ABSTRACT
Introduction
Differential diagnosis of pediatric neck pain requires age-appropriate communication and assessment tools. Recognizing these age-related nuances is critical, emphasizing the role of physical therapists in assessing and managing pediatric patients while ruling out severe pathologies.
Case Description
A 10-year-old male presented to physical therapy with a five-week history of increasing neck pain. A thorough history and segmental cervical examination considering the patient’s age and development, led to patient referral to the emergency department. This case underscores the significance of comprehensive evaluation in pediatric neck pain management.
Outcomes
The patient was diagnosed with Langerhans Cell Histiocytosis (LCH). LCH primarily affects children and is treated with chemotherapy. Chemotherapy reduced the tumor, revealing C2 vertebral body damage. The patient underwent C1-C3 fusion surgery, a standard procedure for atlanto-occipital region stabilization in children. The patient was advised to restrict motion for 6 months while monitoring for tumor growth.
Discussion-conclusion
Pediatric neck cancer presents diagnostic challenges due to varied symptoms, but research highlights specific indicators to assist with differential diagnosis. This case emphasizes the need to recognize the complexities of pediatric neck pain and perform a thorough age-appropriate evaluation.
KEYWORDS: Cancer, Langerhans Cell Histiocytosis, red flags, spinal fusion, case report
Introduction
The cervical spine is an anatomically complex region. Neck pain point prevalence is higher in adults (8%) than in children (.3%) and adolescents (.7%) [1]. This is primarily due to degenerative changes and the presence of intervertebral disc pathology [1,2]. Frequent pain generators reported in the adult cervical spine differ from that in pediatric patients [3]. Therefore, it is critical to distinguish between pediatric and adult sources of neck pain when evaluating a patient and considering the contributing variables of anatomy, biomechanics, diagnosis, and management [2,4].
Another compelling reason to consider the patient’s age is the variation in degree of ossification and the amount of ligamentous laxity, the horizontal inclination of the articular surfaces, underdeveloped spinous processes, and a mild reduction in cervical lordosis [3–5]. The pediatric spine undergoes proportional development and ossification for the first eight years of life with the pediatric head-to-neck ratio being greater than that in adults. This results in a higher movement fulcrum and can contribute to cervical muscle insufficiency with poor motor control [3,4,6–8]. Following trauma, patients younger than eight years old exhibit higher susceptibility to upper cervical injuries, while older children sustain injuries that align more with those of adults, often involving the lower cervical spine [4,7,8]. The pediatric cervical spine ligaments exhibit more laxity, which underscores the potential for spinal cord injury in the absence of bony involvement [3]. This ligamentous laxity can contribute to hypermobility and segmental translation [4].
One of the most frequent pediatric cervical spine condition, atlanto-axial rotatory fixation, presents as a torticollis [4]. Furthermore, 22% of patients under the age of 13 years with cervical or posterior fossa tumors present with a torticollis [9].This underscores a scenario in which a movement impairment commonly associated with adults can hold a different diagnostic implication in pediatric patients [10].
Pediatric differential diagnosis includes a broad spectrum of potential conditions, such as musculoskeletal disorders, fractures, central and peripheral neural involvement, neoplastic disorders, vascular compromise, infections, and inflammatory conditions [2]. The evaluation process includes a detailed patient history, objective assessment, and when deemed necessary, appropriate imaging [11]. When assessing neck pain, the initial focus is on eliminating red flags. The literature states that pediatric red flags for head or neck cancer include midline cervical tenderness, unrelenting or severe pain, night pain, restricted neck range of motion, palpable swelling or lump in the neck, unexplained weight loss, neurological abnormality, unexplained bruising, and others depending upon the specific location [2,12–16]. The findings of two studies reporting on the symptom prevalence for pediatric head and neck cancer are summarized in Table 1.
Table 1.
Symptoms prevalence of pediatric head and neck cancer.
| Symptoms Prevalence [17] | 95% CI | Symptoms Prevalence [16] | p-values | |
|---|---|---|---|---|
| Patient report of tumor or swelling | 73% | 62–82% | 68.4% | .034 |
| Presence of visible or palpable tumor noted by clinician | 87% | 78–93% | ||
| Fatigue | 35% | 25–46% | ||
| Cervical Lymphadenopathy | 28.9% | .096 | ||
| Fever | 27% | 18–38% | 15% | .004 |
| Pain | 22% | 14–33% | 57% | .031 |
| Weight Loss | 18% | 10–27% | 22.5% | .004 |
| Pallor | 16% | 9–26% | 4.7% | .004 |
| Epistaxis | 11% | 5–19% | 9.5% | .012 |
| Sweating | 6% | 2–13% | ||
| Nasal Discharge or obstruction | 6% | 2–13% | 15.4% | .012 |
| Headache | 14.6% | .004 | ||
| Dyspnea | 10.3% | .012 | ||
| Speaking Alterations | 11.9% | .619 |
a10-year symptoms’ prevalence of 85 patients with head and neck cancer including leukemia, lymphoma, Langerhans Cell Histiocytosis, neuroblastoma, sarcoma, and carcinoma [16]; 30-year symptoms’ prevalence of 253 patients with head and neck cancer including lymphoma, carcinoma, sarcoma, embryonal and germ tumors, melanoma; p-values indicate statistically significant associations between the variables of symptoms and the diagnosis of pediatric head or neck cancer.
When gathering a patient history, it is important to determine if there was a mechanism of injury, if there is any neural involvement, and what aggravates and relieves the patient’s symptoms [11]. Obtaining an accurate symptom report from children can be challenging due to their limited ability to communicate what they are feeling. Using age-appropriate communication strategies and utilizing validated tools such as the Wong-Baker faces (WBF), and color scales can facilitate symptoms’ nature and intensity communication [4,15,18,19]. The WBF scale has excellent agreement with the validated Visual Analog Scale for pain (q = 0.90; 95% CI = 0.86 to 0.93) in pediatric patients [20]. Furthermore, observation, palpation, and ligamentous testing are important as the stability of the upper cervical spine is largely contingent on the integrity of supporting ligaments, and pediatric patients are prone to upper cervical injury [4,7,8]. The side-bending alar ligament test has a specificity 90.9% and sensitivity of 85.7% in adults when compared to an upright MRI. Alar ligament testing is reported to be most accurate when evaluated with a cluster of tests [21]. Manual testing of the transverse ligament in adults compared to MRI has a specificity of 99% and sensitivity of 65% [22]. Additional reliability and validity studies are needed for upper cervical ligament testing in the pediatric population.
The purpose of this case report is to demonstrate the importance of a thorough pediatric informed examination when evaluating a pediatric patient with neck pain.
Case description
Patient characteristics and history: systems review
An active 10-year-old male child, previously in good health with no family history of cancer, visited a fellowship- trained pediatric physical therapist with 23 years of clinical experience. This was a direct-access visit prompted by the parent’s desire to have the child evaluated for neck pain, three days before departing for a sleep away tennis camp. Five weeks before seeking treatment, the patient acquired a rope burn on his neck while playing on a rope swing. He began complaining of intermittent neck pain, his parents linking it to the rope swing incident. Three weeks before his physical therapy visit, he returned from tennis day camp and complained of neck discomfort. His mother managed his pain with Advil. Two weeks before his physical therapy visit, his mother took him to see his pediatrician, who diagnosed him with musculoskeletal pain. The pediatrician recommended a regimen of 200 mg of Ibuprofen every six hours and neck massage but did not order imaging. Over the following week, the patient began requesting Advil as soon as he woke up. He attended a golf day camp and returned home each day to rest his head on a pillow or stuffed animal. Although he continued to engage with his friends, he increasingly sought rest and requested more Advil. He also started to use a pillow to support his head while riding in the car and became more sensitive to being touched. Concerned about worsening symptoms, his mother contacted the pediatrician, who advised her to massage the patient’s neck, use icy hot, and increase his Advil to 300 mg. When the earliest appointment available with a pediatric orthopedist was a month away, she reached out to a physical therapist due to concerns about the upcoming sleep away tennis camp. The patient’s subjective report of progressive neck pain in an active and previously healthy child, without a known mechanism of injury, was inconsistent with the physical therapist’s experience with previous pediatric patients. Knowing that children do not experience change that follows a degenerative pattern, it was concerning that his pain was not responding to Advil and that he now was having difficulty supporting the weight of his head. The patient’s primary subjective concern was the persistence of day and night pain unrelieved with rest, which raised the physical therapist’s suspicion of a possible tumor. The goals of the first visit were to screen the patient and appropriately advise the family on the next step of patient care.
The PTs differential diagnosis also included possible segmental dysfunction that was contributing to progressive facet impingement and muscle guarding. Her hope was to rule out more serious diagnosis so she could clear his cervical spine and resolve his pain generator. His mother wanted to alleviate his pain so he could attend camp pain- free. The patient’s parents consented to the publication of this report to help other physical therapists evaluate and treat patients like their son.
Examination
The patient presented to physical therapy with complaints of pain localized to his upper cervical spine and concurrent occipital headache. He denied numbness, tingling, loss of sensation, or radiating pain, suggesting no peripheral neural involvement. There were no symptoms such as fever, fatigue, or rashes indicative of infection. The absence of gait changes or bowel and bladder difficulties reduced concern for myelopathy. Additionally, he denied experiencing serious vascular issues such as drop attacks, vertigo, or vision changes.
His resting position was right cervical side-bending with a more pronounced reduction in cervical lordosis than typically observed in children. He reported increased pain when trying to look up and when supporting his head in an upright position for longer than a few minutes. To facilitate the evaluation and attempt to reduce guarding, the patient was placed in a supine position. Palpation revealed normal tone in the sternocleidomastoid and scalene muscles, with both first ribs exhibiting normal mobility and resting position. However, there was increased tone at the suboccipital muscles.
During the evaluation, the patient became visibly agitated and struggled to communicate his symptoms effectively. The therapist introduced the red, yellow, and green symptom scale and asked him to use those three words to convey his pain as she assessed his motion [20]. Both right and left side-bending and right and left rotation were significantly limited (>50%) with the patient reporting ‘yellow’ on the symptoms scale. Alar and transverse ligament of atlas ligaments laxity were ruled out [21,22]. Subsequently, when the therapist attempted a combination of extension and right side-bending to test the vertebral artery, the patient reported ‘red’ but did not exhibit signs of vertebral artery insufficiency within the testable range [23]. To further investigate, the physical therapist gently performed palpation and identified bony tenderness at C2. She was unable to discriminate between muscle guarding and the presence of a palpable mass. Subsequently, cervical flexion was attempted (<30 degrees), and the patient reported, ‘red red red’ mentioning that the pain moved up the back of his head, which could indicate involvement of the atlanto-axial joint or upper cervical facet joints [2,24].
The patient presented to physical therapy with progressive, multidirectional, and unrelenting neck pain without a clear mechanism of injury. His clinical signs of bony tenderness, reduced range of motion > 50% in multiple directions, and weakness to the degree of needing external support to hold his head in an upright position raised suspicion of more serious pathology. The combination of subjective and objective findings led to the therapist to stop the exam. It was clear that the patient’s presentation did not follow a clinical pattern and he needed imaging prior to proceeding with the physical therapy evaluation. She placed the patient in an Aspen Cervical Collar as both an act of precaution and means to help the patient support his head in neutral. The patient was referred to the nearest emergency room for imaging.
Medical diagnostics and intervention
The patient was promptly taken to the emergency room, where a Computed Tomography (CT) scan revealed a paravertebral abscess with osteomyelitis at C2 and narrowing of the right V3 segment vertebral artery. Subsequently, the patient was transferred via ambulance to the regional children’s hospital. Magnetic Resonance Imaging (MRI) was performed, demonstrating an abnormal STIR hyperintense signal (Figure 1).
Figure 1.

Multiplanar, multisequence MRI revealed reduced diffusion, and enhancement involving the C2 vertebrae (odontoid, vertebral body, right pedicle, right lamina, and spinous process) with soft tissue extension into the right prevertebral space, right.
posterior paraspinal space, right C2–3 foramen and right C2 lateral epidural space. The white arrow points to the cervical tumor. The mass measured 4.1 × 2.6 × 3.0 cm (AP, craniocaudal, transverse).
A biopsy and full-body scan confirmed the presence of Langerhans Cell Histiocytosis (LCH), which was isolated to the cervical spine. LCH originates in the bone marrow and can present as a single-system disease or multisystem. When it involves a single bone lesion, it primarily affects children between the ages of 5 and 15. It is characterized by the potential for significant pain and destruction of the affected bone. Vertebral body involvement is the most prevalent manifestation of this condition [25,26].
Chemotherapy was promptly initiated. Following the first cycle, the patient’s pain escalated to where he was unable to roll over or bear any spinal load by adjusting the incline of his bed. A subsequent CT scan was performed, revealing damage to the C2 vertebrae (Figure 2). In response to these findings, the patient underwent C1 to C3 fusion (Figure 3). This procedure is standard for stabilizing the atlanto-occipital region in children [4]. Once the patient’s pain was effectively managed post-operatively, he was discharged from the hospital, instructed to wear a neck brace for three months, and to follow a prescribed chemotherapy regimen.
Figure 2.
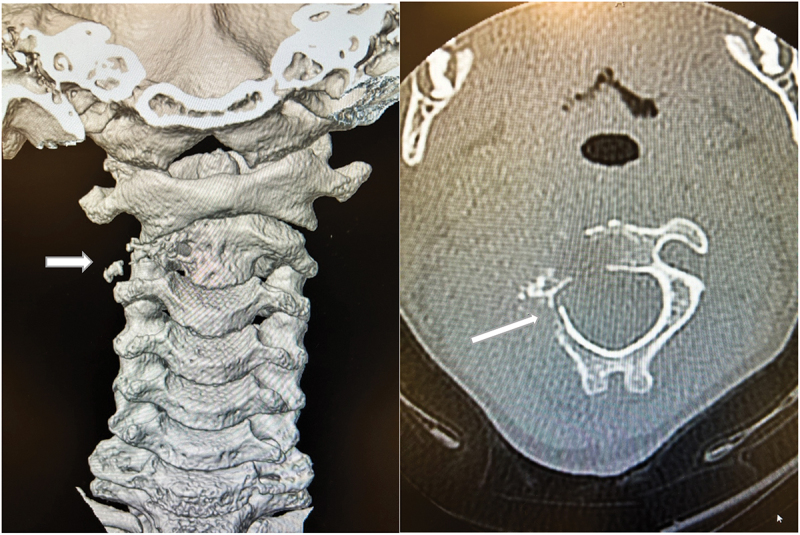
CT image revealing collapse of C2 vertebral body (white arrow) with increased narrowing of right atlantoaxial joint and new pathologic fracture in a posterior neural arch.
Figure 3.
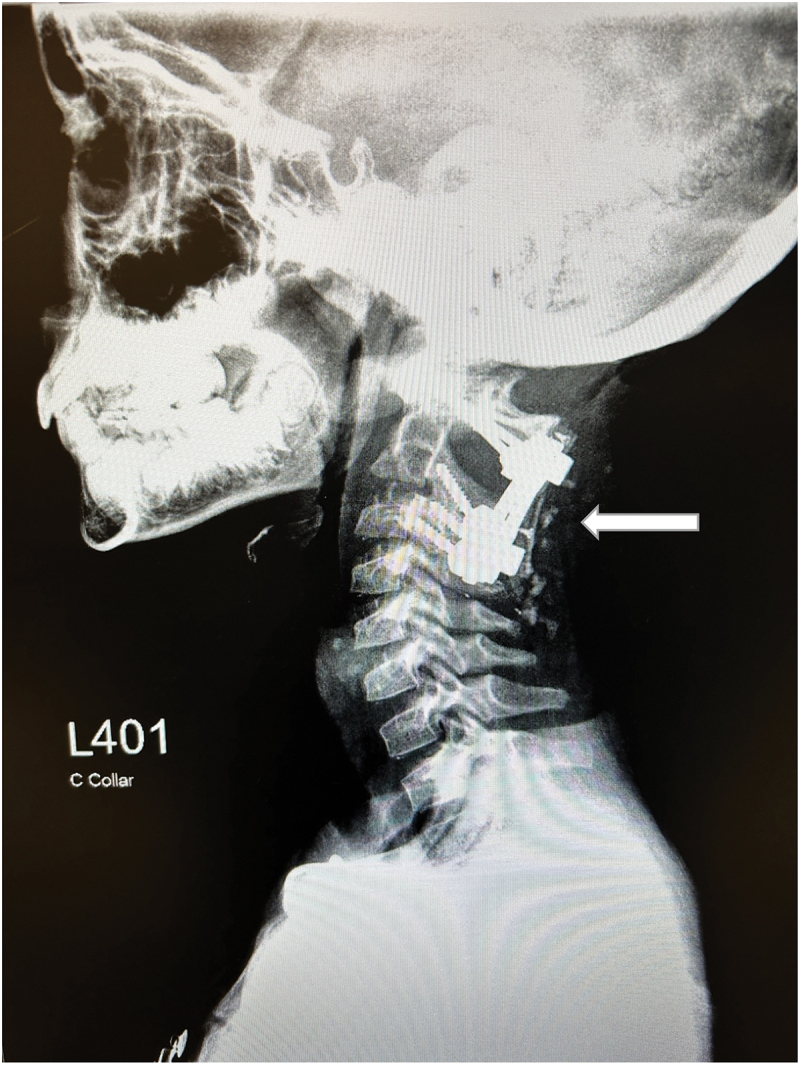
Status post posterior fusion of C1-C3.
Follow-up visits and assessment of outcomes and interventions
At 3 months post-op, the patient reported being pain-free most days with no noticeable weakness or numbness. He reported frequent headaches that were greatest after school but were improving weekly. Since weaning off the brace, he needed to occasionally support his head with his hand or a stuffed animal. He returned to school full time but was not cleared to run or participate in sports. He missed school frequently for doctors’ appointments and stated that since his diagnosis he felt angrier and more afraid. He reported that he was ready to ‘be silly with his friends again.’
He demonstrated ongoing tenderness to occiput palpation. His neck was resting in neutral with no signs of ongoing torticollis. He had 62 degrees of pain-free neck flexion, 36 degrees of extension, 25 degrees of side-bending in each direction, 35 degrees of rotation in each direction. His most significant improvement was in cervical flexion. While his side-bending and rotation were still limited, they were now pain-free and likely related to his period of immobilization in the post-op collar. His upper extremity manual muscle testing was normal and equal bilaterally. Finally, upper limb tension tests for adverse neural tissue tension were negative. He had not been cleared to begin physical therapy treatment at that time. At 6 months post-op his neurosurgeon released him to return to sports. He is now pain-free and able to fully engage in his previous social activities. He reports that he is no longer angry or afraid and that he is happy to be able to play tennis again. He has transitioned from being monitored monthly for tumor growth to being monitored every 3 months. The patient care timeline is summarized in Figure 4.
Figure 4.

Patient care timeline- WNL = within normal limits; ROM = range of motion.
Discussion
This case report described the evaluation of a 10-year-old male evaluated in physical therapy direct access. It demonstrates the need for physical therapy screening of serious red flags, diagnostic reasoning, and the need to expedite a referral to emergency room for further medical diagnosis and intervention. As direct access becomes more and more prevalent, physical therapists assume a critical role in treating musculoskeletal conditions in a primary care role. With this expanded access, physical therapists shoulder the added responsibility of differentially diagnosing conditions such as pediatric neck pain while remaining vigilant to more serious pathologies, including fracture, myelopathy, infection, and cancer. To our knowledge, this is the first case report of a child evaluated in physical therapy direct access care with LCH referred by a physical therapist to the emergency department.
Pediatric neck cancer and similar conditions can manifest through an array of symptoms, resulting in multiple diagnostic challenges, which makes early detection difficult [16]. Research underscores the significance of specific signs and symptoms that serve as stronger indicators for pediatric cancer. A British study looked at pediatric symptoms with the greatest positive predictive value for cancer, which included swelling in the neck, a lump or mass, palor, lymphadenopathy, abnormal movement, bruising, bleeding, fatigue, headache, pain, visual symptoms, and musculoskeletal symptoms. Combinations of these symptoms suggest a higher suspicion for a cancer diagnosis [27].
While LCH presents like cancer and is treated by oncology, there is an ongoing debate as to whether LCH is reactive or neoplastic. LCH can be found in almost any organ, but is most commonly located in bone, skin, central nervous system, the pituitary gland, the liver, bone marrow, the spleen, lymph nodes, and lungs [26]. One difference from most ‘cancers’ is that LCH does not require complete removal that includes margin. Post-surgical treatment consists of chemotherapy and prednisone and typically lasts for 6 months to one year [28,29]. Although the medical community disagrees on exactly how to classify LCH, for physical therapists, it presents like cancer with potential for bone tenderness, a palpable mass, pain, headache, and abnormal bleeding or bruising [29].
In this case, the patient presented to physical therapy with symptoms that, on the surface, might be attributed to a less serious pathology such as facet dysfunction with muscle guarding. Although a prior case study suggested the potential use of manual therapy in treating a patient with a similar presentation, it was only after ruling out more serious conditions with an x-ray [5]. Ultimately, recognizing the unique complexities of pediatric neck pain and the potential for serious underlying pathologies is paramount in ensuring early diagnosis and appropriate care, thereby reinforcing the evolving role of physical therapists in comprehensive patient management.
Limitations for this case study include that we cannot infer causation from the findings from this case and the generalizability of the findings is limited.
Conclusion
Research supports the use of manual therapy to treat pain and movement disorders [30]. Nevertheless, when the possibility of a more severe pathology cannot be ruled out in the clinic, it is imperative to request imaging prior to performing manual therapy on pediatric neck pain patients. This case report highlights the critical role of physical therapists in evaluating and managing pediatric neck pain while emphasizing the necessity of excluding severe pathologies before proceeding with manual therapy.
Clinical Implications: Physical therapists and other healthcare providers in direct access care should be vigilant on cancer related signs and symptoms and expedite referral to medical care for medical diagnosis and intervention.
Research Implications: Automated alert systems within primary care computer systems for physical therapy diagnosis could be beneficial. Further research is warranted on this topic.
Biographies
Virginia K. Henderson PT, DPT is a pediatric physical therapist in the department of orthopedics and sports medicine at Texas Children’s Hospital. She completed her Master’s of Physical Therapy at The University of Texas Health Science Center in San Antonio, and her transitional Doctorate of Physical Therapy at Texas Women’s University. She completed her manual therapy fellowship with The Manual Therapy Institute. Dr. Henderson is currently an ScD student at Texas Tech University Health Sciences Center and is studying the application of joint mobilization in the pediatric population.
Jean-Michel Brismée PT, ScD is professor in the Doctor of Science and Doctor of Philosophy programs in Rehabilitation Science at Texas Tech University Health Sciences Center in Lubbock, Texas, and teaches orthopedic manual therapy related continuing education courses with the International Academy of Orthopedic Medicine (IAOM-US).
Funding Statement
This project was not supported by grant funding
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Kazeminasab S, Nejadghaderi SA, Amiri P, et al. Neck pain: global epidemiology, trends and risk factors. BMC Musculoskelet Disord. 2022;23(1):26. doi: 10.1186/s12891-021-04957-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cohen SP. Epidemiology, diagnosis, and treatment of neck pain. Mayo Clinic Proceedings. 2015;90(2):284–299. doi: 10.1016/j.mayocp.2014.09.008 [DOI] [PubMed] [Google Scholar]
- [3].David EH, William Boydston MD. Pediatric neck injuries. Pediatr Rev. 1999;20(1):13–19. doi: 10.1542/pir.20.1.13 [DOI] [PubMed] [Google Scholar]
- [4].Baumann F, Ernstberger T, Neumann C, et al. Pediatric cervical spine injuries. J Spinal Disord Tech. 2015;28(7):E377–E384. doi: 10.1097/BSD.0000000000000307 [DOI] [PubMed] [Google Scholar]
- [5].Norton TC, Oakley PA, Harrison DE. Improving the cervical lordosis relieves neck pain and chronic headaches in a pediatric: a Chiropractic Biophysics® (CBP®) case report with a 17-month follow-up. J Phys Ther Sci. 2022;34(1):71–75. doi: 10.1589/jpts.34.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schöneberg C, Schweiger B, Hussmann B, et al. Diagnosis of cervical spine injuries in children: a systematic review. Eur J Trauma Emerg Surg. 2013;39(6):653–665. doi: 10.1007/s00068-013-0295-1 [DOI] [PubMed] [Google Scholar]
- [7].Murphy RF, Davidson AR, Kelly DM, et al. Subaxial cervical spine injuries in children and adolescents. J Pediatr Orthop. 2015;35(2):136–139. doi: 10.1097/BPO.0000000000000341 [DOI] [PubMed] [Google Scholar]
- [8].Fesmire FM, Luten RC. The pediatric cervical spine: developmental anatomy and clinical aspects. J Emerg Med. 1989;7(2):133–142. doi: 10.1016/0736-4679(89)90258-8 [DOI] [PubMed] [Google Scholar]
- [9].Fąfara-Leś A, Kwiatkowski S, Maryńczak L, et al. Torticollis as a first sign of posterior fossa and cervical spinal cord tumors in children. Childs Nerv Syst. 2014;30(3):425–430. doi: 10.1007/s00381-013-2255-9 [DOI] [PubMed] [Google Scholar]
- [10].Jackson R. The classic: the cervical syndrome. Clin Orthop. 2010;468(7):1739–1745. doi: 10.1007/s11999-010-1278-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Childress MA, Stuek SJ. Neck Pain: Initial Evaluation and Management. American Family Physician. 2020;102(3):150–156. [PubMed] [Google Scholar]
- [12].Maxwell MJ, Jardine AD. Paediatric cervical spine injury but NEXUS negative. Emerg med J. 2007;24(9):676–676. doi: 10.1136/emj.2007.046912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hannon M, Mannix R, Dorney K, et al. Pediatric cervical spine injury evaluation after blunt trauma: a clinical decision analysis. Ann Emerg Med. 2015;65(3):239–247. doi: 10.1016/j.annemergmed.2014.09.002 [DOI] [PubMed] [Google Scholar]
- [14].Viccellio P, Simon H, Pressman BD, et al. A prospective multicenter study of cervical spine injury in children. Pediatrics. 2001;108(2):e20–e20. doi: 10.1542/peds.108.2.e20 [DOI] [PubMed] [Google Scholar]
- [15].Hawk C, Schneider MJ, Vallone S, et al. Best practices for chiropractic care of children: a consensus update. J Manipulative Physiol Ther. 2016;39(3):158–168. doi: 10.1016/j.jmpt.2016.02.015 [DOI] [PubMed] [Google Scholar]
- [16].Arboleda L, Pérez-de-Oliveira M, Hoffmann I, et al. Clinical manifestations of head and neck cancer in pediatric patients, an analysis of 253 cases in a single Brazilian center. Med Oral Patol Oral Cirugia Bucal. Published online 2022. 2022;e285–e293. doi: 10.4317/medoral.25255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lilja-Fischer JK, Schrøder H, Nielsen VE. Pediatric malignancies presenting in the head and neck. Int J Pediatr Otorhinolaryngol. 2019;118:36–41. doi: 10.1016/j.ijporl.2018.12.009 [DOI] [PubMed] [Google Scholar]
- [18].Douglas GP, McNickle AG, Jones SA, et al. A pediatric cervical spine clearance guideline leads to fewer unnecessary computed tomography scans and decreased radiation exposure. Pediatr Emerg Care. 2023;39(5):318–323. doi: 10.1097/PEC.0000000000002867 [DOI] [PubMed] [Google Scholar]
- [19].Wylde V, Wells V, Dixon S, et al. The colour of pain: can patients use colour to describe osteoarthritis pain? Musculoskelet Care. 2014;12(1):34–46. doi: 10.1002/msc.1048 [DOI] [PubMed] [Google Scholar]
- [20].Garra G, Singer AJ, Taira BR, et al. Validation of the Wong‐Baker FACES pain rating scale in pediatric emergency department patients. Acad Emerg Med. 2010;17(1):50–54. doi: 10.1111/j.1553-2712.2009.00620.x [DOI] [PubMed] [Google Scholar]
- [21].Harry Von P, Maloul R, Hoffmann M, et al. Diagnostic accuracy and validity of three manual examination tests to identify alar ligament lesions: results of a blinded case-control study. J Man Manip Ther. 2019;27(2):83–91. doi: 10.1080/10669817.2018.1539434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kaale BR, Krakenes J, Albrektsen G, et al. Clinical assessment techniques for detecting ligament and membrane injuries in the upper cervical spine region—A comparison with MRI results. Man Ther. 2008;13(5):397–403. doi: 10.1016/j.math.2007.03.007 [DOI] [PubMed] [Google Scholar]
- [23].Rushton A, Rivett D, Carlesso L, et al. International framework for examination of the cervical region for potential of cervical arterial dysfunction prior to orthopaedic manual therapy intervention. Man Ther. 2014;19(3):222–228. doi: 10.1016/j.math.2013.11.005 [DOI] [PubMed] [Google Scholar]
- [24].Dreyfuss P, Michaelsen M, Fletcher D. Atlanto-Occipital and Lateral Atlanto-Axial Joint Pain Patterns. Spine. 1994;19(Supplement):1125–1131. doi: 10.1097/00007632-199405001-00005 [DOI] [PubMed] [Google Scholar]
- [25].Vielgut I, Liegl-Atzwanger B, Bratschitsch G, et al. Langerhans-cell histiocytosis of the cervical spine in an adult patient: case report and review of the literature. J Orthop. 2017;14(2):264–267. doi: 10.1016/j.jor.2017.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Leung AKC, Lam JM, Leong KF. Childhood Langerhans cell histiocytosis: a disease with many faces. World J Pediatr. 2019;15(6):536–545. doi: 10.1007/s12519-019-00304-9 [DOI] [PubMed] [Google Scholar]
- [27].Dommett RM, Redaniel T, Stevens MC, et al. Risk of childhood cancer with symptoms in primary care: a population-based case-control study. Br J Gen Pract. 2013;63(606):e22–e29. doi: 10.3399/bjgp13X660742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gulati N, Allen CE. Langerhans cell histiocytosis: Version 2021. Hematol Oncol. 2021;39(S1):15–23. doi: 10.1002/hon.2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Eckstein OS, Picarsic J, Allen CE. Histiocytic disorders of childhood. Pediatr Rev. 2022;43(10):561–571. doi: 10.1542/pir.2021-005367 [DOI] [PubMed] [Google Scholar]
- [30].Li LL, Hu XJ, Di YH, et al. Effectiveness of Maitland and Mulligan mobilization methods for adults with knee osteoarthritis: a systematic review and meta-analysis. World J Clin Cases. 2022;10(3):954–965. doi: 10.12998/wjcc.v10.i3.954 [DOI] [PMC free article] [PubMed] [Google Scholar]


