Abstract
Purpose:
Simlukafusp alfa [fibroblast activation protein α–targeted IL2 variant (FAP-IL2v)], a tumor-targeted immunocytokine, comprising an IL2 variant moiety with abolished CD25 binding fused to human IgG1, is directed against fibroblast activation protein α. This phase I, open-label, multicenter, dose-escalation, and extension study (NCT02627274) evaluated the safety, pharmacokinetics, pharmacodynamics, and antitumor activity of FAP-IL2v in patients with advanced/metastatic solid tumors.
Patients and Methods:
Participants received FAP-IL2v intravenously once weekly. Dose escalation started at 5 mg; flat dosing (≤25 mg) and intraparticipant uptitration regimens (15/20, 20/25, 20/20/35, and 20/35/35 mg) were evaluated. Primary objectives were dose-limiting toxicities, maximum tolerated dose, recommended expansion dose, and pharmacokinetics.
Results:
Sixty-one participants were enrolled. Dose-limiting toxicities included fatigue (flat dose 20 mg: n = 1), asthenia (25 mg: n = 1), drug-induced liver injury (uptitration regimen 20/25 mg: n = 1), transaminase increase (20/25 mg: n = 1), and pneumonia (20/35/35 mg: n = 1). The uptitration regimen 15/20 mg was determined as the maximum tolerated dose and was selected as the recommended expansion dose. Increases in peripheral blood absolute immune cell counts were seen for all tested doses [NK cells, 13-fold; CD4+ T cells (including regulatory T cells), 2-fold; CD8+ T cells, 3.5-fold] but without any percentage change in regulatory T cells. Clinical activity was observed from 5 mg [objective response rate, 5.1% (n = 3); disease control rate, 27.1% (n = 16)]. Responses were durable [n = 3, 2.8 (censored), 6.3, and 43.4 months].
Conclusions:
FAP-IL2v had a manageable safety profile and showed initial signs of antitumor activity in advanced/metastatic solid tumors.
Translational Relevance
Simlukafusp alfa [fibroblast activation protein α (FAP)–targeted IL2 variant (FAP-IL2v)] with abolished CD25 binding is a novel immunocytokine developed to overcome the limitations of wild-type IL2 by selectively promoting immune responses in the microenvironment of tumors that overexpress FAP, while minimizing known side effects by abrogating CD25 binding. Targeting FAP-overexpressing tumors with IL2v therapy has the potential to provide augmented antitumor responses accompanied by a more manageable safety profile compared with wild-type IL2 therapies. In this phase I study, FAP-IL2v had an acceptable safety profile in participants with advanced/metastatic solid tumors. The early and favorable signs of clinical activity provide a rationale for further investigation of FAP-IL2v, in combination with other agents, in patients with advanced cancers who have progressed on a previous cancer therapy or for whom no standard and effective therapy exists.
Introduction
IL2 (aldesleukin) has been approved for the treatment of metastatic melanoma and renal cell carcinoma (1). However, systemic IL2 therapy is limited by its short half-life, modest efficacy, and challenging safety profile, which requires close patient monitoring and hospitalization (2). Several tumor-targeted IL2 immunocytokines have been developed to improve the efficacy and safety of IL2 by fusing wild-type (WT) IL2 to tumor-targeting antibodies (3, 4). However, all have failed to demonstrate a favorable risk–benefit profile in the clinic, mainly due to IL2 toxicity (5, 6) and the activation of immunosuppressive CD25+ regulatory T cells (Treg; refs. 6, 7).
Simlukafusp alfa [fibroblast activation protein α (FAP)–targeted IL2 variant (IL2v; FAP-IL2v); RO6874281] is a novel, monomeric immunocytokine developed to overcome the limitations of WT IL2 through selectively promoting immune responses in the microenvironment of FAP-overexpressing tumors (8). FAP-IL2v comprises a single IL2v moiety with abolished CD25 binding which is fused to human IgG1 and directed against FAP (a transmembrane glycoprotein with proteolytic activity; ref. 8). Binding of IL2v to CD25 was abolished to reduce the expansion and/or activation of Tregs. FAP is rarely expressed in healthy tissue but is needed for tissue remodeling processes (9). Hence, FAP has been found to be highly expressed on the surface of cancer-associated fibroblasts, which can be found in the stroma in more than 90% of human epithelial tumors (9, 10).
Due to the full activation of CD8+ T cells and NK cells, FAP-IL2v has enhanced the activity of several therapeutic antibodies in preclinical models, including checkpoint inhibitors like anti-PDL1 antibodies, or other antibodies which induce antibody-dependent cellular cytotoxicity (8); we thus hypothesized that FAP-IL2v may serve as a versatile combination partner in cancer immunotherapy. In this study, we report the results of a first-in-human phase I study evaluating the safety, pharmacokinetics (PK), pharmacodynamics (PD), and antitumor activity of FAP-IL2v in patients with advanced/metastatic solid tumors.
Patients and Methods
Study design and participants
This was an open-label, multicenter, dose-escalation and extension phase I study of FAP-IL2v in patients with advanced/metastatic solid tumors (ClinicalTrials.gov: BP29842; NCT02627274; Supplementary Fig. S1). The study consisted of parts A, B, and C; we report data from the monotherapy dose-escalation and expansion cohorts (part A). Eligible participants were of ages ≥18 years, with measurable disease as defined by Response Evaluation Criteria in Solid Tumors (RECIST) v1.1, had an Eastern Cooperative Oncology Group performance status of 0/1, and had been confirmed to have advanced/metastatic solid tumors with at least one tumor lesion accessible to biopsy per clinical judgment of the treating physician. Ineligible participants were those with symptomatic or untreated central nervous system lesions, an active second malignancy, known autoimmune diseases, or disease with ongoing tissue remodeling. The use of concurrent therapy with immune-modulating agents was not allowed. Full eligibility criteria and study representation of underserved communities (Supplementary Table S1) are included in the Supplementary Data.
This study was approved by each center’s ethics committee or institutional review board, and the study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. All participants provided written informed consent.
Study treatment
FAP-IL2v monotherapy was administered as an i.v. infusion once weekly at a starting dose of 5 mg using flat dosing and intraparticipant uptitration regimens. The first administration of FAP-IL2v was given without premedication. Premedication with H1 antihistamines, NSAIDs, and crystalloid fluids could be considered for subsequent administrations at the investigator’s discretion to attenuate IL2-mediated infusion reactions.
The first participant was observed for safety for 1 week, followed by additional participants. Safety data from a previous phase I study (Lassen and colleagues; manuscript in preparation) of cergutuzumab amunaleukin (CEA-IL2v), an immunocytokine with the same active moiety as IL2v and fused to a bivalent carcinoembryonic antigen (CEA)–targeted antibody (11), were used to inform the early dose-escalation steps, as CEA-IL2v demonstrated a manageable safety profile. A modified continual reassessment escalation method with overdose control design was used to guide the dose escalation (Supplementary Fig. S1). The decision to escalate to the next dose level was made when three or more participants in each cohort had completed the dose-limiting toxicity (DLT) period of 14 days (see Supplementary Data for the full definitions of DLTs). Uptitration at the second or third dosing was implemented to counteract the self-induced clearance observed with FAP-IL2v due to its mode of action. Treatment continued for a maximum of 24 months or until disease progression, unacceptable toxicity, or withdrawal of consent.
Study objectives
The primary objectives of this study were to evaluate the safety of FAP-IL2v monotherapy, identify the maximum tolerated dose (MTD) and define a recommended dose for development, and to determine the PK profile. Secondary objectives included the investigation of treatment-induced PD effects on peripheral blood cells and antitumor activity.
Assessments
Safety was assessed by monitoring DLTs and the incidence of adverse events (AE), serious AEs (SAE), laboratory and cardiac abnormalities, antidrug antibodies (ADA), and physical examinations. AEs were evaluated using NCI Common Terminology Criteria for Adverse Events (v4.03 until November 2017; v5.0 following release). The PK parameters of FAP-IL2v in serum were determined using noncompartmental analysis and nonlinear mixed-effect modeling. The PD effects of FAP-IL2v were determined by absolute immune cell counts (CD4+ T cells, CD8+ T cells, NK cells, and Tregs) in peripheral blood. Tumor archival or fresh tumor biopsies were collected and analyzed centrally for PDL1 expression using clone SP142 by IHC (F. Hoffmann-La Roche Ltd. Tissue Diagnostics). Antitumor activity was evaluated according to RECIST v1.1 using CT or MRI after 8 weeks of treatment, and responses were confirmed at ≥4 weeks later. Outcome measures included the objective response rate [ORR, defined as the proportion of participants with a complete response (CR) or partial response (PR)] and disease control rate [defined as CR, PR, or stable disease (SD)].
Statistical analyses
Participants who received one or more doses of FAP-IL2v were included in the safety and PK analysis population. The PD analysis population included all participants who had paired blood samples at baseline and on-treatment (cycle 4 day 1). The preliminary antitumor activity of single-agent FAP-IL2v was evaluated in all participants within the safety population who had a baseline response and one or more on-study response assessment. The 90% confidence interval (CI) for ORR was constructed using the Wilson method, whereas the 95% CI for median duration of response was computed using the Brookmeyer and Crowley method. Data cut-off was January 16, 2023.
Data availability
Qualified researchers may request access to individual participant–level data through the clinical study data request platform (https://vivli.org/ourmember/roche/). Further details on F. Hoffmann-La Roche Ltd's criteria for eligible studies are available at https://vivli.org/members/ourmembers/. For further details on F. Hoffmann-La Roche Ltd's Global Policy on the Sharing of Clinical Information and the procedure to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
Results
Participants
Between November 2015 and October 2019, 61 participants were enrolled to receive FAP-IL2v in weekly doses of flat 5 mg (n = 3), 10 mg (n = 4), 20 mg (n = 4), and 25 mg (n = 5) or intraparticipant uptitration regimens of 15/20 mg (n = 11), 20/25 mg (n = 5), 20/20/35 mg (n = 5), and 20/35/35 mg (n = 1). The 15/20 mg uptitration regimen was determined as the MTD and was chosen for the expansion cohort (n = 23). Participant baseline characteristics were similar across doses (Table 1); the median age was 60 years (range, 38–78); and the most common cancer diagnoses at study entry were melanoma [n = 19 (31%)] and squamous cell carcinoma [SCC; n = 11 (18%)]. Three participants (5%) completed the study, and 58 (95%) discontinued treatment, primarily due to disease progression [n = 36 (59%)] or AEs [n = 6 (10%)]. The median treatment duration was 1.6 months (range, 0–24).
Table 1.
Participant demographics and baseline characteristics.
| Characteristic | All participants (N = 61) |
|---|---|
| Median age (range) | 60 (38–78) |
| Sex, n (%) | |
| Male | 31 (51) |
| Female | 30 (49) |
| Primary tumor type, n (%) | |
| Melanoma | 19 (31) |
| SCCa | 11 (18) |
| Adenocarcinomaa | 7 (12) |
| Head and neck cancer | 7 (12) |
| Breast cancer | 3 (5) |
| Lung cancer | 3 (5) |
| Colorectal carcinoma | 3 (5) |
| Pancreatic cancer | 2 (3) |
| Prostate cancer | 2 (3) |
| Chondrosarcoma | 1 (2) |
| Gastric cancer | 1 (2) |
| Mesothelioma | 1 (2) |
| Ovarian cancer | 1 (2) |
| Stage at study entry, n (%) | |
| III | 3 (5) |
| IV | 56 (92) |
| Missing | 2 (3) |
| ECOG performance status, n (%) | |
| 0 | 22 (36) |
| 1 | 39 (64) |
| Prior lines of therapy, n (%) | |
| 0 | 9 (15) |
| 1 | 15 (25) |
| 2 | 13 (21) |
| 3 | 11 (18) |
| >3 | 13 (21) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Unknown primary origin.
Safety
All 61 participants in the safety population experienced at least one AE (Supplementary Table S2); 98% of the participants had FAP-IL2v–related AEs (Table 2). Any grade/class-specific AEs expected with IL2 therapy included pyrexia (71%), liver function test abnormalities (57%), edema (39%), and infusion-related reactions (IRR; 67%). Seven participants (12%) experienced FAP-IL2v–related grade 2 capillary leak syndrome, which all resolved. Most reported any-grade AEs across all cohorts included pyrexia (71%), IRR (67%), nausea (49%), chills (48%), fatigue (44%), and decreased appetite (41%; Supplementary Table S2). Grade ≥3 AEs were reported in 79% of the participants. Most reported grade 3/4 AEs (≥10% of participants across all doses) were IRR (21%), anemia (16%), aspartate transaminase increase (13%), pyrexia (12%), and hypophosphatemia (12%). The percentage of patients experiencing at least 1 AE of any grade did not change over time. However, the incidence of grade 3/4 AEs showed a substantial reduction over the course of treatment cycles [cycles 1–3, n = 40 (65.6%); cycles 4–6, n = 21 (40.4%); cycles 6+, n = 13 (33.3%)]. Class-specific AEs were predominantly grade 1/2, and although pyrexia was seen at a relatively constant frequency throughout cycles, all the other AEs were noted to decrease. A total of 42 participants (69%) experienced one or more SAEs; 37 participants (61%) had SAEs considered to be FAP-IL2v–related (Table 2). AEs leading to dose modification/interruption of FAP-IL2v occurred in 42 participants (69%; Table 2), and AEs leading to FAP-IL2v withdrawal were reported in six participants [10%; flat dose 20 mg (n = 1); uptitration regimen 15/20 mg (n = 2); 20/25 mg (n = 1); 20/35/35 mg (n = 1; Table 2)].
Table 2.
Overview of AEs during dose escalation and treatment-related AEs at the recommended weekly uptitration regimen of 15/20 mg.
| AE, n (%) | AEs during dose escalation—FAP-IL2v once weekly dosing | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Flat dosing | Uptitration regimen | Expansion | Combined | |||||||
| Cohort 1: 5 mg (n = 3) | Cohort 2: 10 mg (n = 4) | Cohort 3: 20 mg (n = 4) | Cohort 4: 25 mg (n = 5) | Cohort 5: 20/20/35 mg (n = 5) | Cohort 6: 20/35/35 mg (n = 1) | Cohort 7: 20/25 mg (n = 5) | Cohort 8: 15/20 mg (n = 11) | Cohort 9: 15/20 mg (n = 23) | All part A participants (N = 61) | |
| Participants with ≥1 event, n (%) | ||||||||||
| AE | 3 (100) | 4 (100) | 4 (100) | 5 (100) | 5 (100) | 1 (100) | 5 (100) | 11 (100) | 23 (100) | 61 (100) |
| Treatment-related | 3 (100) | 3 (75) | 4 (100) | 5 (100) | 5 (100) | 1 (100) | 5 (100) | 11 (100) | 23 (100) | 60 (98) |
| SAE | 1 (33) | 1 (25) | 2 (50) | 4 (80) | 4 (80) | 1 (100) | 3 (60) | 9 (82) | 17 (74) | 42 (69) |
| Treatment-related | 1 (33) | 0 | 2 (50) | 2 (40) | 4 (80) | 1 (100) | 2 (40) | 9 (82) | 16 (70) | 37 (61) |
| AEs leading to dose modification/interruption | 1 (33) | 2 (50) | 1 (25) | 4 (80) | 5 (100) | 1 (100) | 3 (60) | 9 (82) | 16 (70) | 42 (69) |
| AEs leading to treatment withdrawal | 0 | 0 | 1 (25) | 0 | 0 | 1 (100) | 1 (20) | 2 (18) | 1 (4) | 6 (10) |
| Grade ≥3 AE | 2 (67) | 1 (25) | 2 (50) | 5 (100) | 5 (100) | 1 (100) | 4 (80) | 10 (91) | 23 (100) | 54 (89) |
| DLT | 0 | 0 | 1 (25) | 1 (20) | 0 | 1 (100) | 2 (40) | 0 | 0 | 5 (8) |
| Most common treatment-related AEs (≥20% of the participants) at the recommended weekly dose of 15/20 mg (n = 34) | ||
|---|---|---|
| AE, n (%) | All grades | Grade 3 or 4 |
| Pyrexia | 25 (74) | 5 (15) |
| IRR | 22 (65) | 7 (21) |
| Chills | 20 (59) | 0 |
| Fatigue | 15 (44) | 2 (6) |
| Nausea | 14 (41) | 0 |
| Asthenia | 14 (41) | 4 (12) |
| Decreased appetite | 10 (29) | 1 (3) |
| Peripheral edema | 9 (26) | 0 |
| Diarrhea | 8 (24) | 0 |
| Vomiting | 8 (24) | 0 |
| AST increased | 8 (24) | 2 (6) |
| ALT increased | 8 (24) | 2 (6) |
| Blood bilirubin increased | 8 (24) | 0 |
| Hypotension | 8 (24) | 2 (6) |
| Rash | 7 (21) | 2 (6) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate transaminase.
DLTs among participants receiving flat dosing were as follows: 5 mg (n = 0); 10 mg (n = 0); 20 mg [n = 1 (fatigue)]; and 25 mg [n = 1 (asthenia)]. Among participants on uptitration dosing regimens, DLTs were 15/20 mg (n = 0), 20/25 mg [n = 2 (drug-induced liver injury and increased aspartate aminotransferase)], 20/20/35 mg (n = 0), and 20/35/35 mg [n = 1 (pneumonia); Table 2]. The 15/20 mg uptitration regimen was determined as the MTD and was chosen for expansion to compensate for reduced exposure upon multiple dosing. In the 34 participants who received the recommended uptitration regimen of 15/20 mg, the six most common all-grade FAP-IL2v–related AEs were pyrexia (74%), IRR (65%), chills (59%), fatigue (44%), nausea (41%), and asthenia (41%). The most frequent grade 3/4 FAP-IL2v–related AEs at the recommended dose were IRR (21%), pyrexia (15%), asthenia (12%), and hypophosphatemia (12%; Table 2). AEs leading to dose interruption/modifications were seen in 74% (n = 25/34) of the participants receiving the uptitration regimen 15/20 mg.
In total, 44 participants died, 42 deaths of which were due to disease progression and the cause of death was unknown for 2 participants. No deaths were considered related to FAP-IL2v.
PK analysis
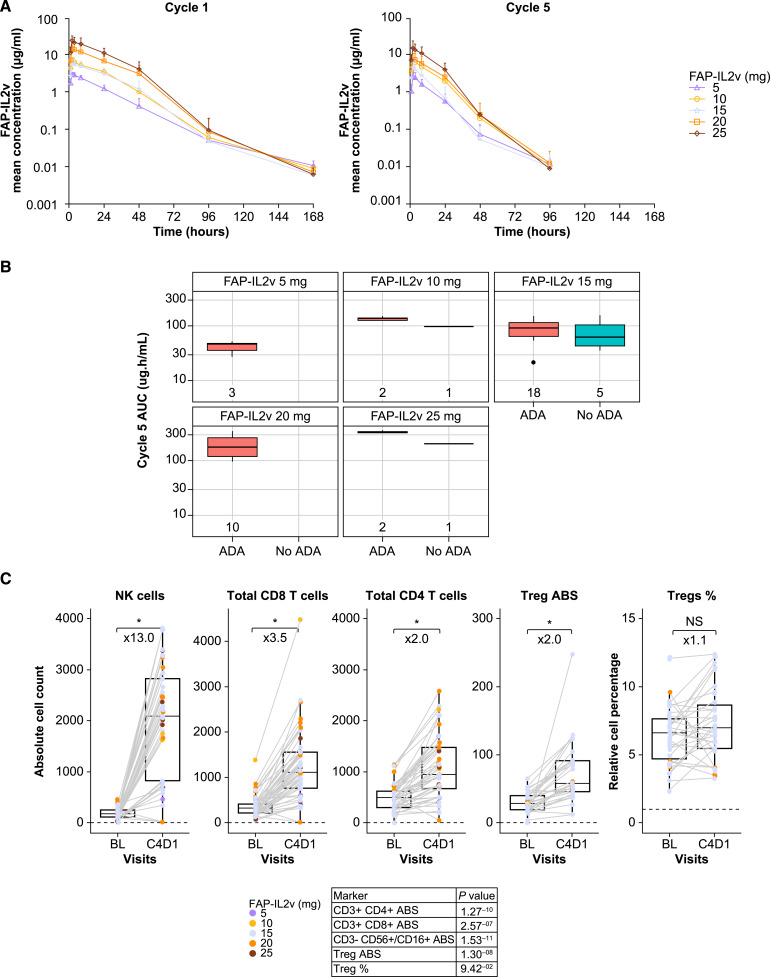
PK analyses were conducted in all participants, and characteristics were followed longitudinally within each dose cohort. FAP-IL2v concentrations exhibited nonlinear elimination [typical of target-mediated drug disposition (TMDD)], consistent with clearance via a nonlinear pathway and via a linear pathway after single-dose administration (Fig. 1A). The nonlinearity was attributed to the FAP-IL2v clearance pathway via a saturable, target-mediated mechanism [like IL2 receptor (IL2R)]. After multiple dose administrations, concentration–time profiles were similar in shape, with lower exposures than those observed after a single dose. No serum accumulation of FAP-IL2v was observed following multiple treatment cycles; rather, serum exposure decreased in most participants (this observation occurred as soon as cycle 2). A reduction in FAP-IL2v exposure correlated with the number of IL2R-expressing cells. FAP-IL2v was described with a TMDD model using an expansion of the target pool to explain the reduced exposure following multiple doses. The expansion of the target pool was in line with the mode of action and was supported by IL2R-positive immune cell expansion in response to treatment. To determine the uptitration regimen to compensate for the reduced exposure on the second dose, population PK simulations were performed to decide dose increases, and ADA development was observed at all doses, but no significant effect on FAP-IL2v exposure was noted (Fig. 1B).
Figure 1.
FAP-IL2v PK profile on cycles 1 and 5 (A), exposure and effects of ADA development (B), and PD effects in peripheral blood (paired samples) following weekly administration (C). *, P < 0.01. ABS, absolute; AUC, area under the concentration curve; BL, baseline; C, cycle; D, day; NS, not significant.
PD analysis
The strongest expansion following FAP-IL2v treatment was seen in NK cells, followed by CD8+ and CD4+ T cells. Increases in absolute cell counts were seen from baseline to cycle 4 day 1 for CD4+ (including Tregs, 2-fold), CD8+ (3.5-fold), and NK cells (13-fold), but without any significant change in the overall Treg percentage (Fig. 1C).
Antitumor activity
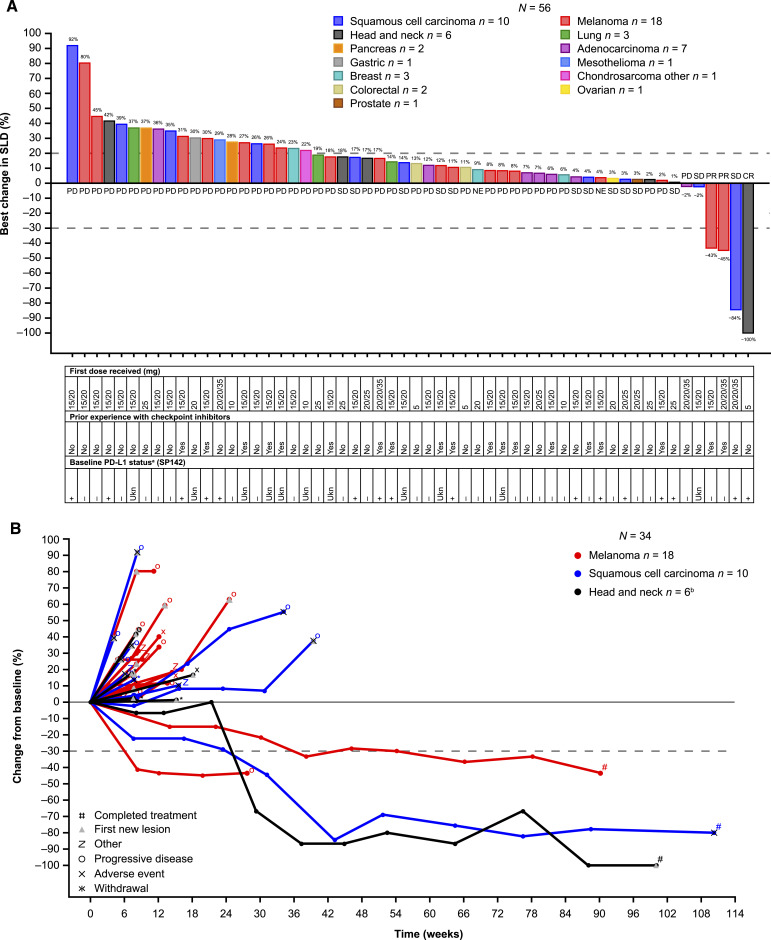
Fifty-nine participants were evaluable for antitumor activity per RECIST v1:1; three participants responded to treatment. The confirmed ORR was 5.1% (90% CI, 2.05–12.06) with a disease control rate of 27.1% (CR = 1.7%; PR = 3.4%; SD = 22.0%; progressive disease = 66.1%; response was missing/not evaluable for 6.8%; Fig. 2A); responses were observed irrespective of PDL1 status. The rate of progression-free survival at 24 weeks was 13% (95% CI, 6.4–25.6). The participant who achieved CR was a 58-year-old male with SCC of the head and neck. Two participants who achieved a PR had melanoma: the first was a 38-year-old female who had progressed after five previous lines of therapy (including nivolumab plus ipilimumab), and the second was a 52-year-old female who had progressed after previous first-line nivolumab plus ipilimumab. The median duration of response was 24.9 months [95% CI, 6.3 (not evaluable)]; responses were durable for three participants [2.8 months (censored), 6.3 months, and 43.4 months]. Although the PR was not confirmed, one participant with SD as their best overall response, due to a nonevaluable RECIST assessment after experiencing a PR in cycle 32, remained on treatment for 24 months. Percentage change in the target lesion from baseline for participants with melanoma, head or neck cancer, or other SCC is illustrated in Fig. 2B.
Figure 2.
Waterfall plot of all evaluable participants according to indication (A) and spider plot of participants with head and neck tumors (including SCC and adenocarcinoma), SCC with various primary origins, and melanoma (B). Sum of the diameter of target lesions post-baseline was not available for three patients. aPositive PDL1 status was defined as TC or IC > 0; bthe participant with head and neck cancer who experienced a CR had SCC of the head and neck; the other five cases of head and neck cancer were adenocarcinomas. NE, not evaluable; PD, progressive disease; SLD, sum of (target) lesion; Ukn, unknown.
Discussion
FAP-IL2v was designed to overcome the limitations of high-dose IL2 therapy by selectively promoting immune responses in the microenvironment of FAP-overexpressing tumors while minimizing known side effects. In this phase I study using patients with advanced/metastatic solid tumors, FAP-IL2v had an acceptable safety profile at the tested doses. Despite targeting, systemic IL2 effects were evident from the moment of infusion. Importantly, central nervous system and skin toxicities were not observed. The MTD was reached at 15/20 mg with an uptitration regimen and was selected for dose expansion. AEs consistent with the class-specific effects of IL2 were manageable, and no unexpected safety signals were observed.
FAP-IL2v delivered a systemically higher exposure than high-dose IL2. Due to its prolonged half-life, a sevenfold higher exposure (defined by area under the concentration–time curve) was seen with the 10 mg flat dose compared with a full cycle of conventional high-dose IL2 therapy (720 kIU/kg every 8 hours, days 1–5; ref. 12). Serum concentrations of FAP-IL2v follow TMDD with a time effect likely due to target induction, while serum exposure to FAP-IL2v decreased following multiple cycles of administration (likely because of the PD effect of IL2 increasing the number of IL2Rs during treatment). In contrast to previously reported observations seen with CEA-IL2v (4), no significant effect on ADAs (including at high titers) from FAP-IL2v exposure was noted.
Treatment and management of advanced solid tumors remain challenging despite the development of new therapeutic agents. IL2 has been used alone or in combination with other cancer therapies and has been shown to induce durable responses (2, 13, 14). Although objective antitumor activity in this study was only demonstrated in three participants (melanoma and SCC), confirmed responses were deep and durable, highlighting the potential benefit of IL2 therapy in certain patients.
PDL1 expression can indicate that a sufficient immune cell tumor infiltration exists in the microenvironment (15), and that antitumor immune responses might be augmented with FAP-IL2v. Notably, there was no obvious relationship between antitumor activity and PDL1 status.
Preclinical models and early-phase clinical trials suggest a distinct mechanism of action (through the activation and proliferation of T and NK cells) by which IL2 treatment exerts its effects (16). We report changes in the median density of NK and CD8 T cells, but not in the percentage of Tregs, in the peripheral blood that were detected following FAP-IL2v administration, highlighting the benefits of IL2v over WT IL2 (1). The tolerability of FAP-IL2v seems better than that reported for high-dose IL2; this is mainly due to a lower incidence and severity of AEs, particularly capillary leak syndrome, which requires close patient monitoring in hospital (2). Although reducing the dose of IL2 could result in reduced toxicity, low-dose regimens are not as clinically active and effective, possibly because in low abundancies, IL2 binds with high affinity to receptors expressed on Tregs (2). Unlike the preferential increase in Tregs among CD4+ T cells that was described for patients treated with IL2 (17, 18), FAP-IL2v preferentially expanded CD8+ T cells and, to a lesser extent, CD4+ T cells including Tregs but did not increase the percentage of Tregs among CD4+ T cells. The benefit of targeting FAP-positive cells in the tumor microenvironment cannot be defined from this study. The development of ADAs may impact tumor uptake and retention in the tumor microenvironment, resulting in reduced intratumoral IL2v exposure and dampened PD effects. Although FAP-specific probes are under clinical investigation for tumor imaging, insights into the FAP-IL2v retention pattern in patients will require dedicated biodistribution studies like those performed with the CEA-targeted IL2v construct (Lassen and colleagues, manuscript in preparation; refs. 4, 11).
Conclusion
Targeting FAP-expressing tumors with IL2v therapy has the potential to provide an augmented antitumor immune response alongside an improved PK profile for convenient dosing schedules, and a manageable safety profile that seems favorable compared with conventional high-dose IL2 therapies.
Early signs of clinical activity in melanoma and SCC provide a rationale for further investigation of FAP-IL2v in combination with other agents in patients with advanced cancers. In particular, the ability of FAP-IL2v to activate T and NK cells is expected to enhance the activity of checkpoint inhibitors and tumor-directed antibody therapies (including cetuximab, rituximab, and trastuzumab; refs. 19, 20, 21) in participants with advanced cancer.
At the time of submission of this article, the sponsor has decided to discontinue the development of FAP-IL2v for strategic reasons.
Supplementary Material
Supplementary Methods
Supplementary Figure S1. Figure design. FAP-IL2v, fibroblast activation protein-α interleukin-2 variant; PD, progressive disease; QW, weekly.
Supplementary Table S1. Study representation of underserved communities.
Supplementary Table S2. Most frequent any-grade AEs (≥20%) across all doses and combined. AE, adverse event; ALT, alanine transaminase; AST, aspartate transaminase; FAP-IL2v, fibroblast activation protein-α interleukin-2 variant.
Acknowledgments
This study was funded by F. Hoffmann-La Roche Ltd. The authors would like to thank the participants, their families, and the participating study centers. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Rick Burgon, MRes, and Neave Baldwin, BSc, at Ashfield MedComms, an Inizio Company, and funded by F. Hoffmann-La Roche Ltd.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors’ Disclosures
N. Steeghs reports grants from Roche to N. Steeghs's institution during the conduct of the study; providing consultation or attending advisory boards for Boehringer Ingelheim, Bristol Myers Squibb, Ellipses Pharma, GlaxoSmithKline, and Incyte; and research grants from AbbVie, Actuate Therapeutics, Amgen, Anaveon, Ascendis Pharma, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, CellCentric, Cogent Biosciences, Crescendo Biologics, Deciphera, Exelixis, Genentech, GlaxoSmithKline, IDRx, Immunocore, Incyte, Janssen, Kling Biotherapeutics, Lixte, Merck, Merck Sharp & Dohme, Merus, Molecular Partners, Novartis, Pfizer, Revolution Medicines, Roche, Sanofi, Seattle Genetics, Taiho, and Zentalis outside the submitted work, all payment to the Netherlands Cancer Institute. C. Gomez-Roca reports grants from Roche/Genentech during the conduct of the study, as well as personal fees from Bristol Myers Squibb, Ellipses Pharma, PharmaMar, Macomics, Pierre Fabre, and PSAD, grants from Amgen, and other support from Amunix, IDEAYA, and Kazia outside the submitted work. K.S. Rohrberg reports grants from Roche during the conduct of the study, as well as grants from Eli Lilly and Company, Bristol Myers Squibb, Symphogen, Pfizer, Incyte, Genmab, Puma Biotechnology, Orion Clinical, BioInvent, MonTa Biosciences, Amgen, Navire, Genentech, and Roche, personal fees from AbbVie, and grants and personal fees from MSD, Bayer, and GSK outside the submitted work. M. Mau-Sørensen reports personal fees from Roche and grants from Roche during the conduct of the study, as well as personal fees from Astellas, Bristol Myers Squibb, Daiichi Sankyo Nordics, and Takeda, grants from Astellas and MSD, and nonfinancial support from Roche Diagnostics, Agilent, and MSD outside the submitted work. D. Robbrecht reports other support from Merck AG, MSD, Pfizer, Astellas, and AstraZeneca, grants from Merck AG, and other support from Janssen outside the submitted work. J. Tabernero reports personal fees from Alentis Therapeutics, AstraZeneca, Aveo Oncology, Boehringer Ingelheim, Cardiff Oncology, CARSgen Therapeutics, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd., Genentech, Inc., hC Bioscience, Ikena Oncology, Immodulon Therapeutics, Inspirna, Inc., Eli Lilly and Company, Menarini, Merck Serono, Merus, MSD, Mirati, NeoPhore, Novartis, ONA Therapeutics, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Scandion Oncology, Scorpion Therapeutics, Seattle Genetics, Servier, SOTIO Biotech, Taiho, Takeda Oncology, TOLREMO Therapeutics, Oniria Therapeutics, Alentis Therapeutics, Pangaea Oncology, 1TRIALSP, Medscape Education, and PeerView Institute for Medical Education and Physicians Education Resource outside the submitted work. C. Ardeshir reports other support from Roche Products Ltd. during the conduct of the study, as well as other support from Roche Products Ltd. outside the submitted work. D. Schmid reports employment with Roche. H. Piper-Lepoutre reports previous employment with Roche. D. Dejardin reports personal fees from Roche during the conduct of the study, as well as personal fees from Roche outside the submitted work. S. Evers reports employment and ownership of stock with F. Hoffmann-La Roche AG. C. Boetsch reports personal fees from F. Hoffmann-La Roche during the conduct of the study, as well as personal fees from F. Hoffmann-La Roche outside the submitted work. J. Charo reports personal fees from Roche during the conduct of the study; personal fees from Roche outside the submitted work; a patent for EPO. 22207100.3 pending; and ownership of Roche stock. V. Teichgräber reports other support from Roche during the conduct of the study, as well as other support from Roche outside the submitted work. I. Melero reports grants and personal fees from Roche during the conduct of the study, as well as grants and personal fees from Bristol Myers Squibb, AstraZeneca, and Genmab and personal fees from PharmaMar, Curon, Pioneer, Pierre Fabre, Boehringer Ingelheim, HotSpot, Highlight Therapeutics, Bright Peak, Amunix, Boston Therapeutics, F-star, and Servier outside the submitted work. No disclosures were reported by the other authors.
Authors’ Contributions
N. Steeghs: Investigation, writing–review and editing, critically reviewed the manuscript and approved it for submission. C. Gomez-Roca: Writing–review and editing, involved in the data interpretation, critically reviewed the manuscript and approved it for submission. K.S. Rohrberg: Investigation, writing–review and editing, involved in the data interpretation, critically reviewed the manuscript and approved it for submission. M. Mau-Sørensen: Writing–review and editing, involved in the data interpretation, critically reviewed the manuscript and approved it for submission. D. Robbrecht: Investigation, writing–review and editing, involved in the data interpretation, critically reviewed the manuscript and approved it for submission. J. Tabernero: Conceptualization, investigation, writing–review and editing, critically reviewed the manuscript and approved it for submission. S. Ahmed: Writing–review and editing, involved in the data interpretation, critically reviewed the manuscript and approved it for submission. M.E. Rodriguez-Ruiz: Writing–review and editing, involved in the data interpretation, critically reviewed the manuscript and approved it for submission. C. Ardeshir: Writing–review and editing, involved in the data interpretation,critically reviewed the manuscript and approved it for submission. D. Schmid: Writing–review and editing, involved in the data interpretation, critically reviewed the manuscript and approved it for submission. N. Sleiman: Writing–review and editing, involved in the data interpretation, critically reviewed the manuscript and approved it for submission. C. Watson: Writing–review and editing, involved in the data interpretation, critically reviewed the manuscript and approved it for submission. H. Piper-Lepoutre: Writing–review and editing, involved in the data interpretation, critically reviewed the manuscript and approved it for submission. D. Dejardin: Writing–review and editing, involved in the data interpretation, critically reviewed the manuscript and approved it for submission. S. Evers: Writing–review and editing, involved in the data interpretation, critically reviewed the manuscript and approved it for submission. C. Boetsch: Writing–review and editing, involved in the data interpretation, critically reviewed the manuscript and approved it for submission. J. Charo: Writing–review and editing, involved in the data interpretation, critically reviewed the manuscript and approved it for submission. V. Teichgräber: Writing–review and editing, involved in the data interpretation, critically reviewed the manuscript and approved it for submission. I. Melero: Investigation, writing–review and editing, involved in the data interpretation, critically reviewed the manuscript and approved it for submission.
References
- 1. PROLEUKIN® (aldesleukin) . Prescribing information. Clinigen, Inc. [Cited 2023 Sep 25]. Available from: https://www.proleukin.com/PI/Proleukin%20Prescribing%20Information.pdf. [Google Scholar]
- 2. Mullard A. Restoring IL-2 to its cancer immunotherapy glory. Nat Rev Drug Discov 2021;20:163–5. [DOI] [PubMed] [Google Scholar]
- 3. Kontermann RE. Antibody-cytokine fusion proteins. Arch Biochem Biophys 2012;526:194–205. [DOI] [PubMed] [Google Scholar]
- 4. Müller D. Antibody-cytokine fusion proteins for cancer immunotherapy: an update on recent developments. BioDrugs 2014;28:123–31. [DOI] [PubMed] [Google Scholar]
- 5. King DM, Albertini MR, Schalch H, Hank JA, Gan J, Surfus J, et al. Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J Clin Oncol 2004;22:4463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weide B, Eigentler TK, Pflugfelder A, Zelba H, Martens A, Pawelec G, et al. Intralesional treatment of stage III metastatic melanoma patients with L19-IL2 results in sustained clinical and systemic immunologic responses. Cancer Immunol Res 2014;2:668–78. [DOI] [PubMed] [Google Scholar]
- 7. Connor JP, Cristea MC, Lewis NL, Lewis LD, Komarnitsky PB, Mattiacci MR, et al. A phase Ib study of humanized KS-interleukin-2 (huKS-IL2) immunocytokine with cyclophosphamide in patients with EpCAM-positive advanced solid tumors. BMC Cancer 2013;13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Waldhauer I, Gonzalez-Nicolini V, Freimoser-Grundschober A, Nayak TK, Fahrni L, Hosse RJ, et al. Simlukafusp alfa (FAP-IL2v) immunocytokine is a versatile combination partner for cancer immunotherapy. MAbs 2021;13:e1913791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu R, Li H, Liu L, Yu J, Ren X. Fibroblast activation protein: a potential therapeutic target in cancer. Cancer Biol Ther 2012;13:123–9. [DOI] [PubMed] [Google Scholar]
- 10. Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med 2019;60:801–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klein C, Waldhauer I, Nicolini VG, Freimoser-Grundschober A, Nayak T, Vugts DJ, et al. Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. Oncoimmunology 2017;6:e1277306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Piscitelli SC, Forrest A, Chaitt D, Metcalf J, Stevens R, Baseler M, et al. Pharmacokinetic modeling of recombinant interleukin-2 in patients with human immunodeficiency virus infection. Clin Pharmacol Ther 1998;64:492–8. [DOI] [PubMed] [Google Scholar]
- 13. Jiang T, Zhou C, Ren S. Role of IL-2 in cancer immunotherapy. Oncoimmunology 2016;5:e1163462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenberg SA. Immersion in the search for effective cancer immunotherapies. Mol Med 2021;27:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mulé JJ, Yang JC, Afreniere RL, Shu SY, Rosenberg SA. Identification of cellular mechanisms operational in vivo during the regression of established pulmonary metastases by the systemic administration of high-dose recombinant interleukin 2. J Immunol 1987;139:285–94. [PubMed] [Google Scholar]
- 16. Ribas A, Hu-Lieskovan S. What does PD-L1 positive or negative mean? J Exp Med 2016;213:2835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmadzadeh M, Rosenberg S. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood 2006;107:2409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mortara L, Balza E, Bruno A, Poggi A, Orecchia P, Carnemolla B. Anti-cancer therapies employing IL-2 cytokine tumor targeting: contribution of innate, adaptive and immunosuppressive cells in the anti-tumor efficacy. Front Immunol 2018;9:2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mimura K, Kono K, Hanawa M, Kanzaki M, Nakao A, Ooi A, et al. Trastuzumab-mediated antibody-dependent cellular cytotoxicity against esophageal squamous cell carcinoma. Clin Cancer Res 2005;11:4898–904. [DOI] [PubMed] [Google Scholar]
- 20. Srivastava S, Lundqvist A, Childs RW. Natural killer cell immunotherapy for cancer: a new hope. Cytotherapy 2008;10:775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawaguchi Y, Kono K, Mimura K, Sugai H, Akaike H, Fujii H. Cetuximab induce antibody-dependent cellular cytotoxicity against EGFR-expressing esophageal squamous cell carcinoma. Int J Cancer 2007;120:781–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods
Supplementary Figure S1. Figure design. FAP-IL2v, fibroblast activation protein-α interleukin-2 variant; PD, progressive disease; QW, weekly.
Supplementary Table S1. Study representation of underserved communities.
Supplementary Table S2. Most frequent any-grade AEs (≥20%) across all doses and combined. AE, adverse event; ALT, alanine transaminase; AST, aspartate transaminase; FAP-IL2v, fibroblast activation protein-α interleukin-2 variant.
Data Availability Statement
Qualified researchers may request access to individual participant–level data through the clinical study data request platform (https://vivli.org/ourmember/roche/). Further details on F. Hoffmann-La Roche Ltd's criteria for eligible studies are available at https://vivli.org/members/ourmembers/. For further details on F. Hoffmann-La Roche Ltd's Global Policy on the Sharing of Clinical Information and the procedure to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.