Abstract
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), a cellular enzyme involved in glycolysis, binds specifically to several viral RNAs, but the functional significance of this interaction is uncertain. Both GAPDH and polypyrimidine tract binding protein (PTB) bind to overlapping sites in stem-loop IIIa of the internal ribosome entry site (IRES) of Hepatitis A virus (HAV), a picornavirus. Since the binding of GAPDH destabilizes the RNA secondary structure, we reasoned that GAPDH may suppress the ability of the IRES to direct cap-independent translation, making its effects antagonistic to the translation-enhancing activity of PTB (D. E. Schultz, C. C. Hardin, and S. M. Lemon, J. Biol. Chem. 271:14134–14142, 1996). To test this hypothesis, we constructed plasmids containing a dicistronic transcriptional unit in which the HAV IRES was placed between an upstream GAPDH-coding sequence and a downstream Renilla luciferase (RLuc) sequence. Transfection with this plasmid results in overexpression of GAPDH and in RLuc production as a measure of IRES activity. RLuc activity was compared with that from a control, null-expression plasmid that was identical except for a frameshift mutation within the 5′ GAPDH coding sequence. In transfection experiments, GAPDH overexpression significantly suppressed HAV IRES activity in BSC-1 and FRhK-4 cells but not in Huh-7 cells, which have a significantly greater cytoplasmic abundance of PTB. GAPDH suppression of HAV translation was greater with the wild-type HAV IRES than with the IRES from a cell culture-adapted virus (HM175/P16) that has reproducibly higher basal translational activity in BSC-1 cells. Stem-loop IIIa RNA from the latter IRES had significantly lower affinity for GAPDH in filter binding experiments. Thus, the binding of GAPDH to the IRES of HAV suppresses cap-independent viral translation in vivo in African green monkey kidney cells. The enhanced replication capacity of cell culture-adapted HAV in such cells may be due in part to reduced affinity of the viral IRES for GAPDH.
Hepatitis A virus (HAV) is a plus-strand RNA virus that is classified within the genus Hepatovirus of the family Picornaviridae. The genomic RNA of HAV contains a single long open reading frame encoding a polyprotein which is proteolytically processed by a virus-encoded proteinase (27). Like other picornavirus RNAs, it has a relatively lengthy 5′-terminal nontranslated RNA segment (5′NTR) and a shorter 3′NTR with a 3′ terminal poly(A) tail. As in all picornaviruses, the 5′NTR of HAV forms a highly ordered RNA structural element (the internal ribosome entry site [IRES]) that is responsible for directing the cap-independent translation of the polyprotein (5, 23, 32). This occurs by a unique mechanism that involves internal entry of the ribosome on the RNA hundreds of bases downstream of the 5′ end and is absolutely dependent on the secondary and probably the tertiary structure of the 5′NTR. Although HAV can be propagated in conventional cell culture systems, the replication of wild-type (wt) virus is slow and leads to persistent infection in most cases without a cytopathic effect (1, 9, 35). However, cell culture adaptation occurs as the result of continued passage of virus (7, 19, 24). Generally, it is accompanied by more efficient replication and increased yields of virus. Several cell culture-adapted HAV variants are attenuated for their ability to induce liver injury in primates, although cell culture adaptation and attenuation are distinct phenotypes (29, 40, 42).
The genomes of several cell culture-adapted HAV strains have been completely sequenced (7, 18, 24). One of these cell culture-adapted viruses, HM175/P16, differs from its wt parent, HM175/wt, by only 19 nucleotide (nt) substitutions scattered throughout the entire genome (24). In general, mutations within the P2 region of the genome (nonstructural proteins) and the 5′NTR of cell culture-adapted viruses are important in enhancing the capacity of these viruses to replicate in cultured primate cells (11, 13, 15). In particular, mutations within the 5′NTR of HM175/P16 (at nt 152, 203 to 204, and 687) confer cell-type-specific enhancement in viral translation and replication (10, 11, 37). A number of studies suggest that efficient translation by IRES elements requires not only canonical translation initiation factors but also noncanonical host factors (2–4, 17, 20, 22, 28, 31). Therefore, it is likely that these 5′NTR mutations enhance translation by altering the affinity of the RNA for cellular proteins that either positively or negatively influence the activity of the IRES.
In previous studies, we identified cellular proteins of 30, 39, 57, and 110 kDa (p30, p39, p57, and p110) which interact specifically with RNA segments within the HAV IRES (6). p39 was the dominant RNA binding protein present in the ribosomal salt wash fraction (RSW) of HAV-permissive BSC-1 cells (African green monkey kidney cells) and FrhK-4 cells (fetal rhesus kidney cells). In contrast, p57 rather than p39 was the dominant RNA binding protein in rabbit reticulocyte lysates as well as RSW prepared from HeLa cells and Huh-7 (human hepatocellular carcinoma) cells (6; K. H. Chang, W. Klymstra, and S. M. Lemon, unpublished data). p57 was shown to be polypyrimidine tract binding protein (PTB), since it reacted with anti-PTB antibody in an immunoblot analysis (6), while the p39 protein was subsequently purified and identified as the cellular glycolytic enzyme, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (36). GAPDH and PTB compete with each other for binding to overlapping sites on this RNA segment, which forms the 5′-most RNA structure in the HAV IRES (5).
GAPDH has generally been considered to be a housekeeping protein involved mainly in glycolysis, but a number of recent studies indicate that it is multifunctional, possibly also playing roles in endocytosis, DNA replication, DNA repair, and RNA transport and/or translation (39). GAPDH specifically binds several cellular RNAs including the AU-rich 3′NTR of human lymphokine mRNA, as well as human tRNA (30, 38). In addition, after our demonstration of the specific binding of GAPDH to the 5′NTR of HAV, the enzyme was shown to bind specifically to 3′NTR sequences of human parainfluenza virus and hepatitis C virus as well as the RNA pregenome of hepatitis B virus (12, 34, 45). All of these RNAs appear to bind to GAPDH within the NAD+ binding groove of the enzyme (30, 36). Using circular dichroism spectropolarimetry, we found that the binding of GAPDH to stem-loop IIIa RNA of HAV resulted in significant destabilization of RNA secondary structure, suggesting that this interaction would be detrimental to IRES-directed translation (36). However, the functional impact of the binding of GAPDH to the HAV IRES has been difficult to demonstrate due to the ubiquitous nature and high constitutive expression levels of the enzyme.
Stem-loop IIIa of the cell culture-adapted HM175/P16 virus contains a 2-nt UU deletion within a 5-nt oligo(U) tract in the wt virus (nt 200 to 204), which contributes to both the enhanced IRES activity and greater replication capacity that characterizes this virus in BSC-1 cells (11, 37). GAPDH preferentially binds U-rich segments of RNA (31, 37), making it likely that this mutation reduces the affinity of this RNA segment for GAPDH. Since GAPDH is the dominant protein in lysates of BSC-1 cells that is bound by this stem-loop of the IRES (36), we have reasoned that this mutation may be selected during the adaptation of the virus to growth in BSC-1 cells because it protects the IRES against the structure-destabilizing effects of GAPDH and thus promotes both cap-independent translation and, secondarily, viral replication.
Here, we report experiments that provide insight into how the binding of GAPDH to HAV RNA influences IRES function and how this phenomenon might relate to mutations that are selected for within the IRES during the adaptation of HAV to growth in cultured cells. We constructed plasmids containing a dicistronic transcriptional unit in which the HAV IRES was placed between an upstream GAPDH-coding sequence and downstream Renilla luciferase (RLuc) sequence. Transfection with this plasmid results in overexpression of GAPDH and in RLuc production as a measure of IRES activity. As a control, cells were transfected in parallel with a null-expression plasmid that was identical except for a frameshift mutation within the 5′ GAPDH coding sequence. The results of these experiments indicate that GAPDH overexpression significantly suppresses the ability of the HAV 5′NTR to direct cap-independent translation in transfected cells. We also demonstrate that the mutation at nt 203 to 204 in the cell culture-adapted virus specifically reduces the affinity of stem-loop IIIa for GAPDH, supporting the hypothesis that this mutation was selected because it reduces the negative impact of GAPDH on IRES-dependent translation and viral replication.
MATERIALS AND METHODS
Plasmids.
pLuc-wt-RLuc and pLuc-P16-RLuc are plasmids with dicistronic T7 transcriptional units under control of a T7 promoter containing the firefly luciferase (FLuc) coding sequence in the first cistron, followed by the 5′ NTRs of wt HAV or cell culture-adapted HM175/P16 virus fused to the RLuc sequence in the second cistron. These were constructed by digesting pLUC-wt-CAT and pLUC-HAV-CAT (37) with XbaI and NotI and inserting a PCR-derived RLuc coding sequence originating from pRLHL (21) in lieu of the chloramphenicol acetyltransferase (CAT) gene. pPTB-wt-RLuc and pPTB-P16-RLuc contain a similar dicistronic transcriptional unit under the control of the same CMV/T7 promoter but with the PTB coding sequence in the first cistron. To construct these, the wt-RLuc and P16-RLuc sequences from pLuc-wt-RLuc and pLuc-P16-RLuc were cloned into HindIII and NotI sites downstream of the PTB coding sequence in pPwt/AC (17). pfsPTB-wt-RLuc and pfsPTB-P16-RLuc are identical to these constructs except for a frameshift mutation at codon 87 of the PTB sequence, and they were constructed in a similar fashion using pΔ87-531/AC in lieu of pPwt/AC. pPTB and pfsPTB are monocistronic plasmids expressing the wt and mutated PTB sequences, respectively, under control of the CMV/T7 promoter. These were generated by inserting PTB and fsPTB sequences excised by partial HindIII digestion into the HindIII site of the mammalian expression vector pRc/CMV (InVitrogen).
A plasmid containing the cDNA sequence of human GAPDH (phGAPDH) was obtained from the American Type Culture Collection. The GAPDH sequence was excised by partial digestion with HindIII and NotI and inserted into the HindIII and NotI sites of pRc/CMV. The SacI-SphI fragment from this plasmid, containing the GAPDH sequence and upstream composite T7/CMV promoter, was inserted into pGEM 3Z f(−) (Promega) to construct pGEM-GAPDH. A deletion-frameshift mutation was created following codon 8 of the GAPDH sequence by HincII-BstEII digestion (removing nt 25 to 56 of the GAPDH sequence) followed by blunt ending with mung bean nuclease prior to religation to create pGEM-fsGAPDH. pGAPDH-wt-RLuc, pGAPDH-P16-RLuc, pfsGAPDH-wt-RLuc, and pfsGAPDH-P16-RLuc are dicistronic constructs containing these wt and mutated GAPDH sequences under control of the CMV/T7 promoter upstream of the HAV IRES (wild type or P16) and RLuc sequence in the second cistron. These were constructed by replacing the PTB sequence of pPTB-wt-RLuc and pPTB-P16-RLuc with a fragment containing the appropriate GAPDH coding sequences excised from pGEM-GAPDH and pGEM-fsGAPDH by partial HindIII digestion. Monocistronic variants of these constructs, pGAPDH and pfsGAPDH, were generated by NotI digestion of pGAPDH-wt-RLuc and pfsGAPDH-wt-RLuc followed by religation leading to the removal of the wt-RLuc sequence. The mutations created within the GAPDH and PTB sequences were confirmed by DNA sequence analysis.
pT7-H6TK is a modified vector generated by inserting linker sequences encoding a six-histidine thrombin cleavage site into the NdeI and EcoRI site of pT7-7 (41). For the construction of p(His)6-PTB, pGST-PTB (kindly provided by Mariano Garcia Blanco, Duke University) was digested with BamHI and TthIII and the TthIII site was filled in with Klenow. The resulting fragment was inserted into the BamHI and HindIII sites of pT7-H6TK. The construction of pT7-sIIIa(P16) and pHAVs3a has been described previously (36). pT7-sIIIa(wt) was constructed in a similar fashion, except that the template for the PCR was cDNA derived from the HM175/wt virus (14). Cloning was accomplished using standard methods, and the products of these manipulations were validated by restriction digestion and/or DNA sequencing.
Cells.
The BSC-1 cells used in these experiments were obtained from David Anderson (MacFarlane-Burnett Centre, Melbourne, Australia) because they proved more readily transfectable than BSC-1 cells that had been obtained directly from the American Type Culture Collection. BSC-1 cells were grown in minimal essential medium supplemented with 10% fetal bovine serum and antibiotics. FRhK-4 and Huh-7 cells (37) were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics.
In vitro translation.
In vitro translation reactions were carried out using the TnT Quick Coupled Transcription-Translation system (Promega). Briefly, 1 μg of the plasmid DNA and 2 μl of [35S]methionine (1,000 Ci/mmol at 10 mCi/ml) were added to each 50-μl reaction mix, which was then incubated at 30°C for 90 min. Translated products were analyzed either by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by autoradiography or PhosphorImager (Molecular Dynamics) analysis or by an assay for RLuc enzymatic activity (see below).
DNA transfections and reporter gene assays.
DNA transfections were carried out using FuGENE 6 transfection reagent (Boehringer Mannheim) under conditions recommended by the manufacturer. About 2 × 105 cells in 2 ml of medium were seeded into each well of a six-well plate 1 day prior to transfection. For each transfection, 2 μg of DNA was mixed with 6 μl of FuGENE reagent diluted with 94 μl of OptiMEM (Gibco-BRL) and incubated for 15 min at room temperature. The DNA-FuGENE complex was then added to cells directly. At 24 to 48 h following transfection, the cells were harvested and the reporter protein activity was assayed. RLuc activity was assayed using the Dual-Luciferase reporter assay system (Promega). Briefly, cells were washed twice with phosphate-buffered saline, 500 μl of passive lysis buffer (provided by the manufacturer) was added to each well, and the culture plates were placed at room temperature for 30 min prior to collection of the lysate. A 20-μl aliquot of the lysate was assayed for RLuc activity, with the luminescent signal read using a TD-20/20 luminometer (Turner Designs, Inc.).
Northern analysis.
Total RNA was extracted from BSC-1 cells 48 h posttransfection using RNAqueous (Ambion, Austin, Tex.) as recommended by the manufacturer. The poly(A)+ RNA fraction was purified on Oligotex spin columns (Oligotex mRNA; Qiagen), separated by electrophoresis in a formaldehyde-agarose gel, and blotted onto a BrightStar-Plus nylon membrane (NothernMax; Ambion). The membrane was subsequently hybridized with a GAPDH-specific probe (nt 570 to 980), which was labeled using BrightStar psorlen-biotin nonisotopic labeling kit (Ambion) reagents. The hybridized products were detected using BrightStar Biodetect nonisotopic detection kit (Ambion) reagents after exposure to film (Kodak).
Cell fractionation and immunoblot analysis.
BSC-1, FRhK-4, and Huh-7 cells were transfected with the monocistronic plasmids pGAPDH, pfsGAPDH, pPTB, or pfsPTB, as described above. At 48 h following transfection, cells were removed with a cell scraper and collected by centrifugation at 500 × g. Cell pellets were resuspended in NP-40 lysis buffer (10 mM Tris [pH 7.4], 10 mM NaCl, 3 mM MgCl2, 0.5% [vol/vol] NP-40), vortexed for 10 s, and kept on ice for 10 min. After separation of the nuclei by centrifugation at 500 × g, the supernatant fluid was collected as the cytoplasmic fraction. The nuclear pellet was washed in NP-40 lysis buffer once and centrifuged at 500 × g. The pelleted fraction was used in subsequent analyses.
For immunoblot analysis of transfected cells, 10 μg of the cytoplasmic extract and corresponding nuclear fraction were separated by SDS-PAGE (12.5% polyacrylamide). Following electrotransfer to polyvinylidene difluoride membranes at 100 V for 2 h, membranes were blocked with 5% skim milk in 0.1% Tween in phosphate-buffered saline for 1 h. Following two washes with the same buffer, membranes were probed with either monoclonal anti-GAPDH antibody (Bio Design International) or polyclonal anti-PTB antibody (Intronn LLC) at 1 or 3.2 μg/ml, respectively, for 1 h. The membranes were washed twice and incubated for 40 min with horseradish peroxidase-conjugated secondary antibodies to mouse (anti-GAPDH) or rabbit (anti-PTB) immunoglobulin G. After the membrane was washed thoroughly, proteins were visualized with an enhanced chemiluminescence reagent kit (Amersham International Plc.) as recommended by the manufacturer.
GAPDH and PTB proteins.
Human GAPDH was purchased from Sigma. For the preparation of PTB, Escherichia coli strain BL21(DE3) was transformed with p(His)6-PTB and the recombinant protein was induced by the addition of 250 μM isopropyl-β-d-thiogalactopyranoside (IPTG) for 2.5 h at 30°C. After being harvested, cells were resuspended in a buffer containing 20 mM HEPES (pH 7.9), 0.5 M NaCl, 10% glycerol, 0.2 mM phenylmethylsulfonyl fluoride and lysed at 1,000 lb/in2 in a French press. Lysates were centrifuged at 40,000 rpm for 90 min in a Ti70 rotor (Beckman) to remove membranes. The supernatant fraction was then applied to an Ni-nitrilotriacetic acid resin and subjected to imidazole elution. Purified recombinant PTB was concentrated 40-fold in a buffer containing 80 mM sodium phosphate (pH 7.7), 0.1 mM EDTA, and 10% glycerol. Proteins were kept frozen at −80°C until use.
Stem-loop IIIa RNA probes.
pT7-sIIIa(P16) and pT7-sIIIa(wt) were digested with XbaI, and RNA was transcribed in a runoff reaction with T7 RNA polymerase (Promega). RNA was radiolabeled by the incorporation of [32P]UTP (8,000 Ci/mmol; Amersham). Following digestion with RNase-free RQ1 DNase, the RNA probe was purified on a G-50 column (Boehringer Mannheim).
Filter binding assay.
A filter binding assay, measuring the affinity of the stem-loop IIIa RNA probes for GAPDH and PTB, was developed from similar assays described by Wong and Lohman (44). Briefly, the binding reaction was carried out in binding buffer A (20 mM HEPES [pH 7.6], 10 mM KCl, 10% glycerol, 0.1 mM EDTA, 0.2 mM dithiothreitol, 100 μg of bovine serum albumin per ml) with 10−11 M 32P-labeled HAV stem-loop IIIa RNA (wild type [wt] or P16) and 10−7 to 10−10 M human GAPDH or PTB. The binding-reaction mixtures were incubated for 15 min at room temperature and then passed through a nitrocellulose filter that preferentially retains protein and protein-RNA complexes and a Hybond N+ filter that retains RNA, previously equilibrated in binding buffer B (binding buffer A without bovine serum albumin). The filters were washed with 50 μl of ice-cold binding buffer B, dried, and exposed to X-ray film or subjected to PhosphorImager analysis. Dissociation constants for GAPDH (tetramer form) and PTB (dimer form) were calculated as described by Weeks and Cech (43).
RESULTS
Dicistronic IRES reporter constructs that overexpress GAPDH or PTB.
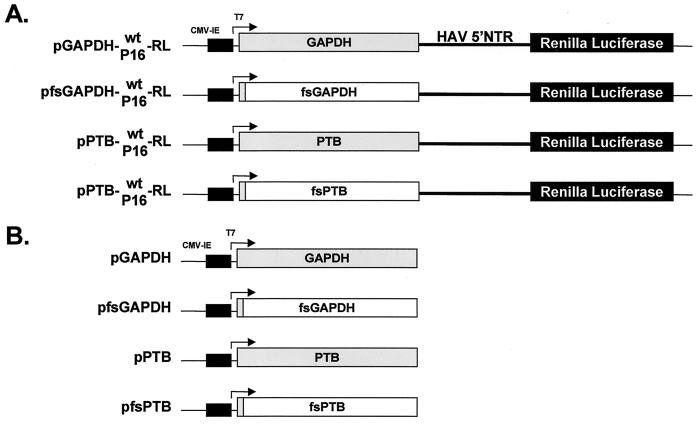
To assess the potential functional significance of the binding of GAPDH to the HAV IRES, we constructed a series of plasmids containing dicistronic transcriptional units under control of the CMV/T7 composite promoter, as depicted in Fig. 1A (pGAPDH-wt-RL and pGAPDH-P16-RL). GAPDH is translated in a cap-dependent manner from the 5′-most open reading frame of the transcripts produced in cells transfected with these plasmids, while RLuc is translated from the downstream reading frame, under control of the HAV IRES, which is placed within the intercistronic space. The proximity of the GAPDH reading frame to the RNA segment that comprises the IRES leads to the synthesis of the protein within the immediate microenvironment of the IRES. This enhances the likelihood that a functional effect of the enzyme on IRES activity would be detected in transient-expression assays. The two dicistronic GAPDH expression vectors (pGAPDH-wt-RL and pGAPDH-P16-RL [Fig. 1A]) contained either the wt or cell culture-adapted HM175/P16 IRES elements, allowing a comparison of the effects of GAPDH overexpression from the upstream reading frame on these two functionally different IRES sequences. As controls for transient-expression experiments, we created two related plasmids, each containing a frameshift mutation following codon 8 of the GAPDH coding sequence (pfsGAPDH-wt-RL and pfsGAPDH-P16-RL) (Fig. 1A). The mutations in these GAPDH “null mutants” ablate the expression of GAPDH but should have little impact on the higher-order structures of the dicistronic RNAs. Using a similar approach but a different reporter protein, we have recently shown that PTB overexpression in BSC-1 cells stimulates cap-independent translation directed by the cell culture-adapted HM175/P16 virus IRES (17). Thus, as a positive control for these experiments, we constructed similar dicistronic reporter plasmids in which the PTB sequence or a frameshift deletion PTB-null mutant replaced the upstream GAPDH reading frame (Fig. 1A). We also constructed monocistronic plasmids expressing only the GAPDH or PTB protein or their frameshift null mutants (Fig. 1B).
FIG. 1.
Schematic representation of GAPDH and PTB expression constructs. Transcription is under control of a composite T7/CMV promoter in all plasmids. (A) Organization of dicistronic plasmids that express GAPDH or PTB from the 5′ reading frame by a cap-dependent translation mechanism and the reporter RLuc from the 3′ reading frame under the translational control of the HAV IRES (wt or HM175/P16), which is placed within the intercistronic space. Frameshift (fs) deletion mutants (“null-expression” vectors) express only a small, N-terminal fragment of GAPDH or PTB but differ by only a few nucleotides from the other constructs (shaded boxes indicate open reading frame segments that undergo translation). (B) Organization of monocistronic plasmids expressing GAPDH or PTB and of related frameshift mutants.
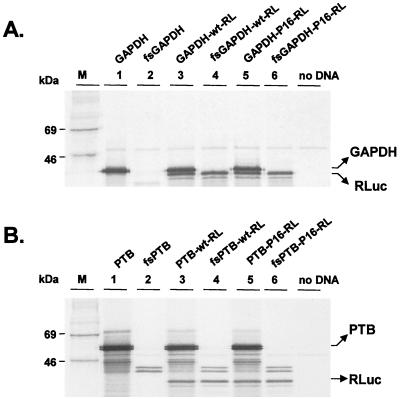
The products of coupled in vitro transcription-translation reactions that were programmed with these plasmid DNAs (20 μg/ml) are shown in Fig. 2. A protein migrating with an apparent mass of about 37 kDa and thus consistent with GAPDH was produced from each of the monocistronic or dicistronic plasmids containing an intact GAPDH reading frame (Fig. 2A, lanes 1, 3, and 5) but not from plasmids containing the frameshift mutation within GAPDH (lanes 2, 4, and 6). A second major protein product that migrated with an apparent mass slightly smaller than that of GAPDH, consistent with RLuc, was also produced from each of the GAPDH-(IRES)-RL dicistronic constructs (lanes 3 to 6). Similar coupled in vitro transcription-translation reactions demonstrated the expected production of a ∼57-kDa protein that migrated in SDS-PAGE as a doublet when reactions were programmed with monocistronic or dicistronic constructs containing an intact PTB reading frame (Fig. 2B, lanes 1, 3, and 5). Much smaller quantities of a smaller protein doublet (∼42 kDa) were evident in reaction mixtures programmed with plasmids containing the PTB frameshift mutation (lanes 2, 4, and 6). This product is likely to reflect the inefficient aberrant initiation of translation at a downstream AUG codon within the PTB reading frame. As with the GAPDH constructs, RLuc was produced under the translational control of the HAV IRES when reaction mixtures were programmed with each of the PTB-(IRES)-RL dicistronic constructs (lanes 3 to 6). These results are thus consistent with the protein products that were expected from these plasmids.
FIG. 2.
SDS-PAGE of protein products of in vitro-coupled transcription and translation reactions carried out in rabbit reticulocyte lysates programmed with the monocistronic and dicistronic plasmids depicted in Fig. 1. (A) Products of translation of the GAPDH expression vectors. The positions of the GAPDH and RLuc proteins are shown on the right. No GAPDH product is evident from translation of fsGAPDH constructs. (B) Translation products from the PTB expression vectors. PTB appeared as a doublet band of about 57 kDa. No PTB product is evident from translation of fsPTB constructs. The intensity of the image in panel B is reduced relative to that in panel A to demonstrate the doublet nature of the PTB bands. Equivalent amounts of RLuc were produced from the two sets of constructs.
Importantly, these studies demonstrated that the expression of neither GAPDH nor PTB from the upstream cistron of these dicistronic transcripts had any functional effect on the activity of the downstream IRES in vitro, since the quantities of the reporter protein produced were not influenced by the frameshift mutation within the upstream GAPDH or PTB reading frame (compare RLuc bands in lanes 3 and 4 and in lanes 5 and 6 in Fig. 2). This is consistent with previous in vitro studies in which we found that IRES activity was not influenced by the expression of PTB from the upstream reading frame of a dicistronic reporter transcript in which bacterial CAT was translated from the downstream reading frame (17). The absence of any impact of GAPDH or PTB overexpression on IRES function may reflect the high endogenous abundance of both proteins in reticulocyte lysates.
GAPDH and PTB have opposing effects on HAV IRES-dependent translation in BSC-1 cells.
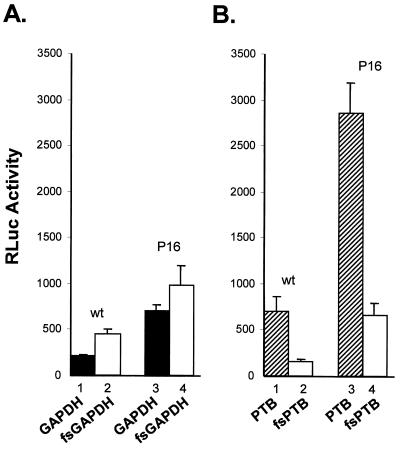
To determine whether GAPDH overexpression would have any effect on IRES-dependent translation in vivo, we transfected BSC-1 cells with the plasmids shown in Fig. 1A. The quantity of RLuc expressed by cells transfected with plasmids containing the wt HAV IRES, pGAPDH-wt-RL, was approximately twofold lower than that expressed by cells transfected with the cognate null mutant, pfsGAPDH-wt-RL (Fig. 3A, compare bars 1 and 2). The suppression of translation was reproducible and highly statistically significant in replicate experiments (relative to the null mutant, mean translation and standard deviation [SD] = 46.7% ± 4.2%; P < 0.001 by the t test). Thus, the overexpression of GAPDH has a negative effect on IRES function in vivo, consistent with the previously noted helix-destabilizing activity of the enzyme in vitro (36). A lesser suppressive effect of GAPDH was evident when cells were transfected with plasmids containing the cell culture-adapted HM175/P16 IRES. However, there was still a significant reduction in IRES activity when GAPDH was expressed (relative to the null mutant, mean translation and SD = 71.3% ± 6.4%; P = 0.02 by the t test) (Fig. 3A, compare bars 3 and 4). Consistent with the greater translational activity of the cell culture-adapted IRES in BSC-1 cells (37), the production of RLuc was significantly greater than in cells transfected with plasmids containing the wt IRES (Fig. 3A, compare bars 1 and 3 and compare bars 2 and 4). These differences, which were also highly statistically significant in repeat experiments, are in good agreement with results of previous studies (37). Since GAPDH is a very abundant cellular protein, the lesser effect of GAPDH overexpression on the cell culture-adapted IRES raises the possibility that its greater basal translational activity in BSC-1 cells may reflect a reduced susceptibility to suppression by endogenous GAPDH, compared with the wt IRES.
FIG. 3.
GAPDH suppresses HAV IRES-dependent translation in BSC-1 cells. BSC-1 cells were transfected with dicistronic plasmids expressing GAPDH (solid bars) or the frameshift GAPDH mutant (open bars) (A) and PTB (shaded bars) or the frameshift PTB mutant (open bars) (B). RLuc was expressed from each construct under control of the wt or HM175/P16 (P16) IRES located within the intercistronic space. At 48 h following transfection, cells were lysed and assayed for RLuc activity. The results shown are means of two independent transfection experiments, each carried out in triplicate (a total of six transfections). The error bars indicate SD.
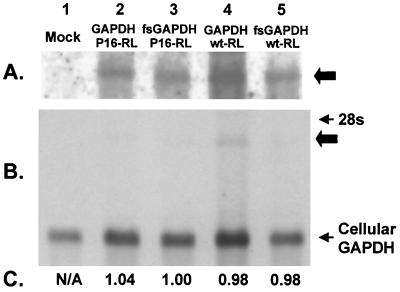
The differences in RLuc expression among the various constructs in Fig. 3A were not likely to reflect differences in transcript abundance, since all four plasmids differ minimally in their primary nucleotide sequence and were prepared, purified, and transfected in parallel. To demonstrate that this is the case, however, we carried out Northern analyses of the poly(A) fraction of RNA extracted from BSC-1 cells that had been transfected with the dicistronic GAPDH expression vectors. These results are shown in Fig. 4. A transcript of the appropriate length that hybridized with a GAPDH-specific probe was present in transfected BSC-1 cells (Fig. 4A and B, lanes 2 to 5) but not in mock-transfected cells (lane 1). This was quantified by densitometry and compared with the endogenous GAPDH transcript (which was present in far greater abundance) as a control for sample loading. These results (Fig. 4C) revealed no differences in the abundance of the GAPDH transcripts produced from the four expression constructs in the transfected cells, confirming that the reduction in RLuc activity observed in Fig. 3A was due to a suppressive effect of GAPDH on IRES-directed translation. In addition, no difference could be detected in the abundance of the reporter transcripts in cells transfected with the expression vector and its paired null expression mutant by using a highly quantitative, real-time reverse transcription-PCR assay (LightCycler; Boehringer) (data not shown).
FIG. 4.
Northern analysis of the poly(A) RNA fraction recovered from BSC-1 cells transfected previously with the GAPDH expression vectors (lanes 2 and 4), their related null-expression mutants (lanes 3 and 5), or mock-transfected cells (lane 1). (A and B) The 24-h (A) and 3-h (B) exposures of the blot, respectively. The thick arrows indicate the location of the dicistronic GAPDH-IRES-RLuc transcript. (C) Normalized ratio of the hybridization signal specific for the endogenous GAPDH transcript (loading control) relative to the dicistronic GAPDH mRNA. N/A, not applicable, since there is no dicistronic transcript present.
These results contrast sharply with previous work in which we demonstrated that PTB overexpression from similar dicistronic transcripts results in an increase in the activity of the cell culture-adapted IRES in BSC-1 cells (17). This is interesting, since PTB and GAPDH compete for binding to identical or overlapping sites on the viral RNA (36). To confirm these earlier results and to determine whether the wt and cell culture-adapted IRES elements respond to PTB differently, as appears to be the case with GAPDH (Fig. 3A), we transfected BSC-1 cells with the dicistronic PTB expression vectors shown in Fig. 1A. Cells transfected with pPTB-wt-RL produced 4.3-fold more RLuc than did cells transfected with the PTB-null mutant, pfsPTB-wt-RL (mean increase and SD = 426% ± 98%; P < 0.001 by the t test) (compare bars 1 and 2 in Fig. 3B), indicating that PTB also stimulates the wt IRES. As with the GAPDH constructs, the cell culture-adapted IRES was severalfold more active than the wt IRES within the context of the PTB constructs (Fig. 3B, compare bars 1 and 3 and compare bars 2 and 4). However, the increase in the activity of the cell culture-adapted IRES when PTB was expressed from the upstream reading frame (compare bars 3 and 4) was proportionate to that observed with the wt IRES (mean increase and SD = 427% ± 50%; P < 0.001 by the t test). These results confirm that GAPDH and PTB have opposing effects on HAV translation but that there is no difference in the effect of PTB on the wt or the cell culture-adapted IRES in transfected BSC-1 cells.
Effect of GAPDH on HAV IRES activity in other cell types.
The experiments summarized in Fig. 3 were carried out with BSC-1 cells, which are derived from African green monkey kidney cells. This is the cell type used for adaptation of the HM175/P16 virus to growth in cell culture, and these cells are maximally permissive for the replication of this virus. To compare the effects of GAPDH and PTB on IRES activity in other cell types, we carried out similar experiments with FRhK-4 and Huh-7 cells. These cell lines are derived from fetal rhesus kidney and human hepatocellular carcinoma, respectively, and both are also permissive for replication of the HM175/P16 virus. The results of these experiments are depicted in Fig. 5, which shows the relative activity of RLuc expressed from each of the dicistronic transcripts in relation to the RLuc activity expressed from the cognate frameshift mutant in each cell type. In this analysis, a relative RLuc activity greater than 1.0 indicates that overexpression of the protein encoded by the upstream cistron (PTB or GAPDH) resulted in a positive effect on translation while a value less than 1.0 indicates translational suppression. As in BSC-1 cells, GAPDH overexpression reduced translation directed by the wt IRES by slightly more than 50% in FRhK-4 cells but had no effect on translation by this IRES in Huh-7 cells (Fig. 5A). On the other hand, PTB expression enhanced translation directed by the wt IRES in both Huh-7 cells and FRhK-4 cells but the extent of enhancement in each cell line was substantially less than in BSC-1 cells (Fig. 5B). The results with Huh-7 cells may reflect a greater abundance of PTB within the cytoplasm of these cells (see below). This could potentially mask the impact of PTB overexpression on IRES activity and could reduce (through competition) the negative effect of GAPDH on IRES activity (see Discussion).
FIG. 5.
Effects of GAPDH overexpression (A) or PTB expression (B) on either the wt (bars 1, 3, and 5) or the HM175/P16 (bars 2, 4, and 6) IRES elements in three different cell lines: BSC-1 (bars 1 and 2), FRhK-4 (bars 3 and 4), and Huh-7 (bars 5 and 6). RLuc activities in cells transfected with dicistronic expression constructs containing either the wt or P16 IRES were normalized to those present in cells transfected in parallel with the corresponding fsGAPDH or fsPTB null-expression plasmids. The results shown are means of the values obtained in two independent transfection experiments, each done in triplicate (a total of six transfections). Error bars indicate SD.
The enhanced translational activity of the HM175/P16 IRES represents one of the mechanisms by which this virus has adapted to growth in African green monkey kidney cells (37). However, previous studies have shown that there is no difference in the activities of these two IRES elements in either FRhK-T7 cells or Huh-T7 cells, which are clonally derived from FRhK-4 and Huh-7 cells, respectively (37). Therefore, it was of interest to compare the effects of GAPDH and PTB overexpression on the function of the wt and cell culture-adapted IRES elements in these different cell lines. As in BSC-1 cells, the overexpression of GAPDH resulted in a greater suppression of the wild-type than the HM175/P16 virus IRES in FRhK-4 cells (Fig. 5A, compare bars 1 and 2 and compare bars 3 and 4). However, this is not the case in Huh-7 cells. Only slight suppression was noted with the HM175/P16 IRES, while no effect was observed with the wt IRES (Fig. 5A, compare bars 5 and 6). On the other hand, in both BSC-1 and FRhK-4 cells, PTB overexpression had similar effects on the wt and cell culture-adapted IRES elements (Fig. 5B, compare bars 1 and 2, and compare bars 3 and 4, respectively). Somewhat greater stimulation of the wt IRES was noted in Huh-7 cells (Fig. 5B, compare bars 5 and 6).
Immunoblot detection of GAPDH and PTB in transfected cells.
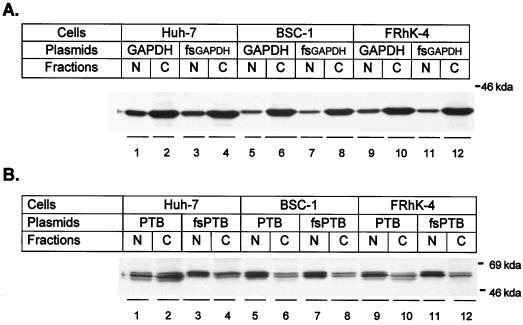
Because of the differences we observed in the effects of GAPDH and PTB overexpression on IRES activities in these three cell lines, we sought to determine the basal abundance and intracellular distribution of these proteins in these cells, as well as the degree of GAPDH and PTB overexpression in transfected cells. This was accomplished by an immunoblot analysis of nuclear and cytoplasmic cell fractions from cells that had been transfected with the monocistronic plasmids shown in Fig. 1B that encode either GAPDH (pGAPDH) or PTB (pPTB) or the frameshift variants of each (pfsGAPDH or pfsPTB). These results are shown in Fig. 6.
FIG. 6.
Enhanced chemiluminescence visualization of immunoblots of nuclear (N) and cytoplasmic (C) extracts prepared from cells that had been transfected 48 h previously with pGAPDH (A) or pPTB (B) or the related null-expression plasmids, pfsGAPDH and pfsPTB. Immunoblots were reacted with either anti-GAPDH antibody (A) or anti-PTB antibody (B).
In all three cell lines (BSC-1, FRhK-4, and Huh-7), GAPDH was localized primarily in the cytoplasmic fraction, although it was also readily detected in the nuclear fractions as well (Fig. 6A). There may have been a slightly greater abundance of GAPDH in the nuclear fraction of Huh-7 cells relative to the other two cell lines. Significantly, there was no noticeable difference in the GAPDH band intensity or the distribution of GAPDH between nuclear and cytoplasmic fractions in lysates of cells that had been transfected with pGAPDH or pfsGAPDH. This probably reflects the large pool of endogenous GAPDH that is present in normal cells (39) and indicates that the GAPDH suppression of IRES activity observed in transfected cells (Fig. 3 and 5) did not result from the expression of supraphysiologic levels of the protein.
PTB was identified in these fractions as a doublet band with an apparent mass of ∼57 kDa (Fig. 6B). Endogenous PTB was localized mainly to the nucleus, as indicated by immunoblots of fractions from cells transfected with the frameshift variant, pfsPTB. This is as expected (16). Significant levels of this protein were also present in cytoplasmic fractions from all three cell types, but Huh-7 cells appeared to have a significantly greater cytoplasmic abundance than did the other cell types (Fig. 6B). Consistent with these results, immunofluorescence microscopy of BSC-1 or Huh-7 cells using anti-PTB as a primary antibody demonstrated bright nuclear staining and much fainter cytoplasmic staining (17), except for dividing cells, in which bright cytoplasmic staining was observed (data not shown). Interestingly, the relative abundance of the individual doublet bands varied among the different cell lines. This difference was particularly evident in the cytoplasmic fractions, in which the doublet bands were of comparable intensity in BSC-1 cells but different intensities in FRhK-4 and Huh-7 cells. The more rapidly migrating PTB species was more abundant in the FRhK-4 cells, while the reverse was the case in Huh-7 cells (Fig. 6B, compare lanes 4 and 12).
In cells that had been transfected with pPTB, a third PTB species was detected in both cytoplasmic and nuclear fractions. This migrated with a slightly smaller apparent mass than did the normal doublet bands (Fig. 6B). This novel PTB band was especially evident within the cytoplasmic fractions of the Huh-7 and FRhK-4 cells but appeared to be mostly translocated to the nucleus in BSC-1 cells (Fig. 6B). Since the human PTB expression vectors used in these studies produced doublet bands when used to program rabbit reticulocyte lysates for translation (Fig. 2B), it is possible that a second, more slowly migrating PTB species expressed from these vectors may have been obscured by the endogenous PTB species. It is not clear why the human PTB expressed from the transfected plasmids migrates slightly more rapidly than the endogenous PTB in both the human and nonhuman primate cells we studied. However, as with GAPDH, these immunoblot results clearly demonstrate that the effects of PTB on IRES-directed translation observed in the preceding experiments (Fig. 3 and 5) are not due to the expression of a supraphysiologic abundance of this protein within the cytoplasmic compartment. The results shown for PTB expression in Huh-7 and BSC-1 cells (Fig. 6B) are identical to those published recently (17).
Stem-loop IIIa of the cell culture-adapted IRES has reduced affinity for GAPDH but not PTB.
The mutations in the 5′NTR of HAV that were selected during the adaptation of the wt HM175 virus to growth in African green monkey kidney cells include two changes within segments of the IRES that bind GAPDH (6, 24, 36). Both the deletion of two U residues (nt 203 and 204) from stem-loop IIIa and the U-to-G substitution at nt 687 within stem-loop V result in enhanced activity of the HM175/P16 virus IRES in BSC-1 cells (37). It is notable that both of these translation-enhancing mutations result in the loss of U residues from RNA structures that bind GAPDH, since GAPDH binds preferentially to U-rich RNA sequences (30, 36). Furthermore, as shown in Fig. 5A, overexpression of GAPDH had a less suppressive effect on the HM175/P16 IRES than on the wt IRES in BSC-1 cells. These observations led us to consider the possibility that these mutations might enhance IRES activity by reducing the affinity of the viral RNA for GAPDH, since this would prevent GAPDH-mediated destabilization of secondary RNA structure and preserve IRES function (36). Such a hypothesis is particularly attractive for the mutation in stem-loop IIIa, since the deletion of the two unpaired U residues occurred within a string of five consecutive U residues in the wt sequence (24).
To test this hypothesis, we studied the GAPDH binding activities of the stem-loop IIIa RNA sequences of both the wt and HM175/P16 virus IRES elements in filter binding assays (Fig. 7A). These results confirmed that GAPDH has a lower affinity for the stem-loop IIIa RNA of the cell culture-adapted HM175/P16 virus than for the comparable wt IIIa RNA probe. The binding isotherms obtained in these studies indicated a kd of 1.86 × 10−10 for the wt RNA probe compared with a kd of 2.35 × 10−10 for the HM175/P16 stem-loop IIIa probe, assuming that GAPDH exists and binds these RNAs in its tetramer form.
FIG. 7.
Results of filter binding experiments assessing the affinities of GAPDH (A) or PTB (B) proteins to synthetic RNA probes representing corresponding stem-loop IIIa segments of wt HM175 virus (solid line) or the cell culture-adapted HM175/P16 virus (dashed lines). The 32P-labeled RNA probes were mixed with purified GAPDH or His6-PTB recombinant proteins in a binding buffer and then passed through sequential filters that either specifically retain protein and protein-RNA complexes or RNA. The results shown represent the fraction of probe bound to protein at different protein concentrations.
In contrast, similar filter binding assays with these RNA probes showed no significant difference in their affinities for recombinant human PTB: kd = 1.83 × 10−10 for the wt RNA and 1.87 × 10−10 for the HM175/P16 probe, assuming that PTB exists as a dimer (33) (Fig. 7B). These results are consistent with those of previous competitive UV cross-linking studies that suggested that PTB has a higher affinity than GAPDH for the HM175/P16 virus stem-loop IIIa RNA (36). However, the results shown in Fig. 7 indicate that PTB and GAPDH have comparable affinities for the wt stem loop IIIa RNA. The filter binding results were reproducible in separate experiments.
DISCUSSION
HAV is highly hepatotropic and replicates poorly in all types of cultured cells. However, during repeated passage, wt virus becomes progressively adapted to replication in cultured African green monkey kidney cells. This process is multifactorial and results in the accumulation within the nonstructural P2 proteins of the virus of mutations that are likely to contribute to viral replicase activity (13, 24). In addition, other mutations accumulate within the viral IRES that facilitate cap-independent translation in a cell-type-specific fashion, reflecting differences that exist between the intracellular environment of these cells and within hepatocytes in situ (37). The experiments described in this report focus on this second aspect of cell culture adaptation and attempt to address the molecular basis for the enhanced translational activity associated with these IRES mutations in African green monkey kidney cells.
The cell-type-specific changes in IRES activity that accompany such mutations are likely to be due to differences in RNA binding proteins that are present in the cytoplasmic compartment of these cells and serve as noncanonical translation initiation factors. Consistent with this hypothesis, there are marked qualitative differences in the RNA binding proteins present in RSW preparations made from African green monkey kidney cells and other cultured cell types (6). A ∼39-kDa protein, subsequently identified as GAPDH, was the predominant protein cross-linking to RNA segments within the HAV IRES in RSW from BSC-1 and FRhK-4 cells, while a ∼57-kDa protein doublet, identified as PTB, was predominant in RSW prepared from HeLa and Huh-7 cells (6; Chang et al., unpublished). GAPDH and PTB compete for binding to overlapping sites on stem-loop IIIa of the viral RNA, the 5′-most RNA structure within the IRES (36).
PTB facilitates translation directed by picornavirus IRES elements in rabbit reticulocyte lysates in vitro (22, 25, 26). In addition, we have recently demonstrated that the overexpression of PTB stimulates translation directed by IRES elements derived from HAV, poliovirus, and even hepatitis C virus in several different types of cells (17). These IRES elements have little if any common secondary or tertiary RNA structure, and so these results suggest that PTB may act to stabilize RNA structure in a nonspecific fashion and that it may function as a general RNA-folding chaperone (17, 25). GAPDH, on the other hand, appears to have an opposite effect on RNA structure. We previously demonstrated changes in the circular dichroism spectra of stem-loop IIIa RNA, following the binding of GAPDH to this RNA, that are indicative of a melting of the secondary structure of the stem-loop formed by this probe (36). We predicted from these results that GAPDH would have a suppressive effect on IRES-directed translation, a hypothesis that is supported by the results of the studies reported here. Thus, GAPDH and PTB have opposing effects on both higher-order RNA structure and IRES-directed translation. GAPDH suppresses translation by destabilizing essential secondary RNA structures within the IRES, while PTB may reverse this effect by stabilizing structures or by effectively competing with GAPDH for the overlapping sites on the IRES to which these proteins bind.
GAPDH is an abundant protein that is located mainly but not exclusively within the cytoplasm of cells. It is essential for glycolysis, but recent evidence suggests it may have several other important cellular functions (39). GAPDH has RNA binding properties and binds to the untranslated RNA sequences of several different viruses, including human parainfluenza virus type 3, hepatitis B virus, and hepatitis C virus, as well as HAV (12, 34, 45). The data presented here are the first to address the functional consequences of the interaction of GAPDH with any of these viral RNAs. Such studies are complicated by the ubiquitous nature and relatively high abundance of GAPDH. However, the use of dicistronic constructs encoding GAPDH within the upstream reading frame (Fig. 1) is likely to have succeeded because of the ability of these transcripts to increase the GAPDH abundance within the microenvironment of an IRES placed immediately 3′ of the GAPDH-coding sequence. This notion is consistent with the fact that there was no detectable increase in the total cellular abundance of GAPDH in cells transfected with these plasmids (Fig. 6A) despite the presence of a significant suppressive effect on IRES function. It also emphasizes the importance of the local concentration of cellular factors such as GAPDH at the site of translation. Although we attempted to assess the impact of GAPDH on HAV translation by cotransfection of the monocistronic GAPDH expression vector (pGAPDH) shown in Fig. 1B and a plasmid encoding a reporter protein under control of the IRES, GAPDH overexpression did not influence IRES-directed translation under these conditions (data not shown).
The results of the GAPDH filter binding experiments (Fig. 7A) indicate that stem-loop IIIa of the cell culture-adapted HM175/P16 viral IRES binds GAPDH with a significantly lower affinity than does the comparable structure in the wt virus. This is consistent with the loss of two uridine residues from a five-residue oligo(U) track that is present at nt 200 to 204 of the wild-type viral RNA, as well as prior observations from our laboratory and others that indicate that GAPDH binds preferentially to U-rich RNA sequences (30, 36). The loss of one or two uridine residues from this oligo(U) track has been noted in at least two independently isolated HAV variants that have been adapted to growth in African green monkey kidney cells, indicating the importance of this mutation to the cell culture adaptation process (8, 24). From a functional perspective, the deletion of the unpaired uridines at nt 203 and 204 results in a significant increase in IRES activity in BSC-1 cells (37). These cells have a relatively low cytoplasmic abundance of PTB (Fig. 6), and GAPDH is the predominant protein in BSC-1 RSW or cytoplasmic extracts that is bound by the viral RNA (6, 37). It is likely that the helix-destabilizing effects of GAPDH would be most evident in a cellular environment with low PTB abundance, due to the ability of PTB to compete with GAPDH for binding to the IRES. In fact, we found GAPDH to have a greater suppressive effect on IRES-directed translation in BSC-1 and FRhK-4 cells than in Huh-7 cells, which have a higher cytoplasmic abundance of PTB (Fig. 5A).
It is intriguing to speculate that the greater activity of HM175/P16 IRES in BSC-1 cells results from a loss of affinity of the viral RNA for GAPDH. This hypothesis is supported by the reduced affinity of the HM175/P16 stem-loop IIIa RNA for GAPDH (Fig. 7) and by the lesser effect of GAPDH overexpression on the HM175/P16 IRES than on the wt IRES in BSC-1 cells (Fig. 5A). The selection of the U-deletion mutation within stem-loop IIIa is also consistent with the greater effect of PTB overexpression on IRES function in BSC-1 cells than in the other cell types studied (Fig. 5B). Notably, PTB overexpression has a lesser translation-enhancing effect in Huh-7 cells (Fig. 5B), presumably because of the greater endogenous cytoplasmic abundance of PTB in this cell type (Fig. 6B) (17). Consistent with this higher PTB abundance, GAPDH overexpression had no demonstrable inhibitory effects on IRES-directed translation in Huh-7 cells and there was no difference in the effect of GAPDH on the wt IRES and on the HM175/P16 IRES in this cell type (Fig. 5A).
While this hypothesis fits the findings with BSC-1 cells particularly well, it does not explain all of the observations with FRhK-4 cells. These cells appear to have a cytoplasmic abundance of PTB comparable to that in BSC-1 cells (Fig. 7B), and, as in BSC-1 cells, GAPDH was the dominant RNA binding protein when RSW from these cells were studied in UV cross-linking experiments (6). Consistent with a similar PTB abundance, GAPDH overexpression had an inhibitory effect on IRES-directed translation that was similar in magnitude to that observed in BSC-1 cells, with a greater inhibition of the wt IRES than of the HM175/P16 IRES (Fig. 5A). Despite this, previous studies indicate that the mutations present in the HM175/P16 IRES (including the deletion of the two uridine residues from stem-loop IIIa) neither enhance IRES-directed translation in these cells nor promote the replication of the virus in this cell type (37). Thus, the translational microenvironment must differ significantly between FRhK-4 and BSC-1 cells, despite similar outcomes in UV-cross-linking studies and apparently similar cellular abundances of both PTB and GAPDH (Fig. 6A). That this is the case is further supported by the relatively low stimulation of IRES-directed translation observed with PTB overexpression (Fig. 5B), which paralleled the results obtained with Huh-7 cells and was strikingly different from the results obtained with BSC-1 cells.
These data are important because they begin to provide a molecular explanation for one aspect of the adaptation of HAV to growth in cell culture, a change in the viral phenotype that is essential for economical production of virus in cell culture for use in vaccine manufacture. Our results indicate that the binding of GAPDH to the viral RNA suppresses cap-independent translation due to destabilization of RNA secondary structure. They further suggest that variations in the local abundance of PTB within the microenvironment of the IRES modulate the negative effects of GAPDH on the viral translational machinery. However, previous UV-cross-linking studies demonstrated that additional, as yet unidentified proteins of ∼110 and ∼30 kDa also bind viral RNA segments that have affinity for PTB and GAPDH (6). Although these unidentified proteins appear to be universally present in different cell types, their relative abundance and the functional impact of their binding to the viral RNA, if any, are not known. It will be extraordinarily difficult to dissect the complex interplay of these factors with the viral IRES in experiments carried out with cell-free systems in which the microenvironment of the IRES is radically changed. Future studies will need to examine the impact of these proteins on IRES function within specific intracellular environments.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (RO1-AI32599) and the Advanced Technology Program of the Texas Higher Education Coordinating Board.
We are grateful to Mariano A. Garcia-Blanco for providing the pGST-PTB plasmid and David V. Sangar for his critical review of the manuscript.
REFERENCES
- 1.Binn L N, Lemon S M, Marchwicki R H, Redfield R R, Gates N L, Bancroft W H. Primary isolation and serial passage of hepatitis A virus strains in primate cell cultures. J Clin Microbiol. 1984;20:28–33. doi: 10.1128/jcm.20.1.28-33.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blyn L B, Towner J S, Semler B L, Ehrenfeld E. Requirement of poly(rC) binding protein 2 for translation of poliovirus RNA. J Virol. 1997;71:6243–6246. doi: 10.1128/jvi.71.8.6243-6246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borman A, Howell M T, Patton J G, Jackson R J. The involvement of a spliceosome component in internal initiation of human rhinovirus RNA translation. J Gen Virol. 1993;74:1775–1788. doi: 10.1099/0022-1317-74-9-1775. [DOI] [PubMed] [Google Scholar]
- 4.Borovjagin A, Pestova T, Shatsky I. Pyrimidine tract binding protein strongly stimulates in vitro encephalomyocarditis virus RNA translation at the level of preinitiation complex formation. FEBS Lett. 1994;351:299–302. doi: 10.1016/0014-5793(94)00848-5. [DOI] [PubMed] [Google Scholar]
- 5.Brown E A, Day S P, Jansen R W, Lemon S M. The 5′ nontranslated region of hepatitis A virus RNA: secondary structure and elements required for translation in vitro. J Virol. 1991;65:5828–5838. doi: 10.1128/jvi.65.11.5828-5838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang K H, Brown E A, Lemon S M. Cell type-specific proteins which interact with the 5′ nontranslated region of hepatitis A virus RNA. J Virol. 1993;67:6716–6725. doi: 10.1128/jvi.67.11.6716-6725.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen J I, Rosenblum B, Feinstone S M, Ticehurst J, Purcell R H. Attenuation and cell culture adaptation of hepatitis A virus (HAV): a genetic analysis with HAV cDNA. J Virol. 1989;63:5364–5370. doi: 10.1128/jvi.63.12.5364-5370.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen J I, Rosenblum B, Ticehurst J R, Daemer R J, Feinstone S M, Purcell R H. Complete nucleotide sequence of an attenuated hepatitis A virus: comparison with wild-type virus. Proc Natl Acad Sci USA. 1987;84:2497–2501. doi: 10.1073/pnas.84.8.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daemer R J, Feinstone S M, Gust I D, Purcell R H. Propagation of human hepatitis A virus in African green monkey kidney cell culture: primary isolation and serial passage. Infect Immun. 1981;32:388–393. doi: 10.1128/iai.32.1.388-393.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day S P, Lemon S M. A single base mutation in the 5′ noncoding region of HAV enhances replication of virus in vitro. In: Brown F, Chanock R M, Ginsberg H S, Lerner R A, editors. Vaccines 90: modern approaches to new vaccines including prevention of AIDS. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. pp. 175–178. [Google Scholar]
- 11.Day S P, Murphy P, Brown E A, Lemon S M. Mutations within the 5′ nontranslated region of hepatitis A virus RNA which enhance replication in BS-C-1 cells. J Virol. 1992;66:6533–6540. doi: 10.1128/jvi.66.11.6533-6540.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De B P, Gupta S, Zhao H, Drazba J A, Banerjee A K. Specific interaction in vitro and in vivo of glyceraldehyde-3-phosphate dehydrogenase and LA protein with cis-acting RNAs of human parainfluenza virus type 3. J Biol Chem. 1996;271:24728–24735. doi: 10.1074/jbc.271.40.24728. [DOI] [PubMed] [Google Scholar]
- 13.Emerson S U, Huang Y K, Purcell R H. 2B and 2C mutations are essential but mutations throughout the genome of HAV contribute to adaptation to cell culture. Virology. 1993;194:475–480. doi: 10.1006/viro.1993.1286. [DOI] [PubMed] [Google Scholar]
- 14.Emerson S U, Lewis M, Govindarajan S, Shapiro M, Moskal T, Purcell R H. cDNA clone of hepatitis A virus encoding a virulent virus: induction of viral hepatitis by direct nucleic acid transfection of marmosets. J Virol. 1992;66:6649–6654. doi: 10.1128/jvi.66.11.6649-6654.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emerson S U, McRill C, Rosenblum B, Feinstone S, Purcell R H. Mutations responsible for adaptation of hepatitis A virus to efficient growth in cell culture. J Virol. 1991;65:4882–4886. doi: 10.1128/jvi.65.9.4882-4886.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghetti A, Pinol-Roma S, Michael W M, Morandi C, Dreyfuss G. hnRNP I, the polypyrimidine tract-binding protein: distinct nuclear localization and association with hnRNAs. Nucleic Acids Res. 1992;20:3671–3678. doi: 10.1093/nar/20.14.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gosert R, Chang K H, Rijnbrand R, Yi M K, Sangar D V, Lemon S M. Transient overexpression of cellular polypyrimidine tract-binding protein stimulates cap-independent translation directed by both picornaviral and flaviviral internal ribosome entry sites in vivo. Mol Cell Biol. 2000;20:1583–1595. doi: 10.1128/mcb.20.5.1583-1595.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graff J, Normann A, Feinstone S M, Flehmig B. Nucleotide sequence of wild-type hepatitis A virus GBM in comparison with two cell culture-adapted variants. J Virol. 1994;68:548–554. doi: 10.1128/jvi.68.1.548-554.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregersen J P, Mehdi S, Mauler R. Adaptation of hepatitis A virus to high titre growth in diploid and permanent cell cultures. Med Microbiol Immunol. 1988;177:91–100. doi: 10.1007/BF00189530. [DOI] [PubMed] [Google Scholar]
- 20.Hellen C U, Witherell G W, Schmid M, Shin S H, Pestova T V, Gil A, Wimmer E. A cytoplasmic 57-kDa protein that is required for translation of picornavirus RNA by internal ribosomal entry is identical to the nuclear pyrimidine tract-binding protein. Proc Natl Acad Sci USA. 1993;90:7642–7646. doi: 10.1073/pnas.90.16.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honda M, Kaneko S, Matsushita E, Kobayashi K, Abell G, Lemon S M. Cell cycle regulation of hepatitis C virus IRES-directed translation. Gastroenterology. 2000;118:152–162. doi: 10.1016/s0016-5085(00)70424-0. [DOI] [PubMed] [Google Scholar]
- 22.Hunt S L, Jackson R J. Polypyrimidine-tract binding protein (PTB) is necessary, but not sufficient, for efficient internal initiation of translation of human rhinovirus-2 RNA. RNA. 1999;5:344–359. doi: 10.1017/s1355838299981414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang S K, Krausslich H-G, Nicklin M J H, Duke G M, Palmenberg A C, Wimmer E C. A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988;62:2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansen R W, Newbold J E, Lemon S M. Complete nucleotide sequence of a cell culture-adapted variant of hepatitis A virus: comparison with wild-type virus with restricted capacity for in vitro replication. Virology. 1988;163:299–307. doi: 10.1016/0042-6822(88)90270-x. [DOI] [PubMed] [Google Scholar]
- 25.Kaminski A, Hunt S L, Patton J G, Jackson R J. Direct evidence that polypyrimidine tract binding protein (PTB) is essential for internal initiation of translation of encephalomyocarditis virus RNA. RNA. 1995;1:924–938. [PMC free article] [PubMed] [Google Scholar]
- 26.Kaminski A, Jackson R J. The polypyrimidine tract binding protein (PTB) requirement for internal initiation of translation of cardiovirus RNAs is conditional rather than absolute. RNA. 1998;4:626–638. doi: 10.1017/s1355838298971898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemon S M, Robertson B H. Current perspectives in the virology and molecular biology of hepatitis A virus. Semin Virol. 1993;4:285–295. [Google Scholar]
- 28.Meerovitch K, Svitkin Y V, Lee H S, Lejbkowicz F, Kenan D J, Chan E K, Agol V I, Keene J D, Sonenberg N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J Virol. 1993;67:3798–3807. doi: 10.1128/jvi.67.7.3798-3807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Midthun K, Ellerbeck E, Gershman K, Calandra G, Krah D, McCaughtry M, Nalin D, Provost P. Safety and immunogenicity of a live attenuated hepatitis A virus vaccine in seronegative volunteers. J Infect Dis. 1991;163:735–739. doi: 10.1093/infdis/163.4.735. [DOI] [PubMed] [Google Scholar]
- 30.Nagy E, Rigby W F. Glyceraldehyde-3-phosphate dehydrogenase selectively binds AU-rich RNA in the NAD(+)-binding region (Rossmann fold) J Biol Chem. 1995;270:2755–2763. doi: 10.1074/jbc.270.6.2755. [DOI] [PubMed] [Google Scholar]
- 31.Niepmann M, Petersen A, Meyer K, Beck E. Functional involvement of polypyrimidine tract-binding protein in translation initiation complexes with the internal ribosome entry site of foot-and-mouth disease virus. J Virol. 1997;71:8330–8339. doi: 10.1128/jvi.71.11.8330-8339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334:320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- 33.Perez I, McAfee J G, Patton J G. Multiple RRMs contribute to RNA binding specificity and affinity for polypyrimidine tract binding protein. Biochemistry. 1997;36:11881–11890. doi: 10.1021/bi9711745. [DOI] [PubMed] [Google Scholar]
- 34.Petrik J, Parker H, Alexander G J. Human hepatic glyceraldehyde-3-phosphate dehydrogenase binds to the poly(U) tract of the 3′ non-coding region of hepatitis C virus genomic RNA. J Gen Virol. 1999;80:3109–3113. doi: 10.1099/0022-1317-80-12-3109. [DOI] [PubMed] [Google Scholar]
- 35.Provost P J, Hilleman M R. Propagation of human hepatitis A virus in cell culture in vitro. Proc Soc Exp Biol Med. 1979;160:213–221. doi: 10.3181/00379727-160-40422. [DOI] [PubMed] [Google Scholar]
- 36.Schultz D E, Hardin C C, Lemon S M. Specific interaction of glyceraldehyde 3-phosphate dehydrogenase with the 5′-nontranslated RNA of hepatitis A virus. J Biol Chem. 1996;271:14134–14142. doi: 10.1074/jbc.271.24.14134. [DOI] [PubMed] [Google Scholar]
- 37.Schultz D E, Honda M, Whetter L E, McKnight K L, Lemon S M. Mutations within the 5′ nontranslated RNA of cell culture-adapted hepatitis A virus which enhance cap-independent translation in cultured African green monkey kidney cells. J Virol. 1996;70:1041–1049. doi: 10.1128/jvi.70.2.1041-1049.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh R, Green M R. Sequence-specific binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science. 1993;259:365–368. doi: 10.1126/science.8420004. [DOI] [PubMed] [Google Scholar]
- 39.Sirover M A. Role of the glycolytic protein, glyceraldehyde-3-phosphate dehydrogenase, in normal cell function and in cell pathology. J Cell Biochem. 1997;66:133–140. [PubMed] [Google Scholar]
- 40.Sjogren M H, Purcell R H, McKee K, Binn L, MacArthy P, Ticehurst J, Feinstone S, Caudill J, See A, Hoke C, Bancroft W, D'Hondt E. Clinical and laboratory observations following oral or intramuscular administration of a live, attenuated hepatitis A vaccine candidate. Vaccine. 1992;10(Suppl. 1):S135–S137. doi: 10.1016/0264-410x(92)90568-5. [DOI] [PubMed] [Google Scholar]
- 41.Stark M J. Multicopy expression vectors carrying the lac repressor gene for regulated high-level expression of genes in Escherichia coli. Gene. 1987;51:255–267. doi: 10.1016/0378-1119(87)90314-3. [DOI] [PubMed] [Google Scholar]
- 42.Taylor K L, Murphy P C, Asher L V, LeDuc J W, Lemon S M. Attenuation phenotype of a cell culture-adapted variant of hepatitis A virus (HM175/p16) in susceptible New World owl monkeys. J Infect Dis. 1993;168:592–601. doi: 10.1093/infdis/168.3.592. [DOI] [PubMed] [Google Scholar]
- 43.Weeks K M, Cech T R. Efficient protein-facilitated splicing of the yeast mitochondrial bl5 intron. Biochemistry. 1995;34:7728–7738. doi: 10.1021/bi00023a020. [DOI] [PubMed] [Google Scholar]
- 44.Wong I, Lohman T M. A double-filter method for nitrocellulose-filter binding: application to protein-nucleic acid interactions. Proc Natl Acad Sci USA. 1993;90:5428–5432. doi: 10.1073/pnas.90.12.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zang W Q, Fieno A M, Grant R A, Yen T S. Identification of glyceraldehyde-3-phosphate dehydrogenase as a cellular protein that binds to the hepatitis B virus posttranscriptional regulatory element. Virology. 1998;248:46–52. doi: 10.1006/viro.1998.9255. [DOI] [PubMed] [Google Scholar]