Abstract
Sarcopenia is a prevalent and clinically significant condition, particularly among older age groups and those with chronic disease. Patients with cancer frequently suffer from sarcopenia and progressive loss of muscle mass, strength, and function. The complex interplay between cancer and its treatment, including medical therapy, radiotherapy, and surgery, significantly contributes to the onset and worsening of sarcopenia. Cancer induces muscle wasting through inflammatory processes, metabolic alterations, and hormonal imbalance. Moreover, medical and radiation therapies exert direct toxic effects on muscles, contributing to the impairment of physical function. Loss of appetite, malnutrition, and physical inactivity further exacerbate muscle wasting in cancer patients. Imaging techniques are the cornerstones for sarcopenia diagnosis. Magnetic resonance imaging, computed tomography, and dual-energy X-ray absorptiometry provide valuable insights into muscle structure and quality. Although each modality has advantages and limitations, magnetic resonance imaging produces high-resolution images and provides dynamic information about muscle function. Despite these challenges, addressing sarcopenia is essential for optimizing treatment outcomes and improving survival rates in patients with cancer. This review explored the factors contributing to sarcopenia in oncologic patients, emphasizing the importance of early detection and comprehensive management strategies.
Keywords: Sarcopenia, cancer patients, muscle wasting, diagnostic techniques, review
Sarcopenia is a common and clinically significant condition, particularly in older patients. Its prevalence rates may differ depending on the diagnostic criteria and demographics of the study population, ranging from 0.2% to 86.5% (1). Sarcopenia is defined as a condition marked by “the progressive loss of muscle mass, strength, and function” and is frequently observed in oncologic patients (2). The presence of cancer, along with medical treatment, radiation therapy, and surgery, can significantly contribute to the onset and worsening of sarcopenia in these patients (3). Cancer can induce muscle wasting via various mechanisms. Tumors often trigger a cascade of inflammatory processes within the body, leading to the breakdown of muscle tissue (4). Additionally, the metabolic demands of proliferating cancer cells may divert essential nutrients away from muscles, accelerating their protein degradation. Moreover, hormonal imbalances associated with certain types of cancer can exacerbate muscle loss (5). Although crucial in treating cancer, radiation therapy can have direct toxic effects on muscles, inducing muscle atrophy and weakness, and ultimately impairing the patient’s physical function (6-8). Furthermore, treatment-related side effects, such as nausea, vomiting, and fatigue can lead to reduced food intake, decreased physical activity, and muscle wasting. Cancer-related surgical procedures may also contribute to the development of sarcopenia. Surgical trauma, coupled with postoperative immobility and reduced dietary intake, can lead to further muscle loss and functional decline (7). Overall, the combination of cancer, its treatments, and their impact on psychological and physical performance creates a perfect storm for the development and worsening of sarcopenia in oncologic patients. Addressing this condition is essential for optimizing treatment outcomes, maintaining the quality of life, and improving overall survival rates.
In this narrative review, we explored various factors contributing to sarcopenia in patients with cancer. We investigated the direct effects of cancer, including inflammatory cytokines and altered metabolism, as well as the impact of cancer treatment. Additionally, this study examined how factors, such as loss of appetite, hormonal changes, and physical inactivity may exacerbate muscle wasting in this population and the role of imaging techniques in evaluating sarcopenia.
Definition and Diagnostics of Sarcopenia
Sarcopenia is a complex condition of growing clinical significance, particularly among aging populations and those affected by chronic diseases such as cancer. The burgeoning interest in sarcopenia has led to a pivotal shift in its conceptualization. In 2018, the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) reconceptualized sarcopenia as a muscle disease, emphasizing muscle failure wherein low muscle strength supersedes low muscle mass as the primary determinant (8). In oncologic patients, sarcopenia presents unique challenges owing to the interplay of cancer itself and its treatments, which can exacerbate muscle wasting.
Imaging techniques for diagnosing sarcopenia are crucial for comprehensively assessing muscle structure and providing insights into both quantitative and qualitative aspects. Dual-energy X-ray absorptiometry (DEXA) is a commonly used method that utilizes X-ray beams to measure bone density and soft tissue composition, including muscle mass (9). The EWGSOP endorsed the utilization of DEXA, specifically for calculating the appendicular lean mass index (ALMI= ALM/height2) to delineate sarcopenia or low muscle mass, with defined cutoff values of <5.5 kg/m2 in women and ALMI <7.0 kg/m2 (10,11). While DEXA offers simultaneous assessment of body composition and bone health, limitations exist, such as the inability to measure intramuscular adipose tissue accurately, which influences muscle quality evaluation (11). Additionally, factors such as body thickness and hydration status can affect the results, potentially leading to overestimation of muscle mass, particularly in obese individuals (12).
Computed tomography (CT) is increasingly employed for sarcopenia screening, effectively assessing muscle mass and quality, and serving as the gold standard for body composition analysis, especially in nutritionally vulnerable patients, even if radiation exposure may represent a limitation (13). Nevertheless, the advantage for oncologic patients lies in the opportunity to analyze the muscle status in routine CT scans. CT allows precise quantitative tissue measurements, including intramuscular fat identification, although manual measurements can be time-consuming and prone to errors, thus requiring expertise for interpretation (10). A straightforward and rapid method for estimating whole-body skeletal muscle mass involves calculating the cross-sectional areas of muscles, such as the psoas or abdominal muscles, at the third (L3) or fourth (L4) lumbar vertebrae to reduce motion artifacts (14). CT measurements can be performed manually by outlining the regions of interest using standardized thresholds on non-contrast-enhanced images. These values can be normalized with respect to height to obtain the Skeletal Muscle Index (SMI). A recent systematic review conducted by Rossi et al. suggested that SMI cutoff values are generally <41 cm2/m2 for men or <38.5 cm2/m2 for women (15).
Magnetic resonance imaging (MRI) stands out because of its ability to produce high-resolution images, which enable a detailed evaluation of both muscle quantity and quality. Unlike other imaging modalities, MRI offers excellent soft tissue contrast, allowing for the precise delineation of muscle boundaries and differentiation between muscle and surrounding tissues, such as fat and connective tissue. This superior imaging capability enables clinicians to accurately measure muscle volume, cross-sectional area, and composition, including the intramuscular fat content (16). Moreover, MRI provides dynamic information on muscle function, such as muscle activation patterns and tissue perfusion, which can offer valuable insights into muscle health and performance. Additionally, advanced MRI techniques, such as diffusion-weighted imaging and magnetic resonance spectroscopy, allow for non-invasive assessment of muscle microstructure and metabolism, providing further depth to evaluate muscle quality (17). Furthermore, MRI is a radiation-free imaging modality, making it particularly suitable for longitudinal studies and repeated assessments, like oncological populations. Despite its advantages, the need for consensus regarding standardized methods, threshold values, and quantification techniques for diagnosing sarcopenia limits much of its utility for research purposes. Finally, recent guidelines from the European Geriatric Medicine Society propose a protocol for using ultrasound (US) to assess muscle mass, including parameters, such as muscle thickness, cross-sectional area, echo intensity, pennation angle, fascicle length, and elastography (18). However, despite its potential, the lack of normative data and standardized protocols for diagnosing sarcopenia using US has limited its clinical application. Furthermore, the absence of established cut-off points adds to these limitations (10).
Clinical and Non-imaging Assessment of Sarcopenia
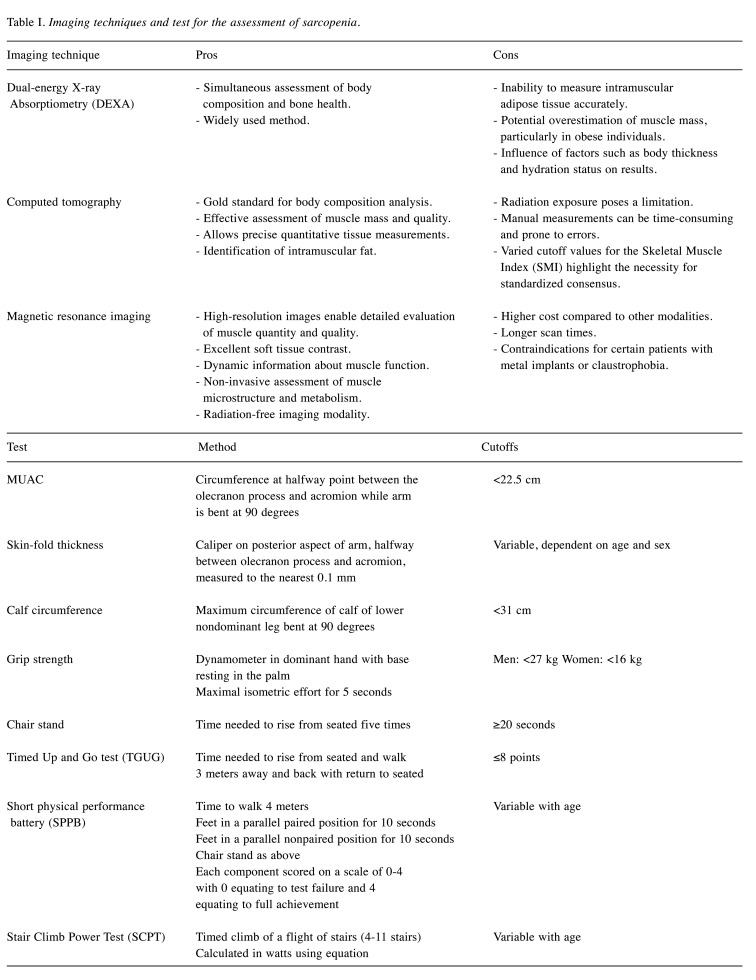
Various clinical and physical tests are available to assess sarcopenia. The gold standard for sarcopenia assessment involves a combination of methods including lean body mass (LBM) imaging, anthropometric measurements such as mid-upper arm circumference (MUAC), and muscle strength assessments. These comprehensive tests are the gold standards for sarcopenia assessment (19). Table I summarizes these assessments. These tests have gained increasing prominence according to the EWGSOP2, which considers low muscle strength as the primary indicator for diagnosing sarcopenia while also introducing muscle quality as a new diagnostic criterion (9,10).
Table I. Imaging techniques and test for the assessment of sarcopenia.
While the tools mentioned above were initially designed for screening individuals, they have been validated and applied in oncology populations (20,21). The SARC-F questionnaire was recently introduced to assess sarcopenia in older adults. It consists of five domains: strength, the need for walking assistance, rising from a chair, climbing stairs, and falling. Each question is scored from 0 to 2, with a maximum total score of 10. Higher scores indicate a higher likelihood of sarcopenia (21). In a cohort study conducted by Williams et al., which primarily involved patients with stage III/IV cancer, approximately 30% of older adults with cancer screened positive for sarcopenia based on the SARC-F questionnaire (21). In this context, bioelectrical impedance analysis (BIA) measurements offer a quick, noninvasive, and relatively inexpensive method for assessing body composition, including muscle mass, in both clinical and research settings (22,23). The BIA is based on measuring the resistance encountered by a low-level electrical current through the body. Because muscles contain more water and electrolytes and conduct electricity better than fat or bone, the measured impedance can be used to estimate various body composition parameters. A systematic review conducted by Aleixo et al. concluded that BIA is endorsed by Asian and European guidelines for objectively assessing body composition (24). However, its utility could be further improved by establishing an international consensus on cutoff points for BIA-assessed sarcopenia across various cancer populations.
Causes Participating in Sarcopenia Development and Progression
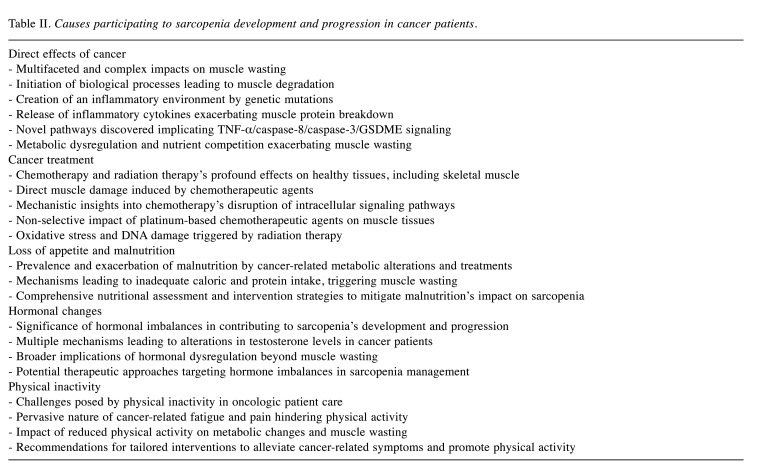
Table II summarizes the factors involved in the development and progression of sarcopenia in cancer patients. These include the direct effects of cancer and oncological treatments as well as anorexia, malnutrition, reduced physical activity, and metabolic/hormonal changes.
Table II. Causes participating to sarcopenia development and progression in cancer patients.
Direct Effects of Cancer
The direct effects of cancer on muscle wasting are complex and multifaceted. Cancer cells initiate a cascade of biological processes that contribute to muscle tissue degradation. Genetic mutations instigate the creation of an inflammatory environment wherein inflammation, particularly in the extrinsic pathway, promotes the onset, progression, and metastasis of cancer, with implications for sarcopenia (2,4). One significant mechanism involves the release of inflammatory cytokines by tumor cells and the tissue microenvironment. Cytokines, such as interleukin-6 (IL-6), interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-α), and transforming growth factor-β (TGF-β) promote a state of chronic inflammation that accelerates muscle protein breakdown and inhibits muscle protein synthesis (25). A recent animal study by Wu et al. uncovered a novel pathway in the development of sarcopenia, implicating TNF-α/ caspase-8/caspase-3/GSDME signaling-mediated pyroptosis, inducing cell death, and exacerbating tissue injury through inflammatory cascades (26,27). Consequently, pyroptosis triggered by TNF-α in the skeletal muscle culminates in the demise of muscle fibers and tissue impairment by releasing inflammatory mediators. Moreover, recent research has revealed that caspase-3-cleaved gastrin E (GSDME-N) can generate pores in the mitochondrial membrane, fostering the release of cytochrome c, which subsequently amplifies caspase-3 activation, thereby establishing a self-perpetuating feedback loop that intensifies cellular and tissue damage (28). Additionally, cancer-induced alterations in metabolism, including increased energy expenditure and changes in hormone levels further exacerbate muscle wasting (29). Furthermore, tumors can compete for nutrients with healthy tissues, diverting essential amino acids and other substrates from the muscle tissue to fuel their growth and proliferation (30). Metabolic dysregulation in cancer is related to the complex interplay between cancer cells and the host environment, and is modulated by pivotal oncogenes, tumor suppressors, and regulatory molecules, including non-coding RNAs. Metabolic alterations in cancer are highly adaptable, reflecting the dynamic changes influenced by the tumor type and the surrounding microenvironment. This complexity has shifted focus from traditional concepts like the Warburg Effect to a broader understanding of metabolic plasticity, encompassing phenomena such as the "reverse Warburg Effect" (31). This evolving perspective highlights the dynamic nature of cancer metabolism and its therapeutic implications. This metabolic hijacking contributes to the progressive loss of muscle mass observed in many cancer patients. Overall, the direct effects of cancer on muscle wasting underscore the importance of addressing this aspect of the disease in the management and treatment of patients with cancer. Metabolic dysfunction significantly contributes to the clinical decline seen in patients with advanced cancer, manifesting as weight loss, skeletal muscle wasting, and adipose tissue atrophy. Known as cancer-associated cachexia (CAC), this systemic syndrome is a pivotal factor in morbidity and mortality rates among cancer patients (32).
Cancer Treatment
Chemotherapy and radiation therapy exert profound effects on tumor cells and healthy tissues, including the skeletal muscle. Chemotherapeutic agents, known for their cytotoxic properties, can directly induce muscle damage, initiating a cascade of molecular events that culminate in muscle atrophy and weakness (33). In an observational cohort study by Best et al., 30% of patients diagnosed with metastatic colorectal cancer who underwent chemotherapy showed a reduction in skeletal muscle mass exceeding 5% within three months. This decline in muscle mass was independently associated with poorer overall survival, irrespective of the mutational status (34). Mechanistically, chemotherapy disrupts intracellular signaling pathways that are vital for muscle homeostasis. For example, tyrosine kinases and immune checkpoint inhibitors represent innovative anticancer therapies that target distinct pathways within cancer cells to impede their growth and survival. However, these treatments can adversely affect the mTOR pathway, which is crucial for the regulation of protein synthesis. Consequently, muscle protein breakdown is promoted, which hampers the natural processes of muscle regeneration (35). In contrast, platinum-based chemotherapeutic agents exhibit non-selective effects, affecting not only cancer cells but also healthy tissues, including muscles. For instance, cisplatin, a commonly used platinum agent, has been demonstrated to activate pathways, such as NF-ĸB, C/EBP-β, and FOXO1, resulting in the increased expression of myostatin (36,37). Furthermore, cisplatin treatment significantly reduced insulin-like growth factor 1 (IGF-1) protein levels by approximately 85% and suppressed the IGF-1/PI3K/Akt signaling pathways. The inclusion of multiple chemotherapeutic agents in treatment protocols frequently intensifies the negative impact on muscle tissues and heightens chemotherapy-induced muscle atrophy. Research indicates an increased breakdown of myofibrillar proteins, leading to muscle weakness and reduced physical performance, particularly in multidrug regimens (38).
Moreover, radiation therapy, while targeting malignant cells, unavoidably irradiates adjacent tissues, including skeletal muscle. This irradiation elicits oxidative stress and DNA damage within muscle fibers, triggering inflammatory responses and impairing muscle contractile function (39). Furthermore, radiation-induced fibrosis and microvascular damage exacerbate treatment-related fatigue and decreased physical activity (40). Patients with cancer undergoing these treatments often experience debilitating fatigue, limiting their capacity for physical exertion, leading to a vicious cycle of muscle disuse and deconditioning (41). Prolonged physical inactivity promotes muscle protein degradation pathways, exacerbating chemotherapy- and radiation-induced muscle wasting (42). Sarcopenia is an independent factor that negatively affects prognosis of patients with gastric carcinoma, advanced biliary cancer, and metastatic renal carcinoma in terms of post-operative complications, treatment failure, time-to-progression, and overall survival (43-45). In a series of 408 patients with gastric cancer treated with gastrectomy post-surgical, CT documented sarcopenia influenced negatively overall survival and was associated with non-tumor-related deaths (46). Interventions targeting muscle maintenance, such as exercise training, nutritional support, and pharmacological agents modulating muscle metabolism, hold promise for mitigating treatment-induced muscle toxicity and improving patient outcomes. Perioperative interventions may also improve outcomes for patients treated with gastrectomy for gastric cancer (47).
The development of muscle fibrosis further compromises muscle architecture and function (48). Several studies have consistently demonstrated a pronounced detrimental effect of sarcopenia on overall survival across various cancer types, such as head and neck cancers, but also in those with tumors affecting the gastrointestinal tract, cervix, and lung (49-54). The negative impact of chemotherapy and radiation therapy on skeletal muscles is exacerbated by treatment-related fatigue and decreased physical activity (40).
Loss of Appetite and Malnutrition
Loss of appetite and malnutrition are prevalent concerns in oncologic patients, stemming from both the disease itself and its treatment. Cancer often induces a cascade of metabolic alterations and systemic inflammation leading to decreased food intake and altered taste perception (55). Chemotherapy, radiation therapy, and surgery further exacerbate these issues by causing nausea, vomiting, and mucositis, which hinder the ability to consume adequate nutrition. In addition, cancer-related fatigue and pain can diminish a patient’s desire or ability to eat. Consequently, inadequate calorie and protein intake ensues, triggering muscle wasting through increased protein breakdown and decreased protein synthesis (56). Moreover, malnutrition compromises the body’s ability to heal and recover from the stress of cancer treatment, exacerbating muscle loss and functional decline (57). Therefore, comprehensive nutritional assessment and intervention strategies are imperative in the management of oncological patients to mitigate the impact of malnutrition on sarcopenia and overall treatment outcomes to reduce cancer mortality (58).
Hormonal Changes
Hormonal changes are a significant factor contributing to the development and progression of sarcopenia in oncologic patients (59). Various cancers and their treatments can disrupt the delicate balance of hormones in the body, with implications for testosterone, which is a hormone crucial for maintaining muscle mass. Testosterone plays a pivotal role in promoting muscle protein synthesis and inhibiting protein breakdown, thus ensuring the integrity and functionality of the skeletal muscle tissue (60). However, in the context of cancer, alterations in testosterone levels can occur via multiple mechanisms. For instance, particular malignancies, such as prostate and testicular cancers, directly affect the production of testosterone, leading to decreased circulating levels of this hormone (61).
Additionally, cancer therapies, including chemotherapy and hormonal treatments, may exacerbate hormonal imbalances by interfering with the normal function of the endocrine system. Chemotherapeutic drugs can induce gonadal dysfunction and disrupt hormone production pathways, resulting in reduced testosterone synthesis (62). Furthermore, treatments targeting hormone receptors, such as androgen deprivation therapy in prostate cancer, deliberately lower testosterone levels to inhibit tumor growth, thereby inadvertently predisposing patients to muscle loss and sarcopenia (63). The consequences of hormonal dysregulation extend beyond muscle wasting, encompassing broader implications for the patient’s overall health and quality of life. Reduced testosterone levels not only compromise muscle integrity, but also contribute to fatigue, decreased exercise tolerance, and impaired physical function, all of which are hallmark features of sarcopenia (64).
Moreover, hormonal changes may synergize with other factors associated with cancer cachexia, such as inflammation and metabolic alterations, to accelerate muscle protein degradation and exacerbate sarcopenia progression (65). Considering these considerations, addressing hormonal imbalances is pivotal for sarcopenia management in oncologic patients. Strategies aimed at restoring or optimizing testosterone levels, such as hormone replacement therapy or targeted interventions to mitigate treatment-induced hormonal disruptions, may hold promise for attenuating muscle loss and improving functional outcomes in this vulnerable population (66). Numerous clinical studies have suggested that selective estrogen receptor modulators (SERMs), selective androgen receptor modulators (SARMs), testosterone, estrogen, and progesterone may play a role in mitigate sarcopenia by reducing muscle loss (67-71). This evidence underscores the potential development of hormone-based therapeutic approaches that could offer substantial benefits to patients with sarcopenia. Nonetheless, the use of sex steroid supplementation for the treatment of sarcopenia remains controversial owing to insufficient evidence or concerns regarding their safety and efficacy (72).
Physical Inactivity
Physical inactivity represents a significant challenge in oncologic patient care, often arising from cancer-related symptoms, such as fatigue, pain, and treatment side effects. Cancer-related fatigue is a pervasive issue, affecting up to 90% of patients undergoing treatment, and persisting even after treatment completion (73). This fatigue, often described as debilitating and overwhelming, significantly impedes a patient’s ability to engage in physical activity, contributing to muscle deconditioning and exacerbating loss of muscle mass. Cancer-related pain, whether due to the disease itself or treatment, can severely limit mobility and physical function. Patients may avoid physical activity to minimize discomfort, leading to a vicious cycle of reduced muscle use and further muscle atrophy (74).
Other symptoms, such as nausea, dyspnea, and neuropathy, can also deter patients from participating in regular exercise, perpetuating the cycle of physical inactivity and muscle loss (75). Moreover, reduced physical activity can lead to metabolic changes, including insulin resistance and alterations in protein metabolism, which further contribute to muscle wasting (76). Prolonged immobility can result in decreased bone density, joint stiffness, and cardiovascular deconditioning, thereby increasing overall morbidity and mortality risk in oncologic patients (77). Healthcare providers should prioritize symptom management, provide tailored interventions to alleviate cancer-related fatigue and pain, and encourage physical activity. Singh et al. conducted a study examining data from 19 clinical trials, where they found that physical activity had a notable impact on reducing fatigue among colorectal cancer patients compared to standard cancer care regimens (78). A study conducted by Hojman et al. found that physical activity has various molecular effects. These effects include enhanced blood circulation, activation of the sympathetic nervous system, regulation of hormone levels, and mobilization of cytotoxic lymphocytes and natural killer (NK) cells, resulting in a potential antitumor effect through these mechanisms (79). Based on this evidence, the World Cancer Research Fund (WCRF) and the American Institute for Cancer Research (AICR) suggest engaging in a minimum of 150 min of moderate-intensity exercise weekly, along with strength training exercises performed at least twice weekly (80).
Conclusion
In conclusion, this review highlights the complexity of the direct effects of cancer on muscle wasting, which involves intricate molecular pathways and metabolic changes. Cancer cells stimulate the inflammatory environment by releasing cytokines, accelerating muscle protein breakdown, and inhibiting muscle protein synthesis. Cancer-induced metabolic dysregulation further exacerbates muscle wasting by altering energy expenditure and nutrient utilization. Moreover, chemotherapy and radiation therapy, which are essential for cancer treatment, can directly induce muscle damage and impair muscle function, thereby contributing to chemotherapy-induced muscle atrophy. These treatment modalities, along with cancer-related symptoms, such as fatigue and pain, often lead to physical inactivity, exacerbating muscle deconditioning and further muscle loss. Furthermore, hormonal imbalances resulting from cancer and its treatments, particularly alterations in testosterone levels, play a significant role in the development and progression of sarcopenia. Addressing these causative factors through targeted interventions, such as exercise training, nutritional support, and hormone replacement therapy may mitigate muscle loss and improve functional outcomes in oncologic patients. However, further research is warranted to better understand the underlying mechanisms of cancer-induced muscle wasting and develop more effective therapeutic strategies to counteract it.
Conflicts of Interest
The Authors have no conflicts of interest to declare in relation to this study.
Authors’ Contributions
GL, MRV, and EM wrote the draft of the review. VG, and GS reviewed all data and prepared the final format. All Authors revised the paper and approved it. GL and MRV equally contributed to this review.
Acknowledgements
The Authors are grateful to Dario Piazza DAP for editorial assistance.
Funding
Funding for editorial expenses was covered by the University of Palermo, Palermo, Italy.
References
- 1.Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, Celis-Morales C. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13(1):86–99. doi: 10.1002/jcsm.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams GR, Dunne RF, Giri S, Shachar SS, Caan BJ. Sarcopenia in the older adult with cancer. J Clin Oncol. 2021;39(19):2068–2078. doi: 10.1200/JCO.21.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anjanappa M, Corden M, Green A, Roberts D, Hoskin P, McWilliam A, Choudhury A. Sarcopenia in cancer: Risking more than muscle loss. Tech Innov Patient Support Radiat Oncol. 2020;16:50–57. doi: 10.1016/j.tipsro.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantovani A, Garlanda C, Allavena P. Molecular pathways and targets in cancer-related inflammation. Ann Med. 2010;42(3):161–170. doi: 10.3109/07853890903405753. [DOI] [PubMed] [Google Scholar]
- 5.Franzoi MA, Agostinetto E, Perachino M, Del Mastro L, de Azambuja E, Vaz-Luis I, Partridge AH, Lambertini M. Evidence-based approaches for the management of side-effects of adjuvant endocrine therapy in patients with breast cancer. Lancet Oncol. 2021;22(7):e303–e313. doi: 10.1016/S1470-2045(20)30666-5. [DOI] [PubMed] [Google Scholar]
- 6.Hariyanto TI, Kurniawan A. Appetite problem in cancer patients: Pathophysiology, diagnosis, and treatment. Cancer Treat Res Commun. 2021;27:100336. doi: 10.1016/j.ctarc.2021.100336. [DOI] [PubMed] [Google Scholar]
- 7.Viana ECRM, Oliveira IDS, Rechinelli AB, Marques IL, Souza VF, Spexoto MCB, Pereira TSS, Guandalini VR. Malnutrition and nutrition impact symptoms (NIS) in surgical patients with cancer. PLoS One. 2020;15(12):e0241305. doi: 10.1371/journal.pone.0241305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albano D, Messina C, Vitale J, Sconfienza LM. Imaging of sarcopenia: old evidence and new insights. Eur Radiol. 2020;30(4):2199–2208. doi: 10.1007/s00330-019-06573-2. [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) and the Extended Group for EWGSOP2 Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601. doi: 10.1093/ageing/afz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tagliafico AS, Bignotti B, Torri L, Rossi F. Sarcopenia: how to measure, when and why. Radiol Med. 2022;127(3):228–237. doi: 10.1007/s11547-022-01450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messina C, Maffi G, Vitale JA, Ulivieri FM, Guglielmi G, Sconfienza LM. Diagnostic imaging of osteoporosis and sarcopenia: a narrative review. Quant Imaging Med Surg. 2018;8(1):86–99. doi: 10.21037/qims.2018.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bredella MA, Ghomi RH, Thomas BJ, Torriani M, Brick DJ, Gerweck AV, Misra M, Klibanski A, Miller KK. Comparison of DXA and CT in the assessment of body composition in premenopausal women with obesity and anorexia nervosa. Obesity (Silver Spring) 2010;18(11):2227–2233. doi: 10.1038/oby.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie H, Gong Y, Kuang J, Yan L, Ruan G, Tang S, Gao F, Gan J. Computed tomography-determined sarcopenia is a useful imaging biomarker for predicting postoperative outcomes in elderly colorectal cancer patients. Cancer Res Treat. 2020;52(3):957–972. doi: 10.4143/crt.2019.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge M, Albu J, Heymsfield SB, Heshka S. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97(6):2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 15.Rossi F, Valdora F, Bignotti B, Torri L, Succio G, Tagliafico AS. Evaluation of body Computed Tomography-determined sarcopenia in breast cancer patients and clinical outcomes: A systematic review. Cancer Treat Res Commun. 2019;21:100154. doi: 10.1016/j.ctarc.2019.100154. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa M, Lester R, Akima H, Gorgey AS. Quantification of intermuscular and intramuscular adipose tissue using magnetic resonance imaging after neurodegenerative disorders. Neural Regen Res. 2017;12(12):2100–2105. doi: 10.4103/1673-5374.221170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelke K, Chaudry O, Gast L, Eldib MA, Wang L, Laredo JD, Schett G, Nagel AM. Magnetic resonance imaging techniques for the quantitative analysis of skeletal muscle: State of the art. J Orthop Translat. 2023;42:57–72. doi: 10.1016/j.jot.2023.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perkisas S, Baudry S, Bauer J, Beckwée D, De Cock AM, Hobbelen H, Jager-Wittenaar H, Kasiukiewicz A, Landi F, Marco E, Merello A, Piotrowicz K, Sanchez E, Sanchez-Rodriguez D, Scafoglieri A, Cruz-Jentoft A, Vandewoude M. Application of ultrasound for muscle assessment in sarcopenia: towards standardized measurements. Eur Geriatr Med. 2018;9(6):739–757. doi: 10.1007/s41999-018-0104-9. [DOI] [PubMed] [Google Scholar]
- 19.Guttikonda D, Smith AL. Sarcopenia assessment techniques. Clin Liver Dis (Hoboken) 2021;18(4):189–192. doi: 10.1002/cld.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ha YC, Won Won C, Kim M, Chun KJ, Yoo JI. SARC-F as a useful tool for screening sarcopenia in elderly patients with hip fractures. J Nutr Health Aging. 2020;24(1):78–82. doi: 10.1007/s12603-019-1307-6. [DOI] [PubMed] [Google Scholar]
- 21.Williams GR, Al-Obaidi M, Dai C, Bhatia S, Giri S. SARC-F for screening of sarcopenia among older adults with cancer. Cancer. 2021;127(9):1469–1475. doi: 10.1002/cncr.33395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishikawa H, Asai A, Fukunishi S, Takeuchi T, Goto M, Ogura T, Nakamura S, Kakimoto K, Miyazaki T, Nishiguchi S, Higuchi K. Screening tools for sarcopenia. In Vivo. 2021;35(6):3001–3009. doi: 10.21873/invivo.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branco MG, Mateus C, Capelas ML, Pimenta N, Santos T, Mäkitie A, Ganhão-Arranhado S, Trabulo C, Ravasco P. Bioelectrical impedance analysis (BIA) for the assessment of body composition in oncology: a scoping review. Nutrients. 2023;15(22):4792. doi: 10.3390/nu15224792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aleixo GFP, Shachar SS, Nyrop KA, Muss HB, Battaglini CL, Williams GR. Bioelectrical impedance analysis for the assessment of sarcopenia in patients with cancer: a systematic review. Oncologist. 2020;25(2):170–182. doi: 10.1634/theoncologist.2019-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019;18(3):121–126. doi: 10.4103/aam.aam_56_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Lin S, Chen W, Lian G, Wu W, Chen A, Sagor MIH, Luo L, Wang H, Xie L. TNF-α contributes to sarcopenia through caspase-8/caspase-3/GSDME-mediated pyroptosis. Cell Death Discov. 2023;9(1):76. doi: 10.1038/s41420-023-01365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou J, Zheng Y, Huang Y, Tang D, Kang R, Chen R. The versatile gasdermin family: their function and roles in diseases. Front Immunol. 2021;12:751533. doi: 10.3389/fimmu.2021.751533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers C, Erkes DA, Nardone A, Aplin AE, Fernandes-Alnemri T, Alnemri ES. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat Commun. 2019;10(1):1689. doi: 10.1038/s41467-019-09397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierucci F, Frati A, Battistini C, Penna F, Costelli P, Meacci E. Control of skeletal muscle atrophy associated to cancer or corticosteroids by ceramide kinase. Cancers (Basel) 2021;13(13):3285. doi: 10.3390/cancers13133285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavlova NN, Zhu J, Thompson CB. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022;34(3):355–377. doi: 10.1016/j.cmet.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiliro C, Firestein BL. Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells. 2021;10(5):1056. doi: 10.3390/cells10051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setiawan T, Sari IN, Wijaya YT, Julianto NM, Muhammad JA, Lee H, Chae JH, Kwon HY. Cancer cachexia: molecular mechanisms and treatment strategies. J Hematol Oncol. 2023;16(1):54. doi: 10.1186/s13045-023-01454-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brierley DI, Harman JR, Giallourou N, Leishman E, Roashan AE, Mellows BAD, Bradshaw HB, Swann JR, Patel K, Whalley BJ, Williams CM. Chemotherapy-induced cachexia dysregulates hypothalamic and systemic lipoamines and is attenuated by cannabigerol. J Cachexia Sarcopenia Muscle. 2019;10(4):844–859. doi: 10.1002/jcsm.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Best TD, Roeland EJ, Horick NK, Van Seventer EE, El-Jawahri A, Troschel AS, Johnson PC, Kanter KN, Fish MG, Marquardt JP, Bridge CP, Temel JS, Corcoran RB, Nipp RD, Fintelmann FJ. Muscle loss is associated with overall survival in patients with metastatic colorectal cancer independent of tumor mutational status and weight loss. Oncologist. 2021;26(6):e963–e970. doi: 10.1002/onco.13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colomba E, Alves Costa Silva C, Le Teuff G, Elmawieh J, Afonso D, Benchimol-Zouari A, Guida A, Derosa L, Flippot R, Raynard B, Escudier B, Bidault F, Albiges L. Weight and skeletal muscle loss with cabozantinib in metastatic renal cell carcinoma. J Cachexia Sarcopenia Muscle. 2022;13(5):2405–2416. doi: 10.1002/jcsm.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damrauer JS, Stadler ME, Acharyya S, Baldwin AS, Couch ME, Guttridge DC. Chemotherapy-induced muscle wasting: association with NF-ĸB and cancer cachexia. Eur J Transl Myol. 2018;28(2):7590. doi: 10.4081/ejtm.2018.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreira-Pais A, Ferreira R, Gil da Costa R. Platinum-induced muscle wasting in cancer chemotherapy: Mechanisms and potential targets for therapeutic intervention. Life Sci. 2018;208:1–9. doi: 10.1016/j.lfs.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 38.van der Meij BS, Deutz NEP, Rodriguez RER, Engelen MPKJ. Increased amino acid turnover and myofibrillar protein breakdown in advanced cancer are associated with muscle weakness and impaired physical function. Clin Nutr. 2019;38(5):2399–2407. doi: 10.1016/j.clnu.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 39.Ganju RG, Morse R, Hoover A, TenNapel M, Lominska CE. The impact of sarcopenia on tolerance of radiation and outcome in patients with head and neck cancer receiving chemoradiation. Radiother Oncol. 2019;137:117–124. doi: 10.1016/j.radonc.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Tsitkanou S, Murach KA, Washington TA, Greene NP. Exercise counteracts the deleterious effects of cancer cachexia. Cancers (Basel) 2022;14(10):2512. doi: 10.3390/cancers14102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mallard J, Hucteau E, Hureau TJ, Pagano AF. Skeletal muscle deconditioning in breast cancer patients undergoing chemotherapy: current knowledge and insights from other cancers. Front Cell Dev Biol. 2021;9:719643. doi: 10.3389/fcell.2021.719643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aversa Z, Costelli P, Muscaritoli M. Cancer-induced muscle wasting: latest findings in prevention and treatment. Ther Adv Med Oncol. 2017;9(5):369–382. doi: 10.1177/1758834017698643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuwada K, Kuroda S, Kikuchi S, Yoshida R, Nishizaki M, Kagawa S, Fujiwara T. Clinical impact of sarcopenia on gastric cancer. Anticancer Res. 2019;39(5):2241–2249. doi: 10.21873/anticanres.13340. [DOI] [PubMed] [Google Scholar]
- 44.Makino T, Izumi K, Iwamoto H, Kadomoto S, Kadono Y, Mizokami A. Sarcopenia is associated with aggressive clinicopathological outcomes and is a poor prognostic indicator for non-metastatic renal cell carcinoma. In Vivo. 2023;37(3):1304–1311. doi: 10.21873/invivo.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meguro K, Hosono K, Sato M, Sugimoto Y, Takai Y, Kurita Y, Kanoshima K, Shimizu T, Sakai E, Nakajima A. Prognostic impact of sarcopenia in patients with biliary tract cancer undergoing chemotherapy. In Vivo. 2021;35(5):2909–2915. doi: 10.21873/invivo.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komori K, Kano K, Aoyama T, Hara K, Nagasawa S, Nakazono M, Shimoda Y, Maezawa Y, Kumazu Y, Kawabe T, Numata M, Hayashi T, Yamada T, Tamagawa H, Sato T, Cho H, Yukawa N, Rino Y, Yoshikawa T, Ogata T, Oshima T. Clinical impact of surgical sarcopenia on long-term survival. Anticancer Res. 2022;42(9):4545–4552. doi: 10.21873/anticanres.15957. [DOI] [PubMed] [Google Scholar]
- 47.Aoyama T, Nakazono M, Nagasawa S, Segami K. Clinical impact of a perioperative exercise program for sarcopenia and overweight/obesity gastric cancer. In Vivo. 2021;35(2):707–712. doi: 10.21873/invivo.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collao N, D’Souza D, Messeiller L, Pilon E, Lloyd J, Larkin J, Ngu M, Cuillerier A, Green AE, Menzies KJ, Burelle Y, De Lisio M. Radiation induces long-term muscle fibrosis and promotes a fibrotic phenotype in fibro-adipogenic progenitors. J Cachexia Sarcopenia Muscle. 2023;14(5):2335–2349. doi: 10.1002/jcsm.13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Rijn-Dekker MI, van den Bosch L, van den Hoek JGM, Bijl HP, van Aken ESM, van der Hoorn A, Oosting SF, Halmos GB, Witjes MJH, van der Laan HP, Langendijk JA, Steenbakkers RJHM. Impact of sarcopenia on survival and late toxicity in head and neck cancer patients treated with radiotherapy. Radiother Oncol. 2020;147:103–110. doi: 10.1016/j.radonc.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 50.Mallet R, Decazes P, Modzelewski R, Lequesne J, Vera P, Dubray B, Thureau S. Prognostic value of low skeletal muscle mass in patient treated by exclusive curative radiochemotherapy for a NSCLC. Sci Rep. 2021;11(1):10628. doi: 10.1038/s41598-021-90187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Nardi P, Giani A, Maggi G, Braga M. Relation between skeletal muscle volume and prognosis in rectal cancer patients undergoing neoadjuvant therapy. World J Gastrointest Oncol. 2022;14(2):423–433. doi: 10.4251/wjgo.v14.i2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhong L, Liu J, Xia M, Zhang Y, Liu S, Tan G. Effect of sarcopenia on survival in patients after pancreatic surgery: a systematic review and meta-analysis. Front Nutr. 2024;10:1315097. doi: 10.3389/fnut.2023.1315097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiyotoki T, Nakamura K, Haraga J, Omichi C, Ida N, Saijo M, Nishida T, Kusumoto T, Masuyama H. Sarcopenia is an important prognostic factor in patients with cervical cancer undergoing concurrent chemoradiotherapy. Int J Gynecol Cancer. 2018;28(1):168–175. doi: 10.1097/IGC.0000000000001127. [DOI] [PubMed] [Google Scholar]
- 54.Katsui K, Ogata T, Sugiyama S, Yoshio K, Kuroda M, Hiraki T, Kiura K, Maeda Y, Toyooka S, Kanazawa S. Sarcopenia is associated with poor prognosis after chemoradiotherapy in patients with stage III non-small-cell lung cancer: a retrospective analysis. Sci Rep. 2021;11(1):11882. doi: 10.1038/s41598-021-91449-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peixoto da Silva S, Santos JMO, Costa E Silva MP, Gil da Costa RM, Medeiros R. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle. 2020;11(3):619–635. doi: 10.1002/jcsm.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Animaw L, Woldegiorgis Abate T, Endeshaw D, Tsegaye D. Fatigue and associated factors among adult cancer patients receiving cancer treatment at oncology unit in Amhara region, Ethiopia. PLoS One. 2023;18(1):e0279628. doi: 10.1371/journal.pone.0279628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clemente-Suárez VJ, Redondo-Flórez L, Rubio-Zarapuz A, Martínez-Guardado I, Navarro-Jiménez E, Tornero-Aguilera JF. Nutritional and exercise interventions in cancer-related cachexia: an extensive narrative review. Int J Environ Res Public Health. 2022;19(8):4604. doi: 10.3390/ijerph19084604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dolgoy ND, O’Krafka P, McNeely ML. Cancer-related fatigue in head and neck cancer survivors: Energy and functional impacts. Cancer Treat Res Commun. 2020;25:100244. doi: 10.1016/j.ctarc.2020.100244. [DOI] [PubMed] [Google Scholar]
- 59.Priego T, Martín AI, González-Hedström D, Granado M, López-Calderón A. Role of hormones in sarcopenia. Vitam Horm. 2021;115:535–570. doi: 10.1016/bs.vh.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 60.Morley JE. Hormones and sarcopenia. Curr Pharm Des. 2017;23(30):4484–4492. doi: 10.2174/1381612823666161123150032. [DOI] [PubMed] [Google Scholar]
- 61.Robertson HL, Michel C, Bartl L, Hamilton-Reeves JM. Sarcopenia in urologic oncology: Identification and strategies to improve patient outcomes. Urol Oncol. 2022;40(11):474–480. doi: 10.1016/j.urolonc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rivkees SA, Crawford JD. The relationship of gonadal activity and chemotherapy-induced gonadal damage. JAMA. 1988;259(14):2123–2125. [PubMed] [Google Scholar]
- 63.Couderc AL, Muracciole X, Nouguerede E, Rey D, Schneider S, Champsaur P, Lechevallier E, Lalys L, Villani P. HoSAGE: Sarcopenia in older patients before and after treatment with androgen deprivation therapy and radiotherapy for prostate cancer. J Nutr Health Aging. 2020;24(2):205–209. doi: 10.1007/s12603-019-1294-7. [DOI] [PubMed] [Google Scholar]
- 64.Shin MJ, Jeon YK, Kim IJ. Testosterone and sarcopenia. World J Mens Health. 2018;36(3):192–198. doi: 10.5534/wjmh.180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neshan M, Tsilimigras DI, Han X, Zhu H, Pawlik TM. Molecular mechanisms of cachexia: a review. Cells. 2024;13(3):252. doi: 10.3390/cells13030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dennison EM, Sayer AA, Cooper C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat Rev Rheumatol. 2017;13(6):340–347. doi: 10.1038/nrrheum.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Urano T, Shiraki M, Kuroda T, Tanaka S, Uenishi K, Inoue S. Preventive effects of raloxifene treatment on agerelated weight loss in postmenopausal women. J Bone Miner Metab. 2017;35(1):108–113. doi: 10.1007/s00774-015-0733-8. [DOI] [PubMed] [Google Scholar]
- 68.Fonsea GWPD, Dworatzek E, Ebner N, Von Haehling S. Selective androgen receptor modulators (SARMs) as pharmacological treatment for muscle wasting in ongoing clinical trials. Expert Opin Investig Drugs. 2020;29(8):881–891. doi: 10.1080/13543784.2020.1777275. [DOI] [PubMed] [Google Scholar]
- 69.Falqueto H, Júnior JLR, Silvério MNO, Farias JCH, Schoenfeld BJ, Manfredi LH. Can conditions of skeletal muscle loss be improved by combining exercise with anabolic–androgenic steroids? A systematic review and meta-analysis of testosterone-based interventions. Rev Endocr Metab Disord. 2021;22(2):161–178. doi: 10.1007/s11154-021-09634-4. [DOI] [PubMed] [Google Scholar]
- 70.Greising SM, Baltgalvis KA, Lowe DA, Warren GL. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64(10):1071–1081. doi: 10.1093/gerona/glp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. J Am Geriatr Soc. 2007;55(1):20–28. doi: 10.1111/j.1532-5415.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 72.Huang LT, Wang JH. The therapeutic intervention of sex steroid hormones for sarcopenia. Front Med (Lausanne) 2021;8:739251. doi: 10.3389/fmed.2021.739251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Campos MPO, Hassan BJ, Riechelmann R, Del Giglio A. Cancer-related fatigue: a practical review. Ann Oncol. 2011;22(6):1273–1279. doi: 10.1093/annonc/mdq458. [DOI] [PubMed] [Google Scholar]
- 74.Wiedmer P, Jung T, Castro JP, Pomatto LC, Sun PY, Davies KJ, Grune T. Sarcopenia – Molecular mechanisms and open questions. Ageing Res Rev. 2021;65:101200. doi: 10.1016/j.arr.2020.101200. [DOI] [PubMed] [Google Scholar]
- 75.Billot M, Calvani R, Urtamo A, Sánchez-Sánchez JL, Ciccolari-Micaldi C, Chang M, Roller-Wirnsberger R, Wirnsberger G, Sinclair A, Vaquero-Pinto N, Jyväkorpi S, Öhman H, Strandberg T, Schols JMGA, Schols AMWJ, Smeets N, Topinkova E, Michalkova H, Bonfigli AR, Lattanzio F, Rodríguez-Mañas L, Coelho-Júnior H, Broccatelli M, D’Elia ME, Biscotti D, Marzetti E, Freiberger E. Preserving mobility in older adults with physical frailty and sarcopenia: opportunities, challenges, and recommendations for physical activity interventions. Clin Interv Aging. 2020;15:1675–1690. doi: 10.2147/CIA.S253535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sarto F, Bottinelli R, Franchi MV, Porcelli S, Simunič B, Pišot R, Narici MV. Pathophysiological mechanisms of reduced physical activity: Insights from the human step reduction model and animal analogues. Acta Physiol (Oxf) 2023;238(3):e13986. doi: 10.1111/apha.13986. [DOI] [PubMed] [Google Scholar]
- 77.Cardoso R, Parola V, Neves H, Bernardes RA, Duque FM, Mendes CA, Pimentel M, Caetano P, Petronilho F, Albuquerque C, Sousa LB, Malça C, Durães R, Xavier W, Parreira P, Apóstolo J, Cruz A. Physical rehabilitation programs for bedridden patients with prolonged immobility: a scoping review. Int J Environ Res Public Health. 2022;19(11):6420. doi: 10.3390/ijerph19116420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh B, Hayes SC, Spence RR, Steele ML, Millet GY, Gergele L. Exercise and colorectal cancer: a systematic review and meta-analysis of exercise safety, feasibility and effectiveness. Int J Behav Nutr Phys Act. 2020;17(1):122. doi: 10.1186/s12966-020-01021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hojman P, Gehl J, Christensen JF, Pedersen BK. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 2018;27(1):10–21. doi: 10.1016/j.cmet.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 80.Clinton SK, Giovannucci EL, Hursting SD. The World Cancer Research Fund/American Institute for Cancer Research third expert report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr. 2020;150(4):663–671. doi: 10.1093/jn/nxz268. [DOI] [PMC free article] [PubMed] [Google Scholar]