Abstract
Type 2 inflammation is characterized by overexpression and heightened activity of type 2 cytokines, mediators, and cells that drive neuroimmune activation and sensitization to previously subthreshold stimuli. The consequences of altered neuroimmune activity differ by tissue type and disease; they include skin inflammation, sensitization to pruritogens, and itch amplification in atopic dermatitis and prurigo nodularis; airway inflammation and/or hyperresponsiveness, loss of expiratory volume, airflow obstruction and increased mucus production in asthma; loss of sense of smell in chronic rhinosinusitis with nasal polyps; and dysphagia in eosinophilic esophagitis. We describe the neuroimmune interactions that underlie the various sensory and autonomic pathologies in type 2 inflammatory diseases and present recent advances in targeted treatment approaches to reduce type 2 inflammation and its associated symptoms in these diseases. Further research is needed to better understand the neuroimmune mechanisms that underlie chronic, sustained inflammation and its related sensory pathologies in diseases associated with type 2 inflammation.
Keywords: Type 2 inflammation, sensory neurons, cytokines, neuropeptides, atopic dermatitis, prurigo nodularis, asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, neuroimmune
Type 2 immunity is a specialized, evolutionarily conserved arm of the immune system that combats ectoparasitic and endoparasitic helminths, expels toxins, and promotes tissue repair.1–4 When the epithelial barrier is breached, alarmin cytokines (eg, thymic stromal lymphopoietin [TSLP], IL-25, IL-33) activate tissue-resident immune cells (such as mast cells, dendritic cells, and group 2 innate lymphoid cells [ILC2s]) while simultaneously recruiting granulocytes, including eosinophils and basophils. Collectively, these cells orchestrate a polarized type 2 immune response through type 2 cytokines, histamine, and other mediators that neutralize and expel parasitic helminths and toxins and repair the barrier through epithelial turnover, remodeling, and fibrosis. Although these processes are protective and intended to restore tissue homeostasis, in the setting of allergy and continuous barrier stress they become pathologic, resulting in a variety of chronic inflammatory diseases.
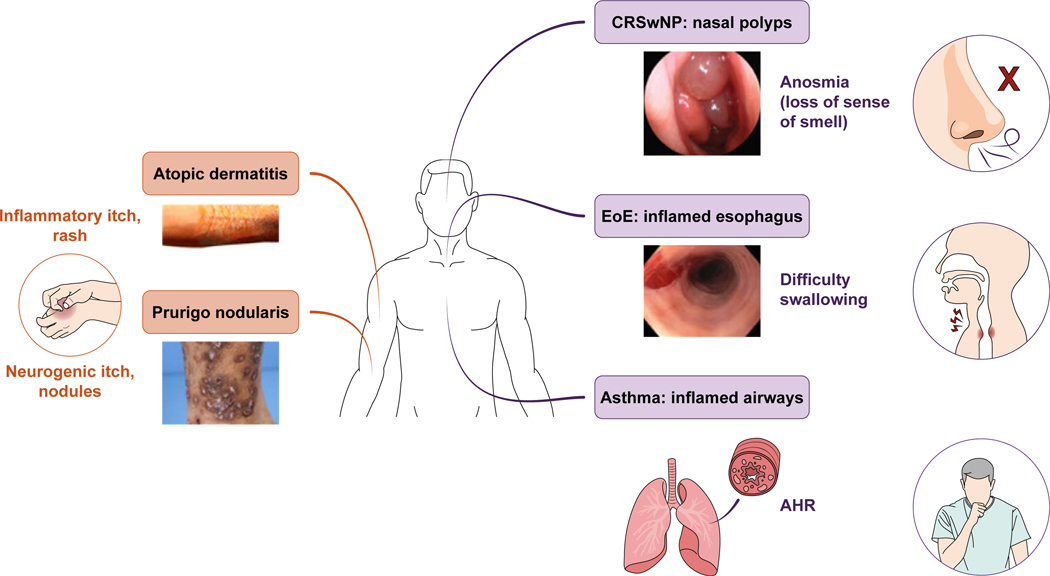
Mechanical reflexes such as scratching, airway constriction, coughing, sneezing, and gastrointestinal motility also protect barrier surfaces and are triggered by direct activation of sensory neurons, often in concert with autonomic input to the target organs. Many of these manifestations are pathologically altered in diseases with type 2 immune dysregulation (Fig 1), including (but not limited to) atopic dermatitis (AD), prurigo nodularis (PN), asthma, food allergy, chronic rhinosinusitis with nasal polyps (CRSwNP), and eosinophilic esophagitis (EoE).1,2,5,6 The extent of similarities among the neuroimmune pathways regulating these mechanical responses remains to be fully defined (Table I7–73).
FIG 1.
Sensory pathologies in atopic diseases.
TABLE I.
Neuroimmune interactions affecting symptoms in AD, PN, asthma, CRSwNP, and EoE
| Condition | Symptoms | Neuroimmune interactions | Type 2 cytokine profile |
|---|---|---|---|
| AD | Pruritus | ● Colocalization of neurons and immune cells7,8
● Type 2 cytokines and OSM promote pruritogen sensitization9 ● Neuropeptide release from sensory neurons induces proinflammatory cytokines and activates immune cells (eg, mast cells, basophils)7,8,10–13 ● Scratching causes release of alarmins (eg, IL-33), which can act directly on sensory neurons9,11,14–17 ● Some sensory neurons express receptors for IL-4, IL-13, IL-31, TRPA1, TRPV118,19 ● Reducing type 2 inflammation reduces itch20–36 ● Inhibition of IL-4–mediated JAK/STAT signaling reduces itch37–41 |
Prominent role: IL-4, IL-13, IL-31 Limited role: IL-15, periostin |
| PN | Pruritus | ● Colocalization of neurons and immune cells7,8 ● Sensory neuron dysfunction and increased density of SP- and CGRP-immunoreactive nerve fibers42–47 ● Neuropeptide release from sensory neurons induces proinflammatory cytokines and activates immune cells (eg, mast cells, basophils)7,8,11,43,45–48 ● Increased numbers of IL-31–positive cells and OSM-positive cells are correlated with itch intensity49,50 ● Reducing type 2 inflammation also reduces itch36,51,52 |
Prominent role: IL-4, IL-13, IL-31, periostin |
| Asthma | AHR | ● Colocalization of neurons and immune cells53 ● Type 2 cytokines can stimulate neuropeptide release from sensory neurons (IL-5) and induce AHR (IL-4 and IL-13)54–56 ● Chronic inflammation sensitizes nerve fibers in airways, promoting AHR53–58 ● Airway neurons can enhance allergen-induced immune cell response, including ILC2 response via NMU59,60 ● Eosinophils increase sensory nerve density and responsiveness, promoting bronchoconstriction61 ● β2AR agonists mediate sympathetic nerve activity, IL-33–induced inflammation, and ILC2 proliferation45,62 ● Pulmonary neuroendocrine cells enhance allergen-induced response by recruiting TH2 immune cells and activating ILC2s63 |
Prominent role: IL-4, IL-5, IL-13, IgE |
| CRSwNP | Anosmia | ● Olfactory sensory neurons express IL-4Rα64 ● Chronic type 2 inflammation inhibits olfactory neuron function and neurogenesis65 ● TRP channels on sensory neurons and immune cells in the upper airway are associated with type 2 inflammatory processes66–68 |
Prominent role: IL-4, IL-5, IL-13, IgE, periostin |
| EoE | Dysphagia | ● Type 2 inflammation sensitizes nociceptors, causing pain, motor dysfunction, and tissue remodeling69,70 ● Type 2 cytokines can induce hypercontractility of smooth muscle cells via STAT6 or MAPK71 ● Infiltrating immune cells can increase vagal sensory neuron responsiveness to acid71,72 ● Pain severity is linked to TRPV1 and mast cells73 |
Prominent role: IL-4, IL-13 Limited role: IL-5, Siglec-8 |
MAPK, Mitogen-activated protein kinase; NMU, neuromedin U; Siglec-8, sialic acid–binding immunoglobulin-like lectin 8; TRP, transient receptor potential.
Type 2 inflammation is characterized by overexpression and increased activity of type 2–associated cytokines (including IL-4, IL-5, IL-13, and IL-31) and alarmins (including IL-25, IL-33, and TSLP). An important property of these cytokines is their ability to activate or sensitize peripheral sensory neurons. For example, IL-4, IL-13, and IL-31 trigger rapid calcium responses in dorsal root ganglion (DRG) neurons and promote itch,18,19,74 and IL-5 has been implicated in the release of vasoactive intestinal peptide (VIP) from sensory neurons in the lungs.75 At the same time, sensory neurons produce neuropeptides such as VIP, substance P (SP), and calcitonin gene–related peptide (CGRP), which act directly on vascular endothelial cells and smooth muscle cells to mediate vascular effects, as well as on immune cells (Fig 2).61,76–80 Neurons and immune cells colocalize in affected areas of the skin in AD and PN and in the airways in asthma.7,8,53 In addition, patients with AD and PN often show alterations in nerve fibers in affected tissue.10,42,43,81 IL-31, a type 2 cytokine associated with itch, has been shown to promote sensory nerve elongation, branching, and density both in vitro and in lesional skin in mice.82 In asthma, sensory neurons also exhibit hyperinnervation; for example, IL-5 exposure in utero enhances nerve sprouting in lungs in mice.83
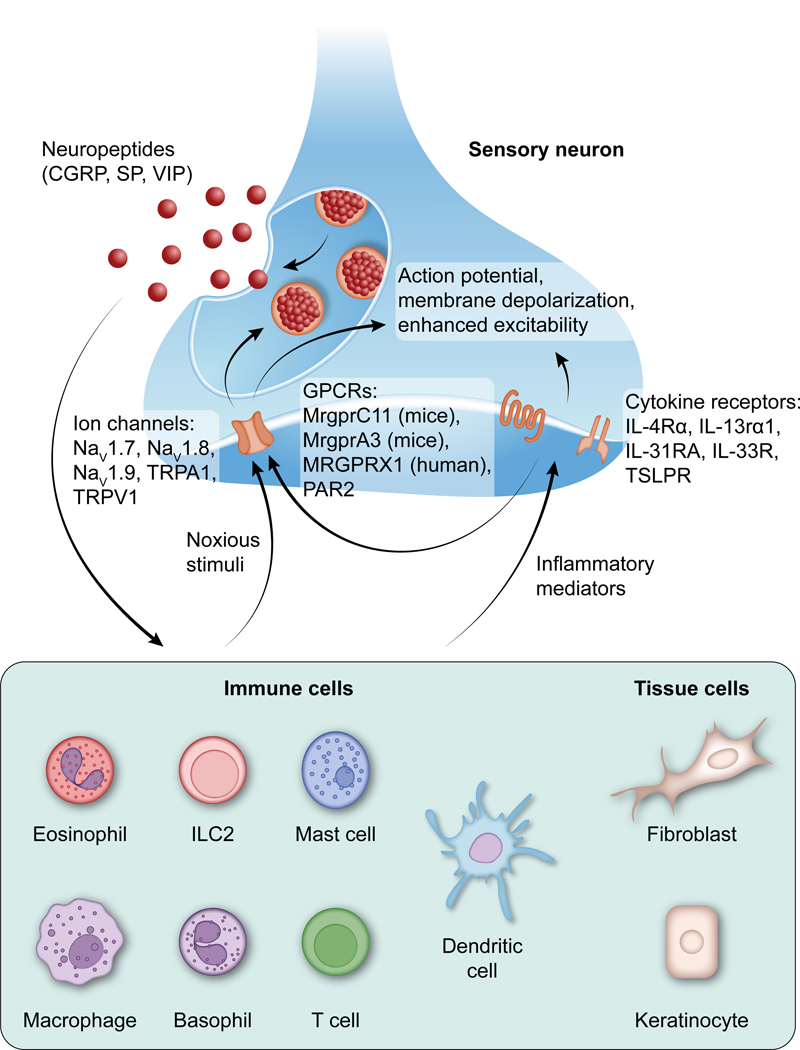
FIG 2.
Type 2 inflammation and sensory neurons: bidirectional cross talk. Stimulated neurons produce neuropeptides that can modulate the activity of immune cells, including the production of inflammatory mediators that can directly affect sensory neurons. IL-13Rα1, IL-13 receptor-α 1; IL-31RA, IL-31 receptor A; IL-33R, IL-33 receptor; MrgprCr11, Mas-related family of G protein-coupled receptor 11; MRGPRX1, Mas-related family of G protein-coupled receptor X1; Nav, voltage-gated sodium channel; NMU, neuromedin U; PAR2, protease activated receptor 2; TSLPR, thymic stromal lymphopoietin receptor.
The mechanisms by which sensory neurons, their associated neuropeptides, and tissue-resident or infiltrated immune cells interact to activate or regulate specific pathways of inflammation, and how these histologic alterations affect the precise sensory response and neuroinflammatory function of organs across diseases featuring type 2 immune dysregulation (including AD, PN, asthma, CRSwNP, and EoE) remains a major area of inquiry. Herein, we review current research on these pathways and present recent advances in targeted treatments to reduce type 2 inflammation and associated sensory pathologies in these diseases.
NEUROIMMUNE CROSS TALK AND SENSORY PATHOLOGY IN CONDITIONS ASSOCIATED WITH TYPE 2 INFLAMMATION
The itch-scratch cycle in AD and PN
AD and PN are chronic inflammatory skin disorders featuring intense pruritus and an itch-scratch cycle.84 Both diseases are often accompanied by pruritic skin, sleep disturbances, and impaired quality of life.44,85 However, there are notable differences. AD is a relapsing-remitting type 2 inflammatory disease characterized by red, scaly, oozing, and crusted lesions, as well as by increased risk for cutaneous infections and intermittent flares.86–90 In contrast, PN is a chronic pruritic condition with type 2 immune dysregulation with or without atopy. In addition to intense pruritus lasting for 6 weeks or longer, PN features discrete papulonodular lesions with firm fibrotic qualities.85,91,92 Although AD is considered a primary inflammatory dermatosis that secondarily results in pruritus, PN may be initiated by sensory neuron dysfunction, resulting in itch that is triggered independently of a pruritogen stimulating the neuron that can cause secondary fibrotic papulonodular lesions as a result of mechanical disruption of the skin from vigorous scratching.44
The itch-scratch cycle refers to a vicious cycle in which pruritus drives repeated scratching, which in turn further exacerbates pruritus either directly by mechanical sensitization of nerves or indirectly via release of trauma-induced inflammatory mediators. In this cycle, abnormal neuroimmune cross talk promotes a positive feedback loop that drives sensitization to pruritogens and itch amplification (Fig 3).11
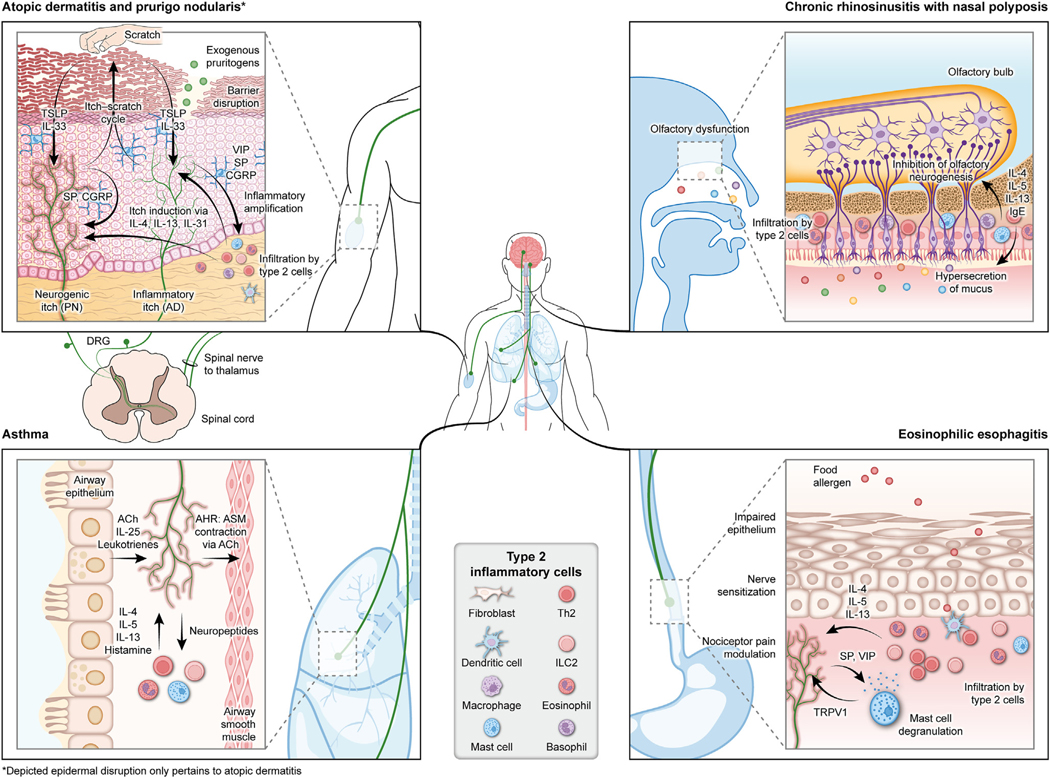
FIG 3.
Neuroimmune mechanisms underlying sensory pathologies in conditions associated with type 2 inflammation. *Depicted epidermal disruption pertains only to AD. ACh, Acetylcholine; ASM, airway smooth muscle.
Multiple and overlapping mechanisms can lead to itch, which can be acute or chronic. Triggers include exogenous pruritogens, endogenous pruritogens produced by keratinocytes and immune cells, and mechanical stimuli (eg, from light touch).93,94 In a genome-wide association study, exogenous itch from mosquito bites showed a strong association with cytokine pathway loci, including interferon regulatory factor 1 (IRF1), IL3, colonystimulating factor 2 (CSF2), and IL21, along with a weaker association with other loci, including IFN-γ–AS1 (IFNG-AS1) and signal transducer and activator of transcription (STAT) 6 (STAT6).95 In addition, acute itch is initiated primarily via histamine release from mast cells and basophils through degranulation following activation by allergens via IgE or via inflammatory mechanisms, as seen in acute urticaria and, less commonly, in chronic urticaria.48 Mechanical stimuli activate Piezo1 channels on itch sensory neurons. In mouse models, stimulation of Piezo1 evoked alloknesis (defined as an itch response evoked by light touching of skin, particularly in dry or aging skin).94 This suggests that itch-specific sensory neurons, which also express IL-4 receptor alpha (IL-4Rα),18 could be pathologically stimulated via type 2 neurogenic inflammation, and itch could be further amplified by excessive touch or scratching via the Piezo1 transducer. Nonhistaminergic mediators of acute itch have also been identified (leukotriene C4, oncostatin M [OSM], bovine adrenal medulla peptide 8–22 [BAM8–22], and SLIGRL-NH2).96–102 Additionally, many of these mediators are variably dependent on canonic transient receptor potential ankyrin 1 (TRPA1) and transient receptor potential vanilloid 1 (TRPV1).99–102 Further, a number of itch-mediating G protein–coupled receptors (GPCRs) have been identified in both mice and humans; they include the Mas-related family of GPCRs (eg, Mas-related family of G-protein-coupled receptor A3 [MrgprA3] and MrgprC11 in mice and Mas-related family of G protein-coupled receptor X1 [MRGPRX1] in humans).96–103
The IL-4– and IL-13–mediated Janus kinase (JAK) and STAT intracellular signaling pathways are critical for TH2 cell differentiation and type 2 inflammatory processes. Inhibition of JAK/STAT signaling can improve clinical symptoms of AD, including itch.37,41,104 Binding of IL-4 and IL-13 to IL-4Rα activates JAK1, JAK3, tyrosine kinase 2, and STAT6. These molecules play an important role in triggering type 2 inflammatory responses, including pruritic responses, as well as acute pruritic responses to stimuli such as insect bites.38,95,105–107 Importantly, STAT6 gain of function is associated with chronic atopic conditions in humans.106,108–110 Although exogenous itch such as that from insect bites has been associated with type 1 and type 2 cytokines, evidence suggests that chronic inflammatory itch is associated with the IL-4/IL-13/JAK1/STAT6 axis.95,105–107
Chronic itch in AD and PN is induced largely via non-histaminergic mediators. Type 2 inflammatory cytokines activate nonhistaminergic itch in multiple ways.11 In response to pruritic stimuli, scratching-induced skin stress or damage (eg, resulting from dry skin) can cause keratinocytes to release alarmins, including TSLP and IL-33, which can trigger pruritus by acting directly on sensory neurons.11,14–16 IL-33 may also play an indirect role in severe itch by initiating the IL-33/ST2/CGRP axis, which has been shown to promote interactions between type 2 inflammatory cells and somatosensory neurons to induce itch in allergic conjunctivitis.111 Notably, a clinical trial of the anti-TSLP mAb tezepelumab in patients with moderate-to-severe AD found little to no efficacy in itch (versus that with placebo), suggesting that TSLP may have only a limited role in pruritus in AD.17 Type 2 cytokines and related factors (such as OSM) may have a prominent role in chronic pruritus and sensitization to pruritogens. Both IL-4 and IL-31 are implicated in the transmission of the pruritic sensation in atopic diseases, with IL-4 promoting TH2 cell polarization, thereby contributing to increased IL-31 expression levels observed in both AD and PN lesional skin.9 When compared with their expression in samples from nonatopic patients, expression of IL-4 and IL-13 (both of which bind to IL-4Rα) is increased in patients with AD lesions.112 A subset of neurons coexpress neuropeptides and receptors for type 2 cytokines, including IL-4Rα and IL-31 receptor A (IL-31RA)18; for example, some sensory neurons in skin coexpress IL-31RA, TRPV1, and TRPA1 in the DRGs.19 These cation channels mediate the release of neuropeptides such as CGRP and SP and are essential for the itch-inducing effects of IL-31, thus providing a direct link between type 2 inflammatory cytokines and neurogenic inflammation.19 IL-4Rα stimulation activates sensory neurons and enhances their response to pruritogens (such as IL-31 and histamine) via transient receptor potential channels and JAK1 pathways.11,18,84,97 In human sensory neurons, exposure to type 2 cytokines (eg, IL-4, IL-13, IL-25, IL-31, IL-33, TSLP) increases sensory neuron excitability, amplifies the neuronal response to pruritogens, and alters activity of downstream gene transcription factors related to AD and itch (including TRPV1 and TPRA1).18,113,114 OSM, which shares a coreceptor with IL-31, also promotes sensitization of itch-specific neurons and is correlated with itch intensity.49,97
The release of neuropeptides from sensory neurons in the skin in response to mechanical or chemical stimuli produces multiple proinflammatory effects, including release of proinflammatory cytokines and neuropeptides from skin cells, and activation of a variety of immune cells.10–12,48 Mast cells are particularly well positioned to act as a link between the nervous and immune systems, as they are often found near afferent nerve fibers.7,8 Many neuropeptides have direct effects on mast cells, promoting mast cell degranulation and the production of type 2 cytokines and chemokines.7 Peptides, including SP, proadrenomedullin peptide, VIP, CGRP, and pituitary adenylate cyclase-activating polypeptide, have been implicated as ligands for MRGPRX2, a key receptor that mediates neuropeptide activation of mast cells.115–118 Basophils can also interact with sensory neurons, mediating allergen-induced itch in the context of AD-associated inflammation.13 Interestingly, basophils have been reported to express MRGPRX2, demonstrating the diversity of ways in which granulocytes engage in a bidirectional positive neuroimmune feedback loop that may facilitate and maintain states of pathologic sensation and chronic inflammation.119
In AD, plasma SP concentrations are linked to pruritus intensity, and in PN, lesional skin demonstrates an elevated density of SP- and CGRP-immunoreactive nerve fibers.42,43,45–47 VIP concentrations are also higher in patients with AD than in individuals without AD.120 Proteases derived from house dust mites are common allergens that trigger the release of SP from TRPV1-expressing sensory neurons, which activates mast cells via MrgprB2 in mice and recruits dendritic cells that prime type 2 differentiation.61,80 In addition, scratching may stimulate the release of neuropeptides, including CGRP, nerve growth factor, and VIP.11,84 Taken together, these findings suggest that allergens and other environmental stimuli may directly stimulate both itch and neurogenic inflammation via neuropeptides such as SP.
The extracellular matrix protein periostin is overexpressed in the skin of patients with AD and may also contribute to itch. TSLP induces periostin secretion in keratinocytes, which promotes JAK/STAT signaling and TH2 cell immunity; it also interacts with integrins to promote immune cell activity and directly stimulate nerve fibers. In PN, periostin expression was reported to be correlated with itch and was independent of IL-31 expression, indicating an independent pathway of PN-related itch.121
Although scratching can temporarily relieve pruritus by activating fibers that block itch sensation in the spine, repeated scratching can cause mechanical disruption of the skin, leading to the aforementioned neural, immunologic, and epithelial changes that further exacerbate pruritus. In normal skin, scratching relieves itch by activating Piezo2 ion channels on Merkel cells, which simulate Ab fibers to reduce itch. In dry skin, the same signaling pathway can activate MRGPRA3-positive pruriceptors that exacerbate itch and drive the itch-scratch cycle.122,123 In addition to miswiring in the periphery, scratching also activates reward regions of the brain. Structural and functional changes have been observed in the brain’s motor and reward regions in patients with chronic pruritus, suggesting that the itch-scratch cycle may be perpetuated at the level of the central nervous system.124 An active area of exploration is the inflammatory cross talk between the periphery and the brain and the way in which inflammatory circuits within peripheral tissue can imprint durable alterations within the nervous system to drive pathologic sensation and neuroinflammation.
The physiologic mechanisms of itch can change with age, which may explain differences in pruritus across a patient’s lifetime. Merkel cells in the skin suppress mechanical itch in pruriceptive C neurons via cutaneous Piezo2 activation; these cells decrease in number with age.122,123,125 In addition, MRGPRA3-positive pruriceptors become more closely associated with Merkel cells in chronic itch models.122,123 An increase in alloknesis arising from a hypersensitive itch response, which is often seen in dry or aging skin, is correlated with the loss of Merkel cells with age.122,123 Similar observations in the oral mucosa may explain loss of ability to effectively chew and swallow with age.126 In addition, immunosenescence is a proinflammatory phenomenon that may, in some cases, be manifested as a type 2 imbalance.127–130 These findings may help explain the higher prevalence of PN in older adults and patients with other chronic pruritic conditions.131,132
Two mAbs, dupilumab and tralokinumab, have been approved for the treatment of AD in several countries,133–135 whereas a third mAb, nemolizumab, has been approved in Japan for treatment of itch associated with AD when other treatments are ineffective.136 In addition, on the basis of the results from 2 phase 3 trials, dupilumab was recently approved by the US Food and Drug Administration and the European Medicines Agency for treatment of PN.133–135,137 Dupilumab is a fully human VelocImmune-derived mAb that blocks IL-4Rα, preventing binding of IL-4 and IL-13.51,138 Multiple randomized placebo-controlled clinical trials of dupilumab (with or without concomitant topical corticosteroids) in adults, adolescents, children, and infants with moderate-to-severe AD have demonstrated that dupilumab significantly improves clinical signs and neuroimmune inflammation-driven symptoms (including itch), as well as other patient-reported outcomes.20–26,139 Tralokinumab and lebrikizumab both bind to IL-13 and have been shown to improve signs and symptoms of moderate-to-severe AD.27–33 Nemolizumab, an anti-IL-31 receptor 1 mAb, has demonstrated activity in AD and PN.34,36,50,136 JAK inhibitors (JAKIs), such as abrocitinib, baricitinib, and upadacitinib, are also approved and have demonstrated efficacy in AD.37–41
Airway type 2 inflammation, hyperresponsiveness, and reduced expiratory volume in asthma
Asthma is a chronic respiratory disease characterized by airflow obstruction, increased mucus production, airway inflammation, and hyperresponsiveness. The pathophysiology of asthma is multifactorial, and multiple types of immune cells have been implicated in its development. Type 2 inflammation plays a critical role in asthma, as IL-4, IL-5, and IL-13 are key drivers of this atopic disease.1,2,140–142 These cytokines promote airway obstruction and tissue remodeling, including smooth muscle contraction, basement membrane thickening, eosinophilia, goblet cell hyperplasia, and mucus production.2,140,142–144 IL-4 and IL-13 induce production of eotaxin, which cooperates with IL-5 in promoting eosinophilia in the respiratory epithelium.2,140 IL-5 is a potent regulator of eosinophil growth, differentiation, migration and survival, and it plays a key role in asthma—particularly the eosinophilic phenotype.1,142,145,146 IL-31 may also contribute to the production of type 2 inflammatory factors and recruitment of immune cells in the lung.147
Similar to the bidirectional neuroimmune cross talk observed with the itch-scratch cycle in AD and PN, reciprocal neuroimmune interactions may perpetuate airway inflammation and sensory pathologies in asthma (Fig 3).148 Sensory neurons of the vagal ganglia densely innervate the conducting airways of the lung; those of the nodose ganglia can extend into the alveolar region.149,150 Very few DRG neurons innervate the lung; those that do project only to extrapulmonary large airways.148–150 Inhaled ovalbumin, a commonly used allergen in animal models of asthma, as well as type 2 inflammatory mediators such as IL-5, can directly activate calcium signaling in cultured nodose neurons.75 In vivo genetic ablation or chemical inactivation of voltage-gated sodium channel 1.8–expressing vagal nociceptor neurons has been shown to blunt allergen-induced increase in immune cells.75 However, in a separate study, in vivo genetic ablation of largely overlapping TRPV1-expressing vagal nociceptor neurons did not affect allergen-induced increases in immune cells.151 Thus, whether vagal sensory neurons are required for regulating allergen-induced TH2 cell airway immune responses remains unclear.
Neuropeptide release from sensory neurons may have a role in asthma. Levels of SP are increased in induced sputum and correlated with airway obstruction, and CGRP level is elevated in bronchoalveolar lavage fluid.152,153 Neuropeptide release by sensory neurons positive for TRPA1 or TRPV1, which are markers of nociceptors, may play a particularly important role in this setting.154,155 For example, in cultured vagal ganglia nociceptive neurons, IL-5 stimulates the release of neuropeptides, such as VIP, that stimulate CD4-positive T cells and ILC2s, which in turn release type 2 cytokines such as IL-5, thereby amplifying allergic inflammation. In contrast, silencing these neurons ameliorates airway inflammation.75 In addition, cholinergic neurons in the gut and lung release neuromedin U, which promotes ILC2 response.59,60,156 Recent studies have suggested that some lung mast cells may also express MRGPRX2, the expression of which is upregulated in patients with asthma and activated by neuropeptide hemokinin-1.157–159 How sensory neurons and autonomic neurons interact to regulate inflammation remains poorly understood, despite the availability of many therapies targeting adrenergic and cholinergic pathways in the airway. For example, CGRP and neuromedin U have divergent effects, demonstrating the complexity of the mechanisms by which neuropeptides regulate type 2 inflammation.160,161 The central nervous system has been shown to exert systemic anti-inflammatory effects via the vagus nerve by inhibiting splenic macrophages in the cholinergic anti-inflammatory pathway, and some evidence suggests that manipulation of the vagus nerve (with cholinergic agonists or vagal nerve stimulation) may modulate lung inflammation.162
Chronic respiratory inflammation can sensitize nerve fibers that innervate the airways, resulting in smooth muscle hypercontraction and airway constriction, ultimately resulting in airway hyperresponsiveness (AHR). AHR is correlated with the severity of asthma and is a cardinal feature associated with the degree of treatment necessary to control symptoms.148 In the human airway, mast cell numbers within the airway smooth muscle bundle are correlated with the severity of AHR.57 A murine model devoid of mast cells shows blunted AHR.58 Eosinophils also play a role in bronchoconstriction and are recruited by eotaxin to cluster at airway nerves; preclinical models suggest that IL-4 and IL-13 increase expression of eotaxin in parasympathetic neurons.53 Additional studies demonstrate a similar effect of both IL-4 and IL-13 in inducing smooth muscle hyperresponsiveness in explanted human lung tissue.54–56 IL-13 has also been shown to upregulate periostin in airway epithelium.163
Sympathetic and parasympathetic neurons (and their reciprocal interactions with sensory neurons and immune cells) contribute to the clinical manifestations of asthma.46 Sympathetic neurons release the neurotransmitter noradrenaline, which induces airway smooth muscle relaxation via β2-adrenergic receptors (β2Ars) on smooth muscle cells.46 β2AR agonists, which are often used as bronchodilators for the treatment of asthma, may influence immune cells. For example, β2AR agonists reduce IL-33–induced airway inflammation and suppress ILC2 proliferation.46,62 Immune cells also influence noradrenergic function: mast cell– derived transforming growth factor β triggers phosphorylation of β2AR on smooth muscle cells, which reduces the response to albuterol, a β2AR agonist.164 Finally, eosinophils increase sensory nerve responsiveness and density in the airway and release mediators that antagonize inhibitory M2 muscarinic receptors on parasympathetic nerves. The effects of eosinophils on both sensory and parasympathetic nerves potentiate bronchoconstriction.165
Rare sensory cell types in the lung epithelium also play important roles in sensing allergens and in asthma pathogenesis.166,167 Pulmonary neuroendocrine cells are activated by allergens to increase their production of neuropeptides, such as CGRP, and neurotransmitters, such as γ-aminobutyric acid. These cells are essential for amplifying allergen-induced responses, including activation of ILC2s, recruitment of TH2 immune cells, and goblet cell metaplasia.63 Similarly, tuft cells can detect allergens and release neurotransmitters (such as acetylcholine), alarmins (such as IL-25), and inflammatory mediators (such as leukotrienes) that can contribute to allergic inflammation in the airways.168,169 Tuft cells also promote respiratory reflexes such as sneezing and coughing.167,169
Six biologics have been approved for treatment of asthma: omalizumab, mepolizumab, reslizumab, benralizumab, tezepelumab, and dupilumab.170,171 Omalizumab is a humanized mAb directed against IgE.170,171 In multiple clinical trials, omalizumab significantly improved signs and symptoms of asthma; for example, it reduced exacerbation rates, improved lung function and symptoms of asthma, and reduced use of inhaled corticosteroids (vs oral corticosteroids).171 Mepolizumab, reslizumab, and benralizumab inhibit the IL-5 pathway (a key regulator of eosinophils) by targeting either IL-5 (mepolizumab and reslizumab) or the IL-5 receptor (benralizumab).172 All 3 were well tolerated and reduced asthma exacerbations in clinical trials in adults and adolescents with severe eosinophilic asthma that was otherwise inadequately controlled with standard therapies.172 Tezepelumab, which targets the epithelial cell–derived cytokine TSLP, reduced asthma exacerbation rates in a phase 3 trial,173 and in a long-term, randomized, placebo-controlled extension study, it continued to reduce exacerbation rates for up to 104 weeks.174 Dupilumab, which inhibits IL-4 and IL-13 signaling, reduced asthma exacerbations, improved lung function, and reduced oral corticosteroid use in clinical trials in patients with moderate-to-severe asthma—particularly in patients with elevated levels of eosinophils and/or fractional exhaled nitric oxide.175–177 Given the neuroimmune effects of eosinophils in asthma, it is possible that blockade of IL-4, IL-5, IL-13, and TSLP may alter these effects, thereby addressing some of the underlying pathophysiologic mechanisms of asthma.
These therapeutics reveal important differences in the tissue-specific effects of type 2 cytokines. Although anti–IL-5 therapies and tezepelumab have been successful and approved for use in asthma, they have not been successful in AD. Therapies specifically targeting IL-13 alone have advanced in AD but have failed in asthma. However, dual blockade of IL-4 and IL-13 has been successful in both diseases. Notably, although the IL-5 receptor has been reported in the nodose ganglia of the lung,75 the DRGs for the skin lack this receptor.18
Loss of smell in CRSwNP
CRSwNP is a chronic inflammatory disease of the nasal mucosa and paranasal sinuses. Clinical manifestations include nasal congestion, rhinorrhea/postnasal drip, facial pain or headache, and impaired sense of smell. Notably, pruritus often accompanies CRSwNP, and increased levels of periostin have been found in the nasal mucosa of patients with CRSwNP.121 Levels of IL-31 are also increased in patients with allergic rhinitis (AR); in this setting, IL-31 is induced by IL-4 and promotes type 2 inflammation.50 Olfactory dysfunction in CRSwNP is common and negatively affects quality of life.178,179 The mechanisms underlying olfactory dysfunction in CRSwNP are multifaceted, involving changes to the olfactory mucosa following (and related to) the type 2 inflammatory response, disruption of synaptic transmission resulting from neuroepithelial edema, and decreased airflow in the olfactory cleft (Fig 3).180,181 Nasal obstruction may contribute to olfactory dysfunction, but it is unlikely to be the primary driver because the sense of smell does not always return after polypectomy.182,183 However, the extent of inflammation in the neuroepithelium is correlated with the severity of olfactory dysfunction.184
CRSwNP has a prominent type 2 inflammatory signature.185 Studies of mucosal tissue in patients with nasal polyps (and other studies using a mouse model of chronic rhinosinusitis) have demonstrated elevated numbers and increased activation of T cells; infiltration of nasal polyps by eosinophils, basophils, and mast cells; and type 2 polarization with elevated levels of IL-4, IL-5, IL-13, and IgE.65,185–187 Release of inflammatory mediators triggers hypersecretion of olfactory mucus, resulting in changes in ion concentration that can affect the activity of olfactory neurons.187 In a mouse model of chronic rhinosinusitis, chronic type 2 inflammation reduced the number of immature neurons, suggesting that chronic inflammation may inhibit olfactory sensory neurogenesis, rendering the olfactory mucosa less able to recover from injury.65 In another mouse study, IL-4Rα was expressed in olfactory sensory neurons; however, although both IL-4 and IL-13 increased calcium uptake (indicating activation) in these neurons, mice developed anosmia following intranasal administration of IL-4, but not IL-13.64
Transient receptor potential channels such as TRPA1, TRPV1, transient receptor potential vanilloid 4 (TRPV4), transient receptor potential cation channel subfamily M member 4 (TRPM4), and transient receptor potential cation channel subfamily M member 8 (TRPM8) are expressed in the upper airway in epithelial cells, sensory neurons, T cells, and mast cells.66 These channels respond to physical stimuli (eg, changes in temperature) and chemical stimuli, and they are associated with type 2 inflammatory processes in the upper airway.66 For example, desensitization or ablation of TRPV1-positive sensory fibers in mice reduced allergy-associated and chemically induced sneezing, which were mediated by neuropeptides such as neuromedin B.67 In another mouse model of AR, knockout or inhibition of TRPV1 suppressed TH2/TH17 cytokine production, eosinophil infiltration, and IgE in nasal mucosa.68 Similarly, patients with AR had higher numbers of TRPV1-positive cells in the nasal mucosa than healthy controls did.68
Recent studies suggest that biologics may improve the olfactory changes associated with CRSwNP. Currently, 3 biologics are approved for CRSwNP: omalizumab, mepolizumab, and dupilumab. Omalizumab improved clinical and endoscopic aspects of CRSwNP, including sense of smell.188,189 Mepolizumab improved nasal obstruction in a phase 3 trial, with modest improvement in sense of smell.190 Dupilumab reduced polyp size, reduced symptom severity, improved sense of smell and quality of life, and was well tolerated in 3 phase 3 trials of adults with CRSwNP.190–193 Notably, improvement in sense of smell was rapid in patients treated with dupilumab.191–193 IL-4Rα is present on olfactory sensory neurons64; however, further research is needed to elucidate the relationship between this receptor and the neuroimmune mechanisms underlying such swift restoration of olfaction.
Dysphagia in EoE
EoE is a chronic inflammatory disease of the esophagus. Primary symptoms include dysphagia and food impaction in adults and failure to thrive, feeding difficulties, and vomiting in infants and children.194,195 These symptoms are consistent with dysregulated neural control of the esophagus and inflammation-driven tissue remodeling.69,70,196 EoE is characterized by a type 2 inflammatory response (Fig 3). Type 2 inflammation–derived nociceptor sensitization of the esophagus leads to pain, motor dysfunction and eventually tissue remodeling.69,70 Following exposure to food-related antigens, esophageal epithelia secrete TSLP and IL-33, causing TH2 cells to release IL-4, IL-5, and IL-13. The presence of IL-4 and IL-13 induce dilated intracellular spaces and basal cell hyperplasia in the esophageal epithelia, whereas IL-5 and eotaxin-3 promote infiltration by eosinophils and possibly by mast cells and basophils.70,195,197 However, some experts suggest that dysphagia is mostly related to tissue remodeling.198–200
Increased infiltration of eosinophils and mast cells in esophageal tissue may increase vagal sensory neuronal responsiveness to acid, promoting barrier dysfunction and impaired epithelial permeability.71,72 Increased epithelial permeability may enhance the ability of luminal acid to stimulate action potential discharge in nociceptive afferent terminals. Type 2 cytokines can also cause hypercontractility of gastrointestinal smooth muscle cells via signal transducer and activator of transcription 6 (STAT6) or mitogen-activated protein kinase (MAPK) signaling pathways, which may contribute to dysphagia.71
TRPV1 and mast cells may modulate pain in EoE. In a study in patients with EoE with pain, pain was positively associated with molecular expression of TRPV1, carboxypeptidase A3, and hematopoietic prostaglandin D synthase but not eosinophilia; mast cells colocalized with neurons, which is suggestive of mast cell specificity.73 Neuropeptides such as SP and VIP promote mast cell degranulation, the production of type 2 cytokines and chemokines, and a resultant immune cascade.7 Because TRPV1-positive sensory neurons are prominent within the vagus nerve and have itch-specific transcriptional identity at the single-cell level,201 symptoms of discomfort and irritation associated with EoE may be defined by molecular and cellular circuits that resemble itch mechanisms rather than traditional pain-associated pathways.
In clinical studies, agents targeting the IL-5 pathway (mepolizumab and reslizumab) have been shown to reduce eosinophil numbers in esophageal tissue but had minimal impact on symptoms.202–204 Similarly, lirentelimab, which targets sialic acid–binding immunoglobulin-like lectin 8 (Siglec-8), reduced eosinophil counts but failed to improve symptoms in patients with EoE.205 Agents targeting IL-13 have produced positive results; they include the anti–IL-13 mAbs QAX576 and RPC4046206,207 and dupilumab, which inhibits both IL-4 and IL-13 signaling.208,209 Dupilumab is the first US Food and Drug Administration–approved drug for the treatment of EoE. In clinical trials in patients with EoE, dupilumab reduced peak intraepithelial eosinophil counts in esophageal tissues and improved clinical, histologic, and endoscopic scores.208,209
Thus, the success of therapeutics targeting the type 2 inflammation spectrum is highly dependent on tissue-specific mechanisms. Future studies will be required to determine how epithelial, stromal, and neural pathways, influenced by type 2 cytokines, imprint unique responses within specific tissues.
DISCUSSION
Multiple links connect neuroimmune cross talk, inflammatory pathways, and sensory pathologies across type 2 inflammatory diseases. Type 2 inflammation promotes alterations in neural and immune effectors and their interactions, leading to both sensory and autonomic dysfunction in AD, PN, asthma, CRSwNP, and EoE. Reciprocal neuroimmune interactions may perpetuate inflammation and sensory pathology in these diseases. For example, in AD, strong evidence supports a role for type 2 inflammation in a positive feedback loop of sustained neural and immunologic changes that drive sensory and behavioral pathology (ie, the itch-scratch cycle), which may contribute to the chronic nature of AD. In contrast, PN exemplifies how a potentially neurogenic process can also be linked to type 2 inflammation and drive inflammatory and fibrotic pathology in the skin. Neuroimmune changes have been well characterized in AD and asthma, but such changes are less well characterized in PN, CRSwNP, and EoE. Interestingly, studies in multiple type 2 inflammatory diseases have demonstrated that mast cells colocalize with afferent nerve terminals in epithelial tissues and sensitize nociceptive C-fibers.7,8,48,57,65,69,73,164,186 As such, these cells may constitute a shared element in type 2 inflammatory diseases at different epithelial surfaces and neuroimmune manifestations. Further research is needed to better understand whether similar feedback loops also occur in other organs susceptible to type 2 inflammatory manifestations.
Traditional systemic treatments for type 2 inflammatory diseases include broad-acting immunosuppressants (such as corticosteroids, cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil); however, their use is limited by safety concerns and a heightened risk of infection.210–214 Further, these broadly immunosuppressive agents do not target neural pathways and do not demonstrate the efficacy seen with more targeted therapeutics. Recent approaches to selective suppression of type 2 inflammation include biologics that target cytokines or their receptors, including dupilumab, lebrikizumab, nemolizumab, tralokinumab, omalizumab, mepolizumab, reslizumab, benralizumab, tezepelumab, and vixarelimab,17,20–36,52,133,134,137,139,170–177,202–209,215–217 as well as JAKIs that target the intracellular JAK/STAT signaling pathways, including abrocitinib, baricitinib, and upadacitinib.37–41 In AD, improvement in pruritus observed with the IL-13 inhibitors tralokinumab27–30 and lebrikizumab31–34 suggests potential modulation of underlying neuroimmune pathology by IL-13 inhibition. In AD and PN, improvements in pruritus with dupilumab20–26,133,134,137,139,215,217 suggest that inhibiting signaling of both IL-4 and IL-13 through IL-4Rα blockade could control multiple manifestations of aberrant neuroimmune activity in the broader context of type 2 inflammation, including IgE-mediated acute itch flares.13,218,219 JAKI treatment has also been shown to improve pruritus in patients with AD, demonstrating the importance of the JAK/STAT pathways in modulating type 2 inflammatory effects on pruritus.37–41 In asthma, the complex interactions among immune cells, type 2 inflammatory cytokines, and sensory neurons indicate that biologics suppressing type 2 inflammation could have neuroimmune effects that improve airway function.170–177 In patients with CRSwNP, improvements in the sense of smell following treatment with biologics (omalizumab, mepolizumab, and dupilumab)188–193 suggest that biologic therapy may have direct neuroimmune effects on olfactory sensory neurons that go beyond simple reductions in nasal obstruction and may offer improvements that are not often achieved with surgery. Lastly, EoE is a type 2 inflammatory disease, and key symptoms of dysphagia and pain suggest a neurologic component. In EoE, persistence of symptoms despite histologic remission suggests that changes to sensory neurologic pathways may also persist and may be epigenetically mediated. The fact that symptom improvement in patients with EoE has been observed in clinical trials of dupilumab,208,209 but not with other biologics (such as IL-5 and sialic acid–binding immunoglobulin-like lectin 8 [Siglec-8] inhibitors),203–205 provides insight into neuroimmune factors that are particularly relevant to EoE symptoms. Additional biologics have been developed, or are under investigation, for the treatment of sensory pathologies in atopic diseases. These biologics and their targets are shown in Fig 4.220
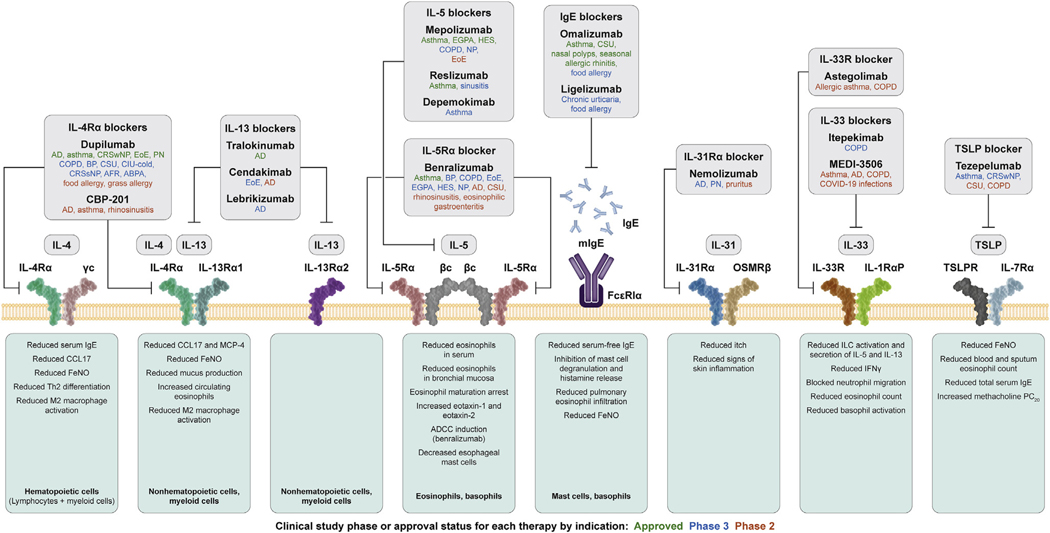
FIG 4.
Biologics that inhibit type 2 inflammation. βc, β-chain; γc, γ-chain; ABPA, allergic bronchopulmonary aspergillosis; ADCC, antibody-dependent cellular cytotoxicity; AFR, allergic fungal rhinosinusitis; BP, bullous pemphigoid; CCL17, C-C motif chemokine ligand 17; CIU, chronic idiopathic urticaria; COPD, chronic obstructive pulmonary disorder; CRSsNP, chronic rhinosinusitis without nasal polyps; CSU, chronic spontaneous urticaria; EGPA, eosinophilic granulomatosis with polyangiitis; FENO, fractional exhaled nitric oxide; HES, hypereosinophilic syndrome; IL-1RαP, IL-1 receptor-α P; IL-5Rα, IL-5 receptor-α; IL-13Rα1/2, IL-13 receptor-α1/2; IL-31RA, IL-31 receptor A; IL-33R, IL-33 receptor; ILC, innate lymphoid cell; MCP-4, monocyte chemoattractant protein-4; mIgE, membrane IgE; NP, nasal polyps; OSMRβ, oncostatin-M specific receptor subunit beta; PC20, provocative concentration causing a 20% drop in forced expiratory volume in 1 second from baseline; TSLPR, thymic stromal lymphopoietin receptor. Figure adapted with permission from Haddad EB et al.220
Further research is needed to better understand the neuroimmune mechanisms underlying chronic, sustained inflammation and its related sensory pathologies in diseases associated with type 2 inflammation, which will undoubtedly inform the development of new treatment strategies and treatment selection for patients with these diseases.
DISCLOSURE STATEMENT
Sponsored by Sanofi and Regeneron Pharmaceuticals Inc.
Abbreviations used
- AD
Atopic dermatitis
- AHR
Airway hyperresponsiveness
- AR
Allergic rhinitis
- β2AR
β2-adrenergic receptor
- CGRP
Calcitonin gene–related peptide
- CRSwNP
Chronic rhinosinusitis with nasal polyps
- DRG
Dorsal root ganglion
- EoE
Eosinophilic esophagitis
- IL-4Rα
IL-4 receptor alpha
- IL-31RA
IL-31 receptor A
- ILC2
Group 2 innate lymphoid cell
- JAK
Janus kinase
- JAKI
JAK inhibitor
- MrgprA
Mas-related family of G-protein-coupled receptor
- MrgprA3
Mas-related family of G-protein-coupled receptor 3
- OSM
Oncostatin M
- PN
Prurigo nodularis
- SP
Substance P
- STAT
Signal transducer and activator of transcription
- TRPA1
Transient receptor potential ankyrin 1
- TRPV1
Transient receptor potential vanilloid 1
- TSLP
Thymic stromal lymphopoietin
- VIP
Vasoactive intestinal peptide
Footnotes
Disclosure of potential conflict of interest:
B. S. Kim is founder of Klirna Biotech; in addition, he has served as a consultant for 23andMe, AbbVie, Almirall, Amagma, Arcutis Biotherapeutics, Arena Pharmaceuticals, Argenx, AstraZeneca, Bellus Health, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Clexio Biosciences, Cymabay Therapeutics, Daewoong Pharmaceutical, Eli Lilly, Escient Pharmaceuticals, Evommune, FIDE, Galderma, Genentech, GSK, Granular Therapeutics, IQVIA, Incyte, Innovaderm Research, Janssen, Kiniksa, LEO Pharma, Maruho, Medicxi, Menlo Therapeutics, Novartis, OM Pharma, Pfizer, Recens Medical, Regeneron Pharmaceuticals Inc, Sanofi, Septerna, Shaperon, Third Harmonic Bio, Vial, and WebMD; he has stock in Abrax Japan, KliRNA Biotech, Locus Biosciences, and RecensMedical; and he holds a patent for the use of JAK1 inhibitors for chronic pruritus. M. E. Rothenberg is a consultant for Allakos, AstraZeneca, Bristol Myers Squibb, Celldex Therapeutics, ClostraBio, Ellodi Pharma, GSK, Guidepoint, Nextstone One, PulmOne, Revolo Biotherapeutics, Sanofi-Regeneron Pharmaceuticals Inc, Serpin Pharma, and Spoon Guru; in addition, he has an equity interest in the first 7 of the aforementioned companies; receives royalties from reslizumab (Teva Pharmaceuticals), PEESSv2 (Mapi Research Trust), and UpToDate; and is an inventor of patents owned by Cincinnati Children’s Hospital. C. Bachert is a consultant for GSK, Mensarini, Novartis, and Sanofi. D. Artis has contributed to scientific advisory boards at FARE, the KRF, Pfizer, and Takeda; research in the Artis laboratory is supported by the National Institutes of Health (grants DK126871, AI151599, AI095466, AI095608, AI142213, AR070116, AI172027, and DK132244), the Crohn’s and Colitis Foundation, Cure for IBD, the Jill Roberts Institute, the Sanders Family, and the Rosanne H. Silbermann Foundation. R. Zaheer and P. Rowe are employees of and may hold stock and/or stock options in Sanofi. Y. Deniz and S. Cyr are employees and shareholders at Regeneron Pharmaceuticals Inc. The remaining author declares that he has no relevant conflicts of interest.
For a video abstract, see Video E1 in the Online Repository at www.jacionline.org. Medical writing and editorial assistance was provided by Jaqui Hodgkinson, DPhil, MBA, and Vicki Schwartz, PhD, of Excerpta Medica, and was funded by Sanofi and Regeneron Pharmaceuticals Inc in accordance with the Good Publication Practice guideline.
REFERENCES
- 1.Gandhi NA, Bennett BL, Graham NMH, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov 2016;15:35–50. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi NA, Pirozzi G, Graham NM. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol 2017;13:425–37. [DOI] [PubMed] [Google Scholar]
- 3.Kopp EB, Agaronyan K, Licona-Limon I, Nish SA, Medzhitov R. Modes of type 2 immune response initiation. Immunity 2023;56:667–94. [DOI] [PubMed] [Google Scholar]
- 4.Molofsky AB, Locksley RM. The ins and outs of innate and adaptive type 2 immunity. Immunity 2023;56:704–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton JD, Harel S, Swanson BN, Brian W, Chen Z, Rice MS, et al. Dupilumab suppresses type 2 inflammatory biomarkers across multiple atopic, allergic diseases. Clin Exp Allergy 2021;51:915–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Floc’h A, Allinne J, Nagashima K, Scott G, Birchard D, Asrat S, et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy 2020;75:1188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008;123:398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang Y, Marcusson JA, Jacobi HH, Haak-Frendscho M, Johansson O. Histamine-containing mast cells and their relationship to NGFr-immunoreactive nerves in prurigo nodularis: a reappraisal. J Cutan Pathol 1998;25:189–98. [DOI] [PubMed] [Google Scholar]
- 9.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol 2006;117:411–7. [DOI] [PubMed] [Google Scholar]
- 10.Tominaga M, Takamori K. Peripheral itch sensitization in atopic dermatitis. Allergol Int 2022;71:265–77. [DOI] [PubMed] [Google Scholar]
- 11.Garcovich S, Maurelli M, Gisondi P, Peris K, Yosipovitch G, Girolomoni G. Pruritus as a distinctive feature of type 2 inflammation. Vaccines (Basel) 2021;9:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YJ, Granstein RD. Roles of calcitonin gene-related peptide in the skin, and other physiological and pathophysiological functions. Brain Behav Immun Health 2021;18:100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F, Trier AM, Li F, Kim S, Chen Z, Chai JN, et al. A basophil-neuronal axis promotes itch. Cell 2021;184:422–40.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu B, Tai Y, Achanta S, Kaelberer MM, Caceres AI, Shao X, et al. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc Natl Acad Sci U S A 2016;113:E7572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013;155:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trier AM, Mack MR, Fredman A, Tamari M, Ver Heul AM, Zhao Y, et al. IL-33 signaling in sensory neurons promoted dry skin itch. J Allergy Clin Immunol 2022;149:1473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson EL, Parnes JR, She D, Crouch S, Rees W, Mo M, et al. Tezepelumab, an anti–thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: a randomized phase 2a clinical trial. J Am Acad Dermatol 2019;80:1013–21. [DOI] [PubMed] [Google Scholar]
- 18.Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 2017;171:217–28.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cevikbas F, Wang X, Akiyama A, Kempkes C, Savinko T, Antal A, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol 2014;133:448–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomized, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017;389:2287–303. [DOI] [PubMed] [Google Scholar]
- 21.Silverberg JI, Simpson EL, Ardeleanu M, Thaçi D, Barbarot S, Bagel J, et al. Dupilumab provides important clinical benefits to patients with atopic dermatitis who do not achieve clear or almost clear skin according to the Investigator’s Global Assessment: a pooled analysis of data from two phase III trials. Br J Dermatol 2019;181:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016;375:2335–48. [DOI] [PubMed] [Google Scholar]
- 23.Thaçi D, Simpson EL, Deleuran M, Kataoka Y, Chen Z, Gadkari A, et al. Efficacy and safety of dupilumab monotherapy in adults with moderate-to-severe atopic dermatitis: a pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2). J Dermatol Sci 2019;94:266–75. [DOI] [PubMed] [Google Scholar]
- 24.Simpson EL, Paller AS, Siegfried EC, Boguniewicz M, Sher L, Gooderham MJ, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol 2020;156:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paller AS, Siegfried EC, Thaçi D, Wollenberg A, Cork MJ, Arkwright PD, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol 2020;83:1282–93. [DOI] [PubMed] [Google Scholar]
- 26.Paller AS, Simpson EL, Siegfried EC, Cork MJ, Wollenberg A, Arkwright PD, et al. Dupilumab in children ages 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2022;400:908–19. [DOI] [PubMed] [Google Scholar]
- 27.Wollenberg A, Blauvelt A, Guttman-Yassky E, Worm M, Lynde C, Lacour JP, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol 2021;184:437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wollenberg A, Howell MD, Guttman-Yassky E, Silverberg JI, Kell C, Ranade K, et al. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J. Allergy Clin. Immunol 2019;143:135–41. [DOI] [PubMed] [Google Scholar]
- 29.Gutermuth J, Pink AE, Worm M, Soldbro L, Bjerregård Øland C, Weidinger S. Tralokinumab plus topical corticosteroids in adults with severe atopic dermatitis and inadequate response to or intolerance of ciclosporin A: a placebo-controlled, randomized, phase III clinical trial (ECZTRA 7). Br J Dermatol 2022;186:440–52. [DOI] [PubMed] [Google Scholar]
- 30.Silverberg JI, Toth D, Bieber T, Alexis AF, Elewski BE, Pink AE, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol 2021;184:450–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson EL, Carsten Flohr C, Eichenfield LF, Bieber T, Sofen H, Taïeb A, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol 2018;78:863–71.e11. [DOI] [PubMed] [Google Scholar]
- 32.Guttman-Yassky E, Blauvelt A, Eichenfield LF, Paller AS, Armstrong AW, Drew J, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis. A phase 2b randomized clinical trial. JAMA Dermatol 2020;156:411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silverberg JI, Guttman-Yassky E, Thaçi D, Irvine AD, Stein Gold L, Blauvelt A, et al. Two phase 3 trials of lebrikizumab for moderate-to-severe atopic dermatitis. N Engl J Med 2023;388:1080–91. [DOI] [PubMed] [Google Scholar]
- 34.Silverberg JI, Pinter A, Pulka G, Poulin Y, Bouaziz JD, Wollenberg A, et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J Allergy Clin Immunol 2020;145:173–82. [DOI] [PubMed] [Google Scholar]
- 35.Kabashima K, Matsumura T, Komazaki H, Kawashima M; Nemolizumab JP01 and JP02 Study Group. Nemolizumab plus topical agents in patients with atopic dermatitis (AD) and moderate-to-severe pruritus provide improvement in pruritus and signs of AD for up to 68 weeks: results from two phase III, long-term studies. Br J Dermatol 2022;186:642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ständer S, Yosipovitch G, Legat FJ, Lacour JP, Paul C, Narbutt J, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med 2020;382: 706–16. [DOI] [PubMed] [Google Scholar]
- 37.Kamata M, Tada Y. Optimal use of Jak inhibitors and biologics for atopic dermatitis on the basis of the current evidence. JID Innov 2023;3:100195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bieber T, Paller AS, Kabashima K, et al. Atopic dermatitis: pathomechanisms and lessons learned from novel systemic therapeutic options. JEADV 2022;36: 1432–49. [DOI] [PubMed] [Google Scholar]
- 39.Lee KP, Plante J, Korte JE, Elston DM. Oral Janus kinase inhibitors in the treatment of atopic dermatitis: A systematic review and meta-analysis. Skin Health Dis 2023;3:e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez-Roy Y, Ficheux A-S, Misery L, Brenaut. Efficacy of topical and systemic treatments for atopic dermatitis on pruritus: A systematic literature review and meta-analysis. Front Med 2022;9:1079323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang I-H, Chung W-H, Wu P-C, Chen C-B. JAK-STAT signaling pathway in the pathogenesis of atopic dermatitis: An updated review. Front Immunol 2022;13: 1068260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haas S, Capellino S, Phan NQ, Böhm M, Luger TA, Straub RH, et al. Low density of sympathetic nerve fibers relative to substance P-positive nerve fibers in lesional skin of chronic pruritus and prurigo nodularis. J Dermatol Sci 2010;58: 193–7. [DOI] [PubMed] [Google Scholar]
- 43.Liang Y, Jacobi HH, Reimert CM, Haak-Frendscho M, Marcusson JA, Johansson O. CGRP-immunoreactive nerves in prurigo nodularis—an exploration of neurogenic inflammation. J Cutan Pathol 2000;27:359–66. [DOI] [PubMed] [Google Scholar]
- 44.Williams KA, Huang AH, Belzberg M, Kwatra SG. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol 2020;83:1567–75. [DOI] [PubMed] [Google Scholar]
- 45.Molina FA, Burrows NP, Jones RR, Terenghi G, Polak JM. Increased sensory neuropeptides in nodular prurigo: a quantitative immunohistochemical analysis. Br J Dermatol 1992;127:344–51. [DOI] [PubMed] [Google Scholar]
- 46.Kabata H, Artis D. Neuro-immune crosstalk and allergic inflammation. J Clin Invest 2019;129:1475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teresiak-Miko1ajczak E, Czarnecka-Operacz M, Jenerowicz D, Silny W. Neurogenic markers of the inflammatory process in atopic dermatitis: relation to the severity and pruritus. Postepy Dermatol Alergol 2013;30:286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang TB, Kim BS. Clinical Review: Pruritus in allergy and immunology. J Allergy Clin Immunol 2019;144:353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hashimoto T, Nattkemper LA, Kim HS, et al. Itch intensity in prurigo nodularis is closely related to dermal interleukin-31, oncostatin M, IL-31 receptor alpha and oncostatin M receptor beta. Exp Dermatol 2021;30:804–10. [DOI] [PubMed] [Google Scholar]
- 50.Stott B, Lavender P, Lehmann S, Pennino D, Durham S, Schmidt-Weber CB. Human IL-31 is induced by IL-4 and promotes Th2-driven inflammation. J Allergy Clin Immunol 2013;132:446–54. [DOI] [PubMed] [Google Scholar]
- 51.Macdonald LE, Karow M, Stevens S, Auerbach W, Poueymirou WT, Jason Yasenchak J, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci U S A 2014;111:5147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ständer S, Yosipovitch G, Lacour JP, Legat FJ, Paul C, Reich A, et al. Nemolizumab efficacy in prurigo nodularis: onset of action on itch and sleep disturbances. J Eur Acad Dermatol Venereol 2022;36:1820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fryer AD, Stein LH, Nie Z, Curtis DE, Evans CM, Hodgson ST, et al. Neuronal eotaxin and the effects of CCR3 antagonist on airway hyperreactivity and M2 receptor dysfunction. J Clin Invest 2006;116:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998;282:2261–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wills-Karp J, Luyimbazi KJ, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science 1998;282: 2258–61. [DOI] [PubMed] [Google Scholar]
- 56.Manson ML, Jesper Säfholm J, James A, Johnsson A, Bergman P, Al-Ameri M, et al. IL-13 and IL-4, but not IL-5 nor IL-17A, induce hyperresponsiveness in isolated human small airways. J Allergy Clin Immunol 2020;145:808–17. [DOI] [PubMed] [Google Scholar]
- 57.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med 2002; 346:1699–705. [DOI] [PubMed] [Google Scholar]
- 58.Weigand LA, Myers AC, Meeker S, Undem BJ. Mast cell-cholinergic nerve interaction in mouse airways. J Physiol 2009;587:3355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klose CSN, Mahlakõiv T, Moeller JB, Rankin LC, Flamar AL, Kabata H, et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 2017;549:282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cardoso V, Chesné J, Ribeiro H, García-Cassani B, Carvalho T, Bouchery T, et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 2017;549:277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perner C, Flayer CH, Zhu X, Aderhold PA, Dewan ZNA, Voisin T, et al. Substance P release by sensory neurons triggers dendritic cell migration and initiates the type-2 immune response to allergens. Immunity 2020;53:1063–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moriyama S, Brestoff JR, Flamar AL, Moeller JB, Klose CSN, Rankin LC, et al. b2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science 2018;359:1056–61. [DOI] [PubMed] [Google Scholar]
- 63.Sui P, Wiesner DL, Xu J, Zhang Y, Lee J, Van Dyken S, et al. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science 2018;360:eaan8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hara Y, Jha MK, Mattoo H, Nash S, Khan A, Orengo J, et al. Interleukin 4 directly activates olfactory neurons and induces loss of smell in mice. J Allergy Clin Immunol 2023;151:AB128. [Google Scholar]
- 65.Rouyar A, Classe M, Gorski R, Bock MD, Le-Guern J, Roche S, et al. Type 2/Th2-driven inflammation impairs olfactory sensory neurogenesis in mouse chronic rhinosinusitis model. Allergy 2019;74:549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Backaert W, Steelant B, Hellings PW, Talavera K, Van Gerven L. A TRiP through the roles of transient receptor potential cation channels in type 2 upper airway inflammation. Curr Allergy Asthma Rep 2021;21:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li F, Jiang H, Shen X, et al. Sneezing reflex is mediated by a peptidergic pathway from nose to brainstem. Cell 2021;184:3762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Samivel R, Kim DW, Son HR, et al. The role of TRPV1 in the CD4+ T cell-mediated inflammatory response of allergic rhinitis. Oncotarget 2015;7:148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu M, Chang C, Undem BJ, Yu S. Capsaicin-sensitive vagal afferent nerve-mediated interoceptive signals in the esophagus. Molecules 2021;26:3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Shea KM, Aceves SS, Dellon ES. Pathophysiology of eosinophilic esophagitis. Gastroenterology 2018;154:333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Akiho H, Ihara E, Motomura Y, Nakamura K. Cytokine-induced alterations of gastrointestinal motility in gastrointestinal disorders. World J Gastrointest Pathophysiol 2011;2:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu Y, Liu Z, Yu X, et al. Increased acid responsiveness in vagal sensory neurons in a guinea pig model of eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol 2014;307:G149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang S, Shoda T, Aceves SS, Arva NC, Chehade M, Collins MH, et al. Mast cell-pain connection in eosinophilic esophagitis. Allergy 2022;77:1895–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Campion M, Smith L, Gatault S, Métais C, Buddenkotte J, Steinhoff M. Interleukin-4 and interleukin-13 evoke scratching behaviour in mice. Exp Dermatol 2019;28:1501–4. [DOI] [PubMed] [Google Scholar]
- 75.Talbot S, Abdulnour REE, Burkett PR, Lee S, Cronin SJF, Pascal MA, et al. Silencing nociceptor neurons reduces allergic airway inflammation. Neuron 2015;87:341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ding W, Wagner JA, Granstein RD. CGRP, PACAP, and VIP modulate Langerhans cell function by inhibiting NF-kappaB activation. J Invest Dermatol 2007; 127:2357–67. [DOI] [PubMed] [Google Scholar]
- 77.Ding W, Stohl LL, Wagner JA, Granstein RD. Calcitonin gene-related peptide biases Langerhans cells toward Th2-type immunity. J Immunol 2008;181:6020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ding W, Stohl LL, Xu L, Zhou XK, Manni M, Wagner JA, et al. Calcitonin gene-related peptide-exposed endothelial cells bias antigen presentation to CD4+ T cells toward a Th17 response. J Immunol 2016;196:2181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lian Z, Qi H, Liu X, Zhang Y, Xu R, Yang X, et al. Ambient ozone, and urban PM2.5 co-exposure, aggravate allergic asthma via transient receptor potential vanilloid 1-mediated neurogenic inflammation. Ecotoxicol Environ Saf 2022;243: 114000. [DOI] [PubMed] [Google Scholar]
- 80.Serhan N, Basso L, Sibilano R, Petitfils C, Meixiong J, Bonnart C, et al. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat Immunol 2019;20:1435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Urashima R, Mihara M. Cutaneous nerves in atopic dermatitis. A histological, immunohistochemical and electron microscopic study. Virchows Arch 1998; 432:363–70. [DOI] [PubMed] [Google Scholar]
- 82.Feld M, Garcia R, Buddenkotte J, Katayama S, Lewis K, Muirhead G, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol 2016;138:500–8.e24. [DOI] [PubMed] [Google Scholar]
- 83.Lebold KM, Drake MG, Hales-Beck LB, Fryer AD, Jacoby DB. IL-5 exposures in utero increases lung nerve density and airway reactivity in adult offspring. Am J Respir Cell Mol Biol 2020;62:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mack MR, Kim BS. The itch-scratch cycle: a neuroimmune perspective. Trends Immunol 2018;39:980–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pereira MP, Ständer S. How to define chronic prurigo? Exp Dermatol 2019;28: 1455–60. [DOI] [PubMed] [Google Scholar]
- 86.Langan SM, Abuabara K, Henrickson SE, Hoffstad O, Margolis DJ. Increased risk of cutaneous and systemic infections in atopic dermatitis—a cohort study. J Invest Dermatol 2017;137:1375–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet 2020;396:345–60. [DOI] [PubMed] [Google Scholar]
- 88.Narla S, Silverberg JI. Association between atopic dermatitis and serious cutaneous, multiorgan and systemic infections in US adults. Ann Allergy Asthma Immunol 2018;120:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ong PY, Leung DY. Bacterial and viral infections in atopic dermatitis: a comprehensive review. Clin Rev Allergy Immunol 2016;51:329–37. [DOI] [PubMed] [Google Scholar]
- 90.Serrano L, Patel KR, Silverberg JI. Association between atopic dermatitis and extracutaneous bacterial and mycobacterial infections: a systematic review and meta-analysis. J Am Acad Dermatol 2019;80:904–12. [DOI] [PubMed] [Google Scholar]
- 91.Doyle JA, Connolly SM, Hunziker N, Winkelmann RK. Prurigo nodularis: a reappraisal of the clinical and histologic features. J Cutan Pathol 1979;6: 392–403. [DOI] [PubMed] [Google Scholar]
- 92.Elmariah S, Kim B, Berger T, Chisolm S, Kwatra S, Mollanazar N, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol 2021;84:747–60. [DOI] [PubMed] [Google Scholar]
- 93.Yosipovitch G, Rosen JD, Hashimoto T. Itch: from mechanism to (novel) therapeutic approaches. J Allergy Clin Immunol 2018;142:1375–90. [DOI] [PubMed] [Google Scholar]
- 94.Hill RZ, Loud MC, Dubin AE, Peet B, Patapoutian A. PIEZO1 transduces mechanical itch in mice. Nature 2022;607:104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jones AV, Tilley M, Gutteridge A, et al. GWAS of self-reported mosquito bite size, itch intensity and attractiveness to mosquitoes implicates immune-related predisposition loci. Hum Mol Gen 2017;26:1391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Voisin T, Perner C, Messou MA, Shiers S, Ualiyeva S, Kanaoka Y, et al. The CysLT2R receptor mediates leukotriene C4-driven acute and chronic itch. Proc Natl Acad Sci U S A 2022;118:e2022087118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tseng PY, Hoon MA. Oncostatin M can sensitize sensory neurons in inflammatory pruritus. Sci Transl Med 2021;13:eabe3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, et al. Activation of mast-cell-expressed mas-related G-protein-coupled receptors drives non-histaminergic itch. Immunity 2019;50:1163–71.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, et al. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci 2011;14:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han L, Ma C, Liu Q, Weng H-J, Cui Y, Tang Z, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 2013;16:174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang C-C, Kim YS, Olson W, Li F, Guo C, Luo W, et al. A histamine-independent itch pathway is required for allergic ocular itch. J Allergy Clin Immunol 2016;137:1267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang C-C, Yang W, Guo C, Jiang H, Li F, Xiao M, et al. Anatomical and functional dichotomy of ocular itch and pain. Nat Med 2018;24:1268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Akiyama T, Nagamine M, Carstens MI, Carstens E. Behavioral model of itch, alloknesis, pain and allodynia in the lower hindlimb and correlative responses of lumbar dorsal horn neurons in the mouse. Neuroscience 2014;266:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAK-STAT 2013;2: e24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shimoda K, van Deursen J, Sangster MY, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature 1996;380:630–3. [DOI] [PubMed] [Google Scholar]
- 106.Takeuchi I, Yanagi K, Takada S, et al. STAT6 gain-of-function variant exacerbates multiple allergic symptoms. J Allergy Clin Immunol 2023;151:1402–9.e6. [DOI] [PubMed] [Google Scholar]
- 107.Sharma M, Leung D, Momenilandi M, et al. Human germline heterozygous gain-of-function STAT6 variants cause severe allergic disease. J Exp Med 2023;220: e20221755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Suratannon N, Ittiwut C, Kik WA, et al. A germline STAT6 gain-of-function variant is associated with early-onset allergies. J Allergy Clin Immuno 2023; 151:565–71. [DOI] [PubMed] [Google Scholar]
- 109.Baris S, Benamar M, Chen Q, et al. Severe allergic dysregulation due to a gain of function mutation in the transcription factor STAT6. J Allergy Clin Immunol 2023;152:182–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Minskaia E, Maimaris J, Jenkins P, et al. Autosomal dominant STAT6 gain of function causes severe atopy associated with lymphoma [e-pub ahead of print]. J Clin Immunol 10.1007/s10875-023-01530-7. Accessed July 11, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Okano M, Hirahara K, Kiuchi M, Onoue M, Iwamura C, Kokubo K, et al. Interleukin-33-activated neuropeptide CGRP-producing memory Th2 cells cooperate with somatosensory neurons to induce conjunctival itch. Immunity 2022;55: 2352–68. [DOI] [PubMed] [Google Scholar]
- 112.Jeong CW, Ahn KS, Rho NK, Park YD, Lee DY, Lee JH, et al. Differential in vivo cytokine mRNA expression in lesional skin of intrinsic vs. extrinsic atopic dermatitis patients using semiquantitative RT-PCR. Clin Exp Allergy 2003;33:1717–24. [DOI] [PubMed] [Google Scholar]
- 113.Miron Y, Miller PE, Hughes C, Indersmitten T, Lerner EA, Cevikbas F. Mechanistic insights into the antipruritic effects of lebrikizumab, an anti-IL-13 mAb. J Allergy Clin Immunol 2022;150:690–700. [DOI] [PubMed] [Google Scholar]
- 114.Meng J, Li Y, Fischer MJM, Steinhoff M, Chen W, Wang J. Th2 modulation of transient receptor potential channels: an unmet therapeutic intervention for atopic dermatitis. Front Immunol 2021;12:696784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cutz E, Chan W, Track NS, Goth A, Said SI. Release of vasoactive intestinal polypeptide in mast cells by histamine liberators. Nature 1978;275:661–2. [DOI] [PubMed] [Google Scholar]
- 116.Nakashima C, Ishida Y, Kitoh A, Otsuka A, Kabashima K. Interaction of peripheral nerves and mast cells, eosinophils, and basophils in the development of pruritus. Exp Dermatol 2019;28:1405–11. [DOI] [PubMed] [Google Scholar]
- 117.Tore F, Tuncel N. Mast cells: target and source of neuropeptides. Curr Pharm Des 2009;15:3433–45. [DOI] [PubMed] [Google Scholar]
- 118.Wang Z, Babina M. MRGPRX2 signals its importance in cutaneous mast cell biology: does MRGPRX2 connect mast cells and atopic dermatitis? Exp Dermatol 2020;29:1104–11. [DOI] [PubMed] [Google Scholar]
- 119.Wedi B, Gehring M, Kapp A. The pseudoallergen receptor MRGPRX2 on peripheral blood basophils and eosinophils: expression and function. Allergy 2020;75: 2229–42. [DOI] [PubMed] [Google Scholar]
- 120.Umemoto N, Kakurai M, Okazaki H, Kiyosawa T, Demitsu T, Nakagawa H. Serum levels of vasoactive intestinal peptide are elevated in patients with atopic dermatitis. J Dermatol Sci 2003;31:161–4. [DOI] [PubMed] [Google Scholar]
- 121.Hashimoto T, Mishra SK, Olivry T, Yosipovitch G. Periostin, an emerging player in itch sensation. J Invest Dermatol 2021;141:2338–43. [DOI] [PubMed] [Google Scholar]
- 122.Feng J, Zhao Y, Xie Z, Zang K, Sviben S, Hu X, et al. Miswiring of Merkel cell and pruriceptive C fiber drives the itch-scratch cycle. Sci Transl Med 2022;14: eabn4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feng J, Hu H. A novel player in the field: Merkel disc in touch, itch and pain. Exp Dermatol 2019;28:1412–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mochizuki H, Papoiu ADP, Nattkemper LA, Lin AC, Kraft RA, Coghill RC, et al. Scratching induces overactivity in motor-related regions and reward system in chronic itch patients. J Invest Dermatol 2015;135:2814–23. [DOI] [PubMed] [Google Scholar]
- 125.Feng J, Luo J, Yang P, Du J, Kim BS, Hu H. Piezo2 channel-Merkel cell signaling modulates the conversion of touch to itch. Science 2018;360:530–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moayedi Y, Duenas-Bianchi LF, Lumpkin EA. Somatosensory innervation of the oral mucosa of adult and aging mice. Sci Rep 2018;8:9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhu X, Chen Z, Shen W, Huang G, Sedivy JM, Wang H, et al. Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: the regulation and intervention. Signal Transduct Target Ther 2021;6:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen B, Yang J, Song Y, Zhang D, Hao F. Skin immunosenescence and type 2 inflammation: a mini-review with an inflammaging perspective. Front Cell Dev Biol 2022;10:835675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu AZ, Tripathi SV, Kau AL, Schaffer A, Kim BS. Immune dysregulation underlies a subset of patients with chronic idiopathic pruritus. J Am Acad Dermatol 2016;74:1017–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dehner C, Chen L, Kim B, Rosman IS. Chronic itch of unknown origin is associated with an enhanced Th2 skin immune profile. Am J Dermatopathol 2021;43: 773–5. [DOI] [PubMed] [Google Scholar]
- 131.Iking A, Grundmann S, Chatzigeorgakidis E, Phan NQ, Klein D, Ständer S. Prurigo as a symptom of atopic and non-atopic diseases: aetiological survey in a consecutive cohort of 108 patients. J Eur Acad Dermatol Venereol 2013;27:550–7. [DOI] [PubMed] [Google Scholar]
- 132.Boozalis E, Tang O, Patel S, Semenov YR, Pereira MP, Stander S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol 2018;79:714–9.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.U.S. Food and Drug Administration. Dupixent (dupilumab) US prescribing information. Available at: https://www.regeneron.com/downloads/dupixent_fpi.pdf. Accessed April 27, 2023.
- 134.Dupixent. European Medicines Agency 2023. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent. Accessed June 27, 2023.
- 135.U.S. Food and Drug Administration. Adbry (tralokinumab-ldrm) US prescribing information. Available at: https://mc-df05ef79-e68e-4c65-8ea2-953494-cdn-endpoint.azureedge.net/-/media/corporatecommunications/us/therapeutic-expertise/our-product/adbrypi.pdf?rev5e283af2c84c546089b1f2994ba0afce1. Accessed April 27, 2023.
- 136.Keam SJ. Nemolizumab: first approval. Drugs 2022;82:1143–50. [DOI] [PubMed] [Google Scholar]
- 137.Yosipovitch G. Mollenazar N, Stander S. Kwatra SG, Kim BS, Laws E, et al. Dupilumab in adult patients with prurigo nodularis: results from two randomized phase 3 trials. Nat Med 2023;29:1180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Murphy AJ, Macdonald LE, Stevens S, Karow M, Dore AT, Pobursky K, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A 2014;111:5153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yosipovitch G, de Bruin-Weller M, Armstrong A, Wu JJ, Herranz P, Thaçi D, et al. Dupilumab treatment provides sustained improvements over 2 years in symptoms and quality of life in adults with atopic dermatitis. Dermatol Ther (Heidelb) 2021;11:2147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scott G, Asrat S, Allinne J, Lim WK, Nagashima N, Birchard D, et al. IL-4 and IL-13, not eosinophils, drive type 2 airway inflammation, remodeling, and lung function decline. Cytokine 2023;162:150691. [DOI] [PubMed] [Google Scholar]
- 141.Busse WW, Katial R, Gossage D, Sari S, Wang B, Kolbeck R, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol 2010;125:1237–44.e2. [DOI] [PubMed] [Google Scholar]
- 142.Maspero J, Adir Y, Al-Ahmad M, Celis-Preciado CA, Colodenco FD, Giavina-Bianchi P, et al. Type 2 inflammation in asthma and other airway diseases. ERJ Open Res 2022;8:00576–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bajbouj K, Abu Jabal R, Sahnoon L, Olivenstein R, Mahboub B, Hamid Q, et al. IL-5 receptor expression in lung fibroblasts: potential role in airway remodeling in asthma. Allergy 2023;78:882–5. [DOI] [PubMed] [Google Scholar]
- 144.Matsuyama T, Matsuyama H, Dotake Y, Takagi K, Machida K, Inoue H, et al. The therapeutic potential for targeting group 2 innate lymphoid cells in asthma. Front Immunol 2022;13:13930862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Palacionyte J, Januskevicius A, Vasyle E, Rimkunas A, Bajoriuniene I, Miliauskas S, et al. IL-5 and GM-CSF, but not IL-3, promote the proliferative properties of inflammatory-like and lung resident-like eosinophils in the blood of asthma patients. Cells 2022;11:3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Principe S, Porsbjerg C, Bolm Ditlev S, Kjaersgaard Klein D, Golebski K, Dyhre-Petersen N. Treating severe asthma: targeting the IL-5 pathway. Clin Exp Allergy 2021;51:992–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Datsi A, Steinhoff M, Ahmad F, Alam M, Buddenkotte J. Interleukin-31: the “itchy” cytokine in inflammation and therapy. Allergy 2020;76:2982–97. [DOI] [PubMed] [Google Scholar]
- 148.Nassenstein C, Krasteva-Christ G, Renz H. New aspects of neuroinflammation and neuroimmune crosstalk in the airways. J Allergy Clin Immunol 2018;142: 1415–22. [DOI] [PubMed] [Google Scholar]
- 149.Kim SH, Patil MJ, Hadley SH, Bahia PK, Butler SG, Madaram M, et al. Mapping of the sensory innervation of the mouse lung by specific vagal and dorsal root ganglion neuronal subsets. eNeuro 2022;9:ENEURO.0026–22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Su Y, Barr J, Jaquish A, Xu J, Verheyden JM, Sun X. Identification of lung innervating sensory neurons and their target specificity. Am J Physiol Lung Cell Mol Physiol 2022;322:L50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tränkner D, Hahne N, Sugino K, Hoon MA, Zuker C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc Natl Acad Sci U S A 2014;111:11515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kay AB, Ali FR, Heaney LG, Benyahia F, Soh CPC, Renz H, et al. Airway expression of calcitonin gene-related peptide in T-cell peptide-induced late asthmatic reactions in atopics. Allergy 2007;62:495–503. [DOI] [PubMed] [Google Scholar]
- 153.Tomaki M, Ichinose M, Miura M, Hirayama Y, Yamauchi H, Nakajima N, et al. Elevated substance P content in induced sputum from patients with asthma and patients with chronic bronchitis. Am J Respir Crit Care Med 1995; 151:613–7. [DOI] [PubMed] [Google Scholar]
- 154.Lee LY, Gu Q. Role of TRPV1 in inflammation-induced airway hypersensitivity. Curr Opin Pharmacol 2009;9:243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D’Amours M, et al. sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci U S A 2009;106:9099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour REE, Nyman J, Dionne D, et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 2017;549:351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thapaliya M, Chompunud Na Ayudhya C, Amponnawarat A, Roy S, Ali H. Mast cell-specific MRGPRX2: a key modulator of neuro-immune interaction in allergic diseases. Curr Allergy Asthma Rep 2021;21:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Plum T, Wang X, Rettel M, Krijgsveld J, Feyerabend TB, Rodewald H-R. Human mast cell proteome reveals unique lineage, putative functions, and structural basis for cell ablation. Immunity 2020;52:404–16. [DOI] [PubMed] [Google Scholar]
- 159.Manorak W, Idahosa C, Gupta K, Roy S, Panettieri R, Ali H. Upregulation of Mas-related G protein coupled receptor X2 in asthmatic lung mast cells and its activation by the novel neuropeptide hemokinin-1. Respir Res 2018;19:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nagashima H, Mahlakõiv T, Shih HY, Davis FP, Meylan F, Huang Y, et al. Neuropeptide CGRP limits group 2 innate lymphoid cell responses and constrains type 2 inflammation. Immunity 2019;51:682–95.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Aoki-Nagase T, Nagase T, Oh-Hashi Y, Shindo T, Kurihara Y, Yamaguchi Y, et al. Attenuation of antigen-induced airway hyperresponsiveness in CGRP-deficient mice. Am J Physiol Lung Cell Mol Physiol 2002;283:L963–70. [DOI] [PubMed] [Google Scholar]
- 162.Kelly MJ, Breathnach C, Tracey KJ, Donnelly SC. Manipulation of the inflammatory reflex as therapeutic strategy. Cell Rep Med 2022;3:100696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Burgess JK, Jonker MR, Berg M, ten Hacken NTH, Meyer KB, van den Berge M, et al. Periostin: contributor to abnormal airway epithelial function in asthma? Eur Respir J 2020;57:2001286. [DOI] [PubMed] [Google Scholar]
- 164.Chachi L, Alzahrani A, Koziol-White C, Biddle M, Bagadood R, Panettieri RA Jr, et al. Increased β2-adrenoceptor phosphorylation in airway smooth muscle in severe asthma: possible role of mast cell-derived growth factors. Clin Exp Immunol 2018;194:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Drake MG, Lebold KM, Roth-Carter QR, Pincus AB, Blum ED, Proskocil BJ, et al. Eosinophil and airway nerve interactions in asthma. J Leukoc Biol 2018; 104:61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xu J, Yu H, Sun X. Less is more: rare pulmonary neuroendocrine cells function as critical sensors in lung. Dev Cell 2020;55:123–32. [DOI] [PubMed] [Google Scholar]
- 167.Hewitt RJ, Lloyd CM. Regulation of immune responses by the airway epithelial cell landscape. Nat Rev Immunol 2021;21:347–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bankova LG, Dwyer DF, Yoshimoto E, Ualiyeva S, McGinty JW, Raff H, et al. The cysteinyl leukotriene 3 receptor regulates expansion of IL-25–producing airway brush cells leading to type 2 inflammation. Sci Immunol 2018;3:eaat9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.O’Leary CE, Schneider C, Locksley RM. Tuft cells—systemically dispersed sensory epithelia integrating immune and neural circuitry. Annu Rev Immunol 2019; 37:47–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Agache I, Akdis CA, Akdis M, Canonica GW, Casale T, Chivato T, et al. EEACI biologicals guidelines – recommendations for severe asthma. Allergy 2021;76: 14–44. [DOI] [PubMed] [Google Scholar]
- 171.Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 2014;13:CD003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev 2017;9:CD010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med 2021;384:1800–9. [DOI] [PubMed] [Google Scholar]
- 174.Menzies-Gow A, Wechsler ME, Brightling CE, Korn S, Corren J, Israel E, et al. Long-term safety and efficacy of tezepelumab in people with severe, uncontrolled asthma (DESTINATION): a randomized, placebo-controlled extension study. Lancet Respir Med 2023;11:425–38. [DOI] [PubMed] [Google Scholar]
- 175.Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe asthma. N Engl J Med 2018;378: 2486–96. [DOI] [PubMed] [Google Scholar]
- 176.Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med 2018;378:2475–85. [DOI] [PubMed] [Google Scholar]
- 177.Wechsler ME, Ford LB, Maspero JF, Pavord ID, Papi A, Bourdin A, et al. Long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma (TRAVERSE): an open-label extension study. Lancet Respir Med 2022;10:11–25. [DOI] [PubMed] [Google Scholar]
- 178.Schleimer RP. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol 2017;12:331–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rombaux P, Huart C, Levie P, Cingi C, Hummel T. Olfaction in chronic rhinosinusitis. Curr Allergy Asthma Rep 2016;16:1–2. [DOI] [PubMed] [Google Scholar]
- 180.Kern RC. Chronic sinusitis and anosmia: pathologic changes in the olfactory mucosa. Laryngoscope 2000;110:1071–7. [DOI] [PubMed] [Google Scholar]
- 181.Bogdanov V, Walliczek-Dworschak U, Whitcroft KL, Landis BN, Hummel T. Response to glucocorticosteroids predicts olfactory outcome after ESS in chronic rhinosinusitis. Laryngoscope 2020;130:1616–21. [DOI] [PubMed] [Google Scholar]
- 182.Nguyen DT, Bonfort G, Arous F, Felix-Ravelo M, Nguyen-Thi PL, Jankowski R. Evaluation of residual symptoms: a method to assess surgical outcomes for nasal polyposis. Am J Rhinol Allergy 2016;30:e36–41. [DOI] [PubMed] [Google Scholar]
- 183.Yan X, Whitcroft KL, Hummel T. Olfaction: sensitive indicator of inflammatory burden in chronic rhinosinusitis. Laryngoscope Investig Otolaryngol 2020;5: 992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 2006;61:1280–9. [DOI] [PubMed] [Google Scholar]
- 185.Mahdavinia M, Carter RG, Ocampo CJ, Stevens W, Kato A, Tan BK, et al. Basophils are elevated in nasal polyps of patients with chronic rhinosinusitis without aspirin sensitivity. J Allergy Clin Immunol 2014;133:1759–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Takabayashi T, Kato A, Peters AT, Suh LA, Carter R, Norton J, et al. Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 2012;130:410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gupta N, Harit A, Taneja HC, Kumar R, Tripathi AK. Olfaction and its correlates in allergic rhinitis: a case control study. Indian J Otolaryngol Head Neck Surg 2019;71:1782–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gevaert P, Omachi TA, Corren J, Mullol J, Han J, Lee SE, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol 2020;146:595–605. [DOI] [PubMed] [Google Scholar]
- 189.Han JK, Bachert C, Fokkens W, Desrosiers M, Wagenmann M, Lee SE, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2021;9:1141–53. [DOI] [PubMed] [Google Scholar]
- 190.Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Gevaert P, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA 2016;315:469–79. [DOI] [PubMed] [Google Scholar]
- 191.Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomized, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019;394:1638–50. [DOI] [PubMed] [Google Scholar]
- 192.Mullol J, Azar A, Buchheit KM, Hopkins C, Bernstein JA. Chronic rhinosinusitis with nasal polyps: quality of life in the biologics era. J Allergy Clin Immunol Pract 2022;10:1434–53. [DOI] [PubMed] [Google Scholar]
- 193.Mullol J, Bachert C, Amin N, Desrosiers M, Hellings PW, Han JK, et al. Olfactory outcomes with dupilumab in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract 2022;10:1086–95.e5. [DOI] [PubMed] [Google Scholar]
- 194.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med 2004;351:940–1. [DOI] [PubMed] [Google Scholar]
- 195.Muir A, Falk GW. Eosinophilic esophagitis: a review. JAMA 2021;326: 1310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rabinowitz SS, Yu L, Geraghty P. EoE behaves as a unique Th2 disease: a narrative review. Transl Gastroenterol Hepatol 2023;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Carlson DA, Shehata C, Gonsalves N, Hirano I, Peterson S, Prescott J, et al. Esophageal dysmotility is associated with disease severity in eosinophilic esophagitis. Clin Gastroenterol Hepatol 2022;20:1719–28.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Reddy SB, Ketchem CJ, Dougherty MK, Eluri S, Dellon ES. Association between eosinophilic esophagitis and esophageal dysmotility: a systematic review and meta-analysis. Neurogastroenterol Motil 2023;35:e14475. [DOI] [PubMed] [Google Scholar]
- 199.Underwood B, Troutman TD, Schwartz JT. Breaking down the complex pathophysiology of eosinophilic esophagitis. Ann Allergy Asthma Immunol 2023; 130:28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Simon D, Straumann A, Schoepfer AM, Simon HU. Current concepts in eosinophilic esophagitis. Allergo J Int 2017;26:258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kupari J, Häring M, Agirre E, Castelo-Branco G, Ernfors P. An atlas of vagal sensory neurons and their molecular specialization. Cell Rep 2019;27:2508–23.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ko E, Chehade M. Biological therapies for eosinophilic esophagitis: where do we stand? Clin Rev Allergy Immunol 2018;55:205–16. [DOI] [PubMed] [Google Scholar]
- 203.Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G 3rd, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol 2012;129:456–63.e1–3. [DOI] [PubMed] [Google Scholar]
- 204.Straumann A, Conus S, Grzonka P, Kita H, Kephart G, Bussmann C, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut 2010;59: 21–30. [DOI] [PubMed] [Google Scholar]
- 205.Allakos Inc. News release. Allakos announces topline phase 3 data from the ENIGMA 2 study and phase 2/3 data from the KRYPTOS study in patients with eosinophilic gastrointestinal diseases. Available at: https://investor.allakos.com/news-releases/news-release-details/allakos-announces-topline-phase-3-data-enigma-2-study-and-phase. Accessed April 27, 2023.
- 206.Rothenberg ME, Wen T, Greenberg A, Alpan O, Enav B, Hirano I, et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol 2015;135:500–7. [DOI] [PubMed] [Google Scholar]
- 207.Hirano I, Collins MH, Assouline-Dayan Y, Evans L, Gupta S, Schoepfer AM, et al. RPC4046, a monoclonal antibody against IL13, reduces histologic and endoscopic activity in patients with eosinophilic esophagitis. Gastroenterology 2019;156:592–603. [DOI] [PubMed] [Google Scholar]
- 208.Hirano I, Dellon ES, Hamilton JD, Collins MH, Peterson K, Chehade M, et al. Efficacy of dupilumab in a phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology 2020;158:111–22. [DOI] [PubMed] [Google Scholar]
- 209.Dellon ES, Rothenberg ME, Collins MH, Hirano I, Chehade M, Bredenoord AJ, et al. Dupilumab in adults and adolescents with eosinophilic esophagitis. N Engl J Med 2022;387:2317–30. [DOI] [PubMed] [Google Scholar]
- 210.Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol 2014;71:327–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Drucker AM, Eyerich K, de Bruin-Weller MS, Thyssen JP, Spuls PI, Irvine AD, et al. Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement. Br J Dermatol 2018;178:768–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2020. Available at: www.ginasthma.org. Accessed April 27, 2023.
- 213.Dalal AA, Duh MS, Gozalo L, Robitaille MN, Albers F, Yancey S, et al. Dose-response relationship between long-term systemic corticosteroid use and related complications in patients with severe asthma. J Manag Care Spec Pharm 2016; 22:833–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yang X, Jin H. Safety of corticosteroids in the treatment of acute respiratory disease in children: a systematic review and meta-analysis. Transl Pediatr 2022;11: 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wollenberg A, Marcoux D, Silverberg JI, Aoki V, Baselga E, Zhang H, et al. Dupilumab provides rapid and sustained improvement in SCORing Atopic Dermatitis outcomes in paediatric patients with atopic dermatitis. Acta Derm Venereol 2022;102:adv00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sofen H, Bissonnette R, Yosipovitch G, et al. Efficacy and safety of vixarelimab, a human monoclonal oncostatin M receptor β antibody, in moderate-to-severe prurigo nodularis: a randomised, double-blind, placebo-controlled, phase 2a study. EClinicalMed 2023;57:101826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Silverberg JI, Simpson EL, Boguniewicz M, de Bruin-Weller M, Foley P, Kataoka Y, et al. Dupilumab provides rapid and sustained clinically meaningful responses in adults with moderate-to-severe atopic dermatitis. Acta Derm Venereol 2021; 101:adv00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bieber T. In search of the Holy Grail in atopic dermatitis: will dupilumab become the first disease-modifying atopic dermatitis drug? J Allergy Clin Immunol 2023;151:694–6. [DOI] [PubMed] [Google Scholar]
- 219.Geba GP, Li D, Xu M, Mohammadi K, Attre R, Ardeleanu M, et al. Attenuating the atopic march: meta-analysis of the dupilumab atopic dermatitis database for incident allergic events. J Allergy Clin Immunol 2023;151:756–66. [DOI] [PubMed] [Google Scholar]
- 220.Haddad EB, Cyr SL, Arima K, McDonald RA, Levit NA, Nestle FO. Current and emerging strategies to inhibit type 2 inflammation in atopic dermatitis. Dermatol Ther (Heidelb) 2022;12:1501–33. [DOI] [PMC free article] [PubMed] [Google Scholar]