Abstract
Background:
Cannabis is the most common illicit drug used in the US and its use has been rising over the past decade, while the historical gap in rates of use between men and women has been decreasing. Sex differences in the effects of cannabinoids have been reported in animal models, but human studies are sparse and inconsistent. We investigated the sex differences in the acute subjective, psychotomimetic, cognitive, and physiological effects of intravenous (IV) delta-9 tetrahydrocannabinol (THC), the main psychoactive constituent of cannabis.
Methods:
Healthy male and female individuals, with limited exposure to cannabis, participated in a double blind, placebo-controlled study of intravenous (IV) placebo or THC at two doses (0.015 mg/kg and 0.03 mg/kg). Visual Analog Scale (VAS) was used to measure subjective effects, Psychotomimetic States Inventory (PSI) and the Clinician-Administered Dissociative Symptoms Scale (CADSS) were used to assess the psychotomimetic effects and perceptual alterations, respectively, and Rey Auditory Verbal Learning Task (RAVLT) was used to evaluate cognitive effects. Outcome variables were represented as the peak change from baseline for each variable, except RAVLT which was used only once per the test day after the subjective effects.
Results:
A total of 42 individuals participated in this study. There were no significant differences between male and female participants in background characteristics. There was a significant main effect of sex on the VAS scores for THC induced “High” (F1,38= 4.27, p<0.05) and a significant dose x sex interaction (F2,77= 3.38, p < 0.05) with female participants having greater “High” scores than male participants at the lower THC dose (0.015 mg/kg). No other sex differences were observed in acute subjective, psychotomimetic, cognitive, or physiological effects of THC.
Conclusion:
There were significant sex differences in subjective effects of feeling “High” at a lower dose of THC. However, there were no other sex related differences in the subjective, physiological, or cognitive effects of THC.
Introduction
Cannabis is the most common illicit drug used in the U.S. and its use has been increasing over the past decade. In 2019, 48.2 million Americans aged 12 or older (17.5% of population) reported use of cannabis over the past 12 months, a 10% increase in just one year [1]. Further, more than 3.5 million individuals reported initiation of cannabis use in 2019, a greater number than any year since 2002 [1]. In parallel, given the trend of cannabis legalization for medicinal and recreational purposes in the United States and abroad, further research into factors influencing the effects of cannabinoids that may affect development of cannabis use disorder is increasingly relevant.
Historically, men have exhibited greater rates of cannabis use compared to women. In the past decade, however, there has been a progressive increase in cannabis use among women, narrowing the prevalence gap between these groups [2, 3]. Interestingly, women show a steeper transition in time from first cannabis use to the development of cannabis use disorder (CUD), known as the “telescopic phenomenon” [4, 5], are more likely to experience physical symptoms of cannabis withdrawal [6], and report a greater impact of cannabis consumption on quality of life [7]. However, the factors underpinning these observed sex differences remain unknown.
The acute effects of cannabis have great implications in the development of CUD. Preclinical data highlights sex differences in the rewarding and reinforcing properties of cannabinoids, extinction, and relapse [8]. Female rats are more sensitive to the reinforcing effects of cannabinoids and show higher rates and more rapid acquisition of cannabis agonist self-administration [9]. They also exhibit stronger drug- and cue-induced reinstatement of cannabinoid seeking behaviors [10]. Interestingly, these behaviors are reduced in bilaterally ovariectomized females, suggesting a critical role of the ovarian hormones in modulating the reinforcing properties of cannabinoid compounds [9, 10].
Human data related to sex differences of the acute rewarding properties of cannabinoids is scant and has shown some discrepancies. Whereas initial studies did not report any differences in the acute subjective effects of cannabinoids [11], or reported more pronounced rewarding effects in men [12, 13], most recent findings are aligned with the previously mentioned preclinical data and have revealed an increased sensitivity to the subjective [14–17][18] effects of cannabis in women, especially in low doses [15]. In a 2020 study of the acute effects of smoked cannabis in men and women, in which participants were permitted to smoke cannabis ad libitum in order to titrate to their desired intoxication, Matheson and colleagues reported similar effects in men and women, despite the women demonstrating significantly lower quantities of cannabis consumed and significantly lower levels of blood THC and THC metabolites [19]. This suggests that women demonstrated acute effects of cannabis equal to those reported by men at a significantly lower dose.
In this study, we examined the dose-dependent subjective, psychotomimetic, cognitive, and physiological effects of acute intravenous (IV) THC in male and female individuals who were not using cannabis regularly. We specifically chose to study the IV route of administration to precisely control the THC administration and be able to investigate the dose related sex differences in THC effects without the confounds of variable delivery, absorption, and pharmacokinetics.
Methods
Data were pooled from two studies (sub-study#1 and Sub-study#2) using similar research methods in healthy human participants. Volunteers participated in three test days in a double blind, placebo-controlled manner, conducted under two overlapping studies designed to examine the effects of THC on psychophysiological outcome measures. Both sub-studies were approved by institutional review board of VA Connecticut Healthcare System and Yale University School of Medicine and had identical methods of participant screening, THC dose and rates of infusion and subjective rating paradigms. Sub-study #1 was designed to examine the effects of THC on electrophysiological signatures of information processing and sub-study #2 on eye blink conditioning.
Participants:
Healthy male and female individuals between the ages of 18 and 55 years were recruited from the community using flyers, digital ads, and word of mouth. Participants first underwent a phone screen and, if suitable, were invited for an in-person screen to determine eligibility. After obtaining informed consent, participants underwent medical and psychiatric examination and laboratory assessment of blood and urine, including urine toxicology for drug use. Participants were excluded if they had substance use disorders except tobacco use disorder (based on SCID-IV) and were required to have negative urine toxicology and pregnancy tests. Individuals with unstable medical or neurological conditions, a DSM-IV Axis I diagnosis (based on SCID-IV), IQ less than 80, history of a hearing deficit, and/or major current or recent (<6 weeks) psychosocial stressors (based on clinical interview) were excluded from participation. Additionally, participants were required to have at least one prior exposure to cannabis in their lifetime (i.e., cannabis naïve individuals were excluded). Individuals who met criteria for cannabis use disorder were excluded. Sex assigned at birth was noted at screening.
Drugs:
THC doses of 0.015 mg/kg and 0.03 mg/kg or placebo were administered intravenously over 10 minutes based on previous data demonstrating robust subjective, psychotomimetic, and cognitive effects and high tolerability at this dose. Based on our previous studies, the peak effects starts around 10–15 minutes after initiation of THC infusion and lasts for up to approximately 60– 80 minutes [20, 21].
Schedule of Testing:
Participants were instructed to fast overnight and report to the test facility at 8 AM. Recent substance use, urine toxicology, and pregnancy (in women) were assessed on each test day. Positive drug screen for any drug other than cannabis or positive pregnancy test resulted in cancellation of the test day. After confirming test day restrictions, nursing staff placed an IV line in the subject’s arm for administration of the study drug (placebo, 0.015, or 0.03 mg/kg THC). Following administration of the study drug, participants completed a series of psychophysiological tasks (results presented separately [22, 23]), measures of subjective and psychotomimetic effects/perceptual alterations, and cognitive tasks to assess drug effects. Table 1 summarizes the timeline of the study assessments. The timing of initiation of subjective assessments was based on the psychophysiological assessments in each sub-study: In sub-study#1, the assessments at +10 began with blood draws, vital signs, free description of subjective effects following by assessment of subjective and perceptual effects using visual analog scale (VAS), Psychotomimetic States Inventory (PSI) and the Clinician-Administered Dissociative Symptoms Scale (CADSS) and then psychophysiological measures at ~+35; In sub-study#2, the +10 timepoint began with blood draws following by psychophysiological assessments that ended around +40 and followed by CADSS and VAS. Based on the duration of other procedures we estimate that CADSS and VAS occurred within the window listed in the table 1. Participants in both sub-studies were asked to rate their overall peak effects. Importantly, since these sub-studies are placebo controlled, each participant served as their own control in the placebo condition. Furthermore, within each sub-study male and female participants had similar timing of assessments and there was no main effect of sub-study on the outcomes. Clinical evaluations including field sobriety test were conducted at the end of each test day to assess the resolution of the drug effects. Participants were discharged approximately 240 min after administration of the study drug, or after resolution of all drug effects after physician evaluation, whichever came last. Participants were not permitted to drive to or from the testing center and were required to arrange alternative transportation. Test days were scheduled at least 3 days apart, to minimize the potential carryover effects of THC [21].
Table 1.
Timeline of assessments over the test days
| Time (minutes) | Assessment |
|---|---|
| −90 | Urine toxicology, breathalyzer, vitals |
| −60 to −10 | Vitals, CADSS1, PSI2, VAS3 |
| −5 | Vitals |
| 0 | THC IV infusion |
| +10 to +452 | Vitals, CADSS, VAS |
| +55 | Vitals, RAVLT4 |
| +80 to +100 | Vitals, CADSS, VAS, |
| +240 | Vitals, CADSS, PSI, VAS |
Clinician-Administered Dissociative Symptoms Scale, 2. Psychotomimetic States Inventory, 3. visual analog scale, 4. Rey Auditory Verbal Learning Task
Of note the timing of “peak” assessments provides a window within which the assessments were conducted to capture the overall peak effects associated with drug administration.
Outcome Measures:
Subjective effects:
Subjective effects of THC were measured on a visual analog scale (VAS) and included subjective states such as “High”, “Calm”, “Happy’ and “Anxious,” scored on a 100 mm line (0= not at all, 100= extremely) [20]. Participants self-rated their subjective states on the VAS before and at several time points after THC/placebo administration on each test day. Rating scales for peak subjective effects and perceptual alterations were administered in the 20–60-minute window after THC administration to capture all effects since drug administration (Table 1).
Psychotomimetic effects and Perceptual Alterations:
Psychotomimetic effects and perceptual alterations were measured using the Psychotomimetic States Inventory (PSI) and the Clinician-Administered Dissociative Symptoms Scale (CADSS), respectively, both of which have been shown previously to be sensitive to the acute effects of THC [21]. CADSS was assessed at the same time points as other subjective effects. PSI was assessed at baseline and at the end of the test day (~240 minutes after THC administration) when participants were asked to rate their peak effects (Table 1).
Cognitive Effects:
Cognitive effects were assessed using a verbal learning and memory task called the Rey Auditory Verbal Learning Task (RAVLT) after assessment of subjective effects and perceptual alterations (Table 1). The RAVLT is a non-semantically organized, 15-item word, list task with 5 alternate versions sensitive to the effects of THC [24]. First, a word list (List A) is presented five times with free immediate recall after each presentation. After the fifth recall, a distractor list (List B) is presented and recalled. Subsequently, short (immediate) and long (~30 minutes) delayed recall of List A are recorded. After completion of the delayed recall, participants are presented with a long list of words from both List A and List B, as well as words that were not presented to the subject that day (List C). Participants are asked to indicate to which list each word belongs (recognition).
Statistical analyses:
The following primary outcome measures represented the most robust THC effects identified from prior studies in order to explore sex-related differences: VAS “High” (THC induced intoxication); CADSS (THC-induced perceptual alterations), and RAVLT total recall (acute effects of THC on verbal memory). Subjective (i.e., VAS, CADSS) and psychotomimetic effects (PSI) outcomes were represented as the change from baseline to the peak THC effect. Cognitive effects (RAVLT) were assessed once per test day. Data were inspected for normality using box plots, normal probability plots, and Kolmogorov-Smirnov tests. Linear mixed models with sex (male/female) as a between subject factor and drug (THC dose) as within-subject factor were employed to assess each outcome. The dose by sex interaction was also modeled and the best-fitting variance-covariance structure was chosen using information criteria. The original sub-study (#1 and #2) was included as a covariate in all analyses. Least-square means were compared post-hoc to interpret significant effects. Physiological effects were measured at several time points throughout the day to monitor safety and were analyzed using the same model above with the inclusion of time as an additional within-subjects factor. Primary outcomes were tested using a two-side alpha threshold of 0.05. All analyses were conducted using SAS, version 9.4 (Cary, NC).
Results
Subject characteristics
The sample consists of 42 individuals with limited lifetime exposure to cannabis who participated in these two studies (19 participants from sub-study #1 and 23 from sub-study #2). There were no significant demographic differences between participants from sub-study #1 and sub-study #2 on demographic variables (data not shown). There were 20 female (7 from sub-study#1 and 13 from sub-study#2) and 22 male participants (12 from study#1 and 10 from sub-study #2) in this study. The difference between the proportion of female to male participants in sub-study#1 (36.8%) vs. sub-study#2 (56.5%) was not statistically significant (p=0.20). There was also no significant effect of sub-study (#1 vs. #2) in any of the models. Overall, there were no significant differences between the demographic factors between male and female participants in this study (Table 2). By far the majority of the participants (n=38, 90%) only had a remote history of cannabis exposure; only four reported cannabis use over the past month, with no significant differences in the last exposure to cannabis between male and female participants (Table 3).
Table 2.
Participants basic characteristics
| Subject Demographics | ||||
|---|---|---|---|---|
|
| ||||
| MALE | FEMALE | |||
| Mean | (S.D) | Mean (S.D) | (S.D) | |
| Total n | 22 | 20 | ||
| Age (years) | 28.4 | 7.8* | 25.0 | 4.6* |
| Education (years) | 16.1 | 2.1 | 15.4 | 1.3 |
|
| ||||
| Race(n) | ||||
| Caucasian | 18 | 18 | ||
| Black | 2 | 1 | ||
| Asian | 2 | 0 | ||
| Mixed | 0 | 1 | ||
| Other | 0 | 0 | ||
|
| ||||
| Ethnicity | ||||
| Non-Hispanic | 21 | 17 | ||
| Hispanic | 1 | 3 | ||
|
| ||||
| BMI | 26.5 | 4.2 | 27.2 | 6.1 |
| Height (in.) | 70.6 | 2.6 | 64.2 | 2.4 |
| Weight (lbs) | 183.2 | 33.1 | 157.0 | 35.6 |
No subjects below the age of 18 years were studied.
No statistically significant difference between studies or between the two groups across these demographic variables.
Table 3.
Cannabis use history in men and women
| Cannabis Use History | ||||
|---|---|---|---|---|
|
| ||||
| Male [N] | Female [N] | |||
| # of subjects | 22 | 20 | ||
| Past Month Cannabis Exposure Days | Male [N (%)] | Female [N (%)] | ||
| None | 20 (90.9%) | 18 (90%) | ||
| 1–3 times | 2 (9%) | 1 (5%) | ||
| 4–8 times | 0 (0%) | 1 (5%) | ||
| Last exposure to cannabis Days ago | Male Mean | (S.D.) | Female Mean | (S.D.) |
| 802.6 | 1238.7 | 538.9 | 443.6 | |
| Lifetime Cannabis Exposure # of days | Male [N (%)] | Female [N (%)] | ||
| Less than 5 times | 3(13.6%) | 1 (5%) | ||
| 5–10 times | 8 (36.3%) | 6 (30%) | ||
| 11–20 times | 2 (9%) | 4 (20%) | ||
| 21–50 times | 4 (18.1%) | 6 (30%) | ||
| 51–100 times | 2 (9%) | 1 (5%) | ||
| >100 times | 3(13.6%) | 2 (10%) | ||
Subjective effects
As expected, the acute administration of I.V. THC (0.015 or 0.03 mg/kg) produced a significant main effect of dose on VAS scores for “High” (F2,77= 114.7, p<0.0001), “Calm” (F2,77= 5.54, p < 0.01), “Happy” (F2,77= 4.52, p < 0.05), and “Anxious” (F2,77= 11.2, p < 0.0001) (full data is presented in supplementary table 1). There was a significant main effect of sex on the VAS scores for “High” (F1,38= 4.27, p<0.05) and a significant dose x sex interaction (F2,77= 3.38, p < 0.05) with female participants having greater “High” scores than male participants at the lower THC dose (0.015 mg/kg (Fig. 1). No other dose by sex interactions were observed.
Figure 1.
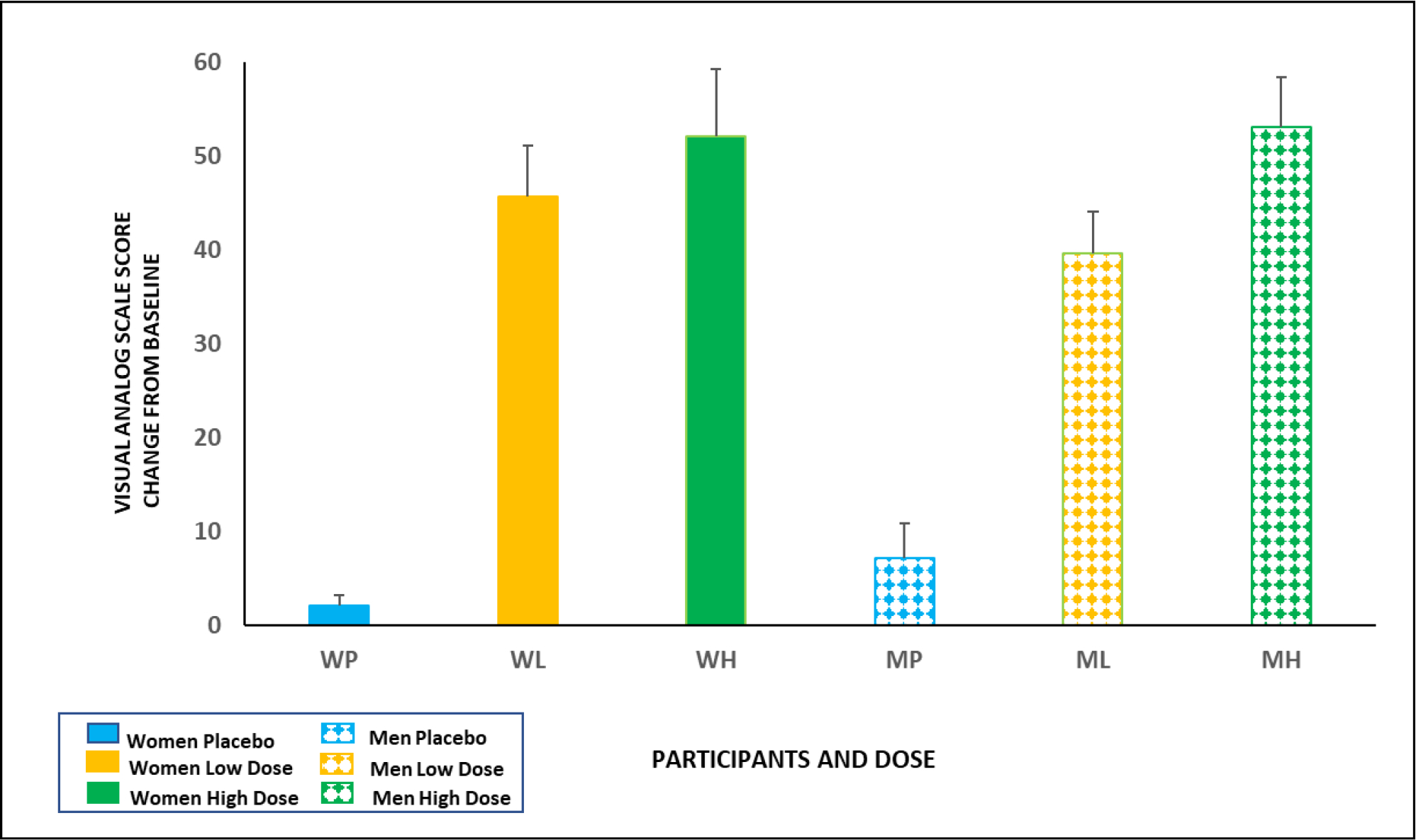
THC induced subjective effects of feeling “High” in male and female participants. The solid bars represent females, and the patterned bars represent males. The Y- Axis represents a change from baseline score on the Visual Analog Scale for “High.” There was a significant main effect of sex on the VAS scores for “High” (F1,38= 4.27, p<0.05) and a significant dose x sex interaction (F2,77= 3.38, p < 0.05) with females having greater “High” scores than males at the lower THC dose (0.015 mg/kg).
Psychotomimetic effects
- Psychotomimetic States Inventory (PSI)
THC induced an increase in psychotomimetic symptoms as measured by the PSI in both males and females, as revealed by main effect of dose (F2,41= 39.79, p < 0.001). THC had no significant main effects on sex or sex x dose interactions on psychotomimetic effects (full data is presented in supplementary table 1).
- Perceptual alterations
THC produced a dose-dependent increase in perceptual alterations measured by the CADSS across both sexes (F2,38= 33.69, p < 0.0001). However, the main effect of sex and the dose x sex interaction were not significant (full data is presented in supplementary table 1).
Cognitive Effects
- Total immediate recall
Males recalled 59.5 ± 2.2 words under the placebo condition, 56.4 ± 2.26 after low dose of THC, and 57.7 ± 2.42 words after the higher dose of THC. Females recalled 61.4 ± 2.15, 58.5 ± 2.62, and 58.7 ± 2.81 during placebo, low, and high dose THC, respectively. While a significant main effect of dose (F2,77= 4.0, p = 0.02) was observed, the main effect of sex and the sex by dose interaction effect were not significant.
- Short delayed recall
Males recalled 12.9 ± 0.58 words under the placebo condition, 13.1 ± 0.52 words after low dose THC, and 13.4 ± 0.42 after the higher dose of THC. Females recalled 13 ± 0.80 words on the placebo condition, 12.3 ± 0.76 on the low THC, and 12.6 ± 0.75 on the higher THC dose. There were no significant main effects of dose, sex, or dose x sex interactions.
- Long delayed recall
On the long-delayed recall, males recalled 11.2 ± 0.92 words on the placebo condition, 12.3 ± 0.74 words on the low dose THC condition, and 11.2 ± 0.80 on the higher dose of THC. Females recalled 11.9 ± 0.84 words on the placebo condition, 12.3 ± 0.66 on the low dose THC condition, and 11.7 ± 0.76 on the higher THC dose. There were no significant main effects of dose, sex, or dose x sex interactions.
Physiological effects
THC administration increased heart rate in a dose dependent manner in both male and female participants (dose: F2,897= 67.3, p < 0.0001; dose x time F14,897= 12.81, p < 0.0001). No effects of sex or dose x sex were observed.
Discussion
Our results demonstrate that female participants demonstrated significantly greater subjective THC induced “High” at the lower dose of THC, compared to male participants at this dose. However, this sex- related difference in “High” was not observed at the higher dose of THC. These results may be explained by a ceiling effect of subjective “High” at the doses studied. However, this is unlikely given that the mean “High” was only 57.55 out of a 100-point scale in female participants after high dose of THC. Alternatively, the results suggest that females were more sensitive to the intoxicating effects of low doses of THC. This pattern of heightened sensitivity may contribute to the differential patterns of cannabis use and faster transition to CUD in women, compared to men. In contrast to the subjective “High”, males and female participants did not show statistically significant differences in the psychotomimetic, perceptual altering, physiological, or cognitive effects of THC. Our results are consistent with the reported higher subjective effects of smoked cannabis [16] or low dose of oral THC [14, 15] in women, though other studies have reported the opposite findings with smoked cannabis [13] or oral THC [12]. However, it is important to note that some of these studies were conducted in individuals who used cannabis frequently [13]. Prior cannabis exposure may affect the potential sex differences associated with acute exposure to THC, given the current evidence from animal studies on faster development of tolerance to the effects of THC in females [25]. Moreover, there are dose-dependent sex differences in the metabolism of THC, which may explain some of the discrepancies in previous studies. In a study of the pharmacokinetic properties of oral THC, women demonstrated a significantly greater peak plasma THC concentration as compared to men after oral administration of 5 mg THC following a fast [26]. Some previous studies that reported lower subjective effects of oral THC in women did not report the blood concentration of THC or its metabolites [12].
In this study, we chose to use IV route of THC administration. While the administration of THC via the smoking, oral, or vaporized route is more ecologically valid, it can introduce substantial inter- and intra-individual variability in bioavailability [23], potentially obscuring or confounding any sex differences in THC effects. Even with standardized paced smoking procedures, participants can titrate the dose of cannabinoids they receive and, in doing so, negate attempts to deliver a uniform dose [24, 26]. The IV route of administration reduces inter- and intra-individual plasma THC variability. Thus, while not generalizable to recreational use, this paradigm permits precise estimation of dose related effects. This is important since variability in the received THC dose can confound any differences attributable to sex. Moreover, while oral THC administration results in production of large amounts of active metabolites (e.g. 11-OH-THC), with reported sex differences, there are low concentrations of these metabolites after IV THC administration, which would minimize the potential confounding effects [27, 28].
Over a wide dose range of IV THC, we have demonstrated robust subjective, cognitive, physiological, endocrine, and psychophysiological effects in a safe and reliable manner [29, 30]. The doses studied here have been shown to produce robust intoxication and cognitive deficits and are well tolerated [30]. Moreover, the IV THC administration has been demonstrated to have a reliable time course of peak effects for subjective feelings and psychotomimetic effects, which is critical in evaluation sex differences [21].
Greater effects of cannabinoids in females are reliably reported in animal studies, and reviewed previously by our group [31]. Female rats are more sensitive to acute and chronic effects of THC on cognition and behavior [32]. Acute THC induces more potent antinociception [33, 34] and locomotor activity [35] in female rats and they develop greater tolerance to these effects with chronic administration [34–36], despite having similar plasma THC levels [36]. Female rats also show higher sensitivity to the reinforcing effects of cannabinoids and have a faster acquisition of cannabinoids self-administration [9], and stronger drug- and cue-induced reinstatement of cannabinoids [10], which are reduced in ovariectomized females [9, 10]. Interestingly, female rats show greater downregulation or desensitization of cannabinoid receptor type 1 (CB1R) in all brain regions after THC exposure [37, 38], which would probably explain their greater withdrawal symptoms, and faster transition to tolerance and dependence [34–36]. In fact, gonadectomized female rats show increased cannabinoid withdrawal behaviors in the presence of estradiol or progesterone administration and gonadectomized male rats show decreased cannabinoid withdrawal behaviors after testosterone administration [39].
Primary sex differences in the endogenous cannabinoid (endocannabinoid, eCB) system could be one of the underlying mechanisms of the observed sex differences in the acute and chronic effects of cannabinoids. Animal studies have demonstrated region-specific sex differences in the levels of CB1R in different brain areas [40–43], which are profoundly affected by sex hormones in both males and females [44]. Similarly, few available human studies also reported sex differences in CB1R availability in different brain regions, but the nature of these differences vary depending on the PET imaging ligands that were used [45–48]. More studies are needed to clarify the sex differences in the eCB system in humans and their interaction with the differentiated acute and chronic effects of cannabinoids in men and women.
This study has some limitations that need to be considered in interpretation of the data. We did not measure sex hormones and did not control for the phase of menstrual periods in female participants. As mentioned above, sex hormones affect the eCB system and cannabinoid effects. More studies with a larger sample size are needed to investigate the magnitude of sex differences in each phase of menstrual period and various levels of sex hormones. We also did not measure THC levels and THC metabolites. Though our method of IV administration of THC would minimize the pharmacokinetic differences, we still may have some residual effects of different metabolism pathways in men and women, which need further evaluations.
Conclusion:
Female participants reported greater subjective effects of feeling “High” at lower dose of THC, compared to male participants. However, there were no other significant sex differences in the subjective, psychotomimetic, physiological, or cognitive effects of THC. More studies are needed to assess the potential underlying mechanism and clinical significance.
Supplementary Material
Funding Sources:
Anahita Bassir Nia was supported by the National Institute of Health K12 DA000167 grant.
Footnotes
Conflict of interests: Anahita Bassir Nia is a member of Scientific Advisory Committee of Synendos Therapeutics AG, Switzerland. Authors have no other conflict of interest to disclose.
Bibliography
- 1.Quality C.f.B.H.S.a., National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration, 2020. [Google Scholar]
- 2.Chapman C, et al. , Evidence for Sex Convergence in Prevalence of Cannabis Use: A Systematic Review and Meta-Regression. J Stud Alcohol Drugs, 2017. 78(3): p. 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson RM, et al. , Past 15-year trends in adolescent marijuana use: Differences by race/ethnicity and sex. Drug Alcohol Depend, 2015. 155: p. 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan SS, et al. , Gender differences in cannabis use disorders: results from the National Epidemiologic Survey of Alcohol and Related Conditions. Drug Alcohol Depend, 2013. 130(1–3): p. 101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernandez-Avila CA, Rounsaville BJ, and Kranzler HR, Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend, 2004. 74(3): p. 265–72. [DOI] [PubMed] [Google Scholar]
- 6.Copersino ML, et al. , Sociodemographic characteristics of cannabis smokers and the experience of cannabis withdrawal. Am J Drug Alcohol Abuse, 2010. 36(6): p. 311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lev-Ran S, et al. , Gender differences in health-related quality of life among cannabis users: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend, 2012. 123(1–3): p. 190–200. [DOI] [PubMed] [Google Scholar]
- 8.Cooper ZD and Craft RM, Sex-Dependent Effects of Cannabis and Cannabinoids: A Translational Perspective. Neuropsychopharmacology, 2018. 43(1): p. 34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fattore L, et al. , Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol, 2007. 152(5): p. 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fattore L, et al. , Drug- and cue-induced reinstatement of cannabinoid-seeking behaviour in male and female rats: influence of ovarian hormones. Br J Pharmacol, 2010. 160(3): p. 724–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cocchetto DM, et al. , Relationship between plasma delta-9-tetrahydrocannabinol concentration and pharmacologic effects in man. Psychopharmacology, 1981. 75(2): p. 158–64. [DOI] [PubMed] [Google Scholar]
- 12.Haney M, Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 2007. 32(6): p. 1391–403. [DOI] [PubMed] [Google Scholar]
- 13.Penetar DM, et al. , Transdermal nicotine alters some of marihuana’s effects in male and female volunteers. Drug Alcohol Depend, 2005. 79(2): p. 211–23. [DOI] [PubMed] [Google Scholar]
- 14.Makela P, et al. , Low Doses of Delta-9 Tetrahydrocannabinol (THC) Have Divergent Effects on Short-Term Spatial Memory in Young, Healthy Adults. Neuropsychopharmacology, 2006. 31(2): p. 462–70. [DOI] [PubMed] [Google Scholar]
- 15.Fogel JS, et al. , Sex differences in the subjective effects of oral Delta(9)-THC in cannabis users. Pharmacol Biochem Behav, 2017. 152: p. 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper ZD and Haney M, Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug Alcohol Depend, 2009. 103(3): p. 107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper ZD and Haney M, Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug and Alcohol Dependence, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathew RJ, Wilson WH, and Davis R, Postural syncope after marijuana: a transcranial Doppler study of the hemodynamics. Pharmacol Biochem Behav, 2003. 75(2): p. 309–18. [DOI] [PubMed] [Google Scholar]
- 19.Matheson J, et al. , Sex differences in the acute effects of smoked cannabis: evidence from a human laboratory study of young adults. Psychopharmacology (Berl), 2020. 237(2): p. 305–316. [DOI] [PubMed] [Google Scholar]
- 20.D’Souza DC, et al. , Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Δ-9-tetrahydrocannabinol in humans. Psychopharmacology, 2008. 198(4): p. 587–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Souza DC, et al. , The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology, 2004. 29(8): p. 1558–72. [DOI] [PubMed] [Google Scholar]
- 22.Cortes-Briones J, et al. , Δ9-THC Disrupts Gamma (γ)-Band Neural Oscillations in Humans. Neuropsychopharmacology, 2015. 40(9): p. 2124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boggs DL, et al. , The dose-dependent psychomotor effects of intravenous delta-9-tetrahydrocannabinol (Δ(9)-THC) in humans. J Psychopharmacol, 2018. 32(12): p. 1308–1318. [DOI] [PubMed] [Google Scholar]
- 24.Ranganathan M, et al. , Tetrahydrocannabinol (THC) impairs encoding but not retrieval of verbal information. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 2017. 79: p. 176–183. [DOI] [PubMed] [Google Scholar]
- 25.Parks C, et al. , Sex and Strain Variation in Initial Sensitivity and Rapid Tolerance to Δ9-Tetrahydrocannabinol. Cannabis Cannabinoid Res, 2020. 5(3): p. 231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lunn S, et al. , Human Pharmacokinetic Parameters of Orally Administered Delta(9)-Tetrahydrocannabinol Capsules Are Altered by Fed Versus Fasted Conditions and Sex Differences. Cannabis Cannabinoid Res, 2019. 4(4): p. 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naef M, et al. , Development and pharmacokinetic characterization of pulmonal and intravenous delta-9-tetrahydrocannabinol (THC) in humans. J Pharm Sci, 2004. 93(5): p. 1176–84. [DOI] [PubMed] [Google Scholar]
- 28.Wall ME, et al. , Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin Pharmacol Ther, 1983. 34(3): p. 352–63. [DOI] [PubMed] [Google Scholar]
- 29.Carbuto M, et al. , The safety of studies with intravenous Delta(9)-tetrahydrocannabinol in humans, with case histories. Psychopharmacology, 2012. 219(3): p. 885–96. [DOI] [PubMed] [Google Scholar]
- 30.D’Souza DC, et al. , Dose-related modulation of event-related potentials to novel and target stimuli by intravenous Delta(9)-THC in humans. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 2012. 37(7): p. 1632–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nia AB, et al. , Cannabis Use: Neurobiological, Behavioral, and Sex/Gender Considerations. Curr Behav Neurosci Rep, 2018. 5(4): p. 271–280. [PMC free article] [PubMed] [Google Scholar]
- 32.Weed PF, et al. , Chronic Δ9-Tetrahydrocannabinol during Adolescence Differentially Modulates Striatal CB1 Receptor Expression and the Acute and Chronic Effects on Learning in Adult Rats. J Pharmacol Exp Ther, 2016. 356(1): p. 20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craft RM, Kandasamy R, and Davis SM, Sex differences in anti-allodynic, anti-hyperalgesic and anti-edema effects of Δ(9)-tetrahydrocannabinol in the rat. Pain, 2013. 154(9): p. 1709–1717. [DOI] [PubMed] [Google Scholar]
- 34.Wakley AA, Wiley JL, and Craft RM, Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat. Drug Alcohol Depend, 2014. 143: p. 22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiley JL, Sex-dependent effects of delta 9-tetrahydrocannabinol on locomotor activity in mice. Neurosci Lett, 2003. 352(2): p. 77–80. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen JD, et al. , Lasting effects of repeated Δ(9) -tetrahydrocannabinol vapour inhalation during adolescence in male and female rats. Br J Pharmacol, 2020. 177(1): p. 188–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farquhar CE, et al. , Sex, THC, and hormones: Effects on density and sensitivity of CB1 cannabinoid receptors in rats. Drug Alcohol Depend, 2019. 194: p. 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burston JJ, et al. , Regional enhancement of cannabinoid CB₁ receptor desensitization in female adolescent rats following repeated Delta-tetrahydrocannabinol exposure. Br J Pharmacol, 2010. 161(1): p. 103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marusich JA, et al. , The impact of gonadal hormones on cannabinoid dependence. Exp Clin Psychopharmacol, 2015. 23(4): p. 206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, et al. , Sexual dimorphic distribution of cannabinoid 1 receptor mRNA in adult C57BL/6J mice. J Comp Neurol, 2020. 528(12): p. 1986–1999. [DOI] [PubMed] [Google Scholar]
- 41.Castelli MP, et al. , Male and female rats differ in brain cannabinoid CB1 receptor density and function and in behavioural traits predisposing to drug addiction: effect of ovarian hormones. Curr Pharm Des, 2014. 20(13): p. 2100–13. [DOI] [PubMed] [Google Scholar]
- 42.Llorente-Berzal A, et al. , Sex-dependent changes in brain CB1R expression and functionality and immune CB2R expression as a consequence of maternal deprivation and adolescent cocaine exposure. Pharmacol Res, 2013. 74: p. 23–33. [DOI] [PubMed] [Google Scholar]
- 43.Farquhar CE, et al. , Sex, THC, and hormones: Effects on density and sensitivity of CB(1) cannabinoid receptors in rats. Drug Alcohol Depend, 2019. 194: p. 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodríguez de Fonseca F, et al. , Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci, 1994. 54(3): p. 159–70. [DOI] [PubMed] [Google Scholar]
- 45.Laurikainen H, et al. , Sex difference in brain CB1 receptor availability in man. Neuroimage, 2019. 184: p. 834–842. [DOI] [PubMed] [Google Scholar]
- 46.Van Laere K, et al. , Gender-dependent increases with healthy aging of the human cerebral cannabinoid-type 1 receptor binding using [(18)F]MK-9470 PET. Neuroimage, 2008. 39(4): p. 1533–41. [DOI] [PubMed] [Google Scholar]
- 47.Neumeister A, et al. , Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol Psychiatry, 2013. 18(9): p. 1034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Normandin MD, et al. , Imaging the cannabinoid CB1 receptor in humans with [11C]OMAR: assessment of kinetic analysis methods, test-retest reproducibility, and gender differences. J Cereb Blood Flow Metab, 2015. 35(8): p. 1313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


