Abstract
Background/Aim
Gliomas are highly heterogeneous malignancies originating from diverse cell types within the brain. Although their precise etiology is frequently unknown, risk factors, such as chemical exposure, radiation, and specific uncommon genetic disorders have been identified. Diagnosis typically entails imaging tests, such as magnetic resonance imaging and computed tomography, complemented by a biopsy for confirmation, which may be further validated through genetic testing.
Case Report
Next-generation sequencing technology revealed germline co-deletion deletion of cyclin-dependent kinase inhibitor 2 A and B genes (CDKN2A and CDKN2B) in a patient diagnosed with pleomorphic xanthoastrocytoma based on the tumor’s molecular characteristics. Following this result, we performed focused genetic analysis with use of multiplex ligation-dependent probe amplification technology for the mother that revealed the same co-deletion. Moreover, due to the father’s neuroendocrine pancreatic cancer, application of the NGS technology detected a pathogenic variant in the BRCA1-interacting helicase 1 (BRIP1) gene. Comprehensive multi-gene testing conducted within the familial context, marked by a varied spectrum of cancer type, revealed a constellation of genetic predispositions.
Conclusion
This case study underscores the critical importance of molecular testing for tumor characterization and highlights the pivotal role of genetic testing in facilitating early intervention and screening for at-risk family members. Furthermore, the identification of germline co-deletions in cancer lays the foundation for the development of targeted therapeutic strategies aimed at restoring normal cellular regulation and improving patient management.
Keywords: Glioma, pleomorphic xanthoastrocytoma, next-generation sequencing, NGS, copy-number variations, CNVs, co-deletion, CDKN2A, CDKN2B
Brain cancer encompasses a diverse range of diseases characterized by the uncontrollable growth of abnormal cells in the brain. These cells cluster together to form tumors, which can disrupt the brain’s normal functioning (1). These tumors can be broadly classified into two main categories: benign and malignant. Benign tumors are non-cancerous growths that tend to grow slowly and do not invade surrounding tissues. They are typically well-defined and can be more easily surgically removed. Despite not being classified as cancerous, their location and size can still lead to significant health issues. In contrast, malignant brain tumors are considered cancerous. They have the potential to grow and spread more aggressively, infiltrating nearby tissues. This aggressive growth can make complete surgical removal more challenging (2). Furthermore, malignant tumors can metastasize, meaning they can spread to other parts of the central nervous system or even distant organs, adding complexity to the treatment process (3).
Various types of brain tumors exist, each arising from different types of brain cells, for instance, gliomas, meningiomas, medulloblastomas and pleomorphic xantho-astrocytoma (PXA) (4,5). Symptoms of brain cancer can vary widely based on factors, such as the tumor’s location, size, and type. Common signs may include persistent headaches, seizures, changes in vision, difficulties with coordination or balance, speech impairments and alterations in cognitive function. These symptoms may emerge gradually or suddenly, contingent on the nature of the tumor (6). While certain risk factors have been identified, such as the exposure to specific chemicals or radiation, along with a genetic predisposition, the majority of cases arise spontaneously without a discernible cause. Some rare genetic disorders have also been linked to an elevated risk of developing specific types of brain tumors (7).
Diagnosing brain cancer involves a comprehensive assessment, typically commencing with imaging tests, such as magnetic resonance imaging and computed tomography. These imaging studies yield detailed images of the brain, allowing healthcare professionals to pinpoint the presence, location, and characteristics of any tumors (8). Additionally, a biopsy, involving the removal of a small sample of tumor tissue, is often performed for a definitive diagnosis and to ascertain the tumor type (9). Treatment approaches for brain cancer are highly tailored and hinge on factors including the type, location, and stage of the tumor. Common treatment methods encompass surgery, aiming to remove as much of the tumor as possible; radiation therapy, employing high-energy rays to target and eliminate cancer cells; chemotherapy, utilizing drugs to destroy cancer cells; targeted therapy, focusing on specific molecular traits of cancer cells; and immunotherapy, mobilizing the body’s immune system to target and eradicate cancer cells. Frequently, a combination of these treatments is employed to optimize outcomes (10). The prognosis for individuals with brain cancer varies widely, contingent on factors, such as tumor type, location, and stage of diagnosis. Certain types of brain cancer have more favorable prognoses than others. Advances in medical science, encompassing enhanced diagnostic techniques and treatment approaches, have significantly improved outcomes for many patients (11).
In the present study, comprehensive multi-gene testing was performed, within a familial context characterized by a diverse spectrum of cancer types, including PXA, pancreatic and breast cancer.
Case Report
After experiencing epileptic episodes, at the age of 10 years, and based on imaging, the patient received a diagnosis of dysembryoplastic neuroepithelial tumor for a 3 cm tumor located within the left temporal lobe of the brain. Over the years, the patient’s condition remained stable, as confirmed through routine annual follow-up imaging, until she reached the age of 20, at which point she began to manifest symptomatic and imaging evidence of disease progression.
In accordance with the current clinical guidelines (12), the patient underwent surgical excision, and the subsequent histological analysis unveiled a PXA categorized as grade III, as per the 2021 World Health Organization central nervous system classification (13). Immunohistochemistry demonstrated a Ki-67 proliferation index of 15% and the presence of a p53 mutation in 90% of cells, while retaining the expression of ATRX chromatin remodeler protein (ATRX). The isocitrate dehydrogenase (IDH) status was confirmed to be wild-type using clones H09 from Dianova (Eching, Germany) and MsMab-1 R132/172 from Merck (Darmstadt, Germany). Additionally, the histone H3.3 status, assessed using clones RM192, RM240, and RM307 from RevMab Biosciences (Burlingame, CA, USA) was also determined to be wild-type. Immunostaining for glial fibrillary acidic protein (GFAP) and forkhead box G1 (FOXG1) was positive, while those for oligodendrocyte transcription factor (OLIG2), CD34 molecule (CD34), and epidermal growth factor receptor (EGFR) displayed negative results. The patient’s B-Raf proto-oncogene serine/threonine kinase (BRAF) status indicated a mutation, with strong positivity for BRAFV600E (VE1; Ventana, Roche, Pleasanton, CA, USA). Furthermore, protein expression for SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 (SMARCB1)/integrase interactor 1 (INI1) (Acris GmbH, Heidelberg, Germany) was preserved. A fluorescent in situ hybridization test revealed a homozygotic deletion of cyclin-dependent kinase inhibitor 2A (CDKN2A) in 81% of cells using the ZytoLight SPEC CDKN2A/CEN 9 dual color probe (ZytoVision GmbH, Bremerhaven, Germany) in 100 cells.
Subsequent to the surgical procedure, the patient underwent radiotherapy, employing volumetric-modulated arc therapy with image-guided radiation therapy techniques, resulting in a total dose of 60 Gy. The patient also received concurrent temozolomide chemotherapy at a daily dose of 75 mg/m2, administered over 7 days per week for a duration of 6 weeks. At the 3-month re-evaluation mark, following brain magnetic resonance imaging, no evidence of disease recurrence was observed.
The mother of the patient presented with an epileptic seizure due to a 3 cm intraventricular mass located at the junction of the choroid plexus and the occipital horn of the left cerebral hemisphere. In accordance with the most recent clinical guidelines, the patient underwent surgical excision, and subsequent histological analysis identified a PXA classified as grade II by the 2021 World Health Organization central nervous system classification. Immunohistochemistry findings included a Ki-67 proliferation index of 4%, one mitotic figure per 10 high-power fields (×40 magnification), mutant p53 in 90% of cells, retained ATRX protein expression, wild-type IDH status (as assessed by clones H09 from Dianova and MsMab-1 R132/172 from Merck), wild-type histone H3.3 status (as evaluated with clones RM192, RM240, and RM307; RevMab Biosciences), and preserved expression of MutL protein homolog 1 (MLH1), PMS1 homolog 2, mismatch repair system component (PMS2), MutS homolog 2 (MSH2), and MutS homolog 6 (MSH6). Additionally, immunostains for CD34, GFAP, FOXG1 and OLIG2 were positive, while the BRAF status was determined to be mutant with strong positivity for BRAFV600E (VE1; Ventana), and EGFR was found to be negative.
Following the surgical procedure, the patient received adjuvant radiotherapy delivered through volumetric-modulated arc therapy with image-guided radiation therapy techniques, resulting in a total dose of 60 Gy. The patient underwent concurrent temozolomide chemotherapy at a dose of 75 mg/m2 per day, administered daily over 7 days per week for a duration of 6 weeks. Subsequent re-evaluation at the 3-month mark, using brain magnetic resonance imaging, revealed no signs of disease recurrence.
We obtained peripheral blood samples for next-generation sequencing (NGS) assessment from the 20-year-old patient with PXA. Genomic DNA was isolated from leukocytes in the peripheral blood using a MagCore® Genomic DNA Whole Blood Kit (RBC Bioscience, New Taipei City, Taiwan, ROC) following the guidelines provided by the manufacturer. A solution-based capture method was employed to study genes linked to hereditary cancer susceptibility. Following a previously outlined protocol (14), targeted NGS was conducted using a 52-gene panel (SeqCap EZ Choice; Roche NimbleGen, Pleasanton, CA, USA). Sample preparation adhered to the SeqCap EZ Choice Library User’s Guide (Roche NimbleGen) from the manufacturer. Sequencing utilized DNBSEQ-G400 technology (MGI Tech Co., Ltd., Shenzhen, P.R. China), and analysis was performed with SeqNext version 4.4.0 (JSI Medical Systems GmbH, Ettenheim, Germany) software. Sequence variations were identified and interpreted within the context of specific clinically relevant transcripts.
The detection of copy number variations was explored through the use of commercial computational algorithms SeqPilot (JSI Medical Systems GmbH), and the multiplex ligation-dependent probe amplification method [MLPA; P419-B1 CDKN2A/2B–cyclin-dependent kinase 4 (CDK4)]. This investigation followed the guidelines outlined by the manufacturer (MLPA General Protocol Version-008; MRC-Holland, DL, Amsterdam, the Netherlands). Electrophoresis was conducted on a SeqStudio Genetic Analyzer (Thermo Fisher Scientific, Applied Biosystems, Waltham, MA, USA), and the analysis was executed using Coffalyser.Net software developed by MRC-Holland (Coffalyser.Net™ version 220513.1739).
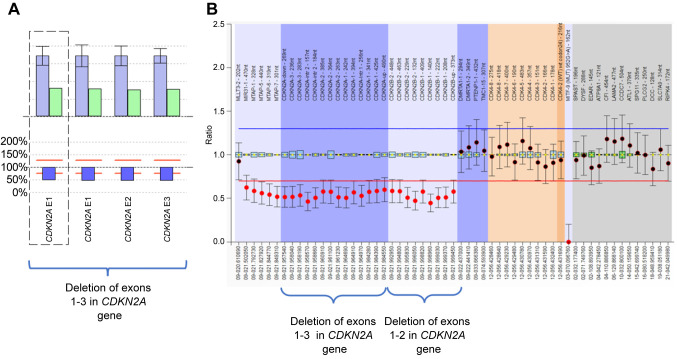
Genetic analysis of the patient’s whole peripheral blood revealed a co-deletion of CDKN2A and CDKN2B genes. This alteration results in the complete deletion of the CDKN2A gene (exons 1-3). This deletion is expected to result in the absence of the protein products p16INK4A and p14ARF from one allele. This specific variation is reported in the ClinVar database (Variation ID: 583687) as pathogenic. Conclusively this variation is characterized as pathogenic. Furthermore, the MLPA technique revealed the deletion of the entire CDKN2B gene (exons 1-2/NM_004936). This is expected to result in the absence of the protein product from one allele (Figure 1).
Figure 1. A: Detection of deletion of exons 1-3 in cyclin-dependent kinase inhibitor 2A (CDKN2A) gene from next-generation sequencing data using SeqNext (JSI) software. The bar plot illustrates the patient sample’s relative coverage of each target region of interest (ROI) in green and the average relative target coverage of control samples in blue. The ratio of the patient sample’s coverage of target ROIs against the controls is shown below each pair of bars. Reds lines correspond to the 75% deletion limit (lower red dotted line) and the 135% duplication limit (upper red dotted line) for the calculated ratio. The average relative target coverage data are reported as means±standard deviations of control samples. B: Sample plots from Coffalyser.net displaying probe ratios with 95% confidence intervals as error bars for CDKN2A and CDKN2B exons. The proband’s MLPA analysis revealed heterozygous deletion of exons 1-3 in CDKN2A and of exons 1-2 in CDKN2B (SALSA MLPA probemix P419; MRC Holland).
Furthermore, we also utilized NGS technology to carry out whole-exome sequencing. This approach enabled us to comprehensively assess the complete exome, encompassing all the regions of the genome responsible for encoding proteins. Our objective was to meticulously investigate the particular genes associated with epilepsy. However, the comprehensive analysis concluded with a negative finding, indicating that no significant genetic mutations or variations in these epilepsy-related genes were identified.
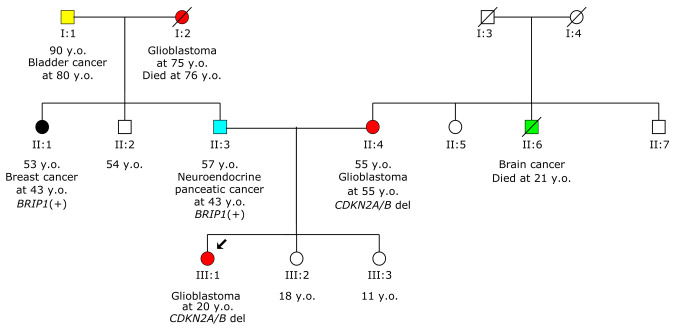
Considering the patient’s family history of cancer (Figure 2), which includes her mother being diagnosed with grade II PXA at 55 years old, and her father with neuroendocrine pancreatic cancer at 43 years old, there is a notable concern for potential hereditary predisposition to cancer in this family. Following the initial genetic testing of the patient, the next step involved performing cascade testing in both parents. This type of testing aims to determine whether the identified genetic mutation is present in other family members. In this case, it was revealed that the patient’s mother carried the same pathogenic variant, whereas the patient’s father tested negative. The confirms that this specific genetic mutation is indeed present in the family and possibly segregates with the disease.
Figure 2. Pedigree of the proband’s family. The arrow indicates the proband. d: Died; y.o: years old. +/− Symbols indicate test result. Different types of cancer are represented by different colors.
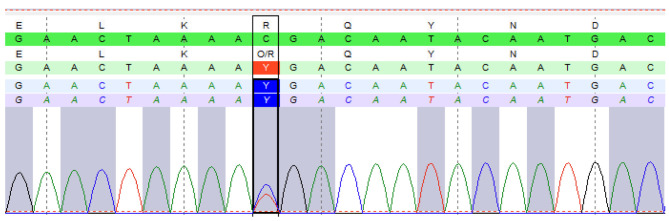
In a subsequent analysis of the father’s genetic material, a pathogenic mutation was discovered in the BRCA1-interacting helicase 1 (BRIP1) gene (c.2392C>T, p.Arg798*) (Figure 3). BRIP1 gene is associated with an increased risk of certain types of cancer, including breast cancer. Remarkably, the same pathogenic mutation in the BRIP1 gene was also identified in the patient’s aunt on the father’s side, who had been diagnosed with breast cancer at 43 years old. These findings shed significant light on the hereditary component of cancer risk within this family. The identification of a shared mutation in both the patient’s father and her aunt suggest a potential genetic link to the development of breast cancer. This information is invaluable for the family members, as it allows for targeted screening strategies. Early detection and intervention can be instrumental in managing the potential risks associated with these genetic mutations, ultimately improving the overall health outcomes for the affected individuals.
Figure 3. Sequencing analysis of genomic DNA from the proband’s father, revealing he carried the c.2392C>T, p.(Arg798*) variant in the BRIP1 gene.
Informed consent was obtained from all individuals involved in the study, which was approved by the Ethics Committee of the ATTIKON University Hospital.
Discussion
PXA is a relatively rare type of brain tumor that predominantly occurs in children and young adults. This particular tumor is identified by its unique cellular characteristics when examined under a microscope. These features include pleomorphic cells, which are irregularly shaped and vary in size and appearance, as well as xanthomatous cells, which are filled with lipid content. Despite being categorized as a form of brain tumor, PXAs are generally classified as low-grade (5). This designation signifies that they exhibit less aggressive behavior in comparison to high-grade malignant brain tumors. In essence, PXAs tend to grow more slowly and are less likely to infiltrate surrounding brain tissue or metastasize to other parts of the body. While still a serious medical condition requiring prompt treatment and management, the prognosis for patients with PXA is generally more favorable compared to individuals with high-grade malignant brain tumors (15).
In this study, we present two patients with PXA who carried germline co-deletion of CDKN2A and CDKN2B genes. CDKN2A and CDKN2B are pivotal genes that exert significant influence over the intricate processes of cell growth and division. They fall into the category of tumor-suppressor genes, a group of genes that serve as natural safeguards against unbridled cell multiplication, which can culminate in the emergence of tumors. These genes regulate the cell cycle to ensure that cells divide and proliferate in an orderly and controlled manner (16). When mutations occur in CDKN2A and CDKN2B, they can lead to a disruption in their normal function. This, in turn, can undermine their ability to effectively inhibit excessive cell growth. Consequently, this may set the stage for the formation of cancerous cells. These aberrant cells can then bypass the usual regulatory checkpoints, leading to uncontrolled proliferation and the potential development of tumors (17). Additionally, the co-deletion of CDKN2A and CDKN2B has been observed in various cancer types and is associated with poor prognosis and aggressive tumor growth (18,19). In glioblastoma multiforme, this genetic alteration may confer resistance to conventional treatments, such as radiation and chemotherapy (20).
In essence, CDKN2A and CDKN2B act as guardians of cellular health, actively working to maintain the balance between cell division and growth restraint. When these genes are compromised by mutations, their protective function is weakened, potentially contributing to the onset and progression of cancer. Understanding the intricate interplay of these genes provides valuable insights into the underlying mechanisms of tumorigenesis, aiding in the development of targeted therapeutic interventions for individuals affected by these genetic alterations (17).
Furthermore, the cancer incidents among the family members of the primary patient adds another layer of complexity to the case. The discovery of a pathogenic variant in the BRIP1 gene in the patient’s father, along with the aunt’s breast cancer diagnosis, further supports the notion of a familial predisposition to cancer. This case underscores the importance of comprehensive genetic testing and analysis in understanding the genetic basis of cancer. The identification of specific mutations not only aids in diagnosis but also provides valuable information for treatment planning and risk assessment for the patient and her family members.
In addition, this case highlights the potential benefits of early intervention and screening for individuals with identified genetic predispositions. Tailored surveillance and preventive measures can significantly impact outcomes, potentially detecting cancer earlier at more treatable stages. Overall, this case serves as a poignant example of how an in-depth genetic analysis can greatly inform clinical decision-making, potentially leading to more targeted and effective interventions for patients with complex cancer profiles. It also underscores the importance of genetic counseling and support for both patients and their family members facing hereditary cancer risks.
Conclusion
In summary, the significance of the CDKN2A and CDKN2B genes as integral components of the cell-cycle regulatory machinery cannot be overstated. Their frequent inactivation or deletion in diverse cancers, particularly in the context of brain cancer, highlights their critical role in preventing tumorigenesis. Understanding the disruption of these genes in the context of cancer provides valuable insights into the molecular mechanisms driving uncontrolled cell growth. This understanding not only contributes to the depth of our knowledge about cancer biology but also lays the foundation for the development of targeted therapeutic strategies aimed at restoring normal cellular regulation and improving outcomes for individuals affected by these genetic anomalies.
Conflicts of Interest
The Authors have no conflicts of interest to declare in relation to this study.
Authors’ Contributions
All Authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Konstantinos Agiannitopoulos, Anastasia Katseli, Kevisa Potska, Christina Ntogka, Georgios N. Tsaousis, Nikolaos Tsoulos, Katerina Kampoli, Anastasios Ntavatzikos, Eirini Papadopoulou, Anna Koumarianou and George Nasioulas. The first draft of the article was written by Konstantinos Agiannitopoulos and all Authors commented on previous versions of the article. All Authors read and approved the final article.
References
- 1.Łazarczyk M, Mickael ME, Skiba D, Kurzejamska E, Ławiński M, Horbańczuk JO, Radziszewski J, Fraczek K, Wolinska R, Paszkiewicz J, Religa P, Sacharczuk M. The journey of cancer cells to the brain: challenges and opportunities. Int J Mol Sci. 2023;24(4):3854. doi: 10.3390/ijms24043854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang LM, Englander ZK, Miller ML, Bruce JN. Malignant Glioma. Adv Exp Med Biol. 2023;1405:1–30. doi: 10.1007/978-3-031-23705-8_1. [DOI] [PubMed] [Google Scholar]
- 3.Kim MM, Mehta MP, Smart DK, Steeg PS, Hong JA, Espey MG, Prasanna PG, Crandon L, Hodgdon C, Kozak N, Armstrong TS, Morikawa A, Willmarth N, Tanner K, Boire A, Gephart MH, Margolin KA, Hattangadi-Gluth J, Tawbi H, Trifiletti DM, Chung C, Basu-Roy U, Burns R, Oliva ICG, Aizer AA, Anders CK, Davis J, Ahluwalia MS, Chiang V, Li J, Kotecha R, Formenti SC, Ellingson BM, Gondi V, Sperduto PW, Barnholtz-Sloan JS, Rodon J, Lee EQ, Khasraw M, Yeboa DN, Brastianos PK, Galanis E, Coleman CN, Ahmed MM. National Cancer Institute Collaborative Workshop on Shaping the Landscape of Brain Metastases Research: challenges and recommended priorities. Lancet Oncol. 2023;24(8):e344–e354. doi: 10.1016/S1470-2045(23)00297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas DL. 2021 updates to the World Health Organization classification of adult-type and pediatric-type diffuse gliomas: a clinical practice review. Chin Clin Oncol. 2023;12(1):7–7. doi: 10.21037/cco-22-120. [DOI] [PubMed] [Google Scholar]
- 5.Shaikh N, Brahmbhatt N, Kruser TJ, Kam KL, Appin CL, Wadhwani N, Chandler J, Kumthekar P, Lukas RV. Pleomorphic xanthoastrocytoma: a brief review. CNS Oncol. 2019;8(3):CNS39. doi: 10.2217/cns-2019-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaff LR, Mellinghoff IK. Glioblastoma and other primary brain malignancies in adults. JAMA. 2023;329(7):574. doi: 10.1001/jama.2023.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristensen BW, Priesterbach-Ackley LP, Petersen JK, Wesseling P. Molecular pathology of tumors of the central nervous system. Ann Oncol. 2019;30(8):1265–1278. doi: 10.1093/annonc/mdz164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villanueva-Meyer JE, Mabray MC, Cha S. Current clinical brain tumor imaging. Neurosurgery. 2017;81(3):397–415. doi: 10.1093/neuros/nyx103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soleman J, Dvir R, Ben-Sira L, Yalon M, Boop F, Constantini S, Roth J. MRI-based diagnosis and treatment of pediatric brain tumors: is tissue sample always needed. Childs Nerv Syst. 2021;37(5):1449–1459. doi: 10.1007/s00381-021-05148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Śledzińska P, Bebyn M, Furtak J, Koper A, Koper K. Current and promising treatment strategies in glioma. Rev Neurosci. 2023;34(5):483–516. doi: 10.1515/revneuro-2022-0060. [DOI] [PubMed] [Google Scholar]
- 11.Smolarska A, Pruszynska I, Wasylko W, Godlewska K, Markowska M, Rybak A Rybak A, Botther J, Kucharzewska P, Nowakowska J, Szeliga J, Kubiak M, Gorczak M, Krol M. Targeted therapies for glioblastoma treatment. J Physiol Pharmacol. 2023;74(3):251–261. doi: 10.26402/jpp.2023.3.01. [DOI] [PubMed] [Google Scholar]
- 12.Segura PP, Quintela NV, García MM, Del Barco Berrón S, Sarrió RG, Gómez JG, Castaño AG, Martín LMN, Rubio OG, Losada EP. SEOM-GEINO clinical guidelines for high-grade gliomas of adulthood (2022) Clin Transl Oncol. 2023;25(9):2634–2646. doi: 10.1007/s12094-023-03245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO Classification of Tumours Editorial Board Central Nervous System Tumours. WHO Classification of Tumours, 5th Edition, Volume 6. IARC, Lyon, France. 2021 [Google Scholar]
- 14.Agiannitopoulos K, Potska K, Katseli A, Ntogka C, Tsaousis GN, Pepe G, Bouzarelou D, Tsoulos N, Papathanasiou A, Ziogas D, Venizelos V, Markopoulos C, Iosifidou R, Karageorgopoulou S, Giassas S, Natsiopoulos I, Papazisis K, Vasilaki-Antonatou M, Psyrri A, Koumarianou A, Matthaios D, Zairi E, Blidaru A, Banu E, Jinga DC, Laçin Ş, Özdoğan M, Papadopoulou E, Nasioulas G. Only 32.3% of breast cancer families with pathogenic variants in cancer genes utilized cascade genetic testing. Cancers (Basel) 2023;15(21):5218. doi: 10.3390/cancers15215218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Z, Yang R, Zheng H, Li Z, Yi G, Wu Q, Yang C, Huang G. Pleomorphic xanthoastrocytoma, anaplastic pleomorphic xanthoastrocytoma, and epithelioid glioblastoma: Case series with clinical characteristics, molecular features and progression relationship. Clin Neurol Neurosurg. 2022;221:107379. doi: 10.1016/j.clineuro.2022.107379. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D, Wang T, Zhou Y, Zhang X. Comprehensive analyses of cuproptosis-related gene CDKN2A on prognosis and immunologic therapy in human tumors. Medicine (Baltimore) 2023;102(14):e33468. doi: 10.1097/MD.0000000000033468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiltbrunner S, Fleischmann Z, Sokol ES, Zoche M, Felley-Bosco E, Curioni-Fontecedro A. Genomic landscape of pleural and peritoneal mesothelioma tumours. Br J Cancer. 2022;127(11):1997–2005. doi: 10.1038/s41416-022-01979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dogan S, Xu B, Rana S, Chen H, Ghossein RA, Berger MF, Ho AL, Katabi N. Loss of CDKN2A/B is a molecular marker of high-grade histology and is associated with aggressive behavior in acinic cell carcinoma. Mod Pathol. 2023;36(7):100150. doi: 10.1016/j.modpat.2023.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Kuang P, Liu T. Prognostic significance of CDKN2A/B deletions in acute lymphoblastic leukaemia: a meta-analysis. Ann Med. 2019;51(1):28–40. doi: 10.1080/07853890.2018.1564359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumari S, Gupta R, Ambasta RK, Kumar P. Multiple therapeutic approaches of glioblastoma multiforme: From terminal to therapy. Biochim Biophys Acta Rev Cancer. 2023;1878(4):188913. doi: 10.1016/j.bbcan.2023.188913. [DOI] [PubMed] [Google Scholar]