Abstract
Numerous agents targeting various phosphatidylinositol 3-kinase (PI3K) pathway components, including PI3K, AKT and mTOR, have been tested in oncology clinical trials, resulting in regulatory approvals for the treatment of selected patients with breast cancer, certain other solid tumours or particular haematological malignancies. However, given the prominence of PI3K signalling in cancer and the crucial role of this pathway in linking cancer growth with metabolism, these clinical results could arguably be improved upon. In this Review, we discuss past and present efforts to overcome the somewhat limited clinical efficacy of PI3Kα pathway inhibitors, including optimization of inhibitor specificity, patient selection and biomarkers across cancer types, with a focus on breast cancer, as well as identification and abrogation of signalling-related and metabolic mechanisms of resistance, and interventions to improve management of prohibitive adverse events. We highlight the advantages and limitations of laboratory-based model systems used to study the PI3K pathway, and propose technologies and experimental inquiries to guide the future clinical deployment of PI3K pathway inhibitors in the treatment of cancer.
Therapeutic targeting of the phosphatidylinositol 3-kinase (PI3K) pathway in cancer is at a crossroads. Decades of preclinical research, drug development, clinical trials and biomarker studies have led to the approval of PI3Kα, PI3Kδ and mTOR inhibitors for the treatment of several different solid tumours and haematological malignancies. However, achieving a therapeutic window that maximizes efficacy and minimizes adverse effects remains the major barrier to developing a new generation of PI3K inhibitors for biomarker-driven disease indications. The toxicity profile of PI3K, AKT and mTOR inhibitors primarily reflects on-target inhibition of insulin signalling. Therefore, modulation of insulin signalling and glucose metabolism has become a necessary strategy to mitigate the adverse effects of these agents and can also directly and indirectly increase their therapeutic efficacy. Several articles provide a comprehensive overview of PI3K signalling in cancer1 as well as of therapeutic targeting of this pathway2–4. Herein, we focus our discussion on the effects of PI3Kα alterations on signalling and metabolism in solid tumours, particularly breast cancers, and on efforts to co-opt these pathways for combination therapies. We also briefly review advances made over the past few years in targeting AKT and mTOR.
PI3K signalling and oncogenesis
Since its discovery in 1985, PI3K5 — and the PI3K pathway more broadly — has been a highly sought drug target. PI3K was first discovered in the retroviral oncogene era as a kinase associated with polyoma middle T antigen, which has an important role in cellular transformation5. It is prescient that, even in this initial report5, the authors noted the role of the activity of this kinase in mitogenesis and oncogenesis given that many retroviral oncogenes were later found to be orthologues involved in human cancers. Later, PI3K was shown to phosphorylate phosphatidylinositol lipids at the 3′ position6 and to act downstream of receptor tyrosine kinases (RTKs) activated by growth factors7, including the insulin receptor8.
Class I PI3Ks are heterodimeric lipid kinases composed of a p110 catalytic subunit and a regulatory subunit1. The human genome encodes three class IA p110 isoforms: p110α (encoded by PIK3CA) and p110β (PIK3CB), which are expressed in all cell types, and p110δ (PIK3CD), which is primarily expressed in immune cells. Five class IA PI3K regulatory subunit isoforms and splice variants (p85α, p85β, p55γ, p55α and p50α) are also found in humans, the most well-characterized of which is p85α (encoded by PIK3R1). Additionally, p110γ (PIK3CG) is a class IB PI3K catalytic subunit that binds to the regulatory subunits p101 or p87. This genetic diversity results in a spectrum of differential signalling propensities varying across cell types.
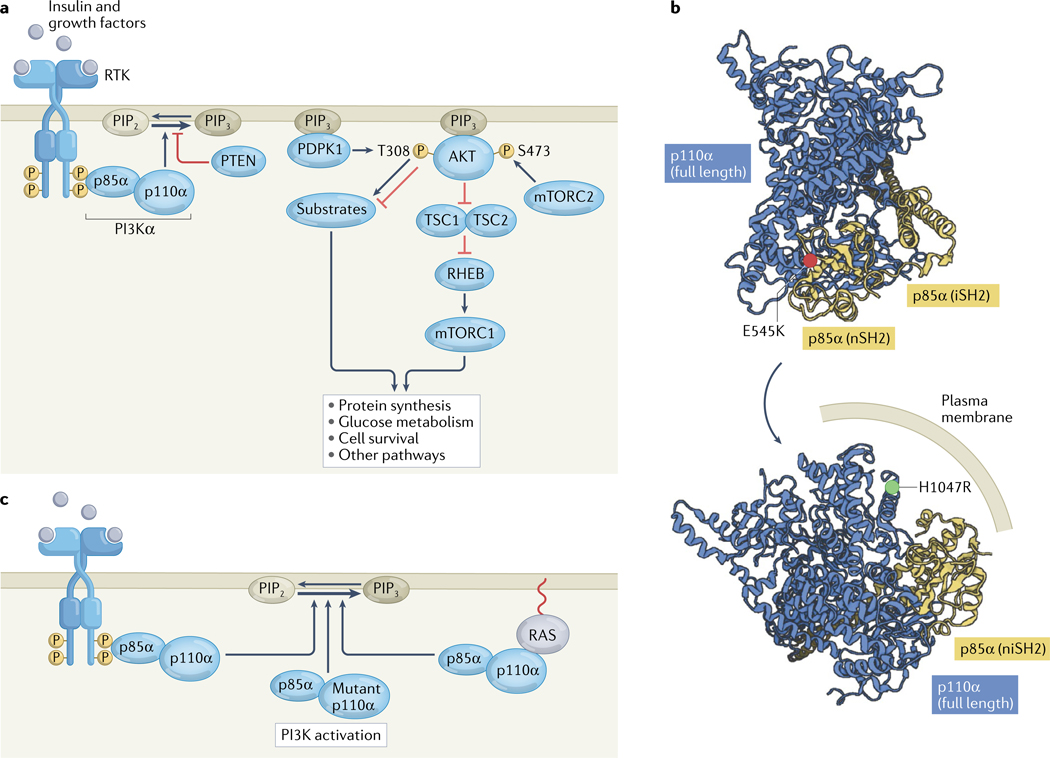
Phosphatidylinositol 3,4,5-trisphosphate (PIP3), the product of the class I PI3K reaction, functions as a potent second messenger molecule by recruiting various kinases, including AKT and PDPK1, to the plasma membrane (FIG. 1a). PDPK1 and mTOR complex 2 (mTORC2) both activate AKT through differential phosphorylation at T308 and S473, respectively9. AKT is an AGC family serine/threonine kinase that phosphorylates a range of substrates that regulate crucial cellular functions, including TSC2 and PRAS40 (protein synthesis), AS160 and TXNIP (glucose metabolism), and BAD and FOXO (apoptosis or cell survival). Given that TSC2 is a negative regulator of mTOR complex 1 (mTORC1) activity, the mTOR kinase functions both upstream and downstream of AKT. PTEN is a lipid phosphatase that antagonizes the function of PI3K by dephosphorylating PIP3 back to PIP2 (FIG. 1a).
Fig. 1 |. The PI3K pathway in non-malignant cells and cancer.
a | Insulin and other growth factors stimulate receptor tyrosine kinases (RTKs), leading to transautophosphorylation at C-terminal domain tyrosine residues. These phosphotyrosines bind to SH2 domains in the regulatory p85α subunit of phosphatidylinositol 3-kinase-α (PI3Kα), activating the enzymatic p110α subunit and thus PI3Kα activity, catalysing the production of phosphatidylinositol 3,4,5-trisphosphate (PIP3) from phosphatidylinositol 4,5-bisphosphate (PIP2) in the inner layer of the cell membrane. The lipid phosphatase PTEN can reverse this process. PIP3 recruits the serine/threonine kinases PDPK1 and AKT to the plasma membrane. Subsequently, PDPK1 and the mTOR complex 2 (mTORC2) phosphorylate AKT at T308 and S473, respectively. AKT then phosphorylates a host of cellular substrates, including tuberin (also known as TSC2), thus inactivating the TSC complex; suppression of the GTPase-activating protein activity of the TSC complex results in the activation of the GTPase RHEB, which in turn activates mTOR complex 1 (mTORC1), which drives numerous necessary processes in normal physiology and cancer. AKT also regulates cellular processes through various targets other than TSC2. b | Structural depiction of the oncogenic activating p110α E545K and H1047R mutations in the crystal structure of PI3Kα (PDB 4OVU)13. E545K is located at the p110α–p85α binding interface, and H1047R is located in the membrane-binding region. c | In cancer, PI3Kα is often activated through RTK phosphopeptide binding to p85α, p110α mutation or RAS stimulation of p110α. iSH2, inter Src homology 2 domain; niSH2, N-terminal and inter Src homology domains; nSH2, N-terminal Src homology 2 domain.
In the 2000s, when cancer genome sequencing was in its infancy, research at Johns Hopkins University found that PIK3CA mutations are frequent events in diverse human cancers, including colorectal, brain, gastric, breast and lung tumours10. This initial report revealed common mutations within the helical domain (E542K and E545K) and the kinase domain (H1047R) as well as rare variants scattered throughout the gene10. Subsequently, using knock-in mutant colorectal cancer cell lines, the PIK3CA E545K and H1047R mutations were shown to activate AKT signalling and to be sufficient for induction of cell growth, migration and invasion11. These effects could be reversed using a PI3K inhibitor tool compound, which did not cause cytotoxicity but stalled cell proliferation11. Notably, serum starvation was required to elucidate these cellular effects owing to the fact that exogenous growth factors can autonomously stimulate PI3K in these cells. These studies culminated in a crystal structure of a truncated PI3K complex consisting of full-length p110α and the N-terminal and inter Src homology 2 (niSH2) domains of p85α, which revealed a globular protein complex with a large membrane-binding surface, where p110α cradles the niSH2 domains of p85α12,13 (FIG. 1b). In this structure, E545 lies at the binding interface between p110α and p85α, such that the E545K mutation disrupts the regulation of p110 by p85 (‘disrupter’ mutation), whereas H1047 lies in the membrane-binding surface, with the H1047R mutation enhancing membrane binding by positioning the positively charged arginine residue in close proximity to the negatively charged plasma membrane (‘binder’ mutation)14 (FIG. 1b).
Mutations in p85α occur rarely in cancer and result in PI3K pathway activation through complex mechanisms, including (1) a p110α-dependent hypermorphic mechanism whereby hemizygous loss of p85α increases p110α–p85α binding to activated RTKs, thus causing oncogenic transformation in mouse models (indicating a tumour-suppressive role for p85)15; (2) a p110α-independent hypermorphic mechanism through the decreased formation of p85α homodimers that usually stabilize PTEN or through disruption of the interface between p85α and PTEN16–18; and (3) a p110α-independent neomorphic mechanism in which mutant p85α activates MAPK signalling in the cytoplasm and translocates to the nucleus, where it acts as a scaffold for activation of JNK signalling19.
These early advances in the genomics, cell biology and structural biology of PI3K have established our contemporary understanding of how this kinase is activated1. Canonically, activation of RTKs, such as the insulin receptor, insulin-like growth factor 1 receptor (IGF1R) and epidermal growth factor receptors (including HER2), results in autophosphorylation of their C-terminal regions at key tyrosine residues within YXXM motifs. The phosphorylated tyrosines can bind to the N-terminal SH2 and inter SH2 domains of p85α, leading to allosteric relief of autoinhibitory contacts with the catalytic p110 subunit and thus to PI3K activation (FIG. 1c). In the context of malignancy, activating mutations in p110α or inactivating mutations in p85α can allosterically activate PI3K through two mechanisms (FIG. 1c): relief of autoinhibitory p85α contacts or increased membrane binding (FIG. 1b). Indeed, PIK3CA is one of the most frequently mutated oncogenes across human cancers20. Alternatively, RAS proteins, which associate with the cell membrane through fatty acid modifications at their C termini, can interact with the RAS-binding domain in the N-terminal region of p110α and activate PI3K through an increase in membrane binding and possibly through structural reorganization of the PI3K active site21,22 (FIG. 1c). Multiple phosphorylation sites have been discovered in both the catalytic and regulatory subunits of PI3K complexes23, the functional effects of which are unknown.
Improvements in next-generation sequencing have provided confirmation that PIK3CA is altered in virtually every cancer type, primarily through mutation20. Modelling the effects of PIK3CA mutations in vivo is challenging; although many reports demonstrate that PIK3CA mutations are sufficient to induce cell proliferation in vitro (in the absence of growth factors), most genetically engineered mouse models with PIK3CA mutations have long tumour latency times, sometimes requiring 1 year for tumour growth. Moreover, the resulting tumours are often sarcomas or have mixed histologies, even when tissue-specific promoters are used24, in contrast to the most frequent PIK3CA-mutant tumour types in patients with cancer, which are adenocarcinomas. Despite stark mechanistic and biochemical differences between the E545K and H1047R mutations, no robust studies have demonstrated profound functional differences between these mutations with regards to oncogenesis or response to therapy; however, differences in downstream protein phosphorylation signatures have been detected in cells bearing different PIK3CA mutations25 and in those with PIK3CA mutations versus PTEN deletions26. PIK3CA can also be amplified across cancer types, causing PI3K pathway activation through an allelic dose-dependent mechanism27. Other genetic alterations of the PI3K pathway found in patients with cancers include frequent PTEN mutations or deletions28,29 and rare AKT mutations9, and these alterations are not necessarily mutually exclusive with PIK3CA mutations. The simplest interpretation of all these findings is that PIK3CA mutation alone constitutes a ‘weak’ oncogenic driver.
Inhibiting the PI3K pathway in cancer
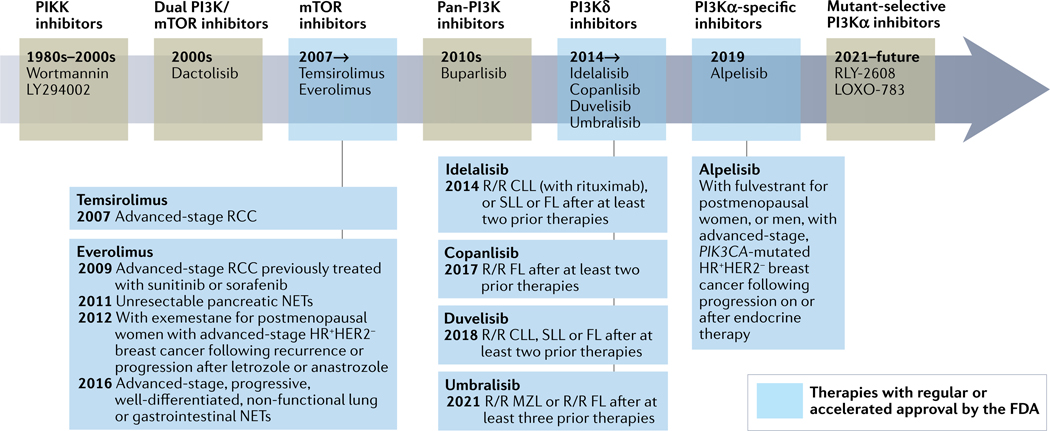
Targeting PI3K and the PI3K pathway has been a challenging undertaking through the decades, with innumerable preclinical studies, large-cohort randomized clinical trials and deep, data-rich correlative analyses resulting in only seven drugs that have received regular or accelerated approval by the FDA: everolimus (an mTOR inhibitor) for renal cell carcinoma, neuroendocrine tumours and oestrogen receptor-positive (ER+) breast cancer in combination with anti-oestrogen therapies; temsirolimus (mTOR inhibitor) in renal cell carcinoma; alpelisib (PI3Kα inhibitor) with fulvestrant in PIK3CA-mutant ER+ breast cancer; and idelalisib (PI3Kδ inhibitor), copanlisib (PI3Kα/δ inhibitor), duvelisib (PI3Kγ/δ inhibitor) and umbralisib (PI3Kδ/CK1ε inhibitor) for B cell chronic lymphocytic leukaemia and/or certain low-grade B cell lymphomas (FIG. 2). One interpretation of this situation is that our therapeutic leveraging of the PI3K pathway has been suboptimal. An alternative reading is that it is remarkable to have identified any therapeutic window for drugs targeting the PI3K pathway given that this pathway has important functions in normal homeostasis and physiology, including insulin signalling. Thus, inhibiting PI3Kα, AKT and/or mTOR would inevitably result in adverse events owing to the inhibition of insulin signalling. Here, we discuss key clinical advances in drugging the PI3K pathway as well as the associated adverse effects and interventions to manage the toxicities.
Fig. 2 |. Drugging the PI3K pathway through the decades.
Timeline summarizing phosphatidylinositol 3-kinase (PI3K) pathway inhibitor drug development. FDA-approved drugs and their indications are boxed. CLL, chronic lymphocytic leukaemia; FL, follicular lymphoma; HR, hormone receptor; MZL, marginal zone lymphoma; NETs, neuroendocrine tumours; PIKK, PI3K-related kinase; RCC, renal cell carcinoma; R/R, relapse and/or refractory; SLL, small lymphocytic lymphoma.
Drugging PI3K.
Targeting PI3K clinically has necessitated strategies to overcome substantial obstacles relating to small-molecule specificity, to identify tumour subsets with PI3K pathway dependence, and to mitigate and/or manage on-target and off-target toxicities. The various PI3Ks are structurally related to each other and to several other members of the PI3K-related kinase (PIKK) family, which includes mTOR as well as ATM and ATR. Non-specific PIKK inhibitors, including the covalent inhibitor wortmannin30,31 and the reversible inhibitor LY294002 (REF.32), were used as tool compounds that led to the development of dual PI3K/mTOR inhibitors such as dactolisib32 and omipalisib (FIG. 2). However, even these selective PI3K/mTOR inhibitors had high levels of toxicity in early phase trials33–35, limiting their later-phase clinical development. Notwithstanding, omipalisib continues to be developed for idiopathic pulmonary fibrosis, a disease in which PI3K inhibition reduces collagen synthesis36,37.
Subsequent development of the pan-PI3K inhibitor buparlisib (FIG. 2), which targets all p110 isoforms, led to the realization that PI3K inhibitor monotherapy has limited clinical efficacy38, spurring efforts to identify the mechanisms of adaptive resistance. Data showing that PI3K inhibitor treatment increases ER-dependent transcription39,40 provided the rationale to investigate buparlisib in combination with fulvestrant in patients with metastatic ER+ breast cancer and also whether PI3K pathway activation (inferred from PIK3CA mutation or PTEN loss) serves as a biomarker of response41. In phase III trials, buparlisib resulted in small statistically significant improvements in progression-free survival (PFS) in patients who had disease progression after treatment with an aromatase inhibitor (that is, in the second line)42 or with an aromatase inhibitor and an mTOR inhibitor (third line)43; however, this agent was also associated with important on-target adverse effects (hyperglycaemia, rash and liver function test abnormalities) as well as psychiatric symptoms (depression and anxiety) attributed to its blood–brain barrier penetration, resulting in frequent treatment discontinuation. Therefore, buparlisib did not move forward in the drug development process. Nevertheless, these investigations of buparlisib remain relevant given that an exploratory analysis provided the first clinical evidence of improved PI3K inhibitor efficacy in the subgroup of patients with PIK3CA-mutant tumours (as detected using circulating tumour DNA)42.
Identification of this mutational biomarker paved the way for trials testing PI3Kα-selective inhibitors and/or PIK3CA-based patient selection. The addition of taselisib, a potent PI3Kα/γ/δ inhibitor, to fulvestrant was evaluated in patients with advanced-stage, previously treated PIK3CA-mutant ER+ breast cancer in the phase III SANDPIPER trial44. In this trial, PIK3CA mutations were detected in tumour tissue using real-time qualitative PCR companion diagnostic testing for 17 mutations, including E542K, E545K and H1047R. Reminiscent of buparlisib, taselisib resulted in a small but significant improvement in PFS (7.4 months versus 5.4 months with placebo plus fulvestrant; HR 0.70; P = 0.0037), with frequent high-grade diarrhoea and hyperglycaemia44. Alpelisib, a PI3Kα-specific inhibitor, was also tested in combination with fulvestrant in patients with advanced-stage, previously treated PIK3CA-mutant ER+ breast cancer in the phase III SOLAR-1 trial45. In SOLAR-1, PIK3CA mutations were detected using an alternative tumour tissue-based companion diagnostic PCR test for 11 mutations, including the most common hotspots. Alpelisib was associated with improved PFS (11.0 months versus 5.7 months with placebo plus fulvestrant; HR 0.65; P <0.001) and frequent high-grade, but manageable, hyperglycaemia and rash45. This favourable benefit to risk ratio led to the FDA approval of alpelisib plus fulvestrant for advanced-stage PIK3CA-mutant ER+ breast cancer in May 2019 (REF.46) (FIG. 2). Alpelisib did not significantly improve overall survival (OS) in SOLAR-1 (although the median OS duration was ~8 months longer than in the fulvestrant-only arm), which is not attributable to crossover given that it was not permitted on trial47. PI3Kα inhibitors have also been tested in other cancer types in early phase trials48,49, with alpelisib producing disease control rates of 68% in patients with PIK3CA-altered head and neck squamous cell carcinoma and 100% in those with PIK3CA-altered cervical cancer49.
Why did alpelisib succeed where other PI3K inhibitors failed? Alpelisib, although less potent than taselisib, is more specific for the p110α isoform, which is a major oncogenic driver of the tumours targeted in the aforementioned studies, and this specificity probably also explains why alpelisib was associated with higher rates of grade ≥3 hyperglycaemia and rash than taselisib (37% and 10% versus 11% and 4%, respectively)44,45. Notably, however, no clinically meaningful decrement in health-related quality of life was reported by patients receiving alpelisib in SOLAR-1 (REF.50). SOLAR-1 initially had a liberal haemoglobin A1c (HbA1c) inclusion criterion (<8%); however, after 50–60% of patients had enrolled, the protocol was amended for improved monitoring and management of hyperglycaemia and rash51. Specifically, the HbA1c inclusion criterion was modified to <6.5%, lifestyle modifications were recommended at patient enrolment, a day 8 clinic visit was added to monitor for hyperglycaemia and health-care consultations were offered to patients with prediabetes; dermatology consultation, topical steroids and oral antihistamines were recommended for any rash of any grade, and a day 8 clinic visit was added to monitor for rash. Moreover, insulin was used primarily to manage acute hyperglycaemia but not as an outpatient maintenance strategy. Together, these pharmacological characteristics and adverse event management strategies might explain why patients receiving alpelisib had better PFS than those receiving other PI3K inhibitors and will guide future clinical trial design.
Drugging mTOR and AKT.
As alluded to above, the mTOR inhibitors everolimus and temsirolimus were the first FDA-approved drugs targeting the PI3K pathway, with multiple indications (FIG. 2). mTOR inhibitors induce complex feedback mechanisms in various cancer types; these effects have been discussed previously in several comprehensive reviews52,53. In patients with breast cancer, PIK3CA mutations are not a biomarker of response to everolimus, which is an allosteric mTORC1-selective inhibitor; however, in other cancer types, rare TSC1 and TSC2 mutations have been reported as predictive biomarkers of response54,55 and rare MTOR mutations as biomarkers of resistance55. Class effects of mTOR inhibitors include stomatitis, hyperglycaemia, rash, hyperlipidaemia, diarrhoea and pneumonitis. Dexamethasone mouthwash has been shown to decrease the incidence of everolimus-induced stomatitis from 33% to 2%56 and is now in standard use. mTOR inhibitors remain an important class of PI3K pathway inhibitors but, given their complex physiological feedback mechanisms52, failure to demonstrate an OS benefit57–60, limited efficacy in prior studies in many disease settings and the lack of robust predictive biomarkers, other targets such as AKT are being more vigorously pursued.
Currently, no AKT inhibitors are approved by the FDA for cancer therapy. Early allosteric pan-AKT inhibitors are safe and tolerable but induce high rates of hyperglycaemia, nausea, fatigue and rash61–64. More recently, two novel active-site pan-AKT inhibitors, capivasertib and ipatasertib, demonstrated favourable toxicity profiles with small and inconsistent efficacy signals in early phase clinical trials. Capivasertib monotherapy was first tested in a phase I trial in patients with advanced-stage solid tumours, including PIK3CA-mutant breast and gynaecological cancers, using three different dosing schedules: continuous dosing; 4 days on, 3 days off; and 2 days on, 5 days off65. Notably, the toxicity profiles of these regimens were different, with dose-limiting rash and diarrhoea for continuous dosing versus hyperglycaemia for the intermittent ‘2/5’ schedule65. Surprisingly, no dose-limiting toxicities were identified for the ‘4/3’ schedule65. Ipatasertib was tested in a phase I trial in patients with various solid tumours at escalating doses on a ‘21/7’ schedule and had a toxicity profile characterized by diarrhoea, nausea and, less frequently, hyperglycaemia66. Overall, ipatasertib and capivasertib have been associated with low objective response rates (0–20%) as monotherapies but with less hyperglycaemia and rash compared with PI3K inhibitors. These compounds are now being tested as combination therapies in randomized phase II–III trials across multiple cancer types but predominantly in breast cancers (Supplementary Table 1).
A randomized phase II trial of capivasertib added to weekly paclitaxel for advanced-stage ER+ breast cancer did not show a PFS benefit in the overall population or the PIK3CA-mutant subgroup67. However, in the phase II FAKTION trial testing fulvestrant with or without capivasertib in this setting, median PFS was 10.3 months in the capivasertib group versus 4.8 months in the placebo group (HR 0.58; P = 0.0018), with the median OS data trending towards a benefit in the capivasertib arm68. Clinical trials with biomarker stratification have not shown a differential survival advantage in patients with ER+ breast cancers harbouring AKT1 E17K mutations69,70 or other PI3K pathway mutations. These findings have led to the ongoing placebo-controlled phase III CAPItello-291 and CAPItello-292 trials testing the addition of capivasertib to fulvestrant71 and to fulvestrant plus palbociclib72, respectively, in patients with previously treated advanced-stage ER+ breast cancer (Supplementary Table 1).
In patients with metastatic triple-negative breast cancer (TNBC), the phase II PAKT study tested paclitaxel with or without capivasertib in the first-line setting73. Capivasertib was tested at 400 mg twice daily, 4 days on, 3 days off. This trial also included a specific subgroup of patients with PI3K pathway activation as defined by either PIK3CA, AKT or PTEN alterations. The median PFS duration was 5.9 months with capivasertib plus paclitaxel versus 4.2 months with paclitaxel alone (HR 0.74; P = 0.06) and the median OS duration was 19.1 months versus 12.6 months (HR 0.61; P = 0.04). In the subgroup with PI3K pathway activation, median PFS was 9.3 months with capivasertib versus 3.7 months with paclitaxel alone (HR 0.30; P = 0.01). These results have led to the phase III CAPItello-290 trial testing capivasertib plus paclitaxel as first-line treatment for metastatic TNBC74 (Supplementary Table 1). Notably, ipatasertib plus paclitaxel resulted in improved PFS versus paclitaxel alone in this setting in the phase II LOTUS trial75, although this was not replicated in the pivotal phase III IPATunity130 trial testing the same combination in patients with advanced-stage PI3K pathway-altered TNBC76.
In patients with metastatic castration-resistant prostate cancer, a phase Ib/II trial showed that combining ipatasertib with abiraterone improved the durability of responses compared to abiraterone alone, although the study was not powered to detect statistically significant differences in survival outcomes77. The subsequent phase III IPATential150 trial in this setting showed a small improvement in radiographic PFS with the addition of ipatasertib (median 18.5 months versus 16.5 months with placebo plus abiraterone; HR 0.77; P = 0.034) in patients with PTEN loss (which was a prespecified co-primary end point) but no improvement in PFS in the intention-to-treat population78.
In summary, mTOR, PI3Kδ and PI3Kα inhibitors have all been established as standard-of-care therapies (FIG. 2). PI3Kα inhibitors have important on-target toxicities that can require reductions in dose intensity for some patients, necessitating careful clinical management. Active-site AKT inhibitors are promising therapies with a favourable toxicity profile compared with PI3Kα inhibitors, albeit with unclear efficacy at present. PIK3CA mutations are biomarkers of a favourable response to PI3Kα inhibitors but not to mTOR or AKT inhibitors.
Mechanisms limiting efficacy
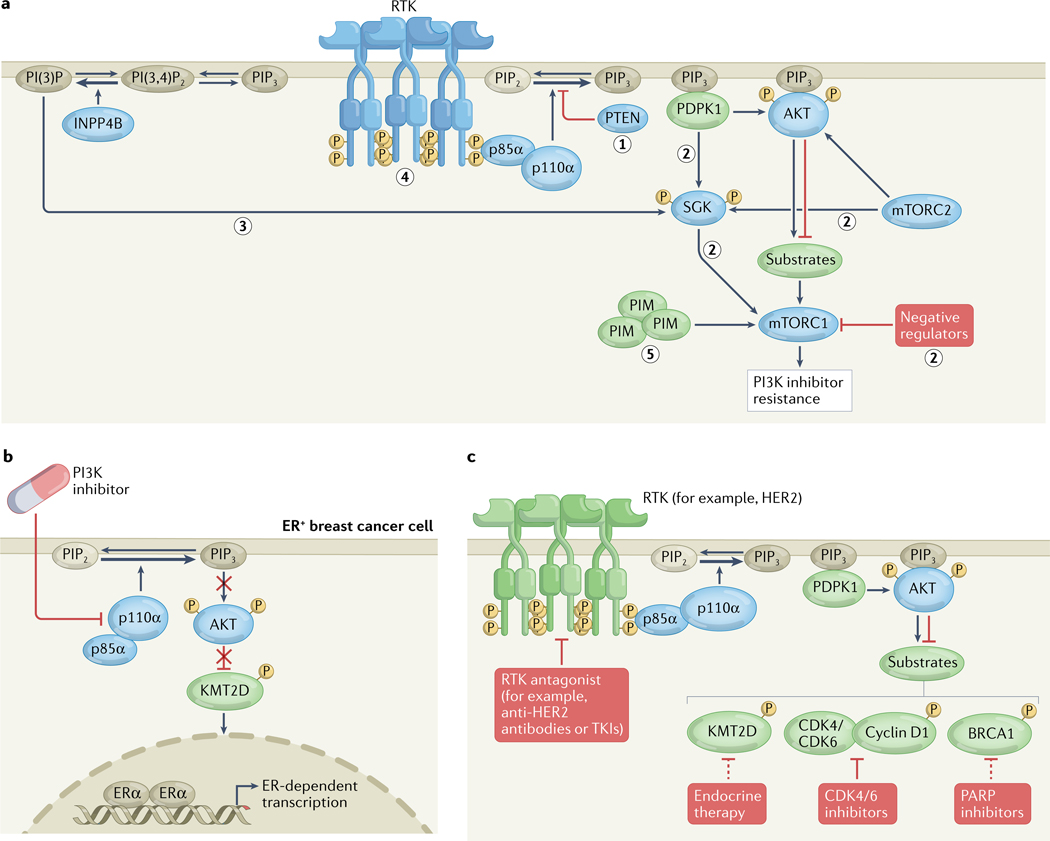
Many PI3K inhibitor resistance mechanisms have been elucidated using preclinical and clinical samples2–4 (FIG. 3a–c). Inactivating PTEN mutations are the most common acquired resistance mutations and were first identified as polyclonal alterations in multiple metastases from a single patient who initially had an exceptional response to alpelisib monotherapy79. Subsequently, PTEN resistance mutations were confirmed in patients in a phase I/II trial of alpelisib combined with an aromatase inhibitor, with a frequency of 25% among alpelisib-resistant tumours80. In preclinical models, acquired PTEN mutations in PIK3CA-mutant breast cancers (FIG. 3a) lead to an increased dependence on PI3Kβ signalling, such that simultaneous treatment with a PI3Kβ inhibitor can resensitize tumours to PI3Kα inhibitors79, although this effect has not been demonstrated in patients. Notably, secondary mutations in PIK3CA have not been reported as a resistance mechanism to PI3Kα inhibitors; this fact, combined with the observation that no oncogenic mutations have been reported in the PI3K active site81, point to the evolutionary constraints on PI3K catalysis.
Fig. 3 |. Signalling and epigenetic mechanisms limiting the efficacy of PI3K inhibitors.
a | Classes of signalling resistance mechanisms to phosphatidylinositol 3-kinase (PI3K) inhibitors include (1) PTEN mutation, (2) AKT-independent activation of mTOR complex 1 (mTORC1) signalling by alternative kinases or via loss of negative regulators (such as subunits of the TSC complex), (3) INPP4B amplification leading to phosphatidylinositol 3-phosphate (PI(3)P)-mediated SGK signalling, (4) receptor tyrosine kinase (RTK) amplification or mutation, and (5) mTORC1 activation through crosstalk with other signalling pathways. b | Adaptive resistance mechanism to PI3K inhibitors in oestrogen receptor-positive (ER+) breast cancer cells, whereby PI3K inhibition decreases AKT phosphorylation of the histone H3 lysine 4 (H3K4) monomethyltransferase KMT2D, leading to KMT2D activation, H3K4 methylation and an open chromatin state at gene enhancers, and thus to increased ER-dependent transcription. c | Key targets (shown in green) upstream and downstream of PI3K–AKT that, when inhibited, might overcome resistance to PI3K pathway inhibitors. mTORC2, mTOR complex 2; PI(3,4)P2, phosphatidylinositol 3,4-bisphosphate; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; TKIs, tyrosine kinase inhibitors.
One straightforward tool to discover mechanisms of pharmacological resistance involves first generating drug-resistant cell lines through months of exposure in vitro and then performing large-scale analyses such as next-generation sequencing82. Nevertheless, cell lines do not become resistant to PI3K inhibitors even over long periods of time; the reasons for this are unclear. Consequently, several groups have used genetic and pharmacological screens to determine signalling-based mechanisms of resistance to PI3K inhibitors in vitro. These alternative mechanisms fall into two main classes (FIG. 3a): (1) AKT-independent mTORC1 activation83, which can occur through PDPK1–SGK1 activation84, INPP4B–SGK3 activation85, PIM kinase overexpression86 or genetic loss of negative regulators of mTORC1 (REF.87) (such as subunits of the TSC, GATOR1 or KICSTOR complexes); or (2) overexpression of upstream RTKs88, including HER3 (REF.88), IGF1R89,90, the insulin receptor88, AXL91 or FGFR1 (REF.92), owing to disruption of negative feedback mechanisms. However, strategies combining PI3K inhibitors with inhibitors of mTORC1/2 (REF.93), IGF1R94 or FGFR1 (REF.95) have not proven clinically effective owing to additive toxicities.
The elucidation of epigenetic mechanisms of resistance to PI3K inhibitors in ER+ breast cancer was crucial to the clinical investigations of PI3Kα inhibitors in combination with anti-oestrogen therapies. As mentioned previously, PI3Kα inhibition was found to lead to increased ER-dependent transcription in ER+ breast cancer cell lines, xenograft models and patient samples, resulting in adaptive resistance39,40. This crosstalk is mediated by the histone H3 lysine 4 monomethyltransferase KMT2D, which is a substrate of AKT and SGK1. Specifically, PI3K inhibitors suppress AKT and SGK phosphorylation, thereby decreasing inhibitory phosphorylation of KMT2D and thus enhancing KMT2D activity, which in turn results in an open chromatin state and increased ER-dependent transcription96,97 (FIG. 3b). This mechanism is conserved throughout the PI3K pathway, such that AKT inhibitors also increase ER-dependent transcription97.
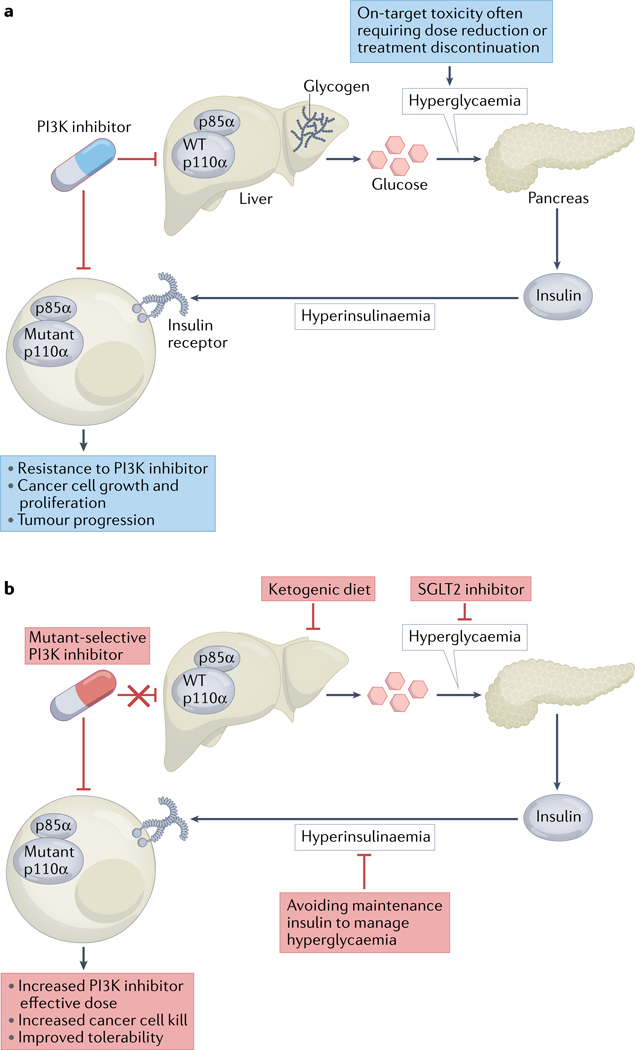
Understanding and co-opting metabolic mechanisms of adaptive resistance to PI3K inhibitors is a major advance that unites the known mechanisms of PI3K in both insulin signalling and cancer cell growth. PI3K inhibitors, including alpelisib, inhibit wild-type PI3K and insulin signalling in non-malignant tissues as well as in cancer cells (FIG. 4a). The inhibition of wild-type PI3K results in decreased glucose transport into hepatocytes and adipocytes, thus causing hyperglycaemia and a subsequent increase in insulin release from the pancreas. Patients with insulin resistance often reach a threshold of hyperglycaemia requiring PI3K inhibitor dose reduction or treatment cessation. Moreover, the compensatory hyperinsulinemia provides an oncogenic growth factor (insulin) that can restimulate the insulin receptor and PI3K signalling in cancer cells, thereby increasing their growth and proliferation, even in tumours treated with PI3K inhibitors98 (FIG. 4a). In preclinical models, co-treatment with sodium–glucose co-transporter 2 (SGLT2) inhibitors, which decrease renal reabsorption of glucose and are approved therapies for type 2 diabetes, leads to decreased hyperglycaemia, PI3K signalling and tumour growth, whereas co-treatment with the anti-hyperglycaemic AMPK activator metformin does not98 (FIG. 4b). Dietary modulation with a ketogenic diet, which can reduce hepatic glycogenolysis, has also been shown to decrease hyperglycaemia and tumour growth in a variety of preclinical models99 (FIG. 4b). Notably, these combination effects with a ketogenic diet were observed irrespective of tumour genotype (that is, in PIK3CA-mutant and PIK3CA-wild-type tumours) and with multiple classes of PI3K inhibitors, including pan-PI3K inhibitors and dual PI3K/mTOR inhibitors98. Ketogenic diets might exert further anticancer effects in addition to disruption of insulin–glucose feedback100. For example, the ketone body β-hydroxybutyrate can bind to and inhibit histone deacetylases101, which might be germane for ER+ breast cancer, a setting in which histone deacetylase inhibitors have demonstrated clinical efficacy102.
Fig. 4 |. Metabolic mechanisms limiting the efficacy of PI3K inhibitors.
a | Phosphatidylinositol 3-kinase (PI3K) inhibitors suppress the activity of not only mutant PI3K in cancer cells but also wild-type (WT) PI3K in non-malignant cells of the liver and other tissues, leading to decreased glucose uptake, hyperglycaemia, and compensatory insulin secretion by the pancreas and thus hyperinsulinaemia. As a result, insulin receptor signalling can restimulate the growth and proliferation of cancer cells, thus attenuating the effects of PI3K inhibitors. b | A ketogenic diet, sodium–glucose co-transporter 2 (SGLT2) inhibitors, mutant-selective PI3K inhibitors and avoidance of maintenance insulin to manage hyperglycaemia are predicted to increase the effective dose, anticancer effects and tolerability of PI3K inhibitors.
Increasing the therapeutic window
Decades of fundamental research have led to the development of PI3K, mTOR and AKT inhibitors; however, the therapeutic window of such agents will need to be increased using combination therapies, mutant-selective inhibitors, biomarker-selected patient populations and adverse effect-mitigation strategies if we are to more effectively target the activated PI3K pathway in cancer. Here, we discuss several new strategies to improve the efficacy of PI3K pathway inhibitors, with emphasis on metabolic mechanisms.
Disrupting insulin signalling.
As outlined above, interventions to disrupt glucose–insulin signalling are a prime mechanism to increase the therapeutic efficacy of PI3K inhibitors (FIG. 4b). The randomized phase II TIFA trial103 is testing whether a ketogenic diet (<35 g of carbohydrates daily), a low-carbohydrate diet (<100 g daily) or the SGLT2 inhibitor canaglifozin can lower the incidence of grade 3–4 hyperglycaemia and might also improve PI3K inhibitor efficacy in patients with metastatic PIK3CA-mutant ER+ breast cancer receiving alpelisib and fulvestrant (Supplementary Table 1). In the clinical trial literature, ketogenic diets vary with respect to carbohydrate content; therefore, determining whether a carbohydrate dose–response relationship exists will be important. Continuous glucose-monitoring devices and patient-reported outcomes might further define the kinetics of hyperglycaemia during PI3K inhibitor treatment as well as additional patient populations for interventions.
While the initial preclinical data supporting the ketogenic diet hypothesis tested PI3K inhibitor monotherapy, data also indicate that dietary modulation might improve response to endocrine therapy in patients with ER+ breast cancer. Caffa et al.104 showed that intermittent fasting or a high-fat, fasting-mimicking diet (FMD) improves the efficacy of tamoxifen and fulvestrant in mouse models. Mechanistically, the FMD led to decreased IGF1 and leptin levels and thus reduced AKT signalling, which resulted in upregulation of the EGR1 transcription factor that in turn increased expression of PTEN104. Feasibility and on-target effects have been demonstrated in patients receiving a 5-day FMD every 3–4 weeks, including decreased IGF1, leptin and blood glucose levels104 as well as systemic and anti-tumour immunomodulatory effects105. Additional preclinical and clinical studies are warranted to further dissect how dietary interventions differentially modulate the PI3K pathway and the response to PI3K inhibitors, and whether this approach could lead to increased PI3K inhibitor efficacy in tumour types beyond breast cancer.
Using mutant-selective PI3K inhibitors.
PI3K pathway dependence leads to mutant-specific PI3K inhibition at the cellular level, even though all PI3K inhibitors suppress the activity of mutant and wild-type PI3Ks equally at the protein level. Nevertheless, inhibition of wild-type PI3K is a liability at the organismal level owing to the disruption of insulin signalling (FIG. 4a). Developing mutant-specific inhibitors that do not target wild-type PI3K would avoid this on-target adverse effect and thereby improve PI3K inhibitor efficacy (FIG. 4b). E545K and H1047R are allosteric mutations that result in increased thermal instability of PI3K in vitro106, which suggests a conformational change compared with the wild-type protein that could be exploited for the development of mutant-specific inhibitors.
Inavolisib is a potent PI3Kα inhibitor107 currently being tested in patients with breast cancer108,109. In addition to inhibiting the PI3K active site, inavolisib has been shown to induce proteasomal degradation of mutant PI3Kα in cells, with the mutant proteins having increased levels of ubiquitination and shorter half-lives than wild-type PI3Kα; these effects were more pronounced for the H1047R mutant than for the E545K mutant110. AKT had previously been shown to exert strong negative feedback on RTK expression, which is released upon PI3K pathway inhibition88. This feedback mechanism might explain the finding that inavolisib-mediated degradation of mutant PI3Kα was more pronounced in HER2+ breast cancer cells, resulting in greater potency compared with alpelisib; in HER2− PIK3CA-mutant cells, the effects were more similar between the two drugs110. Correlative proteomic studies of tumours from patients who have received inavolisib would clinically corroborate this novel mechanism.
The notion that a PI3K inhibitor can cause mutant-specific degradation as well as active-site inhibition, both at the protein level, raises the possibility of alternative drug-binding conformations. Early crystallographic studies of wild-type PI3Kα bound to the tool compound PIK-108 surprisingly revealed a second drug-binding site in a hydrophobic pocket within the C-terminal kinase domain but located far from the active site and close to the frequently mutated H1047 residue111. This second binding site might have an allosteric function given that, in other crystal structures of PI3K, the kinase activation loop (which is necessary for catalysis) occupies the hydrophobic pocket. Recognition of this additional binding site, coupled with the practical need for allosteric inhibitors, has inspired the development of a new generation of mutant-selective PI3Kα inhibitors (FIG. 4b), including RLY-2608 (REF.112) and LOXO-783 (REF.113) (FIG. 2).
Biochemically, RLY-2608 binds specifically and with faster kinetics to mutant PI3Kα (relative to the wild-type form) and can still bind as avidly in the presence of an active-site inhibitor, suggesting a non-competitive mode of action. In contrast to alpelisib or inavolisib, which are equipotent for mutant and wild-type PI3Kα and also have micromolar potency against other PI3K isoforms, RLY-2608 has tenfold less potency for wild-type versus mutant PI3Kα and is inactive against the p110β, p110γ and p110δ isoforms112. Similarly, LOXO-783 can decrease the enzymatic activity of mutant PI3Kα by 95%, compared with 0–15% inhibition of the p110β, p110γ and p110δ isoforms113. Both RLY-2608 and LOXO-783 inhibit PI3K signalling and cell proliferation to a substantially greater extent in PIK3CA-mutant versus PIK3CA-wild-type breast cancer cells112,113. However, these compounds might have differences in variant specificity given that RLY-2608 inhibits signalling and proliferation of ER+HER2– breast cancer cells harbouring either E545K or H1047R mutations112, whereas LOXO-783 has only been reported to inhibit PI3Kα H1047R and does not inhibit the K111N mutant113. In mouse PIK3CA-mutant ER+HER2– breast cancer xenografts, RLY-2608 decreased levels of phosphorylated AKT in a dose-dependent manner and also reduced tumour growth112. LOXO-783 also induced tumour regression at all dose levels tested in a patient-derived xenograft model of ER+HER2– breast cancer with resistance to CDK4/6 inhibitors113. Crucially, for both compounds, these decreases in tumour growth were associated with little to no hyperinsulinaemia, in contrast to alpelisib. LOXO-783 also demonstrates blood–brain barrier penetration and intracranial activity in mouse xenograft models of PIK3CAH1047R-mutant breast cancer113.
Additional structural studies of PI3K would spur the development of alternative mutant-specific inhibitors. For example, a cryoelectron microscopy structure of full-length wild-type PI3Kα suggests that the N-terminal SH3 and RhoGAP domains of p85α, which are not resolved in crystal structures, are stabilized by PI3Kα inhibitors, thus preventing access of the active site to the cell membrane, ATP and phosphoinositides114. How the structure of full-length PI3Kα is altered by different PIK3CA mutations has previously been probed using mass spectrometry to measure solvent exchange115. Together, these studies pave the way for high-resolution structures of full-length mutant PI3Kα.
Exploiting metabolic diagnostics.
Faster tumoural readouts of PI3K inhibitor response and resistance, before changes are detectable on standard surveillance imaging, would enable the streamlining of patients to effective therapies. PI3K inhibitors suppress glycolysis through multiple mechanisms, including by lowering MYC and HIF1α levels, leading to decreased expression of the glycolytic enzymes hexokinase and lactate dehydrogenase (LDH)116. 2-Deoxy-2-[18F]fluoro-D-glucose (FDG) PET scans are often used to stage cancers and monitor response to therapy and are dependent on the activity of hexokinase, which phosphorylates and traps FDG in metabolically active cells, including cancer cells. Other metabolic imaging techniques, such as 13C magnetic resonance spectroscopy (MRS), can be used to measure changes in radiolabelled pyruvate exchange with tumour lactate, which is catalysed by LDH. Both FDG-PET and 13C-MRS have been demonstrated to detect PI3K inhibition in preclinical models and in patients117,118.
Ros et al.119 found that PI3K inhibitor sensitivity of breast cancer cells correlated with decreased 13C exchange, reduced LDH activity and decreased expression of the transcription factor FOXM1 upon drug treatment, whereas resistant cells showed increased 13C exchange and similar levels of LDH activity and FOXM1 expression during treatment. Importantly, these metabolic changes were observed in drug-sensitive and drug-resistant cells before tumour shrinkage or growth, respectively119. By contrast, hexokinase activity was not altered as a function of drug sensitivity or resistance, and no differential changes were observed with FDG-PET imaging119. Notably, AKT phosphorylates FOXO3, which can drive FOXM1 expression, and FOXM1 was found to be upregulated in some patients with PI3K inhibitor-resistant breast cancer119. Thus, 13C-MRS imaging and FOXM1 expression might inform biomarker strategies to identify patients who are not responding to PI3K inhibitor therapy.
Metabolic fingerprinting using a surgical device coupled to rapid mass spectrometry is under active investigation to determine lipid-based signatures in cancers. Koundoros et al.120 used this tool to perform unbiased lipidomics of breast cancers, which revealed that elevated levels of arachidonic acid and its eicosanoid derivatives are enriched in PIK3CA-mutant tumours, and these lipids stimulated cancer cell growth. The authors determined the mechanism of action connecting PI3K to arachidonic acid, whereby mTORC2 phosphorylates PKCζ, which in turn phosphorylates cytosolic/calcium-dependent phospholipase (cPLA2) and thus activates the cleavage of phospholipids to generate arachidonic acid. Importantly, PIK3CA-mutant but not PIK3CA-wild-type TNBC xenografts were selectively sensitive to pharmacological inhibition of cPLA2, and this sensitivity was reversed with arachidonic acid supplementation. Additional unbiased lipidomics analyses of tumours from patients treated with PI3K inhibitors might elucidate other metabolic mechanisms to improve PI3K inhibitor efficacy.
Leveraging metabolic pathways of PI3K.
AKT phosphorylates a host of substrates crucial for both normal physiological and cancer metabolism121. Fundamental research over the past decades has shown that AKT phosphorylates and activates hexokinase122 (glycolysis), 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase123 (glycolysis regulation), transketolase124 (non-oxidative pentose phosphate pathway), NAD kinase125 (oxidative pentose phosphate pathway), ATP citrate lyase126 (de novo lipid synthesis) and PRAS40 (REF.127) (mTORC1 signalling). AKT also phosphorylates and inhibits TXNIP128, thereby increasing glucose uptake, and TSC2 (REF.129), thus increasing protein and pyrimidine synthesis. The majority of these mechanisms have been demonstrated primarily in non-transformed cells, and therefore how these metabolic processes are differentially altered in PI3K pathway-activated cancer cells of treatment-naive tumours versus treatment-resistant tumours remains unknown. Moreover, PI3Ks can mediate metabolic pathways independently of AKT, for example, through Rac GTPases that evict aldolase from the actin cytoskeleton and increase aldolase activity130. Phosphoproteomic and metabolomic analysis of tumours from patients treated with PI3K pathway inhibitors would provide insights into whether these metabolic enzymes might be additional targets for co-inhibition together with PI3K, AKT or mTOR.
Developing heterofunctional drugs.
New drug classes that have greater selectivity and are able to more completely suppress downstream signalling might overcome the efficacy and tolerability issues of current AKT and mTOR inhibitors. Many AKT inhibitors, including ipatasertib and capivasertib, also inhibit other AGC family kinases. Two groups have developed AKT proteolysis targeting chimeras, which consist of an E3 ubiquitin ligase-targeting moiety linked to an AKT inhibitor and, therefore, result in AKT1/2/3 ubiquitination and subsequent degradation. INY-03–041 (REF.131) and MS21 (REF.132) are potent and selective pan-AKT proteolysis targeting chimeras based on ipatasertib and capivasertib, respectively. Both INY-03–041 and MS21 result in an increased amplitude and duration of signalling inhibition and thus in improved growth suppression compared with ipatasertib or capivasertib, respectively131,132. Notably, MS21 did induce hyperglycaemia in mouse xenografts but to lower levels and with slower kinetics compared with capivasertib132.
Hyperglycaemia and disruption of negative feedback control of RTK expression, owing to mTORC2 inhibition and subsequent suppression of AKT activity, are therapeutic liabilities of mTOR inhibitors that target both mTORC1 and mTORC2. mTORC1-selective bi-steric inhibitors of mTOR have been developed by fusing the allosteric inhibitor rapamycin to mTOR active-site inhibitors and decrease S6K and 4EBP1 phosphorylation but not mTORC2 or AKT phosphorylation133. Accordingly, these mTORC1-selective inhibitors result in potent inhibition of tumour growth but do not increase hyperglycaemia or HER3 expression133 and, therefore, have an improved therapeutic index.
Identifying new predictive biomarkers.
Clinical trials have established that PIK3CA mutations are a clinically relevant biomarker of response to PI3K inhibitors in patients with ER+ breast cancer44,45, and indeed alpelisib is approved by the FDA specifically for PIK3CA-mutant ER+ breast cancer. Notably, however, not all PIK3CA mutations are created equal. The most common variants, E542K, E545K and H1047R (accounting for ~60% of PIK3CA-mutant tumours in the COSMIC database134), predict for PI3K inhibitor sensitivity in preclinical models135 and in patients with advanced-stage ER+ breast cancer44,45. Nonetheless, rare variants sum to ~40% of PIK3CA-mutant tumours and have largely unknown effects on PI3K inhibitor sensitivity. Indeed, no published phase III trial or real-world response data link specific rare PIK3CA variants with PI3K inhibitor response. Moreover, preclinical modelling has been limited to date, with few studies investigating sensitivity or resistance to PI3K inhibitors such as alpelisib, and only for small numbers of variants106,136–138. Thus, rare PIK3CA variants (and also PIK3R1 variants) currently have unclear predictive value as biomarkers of PI3K inhibitor sensitivity.
Some patients with PIK3CA-mutant tumours have exceptional responses to PI3K inhibitors on clinical trials79, spurring investigations of additional biomarkers that might predict for increased sensitivity. Interestingly, double PIK3CA mutations in cis are found in 10–15% of PIK3CA-mutant tumours across cancer types106,139. Moreover, these double mutations confer higher levels of PI3K activity, increased oncogene dependence and greater PI3K inhibitor sensitivity in cell lines and in patients with breast cancer relative to single PIK3CA mutations106,139. This ‘gene dose–response’ effect is concordant with data indicating that homozygous PIK3CA mutations cause more transcriptional remodelling and dedifferentiation of stem cells compared with heterozygous mutations and that homozygous mutations are associated with a more-transformed cellular phenotype27. Therefore, a dosage model whereby additional PIK3CA mutations or mutant alleles potentiate PI3K pathway signalling unifies these findings and provides a systems biology framework to investigate pathway additivity and inhibition140. Double PIK3CA mutations define a biomarker-driven cohort targeted using inavolisib in the tumour-agnostic phase II TAPISTRY trial (NCT04589845; Supplementary Table 1) as well as in the first-in-human phase I trial of RLY-2608 (NCT05216432). Such biomarker approaches beyond mere single mutations might also reveal patients with tumours exquisitely dependent on PI3K signalling, who could potentially be treated with lower doses of PI3K pathway inhibitors to increase the therapeutic index141. For example, in adult patients with PIK3CA-related overgrowth spectrum germline genetic disorders, alpelisib doses of even 250 mg daily (the maximum tolerated dose in a phase I trial involving patients with solid tumours was 400 mg)49 are still associated with efficacy142.
Most studies that have adjudicated the effects of PIK3CA mutations in cells and on therapeutic responses have been performed in non-malignant breast or breast cancer models. Testing the effects of mutations in other differentiated cancer models might implicate non-hotspot mutations for targeting, such as C420R, which is the most oncogenic PIK3CA mutation in the context of glioblastoma143.
Combinations with PI3K inhibitors.
Given that PI3K– AKT–mTOR signalling intersects with virtually every notable cellular pathway, combination therapies with PI3K pathway inhibitors remain an area of widespread investigation. Hormone receptor-driven cancers have a high prevalence of PI3K pathway alterations, including PIK3CA mutations in ER+ breast cancers, PTEN mutations in prostate cancers, and both PIK3CA and PTEN mutations in endometrial cancers144. In PIK3CA-mutant ER+ breast cancers, KMT2D is phosphorylated by AKT, which attenuates its methyltransferase activity97 (FIG. 3b). Whether or not KMT2D phosphorylation is enriched in tumours with acquired resistance to PI3K inhibitors, whether this mechanism is relevant for other PIK3CA-mutant cancer types and what effects small-molecule inhibitors of KMT2D have in PIK3CA-mutant tumours all remain open questions. PTEN mutations predict PI3K inhibitor resistance in breast cancers79,80. However, combinations with AKT inhibitors, which would suppress the PI3Kα and PI3Kβ signalling pathways, both of which are active in the setting of PTEN loss, might prevent or overcome this resistance. This approach might also overcome resistance to anti-androgen therapies in patients with prostate cancers with PTEN loss, as suggested by the results of the IPATential150 trial of ipatasertib plus abiraterone78, and this hypothesis is being further tested in the phase III CAPItello-281 trial of capivasertib and abiraterone in patients with metastatic castration-sensitive prostate cancer with PTEN loss (NCT04493853; Supplementary Table 1).
CDK4/6 inhibitors improve PFS and OS in patients with advanced-stage ER+HER2− breast cancer145 and improve disease-free survival as adjuvant therapy in those with high-risk early stage ER+ breast cancer146 when combined with standard endocrine treatments. Alpelisib is effective in patients with breast cancer following first-line treatment with a CDK4/6 inhibitor plus an aromatase inhibitor147. In preclinical models, CDK4/6 inhibitors increase the efficacy of PI3K inhibitors against PIK3CA-mutant breast cancers148, and AKT can regulate cyclin D1 stability transcriptionally and post-transcriptionally via inhibition of GSK3β149 (FIG. 3c). Accordingly, several trials are testing combinations of CDK4/6 inhibitors with PI3K150, AKT and/or mTOR inhibitors151,152 (Supplementary Table 1).
RTK phosphopeptides stimulate wild-type PI3K activity, and RTK amplification (for example, of HER2) enhances PI3K signalling in PIK3CA-mutant tumours153 (FIG. 3c). PIK3CA mutations are a prognostic marker of unfavourable OS in patients with HER2+ breast cancer and predict worse responses to anti-HER2 antibodies, including trastuzumab and pertuzumab154, but not to antibody–drug conjugates such as ado-trastuzumab emtansine155. Therefore, multiple clinical trials are testing alpelisib in combination with anti-HER2 therapies for PIK3CA-mutant HER2+ breast cancer156,157 (Supplementary Table 1). Similar strategies of combining a PI3K inhibitor with RTK-targeted therapies include the addition of alpelisib to the anti-EGFR antibody cetuximab and radiotherapy in patients with head and neck squamous cell carcinomas158, which frequently harbour EGFR amplifications and PIK3CA mutations.
PI3K inhibition reduces BRCA1 expression and thereby increases DNA damage, such that PI3K inhibitor activity is enhanced by combination with the PARP inhibitor olaparib in TNBC159,160, prostate cancer161, small-cell lung cancer162 and ovarian cancer163. The combination of alpelisib and olaparib was tolerable and induced a response rate of ~30% in patients with platinum-resistant/refractory ovarian cancer, including in the group without germline BRCA mutations164. Interestingly, PARP inhibitor monotherapy is only associated with a response rate of ~5% in the germline BRCA-wild-type group164, suggesting enhanced activity when a PI3K inhibitor is added. The same combination has also been tested in a small trial involving patients with metastatic TNBC (n = 17) and demonstrated an 18% response rate; interestingly, none of the three patients who responded had a germline BRCA mutation165. These findings have led to phase III trials investigating alpelisib in combination with olaparib for ovarian cancer (NCT04729387; Supplementary Table 1). AKT and BRCA1 crosstalk mechanisms might explain these additive effects, whereby AKT phosphorylates BRCA1 and prevents its proteasomal degradation (FIG. 3c); in turn, BRCA1 ubiquitinates phosphorylated AKT, causing it to localize to the nucleus166,167.
Translational biomarker platforms
PI3K-dependent phenotypes are necessarily altered by genetic mutations, transcriptional and epigenetic modulation, proteomic changes, and cellular metabolism; therefore, multimodal analyses of human tumours before, during and after treatment are crucial to determining the on-target effects of PI3K pathway inhibitors and to identify new subsets of patients who are candidates for these therapies, across cancer types. Large numbers of studies, many outlined herein, have characterized genomic features of treatment-naive and drug-resistant PIK3CA-mutant tumours48,79,80,168. While PIK3CA mutations predict a response to PI3K inhibitor treatment in patients with ER+ breast cancer, whether this holds true in other cancer types remains unknown. The discovery of non-genomic tumour biomarkers of PI3K inhibitor response and resistance will guide future prospective clinical trials and combination strategies. Indeed, identifying transcriptional changes upon PI3K inhibition in ER+ breast cancers proved crucial to the clinical validation and approval of alpelisib in combination with fulvestrant for this indication39,40,97. Proteomic and phosphoproteomic studies of patient-derived models exposed to PI3K inhibitors have revealed alternative kinases that might mediate PI3K inhibitor resistance, such as NEK9 and MAP2K4 (REF.169). Additional transcriptomic and proteomic analyses could potentially identify new crosstalks between PI3K signalling and other pathways to guide future combination strategies in other tumour types.
Deeper inquiries into the mechanisms underlying the on-target adverse effects of PI3K pathway inhibitors are crucial to widening the therapeutic window. As demonstrated with immunotherapies, vigilant attention to adverse effects through frequent monitoring and the design of novel clinical trials incorporating modified management strategies can lead to improvements in tolerability, ensuring that more patients who are candidates for therapy (for example, patients with PIK3CA-mutant breast cancer) will ultimately receive therapy. To this end, real-world data on the outcomes of patients receiving PI3K pathway inhibitors will be important to determine the best practices that can be realistically implemented.
The metabolic advances in the field of PI3K research discussed herein also warrant reinvestigation and measurement of circulating growth factors and metabolites as paracrine drivers of PI3K signalling in metastatic cancer. Such correlative studies in patients are reminiscent of serum starvation and growth factor-stimulation studies in cellular models. Next-generation versions of the US National Cancer Institute’s Clinical Proteomic Tumour Analysis Consortium (CPTAC)170,171, which involve the analysis of patient-derived samples of not only primary treatment-naive tumours but also metastatic treatment-naive and treated tumours and integrate DNA sequencing, RNA sequencing, proteomics, phosphoproteomics and metabolomics, will generate a wealth of hypotheses for bench-to-bedside validation.
Conclusions
While therapeutic targeting of the PI3K pathway is at a crossroads, similarities are evident between new and old traversed paths. In translational science, the impasse between basic science discoveries and clinical treatments is sometimes referred to as the ‘valley of death’, given the high number of drugs that are efficacious in vitro, in vivo and in early phase clinical trials but ultimately not in late-phase trials. Targeting the PI3K pathway has overcome this valley of death, with multiple drugs having passed phase III trials and providing statistically and clinically meaningful improvements in patient survival. However, the field is now at a therapeutic window precipice, where small changes in drug specificity, adverse event management, drug combinations and biomarker-based patient selection can mean the difference between a successful and a wasted pharmacological expedition. However, an atlas exists to guide us around this barrier, namely, the scores of advances made over the past decades in understanding the metabolic and signalling roles of PI3K. Deeper explorations into these mechanisms will be crucial to moving PI3K pathway inhibitors from specific subsets of certain cancer types into the larger oncology therapeutic landscape.
Supplementary Material
Key points.
PIK3CA is one of the most frequently mutated genes in cancer; the PI3K pathway is altered in a large number of cancer types, driving cell signalling, growth and metabolism, and PI3K inhibitors have been in development for over four decades.
Seven drugs that target the PI3K pathway have received regular or accelerated approval from the FDA, including alpelisib in combination with fulvestrant for advanced-stage, PIK3CA-mutant, oestrogen receptor-positive breast cancer. AKT inhibitors and next-generation mTOR inhibitors are currently being tested in early phase and late-phase clinical trials.
Adaptive signalling, epigenetic and metabolic changes in cancer cells can limit the clinical efficacy of PI3K inhibitors. In addition, PI3K inhibition in non-cancer cells causes hyperglycaemia and other on-target adverse effects that limit patient tolerability, as well as hyperinsulinaemia, which can lead to reactivation of cancer cells; the net effect of these alterations is attenuation of PI3K inhibitor efficacy.
Targeting insulin signalling, improved management of hyperglycaemia, development of mutant-specific PI3K inhibitors, and validation of refined biomarkers of response and resistance are novel translational strategies currently being tested to improve the therapeutic window of PI3K inhibitors in patients with cancer.
Genomic, transcriptomic, proteomic, phosphoproteomic and metabolomic analyses of patients with PIK3CA-mutant tumours receiving PI3K inhibitors are needed to discover new pathways for combination therapies in different cancer types.
Acknowledgements
The authors wish to acknowledge the many seminal papers on this subject that they could not discuss and cite owing to space and editorial limits. The authors acknowledge support from the NIH (grants K08 CA245192 to N.V. and R35 CA197588 to L.C.C.) and the Susan G. Komen Breast Cancer Foundation (to N.V.).
APPENDIX
RELATED LINKS
Footnotes
Competing interests
N.V. reports consulting activities for Novartis and is on the scientific advisory board of Heligenics. L.C.C. is a founder, shareholder and member of the scientific advisory board of Agios Pharmaceuticals, a co-founder and shareholder of Faeth Therapeutics, and a founder and former member of the scientific advisory board of Ravenna Pharmaceuticals (previously Petra Pharmaceuticals); these companies are all developing therapies for cancer. L.C.C. has also received research funding from Ravenna Pharmaceuticals.
Supplementary information
The online version contains supplementary material available at https://doi.org/10.1038/s41571-022-00633-1.
References
- 1.Fruman DA. et al. The PI3K pathway in human disease. Cell 170, 605–635 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanhaesebroeck B, Perry MWD, Brown JR, Andre F. & Okkenhaug K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov 20, 741–769 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castel P, Toska E, Engelman JA & Scaltriti M. The present and future of PI3K inhibitors for cancer therapy. Nat. Cancer 2, 587–597 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanker AB, Kaklamani V. & Arteaga CL Challenges for the clinical development of PI3K inhibitors: strategies to improve their impact in solid tumors. Cancer Discov. 9, 482–491 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitman M, Kaplan DR, Schaffhausen B, Cantley L. & Roberts TM Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature 315, 239–242 (1985). [DOI] [PubMed] [Google Scholar]
- 6.Whitman M, Downes CP, Keeler M, Keller T. & Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature 332, 644–646 (1988). [DOI] [PubMed] [Google Scholar]
- 7.Auger KR, Serunian LA, Soltoff SP, Libby P. & Cantley LC PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell 57, 167–175 (1989). [DOI] [PubMed] [Google Scholar]
- 8.Ruderman NB, Kapeller R, White MF & Cantley LC Activation of phosphatidylinositol 3-kinase by insulin. Proc. Natl Acad. Sci. USA 87, 1411–1415 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manning BD & Toker A. AKT/PKB signaling: navigating the network. Cell 169, 381–405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuels Y. et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 304, 554 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Samuels Y. et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell 7, 561–573 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Huang CH et al. The structure of a human p110alpha/p85alpha complex elucidates the effects of oncogenic PI3Kalpha mutations. Science 318, 1744–1748 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Miller MS et al. Structural basis of nSH2 regulation and lipid binding in PI3Kα. Oncotarget 5, 5198–5208 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandelker D. et al. A frequent kinase domain mutation that changes the interaction between PI3Kalpha and the membrane. Proc. Natl Acad. Sci. USA 106, 16996–17001 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorpe LM et al. PI3K-p110alpha mediates the oncogenic activity induced by loss of the novel tumor suppressor PI3K-p85alpha. Proc. Natl Acad. Sci. USA 114, 7095–7100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chagpar RB et al. Direct positive regulation of PTEN by the p85 subunit of phosphatidylinositol 3-kinase. Proc. Natl Acad. Sci. USA 107, 5471–5476 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung LW et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 1, 170–185 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung LW et al. Regulation of the PI3K pathway through a p85alpha monomer-homodimer equilibrium. eLife 4, e06866 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung LW. et al. Naturally occurring neomorphic PIK3R1 mutations activate the MAPK pathway, dictating therapeutic response to MAPK pathway inhibitors. Cancer Cell 26, 479–494 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey MH et al. Comprehensive characterization of cancer driver genes and mutations. Cell 174, 1034–1035 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nussinov R, Tsai CJ & Jang H. Ras assemblies and signaling at the membrane. Curr. Opin. Struct. Biol 62, 140–148 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Pacold ME et al. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase gamma. Cell 103, 931–943 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Hornbeck PV et al. 15 Years of PhosphoSitePlus(R): integrating post-translationally modified sites, disease variants and isoforms. Nucleic Acids Res. 47, D433–D441 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klarenbeek S, van Miltenburg MH & Jonkers J. Genetically engineered mouse models of PI3K signaling in breast cancer. Mol. Oncol 7, 146–164 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu X. et al. Activation of diverse signalling pathways by oncogenic PIK3CA mutations. Nat. Commun 5, 4961 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moniz LS et al. Phosphoproteomic comparison of Pik3ca and Pten signalling identifies the nucleotidase NT5C as a novel AKT substrate. Sci. Rep 7, 39985 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madsen RR et al. Oncogenic PIK3CA promotes cellular stemness in an allele dose-dependent manner. Proc. Natl Acad. Sci. USA 116, 8380–8389 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsons R. Discovery of the PTEN tumor suppressor and its connection to the PI3K and AKT oncogenes. Cold Spring Harb. Perspect. Med 10.1101/cshperspect.a036129 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YR, Chen M. & Pandolfi PP The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat. Rev. Mol. Cell Biol 19, 547–562 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Wymann MP et al. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol. Cell Biol 16, 1722–1733 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker EH et al. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell 6, 909–919 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Vlahos CJ, Matter WF, Hui KY & Brown RF A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem 269, 5241–5248 (1994). [PubMed] [Google Scholar]
- 33.Bendell JC et al. A phase 1 study of the sachet formulation of the oral dual PI3K/mTOR inhibitor BEZ235 given twice daily (BID) in patients with advanced solid tumors. Invest. New Drugs 33, 463–471 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Rodon J. et al. Phase 1/1b dose escalation and expansion study of BEZ235, a dual PI3K/mTOR inhibitor, in patients with advanced solid tumors including patients with advanced breast cancer. Cancer Chemother. Pharmacol 82, 285–298 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlo MI et al. A phase Ib study of BEZ235, a dual inhibitor of phosphatidylinositol 3-kinase (pi3k) and mammalian target of rapamycin (mTOR), in patients with advanced renal cell carcinoma. Oncologist 21, 787–788 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukey PT et al. A randomised, placebo-controlled study of omipalisib (PI3K/mTOR) in idiopathic pulmonary fibrosis. Eur. Respir. J 10.1183/13993003.01992-2018 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Hettiarachchi SU et al. Targeted inhibition of PI3 kinase/mTOR specifically in fibrotic lung fibroblasts suppresses pulmonary fibrosis in experimental models. Sci. Transl. Med 10.1126/scitranslmed.aay3724 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Rodon J. et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. Invest. New Drugs 32, 670–681 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Miller TW et al. ERalpha-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 1, 338–351 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bosch A. et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci. Transl. Med 7, 283ra251 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma CX et al. A phase I trial of BKM120 (Buparlisib) in combination with fulvestrant in postmenopausal women with estrogen receptor-positive metastatic breast cancer. Clin. Cancer Res 22, 1583–1591 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baselga J. et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18, 904–916 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Leo A. et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 19, 87–100 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Dent S. et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: the SANDPIPER trial. Ann. Oncol 32, 197–207 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andre F. et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med 380, 1929–1940 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Narayan P. et al. FDA approval summary: alpelisib plus fulvestrant for patients with HR-positive, HER2-negative, PIK3CA-mutated, advanced or metastatic breast cancer. Clin. Cancer Res 27, 1842–1849 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andre F. et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann. Oncol 32, 208–217 (2021). [DOI] [PubMed] [Google Scholar]
- 48.Jhaveri K. et al. Phase I basket study of taselisib, an isoform-selective PI3K inhibitor, in patients with PIK3CA-mutant cancers. Clin. Cancer Res 27, 447–459 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Juric D. et al. Phosphatidylinositol 3-kinase alpha-selective inhibition with alpelisib (BYL719) in PIK3CA-altered solid tumors: results from the first-in-human study. J. Clin. Oncol 36, 1291–1299 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ciruelos EM et al. Patient-reported outcomes in patients with PIK3CA-mutated hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer from SOLAR-1. J. Clin. Oncol 39, 2005–2015 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rugo HS et al. Time course and management of key adverse events during the randomized phase III SOLAR-1 study of PI3K inhibitor alpelisib plus fulvestrant in patients with HR-positive advanced breast cancer. Ann. Oncol 31, 1001–1010 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Saxton RA & Sabatini DM mTOR signaling in growth, metabolism, and disease. Cell 168, 960–976 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janku F, Yap TA & Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat. Rev. Clin. Oncol 15, 273–291 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Iyer G. et al. Genome sequencing identifies a basis for everolimus sensitivity. Science 338, 221 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagle N. et al. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N. Engl. J. Med 371, 1426–1433 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rugo HS et al. Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol. 18, 654–662 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Hudes G. et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N. Engl. J. Med 356, 2271–2281 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Motzer RJ et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer 116, 4256–4265 (2010). [DOI] [PubMed] [Google Scholar]
- 59.Piccart M. et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2dagger. Ann. Oncol 25, 2357–2362 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao JC et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: overall survival and circulating biomarkers from the randomized, phase III RADIANT-3 study. J. Clin. Oncol 34, 3906–3913 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biondo A. et al. Phase I clinical trial of an allosteric AKT inhibitor, MK-2206, using a once weekly (QW) dose regimen in patients with advanced solid tumors. J. Clin. Oncol 29, 3037–3037 (2011).21709185 [Google Scholar]
- 62.Hyman D. et al. Abstract CT035: a phase Ib study of miransertib (ARQ 092) in combination with anastrozole in patients with PIK3CA or AKT1-mutant ER+ endometrial or ovarian cancer. Cancer Res. 78, CT035–CT035 (2018). [Google Scholar]
- 63.Schneeweiss A. et al. Phase 1 dose escalation study of the allosteric AKT Inhibitor BAY 1125976 in advanced solid cancer-lack of association between activating AKT mutation and AKT inhibition-derived efficacy. Cancers 10.3390/cancers11121987 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yunokawa M. et al. First-in-human phase I study of TAS-117, an allosteric AKT inhibitor, in patients with advanced solid tumours. Ann. Oncol 30, v169 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banerji U. et al. A Phase I open-label study to identify a dosing regimen of the Pan-AKT inhibitor AZD5363 for evaluation in solid tumors and in PIK3CA-mutated breast and gynecologic cancers. Clin. Cancer Res 24, 2050–2059 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Saura C. et al. A first-in-human phase I study of the ATP-competitive AKT inhibitor ipatasertib demonstrates robust and safe targeting of AKT in patients with solid tumors. Cancer Discov. 7, 102–113 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turner NC et al. BEECH: a dose-finding run-in followed by a randomised phase II study assessing the efficacy of AKT inhibitor capivasertib (AZD5363) combined with paclitaxel in patients with estrogen receptor-positive advanced or metastatic breast cancer, and in a PIK3CA mutant sub-population. Ann. Oncol 30, 774–780 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones RH et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 21, 345–357 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smyth LM et al. Capivasertib, an AKT kinase inhibitor, as monotherapy or in combination with fulvestrant in patients with AKT1 (E17K)-mutant, ER-positive metastatic breast cancer. Clin. Cancer Res 26, 3947–3957 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kalinsky K. et al. Effect of capivasertib in patients with an AKT1 E17K-mutated tumor: NCI-MATCH subprotocol EAY131-Y nonrandomized trial. JAMA Oncol. 7, 271–278 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Turner N. et al. 350TiP A phase III trial of capivasertib and fulvestrant versus placebo and fulvestrant in patients with HR+/HER2− breast cancer (CAPItello-291). Ann. Oncol 31 (Suppl. 4), S388–S389 (2020). [Google Scholar]
- 72.Hamilton E. et al. 338TiP CAPItello-292: a phase 1b/3 study of capivasertib, palbociclib and fulvestrant versus placebo, palbociclib and fulvestrant in HR+/HER2− advanced breast cancer. Ann. Oncol 32, S514 (2021). [Google Scholar]
- 73.Schmid P. et al. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: the PAKT trial. J. Clin. Oncol 38, 423–433 (2020). [DOI] [PubMed] [Google Scholar]
- 74.Schmid P. et al. Abstract OT2–08-02: Capivasertib and paclitaxel in first-line treatment of patients with metastatic triple-negative breast cancer: a phase III trial (CAPItello-290). Cancer Res. 10.1158/1538-7445.SABCS19-OT2-08-02 (2020). [DOI] [Google Scholar]
- 75.Kim SB et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 18, 1360–1372 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dent R. et al. Double-blind placebo-controlled randomized phase III trial evaluating first-line ipatasertib combined with paclitaxel for PIK3CA/AKT1/PTEN-altered locally advanced unresectable or metastatic triple-negative breast cancer: primary results from IPATunity130 Cohort A. 2020 San Antonio Breast Cancer Symposium. Abstract GS3–04. Cancer Res. 10.1158/1538-7445.SABCS20-GS3-04 (2021). [DOI] [Google Scholar]
- 77.de Bono JS et al. Randomized phase II study evaluating akt blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin. Cancer Res. 25, 928–936 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Sweeney C. et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet 398, 131–142 (2021). [DOI] [PubMed] [Google Scholar]
- 79.Juric D. et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kalpha inhibitor. Nature 518, 240–244 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Razavi P. et al. Alterations in PTEN and ESR1 promote clinical resistance to alpelisib plus aromatase inhibitors. Nat. Cancer 1, 382–393 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maheshwari S. et al. Kinetic and structural analyses reveal residues in phosphoinositide 3-kinase alpha that are critical for catalysis and substrate recognition. J. Biol. Chem 292, 13541–13550 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Engelman JA. et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316, 1039–1043 (2007). [DOI] [PubMed] [Google Scholar]
- 83.Elkabets M. et al. mTORC1 inhibition is required for sensitivity to PI3K p110alpha inhibitors in PIK3CA-mutant breast cancer. Sci. Transl. Med 5, 196ra199 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Castel P. et al. PDK1-SGK1 signaling sustains AKT-independent mTORC1 activation and confers resistance to PI3Kalpha inhibition. Cancer Cell 30, 229–242 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gasser JA et al. SGK3 mediates INPP4B-dependent PI3K signaling in breast cancer. Mol. Cell 56, 595–607 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Le X. et al. Systematic functional characterization of resistance to PI3K inhibition in breast cancer. Cancer Discov. 6, 1134–1147 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cai Y. et al. Genomic alterations in PIK3CA-mutated breast cancer result in mTORC1 activation and limit the sensitivity to PI3Kalpha inhibitors. Cancer Res. 81, 2470–2480 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chandarlapaty S. et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 19, 58–71 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zorea J. et al. IGF1R upregulation confers resistance to isoform-specific inhibitors of PI3K in PIK3CA-driven ovarian cancer. Cell Death Dis. 9, 944 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schwartz S. et al. Feedback suppression of PI3Kalpha signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kbeta. Cancer Cell 27, 109–122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elkabets M. et al. AXL mediates resistance to PI3Kalpha inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell 27, 533–546 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Drago JZ et al. FGFR1 amplification mediates endocrine resistance but retains TORC sensitivity in metastatic hormone receptor-positive (HR+) breast cancer. Clin. Cancer Res. 25, 6443–6451 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Curigliano G. et al. Alpelisib in combination with everolimus +/− exemestane in solid tumours: phase Ib randomised, open-label, multicentre study. Eur. J. Cancer 151, 49–62 (2021). [DOI] [PubMed] [Google Scholar]
- 94.Juric D. et al. 342A Phase Ib/II study of alpelisib (BYL719) and ganitumab (AMG 479) in adult patients with selected advanced solid tumors. Eur. J. Cancer 51, S68 (2015). [Google Scholar]
- 95.Hyman DM et al. Combined PIK3CA and FGFR inhibition with alpelisib and infigratinib in patients with PIK3CA-mutant solid tumors, with or without FGFR alterations. JCO Precis. Oncl 10.1200/po.19.00221 (2019). [DOI] [PubMed] [Google Scholar]
- 96.Toska E. et al. PI3K inhibition activates SGK1 via a feedback loop to promote chromatin-based regulation of ER-dependent gene expression. Cell Rep. 27, 294–306.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Toska E. et al. PI3K pathway regulates ER-dependent transcription in breast cancer through the epigenetic regulator KMT2D. Science 355, 1324–1330 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hopkins BD et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560, 499–503 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hopkins BD, Goncalves MD & Cantley LC Obesity and cancer mechanisms: cancer metabolism. J. Clin. Oncol 34, 4277–4283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Newman JC & Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab 25, 42–52 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shimazu T. et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jiang Z. et al. Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer (ACE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 20, 806–815 (2019). [DOI] [PubMed] [Google Scholar]
- 103.U.S. National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT05090358 (2021). [DOI] [PubMed]
- 104.Caffa I. et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 583, 620–624 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vernieri C. et al. Fasting-mimicking diet is safe and reshapes metabolism and antitumor immunity in cancer patients. Cancer Discov. 10.1158/2159-8290.CD-21-0030 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vasan N. et al. Double PIK3CA mutations in cis increase oncogenicity and sensitivity to PI3Kalpha inhibitors. Science 366, 714–723 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hong R. et al. Abstract PD4–14: GDC-0077 is a selective PI3Kalpha inhibitor that demonstrates robust efficacy in PIK3CA mutant breast cancer models as a single agent and in combination with standard of care therapies. Cancer Res. 78 (Suppl. 4), PD4–14 (2018). [Google Scholar]
- 108.Bedard PL et al. Abstract PD1–02: A phase I/Ib study evaluating GDC-0077 + palbociclib (palbo) + fulvestrant in patients (pts) with PIK3CA-mutant (mut), hormone receptor-positive/HER2-negative metastatic breast cancer (HR+/HER2-mBC). Cancer Res. 81 (Suppl. 4), PD1–02 (2021). [Google Scholar]
- 109.U.S. National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT03006172 (2016). [DOI] [PubMed]
- 110.Song KW et al. RTK-dependent inducible degradation of mutant PI3Kalpha drives GDC-0077 (Inavolisib) efficacy. Cancer Discov. 10.1158/2159-8290.CD-21-0072 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hon WC, Berndt A. & Williams RL Regulation of lipid binding underlies the activation mechanism of class IA PI3-kinases. Oncogene 31, 3655–3666 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pazolli E. et al. Discovery and characterization of RLY2608, the first allosteric, mutant, and isoform-selective inhibitor of PI3Kα. In AACR-NCI-EORTC Virtual International Conference on Molecular Targets and Cancer Therapeutics (AACR, NCI, EORTC, 2021). [Google Scholar]
- 113.Klippel A. et al. Preclinical characterization of LOXO-783 (LOX-22783), a highly potent, mutantselective and brain-penetrant allosteric PI3Kα H1047R inhibitor. In AACR-NCI-EORTC Virtual International Conference on Molecular Targets and Cancer Therapeutics (AACR, NCI, EORTC, 2021). [Google Scholar]
- 114.Liu X. et al. Cryo-EM structures of PI3Kalpha reveal conformational changes during inhibition and activation. Proc. Natl Acad. Sci. USA 10.1073/pnas.2109327118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Burke JE, Perisic O, Masson GR, Vadas O. & Williams RL Oncogenic mutations mimic and enhance dynamic events in the natural activation of phosphoinositide 3-kinase p110alpha (PIK3CA). Proc. Natl Acad. Sci. USA 109, 15259–15264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dang CV, Le A. & Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin. Cancer Res 15, 6479–6483 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Venkatesh HS et al. Reduced phosphocholine and hyperpolarized lactate provide magnetic resonance biomarkers of PI3K/Akt/mTOR inhibition in glioblastoma. Neuro-Oncology 14, 315–325 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ward CS et al. Noninvasive detection of target modulation following phosphatidylinositol 3-kinase inhibition using hyperpolarized 13C magnetic resonance spectroscopy. Cancer Res. 70, 1296–1305 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ros S. et al. Metabolic imaging detects resistance to PI3Kalpha inhibition mediated by persistent FOXM1 expression in ER+ breast cancer. Cancer Cell 38, 516–533.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Koundouros N. et al. Metabolic fingerprinting links oncogenic PIK3CA with enhanced arachidonic acid-derived eicosanoids. Cell 181, 1596–1611.e27 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hoxhaj G. & Manning BD The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 20, 74–88 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gottlob K. et al. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 15, 1406–1418 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deprez J, Vertommen D, Alessi DR, Hue L. & Rider MH Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. J. Biol. Chem 272, 17269–17275 (1997). [DOI] [PubMed] [Google Scholar]
- 124.Saha A. et al. Akt phosphorylation and regulation of transketolase is a nodal point for amino acid control of purine synthesis. Mol. Cell 55, 264–276 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hoxhaj G. et al. Direct stimulation of NADP+ synthesis through Akt-mediated phosphorylation of NAD kinase. Science 363, 1088–1092 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Potapova IA, El-Maghrabi MR, Doronin SV & Benjamin WB Phosphorylation of recombinant human ATP:citrate lyase by cAMP-dependent protein kinase abolishes homotropic allosteric regulation of the enzyme by citrate and increases the enzyme activity. Allosteric activation of ATP:citrate lyase by phosphorylated sugars. Biochemistry 39, 1169–1179 (2000). [DOI] [PubMed] [Google Scholar]
- 127.Kovacina KS et al. Identification of a proline-rich Akt substrate as a 14–3-3 binding partner. J. Biol. Chem 278, 10189–10194 (2003). [DOI] [PubMed] [Google Scholar]
- 128.Waldhart AN et al. Phosphorylation of TXNIP by AKT mediates acute influx of glucose in response to insulin. Cell Rep. 19, 2005–2013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Manning BD, Tee AR, Logsdon MN, Blenis J. & Cantley LC Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell 10, 151–162 (2002). [DOI] [PubMed] [Google Scholar]
- 130.Hu H. et al. Phosphoinositide 3-kinase regulates glycolysis through mobilization of aldolase from the actin cytoskeleton. Cell 164, 433–446 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.You I. et al. Discovery of an AKT degrader with prolonged inhibition of downstream signaling. Cell Chem. Biol 27, 66–73.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu J. et al. AKT degradation selectively inhibits the growth of PI3K/PTEN pathway-mutant cancers with wild-type KRAS and BRAF by destabilizing aurora kinase B. Cancer Discov. 10.1158/2159-8290.CD-20-0815 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee BJ et al. Selective inhibitors of mTORC1 activate 4EBP1 and suppress tumor growth. Nat. Chem. Biol 17, 1065–1074 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tate JG et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 47, D941–D947 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gustin JP et al. Knockin of mutant PIK3CA activates multiple oncogenic pathways. Proc. Natl Acad. Sci. USA 106, 2835–2840 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dogruluk T. et al. Identification of variant-specific functions of PIK3CA by rapid phenotyping of rare mutations. Cancer Res. 75, 5341–5354 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Croessmann S. et al. PIK3CA C2 domain deletions hyperactivate phosphoinositide 3-kinase (PI3K), generate oncogene dependence, and are exquisitely sensitive to PI3Kalpha Inhibitors. Clin. Cancer Res 24, 1426–1435 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Spangle JM et al. PIK3CA C-terminal frameshift mutations are novel oncogenic events that sensitize tumors to PI3K-alpha inhibition. Proc. Natl Acad. Sci. USA 117, 24427–24433 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Saito Y. et al. Landscape and function of multiple mutations within individual oncogenes. Nature 582, 95–99 (2020). [DOI] [PubMed] [Google Scholar]
- 140.Madsen RR & Vanhaesebroeck B. Cracking the context-specific PI3K signaling code. Sci. Signal 10.1126/scisignal.aay2940 (2020). [DOI] [PubMed] [Google Scholar]
- 141.Madsen RR, Vanhaesebroeck B. & Semple RK Cancer-associated PIK3CA mutations in overgrowth disorders. Trends Mol. Med 24, 856–870 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Venot Q. et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature 558, 540–546 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yu K. et al. PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis. Nature 578, 166–171 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goncalves MD, Hopkins BD & Cantley LC Phosphatidylinositol 3-kinase, growth disorders, and cancer. N. Engl. J. Med 379, 2052–2062 (2018). [DOI] [PubMed] [Google Scholar]
- 145.Turner NC et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med 379, 1926–1936 (2018). [DOI] [PubMed] [Google Scholar]
- 146.Johnston SRD et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J. Clin. Oncol 38, 3987–3998 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rugo HS et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 22, 489–498 (2021). [DOI] [PubMed] [Google Scholar]
- 148.Vora SR et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell 26, 136–149 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Diehl JA, Cheng M, Roussel MF & Sherr CJ Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 12, 3499–3511 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pascual J. et al. Triplet therapy with palbociclib, taselisib, and fulvestrant in PIK3CA-mutant breast cancer and doublet palbociclib and taselisib in pathway-mutant solid cancers. Cancer Discov. 11, 92–107 (2021). [DOI] [PubMed] [Google Scholar]
- 151.Bardia A. et al. Phase I/II trial of exemestane, ribociclib, and everolimus in women with HR+/HER2− advanced breast cancer after progression on CDK4/6 inhibitors (TRINITI-1). Clin. Cancer Res 27, 4177–4185 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bardia A. et al. Phase Ib dose-escalation/expansion trial of ribociclib in combination with everolimus and exemestane in postmenopausal women with HR+, HER2− advanced breast cancer. Clin. Cancer Res 26, 6417–6428 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hanker AB et al. Mutant PIK3CA accelerates HER2-driven transgenic mammary tumors and induces resistance to combinations of anti-HER2 therapies. Proc. Natl Acad. Sci. USA 110, 14372–14377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Baselga J. et al. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J. Clin. Oncol 32, 3753–3761 (2014). [DOI] [PubMed] [Google Scholar]
- 155.Baselga J. et al. Relationship between tumor biomarkers and efficacy in EMILIA, a phase III study of trastuzumab emtansine in HER2-positive metastatic breast cancer. Clin. Cancer Res 22, 3755–3763 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jain S. et al. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res. Treat 171, 371–381 (2018). [DOI] [PubMed] [Google Scholar]
- 157.Jhaveri K. et al. A phase I study of alpelisib in combination with trastuzumab and LJM716 in patients with PIK3CA-mutated HER2-positive metastatic breast cancer. Clin. Cancer Res 27, 3867–3875 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dunn LA et al. A phase 1b study of cetuximab and BYL719 (Alpelisib) concurrent with intensity modulated radiation therapy in stage III-IVB head and neck squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys 106, 564–570 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ibrahim YH et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2, 1036–1047 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Juvekar A. et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2, 1048–1063 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gonzalez-Billalabeitia E. et al. Vulnerabilities of PTEN-TP53-deficient prostate cancers to compound PARP-PI3K inhibition. Cancer Discov. 4, 896–904 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cardnell RJ et al. Proteomic markers of DNA repair and PI3K pathway activation predict response to the PARP inhibitor BMN 673 in small cell lung cancer. Clin. Cancer Res 19, 6322–6328 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Matulonis UA et al. Phase I dose escalation study of the PI3kinase pathway inhibitor BKM120 and the oral poly (ADP ribose) polymerase (PARP) inhibitor olaparib for the treatment of high-grade serous ovarian and breast cancer. Ann. Oncol 28, 512–518 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Konstantinopoulos PA et al. Olaparib and alpha-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: a dose-escalation and dose-expansion phase 1b trial. Lancet Oncol. 20, 570–580 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Batalini F. et al. Phase 1b clinical trial with alpelisib plus olaparib for patients with advanced triple-negative breast cancer. Clin. Cancer Res 10.1158/1078-0432.CCR-21-3045 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nelson AC et al. AKT regulates BRCA1 stability in response to hormone signaling. Mol. Cell Endocrinol 319, 129–142 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xiang T. et al. Negative regulation of AKT activation by BRCA1. Cancer Res. 68, 10040–10044 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Razavi P. et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34, 427–438.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mundt F. et al. Mass spectrometry-based proteomics reveals potential roles of NEK9 and MAP2K4 in resistance to pi3k inhibition in triple-negative breast cancers. Cancer Res. 78, 2732–2746 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ellis MJ et al. Connecting genomic alterations to cancer biology with proteomics: the NCI clinical proteomic tumor analysis consortium. Cancer Discov. 3, 1108–1112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Edwards NJ et al. The CPTAC data portal: a resource for cancer proteomics research. J. Proteome Res 14, 2707–2713 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.