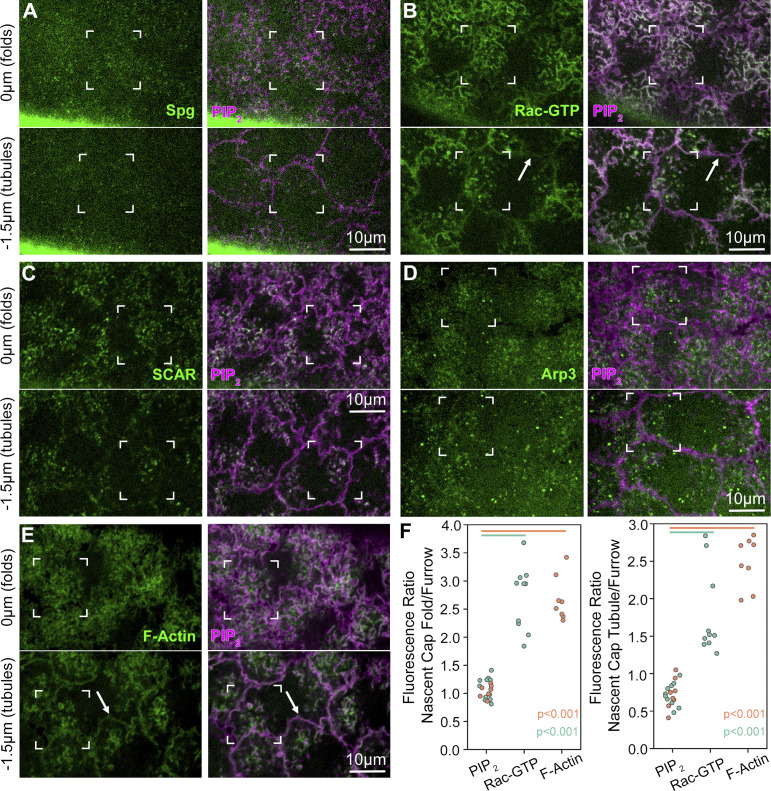
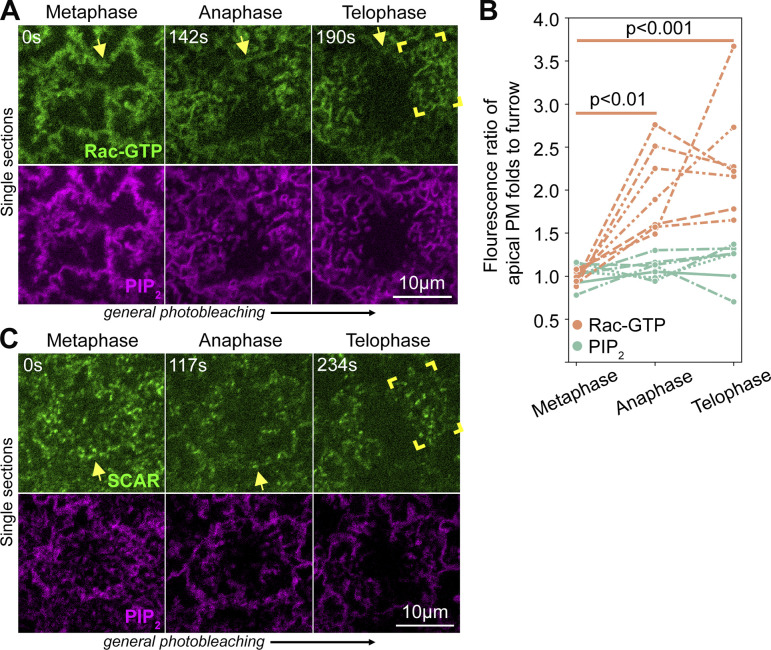
A Rac-based pathway for Arp2/3 actin network induction is needed for cap growth into a dome-like compartment (Tam and Harris, 2024). To test if nascent cap PM folds and tubules are linked to this pathway, we coimaged core pathway members with the PIP2 probe. Embryos were imaged through the cell cycle and then staged at telophase based on morphology changes. To image the GEF Spg, which sits atop the pathway (Postner et al., 1992), we generated a viable and fertile line homozygous for an spg allele with the sequence of Neon Green (NG) inserted downstream by CRISPR-Cas9. Spg-NG signal was weak compared with egg shell autofluorescence, but displayed puncta localized to nascent cap folds (Fig. 3 A, brackets at 0 µm) and tubules (Fig. 3 A, brackets at −1.5 µm). A probe for Rac-GTP, the Rac-binding domain of Pak3 fused to GFP (Abreu-Blanco et al., 2014), which requires Spg to accumulate at the cap (Zhang et al., 2018), strongly labeled nascent cap PM folds (Fig. 3 B, brackets at 0 µm) and tubules (Fig. 3 B, brackets at −1.5 µm). The Rac-GTP probe also labeled double membranes of surrounding pseudocleavage furrows (Fig. 3 B, arrows), but its fluorescence was greater at nascent cap PM folds and tubules (Fig. 3 F). In contrast, coimaged PM marker signal measured at the same sites was indistinguishable between the pseudocleavage furrow and nascent cap PM folds and tubules (Fig. 3 F). Rac-GTP binds and activates the SCAR/WAVE complex, which in turn binds and activates Arp2/3 to nucleate F-actin (Rottner et al., 2021). Endogenously expressed SCAR-NG (Dobramysl et al., 2021) localized as bright puncta overlapping nascent cap PM folds and tubules and with minimal pseudocleavage furrow localization (Fig. 3 C). Endogenously expressed Arp3-GFP (Xie et al., 2021) and a probe for F-actin, the actin-binding domain of Moesin fused to GFP (Kiehart et al., 2000), both localized as puncta enriched at nascent caps and were often between the PM folds and tubules (Fig. 3, D and E, brackets). Arp3-GFP had minimal pseudocleavage furrow localization (Fig. 3 D). F-actin localized at surrounding pseudocleavage furrows (Fig. 3 E, arrows), but was enriched at nascent cap PM folds and tubules, in contrast to coimaged and comeasured PIP2 (Fig. 3 F). Together, these results indicate Arp2/3 induction pathway enrichment and activity at nascent cap PM folds and tubules. Pathway members lacked enrichment to surface PM folds versus surrounding pseudocleavage furrows at metaphase, and the localized enrichment arose as nascent caps formed (Fig. S3).
Figure 3.
Arp2/3 pathway enrichment at PM folds and tubules of the nascent cap. (A–E) Single sections at levels of folds (0 µm) and tubules (−1.5 µm) show pathway members coexpressed with PIP2 probe at telophase 11. Corner brackets indicate nascent cap PM folds and tubules. (A) Spg-NG puncta localize at nascent cap PM folds and tubules, with the minimal signal at surrounding pseudocleavage furrows. Saturated eggshell signal at the base of Spg-NG image. 8/8 embryos. (B) Rac-GTP sensor enriches at nascent cap folds and tubules versus pseudocleavage furrows (arrows). 10/10 embryos. (C) SCAR-NG puncta localize at nascent cap folds and tubules, with minimal signal at pseudocleavage furrows. 6/6 embryos. (D) Arp3-GFP puncta localize to nascent cap folds and tubules, with minimal signal at pseudocleavage furrows. 7/7 embryos. (E) F-actin probe enriched at nascent cap folds and tubules versus lower levels at pseudocleavage furrows (arrows). 8/8 embryos. (F) Signal ratios at nascent cap folds versus pseudocleavage furrows, and tubules versus furrows, were measured at the same sites for PIP2 probe and Rac-GTP probe and for PIP2 probe and F-actin probe. Embryo values plotted.
Figure S3.
Changes to cortical localizations of Rac-GTP and SCAR from metaphase to telophase of cycle 11. (A) Single sections of coexpressed probes for Rac-GTP and PIP2 at metaphase 11, anaphase 11, and telophase 11. Arrows indicate pseudocleavage furrow surrounding the compartment. Corner brackets indicate a nascent cap at telophase. The overall signal decreases from metaphase to telophase due to photobleaching. Enrichment of Rac-GTP at apical PM folds of the nascent cap can be seen by comparing levels at apical folds to levels at pseudocleavage furrows at each stage. Seen in 7/7 embryos. (B) Signal ratios of probes for Rac-GTP and PIP2 at apical PM folds versus surrounding pseudocleavage furrows at metaphase 11, anaphase 11, and telophase 11. Both probes were measured at the same sites. (C) Single sections of co-expressed probes for SCAR and PIP2 at metaphase 11, anaphase 11, and telophase 11. Arrows indicate pseudocleavage furrow surrounding the compartment. Corner brackets indicate nascent cap at telophase. The overall signal decreases from metaphase to telophase due to photobleaching. Enrichment of SCAR at apical PM folds of the nascent cap can be seen by comparing levels at apical folds to levels at pseudocleavage furrows at each stage. Seen in 6/6 embryos.
Centrosomin and dynein are required for PM topography of the telophase compartment
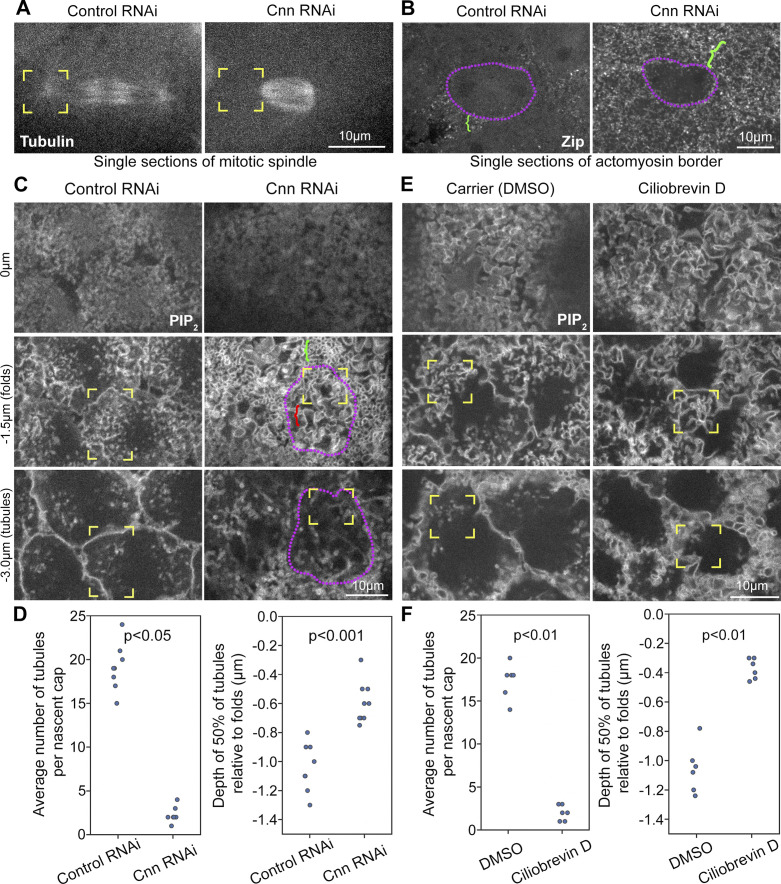
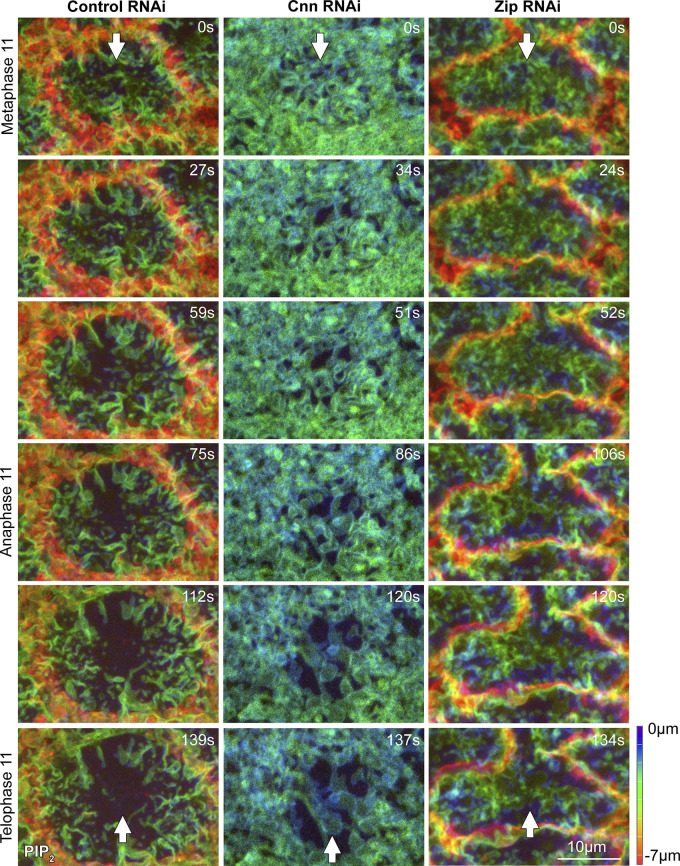
To assess the role of the centrosome in organizing PM topography, we depleted the centrosome component Centrosomin (Cnn). Consistent with disruptions to centrosome integrity and actin cap growth reported for cnn mutants (Megraw et al., 1999; Vaizel-Ohayon and Schejter, 1999), Cnn RNAi resulted in decreased astral centrosomal MTs (Fig. 4 A, corner brackets) and decreased mitotic compartment size (Fig. 4 B, purple outlines), accompanied by expanded surface exposure of the surrounding actomyosin domain (Fig. 4 B, green brackets). To examine telophase 11 compartment PM topography, we imaged the PIP2 probe in control and Cnn RNAi embryos. In Cnn RNAi embryos, the telophase compartment (Fig. 4 C, purple outline) was identified based on distinctive PM organization at its two poles (Fig. 4 C, yellow corner brackets show one pole) and on the development of each pole into an abnormally small compartment by interphase (data not shown). Surrounding the telophase compartment was an abnormal domain of convoluted PM (Fig. 4 C, green bracket), corresponding to the abnormally expanded actomyosin domain (Fig. 4 B, green bracket). Within the telophase compartment, PM folds were across the full apical surface, including the region between the poles (Fig. 4 C, red bracket), in contrast with control RNAi. In Cnn RNAi embryos, PM folds remained abnormally widespread across the mitotic compartment apical surface from metaphase to telophase (Fig. S4), indicating a failure to pattern apical PM topography as nascent caps form. Compared with control RNAi, Cnn RNAi embryos also displayed significantly fewer and shallower PM tubules per nascent cap at telophase (Fig. 4 C, yellow corner brackets at −3.0 µm; quantified in Fig. 4 D). Thus, centrosome activity seems needed to pattern apical PM topography of the telophase compartment. The abnormally widespread PM folding of the telophase Cnn RNAi compartment appears to be an abnormal holdover from metaphase. The reduced PM tubule numbers suggested failed centrosome engagement with the PM folds.
Figure 4.
Perturbation of Cnn or dynein disrupts nascent cap PM tubules. (A) Telophase 11 live imaging of Tubulin-GFP shows reduced astral centrosomal MTs with Cnn RNAi versus control RNAi (corner brackets). Seen in 5/5 embryos each. (B) Telophase 11 live imaging of Zip-GFP shows an expanded actomyosin border (green brackets) around a smaller mitotic compartment (purple outline) of Cnn RNAi embryo versus control RNAi. Seen in 9/9 Cnn RNAi embryos and 8/8 controls. (C) Sections of PIP2 imaging at telophase 11 with control and Cnn RNAi, starting at embryo surface (0 µm). Corner brackets show nascent cap PM folds (−1.5 µm) and tubules (−3.0 µm). The purple outline shows overall telophase compartment in Cnn RNAi. The red bracket shows ectopic folds between nascent caps of Cnn RNAi compartment. Green bracket shows abnormal PM structure outside Cnn RNAi compartment. (D) Quantifications of tubule numbers and depths in embryos corresponding to C. Embryo values plotted. (E) Sections of telophase 11 PIP2 imaging in carrier control (DMSO) and Ciliobrevin D injected embryos, starting at embryo surface (0 µm). Coexpressed Jupiter-GFP (not shown) confirmed cell cycle stage. Corner brackets show nascent cap PM folds (−1.5 µm) and tubules (−3.0 µm). (F) Quantifications of tubule numbers and depths in embryos corresponding to E. Embryo values plotted.
Figure S4.
Mitotic compartment apical PM folds from metaphase 11 to telophase 11 in control RNAi, Cnn RNAi, and Zip RNAi embryos. PIP2 probe color-coded by depth (scale shown). Surrounding pseudocleavage furrows are red-orange (deeper) in control RNAi and Zip RNAi, and green (shallower) in Cnn RNAi. Arrows indicate apical surfaces corresponding to the middle domain between the two nascent caps of the telophase compartment. In control RNAi, folds present in the middle of the apical domain at metaphase are lost by telophase (seen in 10/10 embryos). In Cnn RNAi, the middle of the apical domain remains folded from metaphase to telophase (seen in 8/8 embryos). In Zip RNAi, the middle of the apical domain remains folded from metaphase to telophase (seen in 10/10 embryos).
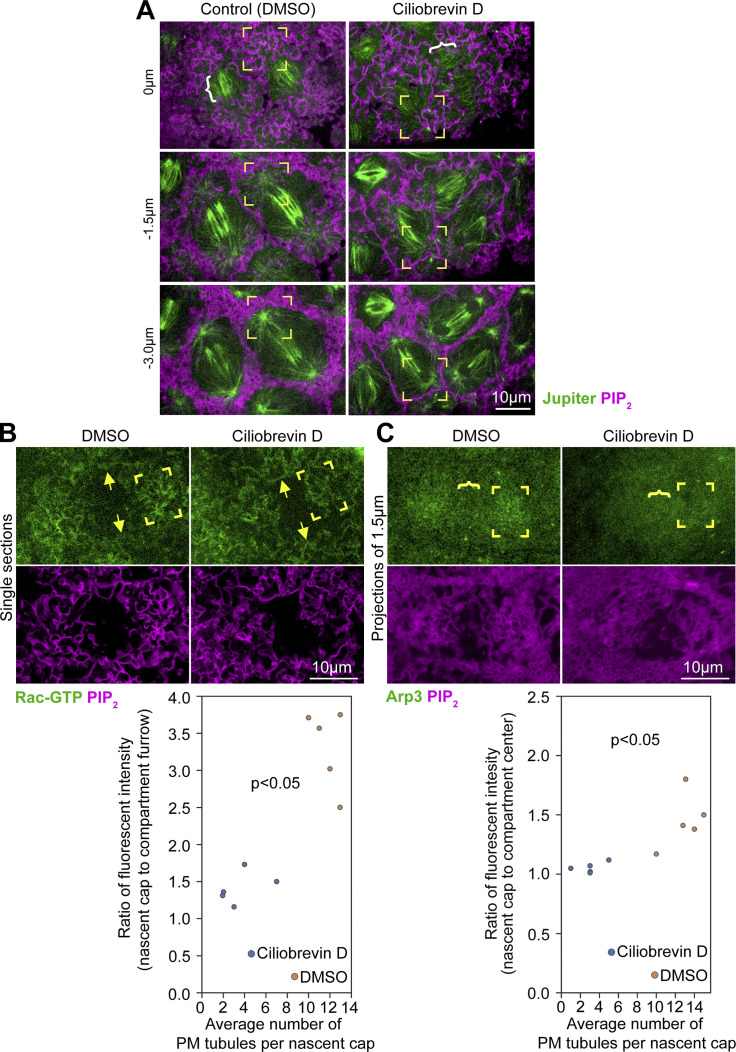
Since the MT motor dynein can associate with the PM and walk along MTs toward their minus ends at centrosomes (Busson et al., 1998; McNally, 2013), we hypothesized that dynein activity pulls PM of the nascent cap toward the centrosome. To assess a dynein requirement, we injected Ciliobrevin D, a dynein inhibitor (Firestone et al., 2012), shown to disrupt dynein-dependent behavior in the early Drosophila embryo (Rollins and Blankenship, 2023). Coimaging of PIP2 and MTs allowed staging at telophase 11 and showed intact centrosomal astral MTs following Ciliobrevin D injection (Fig. S5 A, corner brackets), consistent with Ciliobrevin D not affecting centrosomal γ-tubulin levels in this context (Rollins and Blankenship, 2023). Similar to controls, Ciliobrevin D-injected embryos displayed flat PM domains between nascent caps (Fig. 4 E), regions where the central spindle was closely associated with the PM (Fig. S5 A, white brackets). In contrast to controls, nascent caps of Ciliobrevin D-injected embryos displayed broader spacing of PM folds (Fig. 4 E, corner brackets at −1.5 µm) and significantly fewer and shallower PM tubules (Fig. 4 E, corner brackets at −3.0 µm; quantified in Fig. 4 F). Thus, dynein activity promotes the formation of the nascent cap PM tubules.
Figure S5.
MTs, Rac-GTP, and Arp3 distributions in telophase 11 compartments following Ciliobrevin D and control injections. (A) Single sections of co-imaged PIP2 probe and Jupiter-GFP at telophase 11 following injection of Ciliobrevin D or DMSO carrier control. Yellow corner brackets show astral centrosomal MTs at nascent caps. The white bracket shows the central mitotic spindle. Seen in six embryos each. (B) Single sections of co-imaged PIP2 and Rac-GTP probes at telophase 11 following injection of Ciliobrevin D or DMSO carrier control. Corner brackets indicate nascent caps and arrows indicate surrounding pseudocleavage furrows. Graph plots nascent cap-to-furrow signal ratios versus nascent cap tubule numbers in embryos injected with Ciliobrevin D or DMSO carrier control. P value indicates significant differences both between the signal ratios and between the tubule numbers for the Ciliobrevin D and control injections. (C) 1.5-µm projections of coimaged probes for PIP2 and Arp3 at telophase 11. Corner brackets indicate nascent caps and curly bracket indicates the center domain between caps. Graph plots nascent cap-to-compartment center signal ratios versus nascent cap tubule numbers in embryos injected with Ciliobrevin D or DMSO carrier control. P value indicates significant differences both between the signal ratios and between the tubule numbers for the Ciliobrevin D and control injections.
Centrosomin and dynein are required for recruiting the Arp2/3 pathway to the nascent cap
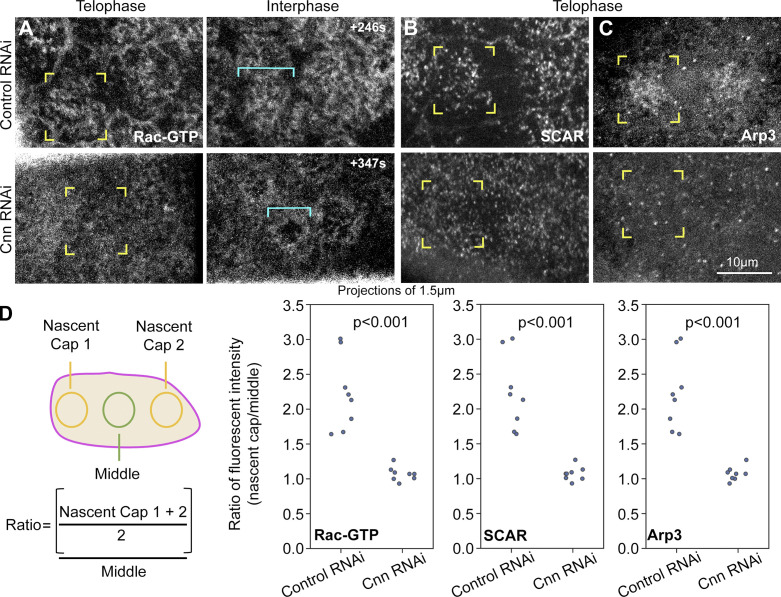
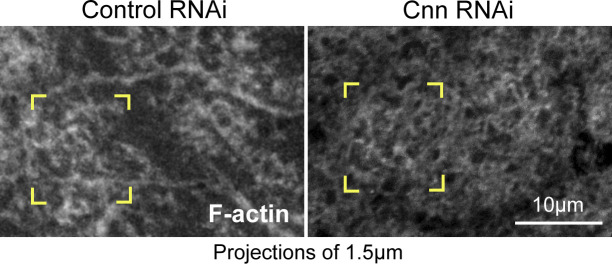
To test whether the centrosome is also needed for Arp2/3 pathway accumulation at the nascent cap, we compared key pathway member localizations between control and Cnn RNAi embryos. In contrast to controls, Cnn RNAi embryos failed to accumulate Rac-GTP at nascent caps (Fig. 5 A, corner brackets), which were detected by subsequent formation of small compartments at the sites (Fig. 5 A, turquoise brackets). Instead, Rac-GTP localized evenly across telophase 11 compartment surfaces in Cnn RNAi embryos. Reduced nascent cap enrichment was quantified by comparing probe levels at nascent caps and at the PM between them (Fig. 5 D). Similarly, SCAR and Arp3 showed significantly less enrichment to Cnn RNAi nascent caps (Fig. 5, B and C, corner brackets; quantified in Fig. 5 D), as did F-actin (Fig. S6). To test whether dynein activity promotes the Arp2/3 pathway accumulation, we compared Ciliobrevin D- and control-injected embryos and found that Ciliobrevin D reduced enrichment of the Rac-GTP probe and Arp3 to nascent caps (Fig. S5, B and C). These results indicate that in addition to being needed for the nascent cap PM tubules, centrosome and dynein activities are required for Arp2/3 pathway recruitment to the nascent cap.
Figure 5.
Cnn is required for Arp2/3 pathway accumulation at the nascent cap. (A) Corner brackets show Rac-GTP sensor at telophase 11 nascent cap in control and Cnn RNAi. Nascent cap position is determined by imaging into interphase when abnormally small compartments form with Cnn RNAi (turquoise brackets). (B and C) Corner brackets show SCAR-NG and Arp3-GFP at telophase 11 nascent caps with control and Cnn RNAi. (D) Enrichment assay and data comparing nascent caps versus middle surface of telophase compartment. Embryo values plotted.
Figure S6.
Cnn is required for F-actin enrichment to nascent caps of telophase 11 compartments. Corner brackets show minimal F-actin probe enrichment to the nascent cap relative to the middle of the telophase compartment in Cnn RNAi, in contrast to the enrichment at the nascent cap in control RNAi. Seen in 8/8 control embryos and 7/10 Cnn RNAi embryos.
Myosin is required for surface tension and PM topography of the telophase compartment
The mitotic compartment surface changes in two main ways from metaphase to telophase. At metaphase, the surface is fully covered with folds (Fig. 1 B and Fig. S1 A). By telophase, PM tubules extend inward from the surface folds of each nascent cap, and folds are lost between the two nascent caps (Fig. 1 B and Fig. S1 A). We hypothesized that centrosome activity is not the only contributor to remodeling of compartment surface topography from metaphase to telophase. Notably, myosin is known to be lost from the cortex at metaphase and regained by telophase (Royou et al., 2002), and caps experience myosin-dependent surface tension as they transform into dome-like compartments (Zhang et al., 2018). By coexpressing endogenously expressed Zip-GFP (zipper [zip] encodes the heavy chain of non-muscle myosin II) with the PIP2 probe, we confirmed that myosin reaccumulation at the base of pseudocleavage furrows coincided with a gain of patterned surface topography by telophase (Fig. S7).
Figure S7.
Myosin localization in metaphase and telophase compartments of cycle 11. Single sections of embryos co-expressing Zip and PIP2 probes. At metaphase, Zip-GFP is non-cortical. At telophase, Zip-GFP accumulates at the base of pseudocleavage furrows (arrows). Seen in 5/5 embryos.
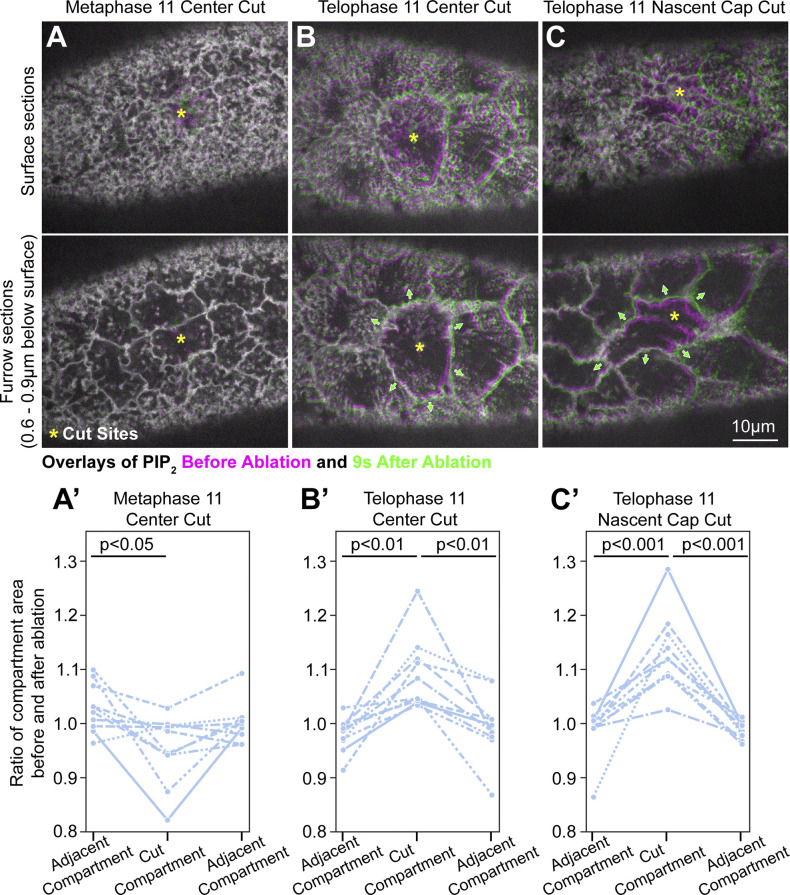
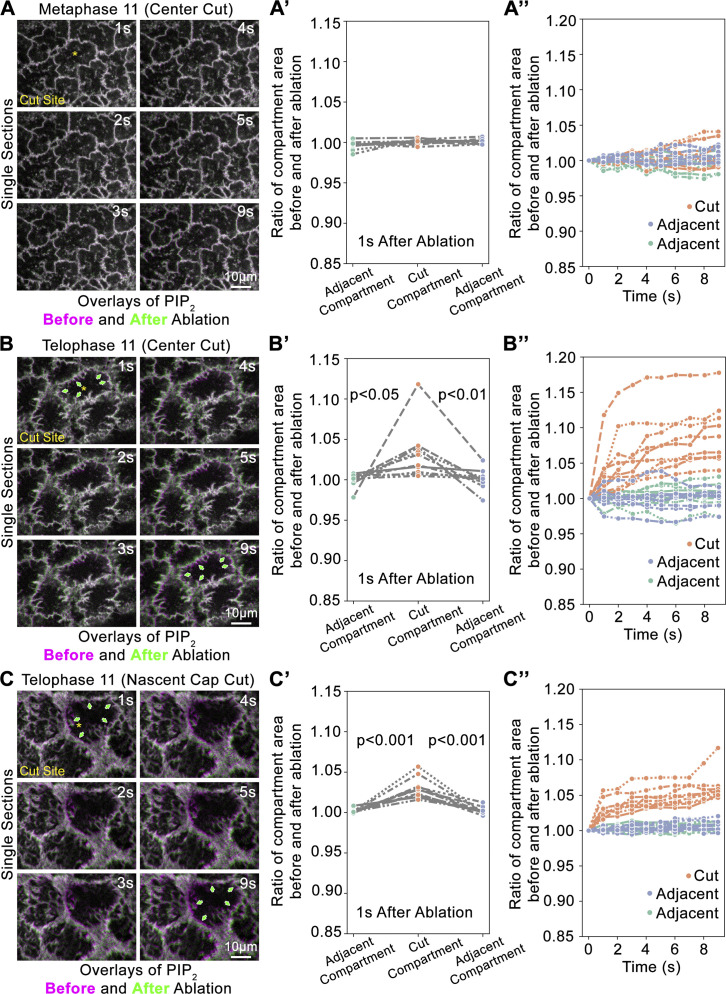
To probe for cortical tension, we cut the compartment surface with a laser and monitored whether the compartment surface and boundary recoiled from the cut site. Coimaging of PIP2 and tubulin probes in 3D allowed precise cell cycle staging and assessments of both the compartment surface and boundary, but reduced time resolution. At metaphase, cutting the center of the compartment surface resulted in no change or a reduction to compartment size (Fig. 6, A and A′), indicating a lack of tensile stress across the surface. At telophase, cutting the flat center of the compartment resulted in movements of the compartment surface and boundary away from the cut site, during which neighboring uncut compartments translocated but maintained their sizes (Fig. 6, B and B′). Cutting of telophase nascent caps produced similar outward movements of the cut compartment in contrast to adjacent compartments that maintained their areas while translocating (Fig. 6, C and C′). These responses to local laser cuts suggested that both the central surface of the telophase compartment and the folded PM of the nascent cap are under tensile stress. To confirm that the observed movements were due to tensile stress present at the compartment locations at the time of ablation, rather than being biological responses to the ablations, we monitored second-by-second responses to local laser cuts by limiting imaging to the PIP2 probe alone in a single confocal plane. Quantifications revealed similar trends as the two-probe, multiplane imaging, confirming that both the flat center and the nascent caps of the telophase compartment are under tensile stress (Fig. S8). To assess the role of myosin in generating cortical tension at telophase, we used a Zip RNAi line previously shown to disrupt myosin-based processes in the early embryo (Jiang and Harris, 2019). Telophase compartment size increases following ablation of the central compartment surface in control RNAi embryos did not occur in Zip RNAi embryos (Fig. 7, A and B), indicating that myosin is required for the tensile stress.
Figure 6.
Mitotic compartment responses to apical surface laser cuts. (A–C) Co-expressed Jupiter-GFP (not shown) confirmed cell cycle stages. (A) Single sections of PIP2 probe before (magenta) and after laser cut (green). A cut of the center of the metaphase 11 compartment (yellow asterisk) produced minimal PM displacements at the apical surface or the pseudocleavage furrows below. Areas encompassed by pseudocleavage furrows of the cut compartment and of adjacent compartments quantified before and 9 s after the cut (A′). (B) Single sections of PIP2 probe before (magenta) and after laser cut (green). A cut of the center of the telophase 11 compartment (yellow asterisk) produced PM displacements away from the cut both at the apical surface and at the pseudocleavage furrows below (green arrows). Note that adjacent compartments translocate without apparent area change. Areas encompassed by pseudocleavage furrows of the cut compartment and of adjacent compartments quantified before and 9 s after the cut (B′). (C) Single sections of PIP2 probe before (magenta) and after laser cut (green). A cut of a nascent cap of the telophase 11 compartment (yellow asterisk) produced PM displacements away from the cut both at the apical surface and at the pseudocleavage furrows below (green arrows). Note that adjacent compartments translocate without apparent area change. Areas encompassed by pseudocleavage furrows of the cut compartment and of adjacent compartments quantified before and 9 s after the cut (C′).
Figure S8.
High-time resolution analyses of mitotic compartment responses to local laser cuts. (A–C″) Single confocal section imaging of the PIP2 probe following local laser ablations, and quantifications of responses. Compartment pseudocleavage furrows overlaid before (purple) and after (green) ablation. Asterisks show cut sites. (A) Minimal response to cutting the center of a metaphase 11 compartment. (A′) Quantifications of minimal compartment area change 1 s after laser cuts of centers of metaphase 11 compartments. Neither the cut compartments nor adjacent compartments changed the area substantially, and the responses of the cut compartments were not significantly different from those of the adjacent compartments. Three interconnected dots of one cut compartment and two adjacent compartments are shown for each of the 10 embryos. (A″) Quantifications of minimal compartment area changes from 1 to 9 s after laser cuts of centers of metaphase 11 compartments. The compartments assessed in A′ were quantified second-by-second until 9 s after ablation. Neither the cut compartments nor adjacent compartments changed area substantially. (B) Green arrows show the increased area of a telophase 11 compartment from 1 to 9 s following a laser cut of its center. Note that adjacent compartments translocate without apparent area change. (B′) Quantifications 1 s after laser cutting show that telophase 11 compartments cut at their center significantly increase in area compared to adjacent compartments with minimal change. Three interconnected dots of one cut compartment and two adjacent compartments are shown for each of the 10 embryos. (B″) Quantifications of compartment area changes from 1 to 9 s after laser cuts of centers of telophase 11 compartments. The compartments assessed in B′ were quantified second-by-second until 9 s after ablation. Cut compartments continued to increase in area from 1 to 9 s, whereas adjacent compartments showed relatively low area changes. (C) Green arrows show increased area of a telophase 11 compartment from 1 to 9 s following a laser cut of one of its nascent caps. Note that adjacent compartments translocate without apparent area change. (C′) Quantifications 1 s after laser cutting show that telophase 11 compartments cut at a nascent cap significantly increase in area compared to adjacent compartments with minimal change. Three interconnected dots of one cut compartment and two adjacent compartments are shown for each of the 10 embryos. (C″) Quantifications of compartment area changes from 1 to 9 s after laser cuts of nascent caps of telophase 11 compartments. The compartments assessed in C′ were quantified second-by-second until 9 s after ablation. Cut compartments continued to increase in area from 1 to 9 s, whereas adjacent compartments showed relatively low area changes.
To test if the reduced cortical tension of Zip RNAi telophase compartments correlated with a change to compartment surface topography, we examined PM organization in control and Zip RNAi embryos. In contrast to controls, Zip RNAi embryos displayed ectopic PM folds between the two nascent caps (Fig. 7 C, red bracket). Imaging Zip RNAi embryos from metaphase to telophase suggested the ectopic PM folds at telophase were an abnormal holdover of folds at metaphase (Fig. S4). Notably, tubules did not extend from the base of the ectopic folds, in contrast to nascent caps of control and Zip RNAi embryos (Fig. 7 D, quantified in Fig. 7 E), suggesting the ectopic folds are not due to centrosomal activity. At nascent caps, the number of tubules was indistinguishable between control and Zip RNAi embryos, but tubules were significantly deeper in Zip RNAi embryos (Fig. 7 D, yellow arrows; quantifications in Fig. 7 E). Overall, these data suggest that actomyosin assembly around the base of the telophase compartment results in surface tension that flattens apical PM in between nascent caps, and that restrains PM tubule depths within the nascent caps.
Ectopic PM folds are insufficient for recruiting the Arp2/3 pathway
The ectopic PM folds between nascent caps of Zip RNAi telophase compartments provided an additional opportunity to test how centrosome activity recruits the Arp2/3 pathway to PM folds at this stage of the cell cycle. Our hypothesis was that centrosome effects on PM topography and Arp2/3 pathway recruitment are locally coupled, but we considered an alternate hypothesis that centrosomes might emit longer-range signals with the potential to promote Arp2/3 pathway localization at more distant PM folds. Imaging of the Rac-GTP and F-actin probes showed signal levels at the ectopic folds that were significantly lower than those at nascent cap folds of the same Zip RNAi compartment (Fig. 7 F, compare red brackets and corner brackets; quantified in Fig. 7 G). The PIP2 probe and another PM probe, Spider-GFP, displayed indistinguishable levels at the two types of folds in Zip RNAi embryos (Fig. 7 F, compare red brackets and corner brackets; quantified in Fig. 7 G). Thus, ectopic PM folds away from the centrosome are unable to recruit the Arp2/3 pathway at telophase, indicating close coupling of centrosome-organized PM topography and centrosome-recruited Arp2/3 activity.
Arp2/3 actin networks are required for PM fold separation and PM tubule numbers in the nascent cap
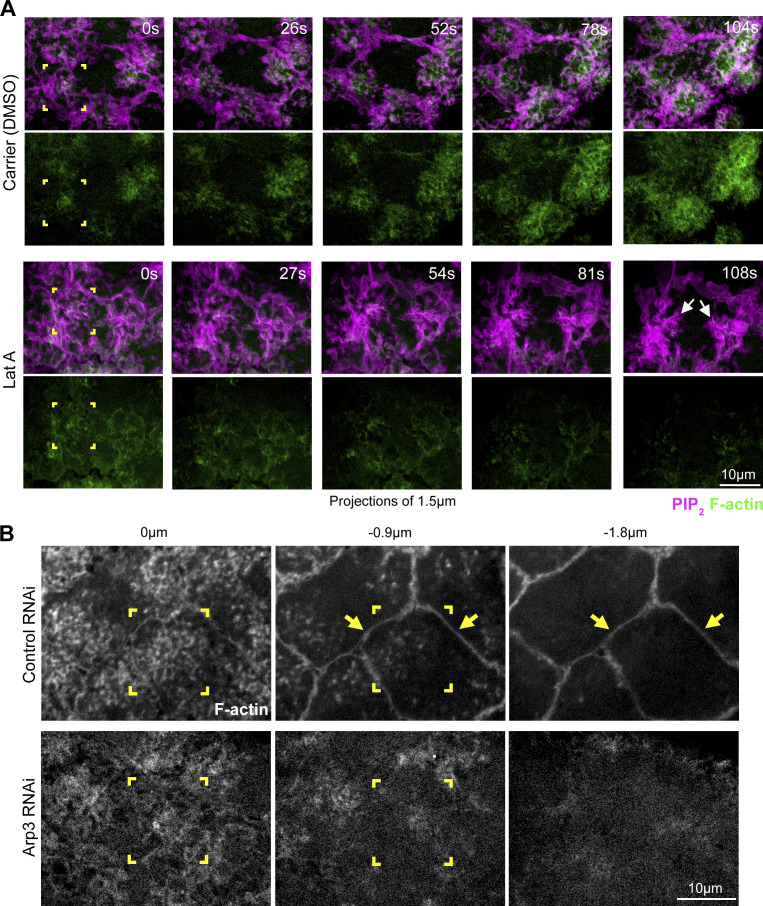
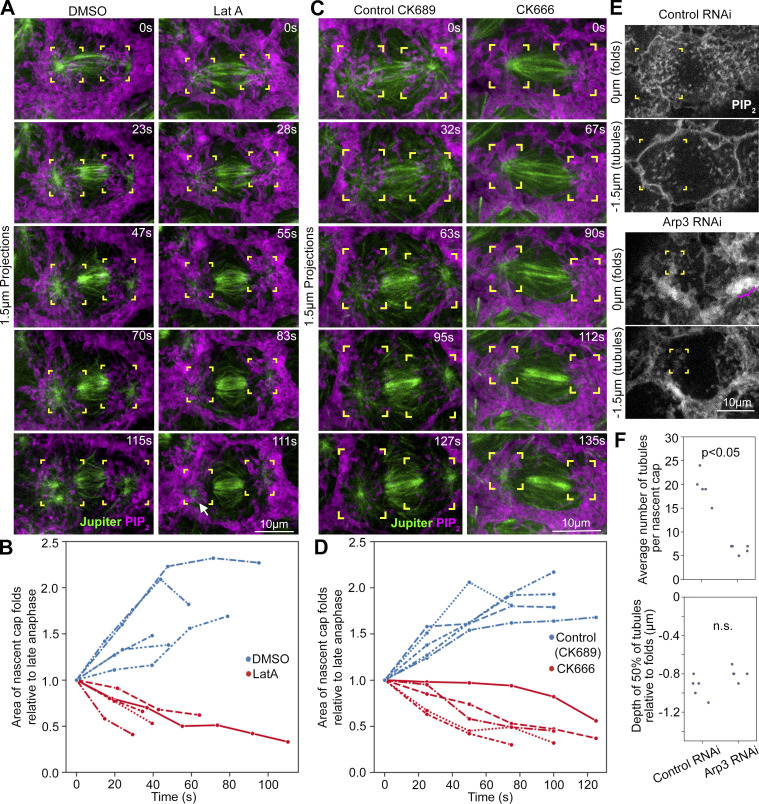
F-actin accumulation between nascent cap PM folds and tubules (Fig. 3 E) suggested that Arp2/3 recruitment might affect them via local induction of F-actin networks. To assess the effect of F-actin on nascent cap PM topography, the general F-actin inhibitor Latrunculin A (Lat A) was injected. Coimaging of F-actin and PIP2 probes following carrier control injections showed growth of F-actin networks at nascent caps and associated movements of PM folds to the periphery of the expanding compartments (Fig. S9 A). In contrast, Lat A injection resulted in rapid loss of F-actin and an abnormal aggregation of PM folds into condensed foci at each nascent cap (Fig. S9 A, arrows). To test if the abnormal aggregation of PM folds was focused at centrosomes, embryos coexpressing the PIP2 probe and Jupiter-GFP were injected. In control injections, nascent cap PM folds redistributed away from the centrosome, whereas Lat A injection resulted in abnormal aggregation of PM folds around the centrosome (Fig. 8 A, compare corner brackets; nascent cap area quantifications in Fig. 8 B). Thus, F-actin networks induced among the nascent cap PM folds appear to push the folds apart in opposition to an attraction to the centrosome. However, disruption to the surrounding actomyosin network could also contribute to the Lat A effect.
Figure S9.
Effects of Lat A and Arp3 RNAi on nascent cap F-actin. (A) Co-expressed PIP2 and F-actin probes at telophase 11 following control (DMSO) or Lat A injection. Corner brackets show similar nascent caps at 0 s. Arrows point to aggregated nascent cap PM in response to Lat A. The Lat A-induced PM aggregation coincided with F-actin loss (27–108 s). In control injections, the nascent cap PM domain expanded and increased in F-actin (26–104 s). Seen in 5/5 embryos for both control and Lat A injections. (B) F-actin probe with control or Arp3 RNAi. Corner brackets show planes of nascent cap folds (0 µm) and tubules (−0.9 µm) with lower F-actin in Arp3 RNAi. Arrows show F-actin at pseudocleavage furrows in control. F-actin-positive pseudocleavage furrows are minimal in Arp3 RNAi. Seen in 10/12 Arp3 RNAi embryos and 10/10 control RNAi embryos.
Figure 8.
Effects of actin polymerization and Arp3 on nascent cap PM folds and tubules. (A) Coimaged PIP2 probe and Jupiter-GFP after injection of Lat A or carrier control (DMSO) from late anaphase 11 (0 s) into telophase 11. Corner brackets show nascent cap PM fold redistributions over time. (B) Quantification of nascent cap PM fold areas relative to anaphase 11 (0 s) into telophase 11 for embryos injected with a carrier (blue) or LatA (red). Embryo values plotted. (C) Coimaged PIP2 probe and Jupiter-GFP after injection of CK666 or control (CK689) from late anaphase 11 (0 s) into telophase 11. Corner brackets show nascent cap PM fold redistributions over time. (D) Quantification of nascent cap PM fold areas relative to anaphase 11 (0 s) into telophase 11 for embryos injected with CK689 control (blue) or CK666 (red). Embryo values plotted. (E) PIP2 probe in control and Arp3 RNAi embryos at telophase 11. Corner brackets show PM folds (0 µm) and PM tubules (−1.5 µm). Pink bracket shows abnormal PIP2 aggregation at pseudocleavage furrow in Arp3 RNAi. (F) Quantifications of nascent cap tubule numbers and depths in control and Arp3 RNAi embryos. Embryo values are plotted.
To specifically test the effects of Arp2/3-based actin networks, we injected embryos with the Arp2/3 inhibitor CK666 and the inactive control molecule CK689 (Nolen et al., 2009). CK666 injection resulted in the collapse of nascent cap folds toward the centrosome, whereas CK689 injection had no apparent effect on nascent cap expansion (Fig. 8 C, compare corner brackets; nascent cap area quantifications in Fig. 8 D). Next, we tested the effect of Arp3 shRNA expression previously shown to disrupt Arp2/3-based actin cap growth (Jiang and Harris, 2019; Zhang et al., 2018). F-actin probe imaging showed a substantial reduction at nascent caps and a greater reduction at surrounding pseudocleavage furrows in Arp3 RNAi embryos versus controls (Fig. S9 B; F-actin remaining at the nascent cap might be due to combined activities of residual Arp2/3 and other actin nucleators). Imaging the PIP2 probe in Arp3 RNAi embryos revealed abnormal aggregation of PM folds and fewer PM tubules at nascent caps (Fig. 8 E, corner brackets; quantified in Fig. 8 F), in addition to abnormal signal aggregation along surrounding pseudocleavage furrows (Fig. 8 E, pink bracket). CK666 injection also resulted in significantly fewer nascent cap tubules versus CK689 control injection (7 ± 2 tubules per cap versus 19 ± 2 tubules per cap, respectively; mean ± SD; 5 embryos each; P < 0.05). Thus, Arp2/3-based actin networks seem to prevent the collapse of nascent cap PM folds toward the centrosome and to promote nascent cap PM tubules.
Links between nascent cap PM topography and exocytic vesicles
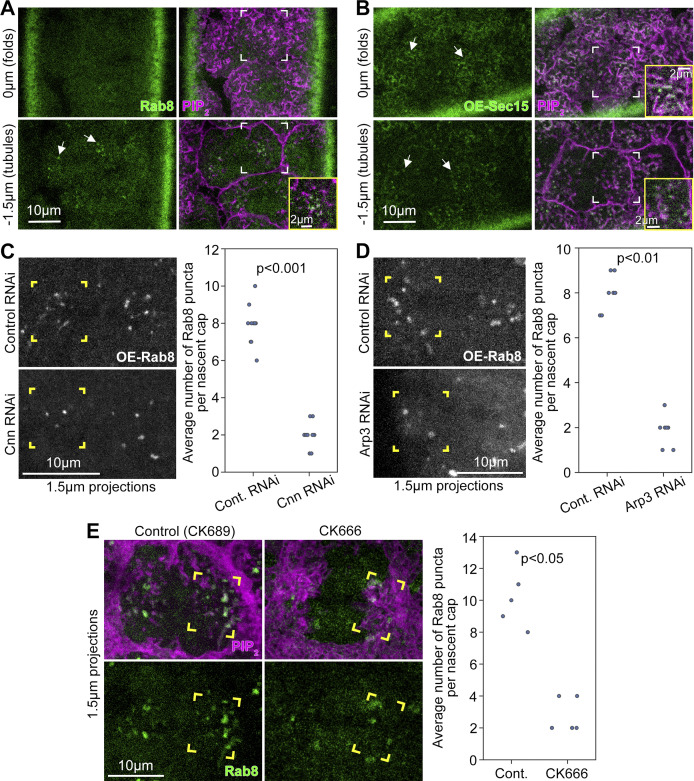
Our results showed a close connection between centrosome-organized PM topography and centrosome-induced Arp2/3 activity at nascent cap PM folds and tubules. In addition to actin polymerization, exocytosis facilitated by Rab8 and the exocyst complex promotes actin cap growth into a dome-like compartment (Holly et al., 2015; Mavor et al., 2016). To test if nascent cap PM folds and tubules are linked to this exocytosis pathway, we imaged endogenously expressed Rab8-YFP (Dunst et al., 2015) and overexpressed Sec15-GFP, a component of the exocyst complex. Rab8 localized as puncta that accumulated at nascent caps more than other regions of the telophase compartment (Fig. 9 A, arrows). Within nascent caps, Rab8 puncta was associated closely with PM tubules (Fig. 9 A, brackets and inset) but associated minimally with PM folds above. Sec15 was enriched at both PM folds and tubules of the nascent cap (Fig. 9 B, arrows, brackets and insets) and localized minimally to pseudocleavage furrows (Fig. 9 B). To test if the nascent cap enrichments of Rab8 and Sec15 were associated with a specific accumulation of the exocytosis pathway, we examined markers of other internal membranes: endogenously expressed Rab11-YFP (Dunst et al., 2015), a marker of recycling endosomes, endogenously expressed Rab5-YFP (Dunst et al., 2015), a marker of early endosomes, and overexpressed Arf1-GFP (Shao et al., 2010), a marker of Golgi membranes. Each localized as puncta distributed throughout the telophase compartment without enrichment at nascent caps (Fig. S10). Thus, nascent cap PM folds and tubules seem specifically associated with exocytosis.
Figure 9.
Localization of Rab8 and Sec15 to the nascent cap. (A and B) Telophase 11 dual live imaging of PIP2 probe with endogenously-expressed Rab8-YFP (A) or with overexpressed Sec15-GFP (B). Single sections at PM folds (0 µm) and tubules (−1.5 µm). Arrows, corner brackets, and magnified insets show nascent caps. Both patterns seen in 9/9 embryos. (C) Corner brackets show Rab8 puncta at telophase 11 nascent caps with control and Cnn RNAi. Rab8 detected as overexpressed Rab8-GFP. Quantifications of Rab8 puncta numbers per nascent cap are shown. Embryo values plotted. (D) Corner brackets show Rab8 puncta at telophase 11 nascent caps with control and Arp3 RNAi. Rab8 detected as over-expressed Rab8-GFP. Quantifications of Rab8 puncta numbers per nascent cap are shown. Embryo values plotted. (E) Imaging of PIP2 probe with endogenously-expressed Rab8-YFP at telophase 11 nascent caps with control CK689 and CK666 injection. Corner brackets show nascent caps. Quantifications of Rab8 puncta numbers per nascent cap are shown. Embryo values plotted.
Figure S10.
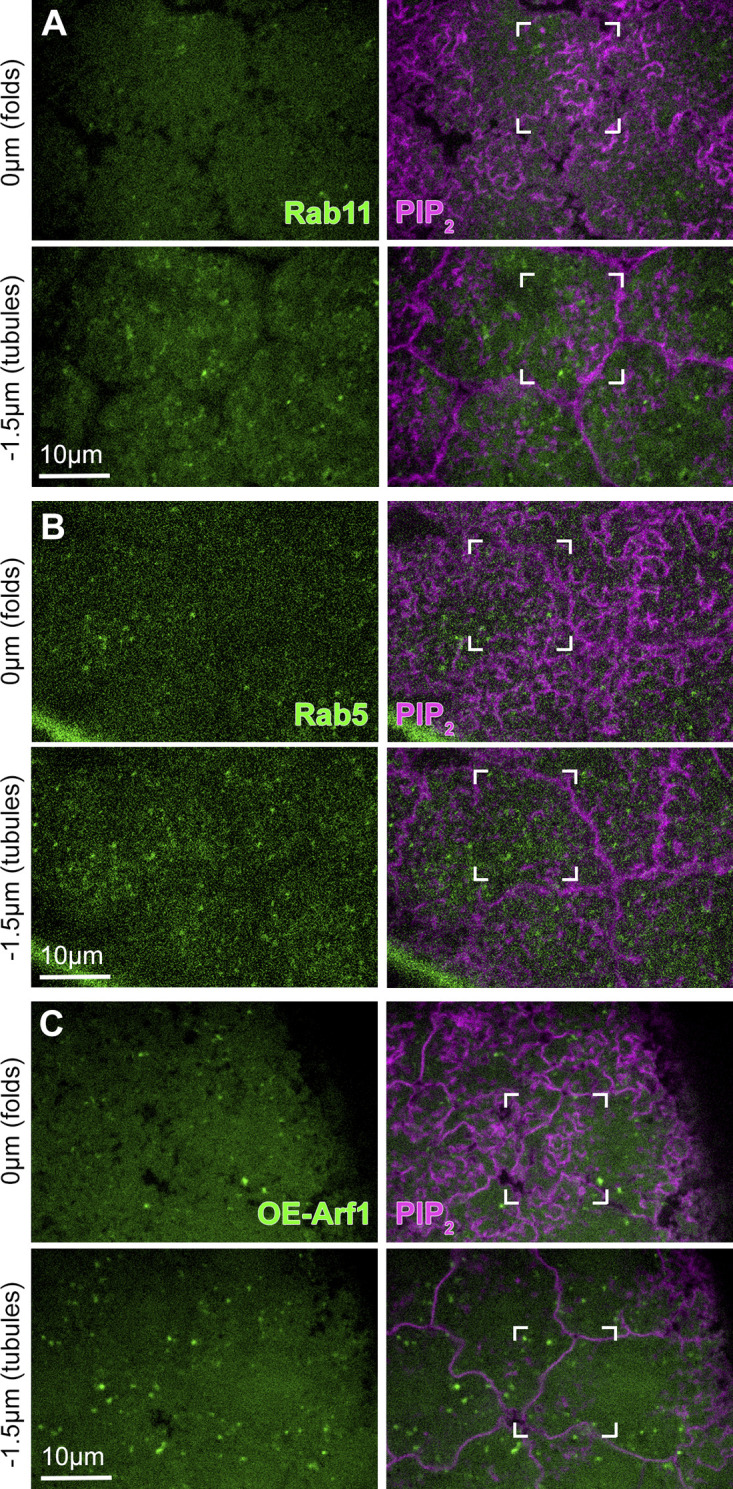
Localizations of Rab11, Rab5, and Arf1 in telophase 11 compartments. (A–C) Telophase 11 dual live imaging of PIP2 probe and internal membrane markers. Single sections at the level of PM folds (0 µm) and tubules (−1.5 µm) of nascent caps (corner brackets). Rab11 (A) and Rab5 (B) were detected as endogenously-expressed YFP-tagged proteins. Arf1 (C) was detected as an over-expressed GFP-tagged protein. Representative of 10/10, 9/9, and 5/5 embryos for Rab11, Rab5, and Arf1, respectively.
To test whether nascent cap PM tubules are relevant to the Rab8 vesicle association, we examined Rab8 puncta localization in two distinct contexts with abnormally few nascent cap PM tubules: Cnn RNAi embryos and Arp3 RNAi embryos. Since the genetic cross only allowed one fluorescent probe to monitor the cell cycle, we used overexpressed Rab8-GFP (Zhang et al., 2007) to maintain sufficient signal with photobleaching. In contrast to control RNAi embryos, both Cnn RNAi embryos and Arp3 RNAi embryos displayed fewer Rab8 puncta per nascent cap (Fig. 9, C and D, corner brackets). These reductions suggested the involvement of nascent cap PM tubules in exocytic vesicle associations. The reduction in Cnn RNAi embryos could also be explained by disrupted centrosomal MTs, but the reduction in Arp3 RNAi embryos may primarily be due to abnormal nascent cap PM topography. Confirming the effect of Arp2/3 complex disruption, embryos coexpressing Rab8-YFP at endogenous levels with the PIP2 probe for staging displayed fewer Rab8 puncta at the nascent cap after CK666 injection in contrast to control CK689 injection (Fig. 9 E, corner brackets).
Discussion
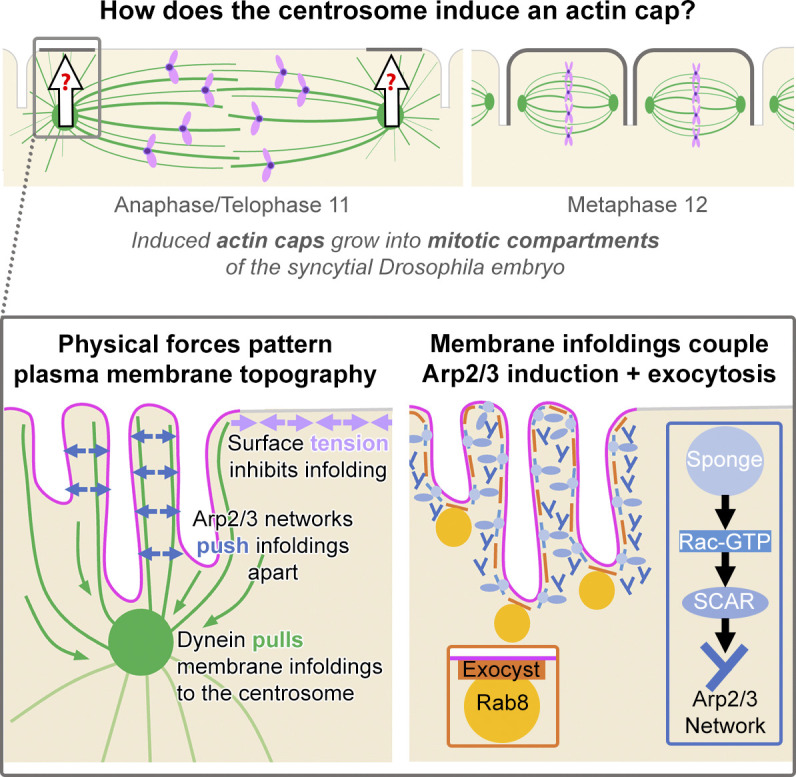
Our data revealed that centrosome organization of local PM topography is linked to actin cap formation in the syncytial Drosophila embryo. At each nascent cap, centrosome- and dynein-dependent PM tubules emanate from upper PM folds into an astral centrosomal MT array. This centrosome-dependent organization of local PM topography is locally coupled with centrosome-dependent recruitment of an Arp2/3 induction pathway. The resulting Arp2/3 actin network growth between nascent cap PM folds and tubules appears to maintain PM tubule numbers and to displace PM folds from the centrosome for actin cap expansion. Abnormal PM topographies in response to either centrosome or Arp2/3 disruptions additionally correlate with fewer exocytic vesicle associations with the nascent cap. Overall, the local organization of PM infoldings by the centrosome appears to coordinate Arp2/3 network growth and exocytosis for cortical domain assembly.
From metaphase to telophase, the mitotic compartment surface is patterned into distinct topographic domains through the activities of conserved force-generating mechanisms. At metaphase, these force-generating mechanisms appear minimal, and PM folding occurs across the entire compartment surface. Myosin is dissociated from the cortex at metaphase (Royou et al., 2002), and minimal surface tension is detected (Fig. 6). Astral centrosomal MTs are also at their lowest abundance (Foe et al., 2000) (Figs. 1 and S2), and centrosome engagement with apical PM folds seems minimal from the few PM tubules emanating inward (Figs. S1 and S2). We speculate that PM folding across the full apical domain at metaphase is due to intrinsic membrane bending properties (Shi and Baumgart, 2014). Into anaphase and telophase, our data show that force-generating cytoskeletal networks are required to remodel apical PM topography into distinct domains. Myosin reaccumulates around the base of pseudocleavage furrows (Royou et al., 2002) (Fig. S7), and myosin-dependent surface tension appears to flatten the apical surface between the nascent caps (Fig. 7), consistent with surface tension inhibiting PM infoldings and protrusions in other cell types (Kessels and Qualmann, 2021; Saha et al., 2018). Astral centrosomal MTs increase at each pole of the compartment (Foe et al., 2000), and this change correlates temporally and spatially with a domain of PM folds at each nascent cap (Figs. 1 and S2). The main indication of centrosome engagement with these local domains is the extension of PM tubules from the PM folds into the astral MT array emanating from the centrosome (Fig. 1). Formation of these PM tubules correlates with increased astral centrosomal MT abundance from metaphase to telophase (Fig. S2) and requires centrosome and dynein activities (Fig. 4). Surface tension in a nascent cap is indistinguishable from the flattened central domain of the compartment (Fig. 6), suggesting a force balance between dynein-based pulling of PM tubules and folds toward the centrosome and myosin-based pulling of the overall compartment from the base of surrounding pseudocleavage furrows, although mechanical properties of the nascent cap may also be affected by Arp2/3 actin network assembly. A force balance is also implicated from the deeper extension of PM tubules into the cell when myosin is depleted (Fig. 7). Notably, centrosome-directed pulling forces also appear to induce local PM infoldings in other cell types, and these infoldings also seem restrained by myosin-based surface tension (Chapa-Y-Lazo et al., 2020; Negishi et al., 2016; Redemann et al., 2010; Yi et al., 2013). As the nascent cap forms, additional counterforces seemingly arise within it. Specifically, centrosome-directed pulling forces appear to be counterbalanced by actin polymerization forces that push nascent cap PM folds apart, as evident from a collapse of the folded domain centered on the centrosome in response to Arp2/3 or F-actin inhibitor injections (Fig. 8). We propose that as a cortical compartment enters anaphase and telophase, centrosome-directed pulling forces overcome myosin-based surface tension to organize a local topographic domain at each nascent cap. Within each folded domain, the centrosome-directed pulling forces are then countered by induced Arp2/3 actin networks.
Nascent cap PM folds and tubules accumulate factors known to promote Arp2/3 actin network polymerization and membrane exocytosis for growth into a dome-like compartment (Tam and Harris, 2024). A canonical pathway of Arp2/3 actin network induction, including the Rac-GEF Spg, Rac-GTP, SCAR, Arp3, and F-actin, is enriched at the nascent cap folds and tubules compared with other PM domains of the telophase compartment (Fig. 3). This enrichment occurs specifically when PM folds become locally engaged with the centrosome at anaphase and telophase (Fig. S3) and depends on both Cnn and dynein (Figs. 5 and S5). In Zip RNAi compartments, ectopically localized PM folds lacking centrosome engagement also lack Arp2/3 pathway enrichment (Fig. 7). Thus, centrosome-based organization of the topographic domain is closely coupled with centrosome-dependent induction of the Arp2/3 pathway in the domain. Indicating local effects of the induced Arp2/3 actin networks, Arp2/3 and F-actin disruptions resulted in the collapse of PM folds toward the centrosome and reduced PM tubule numbers (Fig. 8). Normal expansion of Arp2/3 actin networks appears to push the PM folds apart for cap growth. Nascent cap PM topography also seems functionally relevant to the recruitment of Rab8 exocytic vesicles, as Rab8 puncta specifically associated with PM tubules of the nascent cap, and perturbations of Cnn or Arp2/3 that reduced tubule numbers also reduced Rab8 puncta numbers at the nascent cap (Figs. 4, 8, and 9). The reduced Rab8 puncta numbers with depleted Cnn could be due either to PM tubule disruption or to vesicle transport disruption, but the reductions with reduced Arp2/3 activity may primarily stem from disrupted PM topography. Overall, it is evident that nascent cap PM topography has functional relevance to the growth of the cortical domain. Notably, PM tubules of the nascent cap are distinct from endocytic PM tubules that accumulate around the base of pseudocleavage furrows at metaphase before a period of endocytosis and partial furrow regression during anaphase and telophase (Sokac and Wieschaus, 2008). As pseudocleavage furrows of an existing mitotic compartment regress, the PM infoldings of each nascent cap seem to form a nexus of F-actin and membrane assembly for building the next mitotic compartment of the cycle.
Limitations of the study
As reviewed in the Introduction, folded PM topographies can contribute to cortical domain growth, as can centrosome proximity. Centrosome proximity can also change local PM topography, but a further link to cortical domain growth had not been established. Using an in vivo model system dependent on centrosomes for inducing the growth of new cortical domains, we found that centrosome-organized PM topography is linked to centrosome-dependent pathways of actin polymerization and membrane exocytosis for cortical domain growth. Establishing this link in a naturally evolved, whole-animal system is important, but an inability to directly manipulate PM topography is a limitation. For example, we have not found a way to completely flatten the telophase compartment surface. Also, unknowns remain about how PM folds and tubules of the nascent cap form and function. For example, our model predicts a physical connection between dynein motors and the PM to draw PM tubules toward the centrosome, but this connection is unknown. The biophysics of how centrosome-induced PM tubules may organize the upper domain of PM folds is unclear. Also, a potential contribution of membrane exocytosis to nascent cap PM topography remains untested. Future studies should also address how nascent cap folds and tubules contribute to cap assembly and growth into a dome-like compartment. There are various, non-mutually exclusive possibilities. First, the PM tubules bypass the ER membrane system (Fig. 2), an apparent obstacle to the centrosome-to-PM transfer of signaling molecules and vesicles. Second, local PM folding increases the amount of PM near the centrosome, possibly increasing proximal PM sites for F-actin nucleation events and vesicle docking, and perhaps providing a membrane reservoir to supply growth of the dome-like compartment, as occurs in various other contexts (Figard et al., 2013; Goudarzi et al., 2017; LaFoya and Prehoda, 2023; Masters et al., 2013). Third, local curvatures of the PM folds and tubules might promote protein localization. A protein containing a BAR domain for sensing membrane curvature (Kessels and Qualmann, 2021) could be involved. Scrambled (Stevenson et al., 2001), Centrocortin (Kao and Megraw, 2009), and Par-1 (Jiang and Harris, 2019) each function in cap growth, and each localized to both the centrosome and the actin cap, making them candidates for centrosome-to-cortex signaling, but none contain a BAR domain (FlyBase). The Rac-GEF Spg, and its adaptor protein Engulfment and Cell Motility (ELMO) (Schmidt et al., 2018), also lack BAR domains (FlyBase). However, the SCAR/WAVE complex (Pipathsouk et al., 2021) and Arp2/3-based actin networks (Baldauf et al., 2023) do show preferential association with curved membranes. By avoiding obstruction, increasing local PM area, and/or promoting protein localization, PM infoldings of the nascent cap may enhance centrosome effects and thereby aid the rapid expansion of the cortical actin domain.
Materials and methods
Drosophila stocks, husbandry, and crosses
Fly stocks used in this study are listed in Table 1. Fly stocks were maintained at 25°C, room temperature, or 18°C on fly food provided by the University of Toronto kitchen operated by H. Lipshitz. For embryo collection, flies were kept in a cage at 25°C that allowed flies to lay on an agar plate (25 g agar, 250 ml store-bought apple juice, 12.5 g granulated sugar, 10 ml 10% Tegosept [in ethanol], and distilled H2O to 1,000 ml) and a dab of store-bought baker’s yeast mixed with distilled H2O to create a paste. Adults were not kept for over 1 wk after pupal hatching. No visible health defects were found in caged adults. Embryo sexes were not relevant because the early developmental stages under study rely on maternal gene products, and no phenotypic sex differences exist.
Table 1.
Drosophila stocks used in study
| Stocks | Sources |
|---|---|
| Protein expression by native promoters | |
| Arp3-GFP | Xie et al. (2021) |
| Jupiter-GFP | Bloomington Drosophila stock center (BDSC) #6836 |
| Rab5-YFP | BDSC #62543 |
| Rab8-YFP | BDSC #62546 |
| Rab11-YFP | BDSC #62549 |
| Spider-GFP | BDSC #59025 |
| SCAR-NG | Dobramysl et al. (2021) |
| Spg-NG | This paper |
| Tubulin-GFP | Andrew Wilde, University of Toronto, Toronto, Canada |
| Zip-GFP | BDSC #51564 |
| Protein expression by non-native promoters | |
| Sqh-Gap43-mCherry | Martin et al. (2009) |
| Sqh-Moesin-actin binding domain-GFP | Kiehart et al. (2000) |
| Sqh-Pak3-Rac-GTP binding domain-GFP | BDSC #52304 |
| Tubulin-Steppke-PH domain-GFP (tGPH) | BDSC #8163 |
| Ubi-PLCγ-PH domain-mCherry | Herszterg et al. (2013) |
| Maternal-GAL4-VP16 | Mark Peifer, University of North Carolina at Chapel Hill, USA |
| UAS-driven protein expression | |
| UASp-Arf1-GFP | Shao et al. (2010) |
| UASp-Rab8-GFP | BDSC #9782 |
| UASp-RFP-KDEL | BDSC #30910 |
| UASp-Sec15-GFP | BDSC #39685 |
| UAS-driven shRNA expression | |
| UASp-Arp3 shRNA | BDSC #53972 |
| UASp-ASAP shRNA (ineffective shRNA—control used in combination with mCherry probes) | Rodrigues et al. (2016) |
| UASp-cnn shRNA | BDSC #57149 |
| UASp-mCherry shRNA (control) | BDSC #35785 |
| UASp-zip shRNA | BDSC #36727 |
UAS constructs were expressed maternally using maternal-GAL4-VP16 linked to the X chromosome, and defects were assessed in offspring. Maximum RNAi effects were detected within 3 days of setting up a cage with newly hatched parents. For coexpressing a UAS construct in combination with another genetic element, one component was crossed to maternal-GAL4-VP16 females carrying the balancer chromosomes CyO or TM3. F1 males carrying the element balanced by CyO or TM3 were crossed to females carrying the second element of interest. The F2 generation carrying both elements (selected by lack of CyO or TM3) were self-crossed and their offspring were analyzed. To coexpress non-UAS genes, true breeding stocks were crossed to generate parents heterozygous for both elements, and their offspring were analyzed.
For NG-tagging of the endogenous spg locus by CRISPR/Cas-9, we followed an established approach (Harris et al., 2016). The donor construct was generated using the NEBuilder HIFI DNA Assembly Cloning Kit (Cat #E5520S; New England Biolabs). Phusion High-Fidelity DNA polymerase (Cat #M0530S; New England Biolabs) was used to PCR amplify a portion of the spg genomic locus covering sequences 5′ of the stop codon and replacing the stop codon with a short sequence overlapping with the 5′ end of the NG sequence (forward primer, 5′-CTTGCATGCTAGCCTGTACGACCCG-3′ [all primers from Life Technologies]; reverse primer, 5′-TGGACACCATAAAAGTGCCGCCATC-3′; template, Bac clone of spg genomic DNA [BACR13H19; BACPAC resources center]), as well as the sequence of NG plus overlapping ends with the spg sequence and the pDSRed vector sequence (forward primer, 5′- GGCACTTTTATGGTGTCCAAGGGC-3′; reverse primer, 5′-CCGCGGCCTCACTTGTACAGCTC-3′; template, pUAST-NG-crb [gift of U. Tepass, University of Toronto, Toronto, Canada]). These pieces were assembled into the pDSRed vector (gift of K. O’Connor Giles, University of Wisconsin-Madison, Madison, WI, USA) 5′ of the DSRed sequence with forward primer 5′-CTGTACAAGTGAGGCCGCGGACATA-3′, reverse primer 5′- CCACGGGTCGTACAGGCTAGCATG-3′. In a second assembly step, a sequence of the spg genomic locus 3′ to the stop codon was amplified with ends overlapping the pDSRed vector (forward primer, 5′-GCATAAGGCGCGCCTAGGGTTACAA-3′; reverse primer, 5′-TGCAGAAGTTTGTCGGTGTC-3′; template, Bac clone of spg genomic DNA [BACR13H19]) and assembled into the first assembled construct 3′ of the DSRed sequence using forward primer 5′-CCGACAAACTTCTGCAGCTC-3′ and reverse primer 5′-ATATTGTAACCCTAGGCGCG-3′. The g-RNA sequence was selected with an on-line tool (targetfinder.flycrispr.neuro.brown.edu) and contained a PAM site close to the spg stop codon (g-RNA sequence, 5′-TCTAGTCATCAGCACGATGG-3′). To generate the g-RNA construct, the g-RNA sequence was ligated into the pCDF3 vector (gift of K. O’Connor Giles, University of Wisconsin-Madison, Madison, WI, USA) after cutting with Bbs1. One base pair of the corresponding PAM site in the donor construct was mutated by PCR (forward primer, 5′-CACATCTAGTCATCAGCACGATGGCATCACTTTTATGGTGA-3′; reverse primer, 5′-TCACCATAAAAGTGATGCCATCGTGCTGATGACTAGATGTG-3′). The two constructs were co-injected into yw; attP40(nos-Cas9)/CyO flies, followed by screening for the insertion, removal of the Cas9 transgene, and balancing of the insertion allele (Plan RI; BestGene). Flies homozygous for the insertion were generated and maintained, and the insertion was confirmed by PCR and sequencing.
Embryo imaging, ablations, and injections
For live imaging, embryos were washed with three rounds of 0.1% Triton, dechorionated with 50% bleach for 4 min, and then washed thrice with 0.1% Triton. Embryos were arranged on an agar plate and then transferred to a coverslip using glue (store-bought double-sided tape dissolved in heptane). Embryos were mounted with halocarbon oil (series 700; Cat #9002-83-9; Halocarbon Products) and placed in an imaging dish.
Imaging was done at room temperature with a Ti2 inverted microscope (Nikon), CSU-X Spinning Disk (Yokogawa), sCMOS camera (Teledyne Photometrics Prime 95B), 100X Plan Apochromat VC NA 1.4 oil-immersion objective (Nikon), and NIS-elements software (Nikon). Data was collected as Z-stacks with 300 nm step sizes. Imaging was conducted for at least 10 min to determine the nuclear cycle stage of interest. Images for figures were prepared using NIS-elements, Image J (NIH), and Photoshop (Adobe). Video 1 was made using NIS Elements after deconvolution of the data using the 2D Blind algorithm.
Laser ablations were done with a Optimicroscan UV galvo scanner (Nikon) using a 355 nm laser at 1,000 Hz, with a 200 µs pixel dwell time, and a 4 µm line of ablation. Ablations were conducted with a 60X Plan Apochromat lambda NA 1.4 oil-immersion objective (Nikon). Each embryo was ablated once.
For injections, dechorionated and washed embryos were transferred to a coverslip using glue and dehydrated over Drierite (Cat #23005; W.A Hammond Drierite Company) for 15 min at room temperature in a sealed desiccator chamber (Bel-Art Products). Following coverage with halocarbon oil, injections were done with a glass capillary (4IN THINWALL GL 1.0OD/.75 ID: Cat #TW 100F-4; World Precision Instruments) pulled using a micropipette puller (Sutter Instruments). Dextran (10,000 MW; Anionic) Alexa Fluor 647 (Cat #D22914; Invitrogen) was dissolved to 1 mg/ml in dH20, injected between the vitelline membrane and the plasma membrane of the embryo, and imaged beginning ∼5 min after injection. The following inhibitors were injected at one pole, and imaging of the central region of the embryo began ∼5 min after injection, with the exception of Ciliobrevin D which was injected ∼60 min before imaging to see an effect. Latrunculin A (Cat #428021; Sigma-Aldrich) was dissolved to 250 µM in 25% DMSO (BioShop) in dH20, and 25% DMSO in dH20 was the carrier control. For Ciliobrevin D (Cat #250401; Sigma-Aldrich), the DMSO stock solution was heated to 45°C before dissolving to 7 µM in 32% DMSO in dH20, and the carrier control was 32% DMSO in dH20. CK666 (Cat #182515; Sigma-Aldrich) was dissolved to 10 µM in 50% DMSO in dH20, and the control was CK689 (Cat #182517; Sigma-Aldrich) dissolved to 10 µM in 50% DMSO in dH20.
Quantifications and statistical analyses
Unless noted, quantifications were performed at cycle 11 telophase using Image J. Comparisons were done by both a Student’s t test (two-tailed, unpaired) in Excel (Microsoft) and a Mann–Whitney U Test (https://seaborn.pydata.org/). The highest P value (lowest significance) of the two tests is shown in graphs. N-values are numbers of embryos and points on graphs represent individual embryos. Results were confirmed with at least two separate replicates of the full experiment and individual embryo data points were compiled from the replicates for graphing and statistical tests. Graphs were made in Excel (Microsoft) or seaborn (https://seaborn.pydata.org/).
Areas of astral centrosomal MT arrays
1.5-µm projections of Jupiter-GFP confocal sections centered on the centrosome were used. A line bisecting the mitotic spindle lengthwise was used to draw a perpendicular line at the center of the centrosome. The area of the astral MTs beyond the perpendicular line was measured. Six nascent caps per embryo were measured and averaged for one value per embryo.
Numbers and depths of PM tubules
Tubule numbers were counted as puncta in confocal sections per nascent cap within a 33 µm2 circle placed using the signal-excluded nucleus below as a common reference position. Confocal sections above and below were examined to ensure counted puncta represented tubules continuous with the PM folds above. Tubule depths were quantified by (1) graphing tubule number versus depth below the base of the PM folds and (2) determining the depths where 50% of tubules were lost. Six different nascent caps were analyzed per embryo and averaged for one value per embryo. The same quantifications were done at the ectopic folds of Zip RNAi embryos, except the 33 µm2 circle was placed in the center of the compartment where the ectopic folds formed.
Probe enrichment at the nascent cap versus pseudocleavage furrow
Single sections were analyzed at the PM folds and pseudocleavage furrows. An ROI of 0.943 µm2 was used to measure the fluorescent intensity of the probe. Folds of the nascent cap were compared with pseudocleavage furrows of the same compartment as ratios. Before calculating these ratios, background correction measurements were collected from the same single sections at non-PM sites and subtracted from each PM measurement. Average values for each embryo were calculated from six ratio measurements taken from three different mitotic compartments.
Probe enrichment at the nascent cap versus the central apical surface between nascent caps
Images analyzed were projections of 1.5 µm. Using an ROI of 33 µm2, the fluorescence intensities of two nascent caps were collected, averaged, and divided by a measurement in the middle of the compartment in between the two caps. Before this calculation, measurements were first background-corrected using the first section below the compartment surface that lacked cortical signal. Three compartments were averaged per embryo for one embryo value.
Quantification of Rac-GTP, F-actin, Spider-GFP, and PIP2 enrichment in zip RNAi
In single sections, fluorescence within an ROI 0.943 µm2 was measured at PM folds of the nascent caps and ectopic PM folds in the middle of the compartment in between the caps. Measurements were background corrected with measurements at non-PM locations. Six measurements of nascent caps and ectopic PM folds were taken to determine a ratio per compartment. Three ratios of different compartments were averaged per embryo for one embryo value.
Quantification of Rab8 puncta numbers per nascent cap
Rab8 puncta of 0.3–0.5 µm2 were counted in 33 µm2 circles placed above the signal-excluded nucleus of each nascent cap. The counts were done in 1.5 µm projections, which contained the Rab8 puncta of the nascent cap. Data from six different nascent caps were averaged per embryo for one embryo value.
Quantification of compartment recoil after ablation
In confocal sections immediately before and seconds after the ablation, the largest outlines of the compartment were determined and compared as ratios. One compartment was ablated and measured per embryo. As controls for the behaviors of non-ablated compartments, the same measurements were done to the two neighboring compartments with the maximum contact with the ablated compartment.
Quantification of nascent cap areas following Lat A and CK666 injections
The images analyzed were 1.5 µm projections. The perimeter of the infoldings of nascent caps were traced to calculate nascent cap areas. Six nascent caps per embryo were measured and averaged for one embryo value.
Online supplemental material
Fig. S1 shows changes to PM morphology over the cell cycle from prometaphase 11 to prometaphase 12. Fig. S2 compares astral centrosomal microtubule abundance, PM tubule numbers, and PM tubule depths from metaphase to telophase of cycle 11. Fig. S3 documents changes to cortical localizations of Rac-GTP and SCAR from metaphase to telophase of cycle 11. Fig. S4 compares mitotic compartment apical PM folds from metaphase 11 to telophase 11 in control RNAi, Cnn RNAi, and Zip RNAi embryos. Fig. S5 shows the effects of Ciliobrevin D injection on MT, Rac-GTP, and Arp3 distributions in telophase 11 compartments. Fig. S6 documents the requirement of Cnn for F-actin enrichment to nascent caps of telophase 11 compartments. Fig. S7 shows myosin localization in metaphase and telophase compartments of cycle 11. Fig. S8 compares second-by-second responses to laser ablations of compartments at different stages and different locations. Fig. S9 documents the effects of Lat A and Arp3 RNAi on nascent cap F-actin. Fig. S10 shows localizations of Rab11, Rab5, and Arf1 in telophase 11 compartments. Video 1 shows the intermingling of PM infoldings and astral centrosomal MTs at a telophase 11 nascent cap.
Acknowledgments
We thank Ashley Bruce and Ulrich Tepass for comments on the paper. We thank Cao Guo Yu for technical assistance, and Nicolas Vergara Ruiz for preliminary investigations. We are grateful to members ofthe Drosophila community listed in the Materials and methods section for providing reagents. Reagents were also from the Bloomington Drosophila Stock Center (National Institutes of Health [NIH] P40OD-018537), and the BACPAC Resources Center.
This work was supported by Natural Sciences and Engineering Research Council of Canada Discovery grants (RGPIN-2016–05617 and RGPIN-2023-04378) to T.J.C. Harris.
Author contributions: R. Tam: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing—original draft, Writing—review & editing, T.J.C. Harris: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing—review & editing.
Data availability
The data are available from the corresponding author upon reasonable request.
References
- Abreu-Blanco, M.T., Verboon J.M., and Parkhurst S.M.. 2014. Coordination of Rho family GTPase activities to orchestrate cytoskeleton responses during cell wound repair. Curr. Biol. 24:144–155. 10.1016/j.cub.2013.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldauf, L., Frey F., Arribas Perez M., Idema T., and Koenderink G.H.. 2023. Branched actin cortices reconstituted in vesicles sense membrane curvature. Biophys. J. 122:2311–2324. 10.1016/j.bpj.2023.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake-Hedges, C., and Megraw T.L.. 2019. Coordination of embryogenesis by the centrosome in Drosophila melanogaster. Results Probl. Cell Differ. 67:277–321. 10.1007/978-3-030-23173-6_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor, D.L., Pönisch W., Endres R.G., and Paluch E.K.. 2020. Of cell shapes and motion: The physical basis of animal cell migration. Dev. Cell. 52:550–562. 10.1016/j.devcel.2020.02.013 [DOI] [PubMed] [Google Scholar]
- Britton, J.S., Lockwood W.K., Li L., Cohen S.M., and Edgar B.A.. 2002. Drosophila’s insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev. Cell. 2:239–249. 10.1016/S1534-5807(02)00117-X [DOI] [PubMed] [Google Scholar]
- Busson, S., Dujardin D., Moreau A., Dompierre J., and De Mey J.R.. 1998. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr. Biol. 8:541–544. 10.1016/S0960-9822(98)70208-8 [DOI] [PubMed] [Google Scholar]
- Chapa-Y-Lazo, B., Hamanaka M., Wray A., Balasubramanian M.K., and Mishima M.. 2020. Polar relaxation by dynein-mediated removal of cortical myosin II. J. Cell Biol. 219:e201903080. 10.1083/jcb.201903080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes, P.N., Bhattacharya S., Edwards M., Iglesias P.A., Lampert T., and Miao Y.. 2017. Excitable signal transduction networks in directed cell migration. Annu. Rev. Cell Dev. Biol. 33:103–125. 10.1146/annurev-cellbio-100616-060739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobramysl, U., Jarsch I.K., Inoue Y., Shimo H., Richier B., Gadsby J.R., Mason J., Szałapak A., Ioannou P.S., Correia G.P., et al. 2021. Stochastic combinations of actin regulatory proteins are sufficient to drive filopodia formation. J. Cell Biol. 220:e202003052. 10.1083/jcb.202003052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douanne, T., and Griffiths G.M.. 2021. Cytoskeletal control of the secretory immune synapse. Curr. Opin. Cell Biol. 71:87–94. 10.1016/j.ceb.2021.02.008 [DOI] [PubMed] [Google Scholar]
- Driscoll, M.K., Sun X., Guven C., Fourkas J.T., and Losert W.. 2014. Cellular contact guidance through dynamic sensing of nanotopography. ACS Nano. 8:3546–3555. 10.1021/nn406637c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunst, S., Kazimiers T., von Zadow F., Jambor H., Sagner A., Brankatschk B., Mahmoud A., Spannl S., Tomancak P., Eaton S., and Brankatschk M.. 2015. Endogenously tagged rab proteins: A resource to study membrane trafficking in Drosophila. Dev. Cell. 33:351–365. 10.1016/j.devcel.2015.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elric, J., and Etienne-Manneville S.. 2014. Centrosome positioning in polarized cells: Common themes and variations. Exp. Cell Res. 328:240–248. 10.1016/j.yexcr.2014.09.004 [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez, R., and Harris T.J.C.. 2023. Contractile and expansive actin networks in Drosophila: Developmental cell biology controlled by network polarization and higher-order interactions. Curr. Top. Dev. Biol. 154:99–129. 10.1016/bs.ctdb.2023.02.005 [DOI] [PubMed] [Google Scholar]
- Figard, L., Wang M., Zheng L., Golding I., and Sokac A.M.. 2016. Membrane supply and demand regulates F-actin in a cell surface reservoir. Dev. Cell. 37:267–278. 10.1016/j.devcel.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figard, L., Xu H., Garcia H.G., Golding I., and Sokac A.M.. 2013. The plasma membrane flattens out to fuel cell-surface growth during Drosophila cellularization. Dev. Cell. 27:648–655. 10.1016/j.devcel.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone, A.J., Weinger J.S., Maldonado M., Barlan K., Langston L.D., O’Donnell M., Gelfand V.I., Kapoor T.M., and Chen J.K.. 2012. Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature. 484:125–129. 10.1038/nature10936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe, V.E., Field C.M., and Odell G.M.. 2000. Microtubules and mitotic cycle phase modulate spatiotemporal distributions of F-actin and myosin II in Drosophila syncytial blastoderm embryos. Development. 127:1767–1787. 10.1242/dev.127.9.1767 [DOI] [PubMed] [Google Scholar]
- Frescas, D., Mavrakis M., Lorenz H., Delotto R., and Lippincott-Schwartz J.. 2006. The secretory membrane system in the Drosophila syncytial blastoderm embryo exists as functionally compartmentalized units around individual nuclei. J. Cell Biol. 173:219–230. 10.1083/jcb.200601156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, W.J., and Motegi F.. 2021. Mechanochemical control of symmetry breaking in the Caenorhabditis elegans zygote. Front. Cell Dev. Biol. 8:619869. 10.3389/fcell.2020.619869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi, M., Tarbashevich K., Mildner K., Begemann I., Garcia J., Paksa A., Reichman-Fried M., Mahabaleshwar H., Blaser H., Hartwig J., et al. 2017. Bleb expansion in migrating cells depends on supply of membrane from cell surface invaginations. Dev. Cell. 43:577–587.e5. 10.1016/j.devcel.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, R.A., Paluch E., and Oegema K.. 2012. Cytokinesis in animal cells. Annu. Rev. Cell Dev. Biol. 28:29–58. 10.1146/annurev-cellbio-101011-155718 [DOI] [PubMed] [Google Scholar]
- Harris, K.P., Zhang Y.V., Piccioli Z.D., Perrimon N., and Littleton J.T.. 2016. The postsynaptic t-SNARE Syntaxin 4 controls traffic of Neuroligin 1 and Synaptotagmin 4 to regulate retrograde signaling. Elife. 5:e13881. 10.7554/eLife.13881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, S.M., Xie Y., Rollins K.R., and Blankenship J.T.. 2022. Sponge/DOCK-dependent regulation of F-actin networks directing cortical cap behaviors and syncytial furrow ingression. Dev. Biol. 491:82–93. 10.1016/j.ydbio.2022.08.004 [DOI] [PubMed] [Google Scholar]
- Herszterg, S., Leibfried A., Bosveld F., Martin C., and Bellaiche Y.. 2013. Interplay between the dividing cell and its neighbors regulates adherens junction formation during cytokinesis in epithelial tissue. Dev. Cell. 24:256–270. 10.1016/j.devcel.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Holly, R.M., Mavor L.M., Zuo Z., and Blankenship J.T.. 2015. A rapid, membrane-dependent pathway directs furrow formation through RalA in the early Drosophila embryo. Development. 142:2316–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk, A.R., Jilkine A., Mejean C.O., Boltyanskiy R., Dufresne E.R., Angenent S.B., Altschuler S.J., Wu L.F., and Weiner O.D.. 2012. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell. 148:175–188. 10.1016/j.cell.2011.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, T., and Harris T.J.C.. 2019. Par-1 controls the composition and growth of cortical actin caps during Drosophila embryo cleavage. J. Cell Biol. 218:4195–4214. 10.1083/jcb.201903152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao, L.R., and Megraw T.L.. 2009. Centrocortin cooperates with centrosomin to organize Drosophila embryonic cleavage furrows. Curr. Biol. 19:937–942. 10.1016/j.cub.2009.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova, N., Bobinnec Y., Fouix S., Huitorel P., and Debec A.. 2006. Jupiter, a new Drosophila protein associated with microtubules. Cell Motil. Cytoskeleton. 63:301–312. 10.1002/cm.20124 [DOI] [PubMed] [Google Scholar]
- Kelkar, M., Bohec P., and Charras G.. 2020. Mechanics of the cellular actin cortex: From signalling to shape change. Curr. Opin. Cell Biol. 66:69–78. 10.1016/j.ceb.2020.05.008 [DOI] [PubMed] [Google Scholar]
- Kessels, M.M., and Qualmann B.. 2021. Interplay between membrane curvature and the actin cytoskeleton. Curr. Opin. Cell Biol. 68:10–19. 10.1016/j.ceb.2020.08.008 [DOI] [PubMed] [Google Scholar]
- Kiehart, D.P., Galbraith C.G., Edwards K.A., Rickoll W.L., and Montague R.A.. 2000. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J. Cell Biol. 149:471–490. 10.1083/jcb.149.2.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner, E.J., Ferro L.S., Yu Z., and Bautch V.L.. 2016. Excess centrosomes perturb dynamic endothelial cell repolarization during blood vessel formation. Mol. Biol. Cell. 27:1911–1920. 10.1091/mbc.e15-09-0645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFoya, B., and Prehoda K.E.. 2023. Consumption of a polarized membrane reservoir drives asymmetric membrane expansion during the unequal divisions of neural stem cells. Dev. Cell. 58:993–1003.e3. 10.1016/j.devcel.2023.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, C.D., and Ridley A.J.. 2018. Rho GTPase signaling complexes in cell migration and invasion. J. Cell Biol. 217:447–457. 10.1083/jcb.201612069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou, H.Y., Zhao W., Li X., Duan L., Powers A., Akamatsu M., Santoro F., McGuire A.F., Cui Y., Drubin D.G., and Cui B.. 2019. Membrane curvature underlies actin reorganization in response to nanoscale surface topography. Proc. Natl. Acad. Sci. USA. 116:23143–23151. 10.1073/pnas.1910166116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, A.C., Kaschube M., and Wieschaus E.F.. 2009. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 457:495–499. 10.1038/nature07522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters, T.A., Pontes B., Viasnoff V., Li Y., and Gauthier N.C.. 2013. Plasma membrane tension orchestrates membrane trafficking, cytoskeletal remodeling, and biochemical signaling during phagocytosis. Proc. Natl. Acad. Sci. USA. 110:11875–11880. 10.1073/pnas.1301766110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavor, L.M., Miao H., Zuo Z., Holly R.M., Xie Y., Loerke D., and Blankenship J.T.. 2016. Rab8 directs furrow ingression and membrane addition during epithelial formation in Drosophila melanogaster. Development. 143:892–903. 10.1242/dev.128876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally, F.J. 2013. Mechanisms of spindle positioning. J. Cell Biol. 200:131–140. 10.1083/jcb.201210007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megraw, T.L., Li K., Kao L.R., and Kaufman T.C.. 1999. The centrosomin protein is required for centrosome assembly and function during cleavage in Drosophila. Development. 126:2829–2839. 10.1242/dev.126.13.2829 [DOI] [PubMed] [Google Scholar]
- Negishi, T., Miyazaki N., Murata K., Yasuo H., and Ueno N.. 2016. Physical association between a novel plasma-membrane structure and centrosome orients cell division. Elife. 5:e16550. 10.7554/eLife.16550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen, B.J., Tomasevic N., Russell A., Pierce D.W., Jia Z., McCormick C.D., Hartman J., Sakowicz R., and Pollard T.D.. 2009. Characterization of two classes of small molecule inhibitors of Arp2/3 complex. Nature. 460:1031–1034. 10.1038/nature08231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip, R., Fiorino C., and Harrison R.E.. 2022. Terminally differentiated osteoclasts organize centrosomes into large clusters for microtubule nucleation and bone resorption. Mol. Biol. Cell. 33:ar68. 10.1091/mbc.E22-03-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipathsouk, A., Brunetti R.M., Town J.P., Graziano B.R., Breuer A., Pellett P.A., Marchuk K., Tran N.T., Krummel M.F., Stamou D., and Weiner O.D.. 2021. The WAVE complex associates with sites of saddle membrane curvature. J. Cell Biol. 220:e202003086. 10.1083/jcb.202003086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postner, M.A., Miller K.G., and Wieschaus E.F.. 1992. Maternal effect mutations of the sponge locus affect actin cytoskeletal rearrangements in Drosophila melanogaster embryos. J. Cell Biol. 119:1205–1218. 10.1083/jcb.119.5.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga, X., Walani N., Disanza A., Chavero A., Mittens A., Tebar F., Trepat X., Parton R.G., Geli M.I., Scita G., et al. 2023. A mechanosensing mechanism controls plasma membrane shape homeostasis at the nanoscale. Elife. 12:e72316. 10.7554/eLife.72316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff, J.W., and Glover D.M.. 1989. Centrosomes, and not nuclei, initiate pole cell formation in Drosophila embryos. Cell. 57:611–619. 10.1016/0092-8674(89)90130-X [DOI] [PubMed] [Google Scholar]
- Raucher, D., and Sheetz M.P.. 2000. Cell spreading and lamellipodial extension rate is regulated by membrane tension. J. Cell Biol. 148:127–136. 10.1083/jcb.148.1.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redemann, S., Pecreaux J., Goehring N.W., Khairy K., Stelzer E.H., Hyman A.A., and Howard J.. 2010. Membrane invaginations reveal cortical sites that pull on mitotic spindles in one-cell C. elegans embryos. PLoS One. 5:e12301. 10.1371/journal.pone.0012301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, F.F., Shao W., and Harris T.J.. 2016. The Arf GAP Asap promotes Arf1 function at the Golgi for cleavage furrow biosynthesis in Drosophila. Mol. Biol. Cell. 27:3143–3155. 10.1091/mbc.e16-05-0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins, K.R., and Blankenship J.T.. 2023. Dysregulation of the endoplasmic reticulum blocks recruitment of centrosome-associated proteins resulting in mitotic failure. Development. 150:dev201917. 10.1242/dev.201917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottner, K., Stradal T.E.B., and Chen B.. 2021. WAVE regulatory complex. Curr. Biol. 31:R512–R517. 10.1016/j.cub.2021.01.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royou, A., Sullivan W., and Karess R.. 2002. Cortical recruitment of nonmuscle myosin II in early syncytial Drosophila embryos: Its role in nuclear axial expansion and its regulation by Cdc2 activity. J. Cell Biol. 158:127–137. 10.1083/jcb.200203148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha, S., Nagy T.L., and Weiner O.D.. 2018. Joining forces: Crosstalk between biochemical signalling and physical forces orchestrates cellular polarity and dynamics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373:20170145. 10.1098/rstb.2017.0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, A., and Grosshans J.. 2018. Dynamics of cortical domains in early Drosophila development. J. Cell Sci. 131:jcs212795. 10.1242/jcs.212795 [DOI] [PubMed] [Google Scholar]
- Schmidt, A., Lv Z., and Großhans J.. 2018. ELMO and Sponge specify subapical restriction of Canoe and formation of the subapical domain in early Drosophila embryos. Development. 145:dev157909. 10.1242/dev.157909 [DOI] [PubMed] [Google Scholar]
- Shao, W., Wu J., Chen J., Lee D.M., Tishkina A., and Harris T.J.. 2010. A modifier screen for Bazooka/PAR-3 interacting genes in the Drosophila embryo epithelium. PLoS One. 5:e9938. 10.1371/journal.pone.0009938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Z., and Baumgart T.. 2014. Dynamics and instabilities of lipid bilayer membrane shapes. Adv. Colloid Interf. Sci. 208:76–88. 10.1016/j.cis.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitarska, E., Almeida S.D., Beckwith M.S., Stopp J., Czuchnowski J., Siggel M., Roessner R., Tschanz A., Ejsing C., Schwab Y., et al. 2023. Sensing their plasma membrane curvature allows migrating cells to circumvent obstacles. Nat. Commun. 14:5644. 10.1038/s41467-023-41173-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokac, A.M., and Wieschaus E.. 2008. Local actin-dependent endocytosis is zygotically controlled to initiate Drosophila cellularization. Dev. Cell. 14:775–786. 10.1016/j.devcel.2008.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson, V., Hudson A., Cooley L., and Theurkauf W.E.. 2002. Arp2/3-dependent pseudocleavage [correction of psuedocleavage] furrow assembly in syncytial Drosophila embryos. Curr. Biol. 12:705–711. 10.1016/S0960-9822(02)00807-2 [DOI] [PubMed] [Google Scholar]
- Stevenson, V.A., Kramer J., Kuhn J., and Theurkauf W.E.. 2001. Centrosomes and the Scrambled protein coordinate microtubule-independent actin reorganization. Nat. Cell Biol. 3:68–75. 10.1038/35050579 [DOI] [PubMed] [Google Scholar]
- Svitkina, T.M. 2020. Actin cell cortex: Structure and molecular organization. Trends Cell Biol. 30:556–565. 10.1016/j.tcb.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, R., and Harris T.J.C.. 2024. Reshaping the syncytial Drosophila embryo with cortical actin networks: Four main steps of early development. Results Probl. Cell Differ. 71:67–90. 10.1007/978-3-031-37936-9_4 [DOI] [PubMed] [Google Scholar]
- Tang, N., and Marshall W.F.. 2012. Centrosome positioning in vertebrate development. J. Cell Sci. 125:4951–4961. 10.1242/jcs.038083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaizel-Ohayon, D., and Schejter E.D.. 1999. Mutations in centrosomin reveal requirements for centrosomal function during early Drosophila embryogenesis. Curr. Biol. 9:889–898. 10.1016/S0960-9822(99)80393-5 [DOI] [PubMed] [Google Scholar]
- Warn, R.M., Magrath R., and Webb S.. 1984. Distribution of F-actin during cleavage of the Drosophila syncytial blastoderm. J. Cell Biol. 98:156–162. 10.1083/jcb.98.1.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley, B.A., Rey-Suarez I., Hourwitz M.J., Kerr S., Shroff H., Fourkas J.T., and Upadhyaya A.. 2022. Nanotopography modulates cytoskeletal organization and dynamics during T cell activation. Mol. Biol. Cell. 33:ar88. 10.1091/mbc.E21-12-0601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Y., Budhathoki R., and Blankenship J.T.. 2021. Combinatorial deployment of F-actin regulators to build complex 3D actin structures in vivo. Elife. 10:e63046. 10.7554/eLife.63046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, K.M., Collins J.W., Cruz Walma D.A., Doyle A.D., Morales S.G., Lu J., Matsumoto K., Nazari S.S., Sekiguchi R., Shinsato Y., and Wang S.. 2019. Extracellular matrix dynamics in cell migration, invasion and tissue morphogenesis. Int. J. Exp. Pathol. 100:144–152. 10.1111/iep.12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Q., Miao Y., Banerjee P., Hourwitz M.J., Hu M., Qing Q., Iglesias P.A., Fourkas J.T., Losert W., and Devreotes P.N.. 2023. Nanotopography modulates intracellular excitable systems through cytoskeleton actuation. Proc. Natl. Acad. Sci. USA. 120:e2218906120. 10.1073/pnas.2218906120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, J., Wu X., Chung A.H., Chen J.K., Kapoor T.M., and Hammer J.A.. 2013. Centrosome repositioning in T cells is biphasic and driven by microtubule end-on capture-shrinkage. J. Cell Biol. 202:779–792. 10.1083/jcb.201301004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen, J.A., Cohen Y., Hudson A.M., Cooley L., Wieschaus E., and Schejter E.D.. 2002. SCAR is a primary regulator of Arp2/3-dependent morphological events in Drosophila. J. Cell Biol. 156:689–701. 10.1083/jcb.200109057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., Schulze K.L., Hiesinger P.R., Suyama K., Wang S., Fish M., Acar M., Hoskins R.A., Bellen H.J., and Scott M.P.. 2007. Thirty-one flavors of Drosophila rab proteins. Genetics. 176:1307–1322. 10.1534/genetics.106.066761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Yu J.C., Jiang T., Fernandez-Gonzalez R., and Harris T.J.C.. 2018. Collision of expanding actin caps with actomyosin borders for cortical bending and mitotic rounding in a syncytium. Dev. Cell. 45:551–564.e4. 10.1016/j.devcel.2018.04.024 [DOI] [PubMed] [Google Scholar]
- Zhao, P., Teng X., Tantirimudalige S.N., Nishikawa M., Wohland T., Toyama Y., and Motegi F.. 2019. Aurora-A breaks symmetry in contractile actomyosin networks independently of its role in centrosome maturation. Dev. Cell. 49:651–653. 10.1016/j.devcel.2019.05.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author upon reasonable request.