Abstract
Although hepatitis A virus (HAV) is typically transmitted by the fecal-oral route, little is known of its interactions with cells of the gastrointestinal tract. We studied the replication of HAV in polarized cultures of Caco-2 cells, a human cell line which retains many differentiated functions of small intestinal epithelial cells. Virus uptake was 30- to 40-fold more efficient when the inoculum was placed on the apical rather than the basolateral surface of these cells, suggesting a greater abundance of the cellular receptor for HAV on the apical surface. Infection proceeded without cytopathic effect and did not influence transepithelial resistance or the diffusion of inulin across cell monolayers. Nonetheless, there was extensive release of progeny virus, which occurred almost exclusively into apical supernatant fluids (36.4% ± 12.5% of the total virus yield compared with 0.23% ± 0.13% release into basolateral fluids). Brefeldin A caused a profound inhibition of HAV replication, but also selectively reduced apical release of virus. These results indicate that polarized human epithelial cell cultures undergo vectorial infection with HAV and that virus release is largely restricted to the apical membrane. Virus release occurs in the absence of cytopathic effect and may involve cellular vesicular transport mechanisms.
Human hepatitis A virus (HAV) is a nonenveloped virus with a single-stranded, 7.5-kb positive-sense RNA genome (17, 18). It is classified as the type species of the genus Hepatovirus within the family Picornaviridae and is a common cause of both sporadic and epidemic acute hepatitis in humans (17, 19). The transmission of HAV is generally due to the ingestion of material contaminated with feces containing HAV. However, the pathological sequence of events that begins with entry of the virus via the gastrointestinal tract and ultimately results in hepatitis is not well understood. A primary, extrahepatic site of replication for this highly hepatotropic agent has long been postulated, but has proven difficult to demonstrate. Early experiments involving immunohistologic evaluation of intestinal tissue from infected nonhuman primates provided no evidence for the presence of virus within the gastrointestinal mucosa. Both Mathiesen et al. (25) and Krawczynski et al. (16) were unable to identify viral antigen in the gut of enterically infected primates. However, more recent studies, possibly with better immunologic reagents, have resulted in the demonstration of HAV antigen within cells of the small intestine. Karayiannis et al. (14) found specific HAV antigen within the cytoplasm of ∼3% of cells in duodenal biopsies from two of three tamarins (Saguinus labiatus) infected intravenously with a tamarin-adapted HAV variant. Similarly, Asher et al. (2) demonstrated the presence of HAV antigen in the cytoplasm of epithelial cells lining small intestinal crypts within 3 days of the oral inoculation of New World owl monkeys (Aotus trivirgatus) with virus. In these animals, virus was present in the gut prior to its detection within hepatocytes (2). Thus, studies in two different animal species suggest that small intestinal epithelial cells serve as a primary site of replication for HAV.
The cells which form the small intestinal epithelium are highly polarized, with differential expression of specific proteins on their apical (lumenal) and basolateral (basement membrane) surfaces which contributes to the numerous specialized secretory and absorptive functions of these cells. Relatively little is known of the interactions of nonenveloped viruses with such polarized cells, although it seems likely that these interactions play important roles in determining the pathogenesis of a wide variety of intestinal viral infections. In the case of HAV, such interactions are of special interest because the major cell type which supports replication, the hepatocyte, is also highly polarized and of epithelial origin (6, 11). The apical surface of the hepatocyte forms a well demarcated groove which encircles the cell and provides access to the biliary canaliculi through which components of bile (including HAV during acute hepatitis A) are secreted from the liver into the feces (6, 10, 31). The extended basilar surface of the hepatocyte is exposed to the space of Dissë and through it to the venous sinusoids, via which HAV is likely to reach the liver during the early stages of the infection. HAV appears to infect hepatocytes without cytopathic effect, and much higher virus titers are found in bile and in stool than in blood (17). Thus, a mechanism exists by which progeny viral particles are secreted in a vectorial fashion from the hepatocyte into the bile. It is tempting to speculate that this process may involve either the normal vesicular cellular protein sorting system or perhaps specialized hepatocellular transporter proteins involved in secretion of biliary lipids (phosphotidylcholine) and bile salts at the canalicular membrane (6, 30). However, there are no data available which address this issue.
To establish a system in which we could begin to investigate interactions of HAV with polarized epithelial cells, we sought to determine whether the human colonic epithelial cell line, Caco-2, is permissive for replication of HAV. Caco-2 cells most closely resemble epithelial cells of small intestinal villi and crypts (9, 29) and are thus likely to be similar to cells of the small intestine that are infected by HAV in vivo (2). They were originally derived from a human colonic adenocarcinoma and show evidence of spontaneous differentiation and polarization, especially when grown on a porous support. They express several enzyme activities which are peculiar to human small intestinal epithelial cells and form apical microvilli and prominent tight junctions (9, 28). Furthermore, when grown on a porous support, monolayers of Caco-2 cells transport water and ions to their basolateral surface, generate a high transepithelial electrical resistance, and develop a strong barrier to diffusion of small molecules like inulin.
Here, we present evidence that the uptake of HAV into polarized cultures of Caco-2 cells occurs with greater efficiency on the apical surface of the cells, in contrast to poliovirus, a distantly related picornavirus that is capable of bidirectional entry into these cells (36). Unlike poliovirus, HAV replication occurs without cytopathic effect or disruption of the transepithelial resistance displayed by polarized monolayer cultures. Despite this, there is extensive release of newly replicated progeny HAV in a vectorial fashion across the apical surfaces of Caco-2 cells. The apical release of HAV is selectively inhibited by brefeldin A, suggesting that it is mediated by a vesicular transport mechanism.
MATERIALS AND METHODS
Cells.
Caco-2 cells (HTB-37) were obtained from the American Type Culture Collection, Rockville, Md., and used between passages 18 and 36. The cells were maintained at 35.5°C in a 5% CO2 atmosphere in Dulbecco's modified medium with Earle's salts (DMEM), 4,500 mg of glucose/liter, and 25 mM HEPES (Gibco/BRL, Grand Island, N.Y.) supplemented with 20% fetal bovine serum (Irvine Scientific, Irvine, Calif.), 1% nonessential amino acids (Gibco), streptomycin (100 μg/ml), and penicillin (100 U/ml). Cells were grown in 75-cm2 T-flasks (Corning, Cambridge, Mass.) and passaged every 5 to 7 days at a 1:10 split ratio. Confluency was reached within 5 to 7 days after passage.
For growth of Caco-2 cells on porous supports, we evaluated two types of commercial cell culture inserts. In preliminary studies, we found that BioCoat Collagen I inserts (Collaborative Research, Inc., Bedford, Mass.) with a nominal 0.45-μm pore size were completely impermeable to HAV (data not shown). Furthermore, Caco-2 cells cultured on BioCoat Collagen I inserts demonstrated overgrowth and irregular transepithelial electrical resistance measurements within 4 days of seeding, suggesting that the cells maintained a polarized state for only 2 or 3 days. Thus, the experiments reported herein were carried out by using Transwell-COL tissue culture inserts (Costar Corp., Cambridge, Mass.) with a 0.33-cm2 growth area and 0.4-μm pore size. The permeability of these supports to HAV was confirmed by directly assessing the diffusion of virus across the membrane in the absence of a cell monolayer (see Results). Hydrated Transwell-COL inserts were seeded with 1.4 × 105 Caco-2 cells and incubated at 35.5°C in a 5% CO2 atmosphere for at least 7 days prior to infection. The medium (0.2 ml in the insert and 0.9 ml in the well) was replaced at 2-day intervals. Confluent monolayers contained approximately 3.5 × 105 cells per insert at the time of virus infection.
African green monkey kidney (BS-C-1) cells, between passage levels 93 and 98, were used for propagation and quantitation of HAV and were grown as monolayers in Eagle's minimal essential medium with Earle's salts (Gibco/BRL) supplemented with 100 mM glutamine, streptomycin (100 μg/ml), penicillin (100 U/ml), and 2 to 10% fetal bovine serum.
Assessment of Caco-2 cell monolayer integrity.
The integrity of Caco-2 monolayers was assessed by measurements of transepithelial electrical resistance and permeability of the monolayers to [3H]inulin. The electrical resistance displayed by warm inserts in cell culture medium was measured with a Millicell ERS apparatus (Millipore, Bedford, Mass.) according to the manufacturer's instructions. Only intact monolayers with a resistance of >480 Ω (158 Ω-cm2) were used for infection studies. The background resistance of membranes without cells was approximately 130 Ω (43 Ω-cm2). For measurements of monolayer permeability, 100 μl of DMEM containing 2.5 μCi of [3H]inulin (DuPont NEN Research Products, Boston, Mass.) was added to the insert, and 600 μl of medium was added to the well. Basolateral fluid samples (20 μl) were taken at 90 min. Similar permeability measurements were obtained with 30- and 60-min samplings of the basolateral fluids. A rate of inulin diffusion of less than 1%/h across the insert was considered indicative of intact junctional complexes between cells (36). Diffusion across membranes in the absence of cells was approximately 61%/h.
Virus.
To facilitate preparation of a virus inoculum and quantitation of virus yields, infections of Caco-2 cells were carried out with the HM175/18f variant of human HAV, which is highly adapted to growth in BS-C-1 cells (22). This virus displays a rapid replication/cytopathic (rr/cpe+) phenotype in these cells. The virus inoculum for Caco-2 infections consisted of chloroform-extracted, clarified supernatant fluids, collected 2 weeks following inoculation of a BS-C-1 cell culture with virus, and contained 2.1 × 107 radioimmunofocus-forming units (RFU) of virus per ml (21). For polarized Caco-2 cell infections, equal quantities of virus were allowed to adsorb to either the apical or basolateral cell surface for 2 h at 35.5°C at a multiplicity of infection (MOI) of approximately 6. Since the volumes of the inoculum differed between the apical and basolateral surfaces (0.2 and 0.9 ml, respectively), the concentration of virus present in the basolateral inocula was 22% that of the apical inocula. Following virus adsorption, cells were washed twice on both sides with cell culture medium. The cell culture medium was replaced subsequently on a daily basis. All infections were done in duplicate or triplicate.
Samples were taken for virus titration at daily intervals following inoculation of Caco-2 cells, with the first sample (time 0) collected immediately following adsorption of the virus. Apical and basolateral supernatant fluids were aspirated from the cultures, and the cell sheets were washed on both sides. Lysates of Caco-2 cells were made by the addition of 0.2 ml of 0.1% sodium dodecyl sulfate in Hanks' balanced salt solution to inserts. The supernatant fluids and cell lysates were stored at −70°C until virus titers were measured in BS-C-1 cells by a quantal radioimmunofocus assay, as described previously (21). Results are reported as RFU per milliliter or as RFU per culture (individual cell culture insert). For immunofluorescence detection of HAV antigen, infected Caco-2 monolayers were fixed with acetone at 4°C for 15 min and stained with a 1:250 dilution of a monoclonal anti-HAV antibody (K3-2F2). Nuclei were counterstained with DAPI (4′,6′-diamidino-2-phenylindole). After extensive washing in PBS, cells were incubated with a 1:64 dilution of fluorescein-labeled goat anti-mouse immunoglobulin (Sigma Immunochemicals, St. Louis, Mo.), and examined under a Zeiss epifluorescence microscope.
Inhibition of vesicular transport with brefeldin A and monensin.
A stock solution was prepared by dissolving crystalline brefeldin A (Epicenter Technologies Corp., Madison, Wis.) in ethanol at 10 mg/ml. For treatment of apically infected Caco-2 cells (see Results), cells were fed with medium containing various concentrations of brefeldin A (0.05 to 10 μg/ml) commencing immediately after the 2-h virus adsorption period (time 0) or 18, 36, or 54 h later. Because brefeldin A is rapidly metabolized, it was replenished every 6 h with daily complete changes of medium. Toxic effects resulting in morphological changes and a dramatic decrease in transepithelial resistance occurred within 18 h of exposure to even minimal concentrations of brefeldin A. Monensin (Sigma Chemical Co.) was dissolved in ethanol at a concentration of 1 mM; further dilutions were made in growth medium. At 72 h following infection of Caco-2 cells from the apical side, the medium was replaced with media containing concentrations of 10−7, 10−8, and 10−9 M monensin. After 6 h of additional incubation, cell lysates and apical and basolateral supernatant fluids were collected and assayed for infectious virus.
RESULTS
Caco-2 cells are permissive for replication of cell culture-adapted HAV.
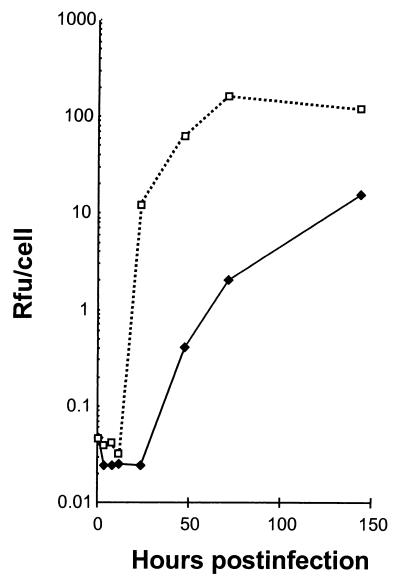
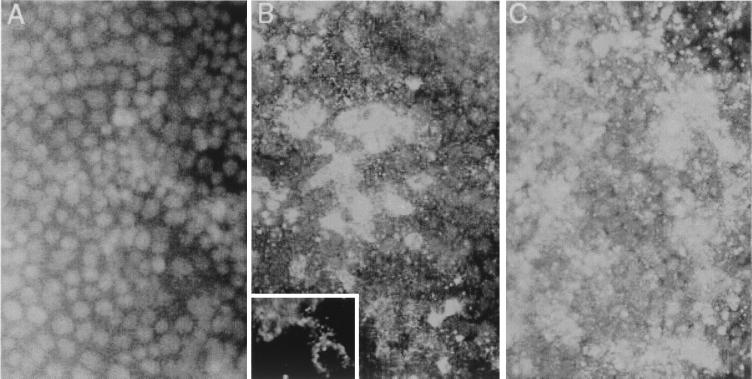
Because there is no published evidence that cultured human colonic epithelial cells are permissive for replication of HAV, we first assessed the ability of monolayer cultures of Caco-2 cells grown on an impermeable polystyrene surface to support replication of the virus under one-step growth conditions. Following inoculation of these cells with the monkey kidney cell culture-adapted HM175/18f virus (22) at an MOI of 4.9, the titer of cell-associated infectious virus increased logarithmically between 24 and 72 h postinoculation (Fig. 1). Thus, Caco-2 cells are permissive for replication of a cell culture-adapted human HAV strain. However, HM175/18f virus replication appeared to occur more slowly than in BS-C-1 cells, in which the period of exponential increase in titer is between 12 and 24 h postinoculation (Fig. 1) (22). Virus yields were also approximately 10-fold less in Caco-2 cells than in BS-C-1 cells at 150 h following inoculation. To determine the proportion of Caco-2 cells that are permissive for HAV replication, monolayer cultures were infected at an MOI of 6.0 and assayed for HAV antigen expression by indirect immunofluorescence 4, 8, and 12 days later (Fig. 2). By 4 days postinfection, approximately 50% of the cells contained detectable levels of HAV antigen (data not shown). Consistent with the one-step growth curve shown in Fig. 1, the proportion of infected cells increased significantly between days 4 and 8. By 8 days after inoculation, the majority (>80%) of cells contained characteristic fine punctate cytoplasmic fluorescence typical of HAV (Fig. 2B). The antigen-specific staining increased in intensity and involved virtually all cells by 12 days following inoculation (Fig. 2C). We conclude from these results that Caco-2 cells are permissive for replication of a cell culture-adapted HAV variant, although replication proceeds relatively slowly and to a lower overall titer in these cells than in monkey kidney cells (Fig. 1).
FIG. 1.
Replication of cell culture-adapted HAV under one-step conditions in cultures of Caco-2 cells grown on an impermeable plastic surface (♦; MOI = 4.9 RFU/cell) and in nonpolarized BS-C-1 cells (□; MOI = 4.5 RFU/cell).
FIG. 2.
Indirect immunofluorescence detection of HAV antigen in infected Caco-2 cells. (A) Normal uninfected Caco-2 monolayer with DAPI nuclear counterstain. (B) Caco-2 cells 8 days following infection with HAV at an MOI of 6.0. The inset shows a high-power view of the cytoplasmic distribution of punctate HAV-specific fluorescence in two cells (no counterstain). (C) Caco-2 cells 12 days following infection with HAV. Viral antigen was visualized with a monoclonal antibody reactive with the virus capsid.
We also assessed the ability of Caco-2 cells to support replication of wild-type virus. For these studies, Caco-2 cells grown on plastic were inoculated directly with a chloroform-extracted suspension of a primate fecal sample containing infectious wild-type human HAV (HM175 strain). Subsequent analysis failed to demonstrate intracellular levels of virus that were detectable by radioimmunoassay (20) or radioimmunofocus assay in BS-C-1 cells, even following three to six serial blind passages of 2 weeks each (data not shown). Thus, subsequent experiments were carried out with the cell culture-adapted HM175/18f virus. The genetic differences that distinguish this virus from its wild-type parent suggest that modifications in viral components that function in cap-independent translation and RNA replication are of the greatest importance to its ability to replicate efficiently in cultured cells (41). The amino acid sequences of the capsid proteins of the cell culture-adapted virus differ minimally from that of wild-type virus (22). This makes it likely that the processes of cellular entry and release are not fundamentally altered by the adaptation of this virus to growth in monkey kidney cell cultures.
HAV entry into polarized Caco-2 colonic epithelial cells occurs in an asymmetric manner.
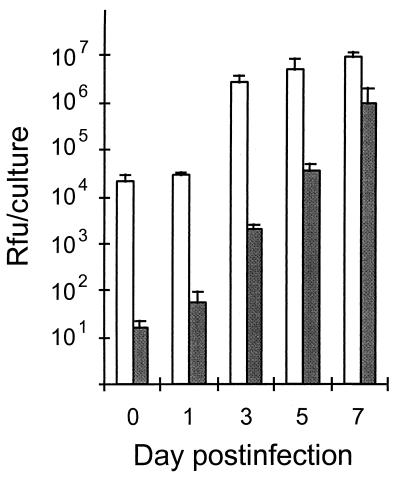
To determine whether polarized cultures of Caco-2 cells could be infected with HAV via either the apical or basolateral cell surfaces, the virus was allowed to adsorb to either domain of well established, polarized Caco-2 cell monolayers grown on porous Transwell-COL membranes. Inocula placed on either side of the membrane contained identical quantities of virus, representing an MOI of approximately 6, but a difference in the volume of the medium resulted in a variance in the concentration of virus in the two chambers (see Materials and Methods). The capacity of HAV to establish a productive infection was determined by measuring cell-associated, infectious HAV by radioimmunofocus assay in BS-C-1 cells. Lysates of inoculated Caco-2 cells were prepared immediately after the 2-h virus adsorption period (time 0) and at periodic intervals up to 7 days. These results demonstrated that HAV infection proceeded much more efficiently following inoculation of the apical surface of polarized Caco-2 cells (Fig. 3). The quantity of cell-associated virus increased exponentially over 3 to 7 days following apical inoculation, with the final virus titer approximately 330-fold greater than the quantity of virus present immediately after adsorption. At 24 and 72 h postinfection, respectively, the titer of cell-associated HAV was 500- to 1,000-fold higher in cells infected via the apical surface compared to that in those infected via the basolateral surface. The magnitude of this difference was progressively reduced at 5 and 7 days postinfection, most likely reflecting second-cycle infections of Caco-2 cells by virus released into the apical supernatant fluids by a small number of cells infected following basolateral inoculation (Fig. 3) (see below).
FIG. 3.
Cell-associated HAV following inoculation of polarized Caco-2 cells on either apical (open bars) or basolateral (shaded bars) cell surfaces at an MOI of approximately 6 RFU/cell. The results shown represent the means of the cell-associated virus content of three replicate infected cultures (± standard deviation).
Uncoating of HAV is thought to occur very slowly following cellular entry (22, 39). Thus, the quantity of infectious virus associated with cells immediately after the 2-h adsorption period may be taken as a reasonable measure of virus attachment and cellular receptor activity, even when virus adsorption is carried out at physiologic temperatures. The proportion of the HAV inoculum remaining associated with the monolayer following adsorption and a series of two washes with medium was approximately 1.1% when the inoculum was placed on the apical cell surface, compared with only 0.0008% following adsorption to the basolateral surface of Caco-2 cells, a 1,375-fold difference (Table 1).
TABLE 1.
Virus adsorption to Caco-2 cells at 35.5°C
| Surface inoculated | Inoculum (RFU) | Amt of cell-associated virus following 2-h adsorption period (RFU ± SD) | % of inoculum associated with Caco-2 monolayer |
|---|---|---|---|
| Apical | 2.1 × 106 (0.2 ml) | 2.3 × 104 (± 6.5 × 103) | 1.1 |
| Basolateral | 2.1 × 106 (0.9 ml) | 1.6 × 101 ± 7 | 0.0008 |
To determine the extent to which the rate of virus diffusion through the Transwell-COL membrane may have limited the uptake of virus from the basolateral medium, we measured the ability of virus to diffuse through the membrane in the absence of a cell monolayer. A virus inoculum was placed on the basolateral side of the membrane, and the titer of virus in fluids on both sides of the membrane following a 2-h mock adsorption period was measured. Virus titers in the apical fluid ranged from 10 to 13% of those in the basolateral fluid at the end of the incubation period and were 5.0 to 6.6% of the original inoculum. Furthermore, although the quantity of virus in the basolateral and apical inocula were kept identical in order to maintain a constant MOI (Fig. 3), the larger volume of the basolateral chamber of the Transwell-COL inserts resulted in a lower concentration of virus in the basolateral medium (22% that of the inoculum in the apical chamber) (see Materials and Methods). Taken together, the limited diffusion across the membrane and the difference in volume in the two chambers suggest that the concentration of virus at the basolateral cell surface may have been only 2.2 to 2.9% of that present at the apical surface in the experiment shown in Fig. 3. While this may explain some of the 1,375-fold difference that was evident in the uptake of virus from the apical versus basolateral inoculum (Table 1), the much smaller fraction of cell-associated virus following basolateral inoculation cannot be explained entirely by differences in virus concentration at the cell surfaces. Thus, we conclude that the cellular uptake of HAV occurs asymmetrically in these polarized cells. Even taking into consideration differences in the virus concentration at the basolateral membrane, virus uptake via the apical membranes of Caco-2 cells is at least 30- to 40-fold (that is, 2.2 to 2.9% of 1,375-fold) more efficient than uptake via the basolateral membranes. These results clearly distinguish HAV from poliovirus, which was capable of bidirectional entry into Caco-2 cells in similar experiments (36).
Infection of Caco-2 cells with HM175/18f virus is noncytopathic and does not disturb the integrity of polarized monolayers.
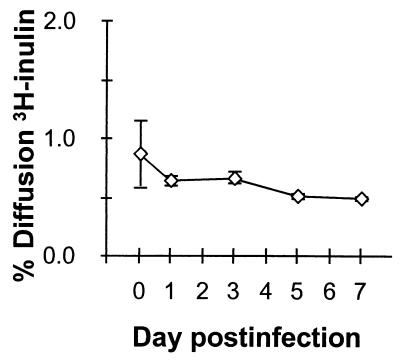
HM175/18f virus has a rapid replication/cytopathic (rr/cpe+) phenotype in BS-C-1 cells that leads to cellular degeneration and visible plaques when the virus is propagated in these cells under an agarose overlay (22). These phenotypic attributes distinguish this virus from its wild-type parent and are due to complex interactions between multiple mutations in the 5′ nontranslated and P2/P3 (nonstructural) regions of the genome which contribute to both enhanced viral translation and RNA replication (41). It was of interest, therefore, to see whether infection of polarized Caco-2 cells would affect the integrity of the monolayer. To address this, we analyzed duplicate monolayer cultures of Caco-2 cells for [3H]inulin permeability following apical infection with this virus under the conditions used for the experiment shown in Fig. 3. As shown in Fig. 4, HM175/18f virus infection did not result in an increase in permeability to [3H]inulin. Immediately following the adsorption period, the mean rate of [3H]-inulin diffusion from apical to basolateral compartments was 0.86%/h (compared with 61%/h in the absence of the cell monolayer). A rate of inulin diffusion of less than 1%/h across the monolayer is indicative of the presence of intact junctional complexes (36). Inulin permeability continued to demonstrate a slow downward trend over the ensuing 7 days of the infection (mean diffusion was 0.49%/h on day 7) (Fig. 4), indicating a strengthening of junctional complexes during the course of the experiment. These results were consistent with transepithelial resistance measurements which were not reduced following infection of Caco-2 monolayers over a similar period (data not shown).
FIG. 4.
Permeability of Caco-2 cells to [3H]inulin following infection with HAV. Duplicate monolayer cultures of polarized Caco-2 cells were infected by apical inoculation of virus under the conditions employed for the experiment shown in Fig. 2. At the intervals noted following infection, the apical-to-basolateral diffusion of [3H]inulin was measured over a 90-min period. The results shown represent the mean hourly rate (± range) of [3H]inulin diffusion. A rate of inulin diffusion of less than 1%/h across the monolayer indicates the presence of intact junctional complexes. Diffusion across the porous support in the absence of cells approximated 61%/h.
The integrity of infected monolayers was also confirmed by transmission electron microscopy. Examination of thin sections of Caco-2 cells which were fixed 72 h after apical inoculation with the virus revealed an intact cellular architecture (data not shown). Tight junctions were well defined, consistent with retention of monolayer integrity. Mitochondria and membranes of the endoplasmic reticulum appeared normal, and virus particles were not identifiable. This is not surprising, given the relatively low yield of HAV in these cells. These studies revealed no evidence of a cytolytic infection.
Release of progeny virions from infected Caco-2 cells occurs in a vectorial fashion through the apical membrane.
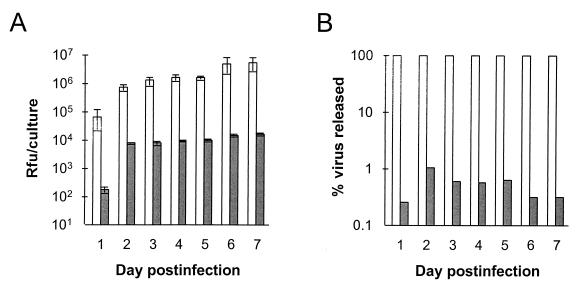
Newly replicated HAV particles were released from apically infected polarized Caco-2 cells almost exclusively through the apical surface (Fig. 5). The amount of virus released into the apical supernatant fluids increased exponentially between 24 and 48 h postinfection and continued to rise slowly, reaching a plateau at approximately 5 × 106 RFU/culture/day by days 6 and 7 (Fig. 5A). In contrast, the maximal amount of virus released into basolateral culture fluids was 1.7 × 104 RFU/culture/day. The proportion of all released virus which was directed into the apical supernatant fluids was remarkably constant throughout the 7-day period of observation, ranging from 98.9% to 99.7% (Fig. 5B), with only 0.26 to 1.1% released into the basolateral supernatant fluids. The release of virus from basolaterally infected cells was similarly restricted to the apical surface (data not shown).
FIG. 5.
Vectorial release of HAV from apically infected polarized Caco-2 cell monolayers. Cells were inoculated as in Fig. 2 and washed prior to refeeding following the 2-h adsorption period. Apical and basolateral supernatant fluids were collected at 24-h intervals for virus titration and replaced with fresh media. (A) Virus content of apical (open bars) and basolateral (shaded bars) supernatant fluids. The values shown represent the mean virus titer of fluids from three replicate infected cultures (± standard deviation). (B) Proportion of all released virus (apical plus basolateral supernatant fluid virus) released into apical (open bars) or basolateral (shaded bars) fluids. The results shown represent the means of three replicate cultures at each time point, as in panel A.
The absence of any decline in the quantity of virus released into the apical supernatant fluids over the 7-day observation period is consistent with the establishment of a persistent infection. This is typical of HAV infection in cultured cells (17, 22). Between 24 and 72 h postinfection, the mean number of infectious virus particles released into the apical supernatant fluids was 2.0 × 106 RFU per culture insert (combined virus contents of the 48- and 72-h supernatant fluids). Because each insert contained 3.5 × 105 cells at the start of the infection, this represents the release of an average of only 6 infectious virus particles per Caco-2 cell during this 48-h period. Since the total cell-associated virus increased from a mean of 2.9 × 104 to 2.9 × 106 RFU/culture, or by an average of approximately 8 RFU per cell, between 24 and 72 h (Fig. 3), it can be deduced that about 40% of newly replicated infectious virus particles were released into the apical supernatant fluids. These are minimal estimates, because they do not take into account the steady loss of viability of virus particles secreted into the medium. Even so, since the experiment shown in Fig. 2 documented that 50 to 80% of Caco-2 cells contain detectable HAV antigen by 4 to 8 days after infection, these data indicate that Caco-2 cells support only a low level of productive infection.
Absence of transcytosis of HAV by polarized Caco-2 cells.
The small proportion of newly replicated virus found in the basolateral supernatant fluids of apically infected Caco-2 cultures (Fig. 5B) could reflect transcytosis of virus present within the apical medium rather than basolateral release of progeny intracellular virions. To address this question, we determined the efficiency of apical-to-basolateral and basolateral-to-apical transcytosis of virus. Polarized cultures of Caco-2 cells were inoculated with HAV on either apical or basolateral surfaces, as described above, and the virus titers in the contralateral supernatant fluid were determined after a 2-h incubation period (Table 2). Less than 0.002% of an apical inoculum was transported (or diffused) into the basolateral fluid, while less than 0.0004% of a basolateral inoculum was transported into the contralateral apical supernatant fluid. The proportion of an apical inoculum transported into basolateral fluids following a 24-h incubation period (without removal of the apical inoculum) was not significantly increased (Table 2). These data do not support the presence of significant transcytosis of HAV by polarized Caco-2 cell cultures and suggest that the small proportion of virus present in basolateral fluids of apically infected cells (0.3 to 1.1% of total released virus [Fig. 5B]) reflects low-level release of intracellularly replicated virus or possibly the presence of a small number of incompletely polarized cells in the culture.
TABLE 2.
Absence of transcytosis of HAV in Caco-2 cells
| Inoculum (2.1 × 106 RFU) | Elapsed time (h)a | Amt of contralateral supernatant virus (RFU) | % Transcytosisb |
|---|---|---|---|
| Apical | 2 | 36 | 0.0017 |
| <36 | <0.0017 | ||
| <36 | <0.0017 | ||
| 24 | 72 | 0.0034 | |
| <36 | <0.0017 | ||
| Basolateral | 2 | <8 | <0.0004 |
| <8 | <0.0004 | ||
| <8 | <0.0004 |
Replicate polarized cultures of Caco-2 cells were inoculated with virus on the indicated surface; after the time indicated, the virus content of the contralateral supernatant fluids was measured.
Transcytosis is reported as the percentage of the original inoculum present within the contralateral medium at 2 or 24 h.
Brefeldin A inhibits replication and vectorial release of HAV from Caco-2 cells.
The nearly exclusive apical release of HAV from infected cells in the absence of significant cellular cytopathology suggests a possible role for normal protein sorting and vesicular transport mechanisms in the movement of progeny virions out of infected Caco-2 cells. To evaluate this possibility, we determined the effects of brefeldin A on the replication and release of HAV from polarized Caco-2 cells. Brefeldin A is a fungal metabolite which disrupts the Golgi complex in many cell types, with resulting inhibition of normal cellular sorting and transport functions (15, 27). It also has been shown to inhibit the replication of some picornaviruses by interfering with the assembly and/or function of cytoplasmic membranous complexes required for viral RNA replication (12, 26, 37). Its effects on replication of members of this genus of the Picornaviridae have not been studied previously.
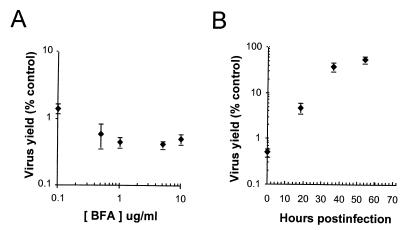
Polarized cultures of Caco-2 cells were inoculated with HAV on the apical surface and fed with medium containing brefeldin A beginning immediately after the 2-h viral adsorption period. Even the lowest concentration of brefeldin A tested (0.1 μg/ml) resulted in a profound reduction in the subsequent total virus yield (cell-associated HAV plus virus present in apical and basolateral supernatant fluids) at 72 h postinfection (1.4% of control virus yield) (Fig. 6A). Somewhat greater inhibition of HAV replication was noted at higher concentrations of the drug (0.41 to 0.59% control yields at concentrations of ≥0.5 μg/ml). When brefeldin A (10 μg/ml) was added at 18 h postinfection, replication was still substantially inhibited (4.7% of control yield), whereas the addition of brefeldin A to medium at 36 h postinfection had a significantly reduced effect (39% of control yield) (Fig. 6B). These data indicate that brefeldin A blocks the logarithmic increases in virus titer that normally occur between 24 and 48 h postinfection in Caco-2 cells (see Fig. 1 and 5A), consistent with an inhibition of viral RNA synthesis, as noted for poliovirus (26). Brefeldin A inhibition of HAV replication in polarized Caco-2 cells coincided with morphological changes involving rounding of the cells, as well as a dramatic decrease in the transepithelial resistance. Resistance decreased within 18 h of treatment and ranged from 43 to 56 Ω-cm2 for infected cell sheets after 72 h of brefeldin A treatment at all concentrations of the drug. Resistance averaged 182 Ω-cm2 for control infected cells.
FIG. 6.
Brefeldin A (BFA) inhibits HAV replication in Caco-2 cells. (A) Replicate cultures of apically inoculated Caco-2 cells were fed with medium containing various concentrations of brefeldin A beginning immediately after the 2-h period of viral adsorption. Brefeldin A was replenished every 6 h, and the medium was replaced daily. The results shown represent the mean total virus yield (cell-associated plus apical and basolateral fluid virus [± range]) at 72 h postinfection as a percentage of the yield from control cells infected in the absence of brefeldin A. (B) Effect of late addition of brefeldin A to apically inoculated Caco-2 cells. Cells were infected as in panel A with brefeldin A (10 μg/ml) added at various times postinfection. As in panel A, the results shown represent the mean total virus yield (± range) at 72 h postinfection as a percentage of virus yield from cells infected in the absence of brefeldin A.
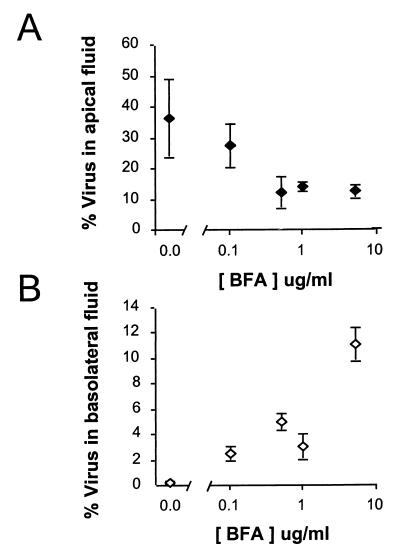
In addition to inhibiting the replication of virus, concentrations of brefeldin A of ≥0.5 μg/ml also resulted in a reduction in the proportion of newly replicated HAV released into apical supernatant fluids (Fig. 7A). While about 36% of the virus yield was released into the apical fluids in the absence of brefeldin A in the experiment shown in Fig. 7, this fell to about 27% at 0.5 μg of brefeldin A per ml and to only 12% at higher concentrations of the drug (5 μg/ml). The proportion of virus released into apical (or basolateral) fluids at the maximal concentration of brefeldin A (10 μg/ml) could not be measured, due to a combination of the low virus yield and the residual antiviral activities of brefeldin A in the supernatant fluid. The decrease in the proportion of the virus yield that was found in the apical supernatant fluids suggests that release of virus may involve vesicular transport pathways that are disrupted by brefeldin A (see Discussion). The effect of brefeldin A (10 μg/ml) on the proportion of virus undergoing apical transport was reduced when the drug was added at 18 h or later postinfection (19 to 28% of virus transported into apical fluids, compared with 48% apical release from control cells) (data not shown). This correlated with somewhat higher transepithelial resistance readings in these cells (60 to 83 Ω-cm2), but suggests that brefeldin A may have a lesser effect on the transport of virus which has already been replicated prior to perturbation of vesicular transport by the drug.
FIG. 7.
Brefeldin A (BFA) inhibits apical release of progeny HAV. Cells were treated with various concentrations of brefeldin A beginning immediately after removal of an apical HAV inoculum, as in Fig. 6A. The proportion of the total virus yield (cell-associated plus apical and basolateral fluid virus) that was present in the apical (A) or basolateral (B) supernatant fluids 72 h postinfection was calculated for each culture. The results shown represent the mean (± range) results for replicate cultures treated with the drug and the mean (± standard deviation) of five cultures maintained in the absence of brefeldin A. Samples of apical and basolateral fluids from cells treated with 10 μg of brefeldin A per ml could not be assayed for HAV due to residual antiviral activity.
In contrast to the inhibition of apical release of HAV observed with higher concentrations of brefeldin A, the release of virus into basolateral fluids was modestly increased. The fraction of the virus yield present in the basolateral supernatant at 72 h was only 0.23% in the absence of the drug, but this rose to as high as 11% at 5 μg/ml (Fig. 7B). At this concentration of the drug, approximately equal proportions of the virus yields were present in the apical and basolateral fluids. While it is possible that this reflects disordered sorting of virus, it may also be due to the impairment of monolayer integrity and increased paracellular leaks suggested by the reductions in transepithelial resistance (see above). Nonetheless, in Caco-2 cells treated with brefeldin A concentrations up to 5 μg/ml for 72 h beginning immediately after HAV inoculation, 77% of the total virus yield remained cell associated, compared with 63% in the absence of drug. This indicates that the cytotoxic effects of brefeldin A were limited in terms of cellular lysis or physical disruption of the monolayer.
Effect of monensin on apical release of virus.
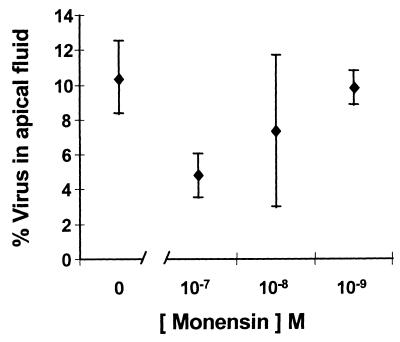
Monensin is a carboxylic ionophore which arrests vesicular transport at a site located distal to the proximal portion of the Golgi complex (5, 35). To determine whether it is capable of causing a dose-related reduction in apical transport of HAV, we infected polarized Caco-2 cells by the apical route. After 72 h, the cells were washed and refed with medium containing monensin (10−7 to 10−9 M). The quantity of virus released into the apical supernatant fluids was then assessed over the ensuing 6-h period. Even when added to cultures this late in the infection cycle, the highest concentration of monensin tested (10−7 M) resulted in an ∼50% decrease in virus released into the apical fluids (Fig. 8). These results are consistent with the involvement of vesicular secretory pathways in the apical release of virus from polarized Caco-2 cells. Under similar experimental conditions, there was no inhibition of the apical release of virus by brefeldin A (0.5 μg/ml).
FIG. 8.
Monensin inhibits apical release of HAV from polarized Caco-2 cells. Cells were treated with the indicated concentration of monensin beginning 72 h after apical infection with HAV. The quantity of virus released into the apical supernatant fluids was then assessed after a 6-h incubation period and is shown as the percentage of virus present in the cell lysate (± range). The titer of virus present in the lysates of cells treated with the highest concentration of monensin (10−7 M) was 93% (range, 87 to 100%) of that present in untreated cells, indicating there was no antiviral effect over this short incubation period.
DISCUSSION
Although the intracellular sorting of viral proteins involved in the morphogenesis of enveloped viruses by polarized cells has received considerable attention, relatively little information is available concerning the interactions of nonenveloped viruses with polarized cells (3). Simian virus 40 (SV40) and poliovirus have been studied most extensively and have contrasting properties in terms of virus entry and release (4, 5, 36, 37). HAV differs from both of these viruses in that its replication is not associated with a demonstrable cytopathic effect in Caco-2 cells, potentially allowing a clearer view of the specific mechanisms controlling release of nonenveloped viruses from infected cells.
We found that HAV infection of Caco-2 cells occurred much more efficiently following inoculation of the apical compared to the basolateral surface of these cells (Fig. 3). In addition, approximately 1,000-fold more virus remained associated with these cells following a 2-h period of apical, versus basolateral, exposure to virus at an equivalent multiplicity (Table 1). Direct measurements of virus diffusion indicated that this difference could not be explained entirely by slow virus diffusion across the support membrane or the 4.5-fold-lower concentration of virus in the basolateral medium that resulted from efforts to keep the MOI constant in these experiments. Taking these variables into account, the uptake of virus was at least 30- to 40-fold more efficient via the apical surface.
The results obtained in these experiments thus suggest that the cellular receptor for HAV may be present in greater abundance on the apical compared to the basolateral surface of Caco-2 cells. However, this does not mean that the receptor is necessarily present at greater density on the apical surface, because the surface area of the apical membrane with its numerous microvilli is much greater than that of the basolateral surface. It is not possible to test this hypothesis directly, since the functional receptor expressed by Caco-2 cells may differ from the HAV receptor identified recently on human liver and kidney cells (8). Nonetheless, the asymmetric attachment and entry we observed with HAV resemble previous observations with SV40 virus (4). The cellular receptor for SV40 virus was found to be expressed only on the apical surfaces of polarized Vero C1008 or Madin-Darby canine kidney cell cultures (4). Significantly, our results distinguish HAV from both poliovirus and rotavirus, which are capable of efficiently initiating infection from either surface of polarized Caco-2 cell cultures (34, 36), and astrovirus, which may infect exclusively from the basolateral surface (40). A striking feature of our studies is the very low proportion of virus that was bound to or taken up by either side of the monolayer at the end of the adsorption period (Table 1).
Release of progeny HAV virions occurred almost exclusively via the apical cellular membrane (Fig. 5). The small proportion of released virus (generally less than 1%) that was present in basolateral culture fluids may reflect either limited basolateral release of virus from polarized cells or the existence of a small proportion of incompletely polarized cells in the cultures (4). This vectorial release of newly replicated HAV is strikingly similar to the apically directed vectorial release of both poliovirus and SV40 from polarized cells (33, 37). With both poliovirus and SV40, apical release of progeny virions appeared to occur prior to the lysis of cells that is induced by these cytopathic viruses (5, 37). However, infection with either of these viruses results in the eventual destruction of the cell monolayer. Thus, it is possible that the apical release of these viruses reflects early disruption of the apical plasma membrane due to virus-induced cytopathology. Since HAV infection of Caco-2 cells has no impact on monolayer integrity as assessed by transepithelial resistance, [3H]inulin permeability (Fig. 4), or transmission electron microscopy, the apical release of this noncytopathic virus provides strong evidence that specific mechanisms exist for vectorial release of nonenveloped viruses that are not dependent upon cellular lysis.
What might be the nature of such a specific virus release mechanism? The involvement of the normal protein sorting and vesicular transport apparatus of the cell is suggested by the inhibition of vectorial HAV release that we observed in cells treated with brefeldin A or monensin (Fig. 7 and 8). Brefeldin A, a fungal metabolite, is known to block the anterograde movement of proteins from the rough endoplasmic reticulum to the proximal Golgi complex (15, 27). This and possibly other actions of brefeldin A result in disruption of the Golgi complex and profound but reversible dysregulation of intracellular vesicular transport in many cell types (15, 23, 27). Brefeldin A also has been shown to have potent antiviral activity against some but not all picornaviruses due to inhibition of viral RNA replication (12, 26). We confirmed that this antiviral activity extends to HAV (Fig. 6), but we also demonstrated that brefeldin A induces substantial dose-related reductions in the transport of newly replicated virions across the apical plasma membrane of Caco-2 cells (Fig. 7A). We also found that monensin, a carboxylic ionophore which arrests vesicular transport at a site located distal to the proximal portion of the Golgi complex (5, 35), caused a moderate dose-related reduction in apical transport of HAV, even when added to cultures late in the infection cycle (Fig. 8). It is interesting that the apical release of poliovirus, which like HAV is a member of the Picornaviridae, was not selectively inhibited by either brefeldin A or monensin (37). This suggests a mechanism of vectorial release that is distinct from that of HAV and not dependent on conventional vesicular transport. Perhaps this is not too surprising, because expression of the poliovirus 2B and 3A proteins during virus infection results in profound disruption of vesicular transport (7), a phenomenon that is not likely to accompany the noncytopathic replication of HAV. Our results also contrast with the lack of inhibition of the apical release of rotavirus by monensin, which appears to exit Caco-2 cells by a noncanonical vesicular transport mechanism that bypasses the Golgi apparatus (13).
It is interesting to consider how these observations of apical entry and release of HAV from Caco-2 cells relate to the pathogenesis of hepatitis A in humans. Caco-2 cells most closely resemble epithelial cells of the small intestinal villi and crypts (9, 29) and are thus closely related to the cells within which HAV replication has been identified in infected owl monkeys (2). The ability of Caco-2 cells to be infected following apical exposure to virus is thus consistent with the observation that intestinal epithelial cells are infected by virus present within the lumen of the gastrointestinal tract (2, 14). The vectorial apical release of newly replicated virus from these cells would result in an increase in the amount of virus present within the lumen of the gastrointestinal tract and an amplification of the inoculum. Thus, it may be necessary to reconsider the generally held view that most if not all of the virus which is shed in feces during hepatitis A is derived from the liver rather than replicated within the intestinal epithelium (17). However, the restricted basolateral release of HAV also suggests that epithelial cell infection is unlikely to result in penetration of the virus beyond the gastrointestinal epithelium. Thus, HAV invasion of deeper tissues, a requirement for its eventual passage to the liver, may be dependent upon alternate mechanisms. One possibility is transcytosis by specialized M cells overlying Peyer's patches in the distal ileum, as appears to be the case for both poliovirus and reovirus (1, 33). Proximal epithelial cell infection could, however, play an important role in amplifying the inoculum ultimately reaching these M cells.
The primary target cell for HAV, the hepatocyte, is also an epithelial cell with defined polarity. Its basolateral membrane faces the venous sinusoids, and its apical surface forms the interface with the biliary canaliculi. The involvement of vesicular transport in the egress of HAV from hepatocytes is consistent with early electron microscopic studies of liver sections from patients and nonhuman primates infected with HAV, in which the virus in hepatocytes was found to be present within cytoplasmic vesicles possibly derived from the rough endoplasmic reticulum (10, 32). However, vesicular transport in hepatocytes differs from that of other polarized cells (including Caco-2 cells) in that hepatocytes are deficient in direct vesicular transport of proteins to the apical plasma membrane (6, 11). Instead, proteins generally reach the apical plasma membrane of hepatocytes via an indirect route, with sorting first to the basolateral surface followed by transport to the apical membrane. For example, dipeptidyl peptidase IV, a type II membrane glycoprotein expressed on the apical surface of various epithelial cells, is known to undergo direct intracellular transport to the apical membrane in some cell types, but indirect transport with transcytosis from the basolateral to the apical membrane in hepatocytes (24). Thus, it is likely that apical secretion of HAV from hepatocytes occurs by a process that is different from apical secretion in infected Caco-2 cells. Nonetheless, some canalicular (apical) glycophosphatidylinositol-linked proteins may reach their final destination by a direct transport mechanism in hepatocytes (38). In addition, cellular transport proteins have been identified which function in the direct transport of biliary lipids and bile salts to the canalicular surface (6). It is possible that HAV in some way parasitizes these cellular pathways to achieve its release from hepatocytes into the bile.
ACKNOWLEDGMENTS
We thank David Rowlands, Kara Stanig, Masao Honda, and Stephen Knight for helpful discussions. We also thank Vicky Madden for outstanding assistance with electron microscopy and Paula Murphy, Geoff Abell, and Terry Chapa for excellent technical assistance.
This work was supported in part by a grant (RO1-AI32599) from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Amerongen H M, Wilson G A, Fields B N, Neutra M R. Proteolytic processing of reovirus is required for adherence to intestinal M cells. J Virol. 1994;68:8428–8432. doi: 10.1128/jvi.68.12.8428-8432.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asher L V S, Binn L N, Mensing T L, Marchwicki R H, Vassell R A, Young G D. Pathogenesis of hepatitis A in orally inoculated owl monkeys (Aotus trivergatus) J Med Virol. 1995;47:260–268. doi: 10.1002/jmv.1890470312. [DOI] [PubMed] [Google Scholar]
- 3.Blau D M, Compans R W. Polarization of viral entry and release in epithelial cells. Semin Virol. 1996;7:245–253. [Google Scholar]
- 4.Clayson E T, Compans R W. Entry of simian virus 40 is restricted to apical surfaces of polarized epithelial cells. Mol Cell Biol. 1988;8:3391–3396. doi: 10.1128/mcb.8.8.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clayson E T, Jones-Brando L V, Compans R W. Release of simian virus 40 virions from epithelial cells is polarized and occurs without cell lysis. J Virol. 1989;63:2278–2288. doi: 10.1128/jvi.63.5.2278-2288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford J M. Role of vesicle-mediated transport pathways in hepatocellular bile secretion. Semin Liver Dis. 1996;16:169–189. doi: 10.1055/s-2007-1007230. [DOI] [PubMed] [Google Scholar]
- 7.Doedens J R, Kirkegaard K. Inhibition of cellular protein secretion by poliovirus proteins 2B and 3A. EMBO J. 1995;14:894–907. doi: 10.1002/j.1460-2075.1995.tb07071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feigelstock D, Thompson P, Mattoo P, Zhang Y, Kaplan G G. The human homolog of HAVcr-1 codes for a hepatitis A virus cellular receptor. J Virol. 1998;72:6621–6628. doi: 10.1128/jvi.72.8.6621-6628.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hidalgo I J, Raub R J, Borchardt R T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–749. [PubMed] [Google Scholar]
- 10.Huang S-N, Lorenz D, Gerety R J. Electron and immunoelectron microscopic study on liver tissues of marmosets infected with hepatitis A virus. Lab Investig. 1979;41:63–71. [PubMed] [Google Scholar]
- 11.Hubbard A L, Barr V A, Scott L J. Hepatocyte surface polarity. In: Arias I M, Boyer J L, Fausto N, Jakoby W B, Schachter D A, Shafritz D A, editors. The liver: biology and pathobiology. New York, N.Y: Raven Press, Ltd.; 1994. pp. 189–213. [Google Scholar]
- 12.Irurzun A, Perez L, Carrasco L. Involvement of membrane traffic in the replication of poliovirus genomes: effects of brefeldin A. Virology. 1992;191:166–175. doi: 10.1016/0042-6822(92)90178-r. [DOI] [PubMed] [Google Scholar]
- 13.Jourdan N, Maurice M, Delautier D, Quero A M, Servin A L, Trugnan G. Rotavirus is released from the apical surface of cultured human intestinal cells through nonconventional vesicular transport that bypasses the Golgi apparatus. J Virol. 1997;71:8268–8278. doi: 10.1128/jvi.71.11.8268-8278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karayiannis P, Jowett T, Enticott M, Moore D, Pignatelli M, Brenes F, Scheuer P J, Thomas H C. Hepatitis A virus replication in tamarins and host immune response in relation to pathogenesis of liver cell damage. J Med Virol. 1986;18:261–276. doi: 10.1002/jmv.1890180308. [DOI] [PubMed] [Google Scholar]
- 15.Klausner R D, Donaldson J G, Lippincott-Schwartz J. Brefeldin A: insights into control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krawczynski K K, Bradley D W, Murphy B L, Ebert J W, Anderson T E, Doto I L, Nowoslawski A, Duermeyer W, Maynard J E. Pathogenetic aspects of hepatitis A virus infection in enterally inoculated marmosets. Am J Clin Pathol. 1981;76:698–706. doi: 10.1093/ajcp/76.5.698. [DOI] [PubMed] [Google Scholar]
- 17.Lemon S M. Type A viral hepatitis: new developments in an old disease. N Engl J Med. 1985;313:1059–1067. doi: 10.1056/NEJM198510243131706. [DOI] [PubMed] [Google Scholar]
- 18.Lemon S M, Robertson B H. Current perspectives in the virology and molecular biology of hepatitis A virus. Semin Virol. 1993;4:285–295. [Google Scholar]
- 19.Lemon S M, Shapiro C N. The value of immunization against hepatitis A. Infect Agents Dis. 1994;3:38–49. [PubMed] [Google Scholar]
- 20.Lemon S M, LeDuc J W, Binn L N, Escajadillo A, Ishak K G. Transmission of hepatitis A virus among recently captured Panamanian owl monkeys. J Med Virol. 1982;10:25–36. doi: 10.1002/jmv.1890100105. [DOI] [PubMed] [Google Scholar]
- 21.Lemon S M, Binn L N, Marchwicki R H. Radioimmunofocus assay for quantitation of hepatitis A virus in cell cultures. J Clin Microbiol. 1983;17:834–839. doi: 10.1128/jcm.17.5.834-839.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemon S M, Murphy P C, Shields P A, Ping L-H, Feinstone S M, Cromeans T, Jansen R W. Antigenic and genetic variation in cytopathic hepatitis A virus variants arising during persistent infection: evidence for genetic recombination. J Virol. 1991;65:2056–2065. doi: 10.1128/jvi.65.4.2056-2065.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu E S, Ou J, Lee A S. Brefeldin A as a regulator of grp78 gene expression in mammalian cells. J Biol Chem. 1992;267:7128–7133. [PubMed] [Google Scholar]
- 24.Low S, Wong S, Tang B L, Subramaniam V N, Hong W. Apical cell surface expression of rat dipeptidyl peptidase IV in transfected Madin-Darby canine kidney cells. J Biol Chem. 1991;266:13391–13396. [PubMed] [Google Scholar]
- 25.Mathiesen L R, Møller A M, Purcell R H, London W T, Feinstone S M. Hepatitis A virus in the liver and intestine of marmosets after oral inoculation. Infect Immun. 1980;28:45–48. doi: 10.1128/iai.28.1.45-48.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maynell L A, Kirkegaard K, Klymkowsky M W. Inhibition of poliovirus RNA synthesis by brefeldin A. J Virol. 1992;66:1985–1994. doi: 10.1128/jvi.66.4.1985-1994.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelham H R B. Multiple targets for brefeldin A. Cell. 1991;67:449–451. doi: 10.1016/0092-8674(91)90517-3. [DOI] [PubMed] [Google Scholar]
- 28.Pinto M, Robine-Leon S, Appay M D, Kedinger M, Triadou N, Dussauix E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colonic carcinoma cell line CaCo-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 29.Quaroni A. Crypt cell antigen expression in human colon tumor cell lines: analysis with a panel of monoclonal antibodies to CaCo-2 luminal membrane components. J Natl Cancer Inst. 1986;76:571–585. doi: 10.1093/jnci/76.4.571. [DOI] [PubMed] [Google Scholar]
- 30.Rothman J E, Wieland F T. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- 31.Schulman A N, Dienstag J L, Jackson D R, Hoofnagle J H, Gerety R J, Purcell R H, Barker L F. Hepatitis A antigen particles in liver, bile, and stool of chimpanzees. J Infect Dis. 1976;134:80–84. doi: 10.1093/infdis/134.1.80. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu Y K, Shikata T, Beninger P R, Sata M, Setoyama H, Abe H, Tanikawa K. Detection of hepatitis A antigen in human liver. Infect Immun. 1982;36:320–324. doi: 10.1128/iai.36.1.320-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sicinski P, Rowinski J, Warchol J B, Jarzcabek Z, Gut W, Szczygiel B, Bielecki K, Koch G. Poliovirus type 1 enters the human host through intestinal M cells. Gastroenterology. 1990;98:56–58. doi: 10.1016/0016-5085(90)91290-m. [DOI] [PubMed] [Google Scholar]
- 34.Svensson L, Finlay B B, Bass D, von Bonsdorff C-H, Greenberg H B. Symmetric infection of rotavirus on polarized human intestinal epithelial (Caco-2) cells. J Virol. 1991;65:4190–4197. doi: 10.1128/jvi.65.8.4190-4197.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tartakoff A M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983;32:1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- 36.Tucker S P, Thornton C L, Wimmer E, Compans R W. Bidirectional entry of poliovirus into polarized epithelial cells. J Virol. 1993;67:29–38. doi: 10.1128/jvi.67.1.29-38.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tucker S P, Thornton C L, Wimmer E, Compans R W. Vectorial release of poliovirus from polarized human intestinal epithelial cells. J Virol. 1993;67:4274–4282. doi: 10.1128/jvi.67.7.4274-4282.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wehner F, Kinne R KH, Petzinger E. Second International Ringberg Conference: Cell Biology and Molecular Basis of Liver Transport. Hepatology. 1996;24:259–267. doi: 10.1002/hep.510240141. [DOI] [PubMed] [Google Scholar]
- 39.Wheeler C M, Fields H A, Schable C A, Meinke W J, Maynard J E. Adsorption, purification, and growth characteristics of hepatitis A virus strain HAS-15 propagated in fetal rhesus monkey kidney cells. J Clin Microbiol. 1986;23:434–440. doi: 10.1128/jcm.23.3.434-440.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willcocks M M, Carter M J, Madeley C R. Astrovirus. Rev Med Virol. 1992;2:97–106. [Google Scholar]
- 41.Zhang H C, Chao S F, Ping L H, Grace K, Clarke B, Lemon S M. An infectious cDNA clone of a cytopathic hepatitis A virus: genomic regions associated with rapid replication and cytopathic effect. Virology. 1995;212:686–697. doi: 10.1006/viro.1995.1526. [DOI] [PubMed] [Google Scholar]