Summary
Forward genetic screens have been a powerful tool for discovering genes involved in various biological processes in Caenorhabditis elegans. Here, we present a protocol for forward genetic screening to identify novel factors involved in a biological process in C.elegans. We describe steps for mutagenesis, screening, and backcrossing. To save time and effort, we also detail procedures for utilizing whole-genome sequencing to exclude mutants of previously characterized genes from crosses for mapping mutations.
For complete details on the use and execution of this protocol, please refer to Yoshida et al.1
Subject areas: sequence analysis, genetics, model organisms
Graphical abstract

Highlights
-
•
Mutagenize worms using EMS to isolate mutants with phenotype of interest
-
•
Selection of weak mutants can help to identify genes with functional redundancy
-
•
Whole-genome sequencing is used to exclude mutants of previously characterized genes
-
•
Map causal mutations by detecting EMS-induced variants after backcrossing
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Forward genetic screens have been a powerful tool for discovering genes involved in various biological processes in Caenorhabditis elegans. Here, we present a protocol for forward genetic screening to identify novel factors involved in a biological process in C.elegans. We describe steps for mutagenesis, screening, and backcrossing. To save time and effort, we also detail procedures for utilizing whole-genome sequencing to exclude mutants of previously characterized genes from crosses for mapping mutations.
Before you begin
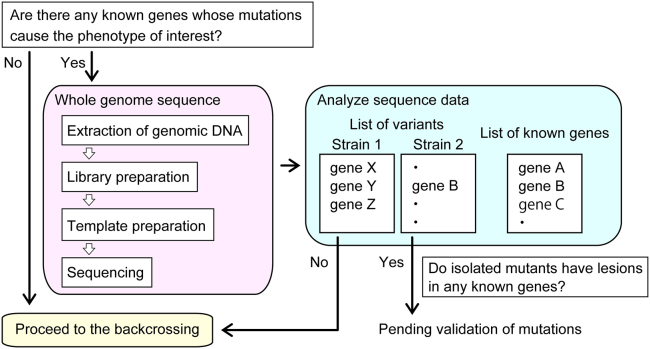
Identifying novel factors, particularly those with redundant roles in a biological process, through forward genetic screening can be challenging. This is because a single mutation in such genes often results in a weak phenotype that is difficult to detect. An increased number of animals may be necessary for screening to identify rare, weak mutants. However, this may also lead to the identification of mutations in previously characterized genes, whose mutants exhibit a relatively stronger phenotype. Here, we describe a strategy to identify previously uncharacterized genes by forward screen in a time and labor efficient manner (Figure 1). In our previous screen to isolate mutants defective in the feeding RNAi, we successfully identified two novel factors that act in parallel to secrete double-stranded RNA during systemic RNAi.1 This strategy can be applied to any type of screening.
Figure 1.
Overview of a strategy for forward genetic screening to identify novel factors involved in a biological process of interest
Preparation for screening
Timing: variable
-
1.
Design a screen to identify mutant worms defective in the biological process of interest. One way to identify novel genes involved in the biological process is to screen for mutant animals with a weak phenotype. Mutant animals with a strong phenotype that are easy to find may have lesions in genes previously identified in preceding screens.
-
2.
List the genes whose mutants are known to show the phenotype that will be the subject of the screen if there are any relevant precedent works. This list will be used at Step 12.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| E. coli OP50-1 | Chalfie Lab | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Ethyl methanesulfonate (EMS) | Sigma | M0880 |
| Sodium chloride | Wako | 191-01665 |
| Monopotassium phosphate | Wako | 169-04245 |
| Disodium phosphate | Wako | 197-02865 |
| Magnesium sulfate | Wako | 131-00405 |
| Gelatin | MP | 232-554-6 |
| Potassium chloride | Wako | 163-0354 |
| 1 M Tris-HCl (pH 8.5) | Nippon Gene | 316-90405 |
| EDTA | Dojindo | N001 |
| Tween 20 | Sigma | P1379 |
| Proteinase K | Wako | 165-21043 |
| Critical commercial assays | ||
| DNeasy Blood & Tissue Kit | QIAGEN | 69504 |
| Deposited data | ||
| Whole-genome sequence data (tm9739_x5) | Yoshida et al.1 | NCBI Trace Archive: SRR24389894 |
| Whole-genome sequence data (tm9742_x5) | Yoshida et al.1 | NCBI Trace Archive: SRR24389893 |
| Whole-genome sequence data (tm9743_x5) | Yoshida et al.1 | NCBI Trace Archive: SRR24389892 |
| Experimental models: Organisms/strains | ||
| C. elegans wild type | Caenorhabditis Genetics Center | N2 |
| Software and algorithms | ||
| DeepVariant | Poplin et al.2 | https://github.com/google/deepvariant |
| Sutoku | Suehiro et al.3 | https://github.com/YujiSue/Sutoku |
| SIFT | Ng and Henikoff4 | https://sift.bii.a-star.edu.sg/www/SIFT4G_vcf_submit.html |
| Other | ||
| AB Library Builder System | Thermo Fisher Scientific | 4463783 |
| Ion Chef Instrument | Thermo Fisher Scientific | 4484178 |
| Ion Proton Sequencer | Thermo Fisher Scientific | 4469949 |
Materials and equipment
M9 buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| Na2HPO4 | 42 mM | 6.0 g |
| KH2PO4 | 22 mM | 3.0 g |
| NaCl | 85 mM | 5.0 g |
| ddH2O | N/A | up to 1 L |
| Total | N/A | 1 L |
| Add 1 mL of 1 M MgSO4 after autoclave | ||
Note on storage conditions: can be stored at room temperature (20°C–25°C) for months.
M9 buffer with gelatin
| Reagent | Final concentration | Amount |
|---|---|---|
| M9 buffer | N/A | 1 L |
| 2% gelatin | 0.02% | 10 mL |
| Total | N/A | 1 L |
Note on storage conditions: can be stored at 4°C for months.
Lysis buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| 2 M KCl | 42 mM | 2.5 mL |
| 1 M Tris-HCl (pH 8.5) | 22 mM | 2.5 mL |
| 0.5 M EDTA | 85 mM | 0.2 mL |
| 10% Tween 20 | N/A | 5 mL |
| ddH2O | N/A | 89.8 mL |
| Total | N/A | 100 mL |
Note on storage conditions: can be stored at room temperature (20°C–25°C) for months.
Lysis buffer with proteinase K
| Reagent | Final concentration | Amount |
|---|---|---|
| Lysis buffer | N/A | 10 mL |
| 50 mg/mL proteinase K | 0.25 mg/mL | 40 μL |
| Total | N/A | 10 mL |
Note on storage conditions: should be freshly prepared before use.
Step-by-step method details
Mutagenesis and screening
Timing: 2 weeks
In this section, we describe how to mutagenize worms according to the Brenner’s protocol.5
-
1.Mutagenize worms.
-
a.Collect synchronized L4 or young-adult hermaphrodites in a sterile 15 mL tube by washing the culture plates with M9 buffer with gelatin (hereafter referred to as M9). Adding gelatin to M9 buffer prevents worms from sticking to the tube wall.
-
b.Wash worms at least twice to remove bacteria.
-
c.Resuspend worms in 4 mL of M9 and add 20 μL of liquid EMS to obtain a final concentration of 50 mM EMS.
-
d.Incubate worms in 50 mM EMS in M9 for 4 h at 20–25°C with constant rotation.
-
e.Wash worms 4x with 15 mL of M9.
-
f.Recover mutagenized worms to NGM plates seeded with E. coli OP50.5
-
a.
CRITICAL: EMS is a strong mutagen. When handling EMS, it is a must to work in a chemical hood and to wear gloves. Waste containing EMS and tubes used to handle EMS must be detoxified by adding 1 M NaOH.
-
2.Screen mutant worms (Figure 2).
-
a.Allow mutagenized adults to lay eggs 16–20 h and then remove them.
-
b.Collect hatched F1 animals by M9, transfer them to new NGM plates, with around 50 animals per plate, and allow them to grow to adulthood.
-
c.Allow F1 adults to lay eggs 16–20 h and then remove them, or bleach gravid F1 animals and plate F2 eggs on NGM plates.
-
d.Screen for the mutants of interest at the appropriate developmental stage.
-
e.Transfer worms that show a phenotype of interest to new plates individually to confirm their phenotype in the next generation.
-
a.
Note: It is recommended to select only one mutant from each F1 plate. Mutants isolated from the same plate may have identical mutations.
Note: Because F2 progeny of mutagenized animals contain a limited number of homozygous mutants, some type of screening, such as screening for mutants with improved survival in a toxic condition (e.g. lethal RNAi1), should be performed in F3 or later generations.
Figure 2.
Overview of mutagenesis and screening
Extraction of genomic DNA for whole-genome sequencing (WGS)
Timing: 1 week
The purpose of the initial sequencing is to exclude mutant strains with lesions in previously characterized genes from crossing for mapping, which is a laborious step in a forward genetic screen (Figure 3).
Note: If there is no known gene involved in the phenotype of interest, proceed to Step 14.
-
3.
Grow isolated mutant strains on 90 mm NGM plates.
-
4.
Collect worms in sterile 1.5 mL tubes by washing freshly starved plate with M9.
Note: Animals should be grown to starvation to minimize the contamination of E. coli.
-
5.
After washing with M9, resuspend worms in lysis buffer with proteinase K and incubate 16–20 h at 50°C.
-
6.
Isolate genomic DNA from worm lysis using a DNeasy Blood & Tissue Kit (QIAGEN) following the manufacturer’s instruction (https://www.qiagen.com/jp/resources/download.aspx?id=aa250d94-fc4b-4e27-bb74-d32391ff8a48&lang=en).
Pause point: Isolated mutant strains can be frozen and stored. We usually make frozen stock of mutants from 200 μL aliquot of worms in M9 before making worm lysis.
Figure 3.
Overview of initial whole-genome sequencing
Prepare DNA library and sequence mutant genome
Timing: 3 days
This section describes the preparation of a DNA library and sequencing using the Ion Proton System (Thermo Fisher Scientific).
CRITICAL: DNA library preparation and sequencing are platform dependent. Please refer to the instructions provided by the sequencing platform that you will be using.
-
7.
Prepare DNA library from genomic DNA with a LibraryBuilder automatic library synthesis machine (Thermo Fisher Scientific).
-
8.
Construct sequencing templates using the DNA library by the ionChef system (Thermo Fisher Scientific).
-
9.
Sequence templates to a target depth of approximately 15–20 using ionProton (Thermo Fisher Scientific).
Analyze initial WGS data
Timing: 2 days
In this section, we describe WGS data analysis using in part our original program, which was previously developed (Figure 4). For details of our original program, please refer to Suehiro et al. 2021.3
Note: For analysis of WGS data, a cloud-based pipeline that requires no software installation is also available.6
-
10.
Search small variants in sequenced data using variantCaller pre-installed as part of the TorrentSuite softwares (v5.2.2 - v5.14.1) for the ionProton system, or GoogleDeepVariant (https://github.com/google/deepvariant).2
-
11.
Detect large variants and annotate genes using Sutoku (https://github.com/YujiSue/Sutoku).
Figure 4.
Schematic for the sequence data analysis
Commands/script used for running the above-mentioned programs.
# For structure variant detection
## Preparation of reference and annotation data (https://github.com/YujiSue/BioInfoTools)
## Genomic sequence, annotation data (gff3) and other resource data are downloaded from WormBase (ftp://ftp.wormbase.org/pub/wormbase/)
> GenomeConverter -l -s "C. elegans" -r <version> -o <path-to-reference>/ce_genome.bin <path-to-reference>/ce_genome.fa
> AnnotDBMake -r <path-to-reference>/ce_genome.bin -p nematode.plugin -g <gff3-file> -d <version> -s "Caenorhabditis elegans" -e "gene:geneIDs.txt,description:functional_descriptions.txt" -o <path-to-reference>/ce_genome.db
## Export template parameter set
> sutoku template --outdir <path-to-export-parameter>
## Prepare a control data
> sutoku summary --bam "<path-to-bam-of-wildtype-animal>" --reference "<path-to-reference>/ce_genome.bin" --param "<path-to-param>/ce_wgs_param.json"
## Run variant detection
> sutoku analyze \
--bam "<torrent-suite-exported.bam>" \
--reference "<path-to-reference>/ce_genome.bin" \
--annotdb "<path-to-reference>/ce_genome.db" \
--param "<path-to-param>/param.json" \
--control "<path-to-param>" \
--oformat tsv
# For torrent variant call
> python2 "<path-to-pipeline>/variant_caller_pipeline.py" \
--input-bam "<torrent-suite-exported.bam>" \
--reference-fasta "<path-to-reference>/ce_genome.fa" \
--parameters-file "<path-to-parameter>/germline_low_stringency.json" \
--error-motifs "<path-to-parameter>/motifset.txt" \
--num-threads <num-of-thread> \
--output-dir <path-to-output>
# For variant call using google deepvariant
> samtools fastq "<torrent-suite-exported.bam>" > "rawseq.fq"
> bwa mem -t <num-of-thread> -M -R "@RG\tID:<read-id>\tSM:<sample-name>\tPL:ionProton" "<path-to-reference>/ce_genome" "rawseq.fq" > "mapped.sam"
> samtools view -@ <num-of-thread> -b -o "raw.bam" "mapped.sam"
> samtools sort -l1 -T tmp -@ <num-of-thread> -O bam -o "sorted.bam" "raw.bam"
> java -jar picard.jar MarkDuplicates -I "sorted.bam" -O "aligned.bam" -M "metric.txt"
> samtools index "aligned.bam"
> docker run --gpus 1 \
-v "<path-to-reference>":"/REF_DIR" \
-v "<path-to-input-directory>":"/INPUT_DIR" \
-v "<path-to-output-directory>":"/OUTPUT_DIR" \
google/deepvariant:"<version>" <path-to-deepvariant>/deepvariant/bin/run_deepvariant \
--model_type=WGS \
--ref /REF_DIR/ce_genome.fa \
--reads /INPUT_DIR/aligned.bam \
--output_gvcf /OUTPUT_DIR/variants.gdv.g \
--output_vcf /OUTPUT_DIR/variants.gdv
Note: Since we performed whole genome sequencing with the ionProton using the BaseCaller program included in TorrentSuite, the VariantCaller in TorrentSuite is considered the most optimized for the data obtained. The Google DeepVariant was used because it can also export GVCF, allowing us to check all the variants that were filtered in the result by the TorrentSuite VariantCaller. Other variant call softwares such as GATK haplotypecaller and Illumina Isaac/Strelka are also available, although recalibration of base qualities may be necessary.
Note: Because approximately 13% of EMS-derived lesions are deletions or other chromosomal rearrangements,7 the search for large variants is recommended. If deletions or structural variants are detected in coding regions, these lesions are considered strong candidates for causal mutations.
-
12.
Search list of affected genes in mutagenized genome for previously characterized genes.
-
13.
If isolated mutants have no lesions in previously identified genes, proceed to the next step.
Mapping and identification of causative mutations by WGS
Timing: variable (at least 3 months)
In this section, we describe the way how to map and identify the causal mutations. The strategy for mapping using EMS-induced nucleotide changes detected by WGS was originally described by Zuryn et al.8
-
14.
Cross the mutagenized strains to the original unmutagenized strain 5 times (Figure 5).
Note: During the backcrossing process, it is recommended to isolate up to five independent strains from a single mutagenized strain.
-
15.Prepare samples for WGS as described in Step 3 to Step 8.
-
a.For sample preparation, mix equal amounts of genomic DNA independently isolated from backcrossed strains derived from a single mutagenized strain.
-
b.Prepare the genomic DNA sample from the original unmutagenized strain to use as the reference genome for variant analysis.
-
a.
Note: Because of the random nature of genetic recombination, EMS-induced mutations that are not linked to the causal mutation may remain after backcrossing. Among independent strains derived from a single mutagenized strain, genomic regions containing such unlinked mutations may vary, although regions associated with the causal mutation must overlap. Mixing genomic DNA from independent strains can decrease the proportion of strain-specific unlinked mutations in the sequenced genomic DNA. This allows for the detection of only EMS-induced mutations that are associated with the causal mutation as homozygous variants in the sequence result.
-
16.
Sequence the genomes of backcrossed strains to a target depth of approximately 35–40.
-
17.
Detect variants in the sequenced data as Step 10 and Step 11.
-
18.
Search the typical EMS-induced G/C to A/T transitions.
-
19.
Predict the effect of amino acid substitution using sorting intolerant from tolerant (SIFT) algorithm.8
-
20.
Select candidate mutations that affect protein functions in the genomic region with high-density of EMS-induced variants (Figure 6).
Note: The priority order for analysis of variants is as follows: deletions, nonsense mutations, missense mutations with deleterious amino acid substitutions and non-deleterious amino acid substitutions.
-
21.
Confirm the causative genes by testing the phenotype of another allele and rescue by introducing wild-type gene.
Figure 5.
Overview of backcrossing
Figure 6.
Results of mapping using EMS-induced variants
The number of EMS-induced variants per megabase is plotted across the genome. Locations of identified causal mutations for each allele are indicated by red arrows.
Expected outcomes
The genomic region with high density of EMS-induced variants linking the causal mutations can be detected as localized peaks on a single or a limited number of chromosomes. In our previous screen that isolated mutants defective in the feeding RNAi, three alleles, tm9739, tm9742 and tm9743, were isolated and subjected to WGS analysis for mapping.1 WGS analysis linked the tm9739 allele to 3 Mb region and 1 Mb region on chromosome III and X, respectively, tm9742 to 8 Mb region on chromosome IV and tm9743 to 6 Mb region and 2 Mb region on chromosome III and X, respectively (Figure 6). We found a nonsense mutation and a missense mutation on the Y39A3CL.1 as the candidates for tm9739 and tm9743, respectively, and a nonsense mutation on tbc-3 as the tm9742 candidate. Subsequent analysis using deletion mutants identified Y39A3CL.1 and TBC-3 as novel systemic RNAi regulators.
Limitations
This protocol uses the variant information detected by WGS to exclude mutant strains with lesions in previously characterized genes from crossing for mapping, saving time and effort. However, mutations are not always associated with phenotype. Thus, experimental confirmation, such as transgenic rescue and complementation assays, is required to conclude that the detected variation is the causal mutation for the phenotype of interest.
Troubleshooting
Problem 1
No mutant of interest is isolated (Step 2).
Potential solution
-
•
Increase the number of genomes to be screened. Using 50 mM EMS, 2000 copies of a gene need to be screened to recover a mutation. Thus, about 1000 F1 animals, each of which has a couple of mutagenized haploid genome, need to be analyzed to find a mutation in the gene of interest.
Problem 2
Low quality of whole genome data output from the sequencer can result in the detection of many false positive mutations (Step 10).
Potential solution
-
•
To detect sequence problems, such as many reads shorter than the expected read length from the machine specifications, many PCR duplications, or many reads with low quality values, use the following commands to check the quality.
# Check quality ( https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ )
fastqc -o <output-directory> <input-fastq> &
If there are low quality reads, use the following commands to filter the data.
# Filter low quality reads and/or regions (https://github.com/OpenGene/fastp)
fastp -i <basecalled-rawdata> -o <filtered-fastq> -e <mean-quality> -l <minimum-read-length> -b <maximumˆread-length> -w <num-of-thread>
Run the quality check again, and if the genome coverage is low, discard the data and resequence.
The above steps control the data quality of whole genome sequencing data.
Problem 3
All isolated mutants have lesions in previously identified genes (Step 13).
Potential solution
-
•
Validate whether the detected variants are causative through experiments, such as transgenic rescue and complementation assays. If variants in previously characterized genes are not causal mutations, a mutation in a novel factor should be responsible.
Problem 4
Peaks of EMS-induced variants are not limited to a single chromosome (Step 20).
Potential solution
-
•
Additional crosses can remove variants that are not physically linked to the causal mutation
Problem 5
No causative mutation is identified from candidate variants (Step 21).
Potential solution
-
•
Change the variant call algorithm (refer to Step 10). Different variant callers sometimes output different lists of variants. It is therefore possible that a list of variants detected by a single variant caller may miss the variant associated with the phenotype.
-
•
In addition to the detection of small variants (SNPs and small indels), we also recommend the detection of large variants (refer to Step 11).
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Shohei Mitani (smitani514@gmail.com).
Technical contact
Technical questions on executing this protocol should be directed to and will be answered by the technical contact, Keita Yoshida (yoshida.keita@twmu.ac.jp).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The whole-genome sequencing data used for creating Figure 6 have been submitted to the NCBI BioProject database under accession number PRJNA964498. Accession numbers are NCBI Trace Archive: SRR24389894, NCBI Trace Archive: SRR24389893 and NCBI Trace Archive: SRR24389892.
This paper does not report original code.
Acknowledgments
This work was supported by JSPS KAKENHI grant number JP20H03422 to S.M.
Author contributions
Conceptualization, K.Y. and S.M.; methodology, K.Y., Y.S., and S.M.; writing – original draft, K.Y.; writing – review and editing, K.Y., Y.S., and S.M.; funding acquisition, S.M.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Keita Yoshida, Email: yoshida.keita@twmu.ac.jp.
Shohei Mitani, Email: smitani514@gmail.com.
References
- 1.Yoshida K., Suehiro Y., Dejima K., Yoshina S., Mitani S. Distinct pathways for export of silencing RNA in Caenorhabditis elegans systemic RNAi. iScience. 2023;26 doi: 10.1016/j.isci.2023.108067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poplin R., Chang P.-C., Alexander D., Schwartz S., Colthurst T., Ku A., Newburger D., Dijamco J., Nguyen N., Afshar P.T., et al. A universal SNP and small-indel variant caller using deep neural networks. Nat. Biotechnol. 2018;36:983–987. doi: 10.1038/nbt.4235. [DOI] [PubMed] [Google Scholar]
- 3.Suehiro Y., Yoshina S., Motohashi T., Iwata S., Dejima K., Mitani S. Efficient collection of a large number of mutations by mutagenesis of DNA damage response defective animals. Sci. Rep. 2021;11:7630. doi: 10.1038/s41598-021-87226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng P.C., Henikoff S. Predicting Deleterious Amino Acid Substitutions. Genome Res. 2001;11:863–874. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner S. The Genetics of CAENORHABDITIS ELEGANS. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minevich G., Park D.S., Blankenberg D., Poole R.J., Hobert O. CloudMap: A Cloud-Based Pipeline for Analysis of Mutant Genome Sequences. Genetics. 2012;192:1249–1269. doi: 10.1534/genetics.112.144204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson P. Mutagenesis. Methods Cell Biol. 1995;48:31–58. [PubMed] [Google Scholar]
- 8.Zuryn S., Le Gras S., Jamet K., Jarriault S. A Strategy for Direct Mapping and Identification of Mutations by Whole-Genome Sequencing. Genetics. 2010;186:427–430. doi: 10.1534/genetics.110.119230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The whole-genome sequencing data used for creating Figure 6 have been submitted to the NCBI BioProject database under accession number PRJNA964498. Accession numbers are NCBI Trace Archive: SRR24389894, NCBI Trace Archive: SRR24389893 and NCBI Trace Archive: SRR24389892.
This paper does not report original code.