Splenic marginal zone lymphoma (SMZL) is a rare histotype of non-Hodgkin lymphoma, accounting for only 2% of all cases.1 At diagnosis, up to 30% of patients may be asymptomatic, while others may present with cytopenia(s), abdominal lymph node swelling, splenomegaly, B-symptoms, and secondary autoimmune diseases.2 SMZL follows an indolent course that, similarly to other indolent lymphomas, can be complicated by the histological evolution into diffuse large B-cell lymphoma (DLBCL).3 After the diagnosis of SMZL, the risk of lymphoma-related death increases significantly, especially in patients who experience progression within the first 24 months.4 However, the 5-year conditional survival of the remaining patients is comparable to that of the general population,3 and the overall survival (OS) exceeds 10 years. Rituximab immunotherapy is a commonly used first-line treatment due to its effectiveness and mild toxicity.5-6 Although splenectomy is an effective procedure, it can lead to severe and potentially fatal acute and late complications.5 Additionally, the role of bendamustine-rituximab chemoimmunotherapy has yet to be defined, and the preferred first-line treatment is undetermined due to the lack of randomized studies.
It is unclear if the quality of clinical response, complete versus partial (CR vs. PR), correlates with the time-related outcomes. Additionally, insufficient evidence prevents evaluating the prognostic role of acquiring an undetectable minimal residual disease (MRD) status.7-8 Given this context, the IELSG conducted the BRISMA-IELSG36 phase II study (EudraCT number: 2011-000880-28; clinicaltrails gov. Identifier: NCT02853370). Approval by local ethics committees and written informed consent by all participants before study entrance was required. Through a series of 56 SMZL patients, the primary study objective aimed to assess the efficacy and toxicity of combining bendamustine with rituximab (BR) as a first-line therapy.9 Herein, we present the updated results integrated with MRD data (median follow-up [FU]: 69 months; 95% confidence interval [CI]: 67-72).
Eligible patients needed to exhibit active/symptomatic disease, and the clinical responses scored according to the criteria described below, proposed by the Splenic Lymphoma Study Group (SLSG)10 for non-splenectomized patients: partial response (PR): ≥50% improvement in the disease manifestations including resolution or decrease in spleen size, improvement on cytopenias and resolution or decrease in lymphadenopathy. Bone marrow (BM) should show a reduction in lymphoid infiltration; complete response (CR): resolution of organomegaly, normalization of the blood counts (Hb>12 g/dL, platelets >100x109/L; neutrophils >1.5x109/L and no evidence of circulating clonal B cells); no evidence of lymphoma in BM through immunohistochemistry. Online Supplementary Table S1 compares the SLSG and Lugano response criteria for non-PET-avid NHL.
In addition, we adopted the term “unconfirmed complete response” (CRu).6;9 CRu describes patients who still exhibit some degree of cytopenia and splenomegaly at the end of treatment (EOT) but who subsequently meet the criteria for CR at the first FU visit.9 The MRD analysis was performed centrally in a EuroMRD standardized laboratory (https:// euromrd.org/) by droplet digital polymerase chain reaction (ddPCR) with ASO primers targeting IGH rearrangements in the BM and peripheral blood (PB) samples, expressed as copies out 250 ng of genomic DNA as previously described.11 The MRD assay was considered reliable when reaching a sensitivity of at least 1x10-4, calculated considering the tumor infiltration at baseline. Accordingly, in each ddPCR experiment, the “10-1” (as control) and the “10-4” dilution points were included.
Patients with a molecular marker underwent MRD assessment after three cycles (early restaging [ER]) at EOT, and yearly at each annual FU assessment.
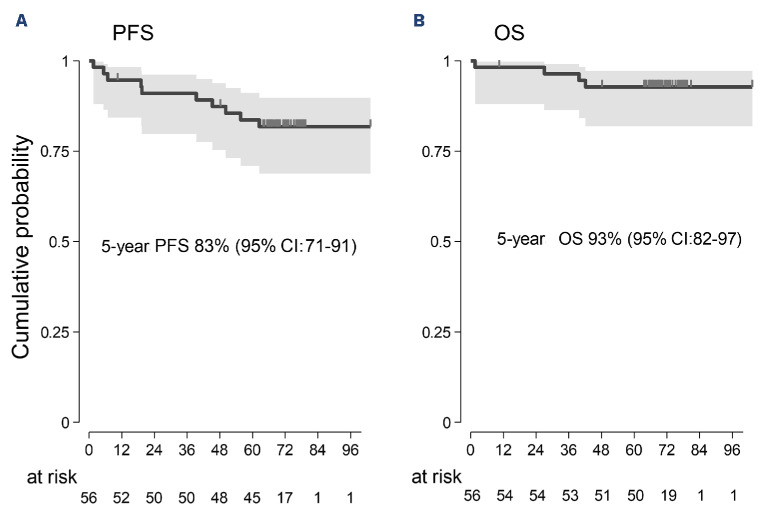
Clinical information has been updated for all living patients except for two. Presenting features are shown in Online Supplementary Table S2. The median age was 66 years, and 41 patients (73%) were older than 60 years. Fifty-one patients (91%) achieved a major response (34 CR; 7 CRu; 10 PR). All the CRu patients and one in stable disease (SD) at EOT improved slowly and steadily, meeting the criteria for CR without additional treatment. Progression-free survival (PFS) events included three progressions documented in patients scored in SD at EOT, six relapses, and one death (due to sepsis at 1,6 months). According to the intention-to-treat analysis, the 5-year PFS was 83% (95% CI: 71-91), and the OS was 93% (95% CI: 82-97) (Figure 1A, B). These results are nearly identical to those achieved with six weekly infusions of rituximab followed by 1-2 years of maintenance therapy.6 None of the ten patients in PR progressed during the median observation time of 69 months (range 64-103). At baseline, 53 of 56 patients assessable for MRD had at least one FU sample and were screened for a monoclonal IGH rearrangement. A reliable MRD molecular marker was found in 42 of 53 (79%) patients, consistent with the literature.8 The ASO assay achieved adequate sensitivity for MRD analysis (at least 10-4) in 37 of 53 (70%) cases. Among these 37 cases, 14 were identified in both BM and PB samples, 18 in PB only, and five in BM only (Figure 2B).
Figure 1.
Progression-free survival and overall survival at 5 years with a median observation time of 69 months. (A) Progression-free survival (PFS). (B) Overall survival (OS). CI: confidence interval.
MRD data were available in five of ten PR patients; at EOT, four were MRD-, and one was MRD+. Four of six CR patients who relapsed had MRD EOT assessment: three were MRD+ and one MRD-. Figure 2A shows the clinical response, MRD status at different time points, and outcome for each of the 37 evaluable patients. The baseline MRD burden was not associated with presenting features, PFS, and OS (P>0.5). Four patients died: one due to sepsis and three due to lymphoma at 1.6, 27, 40 and 42 months, respectively. Two cases of G>2 infections occurred during treatment,9 and three cases (2 acute bronchitis, 1 lung tuberculosis) occurred during the follow-up. Two hundred and eighty-four cycles were administered during the study, with 85% given at full dose. Bendamustine dose was reduced in four patients in cycles 4-6. Relative dose intensity was 0.99 for bendamustine and 0.98 for rituximab.
Seven patients developed a second primary cancer (SPC); the SPC includes one follicular thyroid cancer, one DLBCL, one renal-cell carcinoma, one basal cell carcinoma, one 5q- myelodysplastic syndrome, one malignant peripheral nerve sheath tumor, and one prostate adenocarcinoma. The 5-year cumulative incidence rate of SPC was 11%.
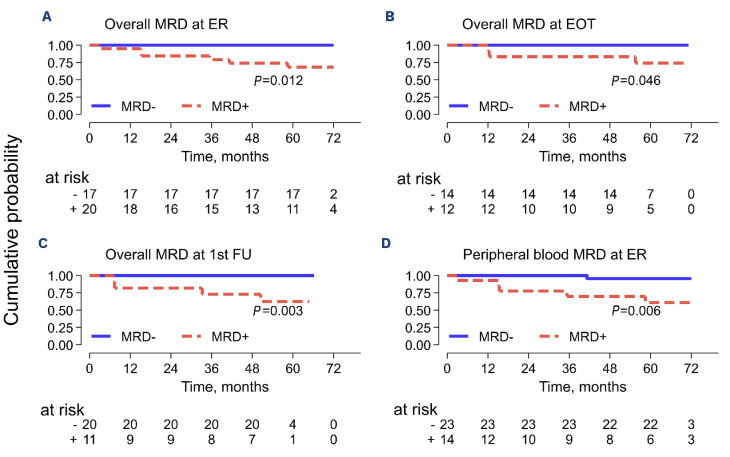
BR treatment resulted in high rates of undetectable MRD: 40% at the ER (17/37), 54% (14/26) at the EOT, and 65% (20/31) after 1 year (Figure 2B). Interestingly, a significant reduction in MRD had already been attained at ER assessment (median 4.7x10-5 in BM and below the quantitative level in PB, respectively). The additional BR courses (1/3 CR patients and 3/9 PR patients) did not contribute to a significant further reduction in residual MRD levels (median 1.4x10-5 in BM and BQL in PB, respectively) (Online Supplementary Figure S1A, B). MRD+ predicted a statistically significant inferior PFS at each evaluated timepoint (ER, EOT, and FU1) (Figure 3A-C). Notably, acquiring early MRD- status in non-invasive peripheral blood samples was associated with a significant improvement in PFS (MRD- vs. MRD+ 100% vs. 68%; 95% CI: 35-86; P=0.006) (Figure 3D). Our analysis of MRD assessment aligns with published data for rituximab-chemotherapy-treated SMZL patients.7-8 Cervetti et al. generated MRD data assessing by real-time quantitative PCR (RQ-PCR) IGH rearrangements in a retrospective series of 50 SMZL (EOT MRD- 48%; PFS: 100% vs. 73%; P=0.023).8 Lyu et al. investigated MRD status by employing multi-color flow cytometry (MFC) in a prospective series of 71 patients (EOT MRD- 77%; PFS: 74,8±6,5% vs. 31,4±12,6%; P<0.001).7 In our study, the EOT MRD status in a landmark analysis was significantly associated with PFS (Figure 3B), while no difference in PFS was observed between CR and PR groups (P=0.438; data not shown). Three MRD+ patients slowly converted to unmeasurable MRD- status, and seven CRu and one SD patient eventually met the criteria for CR at FU1 12 months after completion of treatment. None of the patients received off-protocol treatments or maintenance therapy. A speculative explanation for the spontaneous conversion to MRD- might be the gradual elimination of minimal subsets of residual neoplastic cells by the restored activity of the immune system after EOT. Accordingly, the MRD levels of these three patients were below 1x10-4 (namely 2, 2, and 3 copies/50,000 cells). In conclusion, the BRISMA study substantiates that BR chemoimmunotherapy accomplishes a swift and significant reduction in tumor bulk for patients with symptomatic SMZL, thereby facilitating notably high 5-year PFS and OS rates. Other retrospective studies have reported comparable PFS and OS rates with less toxic treatments.6;13 However, this phase II study with extended FU provides compelling evidence for considering bendamustine-rituximab as an effective treatment for symptomatic SMZL patients.
Figure 2.
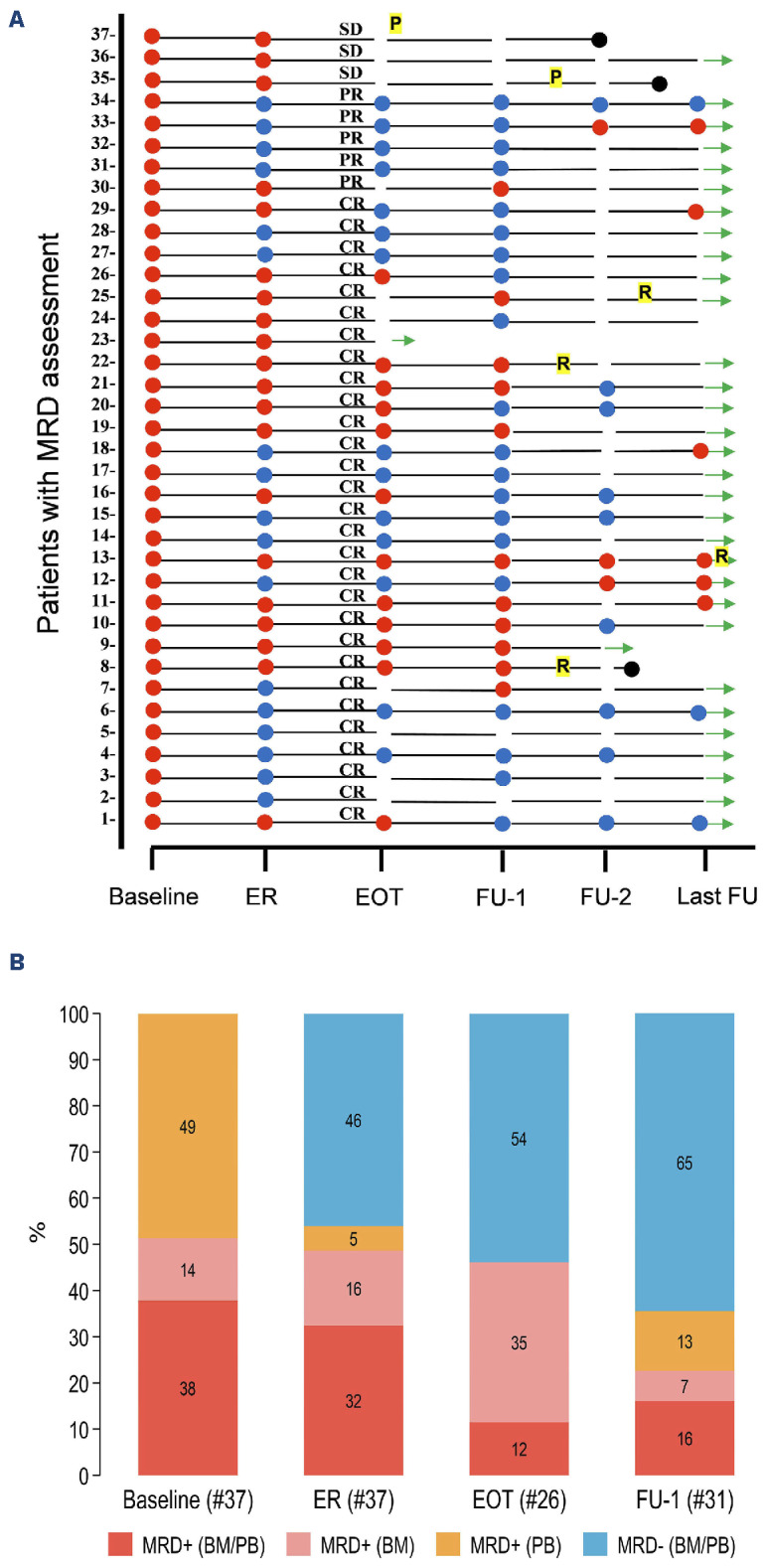
Clinical response and undetectable minimal residual disease rate at different time points. (A) Individual minimal residual disease (MRD) status, clinical response, and outcome at different times. (B) Undetectable MRD rate at different time points. ER: early restaging at 4 months; EOT: end of treatment at 6 months; FU-1: 1-year follow-up; FU2: 2-year follow-up; Last-FU: last available control. Red dot: MRD+ (at least 1 MRD-positive sample, either bone marrow [BM] or peripheral blood [PB]); blue spot: MRD- (unmeasurable MRD); black dot: dead; CR: complete remission; PR: partial remission; SD: stable disease; R: relapse; P: progression; green arrow: alive at last FU.
Figure 3.
Landmark progression-free survival analysis stratified by minimal residual disease evaluated at different time points. (A) Overall minimal residual disease (MRD) at early restaging after 3 bendamustine rituximab courses (ER). (B) Overall MRD at end of treatment (EOT). (C) Overall MRD at 1-year follow-up (FU-1). (D) Peripheral blood MRD at ER. PFS: progression-free survival; overall MRD: at least 1 MRD-positive sample (either bone marrow or peripheral blood); MRD-: unmeasurable MRD.
Three other informative results come from the MRD analysis: i) our findings support the assertion that attaining MRD- after just three courses of treatment is a promising indicator of favorable long-term outcomes, even when monitored in PB.7-8,12 Though data are not yet mature enough to use MRD status in clinical decisions, it could help stratify patients in future trials. Furthermore, ii) integrating MRD results with response criteria is worth exploring due to the imaging limitations in detecting the disease in the spleen.14 Finally, iii) the incidence of additional cancers in this series aligns well with the cumulative incidence rate documented in existing literature.15 Consequently, we recommend conducting an SPC assessment before initiating a BR treatment and throughout the FU period, particularly in elderly patients.
Supplementary Material
Funding Statement
Funding: This research received an unrestricted contribution from Mundipharma.
Data-sharing statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
References
- 1.Piris MA, Isaacson PG, Swerdlow SH, et al Splenic marginal zone lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed. Lyon: IARC Press; 2017. p. 223-225. [Google Scholar]
- 2.Arcaini L, Rossi D, Paulli M. Splenic marginal zone lymphoma: from genetics to management. Blood. 2016;127(17):2072-2081. [DOI] [PubMed] [Google Scholar]
- 3.Kalashnikov I, Tanskanen T, Viisanen L, et al. Transformation and survival in marginal zone lymphoma: a Finnish nationwide population-based study. Blood Cancer J. 2023:13(62):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luminari S, Merli M, Rattotti S, et al. Brief report early progression as a predictor of survival in marginal zone lymphomas: an analysis from the FIL-NF10 Study. Blood. 2019;134(10):798-801. [DOI] [PubMed] [Google Scholar]
- 5.Zucca E, Arcaini L, Buske C, et al. Marginal zone lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(1):17-29. [DOI] [PubMed] [Google Scholar]
- 6.Kalpadakis C, Pangalis GA, Sachanas S, et al. Rituximab monotherapy in splenic marginal zone lymphoma: prolonged responses and potential benefit from maintenance. Blood. 2018;132(6):666-670. [DOI] [PubMed] [Google Scholar]
- 7.Lyu R, Wang T, Wang Y, et al. Undetectable minimal residual disease is an independent prognostic factor in splenic marginal zone lymphoma. Br J Haematol. 2021;194(5):862-869. [DOI] [PubMed] [Google Scholar]
- 8.Cervetti G, Galimberti S, Pelosini M, Ghio F, Cecconi N, Petrini M. Significant efficacy of 2-chlorodeoxyadenosine and rituximab in the treatment of splenic marginal zone lymphoma (SMZL): extended follow-up. Ann Oncol. 2013;24(9):2434-2438. [DOI] [PubMed] [Google Scholar]
- 9.Iannitto E, Bellei M, Amorim S, et al. Efficacy of bendamustine and rituximab in splenic marginal zone lymphoma: results from the phase II BRISMA/IELSG36 study. Br J Haematol. 2018;183(5):755-765. [DOI] [PubMed] [Google Scholar]
- 10.Matutes E, Oscier D, Montalban C, et al. Splenic marginal zone lymphoma proposals for a revision of diagnostic, staging and therapeutic criteria. Leukemia. 2008;22(3):487-495. [DOI] [PubMed] [Google Scholar]
- 11.Drandi D, Alcantara M, Benmaad I, et al. Droplet digital PCR quantification of mantle cell lymphoma follow-up samples from four prospective trials of the European MCL Network. Hemasphere. 2020;4(2):e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bommier C, Lambert J, Nowakowski G, Zucca E, Thieblemont C. What prognostic markers should be evaluated in marginal zone lymphoma? A survey among leading international experts. Hemasphere. 2022;6(2):e680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Else M, Marín-Niebla A, de la Cruz F, et al. Rituximab, when used alone or in combination, is superior to other treatment modalities in splenic marginal zone lymphoma. Br J Haematol. 2012;159(3):322-328. [DOI] [PubMed] [Google Scholar]
- 14.Albano D, Giubbini R, Bertagna F. 18F-FDG PET/CT in splenic marginal zone lymphoma. Abdom Radiol. 2018;43(10):2721-2727. [DOI] [PubMed] [Google Scholar]
- 15.Iannitto E, Minardi V, Callea V, et al. Assessment of the frequency of additional cancers in patients with splenic marginal zone lymphoma. Eur J Haematol. 2006;76(2):134-140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.