Abstract
The descending neurons connecting the fly’s brain to its ventral nerve cord respond to sensory stimuli and evoke motor programs of varying complexity. Anatomical characterization of the descending neurons and their synaptic connections suggests how these circuits organize movements, while optogenetic manipulation of their activity reveals what behaviors they can induce. Monitoring their responses to sensory stimuli or during behavior performance indicates what information they may encode. Recent advances in all three approaches make the descending neurons an excellent place to better understand the sensorimotor integration and transformation required for nervous systems to govern the motor sequences that constitute animal behavior.
Introduction
Descending neurons (DNs) connect the fly’s brain to its ventral nerve cord (VNC). ~1300 DNs are responsible for transmitting sensory and contextual information from the brain (130,000 neurons) to enable detailed execution of motor programs in the VNC (22,000 neurons) [1–3]. Since relatively few DNs pass through the neck connective, they represent a substantially compressed encoding of information, both in terms of the incoming sensory stimuli they integrate and the outgoing behavior responses they elicit.
The organization and function of DNs have been investigated by anatomical characterization, optogenetic analysis of behavioral contributions, and recording of activity. Multiple lines of evidence support the view that, in general, the DNs convey higher-level commands, goals, or decisions about what behavior the fly should perform rather than detailed instructions about the individual actions that compose them. Categorizing the different morphological types of DNs showed that many are single bilateral pairs, while others are small groups with similar shapes and projections [1–3]. These types may reflect command-like and population-coding archetypes, but further causative behavioral and correlative activity measurements will illuminate how these two modes of organizing motor control co-exist.
This review attempts to summarize the recent explosion of research centered around descending neurons and motor control, highlighting the complementarity of different experimental approaches and the value of comparisons among datasets, illustrated by advances in our understanding of the neural control mechanisms for producing more complex behavioral sequences such as courtship and grooming. While most of the work to date has focused on the contributions of DNs to motor circuits, some may affect sensory processing or provide modulatory cues as well. Great progress has been made, but ample room for surprising discoveries remains. Activating combinations of DNs with patterned stimuli, quantifying limb movements with greater resolution, and recording from multiple DNs simultaneously during behaviors, will be needed to assess the full range of potential codes carried by this numerically limited set of neurons.
Anatomical organization of descending neurons
Cross-sections of the neck connective in various fly species, using electron microscopy, showed approximately 3000 neuron profiles, which include both ascending and descending neurons. Dye-filling neurons in the neck and mapping those with cell body locations in the brain, coupled with reagents to assign neurotransmitter identity, led to an estimate of 1100 DNs representing cholinergic, GABAergic, glutamatergic, and aminergic types [4]. This estimate is close to the 1328 DNs counted in the single animal sectioned to produce the MANC connectome [2].
In order to assess how many different functional types of DN might exist, it was useful to ask if each DN is anatomically distinct or if there are sets of similar cells. Some of the DNs are immediately recognizable. For instance, the pair of giant fibers implicated in the jump escape response are recognizable landmarks with huge axon diameters. A large-scale effort to generate genetic reagents to target populations of DNs enabled characterization of the morphology of 350–500 pairs [1], covering approximately 29% of the total number of DNs [2]. Recent electron microscopy (EM) datasets and connectome analyses for the adult fly brain [5–8] and female and male ventral nerve cord [9,10] have identified 376 additional types [2]. The rarest class is single, unique neurons with bilateral projections, reminiscent of aminergic neurons from earlier in development or modulatory neurons associated with conveying contextual state [11]. More DNs occur as morphologically specialized, bilaterally symmetric pairs, with neurites of varying complexity. An appealing hypothesis is that these single pairs are early-born primary neurons establishing axon guidance pathways and foundational functional capacities of the adult animal; most of the experimentally identified command-like neurons described below belong to this type. Other DNs occur in small sets (2–15 neurons/hemisphere) of anatomically similar cells. These may represent population codes, where the number of neurons recruited titrate the intensity of the behavior. Alternatively, subtle differences in their connectivity may permit them to regulate related but distinct functions.
DN nomenclature is coordinated with the rest of the central nervous system [12,13] and based on cell body location, axon tract membership, and VNC target neuropil [1–3,8]. By definition, “descending neurons” receive synaptic inputs in the brain and make synaptic outputs in the ventral nerve cord. Many DNs also make output synapses in the brain, especially in the suboesophageal zone (SEZ), a region in the ventral anterior part of the brain with developmental and evolutionary affiliations to the VNC [14] that has been implicated in a range of sensory and motor functions [15]. Many DNs receive additional synaptic input (onto their axons) from local VNC neurons [2]. DNs also connect to each other [16,17]. Considering partners with more than 10 synapses, a given DN may receive inputs from tens to hundreds of other neurons and send outputs to similar numbers. Individual DNs have different ratios of input and output synapses overall in the brain and VNC. The computational capacity of individual DNs is just beginning to be investigated [18].
The most logical current proposal for functional organization of the DNs is related to their role in motor control. While dendrites of DNs are found in many brain regions, their axons tend to innervate discrete regions of the VNC [1]. The ones targeting the ventral leg neuropils may coordinate walking and grooming; those synapsing into the dorsal tectulum (wing and haltere) may be involved with flight; while others that span multiple VNC regions potentially coordinate leg and wing movements for behaviors involving different appendages, such as escape or courtship [1,2,19]. Connectome analysis in the female shows 209 DNs connect to the (left) legs and 272 to the (left) wing regions [20]; numbers in the male are similar [2]. The significance of the variability in connected partners and synapse numbers should become clearer as more individual samples are analyzed by EM and light microscopy (see below), but some trends are apparent. For example, while some DNs synapse onto motor neurons directly, this accounts for less than 10% of their output [2]. Most DNs influence movement indirectly, via synapses onto pre-motor circuits. Although there are as many as 70 motor neurons (MNs) per leg [9,21], there are thousands of VNC local interneurons associated with each leg, which suggests that the implementation of rhythmic patterns and inter-joint, inter-leg coordination will be both flexible and complex [2,20]. Local premotor neurons are more likely to synapse onto motor neurons innervating a common module (leg segment and muscle type), while DNs can synapse onto motor and premotor neurons belonging to different functional units [2]. Combinatorial, sequential activation of motor neurons could be achieved by synaptic weighting within premotor neurons or by DNs connecting to multiple premotor circuits. The lack of frequent direct DN to MN synapses suggests that DNs probably do not form motor synergies (groups of muscles that work together) themselves but might recruit them into sequences or modify the rhythmic timing of their activity through DN to pre-motor circuit connections. Further investigation into the architecture of DN synaptic outputs–which groups of premotor neurons they target and which sets of motor neurons and muscles those ultimately effect–will guide the behavioral experiments to test these predictions of DN contributions.
To derive insights into DN function or organization from their anatomy, the spatial resolution of electron microscopy for synaptic connectivity, and the ability to assess reproducibility by confocal microscopy in multiple replicates where the same neurons are genetically targeted, make a complementary combination. The EM connectome work was especially challenging because the current datasets from the brain and VNC were collected separately, requiring manual matching of neurons across the divide using landmarks or diagnostic neurite morphology guided by the light-level confocal microscopy images (DN-splitGAL4 lines and multi-color flip-out clones). A complete nervous system, where the neck connective between the brain and ventral nerve cord is intact, is eagerly awaited to help corroborate these deductive identity assignments as well as assess biological variability. And while these anatomical resources provide new insights and powerful resources, our current understanding of neural circuit organization is heavily based on sparser functional studies.
Behavioral changes caused by perturbations of descending neuron activity
In the overarching goal of determining how neural circuits coordinate animal behavior, descending neurons represent an anatomically critical and experimentally tractable control point. The limited behavioral capacities of decapitated flies hint at what descending neurons normally do. A fly without its head displays the righting reflex: if knocked over, it can still coordinate its limbs sufficiently to stand up. Decapitated flies do not walk but can take a few steps if prodded, and they will groom in response to bristle deflections, dust, or the application of certain drugs to the neck connective [22–24]. Thermogenetic activation of some developmental lineages of neurons induces coherent behaviors in decapitated flies, ranging from wing grooming or flapping to jumping, but activation of other lineages causes disorganized or uncoordinated leg movements [25]. These observations indicate that while basic sensory-motor reflexes can be performed by neural circuits located entirely in the VNC [26], more complicated and coordinated behaviors require the sensory apparatus on the head, control neurons in the brain, and the descending neurons connecting them to the VNC.
A survey of the capacity of descending neurons to elicit behavioral programs comes from optogenetic activation screens [27] using splitGAL4 lines [1,28]. Constant redlight stimulation was provided to groups of adult flies expressing UAS-csChrimson [29] in different DN populations, and a range of behaviors were observed, including walking and grooming. Different DNs elicited similar behaviors, suggesting some redundancy. The same neurons could evoke different behaviors depending on what the fly was doing right before activation (context), indicating that neurons can have different effects [27], perhaps depending on the combination of other active neurons.
The following examples highlight DNs with command-like functions, but this may represent experimental design or selection bias: neurons whose activation evokes recognizable behaviors are dramatic. Additionally, some descending neurons that were identified in activation screens as capable of inducing behaviors do not show obvious silencing phenotypes [30]. This may argue for redundancy, where other neurons can also convey the signals essential for normal performance of the behavior [2], or context dependence, where particular environmental conditions are also required to display an effect. Alternatively, we may not be measuring behavioral changes with sufficient resolution to determine unique contributions.
Some descending neurons induce very specific phenotypes (Figure 1). For example, activation of Moonwalker DNs makes flies walk backward [31]. Visual and olfactory cues processed by sensory circuits in the brain [32,33], and tactile cues from the forelegs carried by ascending neurons to the brain [34], trigger the Moonwalker neurons, which are required for flies to back up normally when they reach a dead end. Moonwalker’s postsynaptic targets showed that the precise sequence of muscle and joint movements needed to step backward are elicited by separate premotor neurons [35]. Thus, Moonwalker DNs are command-like, activated by the integration of sensory cues in the brain, and transmitting a signal to perform a motor program whose details are executed by distributed motor control circuits located in the VNC.
Figure 1. Schematic of descending neurons contributing to different behaviors.
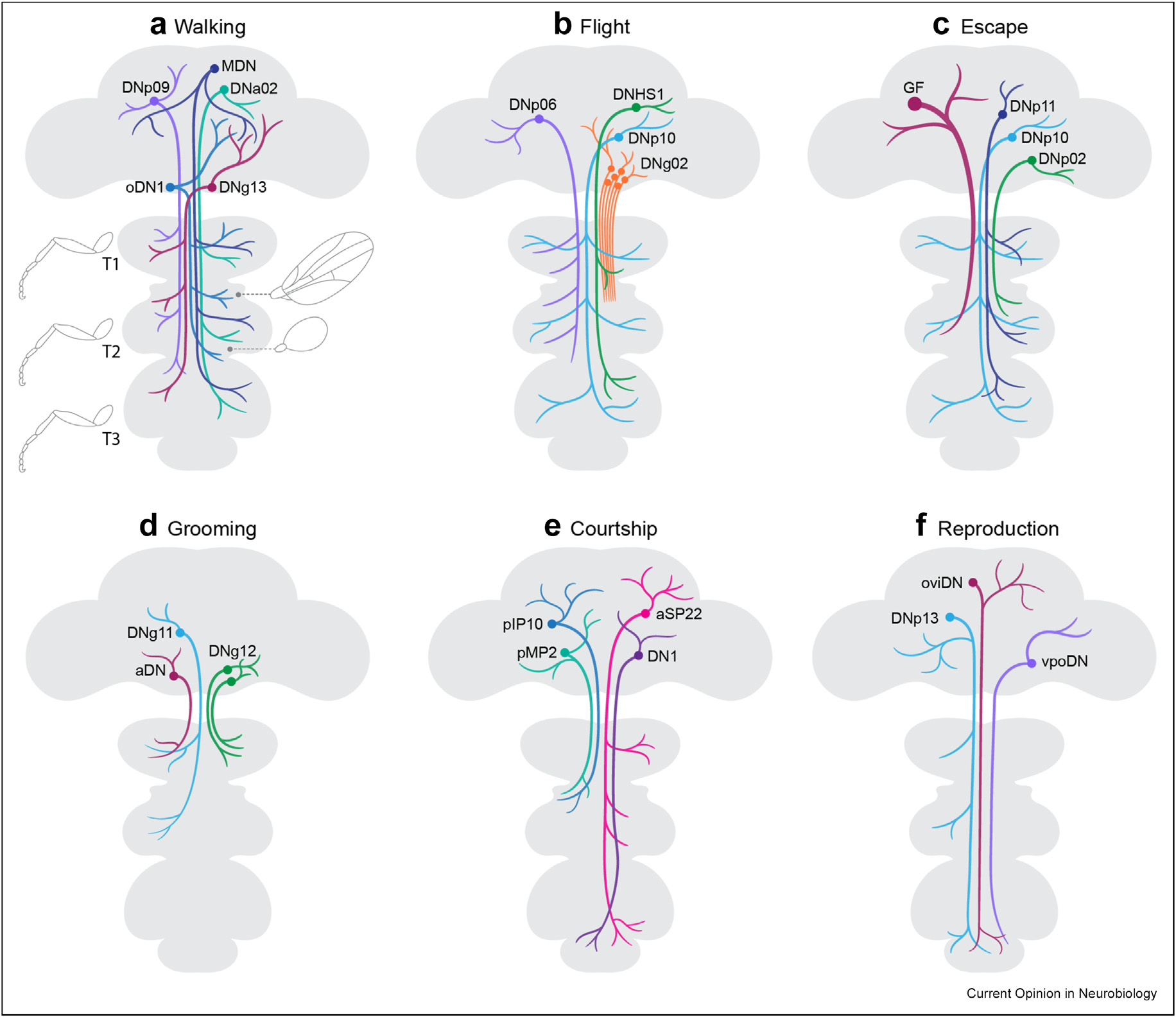
Examples are shown of individual identified neurons or groups with similar morphology that can command behaviors or control specific movements. The outline of the nervous system and the leg and wing drawings were modeled on a figure from Cheong et al., 2023, and show which VNC segmental neuromeres are associated with the sensory and motor circuits for each appendage. Figure by Elfy Chiang, VisLab, MRC Laboratory of Molecular Biology. a. Walking: DNp09 – freeze [62] OR forward walk with ipsilateral turns during courtship [36]; MDN – backward walking [31]; oDN1 – bolt forward walking [37]; DNa02 and DNg13 – steering [39,90] b. Flight: DNg02 – steering [47]; DNHS1 (DNp15) – looming [53]; DNp06 – maintaining straight flight [52]; DNp10 – landing initiation [45]. c. Escape: giant fiber (GF; DNp01) – escape [96]; DNp10 – jump [45]; DNp11 and DNp02 jump [2]; d. Grooming: aDN – antennal grooming [71]; DNg11 – front leg rubbing and DNg12 anterior grooming [73]. e. Courtship: pIP10 – song [78]; pMP2 – song [97] and type [79,80]; pSP22 – proboscis extension, abdominal bending [81]; DN1 – olfactory-initiated chasing [77]. f. Reproduction: OviDN – oviposition [86]; vpoDN – acceptance [82]; DNp13 – rejection [83,84]. VNC, ventral nerve cord
What about walking forward? Is that one behavior or many? An animal might need to walk in a variety of contexts: searching for food, approaching a mate, avoiding a threat, and exploring new territory. Parallel DNs may convey the command to walk under different circumstances, or the range of drives may converge onto a shared circuit, triggering a common motor program. The DNp09 DNs integrate visual cues to mediate pursuit during courtship, while the BPN neurons in the brain connect to other DNs to elicit fast, straight walking [36], arguing for some parallel pathways dedicated to walking for specific purposes. New evidence indicates that there may be distinct circuits that control stopping in different contexts as well [37].
Descending circuits may be required to balance or skew the properties of left and right steps to achieve a directional heading. Since simultaneous manipulation of bilateral pairs of neurons might be expected to promote starts or stops, identification of DNs that control turns requires a different experimental strategy. Detecting differences in neuronal activity between left and right homologs correlated with steering led to the implication of DNa01 and DNa02 [38]: these neurons initiate a common motor command, whether the impetus is an acute sensory cue or a remembered directional goal, illustrating convergence. DNs do not always evoke higher-order behavioral goals. An interesting study of DNs during visually guided steering in walking flies showed activity correlated with specific leg movements and at precise timepoints during the step cycle, suggesting much more fine-grained control [39]. Excellent recent reviews of motor control and sensory feedback in locomotion [40–42] provide additional perspectives.
The probability and speed of walking can be modulated by serotoninergic neurons in the VNC [43], and there are aminergic DNs as well [4]. Dopaminergic DNs promote both locomotion and grooming [22,44], while octopamine has been implicated in flight [45]. Aminergic DNs may modify motor commands by regulating activation thresholds or setting the context for which motor programs are available. Do DNs modulate sensory inputs? Possibly. Connectivity analysis shows that some DNs do synapse onto sensory rather than premotor circuits, and a recent report shows that peptidergic allatostatin-positive Epione DNs can suppress behavioral responses to noxious heat, but the VNC neurons through which these EpiDNs act are still unknown [46].
These examples emphasize the behavioral effects of unique, command-like neurons; evidence for a population code model is rarer. A recent study of descending neurons involved in flight describes the DNg02 neuron type, which encompasses 15 neurons with similar morphology [47]. They connect quite directly between the visual motion circuits and the motor neurons innervating flight muscles. Activation of DNg02 neurons in flying flies increases the wingbeat amplitude, and the more DNg02 neurons that are activated, the greater the increase, resembling a size-principle effect [48] in which both neuron number and activation level contribute to maximum power output. These DNg02 neurons respond to large-field visual motion, and left and right can respond separately, perhaps to balance thrust and maintain straight flight [49]. Silencing DNg02s disrupts the optomotor reflex [50]. Although these neurons are morphologically similar, further assessment of their connectivity reveals some differences. Since these DNs were identified by activating them when the fly was flying, they may contribute to other behaviors in other contexts. Thus, whether DNg02s reflect a population coding for a shared function or similar but distinct visuomotor transforms remains to be seen. The population of DNg02 works with other flight-descending neurons implicated in steering (AX) [51], evasive turns (DNp06) [52], or encoding self-motion (DNOVS1, DNOVS2, DNHS1) [53]. New results identify DNs that can induce rapid saccade turns or straight flight, with an inhibitory neuron located in the brain arbitrating between them [54].
Escape behavior, where a fly responds to an expanding visual stimulus with a jump take-off into flight, is a classic example of a behavioral sequence elicited by descending command neurons [55]. Fast, directionless takeoff is triggered by the activation of the giant fiber neurons (DNp01). These two large-diameter axons are readily visible in the cross-sections of the neck connective in many insects [2,56,57]. They make both chemical and electrical synapses and both direct and indirect connections to motor neurons in the middle leg neuropils needed for leg extension at launch. Once the giant fibers reach their activation threshold, they evoke jump escape [58]. If, however, the visual looming stimulus expands more slowly, perhaps indicating a less urgent threat, an alternative pathway is recruited, and the fly takes off more gracefully, repositioning its legs and jumping away from the approaching object [58,59]. One of the most exciting hypotheses generated from recent connectome analysis is the proposal of candidates for this parallel descending pathway among the LTctDNs that target the region of the VNC spanning the leg and wing neuropils [2]. The short-mode and long-mode escape takeoffs illustrate competing motor sequences initiated in response to similar visual stimuli [60,61] and triggered by parallel descending circuits with different activation thresholds [58].
Escape is not always the best choice. Depending on what a fly’s neighbors are doing and whether the fly itself is still or already moving, the best defensive strategy against an aerial predator might be to freeze instead of flee. This is another example where a DN (DNp09) represents a bridge between sensory integration and motor execution, and its activation instantiates the decision to run or halt [62].
The descending neurons have been a rich vein for exploring mechanisms of context-dependent behavior selection [63]. Looming visual stimuli can activate the motor program for leg extension, which can be used for escape take-off… or for landing! The difference in the fly’s circumstances–earthbound standing or airborne flying–can be encoded by visual and proprioceptive cues that then influence the activity of specific descending neurons DNp07 or DNp10. Ache et al. [45] discovered descending neurons critical for appropriate landing behavior. Silencing them impairs landing, and activating them induces landing. Interestingly, their spike rate determines leg extension amplitude, implying that descending neurons are capable of more than an on-off contribution to this action selection.
Links to larval locomotion
What about descending neurons in larvae? EM connectome analysis identifies 184 DNs connecting the brain to the SEZ and 182 DNs targeting the VNC (of 3016 total neurons) [17]. Although larval and adult flies have very different modes of locomotion, the moonwalker descending neurons (MDNs) are present in both life stages and induce backward movement in both [64,65]. This persistence of neuron and function seems unusual; moonwalker is the only case described in the literature so far, and the nervous system has been extensively remodeled through development [42]. Another neuron, PDM-DN, is an archetypical DN in that it integrates sensory information about changing olfactory signals to induce an appropriate motor sequence of stopping and turning as the larva navigates toward an odor source [66]. A recent analysis of whole brain connectomics in the first instar larva includes a catalog of the anatomy and brain connections of the larval descending neurons [17], including an intriguing “zig-zag” connection between PDM-DN and MDN via an ascending neuron; additional functional studies are eagerly awaited. Larvae also use descending neurons to control escape. Contragoro DNs synapse onto the command-like Goro neuron in the larval VNC that induces rolling escape behavior [67]. The convergence of nociceptive sensory information that triggers escape happens in both the larval brain and ventral nerve cord. Recent work identified peptidergic DNs that dampen the thermal pain response by inhibiting Goro neurons [68], modulating motor circuits rather than sensory ones. But sensory circuits can also be modulated: descending inhibitory neurons may gate the pain response during starvation to sustain feeding [69].
More complex sequences
Behaviors can be subdivided into action sequences of different timescales and complexity. Walking can be decomposed into stance and swing phases, or even further into ordered changes in the angles of individual leg joints [40]. In escape, the fly repositions its legs, lifts its wings, and extends the middle legs to jump [70]. Grooming and courtship behaviors, described below, provide examples of motor sequences of increasing complexity, and ongoing experiments unveil the roles of DNs in coordinating them.
Fly grooming is a sequential combination of motor programs where the legs move in different patterns to remove debris. Descending neurons contribute to its hierarchical control. Command-like DNs for antennal cleaning [71], wing cleaning [72], head cleaning, and front leg rubbing [73] have been identified in activation screens, where they trigger ordered leg muscle contractions that produce targeted grooming movements. Since optogenetic activation of DNs with constant light results in temporally patterned muscle activation, the dynamic structure must be imposed by central pattern-generating circuits in between, feedback loops, or intrinsic electrical properties of the DNs themselves (such as refractory periods). While most of these DNs induce single behavioral subroutines, others induce combinations. Activation of DNg12, for example, evokes the alternation of head sweeps and front leg rubs that constitute anterior grooming, indicating that DNs are capable of inducing complex, goal-directed motor sequences. Current data does not yet address whether DN activation represents the normal decision point about which grooming subroutine to execute when multiple body parts are dirty. Experimental coactivation of DNs results in an alternation of the actions they elicit, indicating that conflicting commands can be resolved by neural circuits in the VNC, but inhibitory neurons connecting sensory inputs to DNs associated with competing actions suggest that the activation of a particular DN may represent the normal decision mechanism [73]. Simultaneous recording from different DNs during behavioral choices will be required to determine if activation of only one represents the physiological mechanism of action selection.
Descending neurons that contribute to reproductive behaviors provide an additional illustration of how complex sensory-motor sequences can be controlled. The neural circuitry that underlies male courtship behavior has been extensively dissected, starting with the seminal discovery of the fruitless mutant and the neurons that express the transcription factor that gene encodes (reviewed in Ref. [74]). Multiple sensory modalities converge to initiate courtship, and the accumulation of excitation induces progressive commitment via a sequence of motor programs from chasing through singing and proboscis contact to copulation (reviewed in Refs. [75,76]). Sensory integration with past social experience is performed in the brain, while coordination of the body and limbs requires the VNC, so as might be expected, DNs are key contributors. A male fly detects an appropriate female by her appearance, movement, and pheromones. The P1 neurons in the brain integrate these sensory inputs and arbitrate between courtship and aggression. There are DNs that receive synapses from the pheromone-detection circuit to initiate chasing [77]. Subsequent courtship songs can be induced by activation of the DN pIP10 [78]; sine and pulse elements of the song can be initiated by overlapping but distinct circuits [79]. Intriguingly, different descending neurons seem to govern the decision to sing and the selection of sine vs. pulse song [80], subdividing modular motor control in surprising ways. Other sequential steps in courtship have a common control point: proboscis extension, abdominal lifting, and attempted copulation occur in response to increased firing of the aSP22 DNs [81]. A single DN can elicit several motor programs in order because different postsynaptic targets are recruited as its firing rate increases.
A virgin female fly can accept a male’s courtship advances by opening her vaginal plates to permit copulation, while a mated female may extrude her ovipositor to reject him. These mutually exclusive motor programs are triggered by the same sensory cues interpreted under different mating state conditions. This information is integrated to activate the appropriate DN: vpoDN for acceptance [82] or DNp13 for rejection [83,84]. A choice is made in the brain and executed by selective activation of a DN (reviewed in Ref. [85]).
The ovipositor is also employed in egg laying, using a motor program that involves some of the same muscles as the rejection response but is controlled by different DNs. In a mated female, the OviDNs that control egg-laying (or oviposition) also integrate sensory cues about the appropriate substrate with the mating state [86]. The state is communicated when these neurons are disinhibited by sex peptide signaling that occurs during sperm transfer via pC1 neurons [86]. The pC1 neurons act as a decision-making center where female flies choose between aggression [87], receptivity to courtship, and egg-laying responses based on internal state and external context. The OviDNs accumulate sensory information over time, and when they reach an excitation threshold, their activation initiates the motor sequence for egg laying [88]. OviDN may encode the overarching command, but sensory and motor feedback contribute to the functional flexibility of the execution of its component steps [89].
Lessons about how descending neurons contribute to the control of motor sequences from courtship and reproductive behaviors include that DNs represent the integration point of sensory and state information, achieve activation thresholds, and tend to initiate or modulate rather than implement detailed motor programs. Temporal or spatial integration may be needed to induce DN activity. In some cases, a DN’s spike pattern is critical, but in others, the fact of their activation may be sufficient to instigate behavior. Courtship, like grooming, can be decomposed into simpler motor programs. Different DNs evoke different courtship subroutines: the neurons that respond to pheromones, drive song, or trigger proboscis extension are distinct. The full courtship sequence includes all of these actions, employed in order but with flexibility in timing and duration that may be enabled by the modularity of descending control and the ability of sensory feedback to tune aspects of sequence execution, as has now been demonstrated for egg-laying.
Measurements of activity in descending neurons during behavior
Interrogating what sensory or contextual information descending neurons respond to requires measuring their activity while providing stimuli; determining which DN activity patterns might be causal requires simultaneously recording the fly’s behavior. Electrophysiological recordings from neurons in the neck connective showed that activity in dopaminergic DNs was correlated with walking [44]. However, activating or inhibiting these DNs did not alter leg movements [44]. Measuring and manipulating neural activity in specific populations of neurons in different environmental contexts and internal states while recording fly behavior will be required to fully assess the coding potential of descending neurons for sensory input and motor output.
In a recent technical tour-de-force, the Ramdya lab developed a dissection and imaging preparation to perform two-photon-based imaging of changes in GCaMP fluorescence, indicating calcium levels commonly thought to correlate with neural activity, in the neck connective and ventral nerve cord of a behaving fly [90]. The tethering required for stable imaging somewhat limits the range of activity-to-behavior correlations that can be assessed, but there is clear potential for discovery, as shown by their recordings of populations of DNs during spontaneous and odor-evoked behaviors [91]. The overall conclusion from this study is that a large fraction of DNs show activity changes during each behavior–and that this activity correlates with higher-order behavioral features (e.g. walking as a whole rather than extension of a particular leg segment). Whether the DNs activity is causing or responding to these behavioral states is not resolved, and the sensory stimuli the fly encountered–other than odor– are undetermined. A new study using the same preparation revealed a surprising degree of connectivity among descending neurons, suggesting that activation of one command-like neuron may recruit other DNs, resulting in a population code [16].
Combinations of neurons may act together to encode sensory inputs or modulate motor outputs. The ability to record from groups of neurons simultaneously–with suitable temporal resolution–will enable researchers to compare the timing of their activity: does whichever command-like DN responds first dictate the behavioral program selected? Do DNs cooperate to produce motor sequences by firing in order? New methods for imaging neural activity in freely-behaving flies, currently employed for the brain [92–94], may be applicable in the descending neurons to expand the range of behaviors and contexts in which correlations can be assessed. Virtual reality experiments, where sensory inputs can be precisely controlled, may also be informative [95]. We need to test what DNs can encode (show activity responses to) by presenting specific combinations of sensory and contextual information in the functional imaging preparation (ideally while also measuring what behaviors the fly performs in response). These correlations will complement the optogenetic experiments where DNs are activated to determine the motor programs they can induce. Together, recording neural activity assays information encoding while inducing neural activity assays motor control capacity, and we will need both to determine how DNs contribute to the normal organization of behavior.
Conclusions
One of the critical jobs of the nervous system is to detect sensory and situational information–do you smell food and are you hungry? Then the detection circuits must integrate this information and determine an appropriate behavioral response: yes, both; let’s eat. The right sequence of movements should be initiated and executed: pick up the fork, skewer the tomato, open the mouth, chew, and swallow. Descending neurons connecting the brain to the ventral nervous system sit at the convergence point, responding to sensory cues and initiating motor programs. They are not passive relays, conveying unfiltered information, but rather specialized for integration. To understand how they do their jobs, we must learn what sensory cues they respond to and what actions they cause. Do the DNs encode the higher-level command “Eat!” or the detail-oriented sequence of actions required to accomplish consumption? And are these orders carried by single neurons or population codes? These debates will likely be resolved by the discovery that both situations coexist. Their computational potential may be extended if we find that the same descending neuron can induce different actions when fired in different temporal patterns, in different environmental contexts, or in conjunction with different peers. Complete anatomical description of DN types and their connectivity, combined with expanding genetic access for functional imaging and optogenetic manipulation, coupled with simultaneous quantitative behavioral assessment, make the Drosophila descending neurons a powerful experimental system to decipher how different parts of the nervous system communicate, and specifically how sensory and contextual information is compressed to initiate higher-order commands that then must be decompressed to execute the complex motor sequences we recognize as behavior.
Acknowledgements
Research in the Simpson Lab on topics related to this review is supported by an NSF Career Award 1943276 and NIH grants R01NS110866 and RF1NS132900. Ideas that influenced this perspective came from discussions at the KITP Neurophysics of Locomotion Program in 2022 supported by NSF PHY-1748958, NIH R25GM067110, and the Gordon and Betty Moore Foundation Grant No. 2919.01. I thank my lab at UCSB, especially Primoz Ravbar, Li Guo, and Durafshan Sakeena Syed, and my sabbatical colleagues at the LMB Lalanti Venkatasubramanian, Tomke Stuerner, Kathi Eichler, and Sebastian Cachero, for constructive feedback. Dr. Gwyneth Card, Dr. Dawn Blitz, and an anonymous reviewer gave critical comments to improve the manuscript.
Funding
The authors report no relevant funding sources associated with this article
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
- 1. Namiki S, Dickinson MH, Wong AM, Korff W, Card GM: The functional organization of descending sensory-motor pathways in Drosophila. Elife 2018, 7. * This core study describes genetic reagents to label different descending neuron types for anatomical and functional experiments.
- 2. Cheong HSJ, Eichler K, Stuerner T, Asinof SK, Champion AS, Marin EC, Oram TB, Sumathipala M, Venkatasubramanian L, Namiki S, et al. : Transforming descending input into behavior: the organization of premotor circuits in the Drosophila Male Adult Nerve Cord connectome. bioRxiv 2023, 543976.2023.2006.2007. * Extensive anatomical connectome analysis of the male electron microscopy dataset for the ventral nerve cord (Takamura et al., 2023) shows how the descending neurons interface with premotor circuits.
- 3.Marin EC, Morris BJ, Stuerner T, Champion AS, Krzeminski D, Badalamente G, Gkantia M, Dunne CR, Eichler K, Takemura S-y, et al. : Systematic annotation of a complete adult male Drosophila nerve cord connectome reveals principles of functional organisation. bioRxiv 2023, 543407. 2023.2006.2005. [Google Scholar]
- 4.Hsu CT, Bhandawat V: Organization of descending neurons in Drosophila melanogaster. Sci Rep 2016, 6, 20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Z, Lauritzen JS, Perlman E, Robinson CG, Nichols M, Milkie D, Torrens O, Price J, Fisher CB, Sharifi N, et al. : A complete electron microscopy volume of the brain of adult Drosophila melanogaster. Cell 2018, 174:730–743 e722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheffer LK, Xu CS, Januszewski M, Lu Z, Takemura S-y, Hayworth KJ, Huang GB, Shinomiya K, Maitlin-Shepard J, Berg S, et al. : A connectome and analysis of the adult Drosophila central brain. Elife 2020, 9, e57443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorkenwald S, Matsliah A, Sterling AR, Schlegel P, Yu S-c, McKellar CE, Lin A, Costa M, Eichler K, Yin Y, et al. : Neuronal wiring diagram of an adult brain. bioRxiv 2023. 2023.2006.2027.546656. [Google Scholar]
- 8.Schlegel P, Yin Y, Bates AS, Dorkenwald S, Eichler K, Brooks P, Han DS, Gkantia M, Santos Md, Munnelly EJ, et al. : A consensus cell type atlas from multiple connectomes reveals principles of circuit stereotypy and variation. bioRxiv 2023. 2023.2006.2027.546055. [Google Scholar]
- 9.Phelps JS, Hildebrand DGC, Graham BJ, Kuan AT, Thomas LA, Nguyen TM, Buhmann J, Azevedo AW, Sustar A, Agrawal S, et al. : Reconstruction of motor control circuits in adult Drosophila using automated transmission electron microscopy. Cell 2021, 184:759–774 e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takemura S-y, Hayworth KJ, Huang GB, Januszewski M, Lu Z, Marin EC, Preibisch S, Xu CS, Bogovic J, Champion AS, et al. : A connectome of the male Drosophila ventral nerve cord. bioRxiv 2023, 543757. 2023.2006.2005. [Google Scholar]
- 11.Busch S, Tanimoto H: Cellular configuration of single octopamine neurons in Drosophila. J Comp Neurol 2010, 518: 2355–2364. [DOI] [PubMed] [Google Scholar]
- 12.Ito K, Shinomiya K, Ito M, Armstrong JD, Boyan G, Hartenstein V, Harzsch S, Heisenberg M, Homberg U, Jenett A, et al. : A systematic nomenclature for the insect brain. Neuron 2014, 81:755–765. [DOI] [PubMed] [Google Scholar]
- 13.Court R, Namiki S, Armstrong JD, Borner J, Card G, Costa M, Dickinson M, Duch C, Korff W, Mann R, et al. : A systematic nomenclature for the Drosophila ventral nerve cord. Neuron 2020, 107:1071–1079 e1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartenstein V, Omoto JJ, Ngo KT, Wong D, Kuert PA, Reichert H, Lovick JK, Younossi-Hartenstein A: Structure and development of the subesophageal zone of the Drosophila brain. I. Segmental architecture, compartmentalization, and lineage anatomy. J Comp Neurol 2018, 526:6–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne GR, Otsuna H, Dickson BJ, Scott K: Classification and genetic targeting of cell types in the primary taste and premotor center of the adult Drosophila brain. Elife 2021, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun J, Hurtak F, Wang-Chen S, Ramdya P: Networks of descending neurons transform command-like signals into population-based behavioral control. bioRxiv 2023, 557103. 2023.2009.2011. [Google Scholar]
- 17.Winding M, Pedigo BD, Barnes CL, Patsolic HG, Park Y, Kazimiers T, Fushiki A, Andrade IV, Khandelwal A, Valdes-Aleman J, et al. : The connectome of an insect brain. Science 2023, 379, eadd9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auth CS, Crickmore MA: Behavioral neuroscience: computation in individual neurons. Curr Biol 2023, 33:R1006–R1008. [DOI] [PubMed] [Google Scholar]
- 19.Louis M, Simpson JH: Disentangling the strings that organize behavior. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lesser E, Azevedo AW, Phelps JS, Elabbady L, Cook A, Mark B, Kuroda S, Sustar A, Moussa A, Dallmann CJ, et al. : Synaptic architecture of leg and wing motor control networks in Drosophila. bioRxiv 2023. 2023.2005.2030.542725. * Anatomical connectome analysis of the female electron microscopy dataset (Phelps et al., 2021) for the ventral nerve cord shows how the descending neurons interface with premotor circuits.
- 21.Azevedo A, Lesser E, Mark B, Phelps J, Elabbady L, Kuroda S, Sustar A, Moussa A, Kandelwal A, Dallmann CJ, et al. : Tools for comprehensive reconstruction and analysis of Drosophila motor circuits. bioRxiv 2022. 2022.2012.2015.520299. [Google Scholar]
- 22.Yellman C, Tao H, He B, Hirsh J: Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc Natl Acad Sci U S A 1997, 94: 4131–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seeds AM, Ravbar P, Chung P, Hampel S, Midgley FM Jr, Mensh BD, Simpson JH: A suppression hierarchy among competing motor programs drives sequential grooming in Drosophila. Elife 2014, 3, e02951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandervorst P, Ghysen A: Genetic control of sensory connections in Drosophila. Nature 1980, 286:65–67. [DOI] [PubMed] [Google Scholar]
- 25.Shepherd D, Sahota V, Court R, Williams DW, Truman JW: Developmental organization of central neurons in the adult Drosophila ventral nervous system. J Comp Neurol 2019, 527: 2573–2598. [DOI] [PubMed] [Google Scholar]
- 26.Venkatasubramanian L, Mann RS: The development and assembly of the Drosophila adult ventral nerve cord. Curr Opin Neurobiol 2019, 56:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cande J, Namiki S, Qiu J, Korff W, Card GM, Shaevitz JW, Stern DL, Berman GJ: Optogenetic dissection of descending behavioral control in Drosophila. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tirian L, Dickson BJ: The VT GAL4, LexA, and split-GAL4 driver line collections for targeted expression in the Drosophila nervous system. bioRxiv 2017, 198648. [Google Scholar]
- 29.Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, et al. : Independent optical excitation of distinct neural populations. Nat Methods 2014, 11:338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshihara M, Yoshihara M: ‘Necessary and sufficient’ in biology is not necessarily necessary - confusions and erroneous conclusions resulting from misapplied logic in the field of biology, especially neuroscience. J Neurogenet 2018, 32:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bidaye SS, Machacek C, Wu Y, Dickson BJ: Neuronal control of Drosophila walking direction. Science 2014, 344:97–101. [DOI] [PubMed] [Google Scholar]
- 32.Sen R, Wu M, Branson K, Robie A, Rubin GM, Dickson BJ: Moonwalker descending neurons mediate visually evoked retreat in Drosophila. Curr Biol 2017, 27:766–771. [DOI] [PubMed] [Google Scholar]
- 33.Israel S, Rozenfeld E, Weber D, Huetteroth W, Parnas M: Olfactory stimuli and moonwalker SEZ neurons can drive backward locomotion in Drosophila. Curr Biol 2022, 32: 1131–1149.e1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sen R, Wang K, Dickson BJ: TwoLumps ascending neurons mediate touch-evoked reversal of walking direction in Drosophila. Curr Biol 2019, 29:4337–4344 e4335. [DOI] [PubMed] [Google Scholar]
- 35. Feng K, Sen R, Minegishi R, Dubbert M, Bockemuhl T, Buschges A, Dickson BJ: Distributed control of motor circuits for backward walking in Drosophila. Nat Commun 2020, 11: 6166. * Characterization of the circuits postsynaptic to the moonwalker descending neuron includes an illustrative example of motor control with a clear discussion of command-type and population-type control models.
- 36.Bidaye SS, Laturney M, Chang AK, Liu Y, Bockemuhl T, Buschges A, Scott K: Two brain pathways initiate distinct forward walking programs in Drosophila. Neuron 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sapkal N, Mancini N, Kumar DS, Spiller N, Murakami K, Vitelli G, Bargeron B, Maier K, Eichler K, Jefferis GSXE, et al. : Neural circuit mechanisms underlying context-specific halting in Drosophila. bioRxiv 2023. 2023.2009.2025.559438. [Google Scholar]
- 38.Rayshubskiy A, Holtz SL, D’Alessandro I, Li AA, Vanderbeck QX, Haber IS, Gibb PW, Wilson RI: Neural circuit mechanisms for steering control in walking Drosophila. bioRxiv 2020. 2020.2004.2004.024703. [Google Scholar]
- 39.Yang HH, Brezovec LE, Capdevila LS, Vanderbeck QX, Adachi A, Mann RS, Wilson RI: Fine-grained descending control of steering in walking Drosophila. bioRxiv 2023. 2023.2010.2015.562426. [Google Scholar]
- 40.Bidaye SS, Bockemuhl T, Buschges A: Six-legged walking in insects: how CPGs, peripheral feedback, and descending signals generate coordinated and adaptive motor rhythms. J Neurophysiol 2018, 119:459–475. [DOI] [PubMed] [Google Scholar]
- 41.Büschges A, Gorostiza EA: Neurons with names: descending control and sensorimotor processing in insect motor control. Curr Opin Neurobiol 2023, 83, 102766. [DOI] [PubMed] [Google Scholar]
- 42.Agrawal S, Tuthill JC: The two-body problem: proprioception and motor control across the metamorphic divide. Curr Opin Neurobiol 2022, 74, 102546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howard CE, Chen CL, Tabachnik T, Hormigo R, Ramdya P, Mann RS: Serotonergic modulation of walking in Drosophila. Curr Biol 2019, 29:4218–4230 e4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tschida K, Bhandawat V: Activity in descending dopaminergic neurons represents but is not required for leg movements in the fruit fly Drosophila. Phys Rep 2015, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ache JM, Namiki S, Lee A, Branson K, Card GM: State-dependent decoupling of sensory and motor circuits underlies behavioral flexibility in Drosophila. Nat Neurosci 2019, 22: 1132–1139. * Command-like circuits that coordinate the motor program for landing act differently when the fly is flying
- 46.Liu J, Liu W, Thakur D, Mack J, Spina A, Montell C: Alleviation of thermal nociception depends on heat-sensitive neurons and a TRP channel in the brain. Curr Biol 2023, 33:2397–2406 e2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Namiki S, Ros IG, Morrow C, Rowell WJ, Card GM, Korff W, Dickinson MH: A population of descending neurons that regulates the flight motor of Drosophila. Curr Biol 2022, 32: 1189–1196 e1186. * A group of anatomically similar descending neurons contribute to steering in flight and may reflect population coding.
- 48.Azevedo AW, Dickinson ES, Gurung P, Venkatasubramanian L, Mann RS, Tuthill JC: A size principle for recruitment of Drosophila leg motor neurons. Elife 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hedrick TL, Dickerson BH: Insect flight: flies use a throttle to steer. Curr Biol 2022, 32:R218–R220. [DOI] [PubMed] [Google Scholar]
- 50. Palmer EH, Omoto JJ, Dickinson MH: The role of a population of descending neurons in the optomotor response in flying Drosophila. bioRxiv 2022. 2022.2012.2005.519224. * There may be subtle anatomical and connectional differences in this group of descending neurons that contribute to the optomotor reflex in flight.
- 51.Schnell B, Ros IG, Dickinson MH: A descending neuron correlated with the rapid steering maneuvers of flying Drosophila. Curr Biol 2017, 27:1200–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim H, Park H, Lee J, Kim AJ: A visuomotor circuit for evasive flight turns in Drosophila. Curr Biol 2023, 33:321–335.e326. [DOI] [PubMed] [Google Scholar]
- 53.Suver MP, Huda A, Iwasaki N, Safarik S, Dickinson MH: An array of descending visual interneurons encoding self-motion in Drosophila. J Neurosci 2016, 36:11768–11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ros IG, Omoto JJ, Dickinson MH: Descending control and regulation of spontaneous flight turns in Drosophila. bioRxiv 2023. 2023.2009.2006.555791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trimarchi JR, Schneiderman AM: Flight initiations in Drosophila melanogaster are mediated by several distinct motor patterns. J Comp Physiol [A 1995, 176:355–364. [DOI] [PubMed] [Google Scholar]
- 56.Koto M, Tanouye MA, Ferrus A, Thomas JB, Wyman RJ: The morphology of the cervical giant fiber neuron of Drosophila. Brain Res 1981, 221:213–217. [DOI] [PubMed] [Google Scholar]
- 57.Nassel DR, Strausfeld NJ: A pair of descending neurons with dendrites in the optic lobes projecting directly to thoracic ganglia of dipterous insects. Cell Tissue Res 1982, 226: 355–362. [DOI] [PubMed] [Google Scholar]
- 58.von Reyn CR, Breads P, Peek MY, Zheng GZ, Williamson WR, Yee AL, Leonardo A, Card GM: A spike-timing mechanism for action selection. Nat Neurosci 2014, 17:962–970. [DOI] [PubMed] [Google Scholar]
- 59.Card G, Dickinson MH: Visually mediated motor planning in the escape response of Drosophila. Curr Biol 2008, 18:1300–1307. [DOI] [PubMed] [Google Scholar]
- 60.Dombrovski M, Peek MY, Park JY, Vaccari A, Sumathipala M, Morrow C, Breads P, Zhao A, Kurmangaliyev YZ, Sanfilippo P, et al. : Synaptic gradients transform object location to action. Nature 2023, 613:534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Reyn CR, Nern A, Williamson WR, Breads P, Wu M, Namiki S, Card GM: Feature integration drives probabilistic behavior in the Drosophila escape response. Neuron 2017, 94: 1190–1204 e1196. [DOI] [PubMed] [Google Scholar]
- 62.Zacarias R, Namiki S, Card GM, Vasconcelos ML, Moita MA: Speed dependent descending control of freezing behavior in Drosophila melanogaster. Nat Commun 2018, 9:3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Devineni AV, Scaplen KM: Neural circuits underlying behavioral flexibility: insights from Drosophila. Front Behav Neurosci 2021, 15, 821680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee K, Doe CQ: A locomotor neural circuit persists and functions similarly in larvae and adult Drosophila. Elife 2021:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carreira-Rosario A, Zarin AA, Clark MQ, Manning L, Fetter RD, Cardona A, Doe CQ: MDN brain descending neurons coordinately activate backward and inhibit forward locomotion. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tastekin I, Khandelwal A, Tadres D, Fessner ND, Truman JW, Zlatic M, Cardona A, Louis M: Sensorimotor pathway controlling stopping behavior during chemotaxis in the Drosophila melanogaster larva. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohyama T, Schneider-Mizell CM, Fetter RD, Aleman JV, Franconville R, Rivera-Alba M, Mensh BD, Branson KM, Simpson JH, Truman JW, et al. : A multilevel multimodal circuit enhances action selection in Drosophila. Nature 2015, 520: 633–639. [DOI] [PubMed] [Google Scholar]
- 68.Oikawa I, Kondo S, Hashimoto K, Yoshida A, Hamajima M, Tanimoto H, Furukubo-Tokunaga K, Honjo K: A descending inhibitory mechanism of nociception mediated by an evolutionarily conserved neuropeptide system in Drosophila. bioRxiv 2023. 2022.2003.2008.483420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakamizo-Dojo M, Ishii K, Yoshino J, Tsuji M, Emoto K: Descending GABAergic pathway links brain sugar-sensing to peripheral nociceptive gating in Drosophila. Nat Commun 2023, 14:6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Card G, Dickinson M: Performance trade-offs in the flight initiation of Drosophila. J Exp Biol 2008, 211:341–353. [DOI] [PubMed] [Google Scholar]
- 71.Hampel S, Franconville R, Simpson JH, Seeds AM: A neural command circuit for grooming movement control. Elife 2015, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang N, Simpson JH: A pair of commissural command neurons induces Drosophila wing grooming. iScience 2022, 25, 103792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo L, Zhang N, Simpson JH: Descending neurons coordinate anterior grooming behavior in Drosophila. Curr Biol 2022, 32: 823–833 e824. [DOI] [PubMed] [Google Scholar]
- 74.Manoli DS, Meissner GW, Baker BS: Blueprints for behavior: genetic specification of neural circuitry for innate behaviors. Trends Neurosci 2006, 29:444–451. [DOI] [PubMed] [Google Scholar]
- 75.Dickson BJ: Wired for sex: the neurobiology of Drosophila mating decisions. Science 2008, 322:904–909. [DOI] [PubMed] [Google Scholar]
- 76.Cobb M: Drosophila courtship: neuronal coordination of behavioural sequences and a 60-year-old hypothesis. Curr Biol 2019, 29:R250–R252. [DOI] [PubMed] [Google Scholar]
- 77.Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R: A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature 2010, 468: 686–690. [DOI] [PubMed] [Google Scholar]
- 78.von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ: Neuronal control of Drosophila courtship song. Neuron 2011, 69:509–522. [DOI] [PubMed] [Google Scholar]
- 79.Lillvis JL, Wang K, Shiozaki HM, Xu M, Stern DL, Dickson BJ: The neural basis of Drosophila courtship song. bioRxiv 2023. 2023.2008.2030.555537. [Google Scholar]
- 80.Shiozaki HM, Wang K, Lillvis JL, Xu M, Dickson BJ, Stern DL: Combinatorial circuit dynamics orchestrate flexible motor patterns in Drosophila. bioRxiv 2023. 2022.2012.2014.520499. [Google Scholar]
- 81.McKellar CE, Lillvis JL, Bath DE, Fitzgerald JE, Cannon JG, Simpson JH, Dickson BJ: Threshold-based ordering of sequential actions during Drosophila courtship. Curr Biol 2019, 29:426–434 e426. [DOI] [PubMed] [Google Scholar]
- 82.Wang K, Wang F, Forknall N, Yang T, Patrick C, Parekh R, Dickson BJ: Neural circuit mechanisms of sexual receptivity in Drosophila females. Nature 2021, 589:577–581. [DOI] [PubMed] [Google Scholar]
- 83.Wang F, Wang K, Forknall N, Parekh R, Dickson BJ: Circuit and behavioral mechanisms of sexual rejection by Drosophila females. Curr Biol 2020, 30:3749–3760 e3743. [DOI] [PubMed] [Google Scholar]
- 84.Mezzera C, Brotas M, Gaspar M, Pavlou HJ, Goodwin SF, Vasconcelos ML: Ovipositor extrusion promotes the transition from courtship to copulation and signals female acceptance in Drosophila melanogaster. Curr Biol 2020, 30:3736–3748 e3735. [DOI] [PubMed] [Google Scholar]
- 85.von Philipsborn AC: Neuroscience: the female art of saying No. Curr Biol 2020, 30:R1080–R1083. [DOI] [PubMed] [Google Scholar]
- 86.Wang F, Wang K, Forknall N, Patrick C, Yang T, Parekh R, Bock D, Dickson BJ: Neural circuitry linking mating and egg laying in Drosophila females. Nature 2020, 579:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schretter CE, Aso Y, Robie AA, Dreher M, Dolan MJ, Chen N, Ito M, Yang T, Parekh R, Branson KM, et al. : Cell types and neuronal circuitry underlying female aggression in Drosophila. Elife 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vijayan V, Wang F, Wang K, Chakravorty A, Adachi A, Akhlaghpour H, Dickson BJ, Maimon G: A rise-to-threshold process for a relative-value decision. Nature 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cury KM, Axel R: Flexible neural control of transition points within the egg-laying behavioral sequence in Drosophila. Nat Neurosci 2023, 26:1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen CL, Hermans L, Viswanathan MC, Fortun D, Aymanns F, Unser M, Cammarato A, Dickinson MH, Ramdya P: Imaging neural activity in the ventral nerve cord of behaving adult Drosophila. Nat Commun 2018, 9:4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Aymanns F, Chen CL, Ramdya P: Descending neuron population dynamics during odor-evoked and spontaneous limb-dependent behaviors. Elife 2022:11. * Functional imaging using GCaMP in the neck connective in tethered, behaving flies shows correlations with higher-order states such as walking or grooming.
- 92.Brezovec LE, Berger AB, Druckmann S, Clandinin TR: Mapping the neural dynamics of locomotion across the Drosophila brain. bioRxiv 2022. 2022.2003.2020.485047. [DOI] [PubMed] [Google Scholar]
- 93.Aimon S, Cheng KY, Gjorgjieva J, Grunwald Kadow IC: Global change in brain state during spontaneous and forced walk in Drosophila is composed of combined activity patterns of different neuron classes. Elife 2023:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aimon S, Katsuki T, Jia T, Grosenick L, Broxton M, Deisseroth K, Sejnowski TJ, Greenspan RJ: Fast near-whole-brain imaging in adult Drosophila during responses to stimuli and behavior. PLoS Biol 2019, 17, e2006732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dombeck DA, Reiser MB: Real neuroscience in virtual worlds. Curr Opin Neurobiol 2012, 22:3–10. [DOI] [PubMed] [Google Scholar]
- 96.Trimarchi JR, Schneiderman AM: Different neural pathways coordinate Drosophila flight initiations evoked by visual and olfactory stimuli. J Exp Biol 1995, 198:1099–1104. [DOI] [PubMed] [Google Scholar]
- 97.Yu JY, Kanai MI, Demir E, Jefferis GS, Dickson BJ: Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol 2010, 20:1602–1614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


