Abstract
Background/Aim
In recent years, switch maintenance after platinum-based chemotherapy has been a standard of care. However, the appropriate number of systemic chemotherapy cycles against advanced-stage urothelial carcinoma (UC) remains unclear. This study assessed the survival outcomes of first-line platinum-based chemotherapy according to treatment cycles in patients with metastatic disease.
Patients and Methods
We retrospectively evaluated patients with metastatic bladder and upper urinary tract cancer who received platinum-based combination therapy. Overall survival (OS) was evaluated using the Kaplan-Meier method and the log-rank test.
Results
Of 179 patients, 47 (26.3%) were women, and 73 (40.8%) had upper urinary tract cancer. Furthermore, 47 (26.3%) who were not eligible for cisplatin received carboplatin. The median number of treatment cycles was 3 (range=1-14 cycles). The rates of progressive disease within two cycles, from two to four cycles, and from four to six cycles were 18.4%, 19.2%, and 30.6%, respectively. The median OS of patients with 2, 3, 4, 5-6, and ≥7 treatment cycles were 8.6, 14.3, 21.3, 24.4, and 26.1 months, respectively. The OS did not significantly differ between patients receiving four treatment cycles and those receiving ≥5 treatment cycles. In patients with disease control (complete or partial response or stable disease) receiving ≥4 treatment cycles, there was no significant difference in terms of OS between patients receiving four cycles and those receiving six cycles.
Conclusion
Four cycles of first-line platinum-based chemotherapy can be effective in patients with metastatic UC.
Keywords: Urothelial carcinoma, chemotherapy, cisplatin, carboplatin, number of cycles, avelumab, switch maintenance
Platinum-based combination chemotherapy is the standard primary treatment for patients with metastatic urothelial carcinoma (UC), which is a progressive disease (PD) with a poor prognosis. The overall survival (OS) rate at five years for metastatic disease in the Surveillance, Epidemiology, and End Results database was 8.3% (1). The JAVERIN Bladder 100 trial showed that patients who received avelumab as maintenance therapy had a longer OS than those receiving best supportive care (BSC) after 4-6 cycles of first-line platinum-based chemotherapy without disease progression (2). Based on this result, the use of avelumab, an anti-programmed death-ligand 1 antibody, for advanced-staged UC was approved in Japan in 2021.
Some immune checkpoint inhibitors, such as avelumab, pembrolizumab, atezolizumab, and durvalumab are not directly comparable. However, previous clinical trials have shown that the OS rate of patients receiving avelumab as switch maintenance is favorable (3-6). The treatment opportunities to switch maintenance using avelumab are relatively increasing. Furthermore, antibody drug conjugates have recently emerged as late-line therapy for advanced-stage UC (7-9). Owing to the advancements of these effective subsequent pharmacotherapies, platinum-based chemotherapy has been the gateway to treatment. Nevertheless, a connecting role has arisen. Over the last three decades, first-line platinum-based chemotherapy has been continually used in cases in which such treatment is effective. Therefore, information on the association between optimal cycles of platinum-based chemotherapy and survival outcomes is still insufficient. Currently, the role of primary chemotherapy should be evaluated to establish novel treatment strategies.
Hence, this study aimed to assess the clinical significance of the number of cycles with respect to the actual circumstances of first-line platinum-based chemotherapy and prognosis in patients with metastatic UC.
Patients and Methods
Patient population. This single-center study was conducted to evaluate patients with metastatic UC in the bladder, ureter, and renal pelvis who received first-line platinum-based chemotherapy between May 2008 and April 2023. All patients presented with a histological diagnosis of UC or a subtype of UC and a radiologically confirmed metastatic disease. The association between the number of cycles of first-line chemotherapy and survival outcomes was retrospectively assessed. This study protocol was approved by the University of Occupational and Environmental Health Institutional Review Board (approval no.: UOEHCRB20-134).
Patient management and evaluation. The GC regimen (gemcitabine: 1,000 mg/m2 on days 1, 8, and 15; cisplatin: 70 mg/m2 on day 2) or the MVAC regimen (methotrexate: 30 mg/m2 on days 1, 15, and 22; vinblastine: 3 mg/m2 on days 2, 15, and 22; doxorubicin: 30 mg/m2 on day 2; and cisplatin: 70 mg/m2 on day 2) was administered intravenously every four weeks. Patients not eligible for cisplatin based on renal function, age, and performance status received carboplatin. Each chemotherapy was repeated until disease progression, development of unacceptable adverse events (AEs), withdrawal according to patient preference, and deterioration of a patient’s condition. The follow-up evaluation included physical examination, laboratory tests, and chest–abdominal–pelvic computed tomography (CT) scan. CT scan was performed at pretreatment and after every 1-3 cycles of chemotherapy according to the discretion of the attending physician. If bone lesions were suspected on CT scan, bone scintigraphy was performed. If pain or symptoms appeared, appropriate additional examinations were conducted. Tumor response was assessed as the best response according to the Response Evaluation Criteria in Solid Tumors version 1.1 (10). The response details were defined as follows: complete response (CR), partial response (PR), stable disease (SD), and PD.
Statistical analysis. All statistical data were analyzed using EZR ver.1.40 (Easy R, Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) (11). Progression-free survival (PFS) was defined as the time from the date of administration of first-line chemotherapy to the date of disease progression or death, whichever occurred earlier. OS was defined as the time from the date of chemotherapy administration to the date of death or the last follow up in patients who survived. PFS and OS were evaluated using the Kaplan-Meier method, and the log-rank test was used to determine between-group differences. p-Values of <0.05 were considered statistically significant.
Results
Characteristics of the patients. In total, 179 patients were enrolled in this study, and Table I shows the baseline characteristics of patients with metastatic UC who received first-line platinum-based chemotherapy. The patients commonly received the GC regimen, and approximately one-quarter of patients not eligible for cisplatin were treated with carboplatin. The median number of cycles of first-line chemotherapy was three (range=1-14 cycles). Briefly, 75 (41.9%), 68 (38.0%), 25 (14.0%), and 11 (6.1%) patients received ≤2, 3-4, 5-6, and ≥7 treatment cycles, respectively. After first-line chemotherapy, 85 (47.5%) patients received BSC. Meanwhile, 38 (21.2%) patients received subsequent pembrolizumab treatment.
Table I. Patient characteristics.
IQR: Interquartile range; UC: urothelial carcinoma; MVAC: methotrexate, vinblastine, doxorubicin, and cisplatin; GC: gemcitabine, cisplatin.
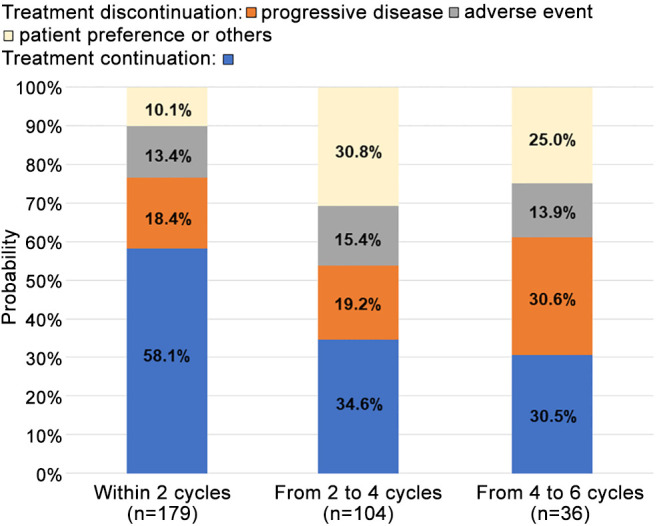
Response to first-line chemotherapy. As shown in Figure 1, the treatment completion rates of patients receiving first-line platinum-based chemotherapy within two cycles, from two to four cycles, and from four to six cycles were 75.9%, 65.4%, and 66.7%, respectively. The objective response rate (CR or PR) and disease control rate (CR, PR, or SD) of patients receiving platinum-based chemotherapy were 35.8% and 63.7%, respectively. The PD rates within two cycles, from two to four cycles, and from four to six cycles were 18.4%, 19.2%, and 30.6%, respectively (Figure 2). The incidence rate of first-line chemotherapy discontinuation due to AE at each cycle was 13.4%-15.4%. The proportions of chemotherapy continuation after the end of two, four, and six cycles were 58.1%, 34.6%, and 30.5%, respectively.
Figure 1. Treatment completion rates in patients receiving first-line platinum-based chemotherapy.
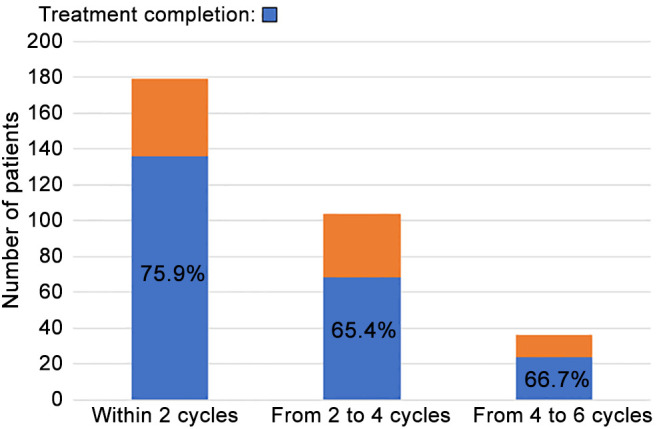
Figure 2. Availability of treatment continuation at each cycle of first-line chemotherapy.
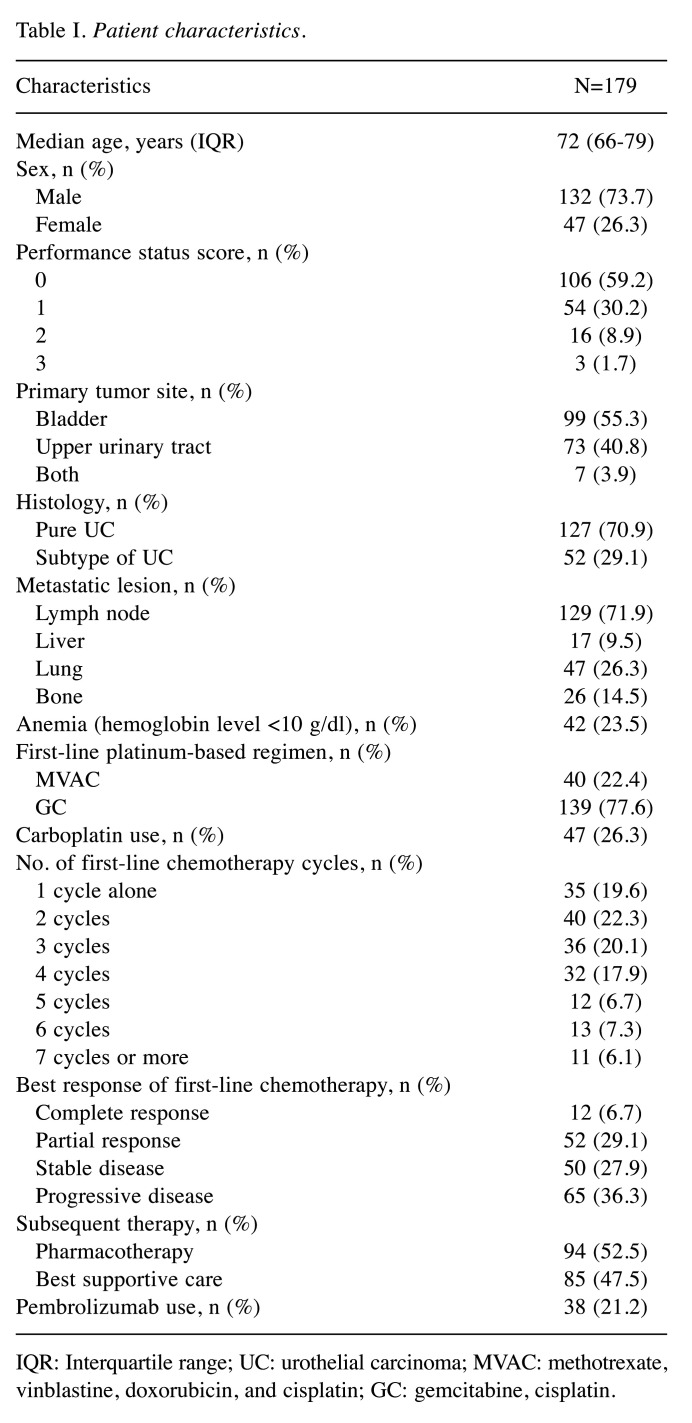
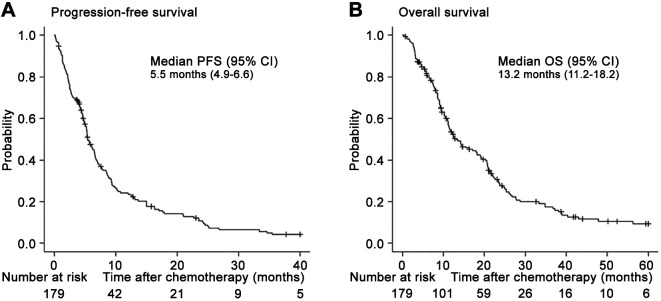
Survival outcomes. The median follow-up time was 13.4 (interquartile range=7.7-24.7) months, during which 145 (81.0%) presented with PD and 139 (77.7%) died. The median durations of PFS (Figure 3A) and OS (Figure 3B) after first-line platinum-based chemotherapy in 179 patients were 5.5 [95% confidence interval (CI)=4.9-6.6] months and 13.2 (95%CI=11.2-18.2) months. The OS between patients receiving the GC regimen (median: 13.2 months, 95%CI=11.1-19.0) and those receiving the MVAC regimen (median: 12.0 months, 95%CI=8.6-21.5) did not significantly differ (Figure 4A). Similarly, the OS did not significantly differ based on the type of platinum agent (cisplatin of carboplatin) (Figure 4B).
Figure 3. Kaplan-Meier curves of (A) progression-free survival (PFS) and (B) overall survival (OS) after the initiation of first-line platinum-based chemotherapy in patients with metastatic urothelial carcinoma. CI: Confidence interval.
Figure 4. Kaplan-Meier curves of overall survival after the initiation of first-line chemotherapy stratified according to regimen (A) and use of cisplatin or carboplatin (B). MVAC: Methotrexate, vinblastine, doxorubicin, and cisplatin; GC: gemcitabine and cisplatin.
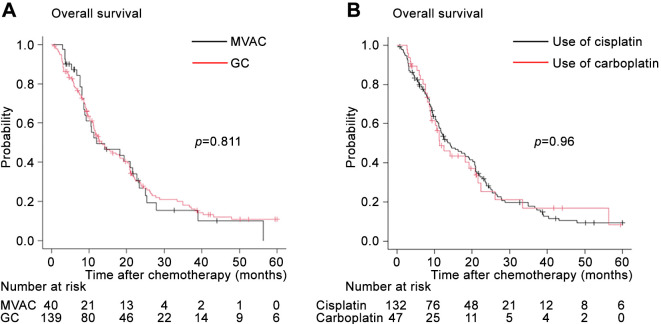
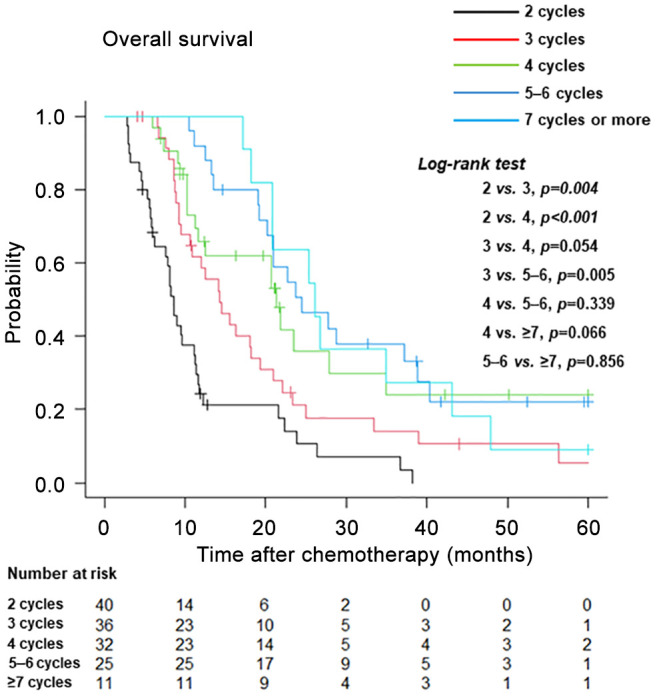
Among the groups stratified according to the number of chemotherapy cycles (Figure 5), the median durations of OS were as follows: 2 cycles, median: 8.6 months (95%CI=6.2-11.1); 3 cycles, median: 14.3 months (95%CI=9.5-18.2); 4 cycles, median: 21.3 months (95%CI=11.2-34.8); 5-6 cycles, median: 24.4 months (95%CI=19.1-38.8); and ≥7 cycles, median: 26.1 months (95%CI=18.2-43.1). Patients receiving two treatment cycles had a poorer OS than those receiving three or four treatment cycles. Patients receiving three treatment cycles had a lower OS than those receiving four treatment cycles (p=0.054). There were no significant differences in terms of OS between patients receiving four treatment cycles and those receiving 5-6 or ≥7 treatment cycles.
Figure 5. Kaplan-Meier curves of overall survival after the initiation of first-line platinum-based chemotherapy stratified according to the number of treatment cycles.
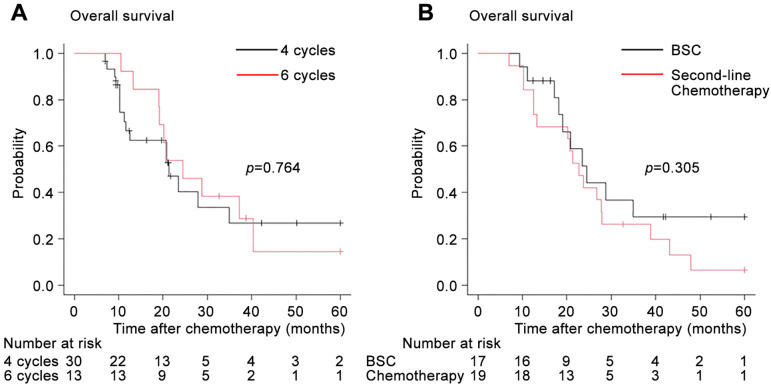
In patients with disease control (CR, PR, or SD) receiving ≥4 treatment cycles, there was no significant difference in terms of OS between patients receiving four cycles and those receiving six cycles (Figure 6A). In patients receiving subsequent chemotherapy or BSC after platinum-based chemotherapy failure, the OS from the start of the first-line chemotherapy showed no significant difference between the two groups (Figure 6B).
Figure 6. In patients with disease control (complete or partial response or stable disease) receiving ≥4 cycles, the Kaplan-Meier curves depict the overall survival after the initiation of first-line platinum-based chemotherapy stratified according to the number of cycles (4 or 6 cycles) (A) and the subsequent chemotherapy or best supportive care (BSC) (B).
Discussion
This study showed that the median duration of PFS and OS of the whole cohort was similar to that in a previous phase III trial (12). In patients with advanced-stage UC, data on survival from first-line chemotherapy according to the number of cycles are limited. In 2018, the Retrospective International Study of Invasive/Advanced Cancer of the Urothelium database in the United States, Europe, Israel, and Canada showed no significant difference in terms of OS between patients receiving 3-5 cycles of platinum-based first-line chemotherapy and those receiving 6-9 treatment cycles (13). In Japan, Yamamoto et al. performed a retrospective study (n=115) and found no difference in terms of OS between patients receiving four treatment cycles and those receiving ≥5 treatment cycles, which is similar to our results (14). Furubayashi et al. reported that early change in therapy should be considered for patients with a therapeutic effect as the number of GC cycles was not an independent prognostic factor based on a multivariate analysis (15). In fact, the current study showed that patients with disease control (CR, PR, or SD) receiving six treatment cycles and those receiving four treatment cycles did not significantly differ with respect to OS. In addition, there was no difference in terms of OS between patients receiving ≥4 cycles of first-line chemotherapy who subsequently had second-line chemotherapy and those who received BSC. Hence, there is a need to switch from platinum-based chemotherapy to immune checkpoint blockade therapy as sequential maintenance therapy or second-line therapy (16). Recently, Shindo et al. reported similar oncological outcomes between maintenance avelumab therapy and pembrolizumab as second-line therapy in patients with advanced-stage UC (17). Previous studies have revealed that immune checkpoint inhibitors are effective in patients who are chemotherapy-sensitive or chemotherapy-resistant (18-20).
In a real-world clinical setting, Okita et al. showed that the median number of cycles of first-line platinum-based chemotherapy leading to the best responses were 4, 4, and 2 in patients with CR, PR, and SD, respectively (21). In 2024, the latest subgroup analysis from the JAVERIN Bladder 100 trial showed that the median durations of OS were 19.9, 19.9, and 24.0 months in the 4, 5, and 6 cycle subgroups of prior chemotherapy, respectively (22). However, this clinical trial did not provide statistical comparisons in terms of OS according to the number of chemotherapy cycles. To determine the optimal duration of chemotherapy in patients with advanced-stage UC, a prospective study of the ongoing randomized DISCUS trial in Europe (EudraCT number: 2021-001975-17) has compared three versus six cycles of platinum-based chemotherapy, followed by avelumab switch maintenance (23). Our study showed that patients receiving three cycles were observed to have a lower OS, nearing significance, compared to those receiving four cycles. However, further research is required to determine whether it is preferable to switch to avelumab after three or four cycles of chemotherapy. A recent meta-analysis on neoadjuvant chemotherapy showed that four cycles of chemotherapy had a significantly better cancer-specific survival than three cycles (24).
Cases were also included during a period of unfamiliarity with chemotherapy management or treated with the highly toxic MVAC therapy. Therefore, the discontinuation rate in our cohort might have been affected. The cumulative toxicity caused by platinum agents should be considered for patients receiving six cycles of chemotherapy compared with those receiving four cycles. In the VESPER trial (NCT01812369), only 60% of patients achieved the planned six cycles in the dose dense-MVAC arm (25).
Considering the duration of PFS with platinum-based chemotherapy (median: approximately 5.2-7.3 months) (12,14,15), the proportion of patients with PD from four to six cycles could increase, similar to our results. Based on the treatment completion rate, PD rate, discontinuation caused by AEs, patient preference, and survival outcomes, we believe that four cycles of platinum-based first-line chemotherapy for metastatic UC are adequate. Moreover, our study showed that the OS did not significantly differ based on the type of platinum agent. Hamada et al. reported that the use of carboplatin can be beneficial for patients with advanced or metastatic UC who have moderate renal dysfunction (26).
Most recently, two randomized phase III clinical trials revealed that the OS of patients with unresectable or metastatic UC improved with novel combination regimens as first-line treatments. In the CheckMate-901 trial (NCT03036098), the median duration of OS in patients with CR treated with GC plus nivolumab (CR rate: 21.7%) was 37.1 months (27). In the EV-302 trial (NCT04223856), the median OS of patients treated with enfortumab vedotin plus pembrolizumab was 31.5 months (28). Based on the results of these trials, the treatment strategy for advanced-stage UC may change significantly in the near future. However, favorable survival outcomes are expected in terms of OS with the sequential therapy approach, transitioning from platinum-based chemotherapy to subsequent avelumab maintenance therapy, and then to enfortumab vedotin monotherapy.
Study limitations. The study had a retrospective nonrandomized design, was performed at a single institution, and had a heterogeneous population (approximately 40% of patients with upper urinary tract cancer). The group who received ≥5 treatment cycles had a small sample size. In addition, the first-line chemotherapy regimens were not standardized, with most patients receiving GC and others receiving MVAC. In a quarter of cases, the use of carboplatin was acceptable based on the status of the patients. Moreover, our study did not evaluate the duration to achieving best response to platinum-based chemotherapy.
Despite these limitations, our data showed that the number of cycles of first-line platinum-based chemotherapy can affect survival outcomes in metastatic disease. Furthermore, actual circumstances, such as the treatment completion rates, availability of treatment continuation, and the proportion of patients with treatment discontinuation can provide useful information for urologists and medical oncologists. We believe that our study can facilitate the optimal timing for switching to maintenance treatment without delay for patients with metastatic UC.
Conclusion
Four cycles of first-line platinum-based chemotherapy can be adequate for patients with metastatic UC. In daily clinical practice, regarding the feasibility of treatment continuation, not only treatment efficacy but also AEs or patient preference must be considered.
Funding
Not applicable.
Conflicts of Interest
The Authors declare that they have no competing interests in relation to this study.
Authors’ Contributions
AM: Conceptualization, methodology, investigation, data curation, statistical analysis, writing of the original draft. DO, KM, YO, and TT: Investigation, data curation. KH and YN: Data curation. IT, KH, and NF: Reviewing, editing, supervision. All Authors discussed, verified, and approved the final version of the article.
Acknowledgements
The Authors thank Enago (www.enago.jp) for the English language editing.
References
- 1.National Cancer Institute SEER cancer stat facts: bladder cancer. Available at: https://seer.cancer.gov/statfacts/html/urinb.html. [Last accessed on April 11, 2024]
- 2.Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, Kalofonos H, Radulović S, Demey W, Ullén A, Loriot Y, Sridhar SS, Tsuchiya N, Kopyltsov E, Sternberg CN, Bellmunt J, Aragon-ching JB, Petrylak DP, Laliberte R, Wang J, Huang B, Davis C, Fowst C, Costa N, Blake-haskins JA, Di Pietro A, Grivas P. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 3.Powles T, Park SH, Caserta C, Valderrama BP, Gurney H, Ullén A, Loriot Y, Sridhar SS, Sternberg CN, Bellmunt J, Aragon-Ching JB, Wang J, Huang B, Laliberte RJ, di Pietro A, Grivas P. Avelumab first-line maintenance for advanced urothelial carcinoma: results from the JAVELIN Bladder 100 trial after ≥2 years of follow-up. J Clin Oncol. 2023;41(19):3486–3492. doi: 10.1200/JCO.22.01792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fradet Y, Bellmunt J, Vaughn DJ, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Nam K, Frenkl TL, Perini RF, de Wit R, Bajorin DF. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol. 2019;30(6):970–976. doi: 10.1093/annonc/mdz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galsky MD, Arija JÁA, Bamias A, Davis ID, De Santis M, Kikuchi E, Garcia-Del-Muro X, De Giorgi U, Mencinger M, Izumi K, Panni S, Gumus M, Özgüroğlu M, Kalebasty AR, Park SH, Alekseev B, Schutz FA, Li JR, Ye D, Vogelzang NJ, Bernhard S, Tayama D, Mariathasan S, Mecke A, Thåström A, Grande E, IMvigor130 Study Group Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10236):1547–1557. doi: 10.1016/S0140-6736(20)30230-0. [DOI] [PubMed] [Google Scholar]
- 6.Powles T, van der Heijden MS, Castellano D, Galsky MD, Loriot Y, Petrylak DP, Ogawa O, Park SH, Lee JL, De Giorgi U, Bögemann M, Bamias A, Eigl BJ, Gurney H, Mukherjee SD, Fradet Y, Skoneczna I, Tsiatas M, Novikov A, Suárez C, Fay AP, Duran I, Necchi A, Wildsmith S, He P, Angra N, Gupta AK, Levin W, Bellmunt J, DANUBE study investigators Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1574–1588. doi: 10.1016/S1470-2045(20)30541-6. [DOI] [PubMed] [Google Scholar]
- 7.Powles T, Rosenberg JE, Sonpavde GP, Loriot Y, Durán I, Lee JL, Matsubara N, Vulsteke C, Castellano D, Wu C, Campbell M, Matsangou M, Petrylak DP. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384(12):1125–1135. doi: 10.1056/NEJMoa2035807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minato A, Kimuro R, Ohno D, Tanigawa K, Kuretake K, Matsukawa T, Takaba T, Jojima K, Harada M, Higashijima K, Nagata Y, Tomisaki I, Harada K, Fujimoto N, Miyamoto H. Efficacy and tolerability of enfortumab vedotin for metastatic urothelial carcinoma: early experience in the real world. Anticancer Res. 2023;43(9):4055–4060. doi: 10.21873/anticanres.16594. [DOI] [PubMed] [Google Scholar]
- 9.Loriot Y, Petrylak DP, Rezazadeh Kalebasty A, Fléchon A, Jain RK, Gupta S, Bupathi M, Beuzeboc P, Palmbos P, Balar AV, Kyriakopoulos CE, Pouessel D, Sternberg CN, Tonelli J, Sierecki M, Zhou H, Grivas P, Barthélémy P, Tagawa ST. TROPHY-U-01, a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors: updated safety and efficacy outcomes. Ann Oncol. 2024;35(4):392–401. doi: 10.1016/j.annonc.2024.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 13.Sonpavde GP, Mariani L, Lo Vullo S, Raggi D, Giannatempo P, Bamias A, Crabb SJ, Bellmunt J, Yu EY, Niegisch G, Vaishampayan UN, Theodore C, Berthold DR, Srinivas S, Sridhar SS, Plimack ER, Rosenberg JE, Powles T, Galsky MD, Necchi A. Impact of the number of cycles of platinum based first line chemotherapy for advanced urothelial carcinoma. J Urol. 2018;200(6):1207–1214. doi: 10.1016/j.juro.2018.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto S, Kato M, Takeyama Y, Yukimatsu N, Hirayama Y, Otoshi T, Yamasaki T, Kuratsukuri K, Uchida J. A retrospective study on optimal number of cycles of the first-line platinum-based chemotherapy for metastatic urothelial carcinoma. Urol Oncol. 2022;40(5):194.e7–194.e14. doi: 10.1016/j.urolonc.2021.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Furubayashi N, Negishi T, Takamatsu D, Ieiri K, Inoue T, Tsukino K, Nakamura M. Timing of changing therapy from gemcitabine and cisplatin chemotherapy based on real-world data of advanced urothelial carcinoma. Oncol Lett. 2020;19(4):2943–2949. doi: 10.3892/ol.2020.11368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minato A, Murooka K, Okumura Y, Takaba T, Higashijima K, Nagata Y, Tomisaki I, Harada K, Fujimoto N. Efficacy of platinum-based chemotherapy in patients with metastatic urothelial carcinoma with variant histology. In Vivo. 2024;38(2):873–880. doi: 10.21873/invivo.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shindo T, Hashimoto K, Takahashi A, Miyamoto S, Kunishima Y, Sato S, Fukuta F, Hiyama Y, Takayanagi A, Kato R, Wanifuchi A, Ueki Y, Okada M, Adachi H, Kobayashi KO, Tanaka T, Masumori N, Sapporo Medical University Urologic Oncology Consortium Comparison of oncological outcomes of pembrolizumab as second-line therapy and maintenance avelumab therapy in advanced urothelial carcinoma after platinum-based chemotherapy. Anticancer Res. 2024;44(3):1271–1279. doi: 10.21873/anticanres.16922. [DOI] [PubMed] [Google Scholar]
- 18.Furubayashi N, Minato A, Tomoda T, Hori Y, Kiyoshima K, Negishi T, Haraguchi Y, Koga T, Kuroiwa K, Fujimoto N, Nakamura M. Organ-specific tumor response to avelumab maintenance therapy for advanced urothelial carcinoma: a multicenter retrospective study. Anticancer Res. 2023;43(12):5689–5698. doi: 10.21873/anticanres.16774. [DOI] [PubMed] [Google Scholar]
- 19.Minato A, Furubayashi N, Harada M, Negishi T, Sakamoto N, Song Y, Hori Y, Tomoda T, Tamura S, Kuroiwa K, Seki N, Tomisaki I, Harada K, Nakamura M, Fujimoto N. Efficacy of pembrolizumab in patients with variant urothelial carcinoma: a multicenter retrospective study. Clin Genitourin Cancer. 2022;20(5):499.e1–499.e8. doi: 10.1016/j.clgc.2022.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Furubayashi N, Negishi T, Sakamoto N, Tamura S, Morokuma F, Song Y, Hori Y, Tomoda T, Seki N, Kuroiwa K, Nakamura M. Clinical outcomes of mixed response to pembrolizumab in advanced urothelial carcinoma after platinum-based chemotherapy. In Vivo. 2021;35(5):2869–2874. doi: 10.21873/invivo.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okita K, Hatakeyama S, Hagiwara K, Suzuki Y, Tanaka T, Noro D, Tokui N, Fujita N, Konishi S, Okamoto T, Yoneyama T, Yamamoto H, Yoneyama T, Hashimoto Y, Ohyama C. The effect of number of treatment cycles of platinum-based first-line chemotherapy on maximum radiological response in patients with advanced urothelial carcinoma. Urol Oncol. 2021;39(12):832.e17–832.e23. doi: 10.1016/j.urolonc.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Sridhar SS, Powles T, Climent Durán M, Park SH, Massari F, Thiery-Vuillemin A, Valderrama BP, Ullén A, Tsuchiya N, Aragon-Ching JB, Gupta S, Petrylak DP, Bellmunt J, Wang J, Laliberte RJ, di Pietro A, Costa N, Grivas P, Sternberg CN, Loriot Y. Avelumab first-line maintenance for advanced urothelial carcinoma: analysis from JAVELIN Bladder 100 by duration of first-line chemotherapy and interval before maintenance. Eur Urol. 2024;85(2):154–163. doi: 10.1016/j.eururo.2023.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Grivas P, Grande E, Davis ID, Moon HH, Grimm MO, Gupta S, Barthélémy P, Thibault C, Guenther S, Hanson S, Sternberg CN. Avelumab first-line maintenance treatment for advanced urothelial carcinoma: review of evidence to guide clinical practice. ESMO Open. 2023;8(6):102050. doi: 10.1016/j.esmoop.2023.102050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Song Y, Qin C, Zhang C, Du Y, Xu T. Three versus four cycles of neoadjuvant chemotherapy for muscle-invasive bladder cancer: a systematic review and meta-analysis. Ann Med. 2023;55(2):2281654. doi: 10.1080/07853890.2023.2281654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfister C, Gravis G, Fléchon A, Chevreau C, Mahammedi H, Laguerre B, Guillot A, Joly F, Soulié M, Allory Y, Harter V, Culine S, VESPER Trial Investigators. Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin or gemcitabine and cisplatin as perioperative chemotherapy for patients with nonmetastatic muscle-invasive bladder cancer: results of the GETUG-AFU V05 VESPER trial. J Clin Oncol. 2022;40(18):2013–2022. doi: 10.1200/JCO.21.02051. [DOI] [PubMed] [Google Scholar]
- 26.Hamada A, Sano T, Matsumoto K, Sakatani T, Nakamura K, Sawada A, Akamatsu S, Matsui Y, Ogawa O, Kobayashi T. Modification of platinum-based systemic chemotherapy for advanced urothelial carcinoma in patients with suboptimal renal function. In Vivo. 2021;35(5):2821–2829. doi: 10.21873/invivo.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Heijden MS, Sonpavde G, Powles T, Necchi A, Burotto M, Schenker M, Sade JP, Bamias A, Beuzeboc P, Bedke J, Oldenburg J, Chatta G, Ürün Y, Ye D, He Z, Valderrama BP, Ku JH, Tomita Y, Filian J, Wang L, Purcea D, Patel MY, Nasroulah F, Galsky MD, CheckMate 901 Trial Investigators Nivolumab plus gemcitabine–cisplatin in advanced urothelial carcinoma. N Engl J Med. 2023;389(19):1778–1789. doi: 10.1056/NEJMoa2309863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, Iyer G, Vulsteke C, Park SH, Shin SJ, Castellano D, Fornarini G, Li JR, Gümüş M, Mar N, Loriot Y, Fléchon A, Duran I, Drakaki A, Narayanan S, Yu X, Gorla S, Homet Moreno B, van der Heijden MS, EV-302 Trial Investigators Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med. 2024;390(10):875–888. doi: 10.1056/NEJMoa2312117. [DOI] [PubMed] [Google Scholar]