Abstract
Objectives
To quantify associations of serum alarmins with risk of rheumatoid arthritis-associated interstitial lung disease (RA-ILD).
Methods
Using serum collected at enrolment, three alarmins (IL-33, thymic stromal lymphopoietin [TSLP] and IL-25) were measured in a multicentre prospective RA cohort. ILD was classified using systematic medical record review. Cross-sectional associations of log-transformed (IL-33, TSLP) or quartile (IL-25) values with RA-ILD at enrolment (prevalent RA-ILD) were examined using logistic regression, while associations with incident RA-ILD developing after enrolment were examined using Cox proportional hazards. Covariates in multivariate models included age, sex, race, smoking status, RA disease activity score and anti-cyclic citrullinated antibody positivity.
Results
Of 2835 study participants, 115 participants (4.1%) had prevalent RA-ILD at baseline and an additional 146 (5.1%) developed incident ILD. There were no associations between serum alarmin concentrations and prevalent ILD in unadjusted or adjusted logistic regression models. In contrast, there was a significant inverse association between IL-33 concentration and the risk of developing incident RA-ILD in unadjusted (hazard ratio [HR] 0.73 per log-fold increase; 95% CI: 0.57, 0.95; P = 0.018) and adjusted (HR 0.77; 95% CI: 0.59, 1.00; P = 0.047) models. No significant associations of TSLP or IL-25 with incident ILD were observed.
Conclusion
In this study, we observed a significant inverse association between serum IL-33 concentration and the risk of developing incident RA-ILD, but no associations with prevalent ILD. Additional investigation is required to better understand the mechanisms driving this relationship and how serum alarmin IL-33 assessment might contribute to clinical risk stratification in patients with RA.
Keywords: rheumatoid arthritis, interstitial lung disease, alarmin, interleukin-33, thymic stromal lymphopoietin, interleukin-25, fibrosis
Rheumatology key messages.
Alarmins are danger signals released with cell/tissue damage, implicated in tissue fibrosis and RA.
Prior studies have not examined associations of circulating alarmins with incident RA-ILD risk.
Serum IL-33 levels are inversely associated with the risk of incident ILD in patients with RA.
Introduction
Clinically relevant interstitial lung disease (ILD) affects up to 10% of individuals with rheumatoid arthritis (RA) [1], a complication that is associated with substantial morbidity, reduced quality of life and higher mortality. In contrast to improvements in all-cause and cardiovascular mortality observed in patients with RA over the last 20 years, similar gains in respiratory-related deaths have not been made [2]. ILD is among the most over-represented causes of death in RA and is associated with a median life expectancy of just 3–7 years [2–4]. Given these prognostic implications and recent advances in management, the early identification of patients at risk for ILD is increasingly paramount. Currently, the identification of RA-related ILD (RA-ILD) relies primarily on advanced imaging and pulmonary function testing in symptomatic patients. It is known, however, from systematic screening studies that up to 20–30% of patients have evidence of subclinical ILD on advanced imaging [5, 6], suggesting that many with RA are at risk of developing progressive lung disease even in the absence of pulmonary symptoms. Numerous peripheral blood biomarkers, including autoantibodies, genetic polymorphisms, lung epithelial-related proteins, and cytokines, among others, have been proposed as screening tools to facilitate earlier identification of patients with RA-ILD [7].
IL-33, a member of the IL-1 cytokine family, is one such biomarker with data suggesting its involvement in the pathogenesis of RA and pulmonary fibrosis [8, 9]. Recognized as an alarmin, IL-33 is constitutively expressed in the nuclei of epithelial and endothelial cells and is released during cellular damage, binding the ST2 receptor (ST2R) to activate pro-inflammatory pathways. However, it is also increasingly recognized that the IL-33/ST2 signalling pathway has a dual role, exerting potential lung protective effects through promotion of type 2 immunity and anti-inflammatory/pro-resolving mediators to regulate tissue homeostasis and repair [10, 11]. IL-25 and thymic stromal lymphopoietin (TSLP) are additional alarmins released by activated epithelial cells that can also drive type 2 immunity as well as promote lung fibrogenesis and inflammation with potential roles in idiopathic pulmonary fibrosis [12]. The role of IL-25 and TSLP in RA-ILD has not been well-described.
Whether circulating IL-33 might serve as a biomarker of RA-ILD has been the subject of limited investigation. There have been no studies examining whether circulating IL-33 concentrations predict the future risk of ILD development in patients with RA. Thus, we sought to examine the association of serum alarmins IL-33, IL-25 and TSLP with RA-ILD. Using a well characterized cohort of patients with RA, we tested the hypothesis that higher alarmin concentrations would be positively associated with ILD risk in patients with RA.
Methods
Study design and patient population
We performed both a cross-sectional study examining associations of biomarkers with prevalent ILD and a cohort study examining associations of alarmin concentrations with incident ILD among participants from 16 enrolment sites in the Veterans Affairs Rheumatoid Arthritis Registry (VARA) [13]. VARA is an ongoing multicentre cohort study with sites across the USA that includes patients >18 years satisfying the 1987 American College of Rheumatology classification criteria for RA [14]. Patients are followed longitudinally during the process of routine rheumatology care. Each site had approval from a site-specific Institutional Review Board and all patients provided written informed consent. This study was approved by the VARA Scientific Ethics and Advisory Committee.
ILD classification
ILD status was identified through standardized medical record review [15–17]. In brief, participants were classified as having RA-ILD if they had a provider diagnosis of ILD in the medical records and either (i) chest CT scan features of ILD or (ii) histopathological abnormalities from a lung biopsy consistent with ILD. Relevant procedures were completed as part of routine care, and clinical reports were reviewed. As previously reported [4], ILD patterns from CT reports are available for less than half of relevant cohort participants, prohibiting meaningful analyses of ILD subtype among those with either prevalent or incident disease. For this analysis, patients were considered to have prevalent RA-ILD if the date of clinical diagnosis was prior to registry enrolment, whereas incident RA-ILD was defined as those whose first clinical diagnosis followed enrolment.
Serum alarmin measurement
Banked serum samples were assayed for IL-33, IL-25 (also recognized as IL-17E) and TSLP using U-PLEX immunoassays (Meso Scale Discovery [MSD], Rockville, MD, USA). Serum samples were collected at enrolment, processed and banked at −70°C for future research. Longitudinal samples are not available for VARA participants. Alarmin assays were performed according to the manufacturer’s protocol and analysed on the MESO QuickPlex SQ 120 imager (MSD). A modification of the IL-33 assay (adding 600 mM acetic acid) was performed to dissociate cytokine complexed to the IL1RL1 (ST2) receptor [18]. Acidified serum was then neutralized with 500 mM Trizma base in buffer 43 (MSD) containing a ST2/IL-33R antibody to remove soluble receptor. Receptor free serum was then added to the immunoassay for quantification of total IL-33 concentrations. IL-33 and TSLP concentrations were log-transformed for analyses, given skewed distributions that were normalized following transformation. As a meaningful proportion of IL-25 values fell below the detectable threshold (n = 454, 16%), IL-25 was examined in categories with undetectable concentrations serving as the referent group. Low, moderate and high categories were defined using tertiles of detectable values.
Measurement of serum cytokines and autoantibodies
Using the same banked serum, additional cytokine concentrations were determined using a separate MSD multiplex panel [19]. Assays were performed on the MESO QuickPlex SQ 120 imager (MSD). Analytes were pre-selected from a larger panel of 33 cytokines/chemokines based on prior reports showing that IL-33 induces Th2 immune responses and its neutralization via monoclonal antibody targeting also leads to changes in Th1 cytokine concentrations [20–22]. Th2 cytokines assessed included IL-2, IL-4, IL-5, IL-6 and IL-10. Th1 cytokines assessed in this study included IFN-γ, TNF-α and IL-12. Rheumatoid factor (RF) and antibodies to cyclic citrullinated peptide (anti-CCP) were quantified as previously described and categorized as positive using manufacturer-defined thresholds [23]. In cases where anti-CCP or RF concentrations were not available, seropositivity status was extracted from the registry database.
Covariables
Baseline demographics, disease-specific characteristics and additional covariables were obtained from the registry database or links available through the national VA Corporate Data Warehouse (CDW). These included age, sex, race (self-reported from a fixed set of categories: White, Black or African American, Other), BMI (kg/m2), smoking status (current, former, never), a diagnosis of chronic obstructive pulmonary disease (COPD), 28-joint Disease Activity Score (DAS28), methotrexate use, prednisone use and use of anti-TNF agents (accounting for the vast majority of biologic use at enrolment). BMI was extracted from the vital sign packages in CDW and the closest BMI value (within 30 days) to the enrolment date was utilized. Enrolment observations with missing BMI data were imputed by carrying forward from the most proximate clinical encounter. The presence of COPD was defined using diagnostic codes at the time of registry enrolment.
Statistical analyses
Characteristics of the study population, overall and by alarmin quartiles/categories, were described at the time of cohort enrolment. Primary analyses utilized logistic regression for cross-sectional analyses examining associations of alarmins with prevalent RA-ILD and Cox proportional hazards regression for longitudinal analyses examining associations with incident RA-ILD. In time-to-event analyses, participants were followed from enrolment until the earliest of ILD diagnosis, death (assessed using linkage to the National Death Index) or end of study period (16 December 2019). For graphical purposes, a Kaplan–Meier estimate of RA-ILD cumulative incidence by quartile of baseline IL-33 was generated. Preselected covariates from enrolment included in multivariable models included age, sex, race, smoking status (never as referent category), DAS28 score (categorized as remission/low disease activity or moderate/high disease activity) and anti-CCP positivity. Using a complete case approach, those with missing values for race (n = 4) were categorized as ‘other’. To account for missing data for smoking status (n = 141) and DAS28 (n = 52), these variables were modelled to include a separate ‘unknown’ category. An additional sensitivity analysis was conducted that included COPD as a covariate as this is a common comorbidity in patients with RA-ILD. In a separate analysis, to reduce effects of possible misclassification in longitudinal analysis, participants developing ILD in the first year of registry follow-up were excluded.
To explore whether associations with RA-ILD might be driven through Th1 or Th2 immunity, correlations between log-transformed alarmins and the aforementioned cytokines (n = 2655) and autoantibodies (n = 2497 for anti-CCP and n = 2501 for RF) were examined using Spearman correlation in subcohorts for whom these data were available. Analyses were completed using Stata v17 (Stata Corp, College Station, TX, USA) within the VA Informatics and Computing Infrastructure (VINCI) environment.
Results
Participant characteristics
Baseline characteristics of 2835 study participants, overall and by IL-33 quartile, are shown in Table 1. Overall, at the time of enrolment, participants were older, predominantly male, seropositive, and had longstanding disease. IL-33 concentrations were higher in younger participants, women and in former/never smokers with RA. Those in the highest IL-33 quartile were more likely to be in low disease activity or remission and less likely to be RF positive, taking anti-TNF therapy, or to have COPD. Characteristics by TSLP quartile and IL-25 category are shown in Supplementary Table S1 and S2, available at Rheumatology online. In contrast to observations with IL-33, TSLP concentrations were higher with older age, self-reported White race, ever smokers, those with higher disease activity, COPD and prednisone use. Anti-TNF use was less frequent in those with higher TSLP values. IL-25 values were higher among participants identifying as White, those with higher disease activity and those without anti-TNF use.
Table 1.
Baseline participant characteristics overall and by IL-33 quartile
| Measure | Overall (n = 2835) | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-value |
|---|---|---|---|---|---|---|
| Age, mean (s.d.), years | 65 (11) | 65 (10) | 65 (11) | 65 (11) | 63 (12) | <0.001 |
| Sex, n (%) | 0.002 | |||||
| Female | 342 (12) | 69 (10) | 88 (12) | 73 (10) | 112 (16) | |
| Male | 2493 (88) | 640 (90) | 621 (88) | 636 (90) | 596 (84) | |
| Race, n (%) | 0.551 | |||||
| White | 2193 (77) | 542 (76) | 546 (77) | 557 (79) | 548 (77) | |
| Black/AA | 447 (16) | 126 (18) | 109 (15) | 105 (15) | 107 (15) | |
| Other | 195 (7) | 41 (6) | 54 (8) | 47 (7) | 53 (8) | |
| Smoking status, n (%) | <0.001 | |||||
| Current | 681 (24) | 184 (26) | 187 (26) | 166 (23) | 144 (20) | |
| Former | 1464 (52) | 374 (53) | 340 (48) | 397 (56) | 353 (50) | |
| Never | 549 (19) | 121 (17) | 124 (17) | 115 (16) | 189 (27) | |
| Unknown | 141 (5) | 30 (4) | 58 (8) | 31 (4) | 22 (3) | |
| BMI, mean (s.d.), kg/m2 | 28 (6) | 29 (5) | 29 (6) | 28 (6) | 28 (6) | 0.335 |
| DAS28, mean (s.d.) | 3.7 (1.5) | 3.8 (1.5) | 3.9 (1.6) | 3.7 (1.6) | 3.5 (1.5) | 0.001 |
| DAS state, n (%) | 0.002 | |||||
| Remission/low | 1152 (41) | 279 (39) | 258 (36) | 286 (40) | 329 (46) | |
| Moderate/high | 1631 (58) | 414 (58) | 435 (61) | 408 (58) | 374 (53) | |
| Unknown | 52 (2) | 16 (2) | 16 (2) | 15 (2) | 5 (1) | |
| Methotrexate use, n (%) | 1239 (54) | 323 (54) | 314 (56) | 311 (56) | 291 (50) | 0.206 |
| Prednisone use, n (%) | 929 (40) | 255 (42) | 244 (43) | 220 (39) | 210 (36) | 0.064 |
| Anti-TNF use, n (%) | 652 (23) | 185 (26) | 173 (24) | 147 (21) | 147 (21) | 0.034 |
| Anti-CCP positive, n (%) | 1998 (77) | 509 (77) | 493 (81) | 487 (77) | 509 (75) | 0.132 |
| RF positive, n (%) | 1933 (77) | 501 (77) | 477 (82) | 468 (77) | 487 (74) | 0.007 |
| RA disease duration, mean (s.d.), years | 11 (11) | 12 (12) | 12 (11) | 11 (11) | 11 (11) | 0.055 |
| COPD, n (%) | 450 (16) | 137 (19) | 120 (17) | 93 (13) | 100 (14) | 0.006 |
P-values generated using Kruskal–Wallis tests for continuous measures, χ2 tests for categorical measures. AA: African American; COPD: chronic obstructive pulmonary disease.
RA-ILD status
At baseline, there were 115 participants (4.1%) with prevalent RA-ILD. Of the remaining 2746 participants, 146 (5.3%) developed incident RA-ILD over 24 355 patient-years of follow-up (28 developed RA-ILD during the first year of follow-up). The median time from enrolment to the development of incident RA-ILD was 3.0 years (interquartile range [IQR] 1.2–6.3 years).
Cross-sectional associations of alarmins with prevalent RA-ILD
Overall median (IQR) serum concentrations of IL-33, TSLP, and IL-25 were 159 (99–224), 2.9 (1.9–4.4) and 0.24 (0.07–0.58) pg/ml, respectively. Among those with prevalent ILD, IL-33, TSLP and IL-25 were 167 (91–214), 3.2 (2.1–4.8) and 0.26 (0.08–0.56) pg/ml, respectively. There were no associations observed between baseline serum concentrations of IL-33, TSLP or IL-25 and prevalent ILD in either unadjusted or adjusted logistic regression models (Table 2). Results of the adjusted model were not changed with the inclusion of COPD as an additional covariate (data not shown).
Table 2.
Associations of alarmin levels with prevalent rheumatoid arthritis associated interstitial lung disease (RA-ILD; present at the time of enrolment)
| Unadjusted |
Adjusted |
|||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| IL-33 | 0.95 (0.71, 1.28) | 0.743 | 1.02 (0.75, 1.38) | 0.906 |
| TSLP | 1.21 (0.95, 1.54) | 0.130 | 1.04 (0.79, 1.36) | 0.787 |
| IL-25 | ||||
| Undetectable | Ref. | Ref. | Ref. | Ref. |
| Low-moderate | 1.20 (0.71, 2.04) | 0.500 | 1.17 (0.68, 1.99) | 0.575 |
| High-moderate | 1.17 (0.68, 1.99) | 0.575 | 1.16 (0.67, 1.99) | 0.596 |
| High | 1.08 (0.63, 1.87) | 0.773 | 1.03 (0.59, 1.78) | 0.926 |
Results of logistic regression models (separate models for each analyte examined); IL-33 and TSLP log-transformed and reported per log-fold increase; IL-25 examined in categories; covariates in adjusted models included age, sex, race, smoking status, 28-joint Disease Activity Score (DAS28) state, and anti-cyclic citrullinated peptide (anti-CCP) antibody positivity. OR: odds ratio; TSLP: thymic stromal lymphopoietin.
Associations of baseline alarmin concentrations with incident RA-ILD
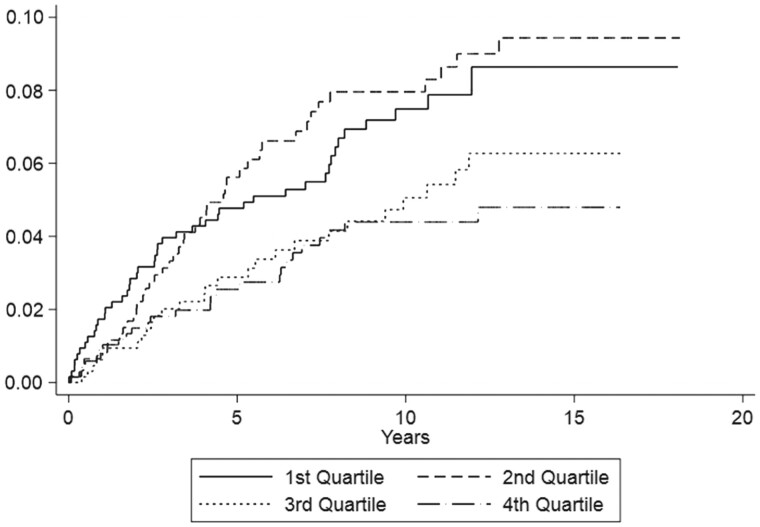
The cumulative incidence of RA-ILD development by IL-33 quartile is shown in Fig. 1. After 10 years of follow-up, ∼8% of patients with RA in the lower two quartiles had developed ILD compared with ∼4% in the upper two quartiles.
Figure 1.
Cumulative incidence of developing incident rheumatoid arthritis associated interstitial lung disease (RA-ILD) based on baseline quartile of IL-33 concentration. Graph depicts Kaplan–Meier estimates of RA-ILD cumulative incidence (or the cumulative probability function), by quartile of serum IL-33 concentration measured at the time of study enrolment
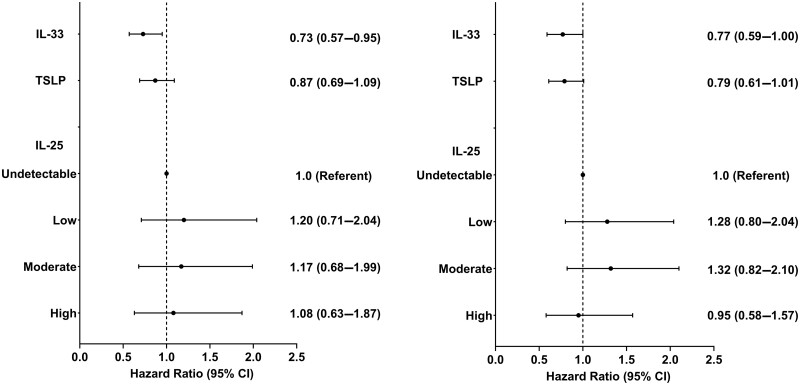
In unadjusted Cox proportional hazards models, there was a significant inverse association between IL-33 concentration and the risk of developing incident RA-ILD (hazard ratio [HR] 0.73 per log-fold increase; 95% CI: 0.57, 0.95) (Fig. 2, left panel). This association was unchanged after multivariable adjustment (HR 0.77; 95% CI: 0.59, 1.00; P = 0.047) (Fig. 2, right panel). Risk estimates were unchanged in sensitivity analyses that included COPD as an additional covariate (HR 0.77), and after excluding those developing RA-ILD during the first year of follow-up (HR 0.77) (Table 3). Although not achieving statistical significance, there were trends suggesting inverse associations between TSLP concentration and RA-ILD risk in models that were unadjusted (HR 0.87; 95% CI: 0.69, 1.09; P = 0.222) or adjusted (HR 0.79; 95% CI: 0.61, 1.01; P = 0.056) (Fig. 2). There were no significant associations between IL-25 concentrations with incident RA-ILD in either unadjusted or adjusted models (Fig. 2).
Figure 2.
Univariate (left) and multivariable (right) associations of alarmin concentrations with the development of incident rheumatoid arthritis-associated interstitial lung disease (RA-ILD). Results of univariate and multivariable Cox proportional hazards regression models (separate models for each analyte examined; multivariable models adjusted for age, sex, race, smoking status, 28-joint Disease Activity Score and anti-cyclic citrullinated peptide antibody). Univariate P-values: IL-33: 0.018; TSLP: 0.222; IL-25 (trend test): 0.930; multivariable P-values: IL-33: 0.047; TSLP: 0.056; IL-25 (trend test): 0.912; IL-33 and TSLP log-transformed, HR reported per log-fold increase. HR: hazard ratio; TSLP, thymic stromal lymphopoietin
Table 3.
Results of sensitivity analyses examining association of IL-33 levels with the development of incident rheumatoid arthritis-associated interstitial lung disease (RA-ILD)
| HR (95% CI) | P-value | |
|---|---|---|
| Model excluding participants developing RA-ILD in first year of follow-up | 0.77 (0.58, 1.03) | 0.076 |
| Model including COPD as additional covariate | 0.77 (0.60, 1.00) | 0.051 |
Results of multivariable Cox proportional hazards regression models adjusted for age, sex, race, smoking status, 28-joint Disease Activity Score and anti-cyclic citrullinated peptide antibody positivity. IL-33 and TSLP log-transformed, HR reported per log-fold increase. COPD: chronic obstructive pulmonary disease; HR: hazard ratio; TSLP: thymic stromal lymphopoietin.
Correlations between baseline alarmin concentrations and Th1/Th2 cytokines/autoantibodies
Correlations between individual alarmins were universally weak, ranging from r = 0.124 to 0.311, albeit statistically significant (P < 0.0001) (Supplementary Table S3, available at Rheumatology online). Additional correlations of baseline IL-33, TSLP and IL-25 with cytokines and autoantibodies, also quantified at baseline, were universally negligible to weak with corresponding correlation coefficients ranging between −0.09 and 0.15.
Discussion
Since its discovery nearly 20 years ago [24], IL-33 has been shown to have pleiotropic functions. In addition to acting as a damage-associated molecular pattern, IL-33 engages the ST2 receptor on a variety of immune cells including T regulatory, Th2, natural killer, dendritic, B lymphocyte, polymorphonuclear cells, and macrophages, eosinophils and basophils, in addition to group 2 innate lymphoid cells. Consequently, IL-33 yields either pro- or anti-inflammatory effects, depending on the microenvironment or disease state [9, 25]. It is suggested, for example, that IL-33 may simultaneously provide anti-inflammatory and anti-proliferative functions during early stages of disease evolution but may paradoxically exacerbate inflammation or fibrosis with more established disease. Potential pro-fibrotic effects have been attributed to IL-33-mediated promotion of M2 macrophage polarization, which promotes tissue repair and wound healing [8]. IL-33 is increased, for example, in the bleomycin-induced lung injury model [26–28]. Moreover, IL-33 mRNA and protein are increased in bronchoalveolar lung fluid from patients with idiopathic pulmonary fibrosis (IPF) and scleroderma-associated ILD compared with controls [29, 30].
Previous research has implicated IL-33 in the pathogenesis of both RA and RA-ILD. In RA, articular cartilage explants produce increased levels of IL-33 along with other pro-inflammatory cytokines [31]. Preclinical experiments have shown that treatments targeting the IL-33/ST2R axis reduce disease severity in both collagen-induced arthritis and autoantibody-induced arthritis [21, 32], two commonly used animal models for RA. In patients with RA, circulating IL-33 has been positively correlated with autoantibody levels of both RF and anti-CCP levels [33, 34] as well as disease severity [34, 35], although at least one study reported no association with measures of disease activity [33]. In reference to lung involvement in RA, our group previously demonstrated that IL-33 expression is increased in lung tissues from a murine model of RA-ILD as well as from patients with RA-associated lung disease compared with lung tissues of controls [36]. Two additional cross-sectional investigations examined the potential utility of serum IL-33 as a biomarker in RA-ILD, both reporting increased concentrations of this biomarker in patients with established RA-ILD compared with RA patients without lung disease or healthy controls [34, 35]. Collectively, these data informed our a priori hypothesis that higher circulating concentrations of IL-33 would be associated with the risk of both prevalent and incident RA-ILD. In contrast to these previous results and our a priori hypothesis, we found no associations of IL-33 or alternative alarmin (i.e. IL-25, TSLP) concentrations with the cross-sectional prevalence of RA-ILD at the time of study enrolment. In contrast to prior reports, we did not detect any meaningful correlations between IL-33 and RF levels. Likewise, rather than detecting positive correlations between IL-33 and disease activity, we identified modest inverse associations between IL-33 concentration and disease activity as defined by the DAS28.
Underscoring a major strength of our approach, the current effort is among the first to examine the relationship between circulating alarmins and the risk of developing new-onset RA-ILD in a prospective fashion. Beyond the availability of longitudinal data, there are other differences in reports that could help to explain discrepant findings. In addition to a much larger study sample available (n = 2835 patients with RA in this study vs n = 121 or n = 50 in others [34, 35]), characteristics of the study populations differed. Reflecting the national veteran population, our study consisted predominantly of older men while prior studies included patients with RA that were younger (mean age of 51 years in both studies) and more often female (76% to 84%). In contrast to the multivariable models used in our study, prior analyses examining the relationship of IL-33 with ILD did not account for confounders. This may be particularly relevant given the associations observed in our study between alarmin concentrations and covariates examined.
Another strength of this study was the inclusion of alternative alarmin analytes, allowing us to examine whether associations observed applied more broadly to other alarmins including TSLP and IL-25. Although the associations observed for these alternative analytes did not reach statistical significance, there were trends suggesting a similar protective association between baseline TSLP concentration and the future risk of RA-ILD. Notably, associations between TSLP with RA-ILD risk were stronger following multivariable adjustment than in unadjusted analyses, reflecting strong associations between TSLP and all of the covariates modelled with the exception of anti-CCP antibody positivity (Supplementary Table S1, available at Rheumatology online). Similar to IL-33, TSLP has been associated with pleiotropic effects. In addition to serving as a danger signal following tissue damage, TSLP has been shown to promote M2 (pro-fibrotic) macrophage polarization and tissue healing following ischaemic injury. In separate studies using the bleomycin-induced injury model of IPF, TSLP has been suggested to exert both pro- and anti-fibrotic effects [37, 38].
Our finding of a statistically significant inverse association between serum IL-33 level and incident ILD risk in patients with RA is both novel and potentially impactful. Given the complex effects of IL-33, more work will be needed to identify mechanisms and to understand whether this association is causal or if circulating IL-33 serves as a surrogate of reduced risk during pre-clinical stages of ILD. If replicated, our findings showing a the lack of correlation between IL-33 and multiple cytokines, including markers of Th1- and Th2-related immunity, as well as autoantibody concentrations suggest that the inverse association observed is independent of pathways involving these analytes. Thus, IL-33 and alarmins represent a potentially novel pathophysiological process contributing to RA-ILD development. Our results, though contrary to our original hypothesis, are perhaps consistent with reports from other disease states that have identified IL-33 as being potentially protective against progressive tissue damage. Using an animal model of myocardial ischaemia and reperfusion (I/R) injury, Ruisong and colleagues found that tissue deposition of IL-33 was increased following I/R [39], akin to increased IL-33 staining observed in lungs of patients with RA-ILD [36]. However, pre-treatment with intravenous/recombinant IL-33 resulted in marked reductions in myocardial injury, myocyte apoptosis and tissue inflammation. Observations of similar IL-33-mediated protection against I/R injury have been replicated by others in relation not only to myocardial infarction, but also to stroke [40, 41]. Whether IL-33 plays a paradoxical and compartmentalized role in RA-ILD wherein circulating IL-33 facilitates tissue homeostasis prior to ILD onset (or early in its evolution) while lung tissue deposition promotes fibrosis [26–29] remains to be elucidated.
Released primarily from damaged epithelial cells, IL-33 is also actively secreted from a variety of immune cells [42]. Whether peripheral immune cells or damaged barrier epithelial cells serve as the primary source of circulating IL-33 detected pre-ILD remains to be defined. It is recognized that the inflammatory microenvironment can modify IL-33 function. Full length IL-33, for example, is cleaved by neutrophil proteases expressed during inflammation and these changes can render a mature, more biologically active form [43]. In the current study, we used a multiplex assay measuring total IL-33, including both full-length and mature forms. Thus, it is unknown to what degree different forms of IL-33 contributed to our findings. If the reduced risk of incident RA-ILD were to be attributable to a specific form of IL-33, our findings would serve as a conservative estimate of this risk.
There are other limitations to this study including our focus on a predominantly older male population with a prevalent history of smoking that could limit generalizability, although these same characteristics are also well-recognized risk factors for RA-ILD and were accounted for as covariates. Although this investigation is among only a few to quantify associations of biomarkers with incident disease, serum samples from this cohort were available only at the time of study enrolment and data from this cohort specific to ILD pattern (e.g. UIP vs non-specific interstitial pneumonia [NSIP]) were not universally available. Given the limited number of incident and prevalent cases with known pattern data, analyses of ILD subtypes were not performed. Such analyses would be informative as stronger associations with UIP might be indicative of mechanisms linked more closely to tissue fibrosis whereas stronger associations with NSIP might implicate anti-inflammatory effects. Data on the severity of ILD by pulmonary function tests or semiquantitative CT scoring were not universally available, which limited the ability to evaluate associations with disease stage. Similarly, there may be misclassification of ILD diagnosis date, which prohibited analyses based on ILD disease duration. Finally, assessment of ILD was retrospective, which likely underestimates the presence of mild or sub-clinical RA-ILD. We would expect such misclassification, in addition to heterogeneity in ILD subtypes and severity represented among cases, to bias findings towards the null.
In summary, we report herein a significant inverse association between serum IL-33 concentrations and the risk of incident RA-ILD. Specifically, for each log-fold increase in serum IL-33 at enrolment, patients were ∼20–30% less likely to develop ILD over follow-up, an association that was independent of other ILD risk factors in this population. Additional work is needed to understand the mechanisms driving this relationship and to better understand how serum IL-33 and related biologic pathways might contribute to the development of ILD in patients with RA.
Supplementary Material
Acknowledgements
Work supported by Center of Excellence for Suicide Prevention, Joint Department of Veterans Affairs (VA) and Department of Defense (DoD) Suicide Data Repository—National Death Index. The identification of specific products or scientific instrumentation does not constitute endorsement or implied endorsement on the part of the authors, U.S. Department of Defense, or any component agency. The views expressed in this presentation are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government. Neither patients nor the public was involved in the design, conduct or reporting of this research.
Contributor Information
Jill A Poole, Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE, USA.
Bryant R England, Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE, USA; Veterans Affairs (VA) Nebraska-Western Iowa Health Care System, Omaha, NE, USA.
Harlan Sayles, Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE, USA.
Tate M Johnson, Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE, USA; Veterans Affairs (VA) Nebraska-Western Iowa Health Care System, Omaha, NE, USA.
Michael J Duryee, Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE, USA; Veterans Affairs (VA) Nebraska-Western Iowa Health Care System, Omaha, NE, USA.
Carlos D Hunter, Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE, USA; Veterans Affairs (VA) Nebraska-Western Iowa Health Care System, Omaha, NE, USA.
Joshua F Baker, Corporal Michael J. Crescenz Veterans Affairs Medical Center, School of Medicine and Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, Philadelphia, PA, USA.
Gail S Kerr, Washington, D.C. VA, Georgetown and Howard University, Washington, DC, USA.
Gary Kunkel, George E. Wahlen Veterans Affairs Medical Center, University of Utah, Salt Lake City, UT, USA.
Grant W Cannon, George E. Wahlen Veterans Affairs Medical Center, University of Utah, Salt Lake City, UT, USA.
Brian C Sauer, George E. Wahlen Veterans Affairs Medical Center, University of Utah, Salt Lake City, UT, USA.
Katherine D Wysham, VA Puget Sound Health Care System, University of Washington, Seattle, WA, USA.
Amy M Joseph, VA St. Louis Health Care System, Washington University School of Medicine, St Louis, MO, USA.
Beth I Wallace, VA Ann Arbor Healthcare System, University of Michigan Medical School, Ann Arbor, MI, USA.
Geoffrey M Thiele, Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE, USA; Veterans Affairs (VA) Nebraska-Western Iowa Health Care System, Omaha, NE, USA.
Ted R Mikuls, Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE, USA; Veterans Affairs (VA) Nebraska-Western Iowa Health Care System, Omaha, NE, USA.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
The data underlying this report cannot be shared publicly based on VA research policy. The data will be shared on reasonable requests made to the corresponding author.
Funding
This work is supported by a grant from the U.S. Department of Defense (PR200793; J.A.P., T.R.M. co-principal investigators). J.A.P. receives research support from National Institute for Occupational Safety and Health R01OH012045 and Central States Center of Agricultural Safety and Health (CS-CASH). T.M.J. is supported by a grant from the Rheumatology Research Foundation. B.R.E. is supported by grants from VA CSR&D (IK2 CX002203) and the Rheumatology Research Foundation. J.F.B. is supported by a VA CSR&D Merit (CX0001703) and an RR&D Merit (RX0003644). K.D.W. is supported by a grant from VA CSR&D (CX002351). B.I.W. is supported by a grant from VA CSR&D (CX002430-01). T.R.M. is supported by grants from the VA (BLR&D Merit I01 BX004660) and National Institutes of Health (2U54GM115458).
Disclosure statement: J.A.P. has received research reagent (anti-IL-33/ST2 antibody, murine studies) from AstraZeneca (no monies) and is a site investigator for clinical allergy/asthma studies for Takeda, GlaxoSmithKline and AstraZeneca (all no monies). B.R.E. has consulted for and received research support from Boehringer-Ingelheim. J.F.B. has received consulting fees from CorEvitas, Cumberland Pharma, and research support from Horizon. G.S.K. has consulted with Janssen, Horizon, UCB, Sanofi and BMS. A.M.J. receives research support from Bristol Myers Squibb. T.R.M. received research support from Horizon Therapeutics and has been a consultant for Horizon, Pfizer, UCB and Sanofi. All other authors report no conflicts.
References
- 1. Olson AL, Swigris JJ, Sprunger DB. et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med 2011;183:372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnson TM, Yang Y, Roul P. et al. A narrowing mortality gap: temporal trends of cause-specific mortality in a national, matched cohort study in U.S. Veterans with Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2022;75:1648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bongartz T, Nannini C, Medina-Velasquez YF. et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 2010;62:1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brooks R, Baker JF, Yang Y. et al. The impact of disease severity measures on survival in U.S. veterans with rheumatoid arthritis-associated interstitial lung disease. Rheumatology (Oxford) 2022;61:4667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR.. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax 2001;56:622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gabbay E, Tarala R, Will R. et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med 1997;156:528–35. [DOI] [PubMed] [Google Scholar]
- 7. Shaver D, Van Kalsbeek D, Ebel A. et al. Biomarkers for rheumatoid arthritis-associated interstitial lung disease: a systematic review. Arthritis Rheumatol 2020;72:s1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kotsiou OS, Gourgoulianis KI, Zarogiannis SG.. IL-33/ST2 axis in organ fibrosis. Front Immunol 2018;9:2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shakerian L, Kolahdooz H, Garousi M. et al. IL-33/ST2 axis in autoimmune disease. Cytokine 2022;158:156015. [DOI] [PubMed] [Google Scholar]
- 10. Krishack PA, Hollinger MK, Kuzel TG. et al. IL-33-mediated eosinophilia protects against acute lung injury. Am J Respir Cell Mol Biol 2021;64:569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zaiss DM, Minutti CM, Knipper JA.. Immune- and non-immune-mediated roles of regulatory T-cells during wound healing. Immunology 2019;157:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Majewski S, Szewczyk K, Bialas AJ. et al. Epithelial alarmins in serum and exhaled breath in patients with idiopathic pulmonary fibrosis: a prospective one-year follow-up cohort study. J Clin Med 2019;8:1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mikuls TR, Reimold A, Kerr GS, Cannon GW.. Insights and implications of the VA Rheumatoid Arthritis registry. Fed Pract 2015;32:24–9. [PMC free article] [PubMed] [Google Scholar]
- 14. Arnett F, Edworthy S, Bloch D. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 15. England BR, Duryee MJ, Roul P. et al. Malondialdehyde-acetaldehyde adducts and antibody responses in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol 2019;71:1483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. England BR, Roul P, Mahajan TD. et al. Performance of administrative algorithms to identify interstitial lung disease in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2020;72:1392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Natalini JG, Baker JF, Singh N. et al. Autoantibody seropositivity and risk for interstitial lung disease in a prospective male-predominant rheumatoid arthritis cohort of U.S. Veterans. Ann Am Thorac Soc 2021;18:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zylstra J, Partridge MA, Sumner G.. Quantitation of reduced IL-33 levels in human serum: mitigating interference from endogenous binding partners. Bioanalysis 2021;13:1751–60. [DOI] [PubMed] [Google Scholar]
- 19. Baker JF, England BR, George M. et al. Disease activity, cytokines, chemokines and the risk of incident diabetes in rheumatoid arthritis. Ann Rheum Dis 2021;80:566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dinarello CA. An IL-1 family member requires caspase-1 processing and signals through the ST2 receptor. Immunity 2005;23:461–2. [DOI] [PubMed] [Google Scholar]
- 21. Li Y, Fu Y, Chen H, Liu X, Li M.. Blocking interleukin-33 alleviates the joint inflammation and inhibits the development of collagen-induced arthritis in mice. J Immunol Res 2020;2020:4297354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmitz J, Owyang A, Oldham E. et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005;23:479–90. [DOI] [PubMed] [Google Scholar]
- 23. Miriovsky BJ, Michaud K, Thiele GM. et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease burden in U.S. veterans with rheumatoid arthritis. Ann Rheum Dis 2010;69:1292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Onda H, Kasuya H, Takakura K. et al. Identification of genes differentially expressed in canine vasospastic cerebral arteries after subarachnoid hemorrhage. J Cereb Blood Flow Metab 1999;19:1279–88. [DOI] [PubMed] [Google Scholar]
- 25. Pei C, Barbour M, Fairlie-Clarke KJ. et al. Emerging role of interleukin-33 in autoimmune diseases. Immunology 2014;141:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gao Q, Li Y, Pan X. et al. Lentivirus expressing soluble ST2 alleviates bleomycin-induced pulmonary fibrosis in mice. Int Immunopharmacol 2016;30:188–93. [DOI] [PubMed] [Google Scholar]
- 27. Luzina IG, Kopach P, Lockatell V. et al. Interleukin-33 potentiates bleomycin-induced lung injury. Am J Respir Cell Mol Biol 2013;49:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu J, Zheng J, Song P, Zhou Y, Guan S.. IL-33/ST2 pathway in a bleomycin-induced pulmonary fibrosis model. Mol Med Rep 2016;14:1704–8. [DOI] [PubMed] [Google Scholar]
- 29. Lee JU, Chang HS, Lee HJ. et al. Upregulation of interleukin-33 and thymic stromal lymphopoietin levels in the lungs of idiopathic pulmonary fibrosis. BMC Pulm Med 2017;17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manetti M, Ibba-Manneschi L, Liakouli V. et al. The IL1-like cytokine IL33 and its receptor ST2 are abnormally expressed in the affected skin and visceral organs of patients with systemic sclerosis. Ann Rheum Dis 2010;69:598–605. [DOI] [PubMed] [Google Scholar]
- 31. Onnheim K, Huang S, Strid Holmertz A. et al. Rheumatoid arthritis chondrocytes produce increased levels of pro-inflammatory proteins. Osteoarthr Cartil Open 2022;4:100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu D, Jiang HR, Li Y. et al. IL-33 exacerbates autoantibody-induced arthritis. J Immunol 2010;184:2620–6. [DOI] [PubMed] [Google Scholar]
- 33. Mu R, Huang HQ, Li YH. et al. Elevated serum interleukin 33 is associated with autoantibody production in patients with rheumatoid arthritis. J Rheumatol 2010;37:2006–13. [DOI] [PubMed] [Google Scholar]
- 34. Xiangyang Z, Lutian Y, Lin Z. et al. Increased levels of interleukin-33 associated with bone erosion and interstitial lung diseases in patients with rheumatoid arthritis. Cytokine 2012;58:6–9. [DOI] [PubMed] [Google Scholar]
- 35. Abdel-Wahab S, Tharwat I, Atta D, El-Sammak A, Atef R.. Serum level of interleukin-33 in rheumatoid arthritis patients and its association with bone erosion and interstitial lung disease. Egypt Rheumatol 2016;38:99–104. [Google Scholar]
- 36. Mikuls TR, Gaurav R, Thiele GM. et al. The impact of airborne endotoxin exposure on rheumatoid arthritis-related joint damage, autoantigen expression, autoimmunity, and lung disease. Int Immunopharmacol 2021;100:108069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li G, Jin F, Du J. et al. Macrophage-secreted TSLP and MMP9 promote bleomycin-induced pulmonary fibrosis. Toxicol Appl Pharmacol 2019;366:10–6. [DOI] [PubMed] [Google Scholar]
- 38. Shubin NJ, Clauson M, Niino K. et al. Thymic stromal lymphopoietin protects in a model of airway damage and inflammation via regulation of caspase-1 activity and apoptosis inhibition. Mucosal Immunol 2020;13:584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ruisong M, Xiaorong H, Gangying H. et al. The protective role of interleukin-33 in myocardial ischemia and reperfusion is associated with decreased HMGB1 expression and up-regulation of the P38 MAPK signaling pathway. PLoS One 2015;10:e0143064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qian L, Yuanshao L, Wensi H. et al. Serum IL-33 is a novel diagnostic and prognostic biomarker in acute ischemic stroke. Aging Dis 2016;7:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seki K, Sanada S, Kudinova AY. et al. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail 2009;2:684–91. [DOI] [PubMed] [Google Scholar]
- 42. Drake LY, Kita H.. IL-33: biological properties, functions, and roles in airway disease. Immunol Rev 2017;278:173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lefrancais E, Roga S, Gautier V. et al. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci USA 2012;109:1673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this report cannot be shared publicly based on VA research policy. The data will be shared on reasonable requests made to the corresponding author.