Hematopoietic stem cells (HSC) are multipotent cells capable of unlimited self-renewal and are essential for production of blood and immune cells throughout life. HSC reside in a quiescent state in the bone marrow and proliferate only after certain stimuli. Failure in wakening these quiescent cells may result in hematologic defects, and, therefore, this process is tightly regulated by multiple signaling pathways. Recent research suggests that Ser/ Thr protein phosphatases may be involved in HSC biology more than previously anticipated.
In this issue, Lu and colleagues show that protein phosphatase PPM1B controls homeostasis of HSC through regulation of the Wnt/b-catenin signaling pathway. Using a transgenic Ppm1bCKO mouse model with VAV-Cre mediated conditional deletion of exon 2 of the Ppm1b gene in hematopoietic cells, they showed that PPM1B is necessary for proliferation of HSC.1 Impaired functionality of HSC in Ppm1bCKO animals was further demonstrated by limiting dilution assays and serial transplantation experiments. Data from the animal model were recapitulated in vitro using the small molecule inhibitor of PPM1B (HN2522), as well as by depletion of PPM1B by RNA interference. In addition, Ppm1bCKO mice also exhibited alterations in common lymphoid progenitors, which resulted in B-cell leukocytopenia, whereas the myeloid lineage was unaffected. Furthermore, RNAseq analysis from Lineage- Sca-1+ c-Kit+ (LSK) hematopoietic stem and progenitor cells revealed that several signaling pathways, including WNT, were dysregulated in Ppm1bCKO animals. In particular, several downstream targets of β-catenin, including Fzd1, Jun, Camk2b, Lrp5, Ccnd1 and Gpc4, were down-regulated upon the deletion of Ppm1b suggesting that the defect in HSC may be caused by suppression of WNT signaling. Indeed, LSK cells from Ppm1bCKO animals showed increased levels of the inactive form of β-catenin, which is phosphorylated at Ser33/37/Thr41. Finally, the Authors nicely demonstrated that stimulation of the WNT pathway by BML-284 rescued the phenotypes in Ppm1bCKO mice, supporting the conclusion that PPM1B controls HSC through stimulation of the WNT pathway.
PPM1B belongs to a conserved PP2C family of Ser/Thr phosphatases that require binding of manganese/magnesium ions for their activity. They function as single subunit enzymes and their substrate specificity is influenced by internal linker regions. Interestingly, other members of this conserved phosphatase family have also been implicated in hematopoiesis. In particular, mitochondrial PPM1K was proposed to regulate branched amino acid catabolism in HSC and loss of PPM1K impaired maintenance of the HSC pool.3 In addition, loss of the nuclear PPM1D (also called WIP1) phosphatase caused mTORC-dependent expansion of the HSC compartment in Ppm1d-/- animals, whereas truncating gain-of-function mutations in PPM1D reduced self-renewal of HSC.4,5 Interestingly, the truncated PPM1D stimulates HSC survival after genotoxic stress by inhibiting the p53 pathway and can promote therapy-induced acute myeloid leukemia.5,6 Altogether, these observations point at the crucial role of Ser/Thr phosphatases in the regulation of HSC properties and their potential contribution to the development of hematologic disorders. In fact, several phosphatases have been implicated in hematologic malignancies as well as in solid tumors, and may represent attractive pharmacological targets in future clinical interventions. The development of small molecule inhibitors to protein phosphatases is challenging, and few compounds show satisfactory specificity and efficiency in cellular and animal models. For example, the selective PPM1D inhibitor GSK2830371 suppressed the growth of p53-positive cancer cells in vitro and in animal models.5 -7 In the present study, Lu and colleagues used a new PPM1B inhibitor (HN252), proved its efficiency in animal models, and elegantly validated its specificity in the Ppm1bCKO animals.1,2 This tool will be invaluable for exploring the functions of PPM1B in several systems. Additionally, considering the B-cell leukocytopenia observed in Ppm1bCKO mice, it might be interesting to investigate the potential of PPM1B inhibitor in suppressing B-cell leukemias.
Figure 1.
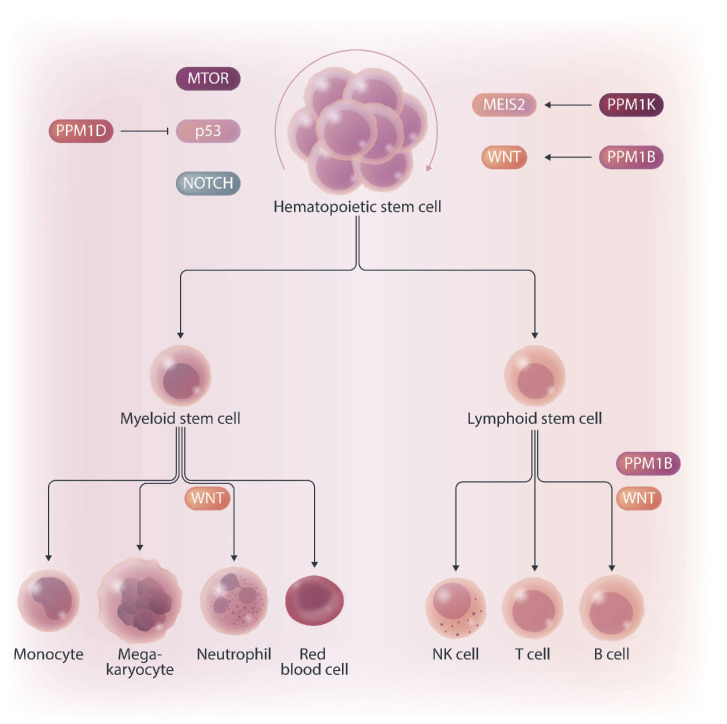
PP2C family protein phosphatases regulating the hematopoiesis. PPM1B and PPM1K promote self-renewal of hematopoietic stem cells (HSC) through activating WNT/b-catenin and MEIS2 pathways, respectively. In contrast, PPM1D promotes differentiation of HSC by modulating MTOR, p53 and NOTCH pathways. In addition, PPM1B regulates production of B cells but, surprisingly, is not needed in myeloid lineage.
Mechanistically, Lu and colleagues point at deregulation of the Wnt/β-catenin signaling pathway as the underlying cause of the B-cell phenotype present in Ppm1bCKO mice. Nevertheless, the role of the Wnt/β-catenin signaling pathway in the hematopoietic system is controversial, and discrepancies are justified by the use of different models and approaches.8 Here, Lu and colleagues bring into the field a new player, PPM1B, which by its dephosphorylating action can promote the active form of β-catenin, and thus enhance the activity of this pathway. However, while β-catenin is critical for T-cell,9 B-cell,10 and granulocytic development,11,12 they report alterations only in the B-cell compartment. Thus, we can hypothesize that PPM1B does not act in myeloid progenitors or T cells, or that it does not regulate β-catenin phosphorylation uniformly in all hematopoietic cells. Ultimately, since the Wnt/β-catenin signaling pathway is regulated at multiple levels and is subjected to spatiotemporal regulations, in the future it will be interesting to investigate the impact of PPM1B on this signaling pathway also in other tissues besides the bone marrow.
In summary, Ser/Thr protein phosphatases of the PP2C family are now emerging as new regulators of hematopoiesis and potential pharmacological targets. Their possible clinical use will depend on the development and careful validation of selective small molecule inhibitors.
References
- 1.Lu Z, Yu H, Li Y, et al. Phosphatase, Mg2+/Mn2+ dependent 1B regulates the hematopoietic stem cells homeostasis via the Wnt/ b-catenin signaling. Haematologica. 2024;109(7):2144-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu Z, Xiao P, Zhou Y, et al. Identification of HN252 as a potent inhibitor of protein phosphatase PPM1B. J Cell Mol Med. 2020;24(22):13463-13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X, Zhang F, Zhang Y, et al. PPM1K regulates hematopoiesis and leukemogenesis through CDC20-mediated ubiquitination of MEIS1 and p21. Cell Rep. 2018;23(5):1461-1475. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Yi W, Morita Y, et al. Wip1 deficiency impairs haematopoietic stem cell function via p53 and mTORC1 pathways. Nat Commun. 2015;6:6808. [DOI] [PubMed] [Google Scholar]
- 5.Burocziova M, Danek P, Oravetzova A, Chalupova Z, Alberich-Jorda M, Macurek L. Ppm1d truncating mutations promote the development of genotoxic stress-induced AML. Leukemia. 2023;37(11):2209-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller PG, Sperling AS, Mayerhofer C, et al. PPM1D modulates hematopoietic cell fitness and response to DNA damage and is a therapeutic target in myeloid malignancy. Blood.2023;142(24):2079-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilmartin AG, Faitg TH, Richter M, et al. Allosteric Wip1 phosphatase inhibition through flap-subdomain interaction. Nat Chem Biol. 2014;10(3):181-187. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter KA, Thurlow KE, Craig SEL, Grainger S. Wnt regulation of hematopoietic stem cell development and disease. Curr Top Dev Biol. 2023;153:255-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen JM. Deletion of beta-catenin impairs T cell development. Nat Immunol. 2003;4(12):1177-1182. [DOI] [PubMed] [Google Scholar]
- 10.Ranheim EA, Kwan HCK, Reya T, Wang Y-K, Weissman IL, Francke U. Frizzled 9 knock-out mice have abnormal B-cell development. Blood. 2005;105(6):2487-2494. [DOI] [PubMed] [Google Scholar]
- 11.Danek P, Kardosova M, Janeckova L, et al. β-Catenin-TCF/LEF signaling promotes steady-state and emergency granulopoiesis via G-CSF receptor upregulation. Blood. 2020;136(22):2574-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hérault A, Binnewies M, Leong S, et al. Myeloid progenitor cluster formation drives emergency and leukaemic myelopoiesis. Nature. 2017;544(7648):53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]