Acute upper gastrointestinal haemorrhage accounts for about 2500 hospital admissions each year in the United Kingdom. The annual incidence varies from 47 to 116 per 100 000 of the population and is higher in socioeconomically deprived areas.
Although hospital mortality has not improved over 50 years and remains at about 10%, older patients who have advanced cardiovascular, respiratory, or cerebrovascular disease that puts them at increased risk of death now comprise a much higher proportion of cases. Many patients' bleeding is associated with use of non-steroidal anti-inflammatory drugs, but there is no evidence that prognosis is worse in patients who are taking these drugs than in those who are not.
Presentation of bleeding
All patients who develop acute gastrointestinal bleeding need urgent assessment. Almost all should be admitted as an emergency to hospital. Only a small minority of young, fit patients who have self limiting bleeding can be managed as outpatients, but even those need urgent investigation. Patients who present with haematemesis tend to have more severe bleeding than those who present with melaena alone.
Risk factors for death after hospital admission for acute upper gastrointestinal haemorrhage
Advanced age
Shock on admission (pulse rate >100 beats/min; systolic blood pressure <100 mm Hg)
Comorbidity (particularly hepatic or renal failure and disseminated cancer)
Diagnosis (worst prognosis for advanced upper gastrointestinal malignancy)
Endoscopic findings (active, spurting haemorrhage from peptic ulcer; non-bleeding, visible blood vessel; large varices with red spots)
Rebleeding (increases mortality 10-fold)
At the initial assessment it is important to define factors that have prognostic importance. The main factors predicting death include increasing age, comorbidity, and endoscopic findings. Mortality is extremely low in patients under 40 years old but thereafter increases steeply with advancing age. Patients who have severe comorbidity—particularly renal insufficiency, hepatic failure, or disseminated malignancy—have a poor prognosis. Hospital admission may be precipitated by gastrointestinal bleeding in many of these patients, and death is often due to disease progression rather than to bleeding.
A risk assessment score has been developed based on the outcome of 4185 patients with acute gastrointestinal bleeding admitted to hospitals in England.1 A series of independent risk factors were scored, and the total score accurately predicts outcome. Patients who score 2 or less have a mortality of 0.1% and a rebleeding rate of 4.3%, but a score in excess of 8 is associated with a 41% mortality and rebleeding rate of 42.1%.
Patients who develop acute upper gastrointestinal haemorrhage after hospitalisation for other serious illness have a much worse prognosis than those who are admitted because of bleeding, with a mortality of about 30%. Endoscopic findings of active, spurting haemorrhage; a non-bleeding blood vessel visible within an ulcer; and red spots on large varices are associated with risk of further bleeding. The absence of these endoscopic stigmata indicates little chance of rebleeding and early discharge from hospital.
Causes of bleeding
The commonest cause of upper gastrointestinal haemorrhage is peptic ulcer. A history of proved ulcer or ulcer-like dyspepsia is absent in about 20% of cases. In these patients consumption of aspirin or non-steroidal anti-inflammatory drugs is common. Infection with Helicobacter pylori is less prevalent in bleeding ulcers than in uncomplicated ulcers. Severe ulcer bleeding is due to erosion of the artery by the ulcer, and the severity of bleeding depends on the size of the ulcer and the size of the arterial defect. Bleeding from a defect greater than 1 mm in diameter is unlikely to stop spontaneously and does not respond to endoscopic treatment. Large ulcers arising from the posterior part of the duodenal cap can erode the gastroduodenal artery and provoke brisk bleeding.
Bleeding from gastric erosions, oesophagitis, or vascular malformations usually stops spontaneously and is not usually life threatening. Mallory-Weiss tears are a consequence of retching, and most patients have a history of alcohol misuse, have features of other gastrointestinal disease such as peptic ulcer or gastroenteritis, or have non-gastrointestinal causes of vomiting. Bleeding usually stops spontaneously, although endoscopic haemostatic treatment is sometimes required.
Bleeding from upper gastrointestinal malignancy is not usually severe, and the prognosis is dictated by the stage of the disease. Patients with extensive upper gastrointestinal cancer have a dismal prognosis, but death is not usually a consequence of gastrointestinal haemorrhage but of disease progression.
Alert for features of liver disease
Ascites • Splenomegaly
Jaundice • Fluid retention
Alcohol misuse
In any patient with acute gastrointestinal bleeding liver disease should be considered because it requires specific management. Oesophageal varices account for a small proportion of cases but have a disproportionate impact on medical resources. Bleeding is often severe, and other features of liver failure—such as fluid retention, hepatic encephalopathy, renal failure, and sepsis—often develop after the bleed. About a third of patients will die, and prognosis is related to the severity of the underlying liver disease rather than the size of variceal haemorrhage.
Blood tests on admission to hospital
Haemoglobin concentration—May be normal during the acute stages until haemodilution occurs
Urea and electrolyte concentrations—Elevated blood urea suggests severe bleeding
Cross match for transfusion—Two units of blood are sufficient unless bleeding is extreme. If transfusion not needed urgently group the blood and save the serum
Liver function tests
Prothrombin time
Aortoduodenal fistula must be considered in patients who develop profuse bleeding and have undergone aortic aneurysm surgery.
Management of bleeding
Primary care management
Initial resuscitation of shocked patients can be started before hospital admission. Intravenous access should be obtained, and infusion of a crystalloid started. Oxygen should be given. Management in primary care is limited, and the priority is to arrange early admission to hospital and to support associated comorbidity, such as that of angina or chest disease.
Hospital management
Resuscitation
The first priority is to support the circulation rather than to identify the source of bleeding. Endoscopy is undertaken once resuscitation has been achieved. At least one large bore cannula is inserted into a substantial vein. When the pulse rate is more than 100 beats/min or the systolic blood pressure falls below 100 mm Hg, infusion with a crystalloid such as normal saline is started. The rate of infusion depends on the severity of shock. A recent meta-analysis showed that crystalloids (such as normal saline) should be used rather than colloids (such as dextrans). If blood transfusion is required the aim is to maintain a haemoglobin concentration of 100 g/l. In patients with suspected liver disease the use of normal saline should be avoided because of the risk of precipitating ascites.
Endoscopy
After resuscitation, endoscopy is undertaken. In most cases this is done electively on the next available routine list but within 24 hours of admission. Only a minority of profusely bleeding patients need “out of hours” emergency endoscopy.
On-call endoscopists must be experienced and be able to apply a range of endoscopic treatments. Endoscopy is necessary to define the cause of bleeding, provide prognostic information, and to apply haemostatic treatment.
Endoscopic treatment for non-variceal bleeding
Thermal
Heater probe • Multipolar electrocoagulation
Injection
Adrenaline (1:10000 to • Sclerosants (ethanolamine,1:100000) 1% polidoconal)
Alcohol (98%) • Procoagulants (thrombin, fibrin glue)
Mechanical
Clips • Sutures • Staples
Diagnosis—It is difficult to prove that diagnostic endoscopy improves outcome, but it is clearly important to define a precise diagnosis in order to plan treatment.
Prognosis—Endoscopic stigmata are extremely useful in defining risk of further bleeding.
Treatment—A range of endoscopic treatments can be administered to patients showing major endoscopic stigmata. Pharmacological, endoscopic, radiological, and surgical treatments are used.
Treatments
Drugs
Non-variceal haemorrhage—There is increasing evidence to support the use of intravenous omeprazole, which in clinical trials reduces the risk of rebleeding and the need for surgical operation. Patients infected with H pylori should undergo eradication treatment after haemostasis has been achieved in order to prevent further ulcer complications. One study has shown that tranexamic acid reduces transfusion requirements in patients presenting with non-variceal haemorrhage.
Variceal haemorrhage—Vasoactive drugs (such as terlipressin) reduce bleeding rates but have little impact on survival. If bleeding continues despite this treatment, a modified Sengstaken-Blakemore (Minnesota) tube is inserted. It must be remembered that both vasoactive drugs and the Minnesota tube are temporising measures used to control active bleeding until definitive endoscopic, surgical, or radiological measures are taken. When varices have been obliterated portal pressure is reduced with propanolol at a dose to decrease the pulse rate by 20%. This diminishes the risk of subsequent rebleeding.
Endoscopic treatment
Non-variceal bleeding—A range of endoscopic haemostatic approaches are available. Each has a similar efficacy, but there is evidence that an injection combined with a thermal method is best. Endoscopic treatment fails in about 20% of patients with bleeding ulcers, most often those with large, actively bleeding posterior duodenal ulcers. Endoscopist and surgeon must work together to identify and treat these patients at an early stage.
Varices—When active variceal bleeding is seen at endoscopy, intravariceal injection of a sclerosant (such as 5% ethanolamine, 1% polidoconal, or sodium tetradecyl sulphate) is attempted. An alternative approach is oesophageal band ligation. Banding obliterates varices more efficiently and has few complications, but it may be more difficult to perform in a patient with active bleeding.
Indications for surgical operation for peptic ulcer bleeding
Active bleeding unresponsive to endoscopic haemostasisProfuse bleeding preventing endoscopic visualisation and treatmentBleeding continues despite application of endoscopic treatment
Endoscopically proved rebleeding despite technically successful endoscopic treatmentPatients at low risk of death, after two unsuccessful attempts at endoscopic treatmentHigh risk patients, after one failure of endoscopic haemostasis
Surgery
Surgery is the best way of stopping active ulcer bleeding and preventing rebleeding, but it carries high morbidity and mortality. It is now reserved for cases in which endoscopic treatment has failed. Specific protocols defining indications for a surgical operation are necessary.
Surgical treatment for acute variceal bleeding, including oesophageal transection with devascularisation and porto-caval shunt, is rarely done because of unacceptable mortality. It has been replaced by TIPPS (transjugular intrahepatic portosystemic shunt). However, both interventions may precipitate encephalopathy.
Figure.



Endoscopic stigmata associated with high risk of further gastrointestinal bleeding. Top left: an active, spurting haemorrhage from a peptic ulcer is associated with an 80% risk of continuing bleeding or rebleeding in shocked patients. Top right: a non-bleeding, visible vessel represents either a pseudoaneurysm of an eroded artery or a closely adherent clot, and 50% of such patients rebleed in hospital. Left: large varices with red spots are also strongly associated with bleeding
Figure.
Causes of acute upper gastrointestinal haemorrhage
Figure.
Gross ascites and distended abdominal veins in advanced cirrhosis
Figure.
Algorithm for diagnosis and management of upper gastrointestinal bleeding (SRH=stigmata of recent haemorrhage, TIPPS=transjugular intrahepatic portosystemic shunt)
Figure.
Minnesota tube
Figure.

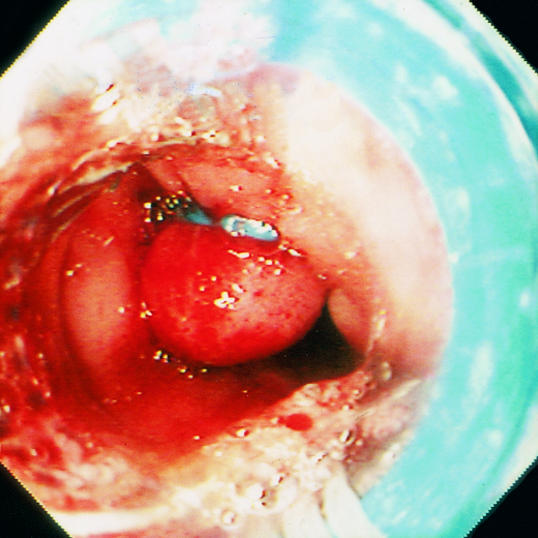
Endoscopic treatment of varices. Intravariceal injection of sclerosant (left) and band ligation of oesophageal varices (right)
Acknowledgments
1 Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut 1996;38:316-21
Footnotes
Helen J Dallal is specialist registrar at Royal Aberdeen Infirmary. K R Palmer is consultant gastroenterologist at Western General Hospital, Edinburgh.
The ABC of the upper gastrointestinal tract is edited by Robert Logan, senior lecturer in the division of gastroenterology, University Hospital, Nottingham; Adam Harris, consultant physician and gastroenterologist, Kent and Sussex Hospital, Tunbridge Wells; J J Misiewicz, honorary consultant physician and joint director of the department of gastroenterology and nutrition, Central Middlesex Hospital, London; and J H Baron, honorary professorial lecturer at Mount Sinai School of Medicine, New York, USA, and former consultant gastroenterologist, St Mary's Hospital, London.






