Abstract
Substance use disorders (SUDs) are seen as a continuum ranging from goal‐directed and hedonic drug use to loss of control over drug intake with aversive consequences for mental and physical health and social functioning. The main goals of our interdisciplinary German collaborative research centre on Losing and Regaining Control over Drug Intake (ReCoDe) are (i) to study triggers (drug cues, stressors, drug priming) and modifying factors (age, gender, physical activity, cognitive functions, childhood adversity, social factors, such as loneliness and social contact/interaction) that longitudinally modulate the trajectories of losing and regaining control over drug consumption under real‐life conditions. (ii) To study underlying behavioural, cognitive and neurobiological mechanisms of disease trajectories and drug‐related behaviours and (iii) to provide non‐invasive mechanism‐based interventions. These goals are achieved by: (A) using innovative mHealth (mobile health) tools to longitudinally monitor the effects of triggers and modifying factors on drug consumption patterns in real life in a cohort of 900 patients with alcohol use disorder. This approach will be complemented by animal models of addiction with 24/7 automated behavioural monitoring across an entire disease trajectory; i.e. from a naïve state to a drug‐taking state to an addiction or resilience‐like state. (B) The identification and, if applicable, computational modelling of key molecular, neurobiological and psychological mechanisms (e.g., reduced cognitive flexibility) mediating the effects of such triggers and modifying factors on disease trajectories. (C) Developing and testing non‐invasive interventions (e.g., Just‐In‐Time‐Adaptive‐Interventions (JITAIs), various non‐invasive brain stimulations (NIBS), individualized physical activity) that specifically target the underlying mechanisms for regaining control over drug intake. Here, we will report on the most important results of the first funding period and outline our future research strategy.
Keywords: addiction, alcohol, alternative rewards, ambulatory assessment (AA), animal models, behavioural control, cocaine, cognitive control, computational models, craving, decision‐making, ecological momentary assessment (EMA), habit formation, relapse, tobacco
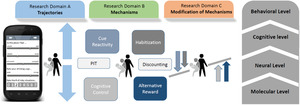
The losing and regaining control over drug intake (ReCoDe) framework. The projects are divided into three research domains. Research domain A relates to trajectories of alcohol and drug use; research domain B relates to mechanisms (e.g. cue reactivity) on different system levels (behavioural, neural and molecular); and research domain C focuses on the modification of mechanisms (e.g., by increasing cognitive control via physical activity or neurofeedback). Pavlovian‐to‐instrumental transfer (PIT).
1. INTRODUCTION
In 2019, we established a collaborative research centre, which brings together three renowned locations (Berlin, Dresden, Mannheim) of addiction research in Germany. Our multidisciplinary ReCoDe ( Regaining Control over Drug Intak e) consortium requires the close interaction of addiction medicine experts, experimental and clinical psychologists, medical engineers specialized in medical sensor development and bioinformatics, geneticists, behavioural pharmacologists and experimental and computational neuroscientists. Our highly inter‐disciplinary team consists of 51 principal investigators (PIs) working on 22 research projects and 2 infrastructure projects on data management (INF) and mobile infrastructure for real‐life assessments. ReCoDe is funded by the German Research Foundation (DFG) with a 12‐year perspective. The first funding period was from 07/2019 to 06/2023 and the second funding period, which was positively evaluated by an international expert panel runs from 07/2023 to 06/2027. Depending on a further evaluation funding for ReCoDe can be extended until 06/2031 with substantial financial support. Here we will first describe the key aims and research domains of our consortium, will then report on some of our key findings from the first funding period, and will finally present our future research strategy.
2. THE KEY AIMS AND RESEARCH DOMAINS OF THE RECODE CONSORTIUM
The main goals of our research consortium during the entire 12‐year funding period are (i) to identify triggers and modifying factors that longitudinally modulate the trajectories of losing and regaining control over drug consumption in real life (Research Domain A), (ii) to study underlying behavioural, cognitive, molecular and neurobiological mechanisms (Research Domain B) and (iii) to develop mechanism‐based interventions (Research Domain C) (Figure 1). We have designed a work plan and developed the methodology to investigate the following core research domains. 1
FIGURE 1.
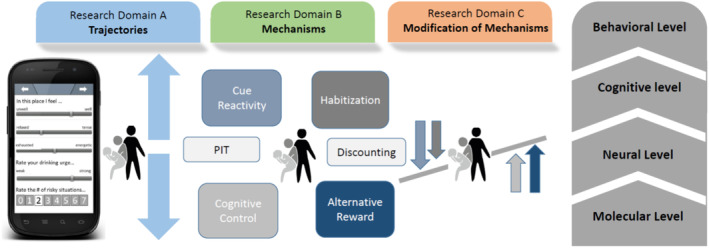
The losing and regaining control over drug intake (ReCoDe) framework. The projects are divided into three research domains. Research domain A relates to trajectories of alcohol and drug use; research domain B relates to mechanisms (e.g. cue reactivity) on different system levels (behavioural, neural and molecular); and research domain C focuses on the modification of mechanisms (e.g., by increasing cognitive control via physical activity or neurofeedback). Pavlovian‐to‐instrumental transfer (PIT).
2.1. Domain A trajectories
Defining individual trajectories of drug intake from voluntary and hedonistic use to habitual and compulsive use requires a holistic approach, which longitudinally assesses the interactions between triggers (drug cues, stressors, drug priming) and modifying factors (age, gender, physical activity, cognitive functions, childhood adversity, social factors such as loneliness and social contact/interaction) in real‐life in SUD subjects and animal models of addiction. Technically, we are using innovative mobile health (mHealth) tools comprising custom‐developed ecological momentary assessment (EMA), smartphone sensing as well as mobile sensors (wearables) and accelerometers. 2 Here, we acquire geolocation and additional data on psychological and physiological cues stress reactivity and alcohol intake, as well as physical activity and movement patterns, and collect thereby intensive longitudinal datasets (ILDs) with high temporal resolution. 3 These data are combined with app‐based tests for key cognitive control and learning mechanisms in real‐life settings. In animal models of addiction 4 ‐ that capture the entire disease trajectories of addictive behaviour ‐ ILDs for drug consumption patterns and motor activity are also sampled. Moreover, the use of animal models allows to study of triggers and modifying factors on drug consumption patterns and measures of habit formation and compulsivity in highly controlled conditions. 5 , 6 We are analysing these large data sets using new multiscale analysis tools from statistical physics and biosignal processing to identify tipping points in substance use trajectories; for example, we are using advanced machine learning techniques such as deep neural networks 7 to analyse our sequential data in order to build prediction models.
2.2. Domain B mechanisms
The effects of the triggers outlined above and modifying factors on goal‐directed, habitual and compulsive aspects of addictive behaviours are mediated by distinct processes. The key behavioural/cognitive processes that we are studying in Domain B in the context of the transition towards losing and regaining control include (i) goal‐directed decision‐making and habitization processes, (ii) enhanced stress and cue reactivity – the latter also involves sign tracking and Pavlovian‐To‐Instrumental Transfer (PIT) effects, (iii) reduced choice of alternative rewards, (iv) heightened reward discounting and aversive discounting and (v) reduced cognitive control. The processes that are studied in Domain B largely overlap with a recent international Delphi study that aimed to reach a consensus among experts in the addiction field on the primary Research Domain Criteria (RDoC constructs) most relevant to SUDs, which include reward learning, reward valuation, action selection, habit formation, response inhibition and compulsivity. 8 In the B projects, we are not only studying those key behavioural processes but also their molecular and neurobiological underpinnings. According to published data and our findings we are building computational models consisting of animal and human data derived from tandem translational projects. The goals of our models are to understand corticolimbic control mechanisms as well as aberrant learning mechanisms and action control and their interaction with triggers and modifying factors, which bias behaviour towards habitual or even compulsive drug‐seeking and ‐intake 9 (Figure 1).
2.3. Domain C interventions
Based on the prediction of individual trajectories of losing and regaining control in Domain A and mechanisms in Domain B, we are developing non‐invasive mechanism‐based interventions that specifically match the key processes outlined above. All these interventions build on the fact that regaining control over drug intake can be achieved by some patients, which suggests that behavioural processes associated with losing control can be partly reversed or compensated. 10 , 11 Regaining behavioural control over drug intake may be attributed to: (i) extinction of drug cue‐induced behavioural tendencies, (ii) mindful selection of goals replacing habitual behaviour or to establish healthy habits, 12 (iii) prioritization of alternative non‐drug rewards, (iv) reducing discounting tendencies and (v) increased cognitive control over behaviour, including inhibitory control, all of which are studied in the B domain (Figure 1).
3. WHAT HAS THE RECODE CONSORTIUM ACHIEVED IN THE FIRST FUNDING PERIOD (2019–2023)?
In the first funding period, we built a functional infrastructure and common “inter‐disciplinary language” across three sites – Berlin, Dresden and Mannheim. To date, we have primarily focused on alcohol use disorder (AUD), as this produces in Germany the largest health and socioeconomic burden of all drugs of abuse (allowing for comorbid tobacco and cannabis use and assessing their respective impact). On all dissemination levels, ranging from collaborative efforts within the ReCoDe consortium to interdisciplinary publication output and media outreach to more than 115 million people to disseminate our findings with hopefully preventive impact ‐ we have already realized added value that is clearly more than simply the sum of all stand‐alone projects (see https://www.trr265.org/).
The trajectories and mechanisms determining substance use and SUDs in everyday life are complex, operate moment‐to‐moment, and are characterized by the dynamic interplay of triggers and modifying factors. Here, methodological developments in everyday life research have greatly expanded the ability of researchers to gain insight into the manner in which triggers and modifying factors act on relevant neurobiological and psychological mechanisms and consequently shape real‐life substance use and the development of addictive behaviour. Recent work by us and others in ecological neuroscience has further demonstrated the value of combining ecological momentary assessment (EMA), sensor and neuroimaging methods to determine the neural bases of momentary changes in behaviour and experiences in everyday life. 2 , 13 , 14 , 15 , 16 One of the key innovative aspects of our ReCoDe consortium is – in addition to the classic clinical‐ and laboratory‐based view on SUDs – the focus on studying the moderating role of real‐life contexts on disease trajectories and underlying mechanisms. Therefore, in the first funding period, we built a robust infrastructure for various mHealth tools and online assessments along with common data infrastructure and applied them to a real‐life cohort study – the so‐called ReCoDe cohort.
3.1. The ReCoDe infrastructure for mHealth tools and online assessment
We are using established EMA and newly‐developed tools to measure key processes engaged in the development of addictive behaviour. These mHealth tools were implemented or newly developed by several projects in close collaboration with the mental mHealth Lab located at the Karlsruhe Institute of Technology (KIT). These mHealth tools and online assessments include:
We custom‐developed and employed a mobile (e‐diary) infrastructure for real‐life assessments and sparse sampling across 365 days and for high‐frequency intense sampling across 2 × 6 weeks, including smartphone sensing and geolocation tracking, in compliance with the General Data Protection Regulation (GDPR) and comprising a software interface for APPs testing cognitive control.
A smartphone‐based gamified assessment battery of cognitive control (response inhibition, working memory) and decision‐making (risk‐taking, information sampling). 17 , 18 A smartphone‐based decision‐making experiment (according to 19 ) in combination with real‐life assessments of electroencephalogram (EEG) and electrocardiogram (ECG) in individuals from the ReCoDe cohort. The codes of smartphone‐based tasks and analyses are publicly available (https://osf.io/n5a2z/).
An online version of the Trier Social Stress Test (TSST ‐ adapted from Gunnar et al, 2021) was developed. In this version, participants interact with the committee via video conference. We confirmed the effectiveness of this online version to induce moderate social stress on physiological (e.g., increase in cortisol) and subjective outcomes.
3.2. The common ReCoDe data infrastructure
Our real‐life assessments produce massive ILDs with high time resolution, many projects conduct multimodal neuroimaging, where also large datasets are generated, and our multi‐omics approaches produce big data as well. These data need to be stored and processed by our ReCoDe data infrastructure, which is located in the Center for Information Services and High Performance Computing (ZIH) in Dresden (Figure 2).
FIGURE 2.
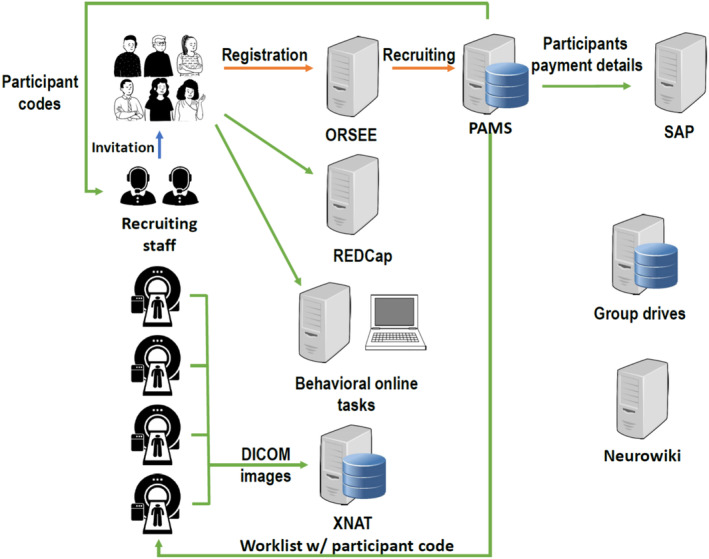
The INF infrastructure provides central storage for all ReCoDe projects. For participant management, PAMS (participant management system) was developed. The PAMS serves as a central system for the administration of personal data and pseudonymization. REDCap, a web‐based system for managing projects, studies, online surveys, interviews, standardized documentation, metadata and other types of data has also been implemented. In addition, an extensible neuroimaging archive toolkit (XNAT) server is used to store all types of image files generated by MRI scanners and the corresponding log files of task‐related fMRI experiments. DICOM = digital imaging and Communications in Medicine; ORSEE = online recruitment system for economic experiments.
The DFG outlines three important points that need to be considered. (i) Research data management must be planned, (ii) access to data should be made possible for others; i.e., to adhere to open science principles and (iii) long‐term archiving of the data is necessary. These three points are considered by FAIR data management (findable, accessible, interoperable and reusable), which is a building block ‐ not only for the reproducibility of research results but also for the reusability of research data to generate new knowledge. 22 Our data infrastructure project ensures the implementation of FAIR and has established a well‐functional information and data infrastructure for the entire ReCoDe consortium (Figure 2).
3.3. The ReCoDe cohort involves a multi‐centre systematic longitudinal assessment of patients with AUD with massive real‐life data
In Domain A, the goal of the first funding period was to recruit a large prospective cohort with 900 patients with AUD and 150 control persons and follow them over the course of 12 months. We were acquiring massive real‐life information with a longitudinal design that will also include follow‐up assessments in the second funding period. All subjects of the ReCoDe cohort underwent four personal and online assessments (baseline and follow‐up every 4 months) and provided ambulatory assessment data over the course of 1 year sampled every 2 days and additionally triggered by alcohol use. Ambulatory assessments have thus allowed us to assess both reported alcohol consumption and intention to alter consumption every 2 days, as well as triggers of alcohol use, including momentary exposure to stress, social isolation, impulsivity and alcohol cues and priming doses. 23 In the ReCoDe cohort a broad age range is covered in three age sub‐cohorts in order to enable conclusions on disease trajectories and mechanisms over the entire lifetime. These sub‐cohorts include currently drinking adolescents and young adults (16‐32y), early middle‐aged adults (33‐49y) and late middle‐aged adults (50‐65y). The control group has a quite similar age range distribution (Figure 3).
FIGURE 3.
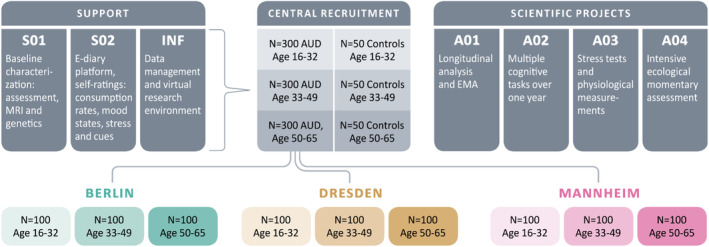
Shows the multi‐site recruitment of the ReCoDe cohort. The recruitment aim during the first funding period was N = 900 patients with AUD. As of today (May 2024), after accounting for drop‐outs we still have 746 (291 female/455 male) patients with AUD and 269 controls (131 female/138 male) for follow‐up assessments. Mostly mild to moderate cases (with 4.1 ± 1.7 SD AUD criteria) were recruited, as those patients are suggested to demonstrate higher disease dynamics in terms of losing and regaining control than severe cases. A broad age range is covered in three age sub‐cohorts in order to draw conclusions on disease trajectories and mechanisms across the entire lifetime. S01, S02, etc., relate to different projects of the ReCoDe consortium.
3.4. The use of the ReCoDe cohort to study the impact of the COVID‐19 pandemic on real‐life alcohol consumption patterns – a showcase study of domain trajectories
The question of whether the pandemic had an impact on alcohol consumption in the general population and on AUD trajectories has received a lot of attention. 24 To minimize the efficacy of the virus spreading, the governments of many countries worldwide initiated several lock‐down phases to repress social interference and therefore hinder the spread of the virus. These strict conditions and the general burdensome situation of the pandemic led to a significant increase in mental health problems and coping strategies such as increased alcohol intake, especially in adolescents. 24 , 25 However, different clusters were observed during the pandemic, with decreased and increased alcohol consumption or no change in drinking behaviour. 26 , 27 Most worrisome was the situation for patients with AUD, as governmental regulations such as lockdowns restricted access to ongoing therapy for extended periods, with unpredictable impact on further disease progression. Hence it is suggested that a lockdown represents a risk factor for increasing alcohol consumption in people with AUD and relapse for those who were previously abstinent. 27 , 28 In summary, there are mixed findings in studies that examined the impact of the pandemic on alcohol consumption in the healthy population as well as in patients with AUD.
By means of our ReCoDe cohort, we were able to study real‐life patterns of alcohol consumption in patients with AUD during the COVID‐19 pandemic and lockdowns in Germany over the course of 1 year. 14 Our real‐time measures revealed surprising results. Contrary to our expectations, during the hard lockdown periods, despite the fact that perceived social isolation was significantly higher, alcohol consumption in the mainly mild to moderate patients with AUD was significantly lower, 14 potentially reflecting reduced social drinking.
3.5. A common molecular pathology for reduced cognitive flexibility and increased craving in AUD – a showcase study of domain mechanisms
A main driver for AUD development is the progressive loss of cognitive control (Figure 1). In a previous study, PIs of our consortium discovered reduced metabotropic glutamate receptor 2 (mGluR2) in the corticostriatal neurocircuitry of alcohol‐dependent humans and rats. 29 This represents a key pathophysiological mechanism mediating impairments in executive functions that facilitates loss of control and craving and can thus lead to relapse. Subsequently, in the first funding period, a causal link for reduced prefrontal mGluR2 function in both cognitive flexibility and alcohol craving was demonstrated using a bi‐directional neuromodulation approach. By means of a neuron‐specific prefrontal knockdown of mGluR2 in rats, a phenotype of reduced cognitive flexibility and excessive alcohol‐seeking was induced. Conversely, viral restoration of prefrontal mGluR2 levels in alcohol‐dependent rats rescued these pathological behaviours. 30 Furthermore, we showed that the serotonergic hallucinogen psilocybin could also normalize mGluR2 expression in alcohol‐dependent rats, which provides a potential mechanism for the long‐lasting action of this drug in the treatment of AUD. 31 In conclusion, we identified a common molecular pathological mechanism for both executive dysfunction and alcohol craving.
Based on this mechanism, we hypothesized that in the first funding period mGlu2 agonists and mGlu2 positive allosteric modulators (PAMs) may be effective in reducing relapse in alcohol‐dependent individuals. We tested several compounds in a well‐established rat model of relapse, the alcohol deprivation effect (ADE) with repeated deprivation phases. 4 All tested compounds significantly and dose‐dependently reduced the expression of the ADE in male and female subjects. No significant changes in water intake, body weight and locomotor activity were observed. mGluR2 PAMs showed a better side effect profile than mGluR2 agonists. 32 Together with other preclinical data showing that PAMs can reduce alcohol‐seeking behaviour we conclude that mGlu2 PAMs should be considered for clinical trials in alcohol‐dependent patients.
Although we intend to further develop mGluR2‐based treatment approaches clinically, in the research context of such a cross‐site DFG‐funded collaborative research centre it will not be possible to aim for a randomized control trial (RCT) with a candidate drug in patients with AUD. RCTs even with registered drugs (also repurposed registered drugs) involve a highly regulatory framework with extensive monitoring and other factors and are thus prohibitively cost‐intensive. Given this limitation to test innovative drugs within the ReCoDe consortium, we have put our focus on non‐invasive interventions in Domain C.
3.6. The application of non‐invasive mechanisms‐based interventions
In the Domain Interventions, we are focusing on the modification of learning and cognitive control mechanisms in subjects with SUD, using the information on risk and protective factors observed under real‐life conditions, as well as the learning and executive control paradigms and their computational modelling. Especially, transcranial magnetic stimulation (TMS), 33 transcranial alternating current stimulation (tACS), 34 or network‐based functional connectivity real‐time fMRI neurofeedback (rt‐fMRI NF) 35 are used as promising non‐invasive techniques to target the key behavioural processes/mechanisms shown in Figure 1. The interventions include the targeting of habitual vs goal‐directed control of behaviour, the modification of Pavlovian and instrumental learning parameters, the modification of cognitive control and the modulation of neural cue reactivity.
For example, during the first funding period, we enhanced cognitive control in smokers who underwent a standard smoking cessation program by using an add‐on chess‐based cognitive remediation treatment (CRT). CRT was developed to improve cognitive skills relating to executive functioning such as inhibition, decision‐making, working memory and cognitive flexibility, and is suggested as a promising tool to improve cognition and treatment outcomes in substance abuse. 36 We used chess‐based CRT (CB‐CRT) conducted smartphone‐based (GYMCHESS®, https://gymchess.com/en/), as cognitive control and inhibitory capacity can be enhanced by chess training and chess trains the frontal brain regions impaired in SUDs. 37 The chess‐based cognitive remediation treatment (CB‐CRT) was applied twice a week over 14 weeks as an add‐on treatment in patients with AUD and tobacco use disorder (TUD). 38 We found increased executive functioning, e.g. in working memory and cognitive flexibility. In conclusion, chess‐based CRT is suggested to improve inhibitory control and cognitive flexibility and thus in turn should positively affect the course of therapy and outcome measures in smokers and patients with AUD (e.g., craving and relapse).
3.7. Professional dissemination of new relevant information from our ReCoDe consortium to the scientific community and the public.
The ReCoDe consortium brings together the three most prominent centres of addiction research in Germany. Some of the PIs of these three centres have cooperated in the addiction field in a highly translational and interdisciplinary fashion over the last 20 years. These collaborations have now been substantially intensified by the DFG‐funded ReCoDe consortium and have resulted in over 720 joint publications to date. Importantly, the added value of ReCoDe collaborations within the first funding period is demonstrated by 63 multi‐PI and multi‐disciplinary published original investigations and reviews. A list of all publications can be found on our website https://TRR265.org. Some key publications of the ReCoDe Consortium are displayed in Box 1.
BOX 1. Selected key publications (original investigations, methodological papers and reviews of the ReCoDe consortium.
Bach et al. 39 published in Biological Psychiatry the original investigation on stress induced sensitization of insula activation predicts alcohol craving and alcohol use in alcohol use disorder. In this study, the effect of stress‐ and alcohol cue‐exposure on alcohol craving and the underlying neurocircuitry were determined. Specifically, results indicate a stress‐induced sensitization of left insula reactivity to alcohol cues as a neurobiological correlate of the effects of psychosocial stress on alcohol craving and alcohol use in AUD, which likely reflects changes in salience attribution and goal‐directed behaviour.
Hoffmann et al. 40 published in the American Journal of Psychiatry the original investigation on associations of menstrual cycle and progesterone‐to‐estradiol ratio with alcohol consumption in alcohol use disorder: a sex‐separated multicentre longitudinal study. The menstrual cycle and the progesterone‐to‐estradiol ratio are associated with problem drinking in females and males with AUD. The results of this study highlight the hormone ratio as a promising future treatment target and provide a basis for the development of therapies tailored to the cycle phases.
Hildebrandt et al. 41 published in Biological Psychiatry the original investigation on dissociating the link of neural correlates of inhibition to the degree of substance use and substance‐related problems: here a large functional magnetic resonance imaging (fMRI) dataset on a stop signal task was combined with detailed and distinguishable assessments of substance use and problems in (poly)substance users. The results support a dual‐process model of SUDs and indicate that frontal hypoactivation during inhibition is linked to substance‐related problems and thus to risk for SUDs. In contrast, frontal hyperactivation during inhibition and the degree of substance use suggest that inhibition network functioning can protect against the risk of developing SUDs.
Chen et al. 42 published in Biological Psychiatry the original investigation on the association of non–drug‐related pavlovian‐to‐instrumental transfer effect in nucleus accumbens with relapse in alcohol dependence: A replication. This study successfully replicated the significant behavioural PIT effects previously reported by Garbusow et al. 43 Furthermore, in both studies, a stronger PIT effect and nucleus accumbens (NAcc) responses were observed among subsequent relapsers compared to abstainers, underscoring the predictive value of PIT‐related mechanisms in alcohol intake and relapse behaviour.
Rane et al. 44 published in Elife an original investigation on structural differences in adolescent brains that can predict alcohol misuse. Here, a general machine‐learning pipeline for analysing structural and functional MRI data involving both classical machine learning and more advanced deep learning methods, such as convolutional neural networks, was developed. In a large dataset of adolescents at ages 14, 19 and 22, alcohol misuse could be predicted with a balanced accuracy of up to 78% using brain structure data from T1‐weighted and diffusion tensor imaging.
Hasanpour et al. 45 published in Cell Reports a method paper on intensive longitudinal characterization of multidimensional biobehavioral dynamics in laboratory rats. One challenge in studying rodents in normal social conditions is a group‐housed setting in which it is difficult to ascertain behaviours of individual rodents concurrently. Here a major methodological breakthrough was made by utilizing wireless tracking technology and videography that allowed us to collect and analyse more than 130 billion data points to characterize at the individual level the evolution of behaviour and physiology of healthy group‐housed male and female rats throughout their development. The resulting high dimensional ILDs reflect and predict strain and sex differences and mark bi‐stable developmental states and the transition indicating the onset of puberty.
Durstewitz et al. 46 published in Nature Reviews Neuroscience a perspective on Reconstructing computational system dynamics from neural data with recurrent neural networks. In this perspective, the author focuses on recent trends in artificial intelligence and machine learning in neuroscience. They discuss formal prerequisites, different model architectures and training approaches for recurrent neural networks (RNN)‐based dynamical system reconstructions, ways to evaluate and validate model performance, how to interpret trained models in a neuroscience context, and current challenges.
Holtz et al. 47 published in Biological Psychiatry a review on early social adversity, altered brain functional connectivity and mental health. Here the authors take an expanded concept of developmentally relevant adverse experiences from infancy over childhood to adolescence as a starting point and focus their review of functional connectivity studies on a selected subset of functional magnetic resonance imaging‐based phenotypes, including connectivity in the limbic and within the frontoparietal as well as default mode networks.
Gianonne et al. 48 published in Translational Psychiatry a review and meta‐analysis on bad habits – good goals? Meta‐analysis and translation of the habit construct to alcoholism. A meta‐analysis of animal data shows a clear bias towards habitual responding after a history of chronic alcohol intake, but the expression of complete and stable habitual control is rarely seen. Moreover, it is difficult to elicit habitual responses in human laboratory tasks and animal paradigms cannot easily be translated to humans. 49 Importantly, in typical habit tests goal‐directed adaptive responses reemerge within the test session already after some minutes. Thus, while these tests are moment‐to‐moment assessments of contingency‐dependent response biases, they are fundamentally different from the concept of compulsivity, which is operationalized as a persistent drug approach despite aversive consequences that are largely independent of specific cues or context. Consequently, our research suggests that habitual response tendencies in a narrow definition may not be causally linked to the development of compulsivity (see also 9 ). Instead, they may act as a moderating factor in the progression from controlled substance use to compulsive drug taking. Taken together, despite the limitations of the traditional habit‐goal dichotomy, enhancing our understanding of decision‐making processes and response biases offers promising avenues for advancing both basic research and clinical interventions in AUD.
Alcohol, tobacco and other drugs of abuse are topics of enormous socio‐economic and health interest and relevance to the public domain and also to politicians. Importantly, the use of alcohol and other drugs during the lockdowns of the COVID‐19 pandemic and the recent legalization of cannabis products in Germany boost interest in these topics that are already of very high awareness in normal day‐to‐day business. Therefore, it is of critical importance that we disseminate our findings from the ReCoDe consortium in all possible ways via classical and social media channels. In Figure 4, we visualize our public outreach efforts by presenting the data from one centre, the Central Institute of Mental Health (CIMH) in Mannheim. The CIMH has a communication centre that provides a precise record of the entire information flow from all CIMH PIs working in the ReCoDe consortium to the public domain. On our website (https://TRR265.org) a detailed list of all media reports can be found.
FIGURE 4.
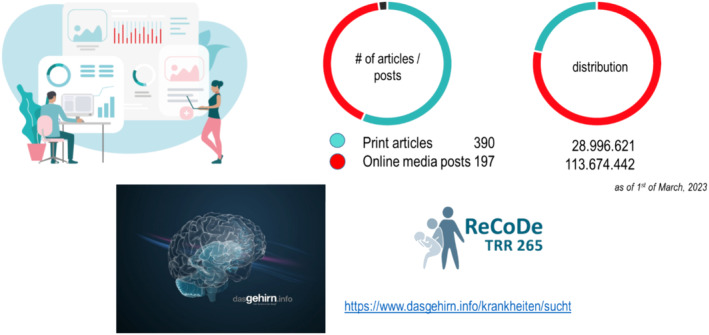
Media reports from the CIMH during the first funding period FP (as of March 2023). The number of printed reports, online reports and social media are shown with a total reach of approx. 140 million interactions. ReCoDe PIs also wrote several posts for the German website “Das Gehirn” – www.dasGehirn.info ‐ which has millions of visits and has set itself the goal of presenting the brain, its functions and its importance for our feelings, thoughts and actions ‐ comprehensively, understandably, attractively and clearly in words, images and sound.
4. THE SECOND FUNDING PERIOD OF THE RECODE CONSORTIUM
In addition to the described key aims in section one, in the second funding period we will study different SUDs (e.g. cocaine use disorder and cannabis use disorder) in a comparative manner in order to generalize findings from the first funding period where the focus was on AUD. We will also put sex/gender differences ‐ in disease trajectories, mechanisms and interventions ‐ more into focus. We will further strengthen our computational expertise for better integration and model building of our multi‐spatial and multi‐temporal big data sets derived from real‐time assessments in the ReCoDe cohort and other big data approaches (e.g. the use of knowledge graphs in addiction research 50 ) within our consortium. This goes along with a further strengthening of our m‐Health infrastructure by implementing more app‐based tasks (e.g., in the cognitive domain) and Ecological Momentary Interventions (EMIs). At the preclinical level, we will test various psychedelic drugs for the treatment of AUD and SUDs. 51 , 52 Recently, we demonstrated that psilocybin produces very similar functional brain signatures in rodents and humans, 53 highlighting the use of preclinical model organisms for a better mechanistic understanding of psychedelic drugs.
4.1. Ecological momentary interventions (EMIs)
Our mHealth infrastructure is currently built for real‐life assessments of physiological, cognitive and behavioural measures and the hereof‐resulting ILDs that will be processed by advanced machine learning 54 to make predictions of future individual events (e.g., relapse to alcohol). However, the ultimate goal of the ReCoDe consortium is to use mHealth tools for mechanism‐based interventions. For doing so, we introduce in the second funding period a real‐life intervention platform that will use Just‐In‐Time‐Adaptive‐Interventions (JITAIs) for patients with AUD from our ReCoDe cohort and in patients with harmful cannabis use. JITAIs use real‐time analyses to determine momentary states, which are best suited for micro‐interventions (e.g., e‐diary ratings of alcohol craving and device‐based read‐outs, such as acceleration signals for physical activity detection), using controlled or randomized intervention‐assignments (e.g., an instruction to engage in physical activity after a couple of high‐craving ratings). The advantages of real‐life interventions are obvious. First, they are non‐invasive, and second, they can provide low‐threshold interventions and thus be used in a preventative way. An additional advantage that is often mentioned in this context is that interventions can be tailored to the time and context, meaning that treatments can be delivered exactly when they are needed, adapted to the context in which a person finds themselves. Validated and ultimately certified EMIs will increasingly play an important role in preserving mental health and counteracting harmful substance use patterns. The reasons for this are three‐fold: (i) The modern digital person has smartphone affinity, (ii) there is a huge treatment gap in AUD in Germany and other countries 55 that can only be captured by low‐threshold interventions and (iii) there is a new target group, completely reliant on smartphone use ‐ as this is often one of few items they possess ‐ that would largely benefit from low‐threshold, language and culturally sensitive smartphone‐based interventions, such as refugees and internally‐displaced persons now residing in Germany and throughout Europe.
4.2. Multi‐omics analysis of biomaterials from the ReCoDe cohort and other sources of biomaterials and functional validation of candidate genes in animal models of addiction
We will give more focus on multi‐omics data exploration and integration (also on the single cell level) from biomaterials of the consortium. A benefit that derives from our ReCoDe cohort is the availability of biomaterials for genetic, epigenetic, gene expression and protein/metabolite expression analyses that we obtained from all subjects and that are stored in our biobank at the CIMH. We will use these biomaterials from the ReCoDe cohort to calculate polygenic scores for each individual and also to generate data for multi‐omics analyses. In particular, we will create pathway‐ and gene‐derived polygenic scores for phenotypes of interest using the genome‐wide genetic data generated for all subjects of the cohort. Evaluations on a single omics level cannot adequately reflect this complexity. Given the rapid developments in the application of multi‐omics in the biomedical field, it is obvious that such an approach should also play a major role in our research activities. In particular, the integration of transcriptomics, chromatin accessibility and epigenomics will allow the identification of molecular patterns associated with the disease, and increase the validity of findings. This is of particular importance in the field of addiction as this disease is the result of cumulative responses to alcohol or drug exposure, the genetic makeup of an individual and the environmental perturbations over time. This complex drug x gene x environment interaction can be best captured on a molecular level by an integrative multi‐omics approach with subsequent functional validation of candidate genes in animal models of addiction.
During the first funding period, we have already demonstrated the applicability of such a multi‐omics approach in brain tissue from patients with AUD where we integrated genome‐wide DNA methylation and gene expression (RNASeq) data from the ventral striatum, caudate nucleus and putamen. We found convergent evidence on the importance of neuroinflammation‐related pathways, and identified a conserved hub gene in AUD‐associated networks, STAT3, demonstrating the value of integrating data from different omics sources. 56 However, bulk brain tissue analysis as it was done in the Zillich et al. 56 study has its limitations as it does not take the heterogeneity of single‐cell populations into consideration. Here, multiome analysis of single cells is the way to go but its application by e.g. 10xGenomics is prohibitively expensive and can thus again only be done in a few samples. Therefore, we take great advantage of previous consortia that we established with the help of the German SysMedAlcoholism consortium 57 (https://www.sysmedsud.org), and the DFG Sequencing grant “Deciphering alcohol addiction‐associated gene regulation changes on a single cell level” a large brain tissue bank and an in‐silico brain bank of numerous AUD and SUD cases that will now be of great help to the here proposed multi‐omics approach. In summary, our multi‐omics approach will retrieve new candidate genes for AUD and cocaine addiction that will then be functionally validated in appropriate rat models of addiction. Given that functional validation in the rat model of addiction is a very time‐consuming task we will also use Drosophila for functional validation studies. This model organism and the large existing genetic toolbox allow for rapid functional validation of human candidate genes ‐ an approach that we have repeatedly applied in a successful way in the past. 58 , 59 , 60
4.3. From 3R to 6R and Open Science
Finally, ReCoDe will further integrate the 3R principles of animal testing, introduce other model organisms (Drosophila) and introduce the 6R principles. Strech and Dirnagl 61 introduced Robustness, Registration and Reporting, in addition to the 3Rs, all of which aim to safeguard and increase the scientific value and reproducibility of animal research.
Science has long been considered “self‐correcting” because it is based on replication of previous work. However, in recent decades the checks and balances that once ensured scientific security have largely disappeared. Fidelity has been questioned throughout the biomedical field. This has affected the ability of today's researchers to reproduce others' results 62 and has led to many translation errors. In recent perspective articles, we provided recommendations according to Open Science principles to improve reproducibility and translation. 22 , 63 However, even if we follow best preclinical practice and open science principles, translation errors will still occur ‐ but probably to a lesser extent. Nevertheless, one can always learn from translation errors. To this end, however, a critical dialogue between basic researchers, preclinical researchers and clinicians is essential.
5. SUMMARY AND FUTURE PERSPECTIVES OF THE RECODE CONSORTIUM
In the first funding period, we examined not only the loss but also the regaining of control over drug use in men and women across the lifespan with different SUDs. In particular, we examined real‐life disease progression in a large cohort of mild to moderate patients with AUD (taking into account comorbid tobacco and cannabis use) and, in some projects, also patients with TUD. For the second funding period, we are moving step by step from the real‐time description of the disease progression to the underlying mechanisms and the mechanism‐based, non‐invasive interventions derived from them. We are also focusing on more severe AUD cases and attempting to generalize our results from AUD to other SUDs, particularly cannabis use disorders. In the third funding period, there will be an increased focus on other drugs, particularly opioids and new psychostimulants to achieve the generalizability of our conclusions and to develop an integrative framework for a better understanding of addictive behaviour.
In the second funding period, we will continue to focus on real‐life assessments and non‐invasive, mechanism‐based interventions. Non‐invasive treatment developments that will mature into the third funding period concern various NIBS approaches and our EMI intervention platform. JITAIs are used to capture momentary states (e.g., mood, stress, impulsivity ratings, etc.) and, where appropriate, controlled or randomized intervention assignments (e.g., exercise instruction after some high‐stress assessments). During the second funding period, the EMI intervention platform will be established and tested in a cohort of AUD patients and in a small exploratory sample of heavy cannabis users. This EMI platform can then be used in future studies to examine larger samples of smokers, and patients with cannabis use disorder, methamphetamine use disorder or opioid use disorder. Additionally, we can use EMIs to target patients with high PIT responses or apply JITAIs to sign trackers to reverse their neural prediction error, to name just a few examples of how we might interfere with mechanisms and potential MRI‐based biomarkers (e.g. B. 41 , 42 ) in real‐life to normalize risky behaviour or even addictive behaviour.
We will investigate the role of oxytocin in social alcohol consumption behaviour and the underlying mechanisms. Oxytocin is a prime candidate for impairment of social drinking behaviour, 64 and intranasal use in at‐risk social drinkers is a viable clinical development process. Another pharmacological intervention that is being developed preclinically is the use of psychedelic medications to treat AUD and SUDs. Here we build on promising preclinical and human findings from the ERANET grant PsiAlc (https://www.psialc.org) and the recent milestone publication by Bogenschutz et al., 65 where it was shown in an RCT that administration of psilocybin in combination with psychotherapy resulted in a significant decrease in the percentage of heavy drinking days compared to those caused by active placebo and psychotherapy. These results support further research into psilocybin‐assisted treatment of AUD.
The ultimate future perspective is the clinical development and EMA approval of innovative pharmacological and NIBS interventions. We also want to develop an app that allows predicting the risk of relapse associated with EMI. Obviously, these cost‐intensive treatment developments can only be carried out in the context of the newly founded German Center for Mental Health (DZPG partner sites Mannheim and Berlin‐Potsdam) and RCTs supported by industry.
CONFLICT OF INTEREST STATEMENT
We do not report any CoI related to the submitted work.
Supporting information
Data S1. Supporting Information
ACKNOWLEDGMENTS
The Collaborative Research Center (CRC) (Transregio ‐ TRR 265 ‐ Losing and regaining control over drug intake – from trajectories to mechanisms and interventions) – the ReCoDe consortium) is funded by the German Research Foundation (Deutsche Forschungsgemeinschaft DFG). The project is carried out using the Mannheim (CIMH)/Charité infrastructure of the German Center for Mental Health (DZPG). Open Access funding enabled and organized by Projekt DEAL.
Spanagel R, Bach P, Banaschewski T, et al. The ReCoDe addiction research consortium: Losing and regaining control over drug intake—Findings and future perspectives. Addiction Biology. 2024;29 (7): e13419. doi: 10.1111/adb.13419
The list of names of the other ReCoDe members can be found in Data S1.
REFERENCES
- 1. Heinz A, Kiefer F, Smolka MN, et al. Addiction research consortium: losing and regaining control over drug intake (ReCoDe)—from trajectories to mechanisms and interventions. Addict Biol. 2020;25(2):e12866. doi: 10.1111/adb.12866 [DOI] [PubMed] [Google Scholar]
- 2. Reichert M, Gan G, Renz M, et al. Ambulatory assessment for precision psychiatry: foundations, current developments and future avenues. Exp Neurol. 2021;345:113807. doi: 10.1016/j.expneurol.2021.113807 [DOI] [PubMed] [Google Scholar]
- 3. Heinz A, Beck A, Halil MG, Pilhatsch M, Smolka MN, Liu S. Addiction as learned behavior patterns. J Clin Med. 2019;8(8):1086. doi: 10.3390/jcm8081086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spanagel R. Animal models of addiction. Dialogues Clin Neurosci. 2017;19(3):247‐258. doi: 10.31887/DCNS.2017.19.3/rspanagel [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Foo JC, Meinhardt MW, Skorodumov I, Spanagel R. Alcohol solution strength preference predicts compulsive‐like drinking behavior in rats. Alcohol Clin Exp Res. 2022;46(9):1710‐1719. doi: 10.1111/acer.14910 [DOI] [PubMed] [Google Scholar]
- 6. Foo JC, Noori HR, Yamaguchi I, et al. Dynamical state transitions into addictive behaviour and their early‐warning signals. Proc: Biol Sci. 2017;284(1860):20170882. doi: 10.1098/rspb.2017.0882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Durstewitz D, Koppe G, Meyer‐Lindenberg A. Deep neural networks in psychiatry. Mol Psychiatry. 2019;24(11):1583‐1598. doi: 10.1038/s41380-019-0365-9 [DOI] [PubMed] [Google Scholar]
- 8. Yücel M, Oldenhof E, Ahmed SH, et al. A transdiagnostic dimensional approach towards a neuropsychological assessment for addiction: an international Delphi consensus study. Addiction. 2019;114(6):1095‐1109. doi: 10.1111/add.14424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heinz A, Gutwinski S, Bahr NS, Spanagel R, Di Chiara G. Does compulsion explain addiction? Addict Biol. 2024;29(4):e13379. doi: 10.1111/adb.13379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hagman BT, Falk D, Litten R, Koob GF. Defining recovery from alcohol use disorder: development of an NIAAA research definition. Am J Psychiatry. 2022;179(11):807‐813. doi: 10.1176/appi.ajp.21090963 [DOI] [PubMed] [Google Scholar]
- 11. Witkiewitz K, Pfund RA, Tucker JA. Mechanisms of behavior change in substance use disorder with and without formal treatment. Annu Rev Clin Psychol. 2022;18(1):497‐525. doi: 10.1146/annurev-clinpsy-072720-014802 [DOI] [PubMed] [Google Scholar]
- 12. Ersche KD, Gillan CM, Jones PS, et al. Carrots and sticks fail to change behavior in cocaine addiction. Science. 2016;352(6292):1468‐1471. doi: 10.1126/science.aaf3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Benedyk A, Reichert M, Giurgiu M, et al. Real‐life behavioral and neural circuit markers of physical activity as a compensatory mechanism for social isolation. Nat Mental Health. 2024;2(3):337‐342. doi: 10.1038/s44220-024-00204-6 [DOI] [Google Scholar]
- 14. Deeken F, Reichert M, Zech H, et al. Patterns of alcohol consumption among individuals with alcohol use disorder during the COVID‐19 pandemic and lockdowns in Germany. JAMA Netw Open. 2022;5(8):e2224641. doi: 10.1001/jamanetworkopen.2022.24641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gan G, Ma R, Reichert M, et al. Neural correlates of affective benefit from real‐life social contact and implications for psychiatric resilience. JAMA Psychiatry. 2021;78(7):790‐792. doi: 10.1001/jamapsychiatry.2021.0560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tost H, Reichert M, Braun U, et al. Neural correlates of individual differences in affective benefit of real‐life urban green space exposure. Nat Neurosci. 2019;22(9):1389‐1393. doi: 10.1038/s41593-019-0451-y [DOI] [PubMed] [Google Scholar]
- 17. Zech HG, Reichert M, Ebner‐Priemer UW, et al. Mobile data collection of cognitive‐behavioral tasks in substance use disorders: where are we now? Neuropsychobiology. 2022;29(5):1‐13. doi: 10.1159/000523697 [DOI] [PubMed] [Google Scholar]
- 18. Zech H, Waltmann M, Lee Y, et al. Measuring self‐regulation in everyday life: reliability and validity of smartphone‐based experiments in alcohol use disorder. Behav Res Methods. 2023;55(8):4329‐4342. doi: 10.3758/s13428-022-02019-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eldar E, Roth C, Dayan P, Dolan RJ. Decodability of reward learning signals predicts mood fluctuations. Curr Biol. 2018;28(9):1433‐1439.e7. doi: 10.1016/j.cub.2018.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thome J, Pinger M, Halli P, et al. A model guided approach to evoke homogeneous behavior during temporal reward and loss discounting. Front Psych. 2022;13:846119. doi: 10.3389/fpsyt.2022.846119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thome J, Pinger M, Durstewitz D, Sommer WH, Kirsch P, Koppe G. Model‐based experimental manipulation of probabilistic behavior in interpretable behavioral latent variable models. Front Neurosci. 2023;16:1077735. doi: 10.3389/fnins.2022.1077735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spanagel R. Ten points to improve reproducibility and translation of animal research. Front Behav Neurosci. 2022;16:869511. doi: 10.3389/fnbeh.2022.869511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deeken F, Banaschewski T, Kluge U, Rapp MA. Risk and protective factors for alcohol use disorders across the lifespan. Curr Addict Rep. 2020;7(3):245‐251. doi: 10.1007/s40429-020-00313-z [DOI] [Google Scholar]
- 24. Henssler J, Stock F, van Bohemen J, Walter H, Heinz A, Brandt L. Mental health effects of infection containment strategies: quarantine and isolation—a systematic review and meta‐analysis. Eur Arch Psychiatry Clin Neurosci. 2021;271(2):223‐234. doi: 10.1007/s00406-020-01196-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jones EAK, Mitra AK, Bhuiyan AR. Impact of COVID‐19 on mental health in adolescents: a systematic review. Int J Environ Res Public Health. 2021;18(5):2470. doi: 10.3390/ijerph18052470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Acuff SF, Strickland JC, Tucker JA, Murphy JG. Changes in alcohol use during COVID‐19 and associations with contextual and individual difference variables: a systematic review and meta‐analysis. Psychol Addict Behav. 2022;36(1):1‐19. doi: 10.1037/adb0000796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Friske MM, Spanagel R. Chronic alcohol consumption and COVID‐19 infection risk: a narrative review. Alcohol Clin Exp Res (Hoboken). 2023;47(4):629‐639. doi: 10.1111/acer.15041 [DOI] [PubMed] [Google Scholar]
- 28. Kim JU, Majid A, Judge R, et al. Effect of COVID‐19 lockdown on alcohol consumption in patients with pre‐existing alcohol use disorder. Lancet Gastroenterol Hepatol. 2020;5(10):886‐887. doi: 10.1016/S2468-1253(20)30251-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meinhardt MW, Hansson AC, Perreau‐Lenz S, et al. Rescue of infralimbic mGluR2 deficit restores control over drug‐seeking behavior in alcohol dependence. J Neurosci. 2013;33(7):2794‐2806. doi: 10.1523/JNEUROSCI.4062-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meinhardt MW, Pfarr S, Fouquet G, et al. Psilocybin targets a common molecular mechanism for cognitive impairment and increased craving in alcoholism. Sci Adv. 2021;7(47):eabh2399. doi: 10.1126/sciadv.abh2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Domanegg K, Sommer WH, Meinhardt MW. Psychedelic targeting of metabotropic glutamate receptor 2 and its implications for the treatment of alcoholism. Cells. 2023;12(6):963. doi: 10.3390/cells12060963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vengeliene V, Spanagel R. mGlu2 mechanism‐based interventions to treat alcohol relapse. Front Pharmacol. 2022;13:985954. doi: 10.3389/fphar.2022.985954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ghin F, Beste C, Stock AK. Neurobiological mechanisms of control in alcohol use disorder—moving towards mechanism‐based non‐invasive brain stimulation treatments. Neurosci Biobehav Rev. 2022;133:104508. doi: 10.1016/j.neubiorev.2021.12.031 [DOI] [PubMed] [Google Scholar]
- 34. Nasr K, Haslacher D, Dayan E, Censor N, Cohen LG, Soekadar SR. Breaking the boundaries of interacting with the human brain using adaptive closed‐loop stimulation. Prog Neurobiol. 2022;216:102311. doi: 10.1016/j.pneurobio.2022.102311 [DOI] [PubMed] [Google Scholar]
- 35. Weiss F, Zhang J, Aslan A, Kirsch P, Gerchen MF. Feasibility of training the dorsolateral prefrontal‐striatal network by real‐time fMRI neurofeedback. Sci Rep. 2022;12(1):1669. doi: 10.1038/s41598-022-05675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nardo T, Batchelor J, Berry J, Francis H, Jafar D, Borchard T. Cognitive remediation as an adjunct treatment for substance use disorders: a systematic review. Neuropsychol Rev. 2022;32(1):161‐191. doi: 10.1007/s11065-021-09506-3 [DOI] [PubMed] [Google Scholar]
- 37. Atherton M, Zhuang J, Bart WM, Hu X, He S. A functional MRI study of high‐level cognition. I. The game of chess. Brain Res Cogn Brain Res. 2013;16(1):26‐31. doi: 10.1016/s0926-6410(02)00207-0 [DOI] [PubMed] [Google Scholar]
- 38. Gerhardt S, Lex G, Holzammer J, Karl D, Wieland A, Schmitt R, Recuero AJ, Montero JA, Weber T, Vollstädt‐Klein S (2022) Effects of chess‐based cognitive remediation training as therapy add‐on in alcohol and tobacco use disorders: protocol of a randomised, controlled clinical fMRI trial. BMJ Open 2022. 12(9):e057707. 10.1136/bmjopen-2021-057707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bach P, Zaiser J, Zimmermann S, et al. Stress‐induced sensitization of insula activation predicts alcohol craving and alcohol use in alcohol use disorder. Biol Psychiatry. 2024;95(3):245‐255. doi: 10.1016/j.biopsych.2023.08.024 [DOI] [PubMed] [Google Scholar]
- 40. Hoffmann S, Gerhardt S, Mühle C, et al. Associations of menstrual cycle and progesterone‐to‐estradiol ratio with alcohol consumption in alcohol use disorder: a sex‐separated multicenter longitudinal study. Am J Psychiatry. 2024;181(5):445‐456. doi: 10.1176/appi.ajp.20230027 [DOI] [PubMed] [Google Scholar]
- 41. Hildebrandt MK, Schwarz K, Dieterich R, Endrass T. Dissociating the link of neural correlates of inhibition to the degree of substance use and substance‐related problems: a preregistered, multimodal, combined cross‐sectional and longitudinal study. Biol Psychiatry. 2023;94(11):898‐905. doi: 10.1016/j.biopsych.2023.06.017 [DOI] [PubMed] [Google Scholar]
- 42. Chen H, Mojtahedzadeh N, Belanger MJ, et al. Model‐based and model‐free control predicts alcohol consumption developmental trajectory in young adults: a 3‐year prospective study. Biol Psychiatry. 2021;89(10):980‐989. doi: 10.1016/j.biopsych.2021.01.009 [DOI] [PubMed] [Google Scholar]
- 43. Garbusow M, Schad DJ, Sebold M, et al. Pavlovian‐to‐instrumental transfer effects in the nucleus accumbens relate to relapse in alcohol dependence. Addict Biol. 2016;21:719‐731. doi:10.1111/adb.12243 [DOI] [PubMed] [Google Scholar]
- 44. Rane RP, de Man EF, Kim J, et al. Structural differences in adolescent brains can predict alcohol misuse. Elife. 2022;11:e77545. doi: 10.7554/eLife.77545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hasanpour M, Mitricheva E, Logothetis N, Noori HR. Intensive longitudinal characterization of multidimensional biobehavioral dynamics in laboratory rats. Cell Rep. 2021;13(2):108987. doi: 10.1016/j.celrep.2021.108987 [DOI] [PubMed] [Google Scholar]
- 46. Durstewitz D, Koppe G, Thurm MI. Reconstructing computational system dynamics from neural data with recurrent neural networks. Nat Rev Neurosci. 2023;24(11):693‐710. doi: 10.1038/s41583-023-00740-7 [DOI] [PubMed] [Google Scholar]
- 47. Holz NE, Berhe O, Sacu S, et al. Early social adversity, altered brain functional connectivity, and mental health. Biol Psychiatry. 2023;93(5):430‐441. doi: 10.1016/j.biopsych.2022.10.019 [DOI] [PubMed] [Google Scholar]
- 48. Giannone F, Ebrahimi C, Endrass T, Hansson AC, Schlagenhauf F, Sommer WH (2024) Bad habits – good goals? Meta‐analysis and translation of the habit construct to alcoholism. Transl Psychiatry (in press). [Google Scholar]
- 49. Doñamayor N, Ebrahimi C, Arndt V, Weiss F, Schlagenhauf F, Endrass T. Goal‐directed and habitual control in substance use: state of the art and future directions. Neuropsychobiology. 2022;81(5):403‐417. doi: 10.1159/000527663 [DOI] [PubMed] [Google Scholar]
- 50. Zhang Y, Sui X, Pan F, Yu K, Li K, Tian S, Erdengasileng A, Han Q, Wang W, Wang J, Wang J, Sun D, Chung H, Zhou J, Zhou E, Lee B, Zhang P, Qiu X, Zhao T, Zhang J (2023) BioKG: a comprehensive, large‐scale biomedical knowledge graph for AI‐powered, data‐driven biomedical research. bioRxiv 10.1101/2023.10.13.562216. [DOI]
- 51. Calleja‐Conde J, Morales‐García JA, Echeverry‐Alzate V, Bühler KM, Giné E, López‐Moreno JA. Classic psychedelics and alcohol use disorders: a systematic review of human and animal studies. Addict Biol. 2022;27(6):e13229. doi: 10.1111/adb.13229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Urban MM, Stingl MR, Meinhardt MW. Mini‐review: the neurobiology of treating substance use disorders with classical psychedelics. Front Neurosci. 2023;17:1156319. doi: 10.3389/fnins.2023.1156319.eCollection [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reinwald JR, Schmitz CN, Skorodumov I, et al. Psilocybin‐induced default mode network hypoconnectivity is blunted in alcohol‐dependent rats. Transl Psychiatry. 2023;13(1):392. doi: 10.1038/s41398-023-02690-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang L, Du Y, Yang W, Liu J. Machine learning with neuroimaging biomarkers: application in the diagnosis and prediction of drug addiction. Addict Biol. 2023;28(2):e13267. doi: 10.1111/adb.13267 [DOI] [PubMed] [Google Scholar]
- 55. Mekonen T, Chan GCK, Connor J, Hall W, Hides L, Leung J. Treatment rates for alcohol use disorders: a systematic review and meta‐analysis. Addiction. 2021;116(10):2617‐2634. doi: 10.1111/add.15357 [DOI] [PubMed] [Google Scholar]
- 56. Zillich L, Poisel E, Frank J, et al. Multi‐omics signatures of alcohol use disorder in the dorsal and ventral striatum. Transl Psychiatry. 2022;12(1):190. doi: 10.1038/s41398-022-01959-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Spanagel R, Durstewitz D, Hansson A, et al. A systems medicine research approach for studying alcohol addiction. Addict Biol. 2013;18(6):883‐896. doi: 10.1111/adb.12109 [DOI] [PubMed] [Google Scholar]
- 58. Juraeva D, Treutlein J, Scholz H, et al. XRCC5 as a risk gene for alcohol dependence: evidence from a genome‐wide gene‐set‐based analysis and follow‐up studies in drosophila and humans. Neuropsychopharmacology. 2015;40(2):361‐371. doi: 10.1038/npp.2014.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Knabbe J, Protzmann J, Schneider N, et al. Single‐dose ethanol intoxication causes acute and lasting neuronal changes in the brain. Proc Natl Acad Sci U S A. 2022;119(25):e2122477119. doi: 10.1073/pnas.2122477119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Velo Escarcena L, Neufeld M, Rietschel M, Spanagel R, Scholz H. ERR and dPECR suggest a link between neuroprotection and the regulation of ethanol consumption preference. Front Psych. 2021;12:655816. doi: 10.3389/fpsyt.2021.655816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Strech D, Dirnagl U. 3Rs missing: animal research without scientific value is unethical. BMJ Open Sci. 2019;3:e000035. doi: 10.1136/bmjos-2018-000048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014;505(7485):612‐613. doi: 10.1038/505612a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Meinhardt MW, Gerlach B, Spanagel R. Good practice guideline for preclinical alcohol research: the STRINGENCY framework. Curr Top Behav Neurosci. 2024. in press. [Google Scholar]
- 64. Potretzke S, Zhang Y, Li J, et al. Male‐selective effects of oxytocin agonism on alcohol intake: behavioral assessment in socially housed prairie voles and involvement of RAGE. Neuropsychopharmacology. 2023;48(6):920‐928. doi: 10.1038/s41386-022-01490-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bogenschutz MP, Ross S, Bhatt S, et al. Percentage of heavy drinking days following psilocybin‐assisted psychotherapy vs placebo in the treatment of adult patients with alcohol use disorder: a randomized clinical trial. JAMA Psychiatry. 2022;79(10):953‐962. doi: 10.1001/jamapsychiatry.2022.2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information


