Abstract
Background:
This longitudinal study examined whether co-occurring stimulant use and HIV disease processes predicted greater risk for depression via dysregulated metabolism of amino acid precursors for neurotransmitters.
Methods:
In total, 110 sexual minority men (i.e., gay, bisexual, and other men who have sex with men) living with HIV who had biologically confirmed recent methamphetamine use were enrolled in a randomized controlled trial. The kynurenine/tryptophan (K/T) and phenylalanine/tyrosine (P/T) ratios were measured over 15 months to index dysregulated metabolism of amino acid precursors for serotonin and catecholamines. Markers of gut-immune dysregulation such as lipopolysaccharide binding protein and soluble CD14 (sCD14), HIV persistence in immune cells (i.e., proviral HIV DNA), and stimulant use were examined as predictors. These bio-behavioral measures, including the K/T and P/T ratios, were also examined as predictors of greater risk for depression over 15 months.
Results:
Higher time-varying sCD14 levels (β = 0.13; p = 0.04) and time-varying detectable viral loads (β = 0.71; p < 0.001) were independent predictors of a higher K/T ratio. Time-varying reactive urine toxicology results for stimulants (β = 0.53; p < 0.001) and greater proviral HIV DNA at baseline (β = 0.34; p < 0.001) independently predicted an increased P/T ratio. Greater time-varying, self-reported methamphetamine use uniquely predicted higher odds of screening positive for depression (Adjusted Odds Ratio = 1.08; 95% CI = 1.01–1.17).
Conclusions:
Ongoing stimulant use and HIV persistence independently predict dysregulated metabolism of amino acid precursors for catecholamines, but this did not explain amplified risk for depression.
Keywords: HIV, Immune Activation, Methamphetamine, Phenylalanine, Reservoir, Tryptophan
Introduction
Despite profound advances in the medical management of HIV infection, people who use stimulants such as methamphetamine and those with depressive disorders remain at elevated risk for faster clinical HIV progression.1-5 In fact, depression is a prevalent comorbidity affecting as many as one third (31%; 95% CI = 28–34%) of people living with HIV (PLWH) that is exacerbated by co-occurring stimulant use.6,7 Depression and stimulant use also fuel difficulties with anti-retroviral therapy (ART) adherence and persistence, leading to elevated viral load and amplified risk of onward HIV transmission.8-11 Although it is clear that depression and stimulant use undermine the benefits of HIV treatment as prevention, few longitudinal studies have examined the bio-behavioral pathways linking the triumvirate of stimulant use, depression, and HIV.
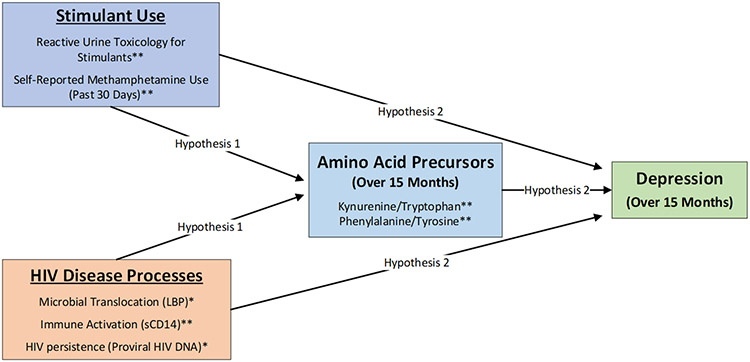
It is well established that PLWH display dysregulated metabolism of essential amino acid precursors for neurotransmitters, which could explain high rates of depression among PLWH who use stimulants.6,7 Dysregulated serotonin synthesis is a risk factor for greater depressive symptoms and clinical HIV progression,12-14 but relatively less is known about the consequences of dysregulated catecholamine synthesis in PLWH. An elevated kynurenine/tryptophan (K/T) ratio indexes decreased serotonin synthesis,15 and a higher phenylalanine/tyrosine (P/T) ratio is indicative of impeded catecholamine synthesis such as dopamine.16 Among PLWH, elevations in the K/T ratio are associated with depletion of T-helper 17 cells in the gastrointestinal tract,17 and a higher K/T ratio predicts faster clinical HIV progression.18 The P/T ratio is also elevated in PLWH,14 which may lead to reduced synthesis of catecholamines such as dopamine.19 This could have clinical implications for the course of stimulant use disorders, because decreased dopamine activity in the central nervous system has been shown to predict relapse.20 As shown in Figure 1, this longitudinal study examined whether co-occurring stimulant use and HIV disease processes predict dysregulated metabolism of essential amino acid precursors for neurotransmitters that could, in turn, explain amplified risk for depression in PLWH who use stimulants.
Figure 1. Hypothesized pathways linking co-occurring stimulant use and HIV disease processes with depression.
*Measured at baseline only; **Measured over 15 months
HIV Disease processes are linked to neuropsychiatric comorbidities
HIV disease processes such as microbial translocation and immune activation could amplify dysregulation of the metabolism of essential amino acid precursors for serotonin and catecholamines that are linked to neuropsychiatric comorbidities in PLWH.21 HIV damages the gastrointestinal tract early in infection,22 causing persistent leakage of products like lipopolysaccharide (LPS). LPS-binding protein (LBP) is a plasma marker for the presence of gram-negative bacteria that translocate more readily from the gastrointestinal tract due HIV-induced epithelial damage.23 Microbial translocation, in turn, amplifies immune activation in those receiving effective HIV treatment.21 For example, soluble CD14 (sCD14) is released by activated monocytes following stimulation by LPS and inflammatory cytokines.23 The clinical relevance of this monocyte activation marker is supported by findings that higher sCD14 levels in plasma and cerebrospinal fluid are associated with neurocognitive impairment and faster clinical HIV progression.24-26 The potentially central importance of immune activation is further supported by prior research documenting an association of greater CD8+ T-cell activation with depression,27,28 and lower CD4+ and CD8+ T-cell activation with greater variation of diurnal salivary cortisol.29 An important gap, addressed in the present longitudinal study, is that relatively little is known about the prospective associations of markers of gut-immune dysregulation such as LBP and sCD14 with risk for depression in PLWH.
It is also clear that HIV persistence in the immune cells of ART-treated individuals is central to HIV pathogenesis.30,31 The amount of HIV DNA integrated into the DNA of the host (referred to as proviral HIV DNA) is one indicator of HIV persistence that measures both replication-competent and defective virus.32 The clinical relevance of this measure is supported by findings that greater proviral HIV DNA in monocytes predicts HIV-associated neurocognitive disorders.33 Although those who achieve an undetectable viral load and remain adherent to ART display decreased immune activation and inflammation,34,35 HIV persistence in immune cells could amplify risk for depressive disorders and more persistent stimulant use disorders by altering the metabolism of essential amino acid precursors for neurotransmitters in those with treated HIV infection.
Stimulant-associated biological alterations could amplify risk for depression
There is increasing evidence that stimulant use amplifies immune dysregulation in treated HIV infection, which may have potent neuropsychiatric consequences. Cross-sectional studies conducted with sexual minority men (i.e., gay men, bisexual men, and other men who have sex with men) have observed greater immune activation and inflammation in those who use stimulants such as methamphetamine.36-38 A cross-sectional study with sexual minority men observed that recent methamphetamine use was associated with elevated rectal interleukin-6 (IL-6) and rectal tumor necrosis factor – alpha (TNF-α) regardless of HIV status.36 Rectal inflammation may be partially attributable to methamphetamine-associated alterations in the gastrointestinal microbiome among those living with HIV.39 In another cross-sectional study of virally suppressed methamphetamine-using sexual minority men living with HIV, those who provided a urine sample that was reactive for stimulants (i.e., methamphetamine or cocaine) displayed differential expression of genes and two-directional perturbation of pathways relevant to immune activation, inflammation, and HIV persistence that were paralleled by elevations in plasma TNF-α.37 Furthermore, recent findings with methamphetamine-using sexual minority men living with HIV who had an undetectable viral load indicate that those providing a urine sample that was reactive for stimulants also displayed elevated sCD14.38 These stimulant-associated alterations in biological processes relevant to HIV pathogenesis may partially explain prior observations of depleted tryptophan in stimulant-using PLWH receiving ART,40 which could have implications for depression and greater risk-taking propensity in this population.41,42
The overarching goal of this longitudinal study was to examine the biological pathways whereby co-occurring stimulant use and HIV disease processes (i.e., microbial translocation, immune activation, and HIV persistence) could amplify risk for depression over 15 months. As shown in Figure 1, we hypothesized that measures of stimulant use and HIV disease processes would predict higher K/T and P/T ratios over 15 months (Hypothesis 1). We also examined the extent to which co-occurring stimulant use and HIV disease processes predicted greater odds of screening positive for depression over 15 months via dysregulated metabolism of amino acid precursors for neurotransmitters (Hypothesis 2).
Methods
The present longitudinal study was conducted as a secondary analysis by leveraging data from randomized controlled trial (RCT) that enrolled 110 sexual minority men living with HIV who had biologically confirmed methamphetamine use.43 Follow-up assessments were conducted at 6, 12, and 15 months. Among the 110 participants randomized, follow-up rates at 6 (88%), 12 (80%), and 15 (71%) months were acceptable with no significant differences between the experimental conditions. In the parent RCT for the present longitudinal study, we previously reported on the efficacy of the positive affect intervention delivered during contingency management for increasing positive affect, reducing self-reported stimulant use, and achieving durable reductions in HIV viral load.44,45 All procedures were approved by the Institutional Review Board for the University of California, San Francisco; with reliance agreements from the University of Miami and Northwestern University. All participants provided written informed consent prior to enrollment, including consent for specimen banking.
Measures
Demographics and health status.
Participants reported their age, race/ethnicity, education level, income, and time since HIV diagnosis in a demographic questionnaire. HIV viral load testing was performed to detect plasma HIV RNA using the Abbott Real Time HIV-1 assay (Abbott Molecular, Inc.; Des Plaines, IL). The lower limit of detection was 40 copies/mL. CD4+ T-cell count was measured with whole blood using flow cytometry by Quest Diagnostics.
ART regimen and adherence.
Participants were asked to list their current ART medications and the date they first started ART. In addition, self-reported ART adherence during the past 30 days was measured using a validated visual analog scale.46
Depressive symptoms.
The 20-item Center for Epidemiologic Studies Depression (CES-D) scale was used to screen for clinically significant depressive symptoms at each assessment over 15 months.47 Those with a CES-D total score of 16 or greater were classified as having greater risk for depression (coded as 1) versus participants with a score of less than 16 (coded as 0). The CES-D had adequate internal consistency (Cronbach’s alpha = 0.90).
Recent stimulant use.
At each assessment over the 15 months, urine samples were collected for on-site toxicology screenings for methamphetamine and cocaine using the iCup (Redwood Biotech, Inc.; Santa Rosa, CA). The iCup can detect any stimulant use (i.e., methamphetamine or cocaine) within the past 72 hours (coded as 1) versus no stimulant use (coded as 0).
Self-reported methamphetamine use.
At each assessment over the 15 months, participants reported the number of days that they used methamphetamine in the last 30 days. Assessment of self-reported methamphetamine use in the last 30 days is consistent with the Addiction Severity Index.48
K/T and P/T ratios.
The K/T and P/T ratios were measured at each assessment over the 15 months. A reverse-phase high performance liquid chromatography method with fluorescence analysis was utilized to measure tryptophan and kynurenine at 286 nm and 360 nm respectively. Concentration of phenylalanine and tyrosine was measured via 210 nm excitation and 302 nm respectively. The detailed methodology has been previously described.49,50
LBP.
LBP was measured at baseline only via the Bio-Techne Human LBP DuoSet ELISA for human LBP (cat# DY870-05) per the manufacturer’s instructions. The samples were diluted 1:2,000, and results were expressed as ng/ml.
sCD14.
At each assessment over the 15 months, plasma levels of sCD14 were determined using Human Quantikine Immunoassay (R&D Systems, Minneapolis, MN) following the manufacturer’s instructions. Units were expressed in ng/ml.
Proviral HIV DNA.
Proviral HIV DNA was measured at baseline only in those with an undetectable plasma HIV viral load. Quantitation of viral DNA was performed on extracted leukocyte DNA by digital droplet polymerase chain reaction (ddPCR) using a Bio-Rad QX200 ddPCR instrument (Bio-Rad Laboratories, Inc., Hercules, CA). These procedures are described elsewhere in detail.51,52
Statistical Methods
Marginal models were constructed to analyze predictors of the time-varying K/T and P/T ratios over 15 months. Generalized Estimating Equations (GEE) were used to predict greater risk for depression (i.e., CES-D ≥ 16) over 15 months. An unstructured covariance matrix was adopted to account for unevenly spaced time points (i.e., baseline, 6, 12 and 15 months). Unadjusted and adjusted models were constructed for each outcome (i.e., K/T ratio, P/T ratio, and CES-D ≥ 16). Unadjusted models provide an aggregate association of each predictor over the 15 months after adjusting for age only. Adjusted models examined the independent associations of each predictor controlling with all other predictors included in the model. Predictors measured only at baseline included age, ART regimen, proviral HIV DNA, and LBP. Time-varying predictors measured over the 15 months included detectable HIV viral loads, sCD14 levels, self-reported ART adherence, any recent stimulant use, and days of self-reported methamphetamine use in the past 30 days. Results provide estimates of associations across all four time points over the 15-month follow-up. The K/T and P/T ratios were log10 transformed prior to conducting these analyses. The continuous dependent and independent variables were standardized to facilitate their interpretation.
The potential confounding effects of ART regimens and adherence were addressed in adjusted models. Specifically, we included whether participants were prescribed Efavirenz or a Protease Inhibitor (PI) at baseline, because these ART medications are independently associated with immune dysregulation and neuropsychiatric symptoms.53-55 Self-reported ART adherence was included for adjusted models examining predictors of the K/T and P/T ratios due to evidence that better ART adherence is associated with lower immune activation and inflammation, even among PLWH who have an undetectable viral load.34,35 In the models examining greater risk for depression, only detectable viral loads were included, because difficulty with ART adherence is more likely a consequence than a cause of depression.56
Results
Among the 110 participants, ages ranged from 24 to 59 years, with a mean of 43.2 (SD = 8.9). Nearly half of the participants were white (43%), 29% were Hispanic/Latino, 16% were African American, 3% were Asian American, and 9% were other ethnic minorities or multiracial. Most participants completed at least some college (75%), and 65% had an income of less than $16,000 USD per year. The median baseline CD4+ T-cell count was 646 (Interquartile Range = 428 – 816) cells/mm3, and 74% had an undetectable HIV viral load (< 40 copies/mL) at baseline. Participants had been living with HIV for an average of 12.9 (SD = 8.6) years. Most participants were currently prescribed ART at baseline (89%), and they had been prescribed ART for a mean of 10.1 (SD = 7.4) years. Approximately 71% of participants had elevated risk for depression at baseline using the CES-D. Table 1 provides detailed descriptive information for this sample at baseline. Longitudinal descriptive information for key independent and dependent variables is provided in Table 2.
Table 1.
Demographics and health status at baseline (N = 110).
| M (SD) | |
|---|---|
| Age | 43.2 (8.9) |
| Time Since HIV Diagnosis (Years) | 12.9 (8.6) |
| Time Since Starting ART (Years) | 10.7 (7.2) |
| CD4+ T-Cell Count (cells/mm3) | 641.4 (292.5) |
| Lipopolysaccharide Binding Protein (ng/ml) | 7183.5 (2958.4) |
| Proviral HIV DNA (copies per 106 cells) | 209.7 (1023.8) |
| N (%) | |
| Race/Ethnicity | |
| Black/African American | 18 (16.4%) |
| White | 47 (42.7%) |
| Hispanic/Latinx | 32 (29.0%) |
| Other Ethnic Minority | 13 (11.8%) |
| Income | |
| Up to $4,999 | 16 (14.7%) |
| $5,000 - $11,999 | 28 (25.7%) |
| $12,000 - $15,999 | 27 (24.8%) |
| $16,000 - $24,999 | 12 (11.0%) |
| $25,000 - $34,999 | 9 (8.3%) |
| $35,000 - $49,000 | 7 (6.4%) |
| $50,000 or greater | 1 (0.9%) |
| Education | |
| Less than High School | 8 (7.3%) |
| High School Graduate | 17 (15.5%) |
| Some College/Trade School | 57 (51.8%) |
| College Graduate | 17 (15.5%) |
| Any Graduate School | 11 (10.0%) |
| ART Regimen | |
| INSTI & NRTI | 26 (24%) |
| Boosted PI & NRTI | 31 (28%) |
| NRTI & NNRTI | 25 (23%) |
| PI, NRTI, & NNRTI | 2 (2%) |
| Other HAART | 9 (9%) |
| Not HAART | 8 (7%) |
| No ART | 8 (7%) |
| Undetectable HIV Viral Load ( < 40 copies/mL) | 80 (74.1%) |
| ART Adherence ≥ 90% | 51 (46.4%) |
Table 2.
Longitudinal measurements of predictors and outcomes from 2013-2017 (N = 110)
| N | M (SD) | |
|---|---|---|
| Kynurenine/Tryptophan (K/T) Ratio | ||
| Baseline | 106 | 42.9 (18.6) |
| 6 Months | 83 | 41.1 (14.1) |
| 12 Months | 73 | 45.6 (23.3) |
| 15 Months | 67 | 42.5 (17.3) |
| Phenylalanine/Tyrosine (P/T) Ratio | ||
| Baseline | 106 | 1.0 (0.2) |
| 6 Months | 83 | 0.9 (0.2) |
| 12 Months | 73 | 1.0 (0.3) |
| 15 Months | 67 | 1.0 (0.3) |
| Soluble CD 14 (sCD14) | ||
| Baseline | 106 | 8,224.7 (2,461.4) |
| 6 Months | 83 | 8124.2 (2,928.9) |
| 12 Months | 73 | 8,273.4 (2,532.9) |
| 15 Months | 67 | 8,108.2 (2,061.5) |
| Self-Reported Methamphetamine Use (Past 30 Days) | ||
| Baseline | 110 | 8.0 (9.2) |
| 6 Months | 96 | 6.2 (8.6) |
| 12 Months | 88 | 7.3 (9.7) |
| 15 Months | 77 | 6.5 (9.2) |
| N | n (%) | |
| Screened Positive for Depression (CES-D ≥ 16) | ||
| Baseline | 110 | 78 (71%) |
| 6 Months | 96 | 64 (67%) |
| 12 Months | 88 | 57 (65%) |
| 15 Months | 78 | 47 (60%) |
| Detectable Viral Load (> 40 copies/mL) | ||
| Baseline | 108 | 28 (26%) |
| 6 Months | 87 | 25 (29%) |
| 12 Months | 84 | 22 (26%) |
| 15 Months | 74 | 20 (27%) |
| Self-Reported ART Adherence ≥ 90% | ||
| Baseline | 98 | 51 (52%) |
| 6 Months | 91 | 60 (66%) |
| 12 Months | 77 | 57 (74%) |
| 15 Months | 66 | 40 (61%) |
| Any Recent Stimulant Use (Methamphetamine or Cocaine) | ||
| Baseline | 110 | 56 (51%) |
| 6 Months | 88 | 51 (58%) |
| 12 Months | 82 | 45 (55%) |
| 15 Months | 76 | 42 (55%) |
Predictors of the K/T ratio.
In the unadjusted models (see Table 3), for every standard deviation unit increase in baseline LBP (p < 0.04) and time-varying sCD14 levels (p < 0.001), the K/T ratio increased by 0.17 and 0.21 standard deviation units respectively. One standard deviation unit increase in the time-varying P/T ratio was associated with a 0.20 standard deviation increase in the K/T ratio (p < 0.001). Participants who were prescribed Efavirenz at baseline had a decrease of 0.64 standard deviation units in the K/T ratio (p = 0.02). Time-varying detectable viral loads were associated with an increase in 0.33 standard deviation units in the K/T ratio (p < 0.001). Because the group x time effect on the K/T ratio was not significant, this was not included in the models. In the adjusted model, time-varying detectable viral loads predicted an increase in 0.71 standard deviation units in the K/T ratio (p < 0.001), while being on Efavirenz at baseline was associated with a decrease of 0.99 standard deviation units in the K/T ratio (p = 0.002). For every standard deviation increase in time-varying sCD14 levels, the K/T ratio increased 0.13 standard deviation units (p = 0.04).
Table 3.
Predictors of dysregulated metabolism of amino acid precursors for neurotransmitters over 15 months (N = 110).
| K/T Ratio | P/T Ratio | |||
|---|---|---|---|---|
| Unadjusted (β) | Adjusted (β) | Unadjusted (β) | Adjusted (β) | |
| Predictors measured at baseline only: | ||||
| Age | 0.010 | 0.016 | 0.002 | −0.006 |
| Prescribed a Protease Inhibitor (PI) | −0.047 | −0.049 | −0.022 | 0.116 |
| Prescribed Efavirenz | −0.640* | −0.993** | −0.507 | −0.175 |
| Proviral HIV DNA | −0.025 | −0.073 | 0.313*** | 0.344*** |
| Lipopolysaccharide Binding Protein (LBP) | 0.173* | 0.048 | 0.052 | −0.092 |
| Time-varying predictors measured over 15 months: | ||||
| Time Point | 0.004 | 0.025 | −0.005 | 0.0215 |
| P/T Ratio | 0.196*** | 0.010 | -- | -- |
| K/T Ratio | -- | -- | 0.278*** | 0.040 |
| Detectable HIV Viral Load | 0.326** | 0.712*** | 0.075 | −0.213 |
| Soluble CD14 (sCD14) | 0.210*** | 0.133* | 0.173** | 0.103 |
| Self-Reported ART Adherence ≥ 90% | −0.016 | −0.041 | −0.051 | 0.087 |
| Any Recent Stimulant use | −0.093 | 0.044 | 0.191 | 0.529*** |
| Self-Reported Methamphetamine Use Days (Past 30 Days) | −0.008 | 0.004 | 0.001 | −0.002 |
K/T = kynurenine/tryptophan; P/T = phenylalanine/tyrosine; Recent methamphetamine use = reactive urine toxicology for methamphetamine or cocaine; * p<0.05, ** p<0.01, ***p<0.001
Predictors of the P/T ratio.
In the unadjusted models (see Table 3), each standard deviation unit increase in baseline proviral HIV DNA (p < 0.001) and the time-varying K/T ratio (p < 0.001) corresponded to a P/T ratio increase of 0.31 and 0.28 standard deviation units, respectively. Likewise, one standard deviation unit increase in time-varying sCD14 levels predicted a 0.17 increase in the P/T ratio (p = 0.002). Because the group x time effect on the P/T ratio was not significant, this was not included in the models. In the adjusted model, each standard deviation unit increase in baseline proviral HIV DNA was associated with an increase of 0.34 standard deviation units in the P/T ratio (p < 0.001). Likewise, time-varying recent stimulant use was independently associated with a 0.53 standard deviation unit increase in the P/T ratio (p = 0.001).
Predictors of elevated risk of depression (i.e., CES-D ≥ 16).
In the unadjusted models (see Table 4), the odds of screening positive for depression were 183% greater for time-varying, recent stimulant users (Odds Ratio = 2.83, 95% CI: 1.86–4.30, p < 0.001). Likewise, each day of time-varying methamphetamine use reported in the past 30 days was associated with 10% greater odds of screening positive for depression (Odds Ratio = 1.10, 95% CI: 1.06–1.15, p < 0.001). Neither the K/T (p = 0.57) nor P/T (p = 0.69) ratios were significantly associated with greater risk of depression in the unadjusted models. In the adjusted model, each day of time-varying, self-reported methamphetamine use was associated with 8% greater odds of screening positive for depression (Adjusted Odds Ratio = 1.08, 95% CI: 1.01–1.17, p = 0.03). Neither the K/T nor the P/T ratios were significantly associated with greater risk of depression (p = 0.63, p = 0.72).
Table 4.
Predictors of screening positive for depression over 15 months (N = 110)
| OR (95% CI) | AOR (95% CI) | |
|---|---|---|
| Predictors measured at baseline only: | ||
| Age | 1.01 (0.98-1.05) | 1.08 (1.02-1.15)* |
| Prescribed a Protease Inhibitor (PI) | 1.14 (0.79-1.66) | 0.62 (0.26 – 1.49) |
| Prescribed Efavirenz | 1.11 (0.30-4.17) | - |
| Proviral HIV DNA | 1.29 (0.87-1.93) | 1.07 (0.66-1.75) |
| Lipopolysaccharide Binding Protein (LBP) | 1.21 (0.90-1.63) | 1.23 (0.79-1.91) |
| Time-varying predictors measured over 15 months: | ||
| Time Point | 0.99 (0.97-1.02) | 0.94 (0.87-1.01) |
| P/T Ratio | 1.05 (0.81-1.37) | 1.44 (0.85-2.42) |
| K/T Ratio | 1.08 (0.85-1.36) | 1.29 (0.78-2.12) |
| Detectable Viral Load | 1.73 (0.92-3.23) | 1.04 (0.37-2.90) |
| Soluble CD14 (sCD14) | 0.93 (0.75-1.16) | 0.98 (0.73-1.32) |
| Any Recent Stimulant Use | 2.83 (1.86-4.30)* | 1.43 (0.62-3.44) |
| Self-Reported Methamphetamine Use (Past 30 Days) | 1.10 (1.06-1.15)* | 1.08 (1.01-1.17)* |
K/T = kynurenine/tryptophan; P/T = phenylalanine/tyrosine; Recent stimulant use = reactive urine toxicology for methamphetamine or cocaine; * p<0.05
Discussion
Findings from this longitudinal study underscore the enduring, deleterious effects of ongoing stimulant use for dysregulated metabolism of amino acid precursors for catecholamines and amplified risk for depression in sexual minority men living with HIV. To our knowledge, this is the first longitudinal study to observe that even in the context of recent stimulant use greater HIV persistence in immune cells could alter the synthetic pathways for catecholamines such as dopamine. Further clinical research is needed to determine whether and how greater HIV persistence in immune cells could dysregulate catecholamine synthesis and potentially complicate recovery from stimulant use disorders in PLWH. Interestingly, dysregulated metabolism of amino acid precursors for neurotransmitters did not mediate the independent association of more frequent methamphetamine use with greater risk of depression over 15 months. Clinical research to identify the neurobehavioral and biological mechanisms linking methamphetamine use and depression is needed to catalyze the development of new treatments for PLWH who use methamphetamine.
Even among individuals with an undetectable viral load, markers of HIV persistence in immune cells such as higher proviral HIV DNA may stimulate intracellular inflammatory mechanisms, such as Toll-Like Receptor 9 and residual transcription of HIV Tat proteins.57 Activation of these pathways can lead to necrosis and the release of HIV DNA, cytokines, and other pro-inflammatory cellular products that induce a positive feedback loop of low-grade, systemic inflammation. Systemic inflammation, in turn, decreases the availability of tetrahydrobiopterin (BH4), an essential substrate in the synthesis of catecholamines that leads to increases in the P/T ratio.16 Further research is needed to determine if these pathways relevant to dysregulated catecholamine synthesis predict clinical HIV progression and more persistent patterns stimulant use in PLWH.
The observed associations of baseline LBP and time-varying sCD14 levels with a higher K/T ratio over 15 months support findings from previous studies. Enduring compromise of intestinal integrity due to co-occurring stimulant use and HIV may increase microbial translocation, leading to elevations in LBP and concomitant increases in sCD14.38 Microbial translocation is partially responsible for elevations in monocyte activation, as indexed by sCD14 levels in this study, which predicted a higher K/T ratio even after adjusting for detectable viral loads. Although a prior cross-sectional study observed greater immune activation and depleted tryptophan in PLWH who use stimulants compared to non-users,40 neither recent stimulant use nor self-reported frequency of methamphetamine use were significantly associated with increases in the K/T ratio over 15 months. It is plausible that stimulant-associated increases in sCD14 levels could indirectly predict a higher K/T ratio,38 and this should be examined in future research.
The association of more frequent methamphetamine use with greater risk of depression was not mediated by dysregulated metabolism of amino acid precursors for neurotransmitters. Prior research has consistently observed that elevations in the K/T and P/T ratios have neuropsychiatric consequences in PLWH, including greater depressive symptoms.12,16 It is noteworthy, however, that many of the studies conducted with PLWH excluded those who use stimulants such as methamphetamine and the estimated prevalence of depression at baseline in the present study was almost double that observed in the general population of PLWH.6 It is also clear that elevations in the K/T and P/T ratios have been most closely linked to the somatic symptoms of depression such as difficulties with sleep and appetite that are also directly affected by stimulant use. Future research should examine if dysregulated amino acid precursor metabolism explains the effects of co-occurring stimulant use and HIV disease processes on other neurobehavioral outcomes, such as impulsivity, that are relevant to the development and maintenance of stimulant use disorders.41
Findings from this longitudinal study should be interpreted in the context of some limitations. First, LBP and proviral HIV DNA were measured at baseline only. Further research is needed to examine whether more comprehensive, longitudinal measures of microbial translocation and HIV persistence predict dysregulated metabolism of amino acid precursors for neurotransmitters and neuropsychiatric comorbidities in PLWH who use stimulants. Second, proviral HIV DNA indexes both replication-competent and defective virus. Other measures, such as cell-associated HIV RNA, may provide a better measure of ongoing HIV replication in immune cells. Although there is some mixed evidence that stimulants may alter HIV persistence in immune cells,51,58,59 more comprehensive measures of HIV persistence should be examined in future studies. Third, measurement of recent stimulant use via urine toxicology screening and self-reported methamphetamine use in the past 30 days did not fully index cumulative exposure. Future research should measure dose-response associations with quantitative levels of methamphetamine metabolites using urine and hair samples while implementing timeline follow-back methods to index cumulative, self-reported exposure. Fourth, key confounders that should also be more carefully examined in future research include nadir CD4+ T-cell count and impaired liver function. Fifth, because this study enrolled sexual minority men living with HIV, we also cannot rule out the potential influence of receptive anal intercourse as a potential driver of key markers of gut-immune dysregulation, such as sCD14 levels, which predicted increases in the K/T ratio.60
Despite these limitations, findings from this longitudinal study underscore the central importance of co-occurring stimulant use and HIV persistence in immune cells for dysregulated metabolism of amino acid precursors for catecholamines such as dopamine. Findings from this longitudinal study will inform subsequent mechanistic research to identify viable targets for pharmacologic treatments that are uniquely tailored to mitigate dysregulated catecholamine synthesis in PLWH who use stimulants.
Acknowledgements
This project was supported by the National Institute on Drug Abuse (R01-DA033854; Carrico, Woods, and Moskowitz, PIs). Additional support for this project was provided by the University of California, San Francisco Center for AIDS Research’s Virology Core (P30-AI027763; Volberding, PI), the Miami Center for AIDS Research (P30-AI073961; Pahwa, PI), the Center for HIV Research and Mental Health (P30-MH116867; Safren, PI), and an HIV reservoirs pilot award funded by the state of Florida.
References
- 1.Carrico AW, Shoptaw S, Cox C, et al. Stimulant use and progression to AIDS or mortality after the initiation of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. Published online 2014. doi: 10.1097/QAI.0000000000000364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrico AW. Substance use and HIV disease progression in the HAART era: Implications for the primary prevention of HIV. Life Sci. Published online 2011. doi: 10.1016/j.lfs.2010.10.002 [DOI] [PubMed] [Google Scholar]
- 3.Cook JA. Associations between use of crack cocaine and HIV-1 disease progression: Research findings and implications for mother-to-infant transmission. Life Sci. Published online 2011. doi: 10.1016/j.lfs.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leserman J. HIV disease progression: Depression, stress, and possible mechanisms. Biol Psychiatry. Published online 2003. doi: 10.1016/S0006-3223(03)00323-8 [DOI] [PubMed] [Google Scholar]
- 5.Ironson G, Fitch C, Stuetzle R. Depression and Survival in a 17-Year Longitudinal Study of People with HIV: Moderating Effects of Race and Education. Psychosom Med. Published online 2017. doi: 10.1097/PSY.0000000000000488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rezaei S, Ahmadi S, Rahmati J, et al. Global prevalence of depression in HIV/AIDS: A systematic review and meta-analysis. BMJ Support Palliat Care. Published online 2019:1–9. doi: 10.1136/bmjspcare-2019-001952 [DOI] [PubMed] [Google Scholar]
- 7.Javanbakht M, Shoptaw S, Ragsdale A, Brookmeyer R, Bolan R, Gorbach PM. Depressive symptoms and substance use: Changes overtime among a cohort of HIV-positive and HIV-negative MSM. Drug Alcohol Depend. Published online 2020. doi: 10.1016/j.drugalcdep.2019.107770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrico AW, Hunt PW, Neilands TB, et al. Stimulant Use and Viral Suppression in the Era of Universal Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2019;80(1). doi: 10.1097/QAI.0000000000001867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang J, Nosova E, Reddon H, et al. Longitudinal patterns of illicit drug use, antiretroviral therapy exposure and plasma HIV-1 RNA viral load among HIV-positive people who use illicit drugs. AIDS. Published online 2020. doi: 10.1097/QAD.0000000000002551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrico AW, Riley ED, Johnson MO, et al. Psychiatric risk factors for HIV disease progression: The role of inconsistent patterns of antiretroviral therapy utilization. J Acquir Immune Defic Syndr. Published online 2011. doi: 10.1097/QAI.0b013e318201df63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer KH, Skeer MR, O’Cleirigh C, Goshe BM, Safren SA. Factors associated with amplified HIV transmission behavior among American men who have sex with men engaged in care: Implications for clinical providers. Ann Behav Med. Published online 2014. doi: 10.1007/s12160-013-9527-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez P, Tsai AC, Muzoora C, et al. Reversal of the kynurenine pathway of tryptophan catabolism may improve depression in ART-treated HIV-infected ugandans. In: Journal of Acquired Immune Deficiency Syndromes. ; 2014. doi: 10.1097/QAI.0000000000000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zangerle R, Widner B, Quirchmair G, Neurauter G, Sarcletti M, Fuchs D. Effective antiretroviral therapy reduces degradation of tryptophan in patients with HIV-1 infection. Clin Immunol. Published online 2002. doi: 10.1006/clim.2002.5231 [DOI] [PubMed] [Google Scholar]
- 14.Zangerle R, Kurz K, Neurauter G, Kitchen M, Sarcletti M, Fuchs D. Increased blood phenylalanine to tyrosine ratio in HIV-1 infection and correction following effective antiretroviral therapy. Brain Behav Immun. Published online 2010. doi: 10.1016/j.bbi.2009.11.004 [DOI] [PubMed] [Google Scholar]
- 15.Strasser B, Gostner JM, Fuchs D. Mood, food, and cognition: Role of tryptophan and serotonin. Curr Opin Clin Nutr Metab Care. Published online 2016. doi: 10.1097/MCO.0000000000000237 [DOI] [PubMed] [Google Scholar]
- 16.Gostner JM, Becker K, Kurz K, Fuchs D. Disturbed amino acid metabolism in HIV: Association with neuropsychiatric symptoms. Front Psychiatry. Published online 2015. doi: 10.3389/fpsyt.2015.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2, 3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. Published online 2010. doi: 10.1126/scitranslmed.3000632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byakwaga H, Boum Y, Huang Y, et al. The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery, and mortality among HIV-infected Ugandans initiating antiretroviral therapy. J Infect Dis. Published online 2014. doi: 10.1093/infdis/jiu115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felger JC, Li L, Marvar PJ, et al. Tyrosine metabolism during interferon-alpha administration: Association with fatigue and CSF dopamine concentrations. Brain Behav Immun. Published online 2013. doi: 10.1016/j.bbi.2012.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang GJ, Smith L, Volkow ND, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2012;17(9):918–925. doi: 10.1038/mp.2011.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. Published online 2013. doi: 10.1016/j.tim.2012.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. Published online 2006. doi: 10.1038/nm1511 [DOI] [PubMed] [Google Scholar]
- 23.Abad-Fernández M, Vallejo A, Hernández-Novoa B, et al. Correlation between different methods to measure microbial translocation and its association with immune activation in long-term suppressed HIV-1-infected individuals. J Acquir Immune Defic Syndr. Published online 2013. doi: 10.1097/QAI.0b013e31829a2f12 [DOI] [PubMed] [Google Scholar]
- 24.Lyons JL, Uno H, Ancuta P, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr. Published online 2011. doi: 10.1097/QAI.0b013e3182237e54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamat A, Lyons JL, Misra V, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. Published online 2012. doi: 10.1097/QAI.0b013e318256f3bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. Published online 2011. doi: 10.1093/infdis/jiq118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maes M, Vandoolaeghe E, Ranjan R, et al. Increased serum soluble CD8 or suppressor/cytotoxic antigen concentrations in depression: Suppressive effects of glucocorticoids. Biol Psychiatry. 1996;40(12):1273–1281. doi: 10.1016/0006-3223(95)00627-3 [DOI] [PubMed] [Google Scholar]
- 28.Evans DL, Ten Have TR, Douglas SD, et al. Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. Am J Psychiatry. Published online 2002. doi: 10.1176/appi.ajp.159.10.1752 [DOI] [PubMed] [Google Scholar]
- 29.Patterson S, Moran P, Epel E, et al. Cortisol Patterns Are Associated with T Cell Activation in HIV. PLoS One. Published online 2013. doi: 10.1371/journal.pone.0063429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chun TW, Fauci AS. HIV reservoirs: Pathogenesis and obstacles to viral eradication and cure. AIDS. Published online 2012. doi: 10.1097/QAD.0b013e328353f3f1 [DOI] [PubMed] [Google Scholar]
- 31.Chun TW, Moir S, Fauci AS. HIV reservoirs as obstacles and opportunities for an HIV cure. Nat Immunol. Published online 2015. doi: 10.1038/ni.3152 [DOI] [PubMed] [Google Scholar]
- 32.Eriksson S, Graf EH, Dahl V, et al. Comparative Analysis of Measures of Viral Reservoirs in HIV-1 Eradication Studies. PLoS Pathog. Published online 2013. doi: 10.1371/journal.ppat.1003174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valcour VG, Ananworanich J, Agsalda M, et al. HIV DNA Reservoir Increases Risk for Cognitive Disorders in cART-Naïve Patients. PLoS One. Published online 2013. doi: 10.1371/journal.pone.0070164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castillo-Mancilla JR, Morrow M, Boum Y, et al. Brief Report: Higher ART Adherence Is Associated with Lower Systemic Inflammation in Treatment-Naive Ugandans Who Achieve Virologic Suppression. In: Journal of Acquired Immune Deficiency Syndromes. ; 2018. doi: 10.1097/QAI.0000000000001629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castillo-Mancilla JR, Brown TT, Erlandson KM, et al. Suboptimal Adherence to Combination Antiretroviral Therapy Is Associated With Higher Levels of Inflammation Despite HIV Suppression. Clin Infect Dis. Published online 2016. doi: 10.1093/cid/ciw650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulcher JA, Shoptaw S, Makgoeng SB, et al. Recent methamphetamine use is associated with increased rectal mucosal inflammatory cytokines, regardless of HIV-1 serostatus. J Acquir Immune Defic Syndr. Published online 2018. doi: 10.1097/QAI.0000000000001643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carrico AW, Flentje A, Kober K, et al. Recent stimulant use and leukocyte gene expression in methamphetamine users with treated HIV infection. Brain Behav Immun. Published online 2018. doi: 10.1016/j.bbi.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carrico AW, Cherenack EM, Roach ME, et al. Substance-associated elevations in monocyte activation among methamphetamine users with treated HIV infection. AIDS. Published online 2018. doi: 10.1097/QAD.0000000000001751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cook RR, Fulcher JA, Tobin NH, et al. Alterations to the Gastrointestinal Microbiome Associated with Methamphetamine Use among Young Men who have Sex with Men. Sci Rep. Published online 2019. doi: 10.1038/s41598-019-51142-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrico AW, Johnson MO, Morin SF, et al. Stimulant use is associated with immune activation and depleted tryptophan among HIV-positive persons on anti-retroviral therapy. Brain Behav Immun. Published online 2008. doi: 10.1016/j.bbi.2008.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J, Lee J, Meade CS, et al. Tryptophan Degradation and Risk-Taking Propensity 1. :1–16. [Google Scholar]
- 42.Carrico AW, Johnson MO, Morin SF, et al. Stimulant use is associated with immune activation and depleted tryptophan among HIV-positive persons on anti-retroviral therapy. Brain Behav Immun. 2008;22(8). doi: 10.1016/j.bbi.2008.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrico AW, Jain J, Discepola MV., et al. A community-engaged randomized controlled trial of an integrative intervention with HIV-positive, methamphetamine-using men who have sex with men. BMC Public Health. Published online 2016. doi: 10.1186/s12889-016-3325-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carrico AW, Gόmez W, Jain J, et al. Randomized controlled trial of a positive affect intervention for methamphetamine users. Drug Alcohol Depend. 2018;192. doi: 10.1016/j.drugalcdep.2018.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carrico AW, Neilands TB, Dilworth SE, et al. Randomized controlled trial of a positive affect intervention to reduce HIV viral load among sexual minority men who use methamphetamine. J Int AIDS Soc. 2019;22(12). doi: 10.1002/jia2.25436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials. Published online 2004. doi: 10.1310/JFXH-G3X2-EYM6-D6UG [DOI] [PubMed] [Google Scholar]
- 47.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. Published online 1977. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 48.Cacciola JS, Alterman AI, McLellan AT, Lin YT, Lynch KG. Initial evidence for the reliability and validity of a “Lite” version of the Addiction Severity Index. Drug Alcohol Depend. Published online 2007. doi: 10.1016/j.drugalcdep.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 49.Laich A, Neurauter G, Widner B, Fuchs D. More rapid method for simultaneous measurement of tryptophan and kynurenine by HPLC. Clin Chem. Published online 2002. doi: 10.1093/clinchem/48.3.579 [DOI] [PubMed] [Google Scholar]
- 50.Neurauter G, Grahmann AV., Klieber M, et al. Serum phenylalanine concentrations in patients with ovarian carcinoma correlate with concentrations of immune activation markers and of isoprostane-8. Cancer Lett. Published online 2008. doi: 10.1016/j.canlet.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 51.Grosgebauer K, Salinas J, Sharkey M, et al. Psychosocial Correlates of Monocyte Activation and HIV Persistence in Methamphetamine Users. J Neuroimmune Pharmacol. Published online 2019. doi: 10.1007/s11481-018-9797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharkey ME, Teo I, Greenough T, et al. Persistence of episomal HIV-1 infection intermediates in patients on highly active anti-retroviral therapy. Nat Med. Published online 2000. doi: 10.1038/71569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leutscher PDC, Stecher C, Storgaard M, Larsen CS. Discontinuation of efavirenz therapy in HIV patients due to neuropsychiatric adverse effects. Scand J Infect Dis. Published online 2013. doi: 10.3109/00365548.2013.773067 [DOI] [PubMed] [Google Scholar]
- 54.Funderburg N, Kalinowska M, Eason J, et al. Effects of maraviroc and efavirenz on markers of immune activation and inflammation and associations with CD4+ cell rises in HIV-infected patients. PLoS One. Published online 2010. doi: 10.1371/journal.pone.0013188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cassol E, Misra V, Holman A, Kamat A, Morgello S, Gabuzda D. Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC Infect Dis. Published online 2013. doi: 10.1186/1471-2334-13-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: A review and meta-analysis. J Acquir Immune Defic Syndr. Published online 2011. doi: 10.1097/QAI.0B013E31822D490A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heil M, Brockmeyer NH. Self-DNA Sensing Fuels HIV-1-Associated Inflammation. Trends Mol Med. Published online 2019. doi: 10.1016/j.molmed.2019.06.004 [DOI] [PubMed] [Google Scholar]
- 58.Massanella M, Gianella S, Schrier R, et al. Methamphetamine Use in HIV-infected Individuals Affects T-cell Function and Viral Outcome during Suppressive Antiretroviral Therapy. Sci Rep. Published online 2015. doi: 10.1038/srep13179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oni O, Glynn TR, Antoni MH, et al. Post-traumatic Stress Disorder, Cocaine Use, and HIV Persistence. Int J Behav Med. 2019;26(5). doi: 10.1007/s12529-019-09804-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palmer CD, Tomassilli J, Sirignano M, et al. Seronegative Men who have Sex with Men. 2015;28(14):2162–2166. doi: 10.1097/QAD.0000000000000386.Enhanced [DOI] [Google Scholar]