Abstract
Objectives
To understand the relative efficacy and safety of bimekizumab, a selective inhibitor of IL-17F in addition to IL-17A, vs other biologic and targeted synthetic DMARDs (b/tsDMARDs) for PsA using network meta-analysis (NMA).
Methods
A systematic literature review (most recent update conducted on 1 January 2023) identified randomized controlled trials (RCTs) of b/tsDMARDs in PsA. Bayesian NMAs were conducted for efficacy outcomes at Weeks 12–24 for b/tsDMARD-naïve and TNF inhibitor (TNFi)-experienced patients. Safety at Weeks 12–24 was analysed in a mixed population. Odds ratios (ORs) and differences of mean change with the associated 95% credible interval (CrI) were calculated for the best-fitting models, and the surface under the cumulative ranking curve (SUCRA) values were calculated to determine relative rank.
Results
The NMA included 41 RCTs for 22 b/tsDMARDs. For minimal disease activity (MDA), bimekizumab ranked 1st in b/tsDMARD-naïve patients and 2nd in TNFi-experienced patients. In b/tsDMARD-naïve patients, bimekizumab ranked 6th, 5th and 3rd for ACR response ACR20/50/70, respectively. In TNFi-experienced patients, bimekizumab ranked 1st, 2nd and 1st for ACR20/50/70, respectively. For Psoriasis Area and Severity Index 90/100, bimekizumab ranked 2nd and 1st in b/tsDMARD-naïve patients, respectively, and 1st and 2nd in TNFi-experienced patients, respectively. Bimekizumab was comparable to b/tsDMARDs for serious adverse events.
Conclusion
Bimekizumab ranked favourably among b/tsDMARDs for efficacy on joint, skin and MDA outcomes, and showed comparable safety, suggesting it may be a beneficial treatment option for patients with PsA.
Keywords: systematic literature review, network meta-analysis, ACR, PASI, MDA, disease-modifying antirheumatic drugs, bimekizumab, psoriatic arthritis
Rheumatology key messages.
For joint efficacy, bimekizumab ranked highly among approved biologic/targeted synthetic DMARDs (b/tsDMARDs).
Bimekizumab provides better skin efficacy (Psoriasis Area and Severity Index, PASI100 and PASI90) than many other available treatments in PsA.
For minimal disease activity, bimekizumab ranked highest of all available b/tsDMARDs in b/tsDMARD-naïve and TNF inhibitor–experienced patients.
Introduction
PsA is a chronic, systemic, inflammatory disease in which patients experience a high burden of illness [1–3]. PsA has multiple articular and extra-articular disease manifestations including peripheral arthritis, axial disease, enthesitis, dactylitis, skin psoriasis (PSO) and psoriatic nail disease [4, 5]. Patients with PsA can also suffer from related inflammatory conditions, uveitis and IBD [4, 5]. Approximately one fifth of all PSO patients, increasing to one quarter of patients with moderate to severe PSO, will develop PsA over time [6, 7].
The goal of treatment is to control inflammation and prevent structural damage to minimize disease burden, normalize function and social participation, and maximize the quality of life of patients [1, 4]. As PsA is a heterogeneous disease, the choice of treatment is guided by individual patient characteristics, efficacy against the broad spectrum of skin and joint symptoms, and varying contraindications to treatments [1, 4]. There are a number of current treatments classed as conventional DMARDs such as MTX, SSZ, LEF; biologic (b) DMARDs such as TNF inhibitors (TNFi), IL inhibitors and cytotoxic T lymphocyte antigen 4 (CTLA4)-immunoglobulin; and targeted synthetic (ts) DMARDs which include phosphodiesterase-4 (PDE4) and Janus kinase (JAK) inhibitors [1, 8].
Despite the number of available treatment options, the majority of patients with PsA report that they do not achieve remission and additional therapeutic options are needed [9, 10]. Thus, the treatment landscape for PsA continues to evolve and treatment decisions increase in complexity, especially as direct comparative data are limited [2].
Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, which is approved for the treatment of adults with active PsA in Europe [11, 12]. Both IL-17A and IL-17F are pro-inflammatory cytokines implicated in PsA [11, 13]. IL-17F is structurally similar to IL-17A and expressed by the same immune cells; however, the mechanisms that regulate expression and kinetics differ [13, 14]. IL-17A and IL-17F are expressed as homodimers and as IL-17A–IL-17F heterodimers that bind to and signal via the same IL-17 receptor A/C complex [13, 15].
In vitro studies have demonstrated that the dual inhibition of both IL-17A and IL-17F with bimekizumab was more effective at suppressing PsA inflammatory genes and T cell and neutrophil migration, and periosteal new bone formation, than blocking IL-17A alone [11, 14, 16, 17]. Furthermore, IL-17A and IL-17F protein levels are elevated in psoriatic lesions and the superiority of bimekizumab 320 mg every 4 weeks (Q4W) or every 8 weeks (Q8W) over the IL-17A inhibitor, secukinumab, in complete clearance of psoriatic skin was demonstrated in a head-to-head trial in PSO [16, 18]. Collectively, this evidence suggests that neutralizing both IL-17F and IL-17A may provide more potent abrogation of IL-17-mediated inflammation than IL-17A alone.
Bimekizumab 160 mg Q4W demonstrated significant improvements in efficacy outcomes compared with placebo, and an acceptable safety profile in adults with PsA in the phase 3 RCTs BE OPTIMAL (NCT03895203) (b/tsDMARD-naïve patients) and BE COMPLETE (NCT03896581) (TNFi inadequate responders) [19, 20].
The objective of this study was to establish the comparative efficacy and safety of bimekizumab 160 mg Q4W vs other available PsA treatments, using network meta-analysis (NMA).
Methods
Search strategy
A systematic literature review (SLR) was conducted according to the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines [21] and adhered to the principles outlined in the Cochrane Handbook for Systematic Reviews of Interventions, Centre for Reviews and Dissemination’s Guidance for Undertaking Reviews in Healthcare, and Methods for the Development of National Institute of Health and Care Excellence (NICE) Public Health Guidance [22–24]. The SLR of English-language publications was originally conducted on 3 December 2015, with updates on 7 January 2020, 2 May 2022 and 1 January 2023 in Medical Literature Analysis and Retrieval System Online (MEDLINE®), Excerpta Medica Database (Embase®) and the Cochrane Central Register of Controlled Trials (CENTRAL) for literature published from January 1991 onward using the Ovid platform. Additionally, bibliographies of SLRs and meta-analyses identified through database searches were reviewed to ensure any publications not identified in the initial search were included in this SLR. Key clinical conference proceedings not indexed in Ovid (from October 2019 to current) and ClinicalTrials.gov were also manually searched. The search strategy is presented in Supplementary Table S1 (available at Rheumatology online).
Study inclusion
Identified records were screened independently and in duplicate by two reviewers and any discrepancies were reconciled via discussion or a third reviewer. The SLR inclusion criteria were defined by the Patient populations, Interventions, Comparators, Outcome measures, and Study designs (PICOS) Statement (Supplementary Table S2, available at Rheumatology online). The SLR included published studies assessing approved therapies for the treatment of PsA. Collected data included study and patient population characteristics, interventions, comparators, and reported clinical and patient-reported outcomes relevant to PsA. For efficacy outcomes, pre-crossover data were extracted in studies where crossover occurred. All publications included in the analysis were evaluated according to the Cochrane risk-of-bias tool for randomized trials as described in the Cochrane Handbook [25].
Network meta-analysis methods
NMA is the quantitative assessment of relative treatment effects and associated uncertainty of two or more interventions [26, 27]. It is used frequently in health technology assessment, guideline development and to inform treatment decision making in clinical practice [26].
Bimekizumab 160 mg Q4W was compared with current b/tsDMARDs at regulatory-approved doses (Table 1) by NMA. All comparators were selected on the basis they were relevant to clinical practice, i.e. recommended by key clinical guidelines, licensed by key regulatory bodies and/or routinely used.
Table 1.
NMA intervention and comparators
| Therapeutic class | Drug dose and frequency of administration |
|---|---|
| Intervention | |
| IL-17A/17Fi | Bimekizumab 160 mg Q4W |
| Comparators | |
| IL-17Ai | Secukinumab 150 mg with or without loading dose Q4W or 300 mg Q4W, ixekizumab 80 mg Q4W |
| IL-23i | Guselkumab 100 mg every Q4W or Q8W, risankizumab 150 mg Q4Wa |
| IL-12/23i | Ustekinumab 45 mg or 90 mg Q12W |
| TNFi | Adalimumab 40 mg Q2W, certolizumab pegol 200 mg Q2W or 400 mg Q4W pooled, etanercept 25 mg twice a week, golimumab 50 mg s.c. Q4W or 2 mg/kg i.v. Q8W, infliximab 5 mg/kg on weeks 0, 2, 6, 14, 22 |
| CTLA4-Ig | Abatacept 150 mg Q1W |
| JAKi | Tofacitinib 5 mg BID, upadacitinib 15 mg QD |
| PDE-4i | Apremilast 30 mg BID |
| Other | Placebo |
See Supplementary Table S4, available at Rheumatology online for additional dosing schedules used in included studies. BID: twice daily; CTLA4-Ig: cytotoxic T lymphocyte antigen 4-immunoglobulin; IL-17A/17Fi: IL-17A/17F inhibitor; IL-17Ai: IL-17A inhibitor; IL-12/23i: IL-12/23 inhibitor; IL-23i: IL-23 inhibitor; JAKi: Janus kinase inhibitor; NMA: network meta-analysis; PDE-4i: phosphodiesterase-4 inhibitor; Q1W: once weekly; Q2W: every 2 weeks; Q4W: every 4 weeks; Q8W: every 8 weeks; Q12W: every 12 weeks; QD: once daily; TNFi: TNF inhibitor.
Two sets of primary analyses were conducted, one for a b/tsDMARD-naïve PsA population and one for a TNFi-experienced PsA population. Prior treatment with TNFis has been shown to impact the response to subsequent bDMARD treatments [28]. In addition, most trials involving b/tsDMARDs for the treatment of PsA (including bimekizumab) report separate data on both b/tsDMARD-naïve and TNFi-experienced subgroups, making NMA in each of these patient populations feasible.
For each population the following outcomes were analysed: American College of Rheumatology response (ACR20/50/70), Psoriasis Area and Severity Index (PASI90/100), and minimal disease activity (MDA). The analysis of serious adverse events (SAE) was conducted using a mixed population (i.e. b/tsDMARD-naïve, TNFi-experienced and mixed population data all were included) as patients’ previous TNFI exposure was not anticipated to impact safety outcomes following discussions with clinicians. The NMA included studies for which data were available at week 16, if 16-week data were not available (or earlier crossover occurred), data available at weeks 12, 14 or 24 were included. Pre-crossover data were included in the analyses for efficacy outcomes to avoid intercurrent events.
Heterogeneity between studies for age, sex, ethnicity, mean time since diagnosis, concomitant MTX, NSAIDs or steroid use was assessed using Grubb’s test, also called the extreme Studentized deviate method, to identify outlier studies.
All univariate analyses involved a 10 000 run-in iteration phase and a 10 000-iteration phase for parameter estimation. All calculations were performed using the R2JAGS package to run Just Another Gibbs Sampler (JAGS) 3.2.3 and the code reported in NICE Decision Support Unit (DSU) Technical Support Document Series [29–33]. Convergence was confirmed through inspection of the ratios of Monte-Carlo error to the standard deviations of the posteriors; values >5% are strong signs of convergence issues [31]. In some cases, trials reported outcome results of zero (ACR70, PASI100, SAE) in one or more arms for which a continuity correction was applied to mitigate the issue, as without the correction most models were not convergent or provided a large posterior distribution making little clinical sense [31].
Four NMA models [fixed effects (FE) unadjusted, FE baseline risk-adjusted, random effects (RE) unadjusted and RE baseline risk-adjusted] were assessed and the best-fit models were chosen using methods described in NICE DSU Technical Support Document 2 [31]. Odds ratios (ORs) and differences of mean change (MC) with the associated 95% credible intervals (CrIs) were calculated for each treatment comparison in the evidence network for the best fitting models and presented in league tables and forest plots. In addition, the probability of bimekizumab 160 mg Q4W being better than other treatments was calculated using surface under the cumulative ranking curve (SUCRA) to determine relative rank. Conclusions (i.e. better/worse or comparable) for bimekizumab 160 mg Q4W vs comparators were based on whether the pairwise 95% CrIs of the ORs/difference of MC include 1 (dichotomous outcomes), 0 (continuous outcomes) or not. In the case where the 95% CrI included 1 or 0, then bimekizumab 160 mg Q4W and the comparator were considered comparable. If the 95% CrI did not include 1 or 0, then bimekizumab 160 mg Q4W was considered either better or worse depending on the direction of the effect.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Study and patient characteristics
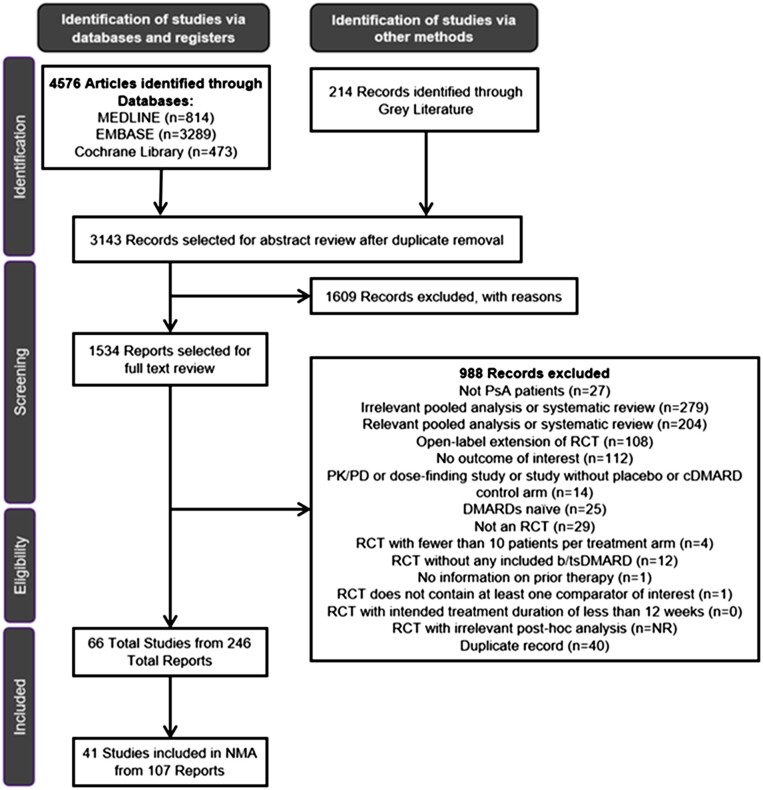
The SLR identified 4576 records through databases and 214 records through grey literature, of which 3143 were included for abstract review. Following the exclusion of a further 1609 records, a total of 1534 records were selected for full-text review. A total of 66 primary studies from 246 records were selected for data extraction. No trial was identified as having a moderate or high risk of bias (Supplementary Table S3, available at Rheumatology online).
Of the 66 studies identified in the SLR, 41 studies reported outcomes at weeks 12, 16 or 24 and met the criteria for inclusion in the NMA in either a b/tsDMARD-naïve population (n = 20), a TNFi-experienced population (n = 5), a mixed population with subgroups (n = 13) or a mixed PsA population without subgroups reported (n = 3). The PRISMA diagram is presented in Fig. 1. Included and excluded studies are presented in Supplementary Tables S4 and S5, respectively (available at Rheumatology online).
Figure 1.
PRISMA flow diagram. The PRISMA flow diagram for the SLR conducted to identify published studies assessing approved treatments for the treatment of PsA. cDMARD: conventional DMARD; NMA: network meta-analysis; NR: not reported; PD: pharmacodynamic; PK: pharmacokinetic; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT: randomized controlled trial; SLR: systematic literature review
The baseline study and patient characteristics (where reported) are presented in Supplementary Table S6 (available at Rheumatology online). There were 20–483 patients included in treatment arms. The median age of patients was 48.9 years, the median percentage of males was 50.3% and a median of 92.3% of patients were Caucasian. Patients had a mean time since diagnosis of 7.6 years and a mean PASI score of 8.7. The mean (range) use of concomitant MTX, NSAIDs and steroids were 53.9% (29.1% to 84.0%), 72.4% (33.3% to 100.0%) and 16.8% (9.2% to 30.0%), respectively. Heterogeneity was generally low across studies except for the concomitant use of MTX, NSAIDs and steroids. Using an approach consistent with established NMA methods in PsA [34–36], a meta-regression model using JAGS code reported in NICE DSU Technical Support Document 3 [33] was used to account for variation in placebo responses when model-fit statistics suggested that baseline risk-adjusted models provided a better fit to the data.
NMA results
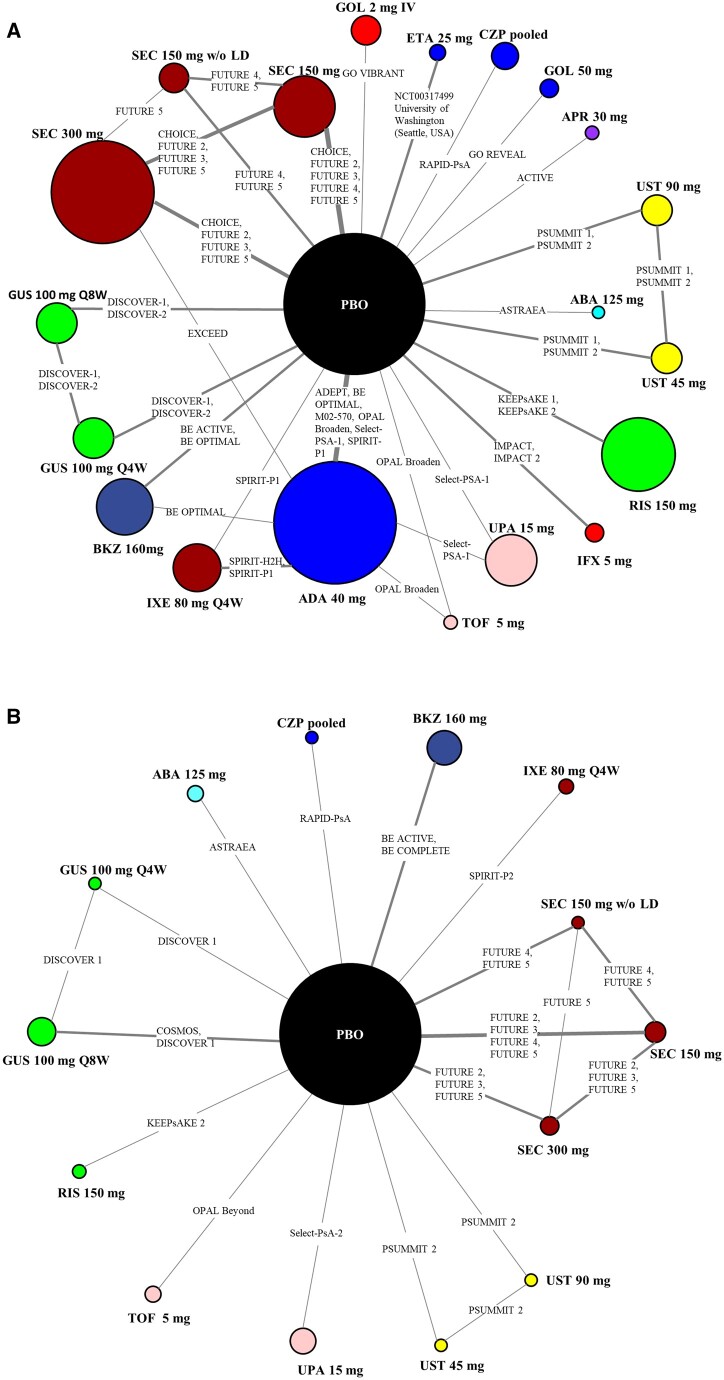
The network diagrams for ACR50 in b/tsDMARD-naïve and TNFi-experienced patients are presented in Fig. 2A and B with network diagrams for other outcomes presented in Supplementary Fig. S1 (available at Rheumatology online). The networks for ACR response were larger, in terms of both number of studies and patients included, than the networks for PASI. Similarly, the networks for b/tsDMARD-naïve patients were larger than TNFi-experienced patients across all outcomes analysed. Placebo was used as a common comparator in all networks and there were a few studies that included more than two arms (OPAL-Broaden, Select-PsA-1, SPIRIT-P1 and BE OPTIMAL) that included adalimumab as the reference arm in b/tsDMARD-naïve patients. Lastly, networks included studies where the primary outcome was evaluated at time points longer than 16 weeks (e.g. EXCEED study at 52 weeks) but as per the methods, 16-week data formed the network.
Figure 2.
Network of evidence for ACR50. (A) b/tsDMARD-naïve patients. (B) TNFi-experienced patients. The size of the circle representing each intervention is proportional to the number of patients included in the analysis. The line width is proportional to the number of studies connecting the interventions. ABA: abatacept; ADA: adalimumab; APR: apremilast; b/tsDMARD-naïve: biologic and targeted synthetic DMARD-naïve; BKZ: bimekizumab; CZP: certolizumab pegol; ETA: etanercept; GOL: golimumab; GUS: guselkumab; IFX: infliximab; IV: intravenous; IXE: ixekizumab; PBO: placebo; Q4W: every 4 weeks; Q8W: every 8 weeks; RIS: risankizumab; SEC: secukinumab; TNFi-experienced: TNF inhibitor–experienced; TOF: tofacitinib; UPA: upadacitinib; UST: ustekinumab; w/o LD: without loading dose
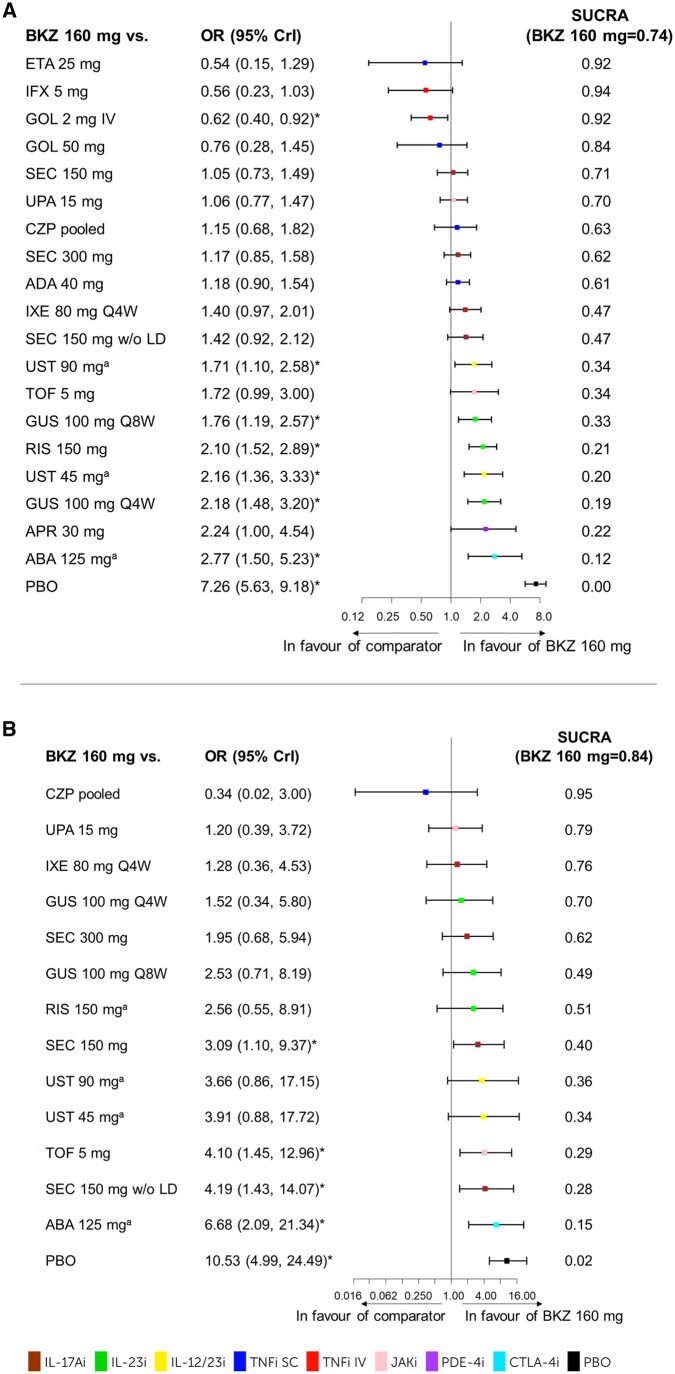
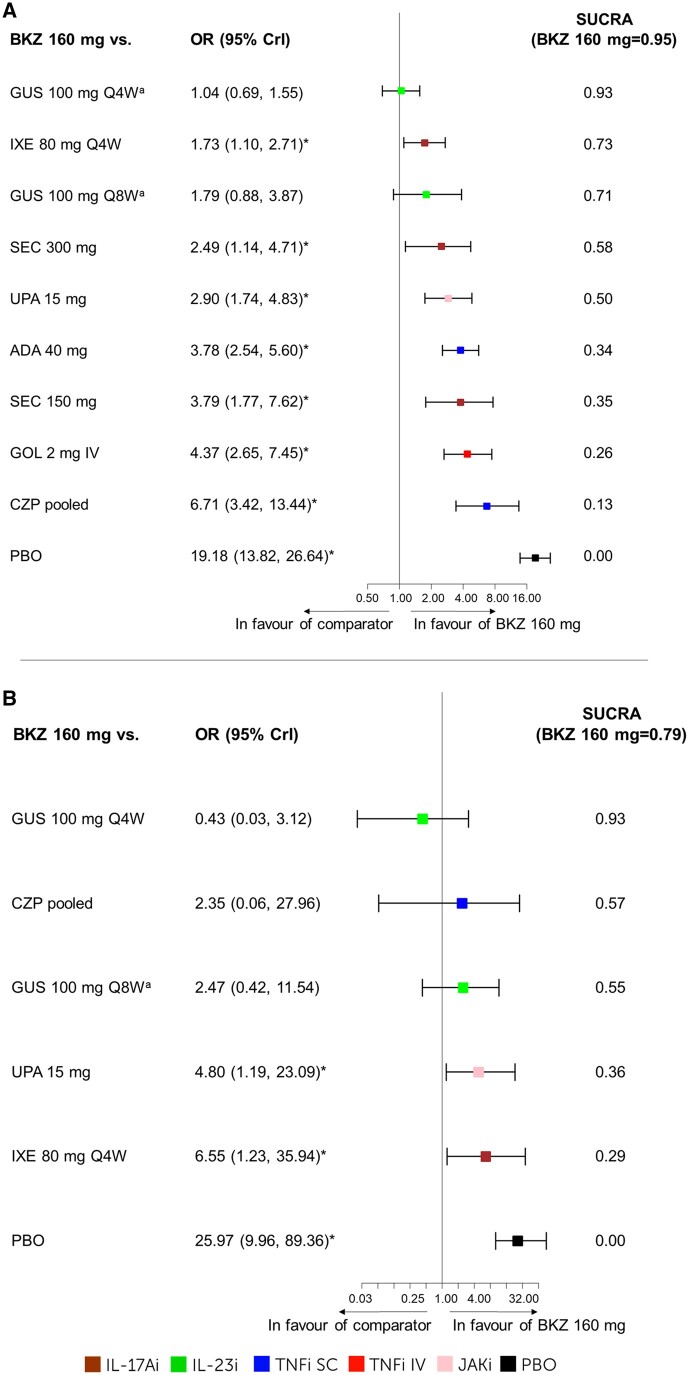
The best-fit model is noted for each outcome with full model fit statistics for all outcomes presented in Supplementary Table S7 (available at Rheumatology online). Forest plots for ACR50 and PASI100 are presented in Figs 3 and 4, with forest plots for other outcomes, along with the league tables in Supplementary Fig. S2 and Table S8, respectively (available at Rheumatology online).
Figure 3.
ACR50. The results for the NMA on ACR50 at week 16. (A) b/tsDMARD-naïve patients including forest plot and SUCRA values. FE baseline–adjusted model DIC = 469.59. (B) TNFi-experienced patients including forest plot and SUCRA values. RE-unadjusted model DIC = 205.33. aWeek 24 data were used as week 16 data was not available. *The 95% CrI does not include 1; bimekizumab 160 mg Q4W is considered either better or worse depending on the direction of the effect. ABA: abatacept; ADA: adalimumab; APR: apremilast; b/tsDMARD-naïve: biologic and targeted synthetic DMARD-naïve; BKZ: bimekizumab; CrI: credible interval; CZP: certolizumab pegol; DIC: deviance information criterion; ETA: etanercept; FE: fixed effects; GOL: golimumab; GUS: guselkumab; IFX: infliximab; IV: intravenous; IXE: ixekizumab; NMA: network meta-analysis; PBO: placebo; Q4W: every 4 weeks; Q8W: every 8 weeks; RE: random effects; RIS: risankizumab; SEC: secukinumab; SUCRA: surface under the cumulative ranking curve; TNFi-experienced: TNF inhibitor–experienced; TOF: tofacitinib; UPA: upadacitinib; UST: ustekinumab; w/o LD: without loading dose
Figure 4.
PASI100. The results for the NMA on PASI100 at week 16: (A) b/tsDMARD-naïve patients including forest plot and SUCRA values. FE baseline–adjusted model DIC = 150.27. (B) TNFi-experienced patients including forest plot and SUCRA values. RE-unadjusted model DIC = 81.76. aWeek 24 data were used as week 16 data was not available. *The 95% CrI does not include 1; bimekizumab 160 mg 4W is considered better. ADA: adalimumab; b/tsDMARD-naïve: biologic and targeted synthetic DMARD-naïve; BKZ, bimekizumab; CrI, credible interval; CZP, certolizumab pegol; DIC, deviance information criterion; FE, fixed effects; GOL, golimumab; GUS, guselkumab; IXE, ixekizumab; NMA, network meta-analysis; PASI, Psoriasis Area and Severity Index; PBO, placebo; Q4W, every 4 weeks; Q8W, every 8 weeks; RE, random effects; SEC, secukinumab; SUCRA, surface under the cumulative ranking curve; TNFi-experienced, TNF inhibitor–experienced; UPA, upadacitinib
Joint outcomes
For ACR50 outcomes, the best-fit models for b/tsDMARD-naïve and TNFi-experienced were the FE baseline–adjusted model and RE-unadjusted model, respectively.
b/tsDMARD-naïve patients
Bimekizumab 160 mg Q4W ranked 6th for ACR20 (SUCRA = 0.75), 5th for ACR50 (SUCRA = 0.74) (Fig. 3A) and 3rd for ACR70 (SUCRA = 0.80) among 21 treatments. For ACR50, bimekizumab 160 mg Q4W was better than placebo, abatacept 125 mg, guselkumab 100 mg Q4W, ustekinumab 45 mg, risankizumab 150 mg, guselkumab 100 mg Q8W and ustekinumab 90 mg; worse than golimumab 2 mg i.v.; and comparable to the remaining treatments in the network (Fig. 3A).
TNFi-experienced patients
Bimekizumab 160 mg Q4W ranked 1st among 16 treatments for ACR20 (SUCRA = 0.96), 2nd among 15 treatments for ACR50 (SUCRA = 0.84) (Fig. 3B) and 1st among 16 treatments for ACR70 (SUCRA = 0.83). Bimekizumab 160 mg Q4W was better than placebo, abatacept 125 mg, secukinumab 150 mg without loading dose, tofacitinib 5 mg and secukinumab 150 mg; and comparable to the remaining treatments in the network on ACR50 (Fig. 3B).
Skin outcomes
For PASI100 outcomes, the best-fit models for b/tsDMARD-naïve and TNFi-experienced were the FE baseline–adjusted model and RE-unadjusted model, respectively.
b/tsDMARD-naïve patients
Bimekizumab 160 mg Q4W ranked 2nd among 15 treatments (SUCRA = 0.89) for PASI90 and 1st among 11 treatments (SUCRA = 0.95) for PASI100 (Fig. 4A). Bimekizumab 160 mg Q4W was better than placebo, certolizumab pegol pooled, golimumab 2 mg i.v., secukinumab 150 mg, adalimumab 40 mg, upadacitinib 15 mg, secukinumab 300 mg and ixekizumab 80 mg Q4W; and comparable to the remaining treatments in the network on PASI100 (Fig. 4A).
TNFi-experienced patients
Bimekizumab 160 mg Q4W ranked 1st among 10 treatments (SUCRA = 0.85) for PASI90 and 2nd among 7 treatments (SUCRA = 0.79) for PASI100 (Fig. 4B). Bimekizumab 160 mg Q4W was better than placebo, ixekizumab 80 mg Q4W and upadacitinib 15 mg; and comparable to the remaining treatments in the network on PASI100 (Fig. 4B).
MDA
For MDA, the best-fit models for b/tsDMARD-naïve and TNFi-experienced were the FE baseline–adjusted model and RE-unadjusted model, respectively.
b/tsDMARD-naïve patients
Bimekizumab 160 mg Q4W ranked 1st among 13 treatments (SUCRA = 0.91) and was better than placebo [OR (95% CrI) 6.31 (4.61–8.20)], guselkumab 100 mg Q4W [2.06 (1.29–3.10)], guselkumab 100 mg Q8W [1.76 (1.09–2.69)], risankizumab 150 mg [1.99 (1.40–2.76)] and adalimumab 40 mg [1.41 (1.01–1.93)]; and comparable to the remaining treatments in the network (Supplementary Fig. S2G, available at Rheumatology online).
TNFi-experienced patients
Bimekizumab 160 mg Q4W ranked 1st among 11 treatments (SUCRA = 0.83) and was better than placebo [12.10 (5.31–28.19)] and tofacitinib 5 mg [6.81 (2.14–21.35)]; and comparable to the remaining treatments in the network (Supplementary Fig. S2H, available at Rheumatology online).
Safety
The network for SAEs for a mixed population included 23 treatments and the best-fit model was an RE-unadjusted model (due to study populations and time point reporting heterogeneity). Bimekizumab 160 mg Q4W showed comparable safety to all treatments in the network (Supplementary Fig. S2I, available at Rheumatology online).
Discussion
The treatment landscape for PsA is complex, with numerous treatment options and limited direct comparative evidence. Bimekizumab 160 mg Q4W has recently been approved for the treatment of active PsA by the European Medicines Agency and recommended by NICE in the UK, and the published phase 3 results warrant comparison with existing therapies by NMA.
This NMA included 41 studies evaluating 22 b/tsDMARDs including the novel IL-17F and IL-17A inhibitor, bimekizumab. Overall, bimekizumab 160 mg Q4W ranked favourably among b/tsDMARDS for efficacy in joint, skin and disease activity outcomes in PsA across both b/tsDMARD-naïve and TNFi-experienced populations. The safety of bimekizumab 160 mg Q4W was similar to the other b/tsDMARDs.
The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and EULAR provide evidence-based recommendations for the treatment of PsA [1, 2]. To treat peripheral arthritis symptoms in PsA, efficacy across the classes of current b/tsDMARDs are considered similar by both GRAPPA and EULAR, in part due to a lack of data comparing licensed therapies in a head-to-head trial setting [1, 2]. EULAR recommends the use of JAK inhibitors in the case of inadequate response, intolerance or when a bDMARD is not appropriate [1]. This recommendation was made when tofacitinib was the only available JAK inhibitor, but reflects current marketing authorizations for tofacitinib and upadacitinib which indicate use in patients with an inadequate response or prior intolerance to TNFis (USA) or bDMARDs (Europe) [37–40]. This NMA suggests that bimekizumab 160 mg Q4W may have an advantage over current treatments, including IL-23 inhibitors in b/tsDMARD naïve patients, and secukinumab 150 mg and tofacitinib in TNFi-experienced patients, as evidenced by our analysis of ACR50 for which the pairwise comparisons were significantly in favour of bimekizumab 160 mg Q4W.
For the treatment of skin symptoms in PsA, IL-23, IL-12/23 and IL-17A inhibitors are currently recommended due to their greater efficacy compared with TNFis [1, 4]. GRAPPA also suggests considering efficacy demonstrated in direct comparative studies in PSO when selecting a treatment for PsA skin symptoms [2]. In our analysis of complete skin clearance as measured by PASI100, bimekizumab 160 mg Q4W demonstrated the likelihood of significantly greater efficacy than IL-17A, JAK inhibitors and TNFis in b/tsDMARD-naïve patients and IL-17A and JAK inhibitors in TNFi-experienced patients. Furthermore, the NMA results for skin clearance in PsA are in alignment with previous studies in PSO that demonstrated superiority of bimekizumab 320 mg Q4W or Q8W vs secukinumab, ustekinumab and adalimumab (P < 0.001) (note that the dosing of bimekizumab in PSO differs from that in PsA) [12, 18, 41, 42].
There are similarities between our results and other recently published NMAs of b/tsDMARDs in PsA, although methodological heterogeneity across all NMAs makes comparisons challenging [34–36, 43–45]. Among recent NMAs, the largest evaluated 21 treatments [34] and only four considered subgroups of b/tsDMARD-naïve and TNFi-experienced patients or those with inadequate response [35, 36, 43, 45]. Furthermore, different or pooled levels of response were evaluated for ACR and PASI outcomes.
Previous NMAs also support IL-17, IL-12/23 and IL-23 inhibitors having greater efficacy for skin symptoms than TNFis [35, 36]. In an overall PsA population, McInnes et al. demonstrated that secukinumab 300 mg, ixekizumab 80 mg Q4W, and ustekinumab 45 mg and 90 mg were likely more efficacious than TNFis for PASI90 [35]. In another NMA by Ruyssen-Witrand et al., results suggested that ixekizumab 80 mg Q4W had significantly greater efficacy than adalimumab, certolizumab pegol pooled, and etanercept 25 mg twice weekly/50 mg once weekly for any PASI score (50%, 75%, 90% and 100% reduction) in bDMARD-naïve patients [36].
For joint outcomes, Mease et al. compared guselkumab Q4W and Q8W with other b/tsDMARDs in a network of 21 treatments in an overall PsA population for ACR50 [34]. Both guselkumab dosing schedules were better than abatacept and apremilast, but golimumab 2 mg i.v. had a higher likelihood of ACR50 response than guselkumab Q8W [34]. Despite MDA being assessed in clinical trials for bDMARD therapies and a treatment target in PsA [46], evidence for comparative efficacy for this outcome is limited. None of the most recent NMAs before this one included an analysis of MDA [34–36]. With regard to safety outcomes, previous NMAs evaluating SAEs also resulted in either no difference between b/tsDMARDs vs placebo or other b/tsDMARDs [34, 36, 44, 45].
This study has a number of strengths. To our knowledge this NMA represents the most comprehensive and in-depth comparative efficacy analysis of approved treatments in PsA to date. The evidence was derived from a recent SLR, ensuring that new RCTs and updated results from previously published RCTs were included. It is also the first NMA to include the phase 3 BE COMPLETE and BE OPTIMAL trials of bimekizumab [19, 20]. Our NMA used robust methods and accounted for variation in placebo response through network meta-regression in accordance with NICE DSU Technical Support Documents [31–33]. As an acknowledgement of the evolution of treatment advances, separate analyses of b/tsDMARD-naïve and TNFi-experienced subgroups were conducted with the intent to assist healthcare decision-making in different clinical settings. In addition, a panel of clinical experts were consulted from project inception and are authors of this paper, ensuring inclusion of a comprehensive set of clinically meaningful outcomes, including the composite, treat-to-target outcome of MDA.
Despite the robust evidence base and methodology, this NMA has limitations. Indirect treatment comparisons such as this NMA are not a substitute for head-to-head trials. There was heterogeneity in the endpoints and reporting in the included studies. Fewer studies reporting PASI outcomes resulted in smaller networks compared with the network of studies evaluating ACR response criteria. Not all trials reported outcomes at the same timepoint, thereby reducing the comparability of trial results, which has been transparently addressed by noting where week 24 data were used vs week 12, 14 or 16 data. The analyses for the TNFi-experienced population were limited by potential heterogeneity, especially in the analyses where fewer studies were included in the networks, as this group could include patients who had an inadequate response to TNFi or discontinued TNFi treatment due to other reasons (e.g. lost access). Also, in the analyses for the TNFi-experienced population, very low patient numbers for some treatments resulted in less statistical power. Additionally, the data included in the analysis were derived exclusively from RCTs, for which the study populations may not reflect a typical patient population seen in real-world practice. For example, trial results may be different in patients with oligoarthritis who are not well-represented in clinical trials.
Over the years covering our SLR, we acknowledge that patient populations and the PsA treatment landscape have evolved. After a thorough review of baseline patient characteristics, no significant differences were observed across the studies included in the NMA. To further mitigate uncertainty, baseline regression was used to actively correct for changes in the placebo rate over time ensuring a consistent and fair comparison across all included treatments. In addition, our analyses were conducted in separate b/tsDMARD-naïve and TNFi-experienced populations that reflect the evolving PsA patient population over time. Radiographic progression was not within the purview of this NMA because the NMA focused on a shorter timeframe than the 52-week duration typically recommended by the literature for investigating radiographic progression. Furthermore, there is existing literature on this topic, as exemplified by the work of Wang et al. in 2022 [47]. Nevertheless, the comprehensive and current evidence base, examination of multiple endpoints, and consistency with previous reported NMAs lend credence to our results.
Conclusion
Overall, the results of this NMA demonstrated the favourable relative efficacy and safety of bimekizumab 160 mg Q4W vs all approved treatments for PsA. Bimekizumab ranked high in terms of efficacy on joint, skin and MDA outcomes in both b/tsDMARD-naïve and TNFi-experienced patient populations, and showed comparable safety to other treatments. In the evolving PsA treatment landscape, bimekizumab 160 mg Q4W is a potentially beneficial treatment option for patients with PsA.
Supplementary material
Supplementary material is available at Rheumatology online.
Supplementary Material
Acknowledgements
The authors acknowledge Leah Wiltshire of Cytel for medical writing and editorial assistance based on the authors’ input and direction, Heather Edens (UCB Pharma, Smyrna, GA, USA) for publication coordination and Costello Medical for review management, which were funded by UCB Pharma. This analysis was funded by UCB Pharma in accordance with Good Publication Practice (GPP 2022) guidelines (http://www.ismpp.org/gpp-2022). Data were previously presented at ISPOR-US 2023 (Boston, MA, USA, 7–10 May 2023).
Contributor Information
Philip J Mease, Swedish Medical Center and Providence St. Joseph Health, University of Washington, Seattle, WA, USA.
Dafna D Gladman, Schroeder Arthritis Institute, Krembil Research Institute, Toronto Western Hospital, University Health Network, University of Toronto, ON, Canada.
Joseph F Merola, Department of Dermatology, Harvard Medical School, Brigham and Women’s Hospital, Boston, MA, USA; Division of Rheumatology, Department of Medicine, Harvard Medical School, Brigham and Women’s Hospital, Boston, MA, USA.
Peter Nash, School of Medicine, Griffith University, Brisbane, QLD, Australia.
Stacy Grieve, Department of RWA Health Economics, Cytel, Inc, Waltham, MA, USA.
Victor Laliman-Khara, Department of RWA Health Economics, Cytel, Inc, Waltham, MA, USA.
Damon Willems, UCB Pharma, Brussels, Belgium.
Vanessa Taieb, UCB Pharma, Colombes, France.
Adam R Prickett, UCB Pharma, Slough, UK.
Laura C Coates, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Science, University of Oxford and Oxford Biomedical Research Centre, Oxford University Hospitals NHS Trust, Oxford, UK.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
This study was funded in full by UCB Pharma.
Disclosure statement: P.J.M.: has received research grants from AbbVie, Amgen, BMS, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Sun Pharma and UCB Pharma; consultancy fees from AbbVie, Acelyrin, Aclaris, Amgen, BMS, Boehringer Ingelheim, Eli Lilly, Galapagos, Gilead, GSK, Janssen, Moonlake Pharma, Novartis, Pfizer, Sun Pharma and UCB Pharma; and speakers’ bureau for AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer and UCB Pharma. L.C.C.: received grants/research support from AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and UCB; worked as a paid consultant for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Gilead, Galapagos, Janssen, Moonlake, Novartis, Pfizer and UCB; and has been paid as a speaker for AbbVie, Amgen, Biogen, Celgene, Eli Lilly, Galapagos, Gilead, GSK, Janssen, Medac, Novartis, Pfizer and UCB. D.D.G.: consultant and/or received grant support from Abbvie, Amgen, BMS, Celgene, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer and UCB. J.F.M.: consultant and/or investigator for AbbVie, Amgen, Biogen, BMS, Dermavant, Eli Lilly, Janssen, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma and UCB Pharma. P.N.: research grants, clinical trials and honoraria for advice and lectures on behalf of AbbVie, Boehringer Ingelheim, BMS, Eli Lilly, Galapagos/Gilead, GSK, Janssen, Novartis, Pfizer, Samsung, Sanofi and UCB Pharma. S.G. and V.L.-K.: employees of Cytel, Inc. which served as a consultant on the project. A.R.P., D.W. and V.T.: employees and stockholders of UCB Pharma.
References
- 1. Gossec L, Baraliakos X, Kerschbaumer A. et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coates LC, Soriano ER, Corp N. et al. ; GRAPPA Treatment Recommendations domain subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol 2022;18:465–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fitzgerald O, Ogdie A, Chandran V. et al. Psoriatic arthritis. Nat Rev Dis Primers 2021;7:59. [DOI] [PubMed] [Google Scholar]
- 4. Coates LC, Soriano ER, Corp N. et al. Treatment recommendations domain subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol 2022;27:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Najm A, Goodyear CS, McInnes IB, Siebert S.. Phenotypic heterogeneity in psoriatic arthritis: towards tissue pathology-based therapy. Nat Rev Rheumatol 2023;19:153–65. [DOI] [PubMed] [Google Scholar]
- 6. Ogdie A, Weiss P.. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am 2015;41:545–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alinaghi F, Calov M, Kristensen LE. et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol 2019;80:251–65.e19. [DOI] [PubMed] [Google Scholar]
- 8. Singh JA, Guyatt G, Ogdie A. et al. Special Article: 2018 American college of rheumatology/national psoriasis foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol 2019;71:5–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coates LC, Robinson DE, Orbai AM. et al. What influences patients' opinion of remission and low disease activity in psoriatic arthritis? Principal component analysis of an international study. Rheumatology (Oxford) 2021;60:5292–9. [DOI] [PubMed] [Google Scholar]
- 10. Gondo G, Mosca M, Hong J. et al. Demographic and clinical factors associated with patient-reported remission in psoriatic arthritis. Dermatol Ther (Heidelb) 2022;12:1885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glatt S, Baeten D, Baker T. et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis 2018;77:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. UCB Pharma S.A. Bimzelx® (bimekizumab): Summary of Product Characteristics. 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/bimzelx (26 June 2023, date last accessed).
- 13. Adams R, Maroof A, Baker T. et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol 2020;11:1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burns LA, Maroof A, Marshall D. et al. Presence, function, and regulation of IL-17F-expressing human CD4(+) T cells. Eur J Immunol 2020;50:568–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuestner R, Taft D, Haran A. et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol 2007;179:5462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johansen C, Usher PA, Kjellerup RB. et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol 2009;160:319–24. [DOI] [PubMed] [Google Scholar]
- 17. Shah M, Maroof A, Gikas P. et al. Dual neutralisation of IL-17F and IL-17A with bimekizumab blocks inflammation-driven osteogenic differentiation of human periosteal cells. RMD Open 2020;6:e001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reich K, Warren RB, Lebwohl M. et al. Bimekizumab versus Secukinumab in Plaque Psoriasis. New Engl J Med 2021;385:142–52. [DOI] [PubMed] [Google Scholar]
- 19. McInnes IB, Asahina A, Coates LC. et al. Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: a randomised, double-blind, placebo-controlled, phase 3 trial (BE OPTIMAL). Lancet 2023;401:25–37. [DOI] [PubMed] [Google Scholar]
- 20. Merola JF, Landewe R, McInnes IB. et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-alpha inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE). Lancet 2023;401:38–48. [DOI] [PubMed] [Google Scholar]
- 21. Page MJ, McKenzie JE, Bossuyt PM. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JP. Cochrane handbook for systematic reviews of interventions. Vol. 2. 2nd edn. Chichester, UK: John Wiley & Sons, 2019.
- 23. National Institute for Health and Care Excellence. The guidelines manual: Process and methods [PMG6]. 2012. https://www.nice.org.uk/process/pmg6/chapter/introduction (3 March 2023, date last accessed).
- 24. Booth AM, Wright KE, Outhwaite H.. Centre for Reviews and Dissemination databases: value, content, and developments. Int J Technol Assess Health Care 2010;26:470–2. [DOI] [PubMed] [Google Scholar]
- 25. Sterne JAC, Savovic J, Page MJ. et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 26. Daly C, Dias S, Welton N, Anwer S, Ades A. NICE Guidelines Technical Support Unit. Meta-Analysis: Guideline Methodology Document 1 (Version 1). 2021.http://www.bristol.ac.uk/population-health-sciences/centres/cresyda/mpes/nice/guideline-methodology-documents-gmds/ (1 March 2023, date last accessed).
- 27. Dias S, Caldwell DM.. Network meta-analysis explained. Archi Dis Childhood Fetal Neonatal Ed 2019;104:F8–F12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Merola JF, Lockshin B, Mody EA.. Switching biologics in the treatment of psoriatic arthritis. Semin Arthritis Rheum 2017;47:29–37. [DOI] [PubMed] [Google Scholar]
- 29.Openbugs (website) 2014. http://www.openbugs.net/w/FrontPage (6 April 2023, date last accessed).
- 30. Lunn D, Spiegelhalter D, Thomas A, Best N.. The BUGS project: evolution, critique and future directions. Stat Med 2009;28:3049–67. [DOI] [PubMed] [Google Scholar]
- 31. Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. London. 2016. https://www.sheffield.ac.uk/nice-dsu/tsds/full-list (25 January 2023, date last accessed). [PubMed]
- 32. Dias S, Welton N, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU technical support document 4: inconsistency in networks of evidence based on randomised controlled trials. 2011. https://www.sheffield.ac.uk/nice-dsu/tsds/full-list (25 January 2023, date last accessed). [PubMed]
- 33. Dias S, Sutton AJ, Welton N, Ades AE. NICE DSU Technical support document 3: heterogeneity: subgroups, meta-regression, bias and bias-adjustment. 2011. https://www.sheffield.ac.uk/nice-dsu/tsds/full-list (25 January 2023, date last accessed).
- 34. Mease PJ, McInnes IB, Tam LS. et al. Comparative effectiveness of guselkumab in psoriatic arthritis: results from systematic literature review and network meta-analysis. Rheumatology (Oxford) 2021;60:2109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McInnes IB, Sawyer LM, Markus K. et al. Targeted systemic therapies for psoriatic arthritis: a systematic review and comparative synthesis of short-term articular, dermatological, enthesitis and dactylitis outcomes. RMD Open 2022;8:e002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ruyssen-Witrand A, Perry R, Watkins C. et al. Efficacy and safety of biologics in psoriatic arthritis: a systematic literature review and network meta-analysis. RMD Open 2020;6:e001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pfizer Inc. XELJANZ® (tofacitinib): Summary of Product Characteristics. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/xeljanz (4 May 2023, date last accessed).
- 38. Pfizer Inc. XELJANZ® (tofacitinib): US Prescribing Information. 2022. https://labeling.pfizer.com/ShowLabeling.aspx?id=959 (4 May 2023, date last accessed).
- 39. Abbvie Inc. RINVOQ®(upadacitinib) extended-release tablets, for oral use: US Prescribing Information. 2023. https://www.rxabbvie.com/pdf/rinvoq_pi.pdf (4 May 2023, date last accessed).
- 40. AbbVie Deutschland GmbH & Co. KG. RINVOQ®(upadacitinib): Summary of Product Characteristics. 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq (4 May 2023, date last accessed).
- 41. Warren RB, Blauvelt A, Bagel J. et al. Bimekizumab versus Adalimumab in Plaque Psoriasis. N Engl J Med 2021;385:130–41. [DOI] [PubMed] [Google Scholar]
- 42. Reich K, Papp KA, Blauvelt A. et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet 2021;397:487–98. [DOI] [PubMed] [Google Scholar]
- 43. Gladman DD, Orbai AM, Gomez-Reino J. et al. Network meta-analysis of tofacitinib, biologic disease-modifying antirheumatic drugs, and apremilast for the treatment of psoriatic arthritis. Curr Ther Res Clin Exp 2020;93:100601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qiu M, Xu Z, Gao W. et al. Fourteen small molecule and biological agents for psoriatic arthritis: a network meta-analysis of randomized controlled trials. Medicine (Baltimore) 2020;99:e21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kawalec P, Holko P, Mocko P, Pilc A.. Comparative effectiveness of abatacept, apremilast, secukinumab and ustekinumab treatment of psoriatic arthritis: a systematic review and network meta-analysis. Rheumatol Int 2018;38:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gossec L, McGonagle D, Korotaeva T. et al. Minimal disease activity as a treatment target in psoriatic arthritis: a review of the literature. J Rheumatol 2018;45:6–13. [DOI] [PubMed] [Google Scholar]
- 47. Wang SH, Yu CL, Wang TY, Yang CH, Chi CC.. Biologic disease-modifying antirheumatic drugs for preventing radiographic progression in psoriatic arthritis: a systematic review and network meta-analysis. Pharmaceutics 2022;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.