Abstract
Background/Aim
The number of available treatment options for urothelial carcinoma has increased recently. Upper tract urothelial carcinoma (UTUC) is relatively rare compared with bladder cancer. There are few reports on the efficacy of immune checkpoint inhibitors (ICIs) for metastatic UTUC, and ICIs may occasionally show less efficacy and cause severe side effects. Therefore, it is important to predict the treatment response and change the treatment strategy as appropriate. We investigated the prognostic factors for treatment response in patients with metastatic UTUC treated with pembrolizumab at our hospital.
Patients and Methods
Patients who received pembrolizumab for UTUC between January 2018 and June 2023 were analyzed. Patients who presented with bladder cancer complications at initial diagnosis were excluded. The primary endpoints assessed were overall survival (OS) and progression-free survival (PFS). Statistical analyses were conducted using laboratory values obtained before and after pembrolizumab administration. The relationship between cancer and inflammation is important. Therefore, we analyzed this relationship using prognostic factors for urothelial carcinoma as previously reported. Specifically, pretreatment C-reactive protein (CRP) level, neutrophil-to-lymphocyte ratio (NLR), and NLR/albumin values were examined.
Results
Forty-seven patients were analyzed. The median PFS was 66 days (24-107 days), and the median OS was 164 days (13-314 days). A CRP level <1 before the first cycle was a useful factor in the multivariate analysis for both OS and PFS [OS: p=0.004, hazard ratio (HR)=3.244, 95% confidence interval (CI)=1.464-7.104; PFS: p=0.003, HR=2.998, 95%CI=1.444-6.225].
Conclusion
CRP level is a prognostic factor for pembrolizumab treatment response in patients with UTUC.
Keywords: Upper tract urothelial carcinoma, prognostic factor, C-reactive protein, pembrolizumab, urothelial carcinoma
The KEYNOTE-045 trial showed that patients with urothelial carcinoma refractory to platinum-based therapy benefit from pembrolizumab administration (1). Although this trial showed benefits for patients with urothelial carcinoma, there have been few studies that focused exclusively on upper tract urothelial carcinoma (UTUC). This may be due to the lower incidence and fewer cases of UTUC compared to bladder cancer (2). Some studies suggest that immune checkpoint inhibitors (ICIs) are less effective than bladder cancer for UTUC; however, this result is not yet definitive (3).
In addition, the recent EV301 trial showed that enfortumab vedotin can successfully treat pembrolizumab-resistant urothelial carcinoma (4). However, real-world data suggest that metastatic urothelial carcinoma has a poor prognosis and that rapid disease progression in some patients prevents effective treatment with enfortumab vedotin (5).
In light of these considerations, it is imperative to undertake early and judicious evaluations of the effectiveness of ICIs in the management of UTUC. However, there is a paucity of literature addressing the predictors of response and prognosis associated with the ICI pembrolizumab in the context of treating UTUC. Therefore, we investigated the prognostic factors in patients undergoing pembrolizumab treatment for metastatic UTUC at our hospital. There are reports that inflammation is closely related to the progression and prognosis of urothelial carcinoma. Markers of the inflammatory system have been widely reported as prognostic factors of ICI treatment (6,7). In this study, we analyzed prognostic factors of inflammatory system markers previously reported in urothelial carcinoma. Specifically, C-reactive protein (CRP), neutrophil-to-lymphocyte ratio (NLR), and NLR/albumin (Alb) values were examined.
Patients and Methods
Patients with unresectable/metastatic UTUC who were treated with pembrolizumab between January 2018 and June 2023 were included in the study. Patients with bladder cancer complications at the time of initial diagnosis were excluded. The primary endpoints were overall survival (OS) and progression-free survival (PFS). Blood samples taken prior to pembrolizumab administration were used for the study. We examined the pretreatment C-reactive protein (CRP), neutrophil-lymphocyte ratio (NLR), and NLR/Albumin (Alb) values, as these have been reported to be prognostic factors for urothelial carcinoma (8-11).
In addition, as a subanalysis, trends in blood analysis values were examined. Blood samples collected three weeks later (before the second cycle of treatment) were examined for CRP, NLR, and NLR/Alb factors. Pre-dose values were used as the baseline. If the dose values in the blood collected before the administration of the second treatment cycle were elevated, the patient was considered a non-responder; otherwise, the patient was considered a responder. PFS was calculated from the start date of pembrolizumab treatment to the date of progression, death, or last follow-up. OS was calculated from the start date of pembrolizumab treatment to the date of death or the last follow-up. Patients who were alive were censored on the date of last contact. PFS and OS were evaluated using the Kaplan-Meier method (log-rank test) and the Cox proportional hazards model. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. p-Values <0.05 were considered significant. Statistical analyses were performed using SPSS (Version 23.0., IBM Corp, Armonk, NY, USA). This study was approved by the Ethics Committee of Kanagawa Cancer Center (#46).
Results
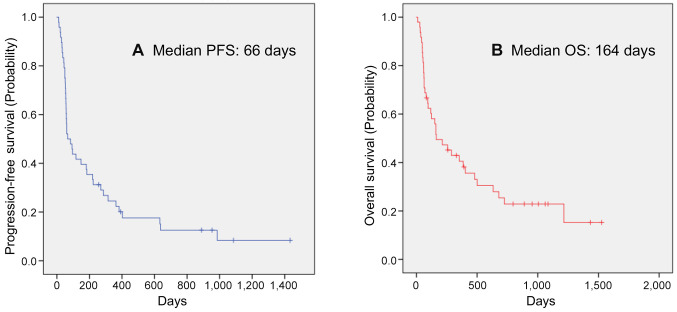
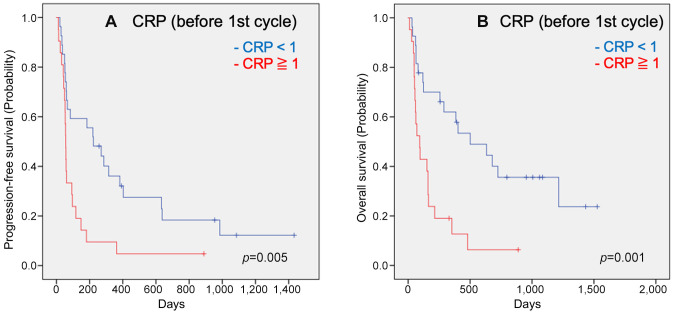
Forty-eight patients with unresectable/metastatic UTUC were treated with pembrolizumab. The patient characteristics are shown in Table I. The median patient age was 76 years (range=47-88 years). This study included 31 males and 17 females. Twenty patients underwent primary tumor resection. Twenty patients had ureteral cancer, and 28 had renal pelvic cancer. ECOG PS0 was assigned to 32 patients, PS1 in 13, and PS2 in two. Pembrolizumab was administered a median of five times (one-29 times). The Kaplan-Meier estimates of PFS and OS for all patients are shown in Figure 1. The median PFS was 66 days (24-107 days), and the median OS was 164 days (13-314 days). We performed a multivariate analysis of three factors (CRP, NLR, and NLR/Alb) that have been reported to be useful in the past. A CRP level <1 before the first cycle was a useful factor in the multivariate analysis for both OS and PFS (OS: p=0.004, HR=3.244, 95%CI=1.464-7.104; PFS: p=0.003, HR=2.998, 95%CI=1.444-6.225). Kaplan-Meier estimates of PFS and OS in patients with CRP<1 before the first cycle are shown in Figure 2.
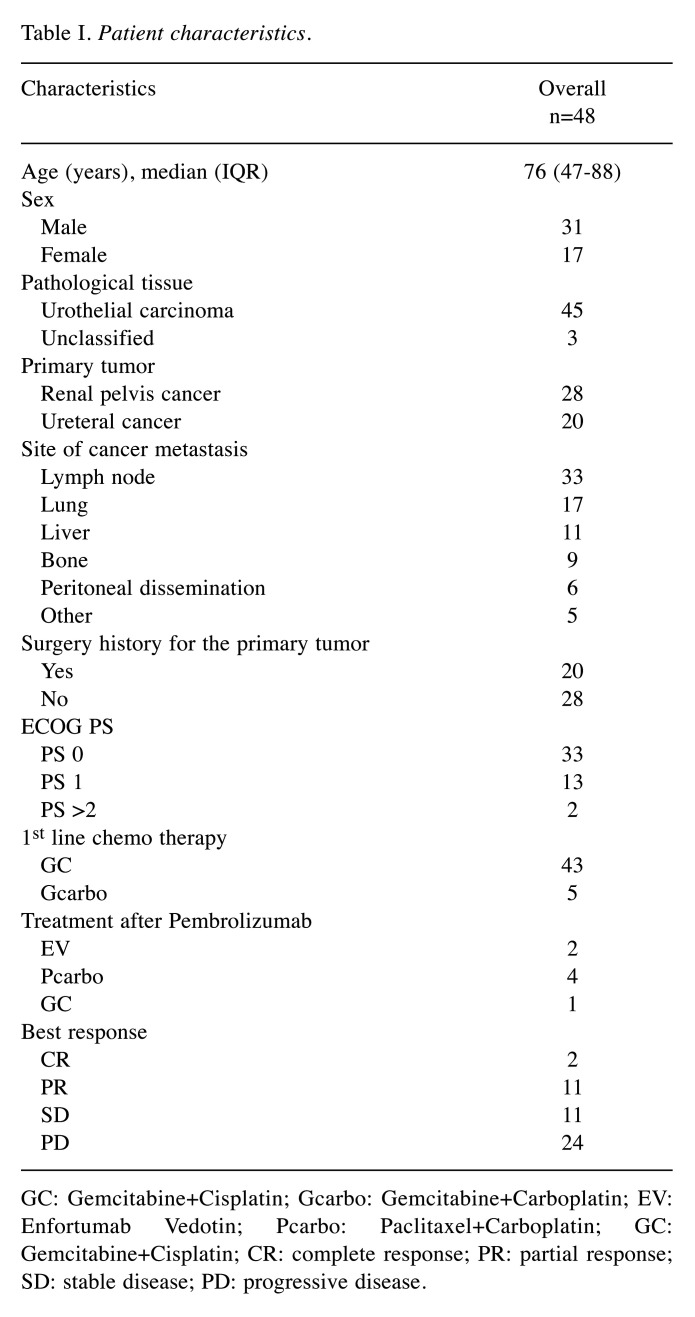
Table I. Patient characteristics.
GC: Gemcitabine+Cisplatin; Gcarbo: Gemcitabine+Carboplatin; EV: Enfortumab Vedotin; Pcarbo: Paclitaxel+Carboplatin; GC: Gemcitabine+Cisplatin; CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease.
Figure 1. Kaplan-Meier estimates of cumulative progression-free (A) and overall survival (B) in all patients.
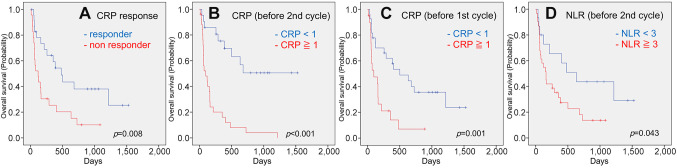
Figure 2. Kaplan-Meier estimates of cumulative overall survival in the groups stratified by CRP response (A), CRP value before the second cycle (B), CRP value before the first cycle (C), and neutrophil-to-lymphocyte ratio (NLR) before the second cycle (D). p-Values (log-rank test) are shown.
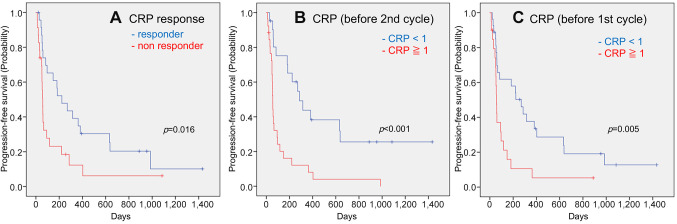
In the subanalysis we focused on trends in blood data values. CRP responders (p=0.016), CRP<1 before two cycles (p<0.001), and CRP<1 before the first cycle (p=0.005) were favorable prognostic factors for PFS in univariate analysis (Figure 3). In the multivariate analysis, CRP responders (p=0.044, HR=2.042, 95%CI=1.021-4.084) and CRP<1 before the second cycle (p=0.031, HR=2.362, 95%CI=1.084-5.145) showed statistically significant differences. In the univariate analysis of OS, CRP responders (p=0.008), CRP<1 before two cycles (p<0.001), CRP<1 before the first cycle (p=0.001), and NLR<3 (p=0.043) were found to be favorable prognostic factors (Figure 4) In the multivariate analysis, CRP level <1 before the second cycle (p=0.031, HR=2.362, 95%CI=1.084-5.145) was a more useful factor.
Figure 3. Kaplan-Meier estimates of cumulative overall survival in the groups stratified by CRP response (A), CRP value before the second cycle (B), CRP value before the first cycle (C), and neutrophil-to-lymphocyte ratio (NLR) before the second cycle (D). p-Values (log-rank test) are shown.
Figure 4. Kaplan-Meier estimates of cumulative progression-free survival in the groups stratified by CRP response (A), CRP value before the second cycle (B), CRP value before the first cycle (C). p-Values (log-rank test) are shown.
Discussion
CRP was statistically superior as a prognostic determinant in metastatic UTUC treated with pembrolizumab. In the past, NLR and NLR/Alb have also been reported as predictors of chemotherapy and postoperative prognosis in UTUC, but no significant differences were found in our study (8,9,12).
Elevated CRP levels may reflect a microenvironment that promotes tumor angiogenesis, proliferation, and metastatic seeding. The CRP level is a known prognostic factor for treatment of various cancer types with ICIs (13). Although there have been several reports, the relationship between CRP levels and ICIs remains uncertain. Primary and acquired resistance to anti-PD1/PDL1 therapy might occur because of insufficient antigen immunogenicity, dysfunction of antigen presentation, irreversible T cell exhaustion, resistance of IFN-γ signaling, and immunosuppression of the tumor microenvironment (14,15). CRP binds to T-cells and inhibits their function in a dose-dependent manner during the early phases of T-cell activation (16,17). CRP has also been reported to be involved in intrinsic immunity in patients with cancer by suppressing dendritic cells, thereby affecting the efficacy of ICIs (18).
In addition, CRP promotes the formation of the tumor microenvironment and contributes to cancer cell invasion (19,20). These findings suggest that high CRP levels may affect the immune response and attenuate the anti-tumor effect of pembrolizumab. The KEYNOTE-045 trial reported a median OS of 10.3 months and median PFS of 2.1 months for pembrolizumab treatment in locally advanced or metastatic urothelial cancer after platinum-based chemotherapy. The incidence of UTUC is 1-2 per 100,000 persons/year, and it accounts for 5-10% of all urothelial carcinomas (2). Although there are few real-world clinical data specific to UTUC owing to the small number of cases, there are reports that the response to ICIs is inferior to that of bladder cancer (3). The median OS and PFS values in our study were also worse than those in the KEYNOTE-045 trial, with a median OS of 5.4 months and PFS of 2.2 months. This may be because UTUC has different oncological characteristics from bladder cancer. UTUC is known to have a higher frequency of FGFR3 mutations and a lower frequency of TP53 mutations than bladder cancer (21). Therefore, it is possible that UTUC may acquire resistance to treatment and have different pharmacokinetics than bladder cancer, even though they are the same type of urothelial carcinoma. Currently, third-line therapies, such as enfortumab vedotin, have emerged and are expected to further extend prognosis. As mentioned above, metastatic UTUC is a cancer with a poor prognosis; therefore, it is important to determine the efficacy of treatment as early as possible and to shift to sequential treatment as soon as possible if the disease does not respond to treatment. CRP levels are relatively noninvasive and easy-to-measure, and may be useful prognostic factor.
In the present study, CRP was found to be useful; however, circulating tumor DNA (ctDNA) may also be a useful prognostic factor. Recent studies have focused on ctDNA as a prognostic factor for urothelial carcinoma (22,23). Moreover, ctDNA has been reported as a prognostic factor in patients with metastatic urothelial carcinoma treated with durvalumab (programmed death-ligand 1 inhibitor) and may be useful in patients with UTUC treated with pembrolizumab (24,25). However, we could not measure this value owing to facility limitations; therefore, further investigation is warranted.
Study limitations. First, this was a single-center, retrospective study. The number of patients was small, and various biases may have contributed to treatment selection. Second, there was variability in the sequential and pre-pembrolizumab treatments. For example, there were periods during the observation period when enfortumab vedotin was available and when it was not.
Conclusion
CRP level <1 before the first treatment cycle was a significant factor for survival in patients with metastatic UTUC receiving pembrolizumab. In addition, CRP trends were a potentially useful factor. CRP contributes to the immune response. High CRP levels in metastatic UTUC may affect the immune response and attenuate the antitumor effects of pembrolizumab.
Funding
The Authors received no funds for this study.
Conflicts of Interest
The Authors have no conflicts of interest to declare in relation to this study.
Authors’ Contributions
Hirotaka Nagasaka: Data collection and manuscript writing. Shotaro Yamamoto, Atsuto Suzuki, Kimitsugu Usui, Hideyuki Terao, Noboru Nakaigawa: Manuscript editing. Takeshi Kishida: Manuscript editing and supervision. All Authors have read and approved the final version of the manuscript.
Acknowledgements
The Authors would like to thank Editage (www.editage.com) for the English language editing.
References
- 1.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Mai Y, Poehlein CH, Perini RF, Bajorin DF, KEYNOTE-045 Investigators Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester RJ, Burger M, Cowan NC, Gontero P, Van Rhijn BW, Mostafid AH, Palou J, Shariat SF. European Association of Urology Guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. 2018;73(1):111–122. doi: 10.1016/j.eururo.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 3.Su R, Chen Z, Hong D, Jiang S, Yuan Y, Cai X, Hu H, Fu C, Huang Z, Wang Z, Zheng B, Huang J, Wang Z, Bao Y, Cai M, Guo J, Chen M, Wei Q, Huang J, Xue W. Effectiveness and safety of immune checkpoint inhibitor monotherapy in advanced upper tract urothelial carcinoma: A multicenter, retrospective, real-world study. Cancer Med. 2023;12(9):10587–10596. doi: 10.1002/cam4.5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg JE, Powles T, Sonpavde GP, Loriot Y, Duran I, Lee JL, Matsubara N, Vulsteke C, Castellano D, Mamtani R, Wu C, Matsangou M, Campbell M, Petrylak DP. EV-301 long-term outcomes: 24-month findings from the phase III trial of enfortumab vedotin versus chemotherapy in patients with previously treated advanced urothelial carcinoma. Ann Oncol. 2023;34(11):1047–1054. doi: 10.1016/j.annonc.2023.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Kawahara T, Hasizume A, Uemura K, Yamaguchi K, Ito H, Takeshima T, Hasumi H, Teranishi JI, Ousaka K, Makiyama K, Uemura H. Administration of enfortumab vedotin after immune-checkpoint inhibitor and the prognosis in Japanese metastatic urothelial carcinoma: a large database study on enfortumab vedotin in metastatic urothelial carcinoma. Cancers (Basel) 2023;15(17):4227. doi: 10.3390/cancers15174227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng J, Peng L, Zhang S, Liao H, Hao J, Wu S, Shen H. Preoperative systemic immune-inflammation index as a prognostic indicator for patients with urothelial carcinoma. Front Immunol. 2023;14:1275033. doi: 10.3389/fimmu.2023.1275033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parent P, Marcq G, Adeleke S, Turpin A, Boussios S, Rassy E, Penel N. Predictive biomarkers for immune checkpoint inhibitor response in urothelial cancer. Ther Adv Med Oncol. 2023;15:17588359231192402. doi: 10.1177/17588359231192402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto M, Fujita K, Nakayama T, Fujimoto S, Hamaguchi M, Nishimoto M, Kikuchi T, Adomi S, Banno E, De Velasco MA, Saito Y, Shimizu N, Mori Y, Minami T, Nozawa M, Nose K, Yoshimura K, Uemura H. Higher neutrophil-to-lymphocyte ratio after the first cycle of the first-line chemotherapy is associated with poor cancer specific survival of upper urinary tract carcinoma patients. Transl Androl Urol. 2021;10(7):2838–2847. doi: 10.21037/tau-21-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Z, Xie S, Feng B, Zhang S, Sun Y, Guo H, Yang R. Preoperative risk classification using neutrophil-to-lymphocyte ratio and albumin for upper tract urothelial carcinoma treated with radical nephroureterectomy. Cancer Manag Res. 2020;12:9023–9032. doi: 10.2147/CMAR.S274332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujiwara M, Fujiwara R, Urasaki T, Oguchi T, Komai Y, Numao N, Yamamoto S, Yonese J, Yuasa T. Early serum and hematological responses to pembrolizumab therapy as predictors of survival in metastatic urothelial cancer. Anticancer Res. 2022;42(4):2045–2051. doi: 10.21873/anticanres.15685. [DOI] [PubMed] [Google Scholar]
- 11.Ishiyama Y, Kondo T, Nemoto Y, Kobari Y, Ishihara H, Tachibana H, Yoshida K, Hashimoto Y, Takagi T, Iizuka J, Tanabe K. Predictive impact of Prognostic Nutritional Index on pembrolizumab for metastatic urothelial carcinoma resistant to platinum-based chemotherapy. Anticancer Res. 2021;41(3):1607–1614. doi: 10.21873/anticanres.14922. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y, Chen Y, Chen J, Chen W, Pan Y, Bao L, Gao X. Combination of systemic inflammation response index and platelet-to-lymphocyte ratio as a novel prognostic marker of upper tract urothelial carcinoma after radical nephroureterectomy. Front Oncol. 2019;9:914. doi: 10.3389/fonc.2019.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han CL, Meng GX, Ding ZN, Dong ZR, Chen ZQ, Hong JG, Yan LJ, Liu H, Tian BW, Yang LS, Xue JS, Li T. The predictive potential of the baseline C-reactive protein levels for the efficiency of immune checkpoint inhibitors in cancer patients: a systematic review and meta-analysis. Front Immunol. 2022;13:827788. doi: 10.3389/fimmu.2022.827788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20(1):25–39. doi: 10.1038/s41577-019-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laino AS, Woods D, Vassallo M, Qian X, Tang H, Wind-Rotolo M, Weber J. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J Immunother Cancer. 2020;8(1):e000842. doi: 10.1136/jitc-2020-000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Lu L, He Z, Xu Z, Xiang Z, Nie RC, Lin W, Chen W, Zhou J, Yin Y, Xie J, Zhang Y, Zheng X, Zhu T, Cai X, Li P, Chao X, Cai MY. C-reactive protein levels predict responses to PD-1 inhibitors in hepatocellular carcinoma patients. Front Immunol. 2022;13:808101. doi: 10.3389/fimmu.2022.808101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida T, Ichikawa J, Giuroiu I, Laino AS, Hao Y, Krogsgaard M, Vassallo M, Woods DM, Stephen Hodi F, Weber J. C reactive protein impairs adaptive immunity in immune cells of patients with melanoma. J Immunother Cancer. 2020;8(1):e000234. doi: 10.1136/jitc-2019-000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilen MA, Martini DJ, Liu Y, Lewis C, Collins HH, Shabto JM, Akce M, Kissick HT, Carthon BC, Shaib WL, Alese OB, Pillai RN, Steuer CE, Wu CS, Lawson DH, Kudchadkar RR, El-Rayes BF, Master VA, Ramalingam SS, Owonikoko TK, Harvey RD. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer. 2019;125(1):127–134. doi: 10.1002/cncr.31778. [DOI] [PubMed] [Google Scholar]
- 19.Hart PC, Rajab IM, Alebraheem M, Potempa LA. C-reactive protein and cancer-diagnostic and therapeutic insights. Front Immunol. 2020;11:595835. doi: 10.3389/fimmu.2020.595835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thouvenin J, Martínez Chanzá N, Alhalabi O, Lang H, Tannir NM, Barthélémy P, Malouf GG. Efficacy of immune checkpoint inhibitors in upper tract urothelial carcinomas: current knowledge and future directions. Cancers (Basel) 2021;13(17):4341. doi: 10.3390/cancers13174341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandekerkhove G, Lavoie JM, Annala M, Murtha AJ, Sundahl N, Walz S, Sano T, Taavitsainen S, Ritch E, Fazli L, Hurtado-Coll A, Wang G, Nykter M, Black PC, Todenhöfer T, Ost P, Gibb EA, Chi KN, Eigl BJ, Wyatt AW. Plasma ctDNA is a tumor tissue surrogate and enables clinical-genomic stratification of metastatic bladder cancer. Nat Commun. 2021;12(1):184. doi: 10.1038/s41467-020-20493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shohdy KS, Villamar DM, Cao Y, Trieu J, Price KS, Nagy R, Tagawa ST, Molina AM, Sternberg CN, Nanus DM, Mosquera JM, Elemento O, Sonpavde GP, Grivas P, Vogelzang NJ, Faltas BM. Serial ctDNA analysis predicts clinical progression in patients with advanced urothelial carcinoma. Br J Cancer. 2022;126(3):430–439. doi: 10.1038/s41416-021-01648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powles T, Assaf ZJ, Davarpanah N, Banchereau R, Szabados BE, Yuen KC, Grivas P, Hussain M, Oudard S, Gschwend JE, Albers P, Castellano D, Nishiyama H, Daneshmand S, Sharma S, Zimmermann BG, Sethi H, Aleshin A, Perdicchio M, Zhang J, Shames DS, Degaonkar V, Shen X, Carter C, Bais C, Bellmunt J, Mariathasan S. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021;595(7867):432–437. doi: 10.1038/s41586-021-03642-9. [DOI] [PubMed] [Google Scholar]
- 25.Powles T, Carroll D, Chowdhury S, Gravis G, Joly F, Carles J, Fléchon A, Maroto P, Petrylak D, Rolland F, Cook N, Balar AV, Sridhar SS, Galsky MD, Grivas P, Ravaud A, Jones R, Cosaert J, Hodgson D, Kozarewa I, Mather R, McEwen R, Mercier F, Landers D. An adaptive, biomarker-directed platform study of durvalumab in combination with targeted therapies in advanced urothelial cancer. Nat Med. 2021;27(5):793–801. doi: 10.1038/s41591-021-01317-6. [DOI] [PubMed] [Google Scholar]