Abstract
Testosterone levels sharply rise during the transition from childhood to adolescence and these changes are known to be associated with changes in human brain structure. During this same developmental window, there are also robust changes in the neural oscillatory dynamics serving verbal working memory processing. Surprisingly, whereas many studies have investigated the effects of chronological age on the neural oscillations supporting verbal working memory, none have probed the impact of endogenous testosterone levels during this developmental period. Using a sample of 89 youth aged 6–14 years‐old, we collected salivary testosterone samples and recorded magnetoencephalography during a modified Sternberg verbal working memory task. Significant oscillatory responses were identified and imaged using a beamforming approach and the resulting maps were subjected to whole‐brain ANCOVAs examining the effects of testosterone and sex, controlling for age, during verbal working memory encoding and maintenance. Our primary results indicated robust testosterone‐related effects in theta (4–7 Hz) and alpha (8–14 Hz) oscillatory activity, controlling for age. During encoding, females exhibited weaker theta oscillations than males in right cerebellar cortices and stronger alpha oscillations in left temporal cortices. During maintenance, youth with greater testosterone exhibited weaker alpha oscillations in right parahippocampal and cerebellar cortices, as well as regions across the left‐lateralized language network. These results extend the existing literature on the development of verbal working memory processing by showing region and sex‐specific effects of testosterone, and are the first results to link endogenous testosterone levels to the neural oscillatory activity serving verbal working memory, above and beyond the effects of chronological age.
Keywords: alpha, development, magnetoencephalography, MEG, neural oscillations, pubertal hormones, puberty, sex differences, theta
During puberty, endogenous testosterone levels rise. We examined whether these increases in testosterone are associated with developmental changes, controlling for age, in the neural oscillatory dynamics serving verbal working memory. Results showed that alpha and theta oscillatory responses were modulated by testosterone levels across multiple regions critical for task performance.
1. INTRODUCTION
Working memory (WM) is the cognitive ability to transiently maintain information for later utilization and increases in capacity from infancy to young adulthood (Fitch et al., 2016; Gathercole, Pickering, & Ambridge, 2004; Gathercole, Pickering, Knight, & Stegmann, 2004; Isbell et al., 2015). The cortical regions supporting WM, such as the parietal and prefrontal cortices (Andre et al., 2016; Emch & von Bastian, 2019; Finn et al., 2010; Montez & Calabro, 2019), undergo immense functional and structural change during childhood and adolescence. Numerous investigations have probed the developmental effects of age on neural activity contributing to WM processing and have reported findings across a distributed cortical network (Brahmbhatt et al., 2008; Embury et al., 2019; Finn et al., 2010; Heinrichs‐Graham et al., 2022; Inguscio et al., 2021; Isbell et al., 2015; Killanin et al., 2022; Kwon & Reiss, 2002; Luciana et al., 2005; Montez & Calabro, 2019; Nagel et al., 2013; Scherf & Sweeney, 2006; Schweinsburg & Nagel, 2005; Thomason et al., 2009; Vogan et al., 2016).
In general, neuroimaging studies have shown that certain WM network hubs are more strongly recruited as a function of development, while other regions appear to become less involved in such processes. Specifically, studies of neural oscillatory activity in adults have shown stronger alpha‐beta (9–16 Hz) oscillations during encoding in left superior temporal, parietal, and prefrontal cortices (Heinrichs‐Graham, 2015), whereas theta (4–7 Hz) activity in prefrontal cortices has been shown to increase with memory load (Jensen, 2002; Proskovec et al., 2019; Proskovec & Heinrichs‐Graham, 2019). In typically‐developing youth, studies suggest that the theta and alpha oscillatory activity exhibited by adults changes as a function of chronological age, with unique differences between males and females (Embury et al., 2019; Heinrichs‐Graham et al., 2021; Heinrichs‐Graham et al., 2022). Still, the timing and other specifics of such development remain incompletely understood. One critical barrier to progress in this area has been the high degree of individual variability, as chronological age and developmental stage are more tightly coupled in some children relative to others, especially during the critical pubertal transition window. Thus, we propose that using pubertal hormones, such as testosterone, as a marker of development may offer unique insight over chronological age alone.
Much of our knowledge regarding the mechanism of testosterone's effects comes from animal models. Studies in rats have shown that neuron exposure to increased levels of testosterone is associated with increased neuronal spine density in the amygdala and hippocampal pyramidal neurons (Cunningham et al., 2007), with mice studies showing that gonadectomy leads to morphological changes in dendritic spines of hippocampal pyramidal neurons (Li et al., 2012). Overall, these studies and others point to testosterone's key role in neuronal plasticity (Chen et al., 2022; Fester, 2021; Muthu et al., 2022). Human work has been largely consistent with this view, although there is some evidence that the effects of pubertal hormones on neural structure and function might be more nuanced with region‐ and/or circuit‐specificity, interactions with sex, and both increases and decreases in plasticity (Dai & Scherf, 2019; Laube et al., 2020). For example, Bramen et al. (2012) reported significant effects of testosterone on cortical thickness in regions known to be involved in WM processing, with higher testosterone being associated with greater cortical thickness in the cuneate, lingual, and frontal cortices of males, while the opposite pattern was observed in these regions in females. In contrast, cortical thickness and testosterone increased together in males and females in the posterior cingulate (Bramen et al., 2012). A later investigation showed smaller anterior cingulate gray matter volumes among males with higher testosterone but no significant relationships in females (Koolschijn & Peper, 2014). Notably, a study investigating the effects of testosterone on the relationship between cortico‐hippocampal structural covariance and executive function found no significant associations between performance on neuropsychological tests of verbal memory and testosterone‐related structural covariance (Nguyen, 2018).
Regarding functional studies, one study used functional magnetic resonance imaging (fMRI) to probe testosterone's mediating role between sex and neural activity during an n‐back task (Alarcón et al., 2014), but found no significant effects of testosterone. However, studies using magnetoencephalography (MEG) have built upon structural studies and shown that testosterone's effects on neural oscillatory activity differ from the effects of chronological age both spatially and spectrally. For example, a study examining gamma activity during visuospatial processing (Fung et al., 2020) found that females with higher testosterone exhibited stronger gamma activity in the right temporal–parietal junction (TPJ) while males with higher testosterone exhibited stronger gamma activity in the right insula. These findings may indicate differential maturational patterns in stimulus detection networks, as the TPJ and insula are both critical for facilitating bottom‐up processing. When age effects were considered, significant relationships were found between chronological age and alpha activity in the right medial prefrontal, precentral, and cingulate cortices. Thus, effects of testosterone and age not only differed in spatial‐specificity but also spectral‐specificity (Fung et al., 2020). Other studies have found testosterone to mediate the relationship between age and the beta oscillations serving motor control (Killanin et al., 2023) and sensorimotor processing more broadly (Fung et al., 2022) in the primary motor cortices. Furthermore, in developing youth, testosterone's relationship with spontaneous cortical activity has been found to differ by sex, with the strongest differences in frontal cortices (Picci, Ott, et al., 2023).
Thus, substantial data indicates that testosterone significantly affects cortical structure and neural oscillatory activity during development, but to date no work has probed the direct effects of testosterone on the neural dynamics serving WM processing, which is surprising given the broad importance of WM to overall cognitive function. As noted above, several previous studies have reported robust chronological age‐related changes in the neural oscillations serving WM (Embury et al., 2019; Heinrichs‐Graham et al., 2022; Killanin et al., 2022), which, to at least some degree, could be related to concomitant puberty‐related alterations in endogenous testosterone. In the current study, we aim to delineate the effects of testosterone on the neural oscillations supporting WM processing in a cross‐sectional sample of developing youth. To this end, we used a verbal WM task during MEG and imaged the resultant neural responses using a time‐frequency resolved beamforming approach. To assess the main effects of testosterone and possible interactive effects with sex, controlling for the effects of age, we used a whole‐brain voxel‐wise ANCOVA approach. Based on the literature, we hypothesized that we would see significant effects of testosterone on alpha and theta oscillatory activity, in left temporal, parietal, and prefrontal cortices supporting WM, with some of these effects differing by sex.
2. MATERIALS AND METHODS
2.1. Participants
Eighty‐nine participants (41 female, 79 right‐handed) ages 6–14 years old completed a modified Sternberg verbal WM task and provided saliva samples for hormone analysis as part of the NIH‐supported Developmental Multimodal Imaging of Neurocognitive Dynamics (Dev‐MIND) study (R01‐MH121101). This project employed an accelerated longitudinal design to investigate brain and cognitive development, and the current investigation utilizes cross‐sectional data collected exclusively during year 1 (of note, because of the accelerated longitudinal design of the study, 10‐year‐olds were not enrolled). Participants were recruited from the community and were all typically‐developing primary English speakers without diagnosed psychiatric or neurological conditions, previous head trauma, learning disabilities, or nonremovable ferromagnetic material (e.g., orthodonture). After a complete description of the study, written informed assent and consent were obtained from the child and the child's parent or legal guardian, respectively. All procedures were completed at the University of Nebraska Medical Center (UNMC) and approved by the UNMC Institutional Review Board (IRB).
2.2. Salivary hormone collection and analysis
Participants were instructed to refrain from chewing gum and consuming any foods or liquids for at least 1 h before providing saliva samples. All saliva samples were collected on average in the afternoon (M = 15:31 h, SD = 2:48 h), and participants were instructed to passively drool into an Oragene DISCOVER (OGR‐500; www.dnagenotek.com) collection tube until liquid saliva exceeded the fill line indicated on the tube, which was 2.0 mL. Before the release of the protease inhibitors for long‐term storage, a single‐channel pipette was used to extract 0.5 mL from the collection tube and placed in a − 20°C freezer for storage. All samples were assayed with duplicate testing using a commercially available assay kit for salivary testosterone (Salimetrics EIA High Sensitivity Salivary Testosterone Kit; www.salimetrics.com). The assay kit had a sensitivity of 0.67 pg/mL, with a range of 6.1–600.0 pg/mL. The intra and inter‐assay coefficients of variation were 5.04% and 3.31%, respectively. The arithmetic means of the duplicate tests exhibited a nonnormal distribution (skewness = 1.89, kurtosis = 4.5), thus values were natural log‐transformed (skewness = −.75, kurtosis = 1.45).
2.3. MEG experimental paradigm
During MEG recording, participants completed a modified Sternberg verbal WM task similar to those used in several previous studies of adults (Embury et al., 2018; Heinrichs‐Graham, 2015; Proskovec et al., 2018; Proskovec et al., 2019; Wilson et al., 2017) and youths (Embury et al., 2019; Killanin et al., 2022). Participants rested their right hand on a custom five‐finger button response pad directly connected to the MEG system so that each button press sent a TTL pulse to the acquisition computer in real time, enabling behavioral responses to be temporally synced with the MEG data. Accuracy and reaction time were calculated offline. Participants were shown a centrally presented fixation cross‐embedded in a 5 × 5 grid for 1000 ms (i.e., baseline period). Four consonants then appeared at fixed locations within the grid for 2000 ms (i.e., encoding period), with the letters then disappearing from the array leaving an empty grid for 2000 ms (i.e., maintenance period). The participants had to maintain the encoded letters when viewing the empty grid and then respond when a probe letter appeared in the grid for 1500 ms (i.e., retrieval period). Participants were instructed to respond with their right index if the probe letter was found in the previous array of letters (i.e., in‐set trials) or with their middle finger if it was not (i.e., out‐of‐set trials). Thus, importantly, the trials were identical up to the retrieval phase and consequently we focus our analyses on the encoding and maintenance phases. We do not examine the retrieval phase because we would have needed to double the number of trials to ensure adequate signal‐to‐noise (i.e., comparable to encoding and maintenance) for each condition (i.e., in‐set and out‐of‐set) during the retrieval phase. Participants completed 128 trials, split equally and pseudorandomized between in‐ and out‐of‐set trials, for a total run time of about 15 min (Figure 1). Visual stimuli were presented using the E‐Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA), and back‐projected onto a semi‐translucent nonmagnetic screen at an approximate distance of 1.07 m, using a Panasonic PT‐D7700U‐K model DLP projector with a refresh rate of 60 Hz and a contrast ratio of 4000:1.
FIGURE 1.
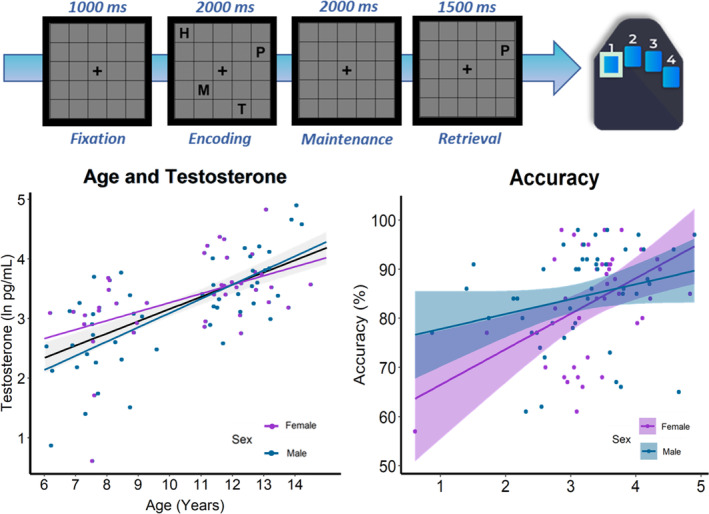
Modified Sternberg verbal working memory task and behavior. (Top) Example trial of the modified Sternberg verbal working memory task and the corresponding correct response on the button response pad. (Bottom left) Testosterone levels were significantly correlated with chronological age. (Bottom right) Participants with higher levels of testosterone were significantly more accurate on the task, with this relationship being stronger among females than males.
2.4. MEG data acquisition
Participants sat in a nonmagnetic chair within a one‐layer magnetically shielded room with active shielding engaged during MEG recording. Neuromagnetic responses were sampled continuously at 1 kHz with an acquisition bandwidth of 0.1–330 Hz using an MEG system with 306 magnetic sensors (MEGIN, Helsinki, Finland). MEG data from each subject were individually corrected for head motion (MaxFilter v2.2; MEGIN) and subjected to noise reduction using the signal‐space separation method with a temporal extension (Taulu, 2006; Taulu & Simola, 2005).
2.5. Structural MRI data acquisition and MEG co‐registration
A 3 T Siemens Prisma scanner equipped with a 32‐channel head coil was used to collect high‐resolution structural T1‐weighted MRI data from each participant (TR: 24.0 ms; TE: 1.96 ms: field of view: 256 mm; slice thickness: 1 mm with no gap; in‐plane resolution: 1.0 × 1.0 mm). Structural volumes were aligned parallel to the anterior and posterior commissures and transformed into standardized space. Each participant's MEG data were co‐registered with their MRI data using BESA MRI (Version 2.0). After source imaging (beamformer), each subject's functional images were also transformed into standardized space using the transform previously applied to the structural MRI volume, and spatially resampled.
2.6. MEG time‐frequency decomposition and statistical analysis
For a more detailed description of our methodological approach, see Wiesman and Wilson (2020). Cardiac and ocular artifacts were visually inspected and removed from the data using signal‐space projection, which was accounted for during source reconstruction (Uusitalo, 1997). The continuous magnetic time series was divided into 5900 ms epochs, with stimulus presentation (i.e., 5 × 5 grid containing four consonants) defined as 0 ms and the baseline defined as the −400 to 0 ms time window (i.e., fixation period; Figure 1). Only correct trials were used for analysis. Epochs containing artifacts were rejected based on a fixed threshold method, supplemented with visual inspection. Briefly, the distributions of amplitude and gradient values per participant were computed using all correct trials, and the highest amplitude/gradient trials relative to the total distribution were excluded. Notably, individual thresholds were set for each participant for both amplitude (M = 2228.93 fT/cm, SD = 983.1) and gradient (M = 553.43 fT/(cm×ms), SD = 251.9) because of differences in head size and sensor proximity among individuals, which strongly affect MEG signal amplitude. An average of 87.06 (SD: 12.2) trials per participant remained for further analysis.
Artifact‐free epochs were then transformed into the time‐frequency domain using complex demodulation with a resolution of 1.0 Hz afree epochs were then nd 50 ms (Kovach, 2016; Papp & Ktonas, 1977). The resulting spectral power estimations per sensor were averaged over trials to generate time‐frequency plots of mean spectral density. These sensor‐level data were then normalized per frequency bin using the mean power during the −400 to 0 ms baseline period. The specific time‐frequency windows used for imaging were determined by statistical analysis of the sensor‐level spectrograms across the entire array of gradiometers. Each data point in the spectrogram was initially evaluated using a mass univariate approach based on the general linear model. To reduce the risk of false‐positive results while maintaining reasonable sensitivity, a two‐stage procedure was followed to control for type 1 error. In the first stage, paired‐sample t‐tests against baseline were conducted on each data point and the output spectrogram of t‐values was thresholded at p < .05 to define time‐frequency bins containing potentially significant oscillatory deviations across all participants. In stage two, time‐frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also below the (p < .05) threshold, and a cluster value was derived by summing all the t‐values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster‐values, and the significance level of the observed clusters (from stage one) was tested directly using this distribution (Ernst, 2004; Maris, 2007). For each comparison, 10,000 permutations were computed to build a distribution of cluster values. Based on these analyses, time‐frequency windows that contained a significant oscillatory event relative to the permutation testing null were subjected to the beamforming analysis.
2.7. MEG source imaging and statistics
Cortical oscillatory activity was imaged through an extension of the linearly constrained minimum variance vector beamformer (Gross et al., 2001; Hillebrand et al., 2005) using the Brain Electrical Source Analysis (BESA 7.1) software. This approach, commonly referred to as dynamic imaging of coherent sources (DICS), applies spatial filters to time‐frequency sensor data to calculate voxel‐wise source power for the entire brain volume. The single images are derived from the cross‐spectral densities of all combinations of MEG gradiometers averaged over the time‐frequency range of interest, and the solution of the forward problem for each location on a 4.0 × 4.0 × 4.0 mm grid specified by input voxel space. Following convention, we computed noise‐normalized, source power per voxel in each participant using active (i.e., task) and passive (i.e., baseline) periods of equal duration and bandwidth. Such images are typically called pseudo‐t maps, with units (pseudo‐t) that reflect noise‐normalized power differences (i.e., active vs. passive) per voxel. This generated participant‐level pseudo‐t maps for each time‐frequency‐specific response identified in the sensor‐level permutation analysis, which were then transformed into standardized space using the transform previously applied to the structural MRI volume and spatially resampled.
Effects of testosterone were analyzed using whole‐brain ANCOVA models in SPM12 (Kiebel & Holmes, 2007). Testosterone (natural log‐transformed), participant sex, and the interactive effect between sex and testosterone were used as variables of interest. Participants' chronological age and time of salivary hormone collection were included in the model as variables of no interest. To account for multiple comparisons, a significance threshold of p < .005 was used in all statistical maps to identify significant clusters, in addition to a cluster threshold of eight contiguous (4 × 4 × 4 mm) voxels (i.e., 512 mm3).
3. RESULTS
3.1. Testosterone and behavioral results
Demographic and behavioral analyses were conducted on the 89 youth who completed the verbal WM task and provided salivary testosterone samples. Average salivary testosterone measured in this sample was 33.09 pg/mL (SD = 24.96). Measures of normality indicated that testosterone values were not normally distributed (skewness = 1.89, kurtosis = 1.50), thus measurements were natural log‐transformed (M = 3.23, SD = 0.79). Testosterone values were significantly correlated with age (r = .64, p < .001; Figure 1), but did not significantly differ between sexes (t(87) = −1.26, p = .21). Average accuracy on the verbal WM task was 83.92% (SD = 10.44), and accuracy was positively correlated with age, such that older participants performed significantly better than younger participants on the task (r = .59, p < .001). An independent samples t‐test showed that males and females did not significantly differ in accuracy (t(87) = 0.29, p = .77), however, linear regression analysis showed that females with higher testosterone had significantly higher accuracy than those with lower testosterone levels, controlling for age (β = 0.99, p = .01; Figure 1). Testosterone levels were not significantly correlated with accuracy among males. Average reaction time on the task was 1209.31 ms (SD = 265.27), and reaction time was negatively correlated with age such that older participants responded significantly faster than younger participants (r = −.65, p < .001). Males and females did not significantly differ in reaction time (t(87) = 1.48, p = .14), and there were no significant effects of testosterone on reaction time, controlling for age (β = 0.11, p = .39).
Of the 89 participants who completed the verbal WM task, we excluded 10 for noisy MEG data. Therefore, the final sample used for neural analyses consisted of 79 participants (M = 10.29 years, SD = 2.50; 36 females; 73 right‐handed), and neither age (t(77) = −.72, p = .48) nor testosterone (t(77) = −1.43, p = .16) significantly differed by sex. To ensure that the MEG data quality of the final sample was not biased by development, we tested for a relationship between age and the number of trials retained for analysis. The number of trials retained was not significantly correlated with age (r = .15, p = .17) or testosterone (r = −.01, p = .96), and did not significantly differ by sex (t(77) = .98, p = .33).
3.2. MEG sensor‐level and source‐level results
Statistical examination of the sensor‐level time‐frequency spectrograms showed a significant increase in theta (4–7 Hz) power relative to baseline from 100 to 500 ms after onset of the encoding grid (p < .0001, permutation corrected), which gradually dissipated over the next 1500 ms. From 400 to 2000 ms, there was a significant decrease in alpha (8–12 Hz) power relative to baseline (p < .0001, permutation corrected). During the maintenance period, there was a strong increase in alpha (9–14 Hz) relative to baseline from 2550 to 2900 ms, which narrowed in bandwidth (8–12 Hz) from 3100 ms to the end of the maintenance period (Figure 2; p < .0001, permutation corrected). For the longer time periods, these significant alpha and theta responses were divided into 400 ms nonoverlapping time bins for source reconstruction. Specifically, we applied a beamformer to the following windows: 4–7 Hz from 100 to 500 ms, 8–12 Hz from 400 to 2000 ms, 9–14 Hz from 2550 to 2900 ms, and 8–12 Hz from 3100 to 3900 ms. Finally, as noted in the MEG experimental paradigm Section, we did not examine the oscillatory neural responses observed during the retrieval period since we used an in‐set/out‐of‐set design.
FIGURE 2.
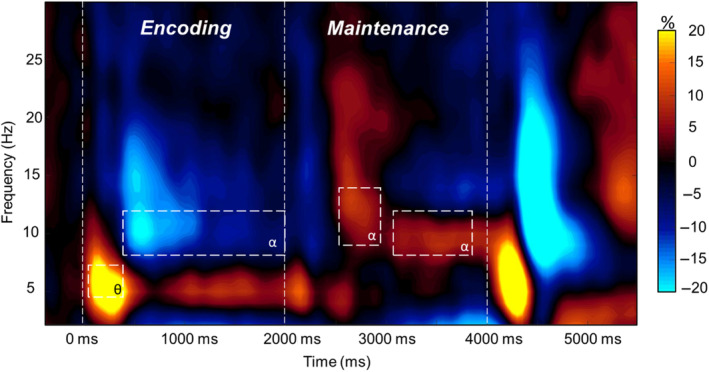
Grand‐Averaged magnetoencephalography (MEG) sensor‐level spectrogram. Grand‐averaged time‐frequency spectrogram taken from a representative sensor (MEG2043). Time is denoted on the x‐axis (0.0 s = encoding onset) and frequency (Hz) is denoted on the y‐axis. Time‐frequency windows that were used for source estimation are denoted by the dashed white boxes. The spectrogram is shown in percent power change from baseline, with the color scale shown to the right of the spectrogram.
Average beamformer images across all participants revealed that the early theta (4–7 Hz) increase in power from baseline was strongest in bilateral occipital cortices, whereas the alpha (8–12 Hz) decrease in power relative to baseline during encoding began in bilateral occipital and cerebellar regions and extended anteriorly to include left temporal regions as encoding progressed. During maintenance, increases in alpha power relative to baseline were detected in right occipitotemporal cortices, whereas decreases in alpha relative to baseline were strongest in the left temporal, left prefrontal, and right cerebellar cortices (see Figure S1).
3.3. Sexually‐divergent effects of testosterone on oscillatory power
Testosterone‐by‐sex interaction effects on theta (4–7 Hz) activity during encoding were found in the right cerebellar cortex (F(1,66) = 11.79, p = .001; Figure 3). Females with greater testosterone exhibited significantly weaker theta increases from baseline, whereas males exhibited the opposite relationship. Interactive effects on alpha (8–12 Hz) activity were found in the left middle temporal gyrus (F(1,73) = 10.58, p = .002; Figure 3). Females with higher testosterone levels showed stronger decreases in alpha power from baseline than those with lower testosterone, with males showing the opposite pattern. There were no significant sex‐by‐testosterone effects found during the maintenance period. All significant sex‐by‐testosterone effects controlled for the main effects of sex, testosterone, age, and time of salivary testosterone collection.
FIGURE 3.
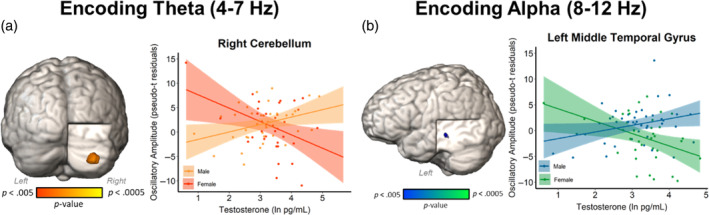
Sexually divergent effects of testosterone on neural oscillations serving working memory encoding. Whole‐brain ANCOVA maps and associated scatterplots showing the interactive effect at the peak voxel, with natural‐log testosterone value (pg/mL) on the x‐axes and residuals of the oscillatory amplitude in pseudo‐t units on the y‐axes, controlling for age and collection time. (a) Females with higher testosterone levels had weaker theta responses than females with lower testosterone levels, with males showing the opposite effect. (b) Females with higher testosterone levels had stronger decreases in alpha power relative to baseline than females with lower testosterone levels. The opposite was true for males.
3.4. Main effects of endogenous testosterone on neural oscillatory responses
Whole‐brain, voxel‐wise ANCOVA results revealed significant main effects of testosterone on early maintenance alpha oscillatory activity (9–14 Hz) in right parahippocampal gyrus (F(1,68) = 14.96, p < .001; Figure 4) and right cerebellum (F(1,68) = 9.05, p = .004; Figure 4), suggesting that participants with greater levels of testosterone had increases in alpha power relative to baseline in these regions. During the latter portion of maintenance, effects of testosterone on alpha oscillatory activity (8–12 Hz) were significant in the left inferior parietal (F(1,61) = 10.31, p = .002; Figure 4) and left occipital cortices (F(1,61) = 11.75, p = .001; Figure 4), indicating that in these two regions participants with higher testosterone levels had greater increases in alpha power relative to baseline. All effects controlled for the effects of chronological age, sex, and time of salivary testosterone collection.
FIGURE 4.
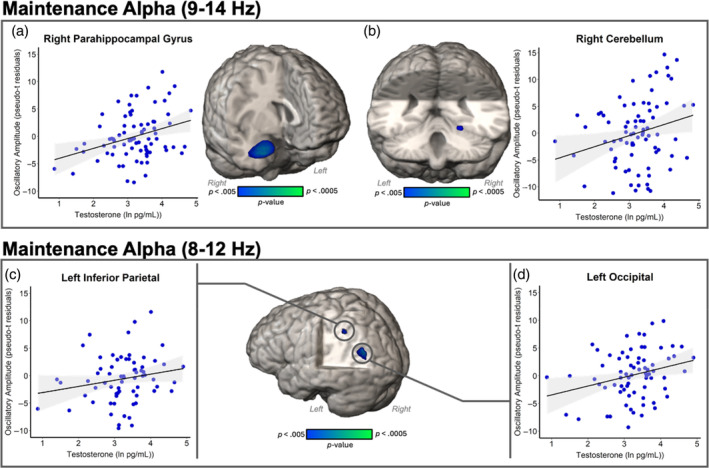
Effects of testosterone on the neural oscillations serving working memory maintenance. Whole‐brain ANCOVA maps and associated scatterplots showing the relationship at the peak voxel, with natural log of testosterone (pg/mL) on the x‐axes and oscillatory amplitude in residuals of pseudo‐t units on the y‐axes. (Top) Alpha power during early maintenance increased relative to baseline with increasing testosterone in the (a) right parahippocampal cortices and (b) right cerebellum. (Bottom) Alpha power during later maintenance became stronger relative to baseline with increasing testosterone in the (c) left inferior parietal cortices and (d) left superior occipital cortices.
4. DISCUSSION
In this cross‐sectional sample of 6–14‐year‐old youth, we examined how endogenous testosterone levels were related to the neural oscillatory underpinnings of verbal WM, above and beyond the effects of chronological age. These results are the first to provide data showing the relationship between endogenous testosterone and neural oscillations serving the encoding and maintenance phases of verbal WM in a sample of typically‐developing youth. As expected, testosterone and chronological age were tightly coupled, such that older youth had higher testosterone levels. Behaviorally, females with higher endogenous testosterone performed better on the task than those with lower testosterone. At the neural level, testosterone's effects on oscillatory activity during encoding significantly differed between males and females. Females with higher testosterone levels exhibited a weaker theta increase from baseline in the right cerebellar cortex during the 100–500 ms period after encoding onset, whereas males exhibited the opposite pattern. Conversely, females with higher testosterone also had significantly stronger alpha oscillations (i.e., decreases from baseline) in the left temporal cortex during the 400–2000 ms encoding period, with males again exhibiting the opposite relationship between testosterone and oscillatory activity. For both of the maintenance responses (early 9–14 Hz and later 8–12 Hz), participants with higher testosterone exhibited increased alpha power relative to baseline in posterior cortices than those with lower testosterone. Our results suggest that testosterone's effects on network maturation are multispectral, involve multiple distinct brain regions, and are distinguishable from those of chronological age.
The adult literature suggests that sex differences in verbal WM performance differ between female or male advantages, depending on the experimental paradigm or neuropsychological test (Reed et al., 2017; Voyer et al., 2021). However developmental sex differences in verbal WM performance have been inconsistent (Andreano, 2009; Barel, 2018; Gathercole, Pickering, & Ambridge, 2004), with no strong agreement on whether sex differences in verbal WM emerge before adolescence, during language acquisition, or during adolescence when cortical networks are being refined (Barel, 2018). Much of this discrepancy is attributable to differences in which neuropsychological test is being used to measure WM (e.g., complex span, California verbal learning test (CVLT), or controlled oral word association test (COWAT)), with slightly different domains of WM being targeted by each measure (i.e., tests of verbal fluency measuring vocabulary and semantic memory and word list tests measuring episodic recall). Our behavioral results suggest that incorporating pubertal hormones such as endogenous testosterone in analyses may be useful for identifying points at which developmental sex differences in WM performance emerge.
Interestingly, during encoding there were only interactive effects of sex and testosterone, with females with higher testosterone having weaker theta increases relative to baseline in the right cerebellar cortices and stronger alpha decreases relative to baseline in the left middle temporal gyrus. Cerebellar oscillatory activity has been implicated in WM (Heinrichs‐Graham, 2015; Proskovec & Heinrichs‐Graham, 2016; Wilson et al., 2017), with theta oscillations thought to reflect cerebellar communication within visuomotor adaptation networks (Tzvi et al., 2022), and serial ordering of stimuli (Tomlinson et al., 2014). On the other hand, regions of the left temporal cortex critically support language processing and sustained alpha decreases in left temporal and inferior frontal cortices have been associated with sub‐vocal rehearsal during encoding and maintenance (Embury et al., 2018; Embury et al., 2019; Emch & von Bastian, 2019; Heinrichs‐Graham, 2015; Proskovec et al., 2019). Sex differences during encoding have been previously reported in youth, with older females exhibiting stronger inferior frontal alpha activity than males (Embury et al., 2019), which potentially reflects differential maturational trends in regions supporting WM in males and females. The regional differences in age‐related and hormone‐related findings could reflect sensitivities in certain networks to the effects of testosterone during adolescence, irrespective of age. Teasing apart these respective effects helps to shed light on different developmental processes that are happening in concert. Such findings likely indicate that maturational processes less related to testosterone are also ongoing and contributing to network refinement, including processes related to DHEA and other hormones (Penhale et al., 2022; Picci, Casagrande, et al., 2023).
Our alpha findings revealed significant effects of testosterone in left‐lateralized language regions only during encoding and late maintenance. In contrast, during early maintenance, we found testosterone alpha effects in the right parahippocampal area and right cerebellar cortices. The parahippocampal gyrus has been shown to facilitate contextual associations for stronger stimulus encoding (Aminoff et al., 2007; Diana, 2017; Li et al., 2016; Luck et al., 2010) and serve as a short‐term memory buffer with increasing memory load (Schon et al., 2016). Sustained alterations in alpha activity within the inferior parietal and occipital cortices, along with portions of the left temporal cortex, have been suggested by previous WM investigations to facilitate active maintenance of verbal stimuli through the phonological loop (Heinrichs‐Graham, 2015; Price, 2012). However, we see that during maintenance, greater testosterone is associated with increases in alpha power relative to baseline in posterior left inferior parietal and occipital cortices, as well as right hippocampal and cerebellar cortices. If testosterone aids in fine‐tuning developmental networks, participants with greater hormones may rely on these specific nodes less than their low testosterone counterparts as a product of increased network specialization. Future research using a longitudinal design should probe associations between testosterone and functional connectivity within verbal WM networks, to better delineate how individual testosterone levels interact with the maturation of these sub‐systems.
Before closing, we must acknowledge the limitations of our investigation and offer suggestions for future work. Primarily, with a cross‐sectional sample it is difficult to predict how testosterone levels will change in each individual over time. Future studies should utilize a longitudinal design, to capture how the timing and tempo of puberty may affect testosterone levels, and in turn, affect the neural oscillatory underpinnings of verbal WM. Salivary hormone collection has proven to be an effective method of analyzing hormonal effects on neural function, but it can be challenging to ensure that collection is done at the same time of day for each participant to account for the cyclical nature of testosterone levels. Although testosterone's diurnal cycle is far less than other hormones, like cortisol, we still used collection time as a covariate of no interest in this study. Nevertheless, future work may benefit from collecting hair samples, to provide an average estimate of hormone levels over a longer period (Short et al., 2016). Lastly, future investigations would benefit from larger sample sizes providing more power to account for variables like race, SES, and body mass index (BMI), which are known to affect hormone levels and puberty onset (Cheng et al., 2021).
The data presented herein was the first to show testosterone's effects, above and beyond the effects of age, on WM encoding and maintenance‐related oscillatory activity in a typically‐developing sample of children and adolescents. There were significant effects of testosterone on task accuracy and alpha and theta activity during encoding that differed by sex, and main effects of testosterone on oscillatory activity in key WM‐related cortices during maintenance. These results shed light on testosterone's region and sex‐specific effects and offer evidence for its role in fine‐tuning developmental processes.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Supporting information
Figure S1. Grand averaged images per time window during encoding (red and blue), early maintenance (green), and late maintenance (orange) show the progression of neural oscillatory responses during task performance. At the beginning of encoding, theta power increased in occipital cortices followed by alpha oscillations (i.e., decreases in power relative to baseline) in temporal, occipital, and cerebellar cortices. Such alpha changes spread to include more anterior cortices during later encoding. Alpha oscillations during maintenance were detected in parietal, temporal, and prefrontal regions. In later maintenance, increases in alpha power relative to baseline were detected in right occipitotemporal cortices, whereas left temporal, left prefrontal, and right cerebellar cortices exhibited decreases in alpha power relative to baseline.
ACKNOWLEDGMENTS
This research was supported by grants R01‐MH121101 (TWW), P20‐GM144641 (TWW), and F30‐MH130150 (ADK) from the National Institutes of Health as well as #2112455 from the National Science Foundation. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. The data presented in this manuscript have not been published or presented elsewhere.
Killanin, A. D. , Ward, T. W. , Embury, C. M. , Calhoun, V. D. , Wang, Y.‐P. , Stephen, J. M. , Picci, G. , Heinrichs‐Graham, E. , & Wilson, T. W. (2024). Effects of endogenous testosterone on oscillatory activity during verbal working memory in youth. Human Brain Mapping, 45(10), e26774. 10.1002/hbm.26774
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alarcón, C. , Cservenka, A. , Fair, D. A. , & Nagel, B. J. (2014). Sex differences in the neural substrates of spatial working memory during adolescence are not mediated by endogenous testosterone. Brain Research, 1593, 40–54. 10.1016/j.brainres.2014.09.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff, G. , Gronau, N. , & Bar, M. (2007). The parahippocampal cortex mediates spatial and nonspatial associations. Cerebral Cortex, 17(7), 1493–1503. 10.1093/cercor/bhl078 [DOI] [PubMed] [Google Scholar]
- Andre, P. , Picchioni, M. , Zhang, R. , & Toulopoulou, T. (2016). Working memory circuit as a function of increasing age in healthy adolescence: A systematic review and meta‐analyses. NeuroImage: Clinical, 12, 940–948. 10.1016/j.nicl.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano, J. M. , & Cahill, L. (2009). Sex influences on the neurobiology of learning and memory. Learning & Memory, 16(4), 248–266. 10.1101/lm.918309 [DOI] [PubMed] [Google Scholar]
- Barel, E. , & Tzischinsky, O. (2018). Age and sex differences in verbal and visuospatial abilities. Advances in Cognitive Psychology, 2(14), 51–61. 10.5709/acp-0238-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmbhatt, M. A. , McAuley, T. , & Barch, D. M. (2008). Functional developmental similarities and differences in the neural correlates of verbal and nonverbal working memory tasks. Neuropsychologia, 46(4), 1020–1031. 10.1016/j.neuropsychologia.2007.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen, H. , Hranilovich, J. A. , Dahl, R. E. , Chen, J. , Rosso, C. , Forbes, E. E. , Dinov, I. D. , Worthman, C. M. , & Sowell, E. R. (2012). Sex matters during adolescence: Testosterone‐related cortical thickness maturation differs between boys and girls. PLoS One, 7(3), e33850. 10.1371/journal.pone.0033850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Q. , Qiao, D. , Si, Y. , He, Z. , Zhang, B. , Wang, C. , Zhang, Y. , Wang, X. , Shi, Y. , Cui, C. , Cui, H. , & Li, S. (2022). Effects of membrane androgen receptor binding on synaptic plasticity in primary hippocampal neurons. Molecular and Cellular Endocrinology, 554, 111711. 10.1016/j.mce.2022.111711 [DOI] [PubMed] [Google Scholar]
- Cheng, M.‐W. , Magis‐Weinberg, L. , Guazzelli Williamson, V. , Ladouceur, C. D. , Whittle, S. L. , Herting, M. M. , Uban, K. A. , Byrne, M. L. , Barendse, M. E. A. , Shirtcliff, E. A. , & Pfeifer, J. H. (2021). A Researcher's guide to the measurement and modeling of puberty in the ABCD Study® at Baseline. Frontiers in Endocrinology, 12, 608575. 10.3389/fendo.2021.608575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, C. , Claiborne, B. J. , & McGinnis, M. Y. (2007). Pubertal exposure to anabolic androgenic steroids increases spine densities on neurons in the limbic system of male rats. Neuroscience, 150(3), 609–615. 10.1016/j.neuroscience.2007.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, J. , & Scherf, K. S. (2019). Puberty and functional brain development in humans: Convergence in findings? Developmental Cognitive Neuroscience, 39, 100690. 10.1016/j.dcn.2019.100690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana, R. A. (2017). Parahippocampal cortex processes the nonspatial context of an event. Cerebral Cortex, 27(3), 1808–1816. 10.1093/cercor/bhw014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embury, C. M. , Wiesman, A. I. , Proskovec, A. L. , Heinrichs‐Graham, E. , McDermott, T. J. , Lord, G. H. , Brau, K. L. , Drincic, A. T. , Desouza, C. V. , & Wilson, T. W. (2018). Altered brain dynamics in patients with type 1 diabetes during working memory processing. Diabetes, 67(6), 1140–1148. 10.2337/db17-1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embury, C. M. , Wiesman, A. I. , Proskovec, A. L. , Mills, M. S. , Heinrichs‐Graham, E. , Wang, Y.‐P. , Calhoun, V. D. , Stephen, J. M. , & Wilson, T. W. (2019). Neural dynamics of verbal working memory processing in children and adolescents. NeuroImage, 185, 191–197. 10.1016/j.neuroimage.2018.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emch, M. , von Bastian, C. C. , & Koch, K. (2019). Neural correlates of verbal working memory: An fMRI meta‐analysis. Frontiers in Human Neuroscience, 13, 180. 10.3389/fnhum.2019.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst, M. D. (2004). Permutation methods: A basis for exact inference. Statistical Science, 19(4), 676–685. 10.1214/088342304000000396 [DOI] [Google Scholar]
- Fester, L. , & Rune, G. M. (2021). Sex neurosteroids: Hormones made by the brain for the brain. Neuroscience Letters, 753, 135849. 10.1016/j.neulet.2021.135849 [DOI] [PubMed] [Google Scholar]
- Finn, A. S. , Sheridan, M. A. , Hudson Kam, C. L. , Hinshaw, S. , & D'Esposito, M. (2010). Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. Journal of Neuroscience, 30(33), 11062–11067. 10.1523/jneurosci.6266-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch, A. , Smith, H. , Guillory, S. B. , & Kaldy, Z. (2016). Off to a good start: The early development of the neural substrates underlying visual working memory. Frontiers in Systems Neuroscience, 10, 68. 10.3389/fnsys.2016.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung, M. H. , Heinrichs‐Graham, E. , Taylor, B. K. , Frenzel, M. R. , Eastman, J. A. , Wang, Y.‐P. , Calhoun, V. D. , Stephen, J. M. , & Wilson, T. W. (2022). The development of sensorimotor cortical oscillations is mediated by pubertal testosterone. NeuroImage, 264, 119745. 10.1016/j.neuroimage.2022.119745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung, M. H. , Taylor, B. K. , Frenzel, M. R. , Eastman, J. A. , Wang, Y.‐P. , Calhoun, V. D. , Stephen, J. M. , & Wilson, T. W. (2020). Pubertal testosterone tracks the developmental trajectory of neural oscillatory activity serving visuospatial processing. Cerebral Cortex, 30(11), 5960–5971. 10.1093/cercor/bhaa169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole, S. E. , Pickering, S. J. , Ambridge, B. , & Wearing, H. (2004). The structure of working memory from 4 to 15 years of age. Developmental Psychology, 40(2), 177–190. 10.1037/0012-1649.40.2.177 [DOI] [PubMed] [Google Scholar]
- Gathercole, S. E. , Pickering, S. J. , Knight, C. , & Stegmann, Z. (2004). Working memory skills and educational attainment: Evidence from national curriculum assessments at 7 and 14 years of age. Applied Cognitive Psychology, 18(1), 1–16. 10.1002/acp.934 [DOI] [Google Scholar]
- Gross, J. , Kujala, J. , Hämäläinen, M. , Timmermann, L. , Schnitzler, A. , & Salmelin, R. (2001). Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proceedings of the National Academy of Sciences, 98(2), 694–699. 10.1073/pnas.98.2.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham, E. , & Wilson, T. W. (2015). Spatiotemporal oscillatory dynamics during the encoding and maintenance phases of a visual working memory task. Cortex, 69, 121–130. 10.1016/j.cortex.2015.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham, E. , Walker, E. A. , Eastman, J. A. , Frenzel, M. R. , & McCreery, R. W. (2022). Amount of hearing aid use impacts neural oscillatory dynamics underlying verbal working memory processing for children with hearing loss. Ear and Hearing, 43(2), 408–419. 10.1097/AUD.0000000000001103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs‐Graham, E. , Walker, E. A. , Eastman, J. A. , Frenzel, M. R. , Joe, T. R. , & McCreery, R. W. (2021). The impact of mild‐to‐severe hearing loss on the neural dynamics serving verbal working memory processing in children. NeuroImage: Clinical, 30, 102647. 10.1016/j.nicl.2021.102647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand, A. , Singh, K. D. , Holliday, I. E. , Furlong, P. L. , & Barnes, G. R. (2005). A new approach to neuroimaging with magnetoencephalography. Human Brain Mapping, 25(2), 199–211. 10.1002/hbm.20102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inguscio, B. M. S. , Cartocci, G. , Sciaraffa, N. , Nasta, C. , Giorgi, A. , Nicastri, M. , Giallini, I. , Greco, A. , Babiloni, F. , & Mancini, P. (2021). Neurophysiological verbal working memory patterns in children: Searching for a benchmark of modality differences in audio/video stimuli processing. Computational Intelligence and Neuroscience, 2021, 1–17. 10.1155/2021/4158580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbell, E. , Fukuda, K. , Neville, H. J. , & Vogel, E. K. (2015). Visual working memory continues to develop through adolescence. Frontiers in Psychology, 6, 696. 10.3389/fpsyg.2015.00696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, O. , & Tesche, C. D. (2002). Frontal theta activity in humans increases with memory load in a working memory task. European Journal of Neuroscience, 15(8), 1395–1399. 10.1046/j.1460-9568.2002.01975.x [DOI] [PubMed] [Google Scholar]
- Kiebel, K. , & Holmes, A. P. (2007). CHAPTER 8–the general linear model. In Friston K., Ashburner J., Kiebel S., Nichols T., & Penny W. (Eds.), Statistical parametric mapping (pp. 101–125). Academic Press. 10.1016/B978-012372560-8/50008-5 [DOI] [Google Scholar]
- Killanin, A. D. , Embury, C. M. , Picci, G. , Heinrichs‐Graham, E. , Wang, Y.‐P. , Calhoun, V. D. , Stephen, J. M. , & Wilson, T. W. (2022). Trauma moderates the development of the oscillatory dynamics serving working memory in a sex‐specific manner. Cerebral Cortex, 32, 5206–5215. 10.1093/cercor/bhac008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killanin, A. D. , Taylor, B. K. , Embury, C. M. , Picci, G. , Wang, Y.‐P. , Calhoun, V. D. , Stephen, J. M. , Heinrichs‐Graham, E. , & Wilson, T. W. (2023). Testosterone levels mediate the dynamics of motor oscillatory coding and behavior in developing youth. Developmental Cognitive Neuroscience, 61, 101257. 10.1016/j.dcn.2023.101257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn, P. C. M. P. , Peper, J. S. , & Crone, E. A. (2014). The influence of sex steroids on structural brain maturation in adolescence. PLoS One, 9(1), e83929. 10.1371/journal.pone.0083929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach, C. K. , & Gander, P. E. (2016). The demodulated band transform. Journal of Neuroscience Methods, 261, 135–154. 10.1016/j.jneumeth.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, H. , Reiss, A. L. , & Menon, V. (2002). Neural basis of protracted developmental changes in visuo‐spatial working memory. Proceedings of the National Academy of Sciences, 99(20), 13336–13341. 10.1073/pnas.162486399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube, C. , van den Bos, W. , & Fandakova, Y. (2020). The relationship between pubertal hormones and brain plasticity: Implications for cognitive training in adolescence. Developmental Cognitive Neuroscience, 42, 100753. 10.1016/j.dcn.2020.100753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. , Masugi‐Tokita, M. , Takanami, K. , Yamada, S. , & Kawata, M. (2012). Testosterone has sublayer‐specific effects on dendritic spine maturation mediated by BDNF and PSD‐95 in pyramidal neurons in the hippocampus CA1 area. Brain Research, 1484, 76–84. 10.1016/j.brainres.2012.09.028 [DOI] [PubMed] [Google Scholar]
- Li, M. , Lu, S. , & Zhong, N. (2016). The parahippocampal cortex mediates contextual associative memory: Evidence from an fMRI study. BioMed Research International, 2016, 1–11. 10.1155/2016/9860604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana, M. , Conklin, H. M. , Hooper, C. J. , & Yarger, R. S. (2005). The development of nonverbal working memory and executive control processes in adolescents. Child Development, 76(3), 697–712. 10.1111/j.1467-8624.2005.00872.x [DOI] [PubMed] [Google Scholar]
- Luck, D. , Danion, J.‐M. , Marrer, C. , Pham, B.‐T. , Gounot, D. , & Foucher, J. (2010). The right parahippocampal gyrus contributes to the formation and maintenance of bound information in working memory. Brain and Cognition, 72(2), 255–263. 10.1016/j.bandc.2009.09.009 [DOI] [PubMed] [Google Scholar]
- Maris, E. , & Oostenveld, R. (2007). Nonparametric statistical testing of EEG‐ and MEG‐data. Journal of Neuroscience Methods, 164(1), 177–190. 10.1016/j.jneumeth.2007.03.024 [DOI] [PubMed] [Google Scholar]
- Montez, D. F. , Calabro, F. J. , & Luna, B. (2019). Working memory improves developmentally as neural processes stabilize. PLoS One, 14(3), e0213010. 10.1371/journal.pone.0213010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthu, S. J. , Lakshmanan, G. , Shimray, K. W. , Kaliyappan, K. , Sathyanathan, S. B. , & Seppan, P. (2022). Testosterone influence on microtubule‐associated proteins and spine density in hippocampus: Implications on learning and memory. Developmental Neuroscience, 44(6), 498–507. 10.1159/000525038 [DOI] [PubMed] [Google Scholar]
- Nagel, B. J. , Herting, M. M. , Maxwell, E. C. , Bruno, R. , & Fair, D. (2013). Hemispheric lateralization of verbal and spatial working memory during adolescence. Brain and Cognition, 82(1), 58–68. 10.1016/j.bandc.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T.‐V. (2018). Developmental effects of androgens in the human brain. Journal of Neuroendocrinology, 30(2), e12486. 10.1111/jne.12486 [DOI] [PubMed] [Google Scholar]
- Papp, N. , & Ktonas, P. (1977). Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomedical Sciences Instrumentation, 13, 135–145. [PubMed] [Google Scholar]
- Penhale, S. H. , Picci, G. , Ott, L. R. , Taylor, B. K. , Frenzel, M. R. , Eastman, J. A. , Wang, Y.‐P. , Calhoun, V. D. , Stephen, J. M. , & Wilson, T. W. (2022). Impacts of adrenarcheal DHEA levels on spontaneous cortical activity during development. Developmental Cognitive Neuroscience, 57, 101153. 10.1016/j.dcn.2022.101153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picci, G. , Casagrande, C. C. , Ott, L. R. , Petro, N. M. , Christopher‐Hayes, N. J. , Johnson, H. J. , Willett, M. P. , Okelberry, H. J. , Wang, Y.‐P. , Stephen, J. M. , Calhoun, V. D. , & Wilson, T. W. (2023). Dehydroepiandrosterone mediates associations between trauma‐related symptoms and anterior pituitary volume in children and adolescents. Human Brain Mapping, 44(18), 6388–6398. 10.1002/hbm.26516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picci, G. , Ott, L. R. , Penhale, S. H. , Taylor, B. K. , Johnson, H. J. , Willett, M. P. , Okelberry, H. J. , Wang, Y.‐P. , Calhoun, V. D. , Stephen, J. M. , & Wilson, T. W. (2023). Developmental changes in endogenous testosterone have sexually‐dimorphic effects on spontaneous cortical dynamics. Human Brain Mapping, 44(17), 6043–6054. 10.1002/hbm.26496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, C. J. (2012). A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage, 62(2), 816–847. 10.1016/j.neuroimage.2012.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskovec, A. L. , Heinrichs‐Graham, E. , & Wilson, T. W. (2016). Aging modulates the oscillatory dynamics underlying successful working memory encoding and maintenance. Human Brain Mapping, 37(6), 2348–2361. 10.1002/hbm.23178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskovec, A. L. , Heinrichs‐Graham, E. , & Wilson, T. W. (2019). Load modulates the alpha and beta oscillatory dynamics serving verbal working memory. NeuroImage, 184, 256–265. 10.1016/j.neuroimage.2018.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskovec, A. L. , Wiesman, A. I. , Heinrichs‐Graham, E. , & Wilson, T. W. (2018). Beta oscillatory dynamics in the prefrontal and superior temporal cortices predict spatial working memory performance. Scientific Reports, 8(1), 8488. 10.1038/s41598-018-26863-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskovec, A. L. , Wiesman, A. I. , Heinrichs‐Graham, E. , & Wilson, T. W. (2019). Load effects on spatial working memory performance are linked to distributed alpha and beta oscillations. Human Brain Mapping, 40(12), 3682–3689. 10.1002/hbm.24625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, J. L. , Gallagher, N. M. , Sullivan, M. , Callicott, J. H. , & Green, A. E. (2017). Sex differences in verbal working memory performance emerge at very high loads of common neuroimaging tasks. Brain and Cognition, 113, 56–64. 10.1016/j.bandc.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Scherf, K. S. , Sweeney, J. A. , & Luna, B. (2006). Brain basis of developmental change in visuospatial working memory. Journal of Cognitive Neuroscience, 18(7), 1045–1058. 10.1162/jocn.2006.18.7.1045 [DOI] [PubMed] [Google Scholar]
- Schon, K. , Newmark, R. E. , Ross, R. S. , & Stern, C. E. (2016). A working memory buffer in Parahippocampal regions: Evidence from a load effect during the delay period. Cerebral Cortex, 26(5), 1965–1974. 10.1093/cercor/bhv013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg, A. D. , Nagel, B. J. , & Tapert, S. F. (2005). fMRI reveals alteration of spatial working memory networks across adolescence. Journal of the International Neuropsychological Society, 11(5), 631–644. 10.1017/s1355617705050757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short, S. J. , Stalder, T. , Marceau, K. , Entringer, S. , Moog, N. K. , Shirtcliff, E. A. , Wadhwa, P. D. , & Buss, C. (2016). Correspondence between hair cortisol concentrations and 30‐day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology, 71, 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu, S. , & Simola, J. (2006). Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Physics in Medicine and Biology, 51(7), 1759–1768. 10.1088/0031-9155/51/7/008 [DOI] [PubMed] [Google Scholar]
- Taulu, S. , Simola, J. , & Kajola, M. (2005). Applications of the signal space separation method. IEEE Transactions on Signal Processing, 53(9), 3359–3372. 10.1109/tsp.2005.853302 [DOI] [Google Scholar]
- Thomason, M. E. , Race, E. , Burrows, B. , Whitfield‐Gabrieli, S. , Glover, G. H. , & Gabrieli, J. D. E. (2009). Development of spatial and verbal working memory capacity in the human brain. Journal of Cognitive Neuroscience, 21(2), 316–332. 10.1162/jocn.2008.21028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson, S. P. , Davis, N. J. , Morgan, H. M. , & Bracewell, R. M. (2014). Cerebellar contributions to spatial memory. Neuroscience Letters, 578, 182–186. 10.1016/j.neulet.2014.06.057 [DOI] [PubMed] [Google Scholar]
- Tzvi, E. , Gajiyeva, L. , Bindel, L. , Hartwigsen, G. , & Classen, J. (2022). Coherent theta oscillations in the cerebellum and supplementary motor area mediate visuomotor adaptation. NeuroImage, 251, 118985. 10.1016/j.neuroimage.2022.118985 [DOI] [PubMed] [Google Scholar]
- Uusitalo, M. A. , & Ilmoniemi, R. J. (1997). Signal‐space projection method for separating MEG or EEG into components. Medical & Biological Engineering & Computing, 35(2), 135–140. 10.1007/bf02534144 [DOI] [PubMed] [Google Scholar]
- Vogan, V. M. , Morgan, B. R. , Powell, T. L. , Smith, M. L. , & Taylor, M. J. (2016). The neurodevelopmental differences of increasing verbal working memory demand in children and adults. Developmental Cognitive Neuroscience, 17, 19–27. 10.1016/j.dcn.2015.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyer, D. , Aubin, J. S. , Altman, K. , & Gallant, G. (2021). Sex differences in verbal working memory: A systematic review and meta‐analysis. Psychological Bulletin, 147(4), 352–398. 10.1037/bul0000320 [DOI] [PubMed] [Google Scholar]
- Wiesman, A. I. , & Wilson, T. W. (2020). Attention modulates the gating of primary somatosensory oscillations. NeuroImage, 211, 116610. 10.1016/j.neuroimage.2020.116610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, T. W. , Proskovec, A. L. , Heinrichs‐Graham, E. , O'Neill, J. , Robertson, K. R. , Fox, H. S. , & Swindells, S. (2017). Aberrant neuronal dynamics during working memory operations in the aging HIV‐infected brain. Scientific Reports, 7, 41568. 10.1038/srep41568 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Grand averaged images per time window during encoding (red and blue), early maintenance (green), and late maintenance (orange) show the progression of neural oscillatory responses during task performance. At the beginning of encoding, theta power increased in occipital cortices followed by alpha oscillations (i.e., decreases in power relative to baseline) in temporal, occipital, and cerebellar cortices. Such alpha changes spread to include more anterior cortices during later encoding. Alpha oscillations during maintenance were detected in parietal, temporal, and prefrontal regions. In later maintenance, increases in alpha power relative to baseline were detected in right occipitotemporal cortices, whereas left temporal, left prefrontal, and right cerebellar cortices exhibited decreases in alpha power relative to baseline.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


