Abstract
Background
Silicosis, characterized by interstitial lung inflammation and fibrosis, poses a significant health threat. ATII cells play a crucial role in alveolar epithelial repair and structural integrity maintenance. Inhibiting ATII cell senescence has shown promise in silicosis treatment.
However, the mechanism behind silica-induced senescence remains elusive.
Methods
The study employed male C57BL/6 N mice and A549 human alveolar epithelial cells to investigate silicosis and its potential treatment. Silicosis was induced in mice via intratracheal instillation of crystalline silica particles, with honokiol administered intraperitoneally for 14 days. Silica-induced senescence in A549 cells was confirmed, and SIRT3 knockout and overexpression cell lines were generated. Various analyses were conducted, including immunoblotting, qRT-PCR, histology, and transmission electron microscopy. Statistical significance was determined using one-way ANOVA with Tukey's post-hoc test.
Results
This study elucidates how silica induces ATII cell senescence, emphasizing mtDNA damage. Notably, honokiol (HKL) emerges as a promising anti-senescence and anti-fibrosis agent, acting through sirt3. honokiol effectively attenuated senescence in ATII cells, dependent on sirt3 expression, while mitigating mtDNA damage. Sirt3, a class III histone deacetylase, regulates senescence and mitochondrial stress. HKL activates sirt3, protecting against pulmonary fibrosis and mitochondrial damage. Additionally, HKL downregulated cGAS expression in senescent ATII cells induced by silica, suggesting sirt3's role as an upstream regulator of the cGAS/STING signaling pathway. Moreover, honokiol treatment inhibited the activation of the NF-κB signaling pathway, associated with reduced oxidative stress and mtDNA damage. Notably, HKL enhanced the activity of SOD2, crucial for mitochondrial function, through sirt3-mediated deacetylation. Additionally, HKL promoted the deacetylation activity of sirt3, further safeguarding mtDNA integrity.
Conclusions
This study uncovers a natural compound, HKL, with significant anti-fibrotic properties through activating sirt3, shedding light on silicosis pathogenesis and treatment avenues.
Keywords: Silicosis, sirtuin3, Mitochondrial DNA damage, Type II alveolar epithelial cell, Senescence
Graphical abstract
1. Introduction
As a life-threatening pulmonary interstitial disease, silicosis has become the most serious occupational disease worldwide. Cellular senescence is an irreversible cell cycle arrest and is one of the hallmarks of a variety of interstitial fibrosis diseases [1,2]. Unlike other forms of growth arrest, senescent cells have a lower replicative capacity but retain metabolic activity [3]. Senescent type II alveolar epithelial cells (AT2s) driven by stress stimulation play a prominent role in the remodeling process of pulmonary fibrosis diseases. ATs have an absolute advantage in the composition of the alveolar area, which makes them more likely to be damaged by silica particles at the first time compared with other cells in lung tissue. Moreover, as important "stem cells" for alveoli restoration, AT2s are responsible for maintaining the integrity of the alveolar structure during the sustained injured-repair process of the alveolar epithelium. When senescent AT2s lose the capacity of stem cells to self-renew and differentiate, it not only impedes the repairment of damaged lung tissues but also constructs the positive feedback loops of contribution to induce senescence in healthy cells depending on a complex senescence-associated secretory phenotype (SASP) [4]. Previous studies have found that eliminating senescent alveolar epithelial cells can effectively mitigate idiopathic pulmonary fibrosis (IPF), and chronic pulmonary diseases including silicosis [5,6]. Hence, understanding the mechanism of senescent alveolar epithelial cells induced by silica may provide a new perspective to clarify the pathogenesis and therapeutic options of silicosis.
Dysregulated mitochondrial integrity and function are the major contributors to cellular senescence [7]. First of all, mitochondria are closely linked to cellular fate, and as the "energy factory" in cells, they are the important places of cellular metabolism [8]. On the other hand, mitochondria are also the major producers of by-production of ATP production, and endogenous reactive oxygen species (ROS) [9]. ROS have been demonstrated to be the crucial factor in driving the aging process based on the mitochondrial free radical theory of aging (MFRTA) [10]. In addition, high levels of pro-inflammatory mediators can be found in the serum and lungs of silicosis patients. Inflammation is a common theme among multiple fibrotic diseases, including silica. In this respect, the release of damaged mitochondrial DNA (mtDNA), as a type of damage-associated molecular patterns (DAMPs) in silicosis and other pulmonary interstitial diseases is proportional to the inflammation level of these diseases. As early as a 2018 study [11], a self-nucleic acid sensing molecule, STING has been experimentally confirmed to be a key mediator of silica-induced lung disease. In addition, the level of dsDNA in the plasma of silicosis patients was significantly higher than that of healthy people, and the cGAS-STING pathway was activated in the lung tissues of silicosis patients and dust-exposed mice. Activated STING translocates from the ER membrane to the Golgi apparatus, induces nuclear factor κB (NF-κB) and interferon regulatory factor 3 (IRF3) transcription factors activation to regulate the host inflammation and convey senescence through the secretion of SASP.
Many natural products with excellent pharmacological properties are reported to be good candidates for resistance senility. Honokiol (HKL) is a natural bioactive phenylpropanoid compound from the Chinese herbal medicine, Magnolia officinalis. Honokiol processes multiple pharmacological activities and health benefits without corresponding toxicity [12]. Previous studies have confirmed that HKL has favorable properties in the treatment of a variety of interstitial fibrosis diseases. Although its role in the antagonism towards senescence has been demonstrated, the anti-aging mechanism of HKL is still unclear. Recently, HKL has been reported to prevent senescence via sirt3 activation [13] and decrease renal inflammation and fibrosis via regulation of mitochondrial dynamics and the NF-κb/TGF-β1/Smad signaling pathway [14]. Sirtuin 3 (sirt3), as a mitochondrial deacetylase, is a vital antiaging molecule and antioxidant through deacetylating and activating SOD2 [15]. The protective effects of sirt3 have been widely confirmed on a variety of age-related diseases, such as insulin resistance, cancer, cardiovascular diseases, and pulmonary fibrosis from in vivo and in vitro models [[16], [17], [18], [19]]. However, the exact molecular mechanism of anti-aging and anti-fibrosis of HKL targeting sirt3 in silicosis has been described. There is a putative idea that a targeted sirt3 HKL is a potential therapy for regulating the ATII aging phenotype. To clarify its functions in silicosis fibrosis, we use a variety of pharmacological, molecular, and genetic approaches to uncover this issue both in vitro and in vivo.
2. Materials and methods
2.1. Silicotic model
Male C57BL/6 N mice (4 weeks) were purchased from the Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). The mice were housed in a specific pathogen-free environment and maintained on standard chow at a temperature of 24 ± 1 °C and a 12/12 h light/dark cycle with ad libitum access to water. The silicotic model was developed in a previous study [20]. Briefly, mice were anesthetized with 0.3 % pentobarbital sodium (2 mL/100 g) and were either administered 50 μL of crystalline silica particles suspension (50 mg/mL, diameter 3−5 μm. SILICA Co., Ltd., USA) by intratracheal instillation as the model group or sterile PBS buffer as the control group. Mice in the HKL group received daily HKL (5 mg/kg body weight) by intraperitoneal injection for 14 days after exposure to silica, then mice were sacrificed on day 28 and day 56 after treatment with silica, separately. The work was conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Laboratory Animal Ethical Committee of the North China University of Science and Technology (LAEC-NCST-2020157), Tangshan, China.
2.2. Cell culture
Human type II alveolar epithelial A549 cells (American Type Culture Collection, Manassas, VA, USA) were cultured in DMEM high glucose medium with 10 % fetal bovine serum supplemented with penicillin-streptomycin (100 U/ml and 100 μg/ml, respectively) at 37 °C in a 5 % CO2 humidified atmosphere. To obtain A549 cells with characteristics of type II alveolar epithelial cells, we subjected A549 cells to long-term culture (passage 30 to 50) at a liquid/air interface for the in vitro experiments. Then A549 was spread in the various cell culture plates at the appropriate density, which were subsequently transformed to senescent cells induced by 50 mg/cm2 silica particles for 120 h.
2.3. Generation of SIRT3−/− A549
CRISPR/Cas9-mediated gene knockout was performed following previously reported methods with minor modifications [21]. In brief, sgRNAs were designed using the website http://crispr.mit.edu. Sequences representing sgRNAs (detailed sequences of sgRNAs shown in Supplementary Table S1) targeting exon 3 of SIRT3 were cloned into pX330-U6-Chimeric_BB-CBh-hSpCas9 vector (Addgene, #42230). When A549 cells in 12-well plates reach approximately 70–80 % confluency, pX330-U6-Chimeric_sirt3_sg1-CBh-hSpCas9 and pX330-U6-Chimeric_sirt3_sg2-CBh-hSpCas9 were simultaneously transfected into A549 cells by Kemix-TRLIP according to the manufacturer's instructions (Kemix, Beijing, China). After the accomplishment of transfection, cells were seeded on 12-well plates at a dilution of 1/10 and cultured with ampicillin medium. After 144 h, A549 cells with the ampicillin-resistant gene were adjusted to an appropriate density and seeded on 96-well plates with one or fewer cells per well. Emerging clones were manually picked out for further verification. Genomic DNAs from these A549 clones were extracted for PCR and DNA sequencing. sgRNA sequences for gene editing and primers for clone identification are listed in Supplementary Table S1. Immunoblotting and PCR were used to verified that sirt3−/− A549 cell line was successfully constructed, and the results were shown in Supplementary Fig. S2G & S2H. The sirt3−/− A549 generated by CRISPR/Cas9-mediated gene editing was used for further analysis in this study.
2.4. Generation of SIRT3 overexpression cell line
The pcDNA3.1-sirt3-EGFP plasmid (Fenghuishengwu, Hunan, China) used to overexpress sirt3, was imported into the A549 in 6-well plates. Firstly, Kemix-TRLIP was diluted in Opti-MEM medium (Thermo Fisher Scientific, Madison, USA). Then, the master mix of pcDNA3.1-sirt3-EGFP/DNA was prepared by diluting pcDNA3.1-sirt3-EGFP/DNA in the Opti-MEM medium. After that, the diluted plasmids/DNA was added into diluted Kemix-TRLIP at a 1:1 ratio and incubated for 20 min at room temperature. Finally, the plasmid/DNA-lipid complex was added into cells incubating for 4–6 h. Immunoblotting was used to analyze the transfection efficiency of the plasmid, and the result was shown in Supplementary Fig. S2D.
2.5. Cell viability analysis
Cell viability was assessed using the Cell Counting Kit-8 (CCK-8) assay (Beyotime, Beijing, China) following the manufacturer's instructions. Following the respective treatments, 100 μL of DMEM high glucose medium supplemented with CCK-8 at a 10:1 dilution ratio was added to the cells and incubated in the dark for 1 h. The absorbance of each well was measured at 450 nm using a Synergy H1M2 microplate reader (BioTek, USA), reflecting the survival rate of the cells.
2.6. Histologic analysis
The inferior lobe of the right lung was collected and washed with phosphate buffer solution to remove blood, then fixed in 4 % formalin for 48h. The following paraffin embedding and staining were performed at Biopathology Institute Co., Ltd. (Kunisaki, Oita, Japan) for histopathological evaluation. Lung samples were embedded in paraffin, and sections were cut into 4 μm and subjected to hematoxylin-eosin (H&E) and Masson tricolor (Masson) staining, separately. Representative images of the four groups were acquired using an upright light microscope (Leica DM3000, Carl).
To assess the level of inflammation in lung tissues of mice (n = 6), we scored on H&E-stained slides based on inflammatory cell counts [22]. Two different methods were conducted for quantitative analysis of pulmonary fibrosis (n = 6). One is to score the degree of pulmonary fibrosis by using the Modified Ashcroft Scale based on the H&E-stained slides [23,24]. Briefly, two observers selected 5 fields from each slice at 200x magnification, randomly, scored on a scale from 0 (normal lung) to 8 (complete fibrosis), and calculated the average score of each slice as its fibrosis score. During the above process, we obey the principle of double-blind. Another one, the digital slices stained by Masson trichrome were used to evaluate pulmonary fibrosis on Image J software detecting and calculating percentages that are the areas of the blue part (collagen) to the area of the lung tissue, termed collagen volume fraction (CVF) [22].
2.7. Transmission electron microscope
The fresh lung tissue blocks, measuring 1 mm3, extracted from mice, were rapidly added to electron microscope fixation solution (Servicebio, Wuhan, China) for fixing 2–4 h at 4 °C. Then post-fixed specimens were sent to Servicebio Co., Ltd (Wuhan, China) for following treatment and observation by transmission electron microscopy (TEM). Briefly, fixed lung tissue blocks were meticulously embedded into Agar 100 Resin® (Agar, Essex, England), an epoxy-based medium, and subjected to polymerization at 60 °C for 3 days. Following this, the embedded tissue blocks underwent precise trimming using a diamond trimmer (Reichert TM 60, Austria). Subsequently, ultrathin sections (80 nm) were skillfully sliced utilizing a Leica Ultracut E ultramicrotome (Leica, Nussloch, Germany). These sections were then treated with uranyl acetate (2 min) and lead citrate (45 s) to enhance contrast. The processed sections were meticulously scrutinized employing a transmission electron microscope (Hitachi TEM system, Tokyo, Japan).
2.8. Immunofluorescence and immunohistochemistry staining
For in vivo immunofluorescence staining, the lung sections were fixed with 4 % paraformaldehyde, and permeabilized with 0.5 % Triton X100 for 30 min. To prevent nonspecific binding, the sections were blocked with 3 % bovine serum albumin for 1 h. Specific primary antibodies were then added and incubated overnight at 4 °C. After washing with PBS 3 times, the tissues were incubated with the corresponding secondary antibodies at room temperature and then washed 3 times again. Finally, DAPI was used for counterstaining the cell nuclei. Images were captured using a Leica Microsystems TCS SP2 confocal microscope (Leica, Wetzlar, Germany). As for in vitro immunofluorescence staining, after different treatments, the cells seeded in confocal dishes were rinsed with PBS, and the subsequent procedures were similar to in vivo experiments.
Immunohistochemical analysis was executed following a well-established methodology [25]. In brief, 5-μm-thick sections of formalin-fixed paraffin-embedded lung tissue blocks underwent deparaffinization utilizing xylene, followed by hydration through a graded series of ethanol solutions. Antigen retrieval was conducted employing a 10 mM Tris/EDTA buffer solution (pH 9.0) at 100 °C for 20 min. The sections underwent a permeabilization process, after which they were blocked and exposed to anti-sirt3 antibody. Chromogenic detection entailed the use of a HRP-conjugated secondary antibody (30 min) and DAB reagents (5 min). The sections were counterstained utilizing 0.1 % hematoxylin. Proficient immunohistochemical staining was conducted by a skilled pathology team at the Servicebio laboratory (Wuhan, China). Immunohistochemical images were captured utilizing an upright light microscope (Leica DM3000, Carl).
2.9. Senescence-associated β-galactosidase staining
Lung tissues or Cells were plated on microscope slides or 12-well plates and then detected with a senescence-associated β-galactosidase Staining Kit (Beyotime, Beijing, China) following the manufacturer's instructions.
2.10. Coimmunoprecipitation and western blotting
The cells were harvested and centrifuged at 3000 rpm (Centrifuge 5424R, Eppendorf, Germany) for 5 min to acquire the total protein samples of cells. For the mitochondrial protein samples, Cell Mitochondria Isolation Kit (Beyotime, Beijing, China) was employed for obtaining mitochondria, before extracting the mitochondrial protein samples. Then cells or mitochondria were cleaved with RIPA buffer containing a 1X protease and phosphatase inhibitor cocktail (Beyotime, Beijing, China) and a 1X deacetylase inhibitor cocktail (Beyotime, Beijing, China) on ice after a 30-min, followed by high-speed centrifugation. A small portion of the supernatant was used for detecting the protein concentration by Pierce™ BCA Protein Assay Kits (Thermo Fisher Scientific, Waltham, USA), and 10 % of the lysate supernatant was reserved as the input sample. The remaining supernatant was then incubated with the specified antibodies. The mixture was gently rotated overnight at 4 °C, and subsequently, 25 μL protein A/G Plus-agarose beads (Beyotime, Beijing, China) were added for an additional 2 h. Following four washes with TBS, the immunocomplexes were boiled in an SDS loading buffer at 98 °C for 10 min for western blotting analysis. Briefly, 30 μg protein samples per well were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE), followed by immunoblotting analysis. The sample proteins were transferred onto PVDF membranes, blocked in 5 % milk, and incubated with primary and secondary antibodies (Supplementary Table S2). Visualization of proteins was accomplished using an ImageQuant 800 imaging station (Cytiva, USA).
2.11. SIRT3 activity assay
A549 cells were cultured in 60-mm plates at a density of 1 × 10^6 cells. After silica or/and HKL treatment, total proteins of cells were acquired for conducting immunoprecipitation with the SIRT3 antibody. For mice tissue samples, the tissue was weighed (approximately 20 mg wet weight) for extracting the total protein samples, then immunoprecipitation with the SIRT3 antibody was conducted to acquire SIRT3 proteins for the subsequent SIRT3 activity assay, as previously described [26].
The SIRT3 Direct Fluorescent Screening Assay Kit (Cayman Chemical, MI, USA) was utilized to assess the SIRT3 deacetylase activity, following to the manufacturer's guidelines. The fluorescence of each well was measured at 355 nm and 460 nm using a Synergy H1M2 microplate reader (BioTek, USA), respectively. Three replicate samples were tested for each group.
2.12. Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from the upper left lung lobe of mice with TRIzol™ Plus RNA Purification Kit (Invitrogen, USA) complying with the instructions. The RNA was reverse transcribed to cDNA based on the SuperScript III Reverse Transcriptase (Thermo Fisher Scientific, USA). Then cDNA was analyzed by SYBR GreenER™ qPCR SuperMix Universal (Invitrogen, USA) with an AriaMx Real-time PCR system (Aglient Technologies, USA). Here we calculated 2−ΔΔCT values to evaluate the relative mRNA expression. β-actin, a housekeeping gene, was used as the internal control for normalization. Results were calculated relative to the control group at each time point. The sequences of corresponding primers are listed in Supplementary Table S3.
2.13. MtDNA damage analysis
To assess mtDNA damage, total DNA was isolated from frozen lung tissue (approximately 25 mg wet weight) or A549 (800, 000 cells) using the Qiagen DNeasy Tissue Kit (Qiagen, China) and quantified using the PicoGreen dsDNA Assay Kit (Invitrogen, USA). Damage to mtDNA was then assessed using a quantitative polymerase chain reaction method, as previously described [27]. Briefly, based on the ratio of the amount of long amplicons (long mitochondrial DNA fragments ∼10 kb, Mus; ∼8.9 kb, Homo) and short amplicons (gene ND1 >160 bp), the extent of DNA damage was calculated. Primers are listed in Supplementary Table S4.
2.14. Immunofluorescence staining of cytosolic dsDNA and 8-OHdG
After treatment with Silica or Silica + HKL, A549 cells were fixed in 4 % paraformaldehyde dissolved in PBS. Cells were permeabilized using 0.1 % Triton X-100 on ice for 30 min; then, cells were blocked with 5 % bovine serum for 1 h at room temperature. Then, cells were incubated with primary anti-dsDNA (1:100, PROGEN) or anti-8-OHdG-FITC (1:100, Santa Cruz Biotechnology) and MitoTrackerRedCMXRos (1: 10000, Beyotime), for 3 h at room temperature, followed by incubation with FITC-labeled anti-IgM or FITC-labeled anti-IgG (1: 500, Beyotime) for an hour. Immunostaining images were collected using confocal microscopy.
2.15. Cytosolic mtDNA quantification
The amount of cytosolic mtDNA leakage was determined by quantification of mtDNA in the cytosolic extract [28]. Briefly, the cells (800, 000 cells) and lung tissues (approximately 25 mg wet weight) were lysed with 1 % NP-40 buffer, incubated on ice for 15min, and then divided into two equal parts, one of which was spun at 4 °C at 13000×g for 15min, and the supernatant was collected as the cytoplasmic component. Cytosolic fraction and corresponding total tissue or cellular homogenate were used to isolate cytosolic and total DNA using DNAeasy Blood & Tissue Kit (Qiagen), respectively. Amplify the mtDNA-specific gene ND1 (NADH dehydrogenase subunit 1) by qPCR method and use β-actin as an internal reference gene to calculate the relative fold change. Primers are listed in Supplementary Table S5. The relative cytosolic mtDNA was calculated and plotted by normalizing the Ct values of cytosolic mtDNA with the Ct values of total DNA.
2.16. Statistical analysis
Statistical analysis was performed using GraphPad Prism version 7.2.0 (GraphPad Software, Inc., California, USA). Data are expressed as the mean ± standard deviation (SD). One-way ANOVA or Two-way ANOVA was used to compare the differences among groups, followed by Tukey's post-hoc test. A p-value <0.05 was considered statistically significant. Graphical abstract was drawn by Figdraw and Adobe Illustrator.
3. Results
3.1. Honokiol (HKL) restored senescent ATII cells induced by silica via activating the deacetylation activity of sirt3
To determine whether silica induces ATII cell senescence, we initially used the CCK-8 assay to detect the cell viability of A549. As shown in Fig. 1A, the cell viability of A549 decreased after exposure to silica for 24 h in a dose-dependent manner. At the same time, there was an obvious difference in the numbers of β-gal positive cells on A549 treated with 50 μg/cm2 silica for 24 h (Fig. 1B and Supplementary Fig. S1A). In addition, the numbers of β-gal positive cells on A549 treated with 50 μg/cm2 silica for 0, 24, 48, 96, or 120 h (Fig. 1C and Supplementary Fig. S1B), have a significant increase in a time-dependent manner. The results of β-gal staining were consistent with the expression of senescence-related marker proteins, phosphorylation P53 (p-P53: Ser15), total P53, and P21, shown in Fig. 1D. The corresponding densitometry data were shown in Supplementary Fig. S1C & D. Therefore, A549 treated with 50 μg/cm2 silica for 120 h was subsequently used.
Fig. 1.
Honokiol (HKL) restored senescent ATII cells induced by silica via activating the deacetylation activity of sirt3. (A) Cell viability of ATII cells exposed to different concentrations of silica was measured by the cell counting kit 8 (CCK-8) assay (n = 5). (B, C) β-Gal staining was performed to evaluate the senescence in ATII cells to identify the concentration and time of silica stimulation for the construction of senescent ATII cells, Scale bars, 200 μm (n = 5). (D, G) Immunoblot analysis of p-P53, P53, and P21 in ATII cells with different treatments. (E, F) Cell viability of ATII cells was measured by the cell counting kit 8 (CCK-8) assay to identify the time for induction senescence of ATII cells and the intervention time of HKL, respectively (n = 5). (H) β-Gal staining was performed to evaluate the anti-aging effect of HKL. Scale bars, 200 μm (n = 5). (I, K) Immunofluorescence (I) and immunoblot (K) analysis of the abundance of sirt3 protein in HKL-treated senescent ATII cells. Scale bar, 50 μm (n = 5). (J, K) Immunoblot analysis of total acetylated-lysine proteins in A549 cells (J), EMT-related proteins, and senescence-related proteins in HKL-treat senescent ATII cells induced by silica (n = 4 or 5). (L–R) Relative mRNA levels of SASP genes, including IL-1α, IL-1β, IL-6, TNF-α, MCP-1, and CXCL1 in silica-treated ATII cells combined with HKL (n = 5). Bar graphs were presented as mean ± SD (n = 5). Statistical analysis was performed using one-way ANOVA, GraphPad Prism 7.2.0. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; n.s. presents no statistical difference. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Emerging experimental evidence has demonstrated that silica-induced alveolar epithelial cell senescence would aggravate the pathogenesis of pulmonary fibrosis [29]. To determine whether HKL protects ATII cells against silica particle injury, we initially used the CCK-8 assay to detect the cell viability of A549. As shown in Fig. 1E, the cell viability of A549 had significantly decreased after exposure to 50 μg/cm2 silica for 120 h and was restored by HKL in a dose-dependent manner. As well as, there was no obvious difference in the cell viability of A549 treated with 5 μmol/L HKL for 6, 12, or 24 h, except 48 h, as demonstrated in Fig. 1F.
To evaluate the effect of HKL on the senescence of ATII cells, β-gal staining, immunoblot, and qPCR analyses were performed to detect the abundance levels of senescent markers. The results of immunoblot analysis showed that the abundance of p-P53, total P53, and P21 were significantly decreased on senescent A549 induced by silica after treatment with 5 μmol/L HKL at 24h time point and 48h time point. (Fig. 1G and Supplementary Fig. S1E&F). To further assess the safety of HKL in the in vitro study, we employed the CCK-8 assay to assess cell viability at various time points. The results shown in Fig. 1F revealed a potential decrease in cell viability at a 48-h time point. Considering both drug safety and effectiveness, hence, we decided on using 5 μmol/L HKL for 24 h as the measure of intervention against senescence of ATII cells induced by silica in vitro. The results manifested that HKL could mitigate the epithelial-mesenchymal transition and senescence of A549 induced by silica (Fig. 1H & Supplementary Fig. S1G, Fig. 1K & Supplementary Fig. S1I-N). Upon senescence induction, it is often accompanied by a unique secretory phenotype that can either promote or suppress immune functions, depending on the expression profile of cytokines and chemokines, termed as senescence-associated secretory phenotype, SASP, which is involved in cell proliferation, inflammation, and induction of epithelial-mesenchymal transition (EMT) and promotion of fibrosis. The results of qPCR reveal that HKL could restrain the mRNA expression of six common SASP, IL-1α, IL-1β, IL-6, TNF-α, MCP-1, and CXCL1 in A549 exposure to silica (Fig. 1L-R).
To detect whether sirt3 was involved in HKL against senescent A549 induced by silica, we initially assessed the effect of HKL on the abundance of total acetylated-lysine and sirt3. The results show that the level of acetylated proteins was upregulated by silica as evidenced by the reduced abundance of sirt3 along with the increased acetylated-lysine abundance. However, they were reversed by HKL (Fig. 1I and J & Supplementary Fig. S1H, and & Supplementary Fig. S1H). Consistent with the results of abundance of sirt3, the decrease in deacetylation activity of sirt3 induced by SiO2 was alleviated by HKL (Supplementary Fig. S1O). The above findings suggest that HKL protected the senescent ATII cells induced by silica, which could be related to the deacetylation activity of sirt3.
3.2. HKL reduced mtDNA damage via a sirt3-dependent manner after exposure to silica
Currently, there are some reports that HKL could mitigate mitochondrial dysfunction by maintaining mitochondrial reactive oxygen species (mtROS) homeostasis [30]. Since mitochondrial DNA is bare, it is highly susceptible to oxidative damage from the attack of reactive oxygen species. Previous studies have suggested that sirt3 could enhance the activity of 8-oxo guanine DNA glycosylase-1 (OGG1) to repair oxidative damage of mtDNA by deacetylating OGG1 [31]. Therefore, we hypothesized that HKL could take effects via promoting the deacetylation activity of sirt3 to activate OGG1 repairing the oxidative damage of mtDNA. We first examined the production of ROS upon HKL treatment in senescent ATII cells induced silicosis using a Dihydroethidium (DHE) assay. ROS were accumulated in senescent ATII cells and were reduced in those treated with HKL, suggesting HKL could effectively reduce the accumulation of ROS induced by silica (Fig. 2A).
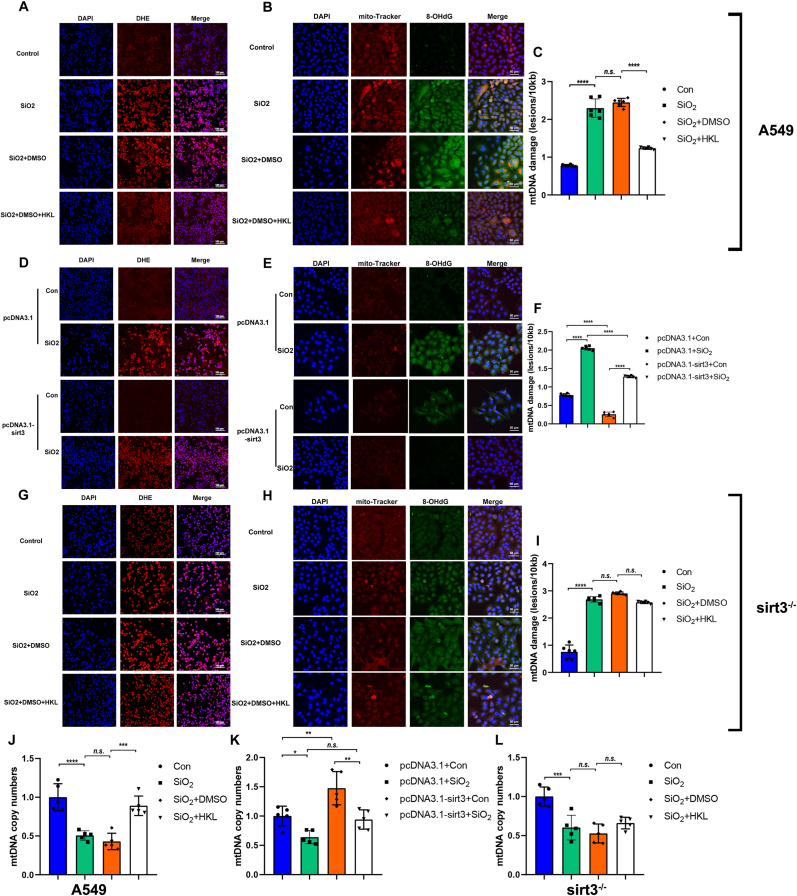
Fig. 2.
HKL reduced mtDNA damage via a sirt3-dependent manner after exposure to silica. (A, D & G) A549 cells (A), sirt3-OE A549 cells (D), and sirt3-KO A549 cells (G) were incubated ex vivo with DHE (red) (5 mM) in DMSO for 30 min before analysis of ROS production by fluorescence microscopy. Scale bar, 100 μm. (B, E & H) Images were taken by confocal microscopy of immunofluorescent staining of the 8-OHdG (green) and MitoTracker™ RED CMXROS (red) and DAPI (blue) in A549 cells (B), sirt3-OE A549 cells (E), and sirt3-KO A549 cells (H). Scale bar, 50 μm. Because permeabilization was not performed, the 8-OHdG in the nucleus was not stained. (C, F & I) Frequency of mtDNA lesions per 10 kb per strand in A549 cells (C), sirt3-OE A549 cells (F), and sirt3-KO A549 cells (I). (J–L) The quantitation of mtDNA copy number in A549 cells (J), sirt3-OE A549 cells (K), and sirt3-KO A549 cells (L). Bar graphs were presented as mean ± SD (n = 5 or 6). Statistical analysis was performed using one-way ANOVA, GraphPad Prism 7.2.0. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; n.s. presents no statistical difference. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Similarly, the 8-hydroxy-deoxyguanine (8-OHdG) as a biomarker for assessing oxidative damage of DNA and mtDNA lesions was also elevated in senescent ATII cells induced by silica, and was reduced in that treatment with HKL (Fig. 2B). Furthermore, the impact of mtDNA oxidative damage on mtDNA replication was assessed through the evaluation of mtDNA copy numbers and long-chain polymerase chain reaction (long-PCR) results. These findings exhibited consistency with those derived from the analysis of 8-OHdG (Fig. 2C and J).
Then to explore the role of sirt3 on the reduction of silica-induced oxidative stress and mtDNA damage by HKL, we generated an A549 cell line overexpressing sirt3 (sirt3oe-A549) and a sirt3 knock-out A549 (sirt3−/− A549) cell line through transfecting pcDNA3.1-sirt3 plasmid or CRISPR/Cas9-based gene editing, separately. As observed in Supplementary Fig. S2D&S2H, the abundance levels of sirt3 in different groups had been significantly changed. To further assess the impact of knockdown and overexpression on the activity of sirt3 and cellular senescence, we examined changes in the levels of total acetylated proteins and markers associated with cellular senescence. As illustrated in Supplementary Figs. 3E and 3F, the total acetylated protein level was significantly up-regulated after sirt3 knockdown, while after sirt3 overexpression, the total acetylated protein level was not significantly changed. Then the ROS and biomarkers of mtDNA damage in the sirt3oe-A549 cell line were significantly decreased compared to those in A549 cells of the negative control group simultaneously transfected in pcDNA3.1 under silica stimulation (Fig. 2D–E, and Fig. 2F). When the sirt3 was knocked out, the accumulation of ROS and degree of mtDNA damage in ATII cells would been aggravated under silica stimulation, and these changes would not been relieved with HKL treatment (Fig. 2G–H and I). Notably, the reduction in mtDNA copy number induced by silica treatment can be recovered by HKL, which also depends on sirt3 activity (Fig. 2J–K and L).
The results of immunoprecipitation of total acetylated-lysine proteins followed by immunoblotting with OGG1 also confirmed that the increase of acetylated OGG1 induced by silica would be attenuated by HKL, whether in A549 or sirt3oe A549. In addition, when the sirt3 was knocked out, the abundance levels of acetylated OGG1 would not be affected by HKL (Fig. 3A–F). As shown in Fig. 3A & B, there is no significant difference in acetylation in the same lane. The result is consistent with other relevant research results [32,33] concerning acetylation protein levels and SIRT3 activity. The consistent profiles suggested that sirt3 had a significant influence over the acetylation status of numerous mitochondrial proteins, and highlight the critical and broad role of SIRT3 in modulating mitochondrial protein acetylation under the current study. Taken together, these results indicated that HKL protects mitochondrial DNA and regulates oxidative stress of ATII cells under exposure to silica in a sirt3-dependent manner.
Fig. 3.
HKL attenuates silica-induced acetylation of OGG1 through sirt3. (A & B) Mitochondrial lysates from ATII cells with different treatments were used for immunoprecipitation with anti-acetylated lysine antibody and immune complexes analyzed by Western blots using anti-acetylated lysine antibody. Membranes were stripped and reprobed with anti-OGG1 antibody to determine the level of immunoprecipitated OGG1. The lower panel shows the OGG1 Western blot of input proteins and COX IV as the internal control. (C–F) The bar graph of immunoblot analysis of relative abundance levels of Ace-OGG1 in ATII cells treated as in Fig. 3A&B. Bar graphs were presented as mean ± SD (n = 3). Statistical analysis was performed using one-way ANOVA, GraphPad Prism 7.2.0. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; n.s. presents no statistical difference. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. HKL inhibited the cGAS-STING pathway by attenuating mtDNA leakage through activating sirt3
In addition, we found that the accumulation of cytosolic dsDNA in senescent ATII cells significantly was more than that in the control group. As well as the increased accumulation of cytosolic dsDNA would be reduced by HKL treatment (Fig. 4A). To confirm the source of the dsDNA, we examined the levels of mitochondrially encoded NADH dehydrogenase 1 (mtND1) of dsDNA in cytoplasm by qPCR. As depicted in Fig. 4B, the cytosolic mtDNA was elevated in ATII cells under silica stimulation, and the increase of cytosolic mtDNA in the SiO2 group was significantly reduced by HKL treatment. The results suggested that HKL could attenuate mtDNA leakage of ATII cells induced by silica. Consistent with the above findings of cytosolic mtDNA, sirt3oe A549 has effectively inhibited the contents of cytosolic dsDNA under silica stimulation compared to them in the pcDNA3.1+SiO2 group, and HKL had not significantly affected the contents of cytosolic dsDNA in sirt3−/− A549 (Fig. 4C–F).
Fig. 4.
HKL inhibited the cGAS-STING pathway by attenuating mtDNA leakage through activating sirt3. (A, C & E) Images taken by confocal microscopy of immunofluorescent staining of the dsDNA (green) and DAPI (blue) in A549 cells (A), sirt3-OE A549 cells (C), and sirt3-KO A549 cells (E). Scale bar, 50 μm. (B, D & F) The quantitation of cytosolic mtDNA in A549 cells (B), sirt3-OE A549 cells (D), and sirt3-KO A549 cells (F). Bar graphs were presented as mean ± SD (n = 5). Statistical analysis was performed using one-way ANOVA, GraphPad Prism 7.2.0. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; n.s. presents no statistical difference. (G, H & I) Immunoblot analysis of cGAS-STING pathway-related proteins in A549 cells (G), sirt3-OE A549 cells (H), and sirt3-KO A549 cells (I) induced by silica (n = 3). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
As a key pathway to regulate innate immune response, the STING pathway would be activated by cytosolic mtDNA following tissue injury to regulate sterile inflammation [34]. Furthermore, emerging reports have confirmed that the STING pathway promoted the production of senescence-associated secretory phenotype factors, relaying cellular senescence [35,36]. As a cytoplasmic DNA sensor, cGAS activation leads to the activation of its downstream target STING, followed by the phosphorylation of interferon regulatory factor 3 (IRF3) and driving the downstream NF-κB signaling pathway through a variety of mechanisms. Therefore, we carried out immunoblotting analysis on the proteins associated with the STING pathway. The results of immunoblotting showed that the abundance of cGAS, STING, p-TBK1, p-IRF3, p–NF–κB p65, p-IκBα were up-expression under the condition of sirt3 decreased or absent, and HKL inhibited the expression of those proteins dependent on the presence of sirt3 (Fig. 4G–I& Supplementary Fig. S4).
3.4. Honokiol (HKL) attenuated fibrosis and senescence in silicotic mice
To further support and extend the application of our data, we assessed the effect of HKL on reducing pulmonary fibrosis induced by silica in vivo. Based on previous experimental studies about silicosis, we established the silicotic mice model by intratracheal instillation 50 μL of 50 mg/mL crystalline silica particles suspension treatment, and then the mice were intraperitoneally administered with HKL at 5 mg/kg for 14 days, as shown in Fig. 5A. After that, H&E, Masson and senescence-associated β-galactosidase (SA β-gal) staining revealed the obvious pulmonary tissue fibrotic lesions in silica-induced mice, and HKL could reduce pulmonary fibrosis and cellular senescence (Fig. 5B & Supplementary Fig. S5A -5D). The corresponding quantitative results are presented in Supplementary Fig. S5. Besides, significant changes in mRNA levels of the well-known proinflammatory and profibrotic senescence-associated secretory phenotype (IL-1α, IL-1β, IL-6, TNF-α, MCP-1 and CXCL1) in lung tissue highlighted the effect of HKL on anti-fibrosis in vivo (Fig. 5C–H). The results of double-label immunofluorescent staining revealed that overlapping expression patterns for ATII cell marker, SP-C (green), and senescent cell biomarker, P21 (red) were increased in mice lung tissues of the SiO2 group, compared to those in the corresponding PBS group, whether at 28-day time point or 56-day time point. Then, HKL significantly inhibited the overlapping expression of SP-C and P21 in mice lung tissues induced by silica treatment at both two-time points (Fig. 6A). Further immunoblotting analysis showed the reduced expression of collagen proteins (collagen I and collagen III), the mitigated EMT (epithelial markers, including E-cadherin and mesenchymal markers, including vimentin and α-smooth muscle actin), cellular senescence (p-P53, P53 and P21) effects of HKL in vivo (Fig. 6B), and the corresponding densitometry data of fibrosis-related proteins and EMT related proteins were quantified using Image J Software, and shown in Fig. 6C–I.
Fig. 5.
Honokiol (HKL) attenuated lung pathological injury and inflammation in silicotic mice. (A) Schematic illustration of mouse silicosis model generation. 4 weeks old of C57BL/6 mice were injured by intratracheal instillation of crystalline silica (SiO2), and lung tissues were harvested at d28 and d56 post injury (n = 6). (B) H&E staining, Masson staining, and β-galactosidase staining of lung tissue in each group at 28 days and 56 days after instillation. Scale bars: 100 μm; (C–H) Relative mRNA levels of SASP genes, including IL-1α, IL-1β, IL-6, TNF-α, MCP-1, and CXCL1 in silica-treated mice combined with HKL (n = 6). Bar graphs were presented as mean ± SD (n = 6). Statistical analysis was performed using one-way ANOVA, GraphPad Prism 7.2.0. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; n.s. presents no statistical difference. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 6.
Honokiol (HKL) attenuated fibrosis and senescence in silicotic mice. (A) Images taken by confocal microscopy of immunofluorescent staining of the SP-C (green) and P21 (red) and DAPI (blue) in paraffin sections from mice lung tissues. Scale bar, 200 μm. (B) Immunoblot analysis of fibrosis-related proteins and EMT-related proteins in lung tissues from mice of different groups. (C–I) Corresponding densitometry data of fibrosis-related proteins and EMT-related proteins were quantified using Image J Software. Bar graphs were presented as mean ± SD (n = 5). Statistical analysis was performed using one-way ANOVA, GraphPad Prism 7.2.0. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; n.s. presents no statistical difference. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.5. HKL improved the inhibition of sirt3 induced by silica and senescence of ATII cells in silicotic mice
As expected, we found that silica significantly reduced the expression of sirt3 around the fibrotic lesions (Fig. 7A&7B), and HKL increased the down-regulation of sirt3 expression during the process of silicosis fibrosis (Fig. 7A–D). Despite the discrepancies in quantification between these two methods in Fig. 7A &B, both demonstrate that silica downregulated the expression of SIRT3 in mouse lung tissues, while intervention with HKL alleviated silica-induced suppression of SIRT3 expression at different degree. Besides, the results of sirt3 activity assay showed that SiO2 could inhibit the deacetylation activity of sirt3 in mice lung tissue, while HKL could effectively enhance the deacetylation activity of sirt3 in the lung tissue of silicosis mice (Supplementary Fig. S5E). For further confirming the effect of sirt3 in the process of silicosis fibrosis and the role of HKL in it, we examined double-label immunofluorescent staining of SP-C (green) and acetylated-lysine (red). The results of the overlapping expression pattern for acetylated-lysine and SP-C had shown that the fluorescence intensity of acetylated proteins was significantly enhanced in the lung tissues of mice exposed to silica, especially in ATII cells, while the fluorescence intensity of acetylated-lysine proteins was significantly decreased in the lung tissues of mice exposed to HKL, compared with the corresponding SiO2 group, whether at 28-day time point or 56-day time point. Consistent with the above findings in vitro experiments, these results suggested that the increase of acetylated protein levels in ATII cells in the lung tissue of silicotic mice could be effectively inhibited by HKL (Fig. 7E).
Fig. 7.
HKL improved the inhibition of sirt3 induced by silica and senescence of ATII cells in silicotic mice. (A) Immunoblot analysis of sirt3 protein in lung tissues from mice of different groups. (B) Representative images of immunohistochemical results of sirt3 in paraffin sections from mice lung tissues. Scale bar:100 μm. (C) Corresponding densitometry data of sirt3 protein were quantified using Image J Software. Bar graphs were presented as mean ± SD (n = 5). (D) Semi-quantification of sirt3 immunohistochemical results using Image J Software (n = 5). Statistical analysis was performed using one-way ANOVA, GraphPad Prism 7.2.0. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; n.s. presents no statistical difference. (E) Representative images of immunofluorescence of SP-C (green) and Acetylated-Lysine (red) and DAPI (blue) in paraffin sections from mice lung tissues. Scale bar, 200 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.6. HKL mitigated mtDNA damage and inhibited ROS accumulation induced by silica in vivo
In the above findings of the vivo experiment, we found that HKL effectively ameliorated silica stimulation-mediated sirt3 protein inhibition and mtDNA damage in ATII cells in vitro. To determine whether the involvement of mtDNA damage and the accumulation of ROS in the anti-fibrosis activity of HKL in vivo, we detected the fluorescence intensity of ROS and 8-OHdG, mtDNA copy numbers, mtDNA lesions frequency and contents of cytosolic mtDNA of different groups of mice using the methods mentioned above. We confirmed that the fluorescence intensity of ROS and 8-OHdG increased markedly in mice exposed to silica at both two time points and were partially decreased in the presence of HKL (Fig. 8A & B). Compared with the mice that received silica stimulation, the mtDNA lesions frequency and contents of cytosolic mtDNA in lung tissue from the mice treated with HKL significantly decreased as assessed by qPCR (Fig. 8C–E). Besides, compared with mice from the PBS group, there was increased mitochondrial damage in mitochondrial ultrastructures, such as fragmentation and ridge disappearance, which was observed in ATII cells of mice exposed to silica. However, HKL effectively decreased the mitochondrial breakdown in ATII cells of mice that received silica (Fig. 8F).
Fig. 8.
HKL mitigated mtDNA damage and inhibited ROS accumulation induced by silica in vivo. (A) The paraffin sections from mice lung tissues were incubated ex vivo with DHE (red) (5 mM) in DMSO for 30 min before analysis of ROS production by fluorescence microscopy. Scale bar, 100 μm. (B) Images taken by confocal microscopy of immunofluorescent staining of the dsDNA (green) and DAPI (blue) in paraffin sections from mice lung tissues. Scale bar, 100 μm. (C) Frequency of mtDNA lesions per 10 kb per strand in paraffin sections from mice lung tissues. (D) The quantitation of mtDNA copy number in paraffin sections from mice lung tissues. (E) The quantitation of cytosolic mtDNA in paraffin sections from mice lung tissues. Bar graphs were presented as mean ± SD (n = 5). Statistical analysis was performed using one-way ANOVA, GraphPad Prism 7.2.0. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; n.s. presents no statistical difference. (F) Morphological changes of the mitochondria and LBs in the ATII cells of mice in each group. The red arrows present the mitochondria and the yellow arrows present the LBs. Scale bar, 2 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.7. cGAS/STING pathway was involved in the anti-fibrosis and anti-aging effect of HKL in vivo
To further address a potential role for self-DNA recognition by the cGAS/STING pathway in the progress of lung fibrosis induced by silica and anti-fibrosis and anti-aging effect of HKL, we examined the expression levels of some of the same key molecules of the pathway and their activated forms of the lung tissues of mice. As shown in Fig. 9A, Western blot analysis showed that silica-induced the increase in cGAS, p-TBK1/TBK1, p-IRF3/IRF3, p–NF–κB p65/NF-κB p65, p-IκBα/IκBα, and STING protein abundance levels compared with the PBS group, which was partly reversed by HKL treatment at both two time points. Moreover, pharmacological intervention with HKL markedly suppressed the silica-induced acetylation of anti-oxidative stress proteins SOD2 when compared with the silica groups on the 28-day and 56-day (Fig. 9B–H). Combined with the above results, these results suggested that cGAS/STING pathway played an important role in the silica-induced lung fibrosis and senescence, and the increase of sirt3 deacetylation activity mediated by HKL may reduce mitochondrial DNA damage and leakage to play a role in anti-fibrosis and cell senescence by activating the antioxidant capacity of SOD2 and the DNA repair capacity of OGG1, thereby regulating the cGAS/STING pathway.
Fig. 9.
cGAS-STING pathway was involved in the anti-fibrosis and anti-aging effect of HKL in vivo. (A–H) Representative blots and quantification of cGAS-STING pathway-related proteins in mice lung tissues. Bar graphs were presented as mean ± SD (n = 5). Statistical analysis was performed using one-way ANOVA, GraphPad Prism 7.2.0. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; n.s. presents no statistical difference. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Silicosis is characterized by interstitial lung inflammation and diffuse fibrosis, and could seriously threaten the health of patients. During the pathological process of silicosis, ATII cells play a vital role in repairing the damaged alveolar epithelial and maintaining the structural and functional integrity of the alveoli [37]. Emerging reports showed that inhibiting ATII cell senescence delays the delivery of SASP which promotes fibrogenesis as a strategy for the treatment of silicosis [38,39]. However, the mechanism of senescent ATII cells induced by silica is still not clear. Therefore, the discovery of anti-senescence and safe drugs is impending but remains a challenge. Here, this work represents a basis for how silica drives ATII cell senescence phenotypes, particularly in the context of mtDNA damage. Importantly, we confirm the therapeutic potential of anti-senescence and anti-fibrosis induced by silica of honokiol targeting sirt3.
Our results show that HKL markedly ameliorated ATII cell senescence dependent on the expression of sirt3, and attenuated the mtDNA damage and leakage. Therefore, HKL has a protective role in silicosis. Sirt3, as class III histone deacetylase, is a novel regulator of senescence and mitochondrial stress [40]. Normally, sirt3 is encoded by human chromosome 11 and exists in two forms: full-length and mature. The full-length form of sirt3 is found predominantly in the nucleus. When cells are under stress, full-length sirt3 can be transferred to mitochondria and cleaved by matrix processing peptidases (MMPs) to form 28-KDa mature sirt3, which then has deacetylase activity [41,42]. Interestingly, HKL has been reported to mitigate pathologic cardiac hypertrophy and fibrosis, and even doxorubicin-induced cardiotoxicity by activating sirt3 [43]. The honokiol analogue treatment (hexafluoro), reduced bleomycin-induced lung and skin fibrosis in a model by upregulation of sirt3 expression [44]. The protective effects of HKL on cardiac fibrosis were lost in sirt3-KO mice [45]. Besides, some reports have pointed out that HKL could exert beneficial effects on alleviating mitochondrial structural damage, repairing mitochondrial dysgenesis, and maintaining mitochondrial quality control [46]. However, the sirt3 response upon HKL treatment in senescent ATII cells induced by silica particles has never been reported. Here we demonstrate that HKL alleviated the mtDNA damage and leakage into cytoplasm in the senescent ATII cells and corresponding activation of sirt3. After sirt3 knockout, the above protective effects of HKL were completely inhibited, and the overexpression of sirt3 could exert similar beneficial effects to those of HKL intervention in vitro.
The expression of cyclic GMP-AMP synthase (cGAS) in senescent ATII cells induced by silica was downregulated by HKL and was partly reversed by overexpression of sirt3, and other previously reported that small molecule inhibition of cGAS, RU.521, mitigated the inflammatory response and the oxidative damage via increased sirt3 expression [47], implicating sirt3 was the upstream regulator of cGAS/STING signaling. This finding is consistent with the results of another diabetic study, which found that nicotinamide riboside (NR), another activator of sirt3, could significantly ameliorate mitochondrial DNA damage and inhibit the cGAS/STING signaling activating in the kidney of db/db mice [48]. Here, we found that the cGAS/STING signaling was activated in the senescent ATII cells induced by silica, compared with the increased mtDNA oxidative damage and leakage, and was mitigated by HKL treatment or the overexpression of sirt3. The cGAS/STING pathway, as the key component to sense cytosolic dsDNA, is an important mechanism of innate immune response to regulate inflammation and senescence [49]. The endosymbiosis theory of the origin of mitochondria suggests that mitochondria were formed by the engulfment of bacteria by primitive eukaryotic organisms over a long period of evolutionary symbiosis, and therefore, like bacterial DNA, mitochondrial DNA is generally poorly methylated, which allows it to act as a heterologous DNA to activate the innate immune response of the organism [50,51]. Once mtDNA leaks into the cytoplasm, under diverse stress circumstances, cGAS would recognize and catalyze the synthesis of a messenger molecule, 2′,3′-cGAMP, which binds and activates STING to trigger its association with TANK-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3). Moreover, the activation of STING can also activate the classical nuclear factor kappa-B (NF-κB) signaling pathway and induce the expression of some SASP such as tumor necrosis factor α (TNF-α), interleukin-6 (IL-6) and so on [52,53]. A previous report had pointed out that silica particles activated the STING signal pathway (NF-κB and IRF3) of AMs through the release of dsDNA, which potentiated the polarization of AMs by mediating the generation of different polarization inducible cytokines, including TNF-α, IL-6, IFN-β, and TGF-β [29]. This study further substantiates the crucial role played by the activation of the STING signaling pathway in the transmission of fibrotic signals. Therefore, we conduct the immunoblotting analysis to explore the role of the classical NF-κB signaling pathway in the senescent ATII cells induced by silica. As expected, we demonstrated that treating C56BL/6 N mice and A549 cells with silica results in increases in p–NF–κB p65/NF-κB p65 and p-IκBα/IκBα expression, which were inhibited following the treatment with HKL.
In addition, the oxidant/antioxidant balance in cell perturbation plays an important role in the pathogenesis of several age-related diseases, including multiple sclerosis [54] and Alzheimer's disease [55]. Mitochondria, as a hub of cellular redox processes and due to the “naked” nature of mtDNA, are susceptible to oxidative damage. Consequently, mitochondrial forms of the superoxide dismutase (SOD2) play an important role in maintaining mitochondrial function to resist oxidative damage [56]. In this study, we found that the increase in sirt3 activity after HKL treatment was associated with decreases in the acetylation of proteins that are important for mitochondrial function including SOD2 and mtOGG1. The increase in the mitochondrial antioxidant SOD2, with the deacetylation of ace-SOD2, is reflected by the ability of HKL to decrease lung oxidative stress and mtDNA oxidative damage. Notably, natural polyphenols with antioxidative properties have attracted more and more attention for their potential in combating oxidative stress-driven degenerative diseases [57]. Previous studies have also reported that HKL, as a polyphenol, could enhance the antioxidant activity of SOD2 by activating sirt3 [58]. However, we further connected the protective effects of HKL on mtDNA damage and leakage in the silicotic mice to its direct target sirt3 by showing the increase in sirt3 deacetylase activity and the decrease of acetylation level in the mitochondria, especially the decrease of ace-OGG1. OGG1 is an important DNA glycosylase for the repair of 8-oxo guanine (8-oxoG), which is one of the major DNA oxidative lesions both of the nDNA and mtDNA, especially in mtDNA [59]. The mtDNA repair functions of mtOGG1 rely on the activities of sirt3 to improve mitochondrial functions [60,61]. The collective findings support the viewpoint that HKL significantly improves the senescence of ATII cells by promoting the deacetylation activity of sirt3.
Although HKL was effective in anti-senescence and mitigating the progress of silicosis based on experimental evidence, the effect of HKL in treating silicosis patients remains to be confirmed. Furthermore, it has been recognized that advanced fibrosis in humans is less reversible than in rodents, likely due to the densely cross-linked and accumulated ECM over decades in patients [62]. The lack of groups treated solely with HKL (without silica exposure) in this study limits the assessment of the safety profile of HKL under normal conditions. Moreover, there is insufficient information and limited evidence of the benefits of HKL in silicosis from clinical studies. Therefore, it is crucial to add more preclinical trials to evaluate the safety of HKL and further validate the role of HKL in silica. Another issue is the duration of anti-senescence therapy for silicosis. It is worth considering to exclude unwanted long-term effects of HKL treatment during anti-senescent.
Taken together, this study demonstrated that activation of sirt3 could alleviate silica-induced fibrosis and mtDNA damage. our study provides new insights into the pathogenesis and progression of silicosis, as well as a preclinical basis that the HKL be a potential lead natural compound with anti-fibrotic properties.
5. Significances
-
1.
The activation of sirt3 may offer a promising avenue for future therapeutic interventions.
-
2.
Honokiol (HKL) is a potential therapeutic agent for silicosis, a debilitating occupational lung disease, by targeting senescence in ATII cells through activation of the sirt3 pathway.
-
3.
It provides crucial mechanistic insights into the progression of silicosis, shedding light on the interplay between cGAS/STING signaling and mitochondrial DNA damage.
Funding
This work was supported by the National Natural Science Foundation of China (Grant numbers [U21A20334]), and the Graduate Student Innovation Fund of North China University of Science and Technology (Grant numbers [CXZZBS2022116]).
CRediT authorship contribution statement
Qiang Zhou: Writing – review & editing, Writing – original draft, Project administration, Methodology, Funding acquisition, Data curation, Conceptualization. Guan Yi: Writing – review & editing, Writing – original draft, Visualization, Methodology, Data curation. Meiyu Chang: Visualization, Validation, Methodology, Investigation. Ning Li: Writing – review & editing, Writing – original draft, Methodology, Investigation. Yichun Bai: Supervision, Project administration, Methodology, Data curation. Haibin Li: Validation, Investigation, Formal analysis, Data curation. Sanqiao Yao: Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103224.
Contributor Information
Qiang Zhou, Email: 18790670206@163.com.
Guan Yi, Email: gy110901@163.com.
Meiyu Chang, Email: changmy0101@163.com.
Ning Li, Email: johnsonln93@163.com.
Yichun Bai, Email: yichun1979@126.com.
Haibin Li, Email: lihaibin@xxmu.edu.cn.
Sanqiao Yao, Email: sanqiaoyao@xxmu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Birch J., Gil J. Senescence and the SASP: many therapeutic avenues. Genes & development. 2020;34:1565–1576. doi: 10.1101/gad.343129.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao C., Guan X., Carraro G., et al. Senescence of alveolar type 2 cells drives progressive pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2021;203(6):707–717. doi: 10.1164/rccm.202004-1274OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calcinotto A., Kohli J., Zagato E., et al. Cellular senescence: aging, cancer, and injury. Physiol. Rev. 2019;99(2):1047–1078. doi: 10.1152/physrev.00020.2018. [DOI] [PubMed] [Google Scholar]
- 4.Lingampally A., Jones M.R., Bagari S., et al. Use of the reversible Myogenic to Lipogenic Transdifferentiation Switch for the design of preclinical drug Screening in idiopathic pulmonary fibrosis. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.569865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li T., Yang X.-y., Xu D.-j., et al. OC-STAMP overexpression drives lung alveolar epithelial cell type II senescence in silicosis. Oxid. Med. Cell. Longev. 2021 doi: 10.1155/2021/4158495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehmann M., Korfei M., Mutze K., et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur. Respir. J. 2017;50(2) doi: 10.1183/13993003.02367-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balasubramanian P., Howell P.R., Anderson R.M. Aging and Caloric Restriction research: a Biological perspective with Translational potential. EBioMedicine. 2017;21:37–44. doi: 10.1016/j.ebiom.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun X., Qian M., Li H., et al. FKBP5 activates mitophagy by ablating PPAR-γ to shape a benign remyeli nation environment. Cell Death Dis. 2023;14(11):736. doi: 10.1038/s41419-023-06260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacedonia D., Carpagnano G.E., Crisetti E., et al. Mitochondrial DNA alteration in obstructive sleep apnea. Respir. Res. 2015;16(1):47. doi: 10.1186/s12931-015-0205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chocron E.S., Munkácsy E., Pickering A.M. Cause or casualty: the role of mitochondrial DNA in aging and age-associated disease. Biochim. Biophys. Acta, Mol. Basis Dis. 2019;1865(2):285–297. doi: 10.1016/j.bbadis.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benmerzoug S., Rose S., Bounab B., et al. STING-dependent sensing of self-DNA drives silica-induced lung inflammation. Nat. Commun. 2018;9(1):5226. doi: 10.1038/s41467-018-07425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarrica A., Kirika N., Romeo M., et al. Safety and Toxicology of Magnolol and honokiol. Planta Med. 2018;84(16):1151–1164. doi: 10.1055/a-0642-1966. [DOI] [PubMed] [Google Scholar]
- 13.Shen T., Wu Y., Wang X., et al. Activating SIRT3 in peritoneal mesothelial cells alleviates postsurgical peritoneal adhesion formation by decreasing oxidative stress and inhibiting the NLRP3 inflammasome. Experimental & molecular medicine. 2022;54(9):1486–1501. doi: 10.1038/s12276-022-00848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan Y., Park W., Jin J., et al. Sirtuin 3 activation by honokiol decreases Unilateral Ureteral Obstruction-induced renal inflammation and fibrosis via regulation of mitochondrial dynamics and the renal NF-κBTGF-β1/Smad signaling pathway. Int. J. Mol. Sci. 2020;21(2) doi: 10.3390/ijms21020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dikalova A.E., Pandey A., Xiao L., et al. Mitochondrial deacetylase Sirt3 reduces Vascular dysfunction and hyper tension while Sirt3 Depletion in Essential Hypertension is linked to V ascular inflammation and oxidative stress. Circ. Res. 2020;126(4):439–452. doi: 10.1161/CIRCRESAHA.119.315767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonnell E., Peterson B.S., Bomze H.M., et al. SIRT3 regulates progression and development of diseases of aging. Trends in Endocrinology & Metabolism. 2015;26(9):486–492. doi: 10.1016/j.tem.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei W., Li T., Chen J., et al. SIRT3/6: an amazing challenge and opportunity in the fight against fibrosis and aging. Cell. Mol. Life Sci. 2024;81(1):69. doi: 10.1007/s00018-023-05093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen T., Li J., Liu J., et al. Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and im proves cardiac function via the TGF-β/Smad3 pathway. Am. J. Physiol. Heart Circ. Physiol. 2015;308(5):H424–H434. doi: 10.1152/ajpheart.00454.2014. [DOI] [PubMed] [Google Scholar]
- 19.Lantier L., Williams A.S., Williams I.M., et al. SIRT3 is crucial for maintaining Skeletal muscle insulin action and protects against Severe insulin resistance in high-Fat-Fed mice. Diabetes. 2015;64(9):3081–3092. doi: 10.2337/db14-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan S., Yang S., Chen M., et al. Lipopolysaccharides promote pulmonary fibrosis in silicosis through the aggravation of apoptosis and inflammation in alveolar macrophages. Open Life Sci. 2020;15(1):598–605. doi: 10.1515/biol-2020-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araki K., Kawauchi K., Sugimoto W., et al. Mitochondrial protein E2F3d, a distinctive E2F3 product, mediates hypoxia-induced mitophagy in cancer cells. Commun. Biol. 2019;2:3. doi: 10.1038/s42003-018-0246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fineschi S., Bongiovanni M., Donati Y., et al. In vivo investigations on anti-fibrotic potential of proteasome inhibition in lung and skin fibrosis. American journal of respiratory cell and molecular biology. 2008;39(4):458–465. doi: 10.1165/rcmb.2007-0320OC. [DOI] [PubMed] [Google Scholar]
- 23.Hübner R.H., Gitter W., El Mokhtari N.E., et al. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques. 2008;44(4):507–511. doi: 10.2144/000112729. 514-511. [DOI] [PubMed] [Google Scholar]
- 24.Ashcroft T., Simpson J.M., Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numeri cal scale. J. Clin. Pathol. 1988;41(4):467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papparella A., Noviello C., Ranucci S., et al. Pneumoperitoneum Modifies serum and tissue CCL2-CCL5 expression in mice. J. Soc. Laparoendosc. Surg. : J. Soc. Laparoendosc. Surg. 2020;24(2) doi: 10.4293/jsls.2020.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H., Gan C., Gao Z., et al. Caffeine targets SIRT3 to enhance SOD2 activity in mitochondria. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C., Le B.H., Xu W., et al. Dual chemical labeling enables nucleotide-resolution mapping of DNA abasic sites and common alkylation damage in human mitochondrial DNA. Nucleic acids research. 2023;51(13):e73. doi: 10.1093/nar/gkad502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bronner D.N., O'Riordan M.X. Measurement of mitochondrial DNA release in response to ER stress. Bio-protocol. 2016;6(12) doi: 10.21769/BioProtoc.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ou L., Zhang P., Huang Z., et al. Targeting STING-mediated pro-inflammatory and pro-fibrotic effects of alveolar macrophages and fibroblasts blunts silicosis caused by silica particles. J. Hazard Mater. 2023;458 doi: 10.1016/j.jhazmat.2023.131907. [DOI] [PubMed] [Google Scholar]
- 30.Wang D., Cao L., Zhou X., et al. Mitigation of honokiol on fluoride-induced mitochondrial oxidative stress, mitochondrial dysfunction, and cognitive deficits through activating AMPK/PGC-1α/Sirt3. J. Hazard Mater. 2022;437 doi: 10.1016/j.jhazmat.2022.129381. [DOI] [PubMed] [Google Scholar]
- 31.Wang H., Su J., Yu M., et al. PGC-1α in osteoarthritic chondrocytes: from mechanism to target of action. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1169019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng A., Yang Y., Zhou Y., et al. Mitochondrial SIRT3 mediates Adaptive responses of Neurons to Exercise and metabolic and Excitatory challenges. Cell Metab. 2016;23(1):128–142. doi: 10.1016/j.cmet.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J., Li W., Xue S., et al. Qishen granule attenuates doxorubicin-induced cardiotoxicity by protecting mitochondrial function and reducing oxidative stress through regulation of Sirtuin3. J. Ethnopharmacol. 2024;319 doi: 10.1016/j.jep.2023.117134. [DOI] [PubMed] [Google Scholar]
- 34.Liao Y., Cheng J., Kong X., et al. HDAC3 inhibition ameliorates ischemia/reperfusion-induced brain injury by regulating the microglial cGAS-STING pathway. Theranostics. 2020;10(21):9644–9662. doi: 10.7150/thno.47651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pu F., Chen F., Liu J., et al. Immune regulation of the cGAS-STING signaling pathway in the tumor Microenvironment and its clinical application. OncoTargets Ther. 2021;14:1501–1516. doi: 10.2147/ott.s298958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J., Chen Y.-J., Dobbs N., et al. STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J. Exp. Med. 2019;216(4):867–883. doi: 10.1084/jem.20182192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X., Xiao C., Liu Y., et al. HUB genes transcriptionally regulate lipid metabolism in alveolar type II cells under LPS stimulation. Heliyon. 2023;9(9) doi: 10.1016/j.heliyon.2023.e19437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J., Li J., Wang J., et al. Oroxylin A relieves intrauterine adhesion in mice through inhibiting macrophage pyroptosis via SIRT3-SOD2-ROS pathway. Int. Immunopharm. 2023;118 doi: 10.1016/j.intimp.2023.110023. [DOI] [PubMed] [Google Scholar]
- 39.Yang J., Wu S., Hu W., et al. Bmi1 signaling maintains the plasticity of airway epithelial progenitors in response to persistent silica exposures. Toxicology. 2022;470 doi: 10.1016/j.tox.2022.153152. [DOI] [PubMed] [Google Scholar]
- 40.Wu J., Yang Y., Gao Y., et al. Melatonin attenuates Anoxia/Reoxygenation injury by inhibiting Excessive mitophagy through the MT2/SIRT3/FoxO3a signaling pathway in H9c2 cells. Drug Des Devel Ther. 2020;14:2047–2060. doi: 10.2147/DDDT.S248628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song Z., Shao H., Chen Y., et al. Expression and significance of NLRP3/IL-1β/TGF-β (1) signal axis in rat model of silicosis pulmonary fibrosis. Chin. J. Ind. Hyg. Occup. Dis. 2018;36(11):819–823. doi: 10.3760/cma.j.issn.1001-9391.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Zeng H., Chen J.-X. Microvascular Rarefaction and Heart Failure with Preserved Ejection fraction. Front Cardiovasc Med. 2019;6:15. doi: 10.3389/fcvm.2019.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang X., Feng X., Du M., et al. Pharmacological effects and mechanisms of paeonol on antitumor and prevention of side effects of cancer therapy. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1194861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mora A.L., Rojas M., Pardo A., et al. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat. Rev. Drug Discov. 2017;16(11):810. doi: 10.1038/nrd.2017.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pillai V.B., Samant S., Sundaresan N.R., et al. Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat. Commun. 2015;6:6656. doi: 10.1038/ncomms7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J., Nisar M., Huang C., et al. Small molecule natural compound agonist of SIRT3 as a therapeutic target for the treatment of intervertebral disc degeneration. Experimental & molecular medicine. 2018;50(11):1–14. doi: 10.1038/s12276-018-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Q., Xiong H., Zhu W., et al. Small molecule inhibition of cyclic GMP-AMP synthase ameliorates sepsis-induced cardiac dysfunction in mice. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118315. [DOI] [PubMed] [Google Scholar]
- 48.Myakala K., Wang X.X., Shults N.V., et al. NAD metabolism modulates inflammation and mitochondria function in diabetic kidney disease. J. Biol. Chem. 2023;299(8) doi: 10.1016/j.jbc.2023.104975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Q., Boire A., Jin X., et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533(7604):493–498. doi: 10.1038/nature18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin M.-K., Cheong J.-H. Mitochondria-centric bioenergetic characteristics in cancer stem-like cells. Arch Pharm. Res. (Seoul) 2019;42(2):113–127. doi: 10.1007/s12272-019-01127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu C., Cao N., Liu W., et al. Crosstalk between mitophagy and innate immunity in viral infection. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.1064045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andreeva L., Wu H. STING condensates on ER limit IFN response. Nat. Cell Biol. 2021;23(4):299–300. doi: 10.1038/s41556-021-00662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Y. Shen, D. Guo, X. Ji, et al., Homotypic targeting of immunomodulatory nanoparticles for enhanced per ipheral and central immunity, Cell Prolif. 55(3) e13192, 10.1111/cpr.13192. [DOI] [PMC free article] [PubMed]
- 54.Calabrese V., Scapagnini G., Ravagna A., et al. Disruption of Thiol homeostasis and Nitrosative stress in the Cerebrospinal Fluid of patients with active multiple sclerosis: evidence for a protective role of Acetylcarnitine. Neurochem. Res. 2003;28(9):1321–1328. doi: 10.1023/A:1024984013069. [DOI] [PubMed] [Google Scholar]
- 55.He Q., Ding G., Zhang M., et al. Trends in the Use of Sphingosine 1 phosphate in age-related diseases: a Scientometric research study (1992-2020) J. Diabetes Res. 2021;2021 doi: 10.1155/2021/4932974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calabrese V., Cornelius C., Dinkova-Kostova A.T., et al. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal. 2010;13(11):1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calabrese V., Mancuso C., Calvani M., et al. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007;8(10):766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 58.Yi X., Guo W., Shi Q., et al. SIRT3-Dependent mitochondrial dynamics remodeling contributes to oxidative stress-induced Melanocyte degeneration in Vitiligo. Theranostics. 2019;9(6):1614–1633. doi: 10.7150/thno.30398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Darbinian N., Darbinyan A., Merabova N., et al. HIV-1 and HIV-1-Tat induce mitochondrial DNA damage in human Neurons. J HIV AIDS. 2020;6(1):176. doi: 10.16966/2380-5536.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng Y., Ren X., Gowda A.S.P., et al. Interaction of Sirt3 with OGG1 contributes to repair of mitochondrial DNA and protects from apoptotic cell death under oxidative stress. Cell Death Dis. 2013;4(7):e731. doi: 10.1038/cddis.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sampath H., Lloyd R.S. Roles of OGG1 in transcriptional regulation and maintenance of metabolic homeostasis. DNA Repair. 2019;81 doi: 10.1016/j.dnarep.2019.102667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xi Y., Li Y., Xu P., et al. The anti-fibrotic drug pirfenidone inhibits liver fibrosis by targeting the small oxidoreductase glutaredoxin-1. Sci. Adv. 2021;7(36) doi: 10.1126/sciadv.abg9241. eabg9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.