Abstract
Influenza virus neuraminidase (NA), a type II transmembrane protein, is directly transported to the apical plasma membrane in polarized MDCK cells. Previously, it was shown that the transmembrane domain (TMD) of NA provides a determinant(s) for apical sorting and raft association (A. Kundu, R. T. Avalos, C. M. Sanderson, and D. P. Nayak, J. Virol. 70:6508–6515, 1996). In this report, we have analyzed the sequences in the NA TMD involved in apical transport and raft association by making chimeric TMDs from NA and human transferring receptor (TR) TMDs and by mutating the NA TMD sequences. Our results show that the COOH-terminal half of the NA TMD (amino acids [aa] 19 to 35) was significantly involved in raft association, as determined by Triton X-100 (TX-100) resistance. However, in addition, the highly conserved residues at the extreme NH2 terminus of the NA TMD were also critical for TX-100 resistance. On the other hand, 19 residues (aa 9 to 27) at the NH2 terminus of the NA TMD were sufficient for apical sorting. Amino acid residues 14 to 18 and 27 to 31 had the least effect on apical transport, whereas mutations in the amino acid residues 11 to 13, 23 to 26, and 32 to 35 resulted in altered polarity for the mutant proteins. These results indicated that multiple regions in the NA TMD were involved in apical transport. Furthermore, these results support the idea that the signals for apical sorting and raft association, although residing in the NA TMD, are not identical and vary independently and that the NA TMD also possesses an apical determinant(s) which can interact with apical sorting machineries outside the lipid raft.
Polarized epithelial cells possess two distinct domains of the plasma membrane, the apical and the basolateral, separated by tight junctions which prevent lateral diffusion and commingling of the proteins and lipids of these domains of the plasma membrane. Each domain has distinct lipids and a distinct protein composition; the lipids and proteins are sorted and directed by separate pathways from the trans-Golgi network (TGN) to the specific plasma membrane (26, 32, 37). Different cells may use different mechanisms and sorting machineries to transport proteins to the apical and basolateral plasma membranes. In Madin-Darby canine kidney (MDCK) cells, the cells most widely used for studying apical and basolateral sorting, proteins and lipids are sorted and transported directly from the TGN to the apical or basolateral membranes (20, 30). In contrast, in hepatocytes, proteins are sorted via the endocytic-transcytotic route, i.e., first, both apical and basolateral proteins are transported to the basolateral side, and subsequently, apical proteins are transported to the apical surface via transcytosis (2). Again, in intestinal epithelium-derived Caco-2 cells, both pathways, i.e., direct delivery and transcytosis, are used for apical transport (17, 21). Mechanisms of protein sorting, including the recognition events that occur at the TGN, leading to separate vesicle formation and eventual delivery and fusion of these vesicles to apical or basolateral membranes, are beginning to be elucidated. For various basolateral proteins, different classes of short peptides present in the cytoplasmic tail of the protein have been identified. These peptides are critical for and capable of targeting the protein to the basolateral surface. However, these classes of peptides do not possess common sequences or structures, suggesting diversity in both the recognition and the binding sites of sorting machineries (4, 6, 11, 22, 28, 29). Furthermore, N-ethylmaleimide-sensitive factor (NSF), soluble NSF attachment proteins, and soluble NSF attachment protein membrane receptors, as well as Rab proteins, have been shown to be involved in the vesicular transport from the TGN to basolateral membranes (12). Also, it has been shown that the AP-1 clatherin adapter complex is involved in basolateral transport (9).
Compared to what is known about the sorting signals and machineries for basolateral proteins, very little is known about the sorting signals or the machineries involved in apical transport. Recently, VIP17/MAL protein has been reported to be involved in apical transport (8). Apical sorting signals are not present in the cytoplasmic tail. For apical membrane proteins, which are anchored by a glycosyl phosphatidylinositol (GPI) moiety and lack the transmembrane and cytoplasmic tail, the GPI anchor is responsible for directing the protein to the apical membrane by associating with the detergent-insoluble lipids in the TGN. Detergent-resistant membranes, or lipid rafts (also known as detergent-insoluble glycolipid-rich complexes), enriched in glycosphingolipids and cholesterol, serve as a platform for the apical transport of GPI-anchored proteins (5, 7, 31, 38). Some secreted and transmembrane proteins are directed apically by glycans (3, 34, 39) although others may not need glycans for apical transport (19, 24, 27). Finally, the transmembrane domain (TMD) has been shown to possess a signal(s) for apical transport. This was first shown for influenza virus neuraminidase (NA), a type II transmembrane protein (16). This observation has now been extended to other type II proteins such as simian virus 5 hemagglutinin-neuraminidase (HN) (10), as well as type I protein influenza virus hemagglutinin (HA) (18), suggesting that the presence of an apical signal in the TMD may be a general feature of many apical proteins. Furthermore, the TMDs of both influenza virus HA and influenza virus NA are also associated with glycosphingolipid- and cholesterol-enriched detergent-resistant lipid rafts (13, 16, 18, 35), suggesting that, like the GPI anchor proteins, raft association with the TMD may provide a critical determinant in apical targeting of these proteins. However, some apical proteins are not associated with detergent resistant lipid rafts (19, 39), indicating that all transmembrane proteins do not have apical signals in the TMD and do not use raft association for apical sorting and that alternate pathway(s) for apical sorting of different transmembrane proteins exist. These studies demonstrate the existence of at least two separate classes of machineries for delivery to the apical and basolateral surfaces and variation even within the apical or basolateral sorting machineries. Earlier, members of our group showed that influenza virus NA, a type II transmembrane protein, possesses two apical determinants: one in the ectodomain (15) and the other in the TMD (16). The TMD of NA is able to direct the ectodomain of human transferrin receptor (TR), a reporter protein, to the apical membrane, demonstrating that the TMD of NA can function independently and is sufficient for apical transport. In this study, we further dissected the NA TMD by using chimeric constructions (with the TR TMD) as well as by using alanine mutations of the NA TMD to investigate the nature of the apical signal(s) and the role of raft association with the NA TMD in apical transport.
MATERIALS AND METHODS
Cell culture.
MDCK cells obtained from the American Type Culture Collection were maintained in Dulbecco modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum. Costar Transwell Clear Insert filter units (Corning Coster, Cambridge, Mass.) with a pore size of 0.4 μm and a diameter of 24 mm were used for growing polarized MDCK cells. Cells (1.5 × 106) were plated and grown for 3 to 4 days prior to the experiments to form tight monolayers on the filters. The polarity of cell monolayer and the formation of tight junctions were determined by measuring the transepithelial resistance (36) with an EVOM voltmeter (World Precision Instruments, New Haven, Conn.).
Construction of chimeric and alanine mutants of NA TMD.
Plasmids containing the influenza virus (WSN/33) NA and TR were used (15, 16). Construction of deletion mutant TRΔ57 and chimeric NA(gs)TR (previously called TRΔ57NATR) containing the NA TMD and the TR ectodomain has been described previously (16). All mutations were carried out using megaprimer PCR mutagenesis (33). For chimeric constructions within the TMDs of NA and TR, specific restriction sites were created and domains were swapped. The sequences of specific primers will be provided upon request. All PCR DNAs including the created restriction sites were sequenced to make sure that additional changes did not occur. Altered amino acids caused by the creation of restriction sites are indicated in Fig. 1.
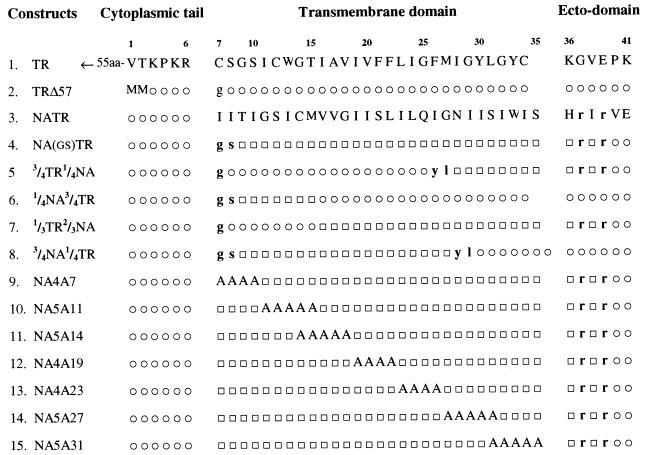
FIG. 1.
Constructs of chimeric and mutant NA TMDs. Portions of the TR and NA TMDs were swapped to construct four chimeric TMDs (construct no. 5 to 8), as described in Materials and Methods. For the mutants (construct no. 9 to 15), sets of 4 or 5 amino acids in the NA TMD were replaced by alanines. The six amino acids at the NH2 terminus of TRΔ57 and the other constructs consist of the first two residues (MM) plus the last four residues (KPKR) of the TR cytoplasmic tail. □, amino acid from NA sequence; ○, amino acid from the TR sequence. The lowercase letters represent amino acids neither from the NA sequence nor from the TR sequence but introduced or replaced due to creation of restriction enzyme sites. The numbers at the top represent amino acid positions with respect to the NA sequence.
Stable expression in MDCK cells.
cDNAs encoding the TR, TRΔ57, NATR, and other constructs containing chimeric and mutated TMDs (Fig. 1) were expressed under the control of the metallothionein promoter using the expression vector pMEP4 containing the hygromycin resistance marker (Invitrogen Corporation, San Diego, Calif.). MDCK cells were transfected by the cDNAs using Lipofectamine Plus (Gibco BRL, Grand Island, N.Y.) according to the manufacturer's protocol. Clones expressing the hygromycin resistance marker were selected in the presence of hygromycin B (300 μg/ml; Sigma Chemical Co., St. Louis, Mo.) in the culture medium. Cells expressing the protein of interest were identified by labeling with [35S]-Easy Tag Express protein-labeling mix (NEN Life Science Products Inc., Boston, Mass.) and immunoprecipitating with anti-TR monoclonal antibodies (Caltag Laboratories, Burlingame, Calif.). Hygromycin-resistant, uncloned cells expressing the desired protein from each cDNA construct were used for experiments to avoid any clonal variation. Furthermore, uncloned cells were obtained from at least three independent transfections. The polarity of each transfected cell line used in the experiments was confirmed by determining the transepithelial resistance (36). For transient expression, T7 promoter and vaccinia virus expressing T7 polymerase were used. Pulse-chase analysis and analysis of endo H resistance of expressed proteins were carried out as described previously (15, 16).
Plasma membrane domain-selective biotinylation.
Cells grown (3 to 4 days) on Costar Transwell Clear Insert filter units were induced with 2 μM CdCl2 for 16 h, metabolically pulse-labeled for 2 h with 300 μCi [35S]-Easy Tag Express protein-labeling mix in serum-free DMEM lacking l-methionine and l-cysteine, and chased for 2 h in DMEM with 10% fetal bovine serum, and cell-surface proteins were assayed by biotinylation (15, 16). Briefly, cell monolayers were washed three times with ice-cold phosphate-buffered saline containing 0.1 mM CaCl2 and 1.0 mM MgCl2 (PBS-CM) and the apical or basolateral surface of parallel culture units was biotinylated twice for 30 min at 4°C with 1 mg of sulfosuccinimidyl 6-(biotinamido) hexanoate (EZ-Link Sulfo-NHS-LC-Biotin; Pierce Chemical Co., Rockford, Ill.) per ml in PBS-CM. The reaction was quenched by washing the cells with PBS-CM and incubating them with serum-free DMEM at 4°C for 10 min (40). The filters were then cut out of the holder, and the cells were lysed in radioimmunoprecipitation assay (RIPA) buffer by constant agitation for 60 min at 4°C. The lysates were centrifuged, and the supernatant was immunoprecipitated with monoclonal anti-TR antibodies. Immunoprecipitates were recovered with protein A-Sepharose (Pharmacia, Uppsala, Sweden) and washed three times with RIPA buffer. The immunoprecipitate was eluted from Sepharose by boiling twice in 100 μl of elution buffer (1% sodium dodecyl sulfate [SDS], 0.2 M Tris-HCl [pH 8.5], 5 mM EGTA) for 2 min and then washed with 300 μl of RIPA buffer. Both the eluates and the wash were pooled and incubated with 50 μl of a slurry of streptavidin-agarose beads (Pierce Chemical Co.) for 40 min at 4°C (15). The beads were then washed three times with RIPA buffer and boiled in sample buffer. The supernatant was analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and autoradiographed. Autoradiograms were quantified by densitometric analysis using a Personal Densitometer SI and the Image-QuaNT software program (Molecular Dynamics Inc., Sunnyvale, Calif.).
Assays for TX-100 insolubility.
For determining Triton X-100 (TX-100) insolubility, stable cell monolayers expressing the desired protein were extracted directly on tissue culture plates by a modification of the procedure described by Morrison and McGinnes (25). Briefly, stable cells expressing each protein were grown on 35-mm petri dishes for 2 days, induced with 2 μM CdCl2 for 16 h, labeled with [35S]-Easy Tag Express protein-labeling mix (150 μCi/ml) for 2 h, and chased for 2 h. The cells were extracted on ice with 0.5 ml of extraction buffer (50 mM NaCl, 3 mM KCl, 10 mM HEPES [pH 7.4], 2.5 mM MgCl2, 0.3 M sucrose) containing 1% TX-100 (Boehringer Mannheim, Mannheim, Germany) for 10 min, as described previously (1). For analysis of total proteins, parallel dishes were used and over 95% of the total proteins were recovered in combined TX-100-soluble and -insoluble fractions. TX-100-soluble and -insoluble proteins were immunoprecipitated using monoclonal anti-TR antibodies, analyzed by SDS-PAGE, and quantified by densitometric analysis.
RESULTS
Construction and expression of proteins containing the chimeric and mutant NA TMDs.
Influenza virus NA, when made into a secretory protein, was shown to be secreted apically, suggesting that the ectodomain of NA possesses an apical signal (15). Therefore, the NA ectodomain could not be used to analyze the apical signal(s) present in the TMD of NA because the presence of a potential apical signal(s) in the NA ectodomain would complicate the analysis of the NA TMD. In order to demonstrate that the NA TMD possessed an apical signal, we used a reporter protein, the TR ectodomain, which did not possess either an apical or basolateral signals. This was demonstrated in TRΔ57, a deletion mutant, which lacked 55 amino acids (aa) (aa 3 to 57) of the cytoplasmic tail possessing the basolateral and endocytic signals (28). TRΔ57 possessed the TMD and ectodomain of TR and only 6 out of the 61 amino acids of the cytoplasmic tail (Fig. 1). This tail-negative mutant was transported randomly to both apical and basolateral membranes in polarized MDCK cells, demonstrating that TRΔ57 lacked apical and basolateral signals in either the TMD or the ectodomain of TR. To characterize the role of the NA TMD, the TR TMD of TRΔ57 was swapped with the NA TMD and the resulting construct NA(gs)TR (no. 4) (Fig. 1), previously called TRΔ57NATR (16), containing only the TMD of NA was targeted to the apical plasma membrane, demonstrating that the NA TMD was capable of directing a reporter protein (i.e., the TR ectodomain) to the apical plasma membrane. This experiment clearly demonstrated the presence of an apical signal(s) in the TMD of NA (16). In the present studies, this NATR construct containing the NA TMD and the TR ectodomain was used for further analysis of the NA TMD to define the signals for apical transport and raft association.
Chimeric constructs within the NA TMD were created by swapping parts of the TMD of NA with that of TR. As mentioned in Materials and Methods, restriction sites were created in both the NA and TR TMDs and specific portions (one-fourth to three-fourths of the TMD from either end) were swapped, creating the TMD chimeras (no. 5 to 8) (Fig. 1). Creation of restriction enzyme sites also caused mutation of one or two amino acids, as noted in Fig. 1. To further investigate the region of NA TMD and the amino acid sequences involved in apical sorting, we systematically mutagenized the entire NA TMD by converting blocks of four or five contiguous amino acids to alanine (no. 9 to 15) (Fig. 1). In earlier experiments, NA(gs)TR (previously called TRΔ57NATR [16]) possessed two altered residues (G7S8) in place of those (I7I8) present in the wild-type NA TMD because of the creation of a restriction site. Although these two residues had no effect on the apical transport of the NA TMD, we mutated these residues back to the wild-type residues I7I8 to avoid any possible effect on alanine mutation. Initially, each construct was transiently expressed, pulse-labeled, and chased and the intracellular transport to Golgi was monitored by the migration behavior in SDS-PAGE. Mature proteins migrated slower than the immature proteins in SDS-PAGE (see Fig. 2, 3, 4). We also noted that endo H resistance mimicked the slow migration behavior of mature proteins in SDS-PAGE (data not shown). Immunoprecipitation data showed that mutant proteins were expressed efficiently, with no more than a two- to threefold difference, and exhibited similar transport behavior, as is evident from the endo H resistance and migration behavior in SDS-PAGE. However, two proteins, NA5A11 (no. 10) and NA4A23 (no. 13) were expressed significantly but rather inefficiently transported. Of these, NA5A11 was severely defective in transport and required 6 h of chase for 40% maturation both in transient and stable expression (Fig. 2). The other protein, NA4A23, also exhibited slower maturation, but to a lesser extent than NA5A11, producing 70% mature forms after a 2-h chase, whereas all other proteins exhibited 90% or more maturation by that time. Similar transport behavior of proteins was also observed in stable cell expression (Fig. 2). Mutation in the TMD could affect conformation, including oligomerization, and cause slow transport. A similar transport defect in a HA TMD mutant was previously observed (2A514 [18]).
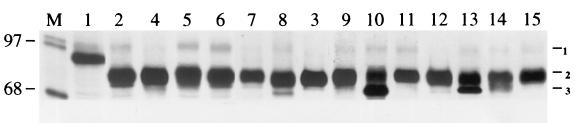
FIG. 2.
Expression of different chimeric and mutant proteins in stable MDCK cells. Stable, transfected MDCK cells were established and cultured for 2 days and induced for 16 h with 2 μM of CdCl2. Cells were then pulse-labeled with 150 μCi of [35S]-Easy Tag Express protein-labeling mix for 1 h, followed by a 2-h chase (except for construct no. 10, which was chased for 6 h). Cells were then lysed, and the lysates were immunoprecipitated, analyzed by SDS-PAGE, and autoradiographed. Lane M, standard protein marker; lanes 1 to 15, constructs 1 to 15, respectively (numbering of constructs is shown in Fig. 1). The numbers on the righthand side represent the following: 1, nonspecific band; 2, mature protein band; 3, immature protein band (construct no. 2 to 15). Note that there is some migration variation among the different chimeric and mutant proteins.
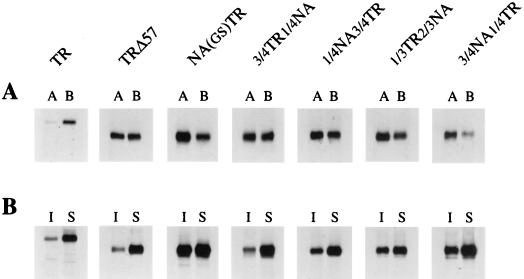
FIG. 3.
Polarized cell surface distribution (A) and TX-100 extraction (B) of chimeric NA TMD proteins. For panel A, confluent monolayers of MDCK cell lines were grown on filters for 3 to 4 days, induced for 16 h with 2 μM CdCl2, pulse-labeled for 2 h with 300 μCi of [35S]-Easy Tag Express protein-labeling mix and chased for 2 h. The apical (lanes A) and basolateral (lanes B) surface proteins from parallel cultures were biotinylated, isolated by anti-TR antibodies, analyzed by SDS-PAGE, and autoradiographed. For panel B, cells were grown on 35-mm petri dishes for 2 days and induced with 2 μM of CdCl2 for 16 h, pulse-labeled with 150 μCi of [35S]-Easy Tag Express protein-labeling mix for 2 h, followed by a 2-h chase. Cells were extracted on ice with extraction buffer containing 1% TX-100 for 10 min, as described in Materials and Methods. TX-100 insoluble (lanes I) and soluble (lanes S) proteins were immunoprecipitated with anti-TR antibodies, analyzed by SDS-PAGE, and autoradiographed.
FIG. 4.
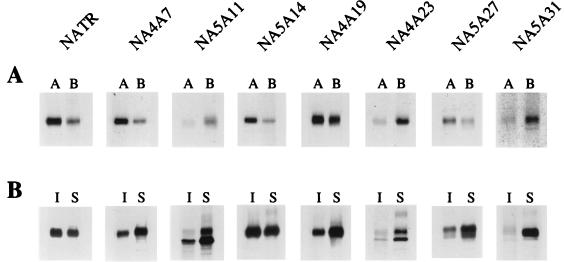
Polarized surface distribution (A) and TX-100 extraction (B) of mutant NA TMD proteins. Experimental conditions were as described for Fig. 3, except that the mutant protein NA5A11 was chased for 6 h. Lanes: A, apical surface proteins; B, basolateral surface proteins; I, insoluble proteins; S, soluble proteins.
Apical targeting of proteins containing chimeric NA TMDs.
To determine the apical and basolateral distribution of chimeric proteins, each chimeric protein was expressed in stable MDCK cell lines using metallothionein promoter. Stable MDCK cell lines expressing each chimeric protein were grown as confluent monolayers on filters and exhibited behavior similar to that of polarized MDCK cells, as determined by transepithelial resistance. The expression level and protein maturation of chimeric proteins (no. 5 to 8) in stable MDCK cell lines, as determined by pulse-chase labeling, immunoprecipitation, and migration in SDS-PAGE, were similar to that observed in transient expression and varied no more than two- to threefold (Fig. 2). The apical and basolateral distribution of each chimeric protein was determined by domain-specific surface biotinylation, as described in Materials and Methods. Apical distribution and basolateral distribution were not affected by expression level. Autoradiographs from typical experiments are shown in Fig. 3A. Quantification by densitometric analysis of bands was done from a number of independent experiments (Table 1). These results show that 3/4TR1/4NA (no. 5) was missorted randomly to both apical and basolateral membranes in polarized MDCK cells and behaved like TRΔ57 (no. 2) containing the TR TMD. Proteins 1/4NA3/4TR (no. 6) and 1/3TR2/3NA (no. 7) exhibited an intermediate behavior with respect to apical transport, and about 60% of the proteins were transported to the apical plasma membrane. On the other hand, protein 3/4NA1/4TR (no. 8) behaved like NATR containing the wild-type NA TMD in apical sorting (≥70% apical). These data taken together would suggest that the entire NA TMD was not needed for apical transport and that the NH2-terminal three-fourths of the NA TMD (aa 9 to 27) could efficiently transport the protein to the apical plasma membrane.
TABLE 1.
Polarized surface distribution and Triton X-100 insolubility of chimeric and mutant proteinsa
| Construct no. | Protein | No. of expts | Avg amt of apically transported protein ± SD (%) | No. of expts | Avg amt of TX-100-insoluble protein ± SD (%) |
|---|---|---|---|---|---|
| 1 | TR | 4 | 18 ± 4 | 5 | 8 ± 3 |
| 2 | TRΔ57 | 4 | 49 ± 3 | 5 | 19 ± 4 |
| 3 | NATR | 5 | 78 ± 4 | 4 | 60 ± 3 |
| 4 | NA(gs)TR | 4 | 74 ± 3 | 5 | 44 ± 3 |
| 5 | 3/4TR1/4NA | 4 | 49 ± 2 | 4 | 15 ± 2 |
| 6 | 1/4NA3/4TR | 4 | 61 ± 5 | 4 | 25 ± 6 |
| 7 | 1/3TR2/3NA | 3 | 59 ± 2 | 5 | 35 ± 4 |
| 8 | 3/4NA1/4TR | 3 | 74 ± 5 | 4 | 30 ± 4 |
| 9 | NA4A7 | 4 | 74 ± 2 | 5 | 32 ± 2 |
| 10 | NA5A11 | 5 | 20 ± 5 | 5 | 20 ± 6 |
| 11 | NA5A14 | 4 | 77 ± 4 | 5 | 54 ± 3 |
| 12 | NA4A19 | 5 | 61 ± 5 | 5 | 31 ± 4 |
| 13 | NA4A23 | 3 | 21 ± 4 | 3 | 20 ± 5 |
| 14 | NA5A27 | 3 | 69 ± 2 | 4 | 22 ± 4 |
| 15 | NA5A31 | 3 | 20 ± 3 | 5 | 8 ± 4 |
Confluent monolayers of MDCK cell lines were grown on filters, induced with CdCl2, pulse-labeled, and chased (Fig. 3 and 4). For polarized surface distribution, apical and basolateral surface proteins were biotinylated, isolated by anti-TR antibodies, analyzed by SDS-PAGE, autoradiographed, and quantified as described in Materials and Methods. For TX-100 insolubility, cells were extracted on ice with extraction buffer containing 1% TX-100 for 10 min and insoluble and soluble proteins were precipitated with anti-TR antibodies, analyzed by SDS-PAGE, and quantified as described in Materials and Methods.
Mutational analysis of the NA TMD in apical sorting.
To further analyze the role of specific sequences in NA TMD, alanine mutants (no. 9 to 15) (Fig. 1) were stably expressed in MDCK cells. The expression of three mutant proteins (no. 10, 13, and 15) varied in stable cell lines. Again, as in transient expression, NA5A11 (no. 10), although expressed efficiently, was partially blocked in transport, as noted before, and therefore, in all experiments, this protein was chased for 6 h before used for surface biotinylation or TX-100 extraction. Slow maturation of this protein was evident from the migration behavior of protein bands in SDS-PAGE (Fig. 2 and 4B). The expression levels of proteins NA4A23 (no. 13) and NA5A31 (no. 15) varied and depended on the passage level in stable MDCK cells. Early-passaged cells expressed these proteins efficiently, but late-passaged cells exhibited poor expression. However, there was no difference in the intracellular transport behavior or the apical and basolateral distribution or TX-100 insolubility of these proteins in either early- or late-passaged polarized MDCK cells. Other mutant proteins behaved like the wild-type protein with respect to expression and maturation in stable MDCK cells. The apical distribution and basolateral distribution of mutant and wild-type proteins were determined by surface biotinylation, as described in Materials and Methods. The results of a typical experiment are depicted in Fig. 4A. Quantification by densitometric analysis of autoradiographs from a number of independent experiments is shown in Table 1. These results demonstrate that mutation in the first four amino acids (NA4A7) did not affect apical targeting. This is also supported from earlier results for NA(gs)TR possessing mutations in the first two residues (16). Both of these proteins (no. 4 and 9) exhibited apical targeting similar to that of NATR containing the wild-type NA TMD. Similarly, NA5A14 (no. 11) and NA5A27 (no. 14) also behaved like the wild-type NA TMD in apical targeting. Protein NA4A19 (no. 12), on the other hand, exhibited an intermediate phenotype, with 61% of the proteins transported to the apical plasma membrane. Surprisingly, however, mutant NA5A11 (no. 10), NA4A23 (no. 13), and NA5A31 (no. 15) proteins behaved differently and sorted efficiently (about 80%) to the basolateral surface (Table 1). These results indicate that amino acids at positions 11 to 13, 23 to 26, and 32 to 35 were critical for apical transport. However, it should be noted that some mutations including alanine substitutions could potentially affect the structure of TMD and thereby indirectly interfere with the polarized sorting of the mutated protein.
Raft association with the NA TMD.
It has been shown that many apical proteins with either GPI anchor or TMD anchor associate with detergent-resistant lipid rafts enriched in glycosphinolipids and cholesterol, while basolateral proteins are excluded from such lipid rafts. The TMD of influenza virus HA was shown to associate with detergent-resistant lipid rafts in both nonpolar fibroblasts (35) and polarized MDCK cells (18). Furthermore, in both types of cells, it was shown that the amino acid sequences of HA TMD spanning the outer leaflet of the lipid bilayer were critical for association with lipid rafts, whereas the sequences spanning the inner leaflet of the lipid bilayer were relatively less important for raft association. Furthermore, Lin et al. (18) have shown that, for influenza virus HA, raft association was necessary but not sufficient for apical transport. In earlier studies, members of our group reported that the NA TMD also associated with detergent insoluble lipid rafts and that chimeric constructs that were missorted or sorted basolaterally, did not associate with rafts, and became largely TX-100 soluble (16). Therefore, we wanted to determine which sequences in the NA TMD were essential for raft association and whether raft association was critical for apical transport. All experiments were done in stable MDCK cell lines expressing the mutant proteins. The cells were labeled for 2 h and chased for 2 h so that ≥90% of the protein acquired mature conformation (except for NA5A11 and NA4A23), as indicated earlier. Since the raft association occurred in the TGN, it was critical that the labeled protein assayed for detergent insolubility was present in the TGN and plasma membrane, as determined by the slow migration of protein in SDS-PAGE (Fig. 2). Furthermore, pulse and chase conditions used for TX-100 extraction were essentially the same as those used for apical and basolateral distribution indicating that the labeled proteins were transported to the apical or basolateral plasma membrane as discussed above (Fig. 3A and 4A). Results of typical TX-100 extraction experiments are depicted in Fig. 3B and 4B. NATR (no. 3) containing the wild-type NA TMD exhibited approximately 60% TX-100 insolubility, whereas TR assayed under similar conditions was consistently less than 10% detergent insoluble. Also, under our conditions for detergent extraction, HA was approximately 60% insoluble (data not shown), as has been reported by others (18, 35). We also observed that 3/4TR1/4NA (no. 5), which was transported randomly to both plasma membranes, was consistently highly TX-100 soluble (Table 1). Among the mutants and chimeras of NA TMD, we observed three classes of detergent insolubility: 44 to 60% (no. 3, 4, and 11), 20 to 35% (no. 6 to 10 and 12 to 14) and ≤15% (no. 5 and 15). None of the proteins containing chimeric or mutant NA TMD exhibited 60% or above detergent insolubility as was found for NATR containing wild-type NA TMD. Proteins NA(gs)TR (no. 4) and NA5A14 (no. 11), containing mutant NA TMD, exhibited variation in TX-100 insolubility (44 and 54% insoluble, respectively), although these proteins transported apically with the same efficiency (i.e., >70%) as NATR (no. 3), suggesting that the efficiency of apical transport may not correlate with raft association. Another group of chimeras or mutants exhibited 20 to 35% TX-100 insolubility. Among these proteins, mutants NA4A7 (no. 9) and NA5A27 (no. 14) exhibited essentially wild-type apical transport, (74 and 69%, respectively) but only intermediate detergent insolubility (32 and 22%, respectively). Similarly, another chimeric construct 3/4NA1/4TR (no. 8), which exhibited 74% apical transport, was only 30% detergent insoluble. Chimeric constructs 3/4TR1/4NA (no. 5) and TRΔ57 (no. 2), which exhibited random distribution to both apical and basolateral membranes, were predominantly TX-100 soluble. These results, taken together, would suggest that apical transport and raft association were not tightly coupled and that factors other than those involved with detergent insolubility may also promote apical transport. However, we observed that all mutant proteins exhibiting missorting and disruption of apical transport consistently exhibited a low level of detergent insolubility (≤20%) and that no missorted proteins became associated with raft, as determined by TX-100 resistance.
DISCUSSION
Our results confirm that neither the TMD nor the ectodomain of TR contains any apical or basolateral signal, as is evident from the random distribution of TRΔ57 and of 3/4TR1/4NA chimera lacking the basolateral signal in its cytoplasmic tail. These data support the idea that neither the amino acid sequence nor glycans present in the ectodomain TR possess any apical signal and that the chimeric NATR (previously called TRΔ57NATR [16]) protein containing the NA TMD and the TR reporter protein provides an excellent construct for studying the apical signal(s) in a transmembrane peptide domain.
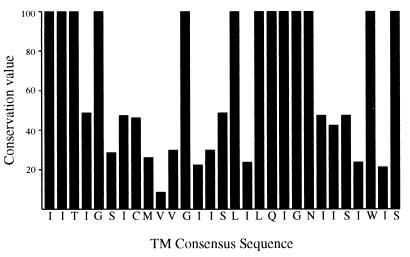
Sequence comparison of the NA TMD shows the presence of some highly conserved residues. These conserved sequences are present in clusters and dispersed predominantly in two separate regions, aa 7 to 14 at the NH2 terminus and aa 22 to 28 in the COOH half of the TMD (Fig. 5). The least conserved residues are present in the middle of the TMD. It should be also noted that the 6-amino-acid cytoplasmic tail just outside the TMD is also highly conserved although no functional significance has been attributed to this region.
FIG. 5.
Conservation of amino acids in NA TMD. TMD sequences of 37 NA (subtype N1) proteins were compared. For each position, a conservation value was calculated by dividing the percentage of sequences having the most common amino acid by the number of different amino acids found at that position as described by Lin et al. (18).
Our results show that raft association, as measured by TX-100 resistance, was the property of the NA TMD and that critical regions in the NA TMD are involved in protein-lipid interactions. TX-100 resistance has been shown to be due to association of the amino acid sequence with the exoplasmic lipid leaflet containing cholesterol and glycosphingolipids (35). Our data showing that 5 amino acids (aa 14 to 18) in the cytoplasmic half of TMD (NA5A14) were not critical for raft interaction support the idea that the mutation of residues interacting with the cytoplasmic lipid leaflet is well tolerated for TX-100 resistance. However, it should be noted that unlike the 18 to 20 TMD residues of type I transmembrane proteins, the type II NA TMD possesses an extended hydrophobic sequence of 29 amino acids and the precise boundary of interaction of these residues to the inner and outer lipid leaflet has not been determined. In the NA TMD, two dispersed regions appear to affect the interaction of NA TMD with the lipid rafts. (i) Mutation in residues 19 to 35 considerably affected the TX-100 resistance as evident from the behavior of 3/4NA1/4TR, NA4A19, NA4A23, NA5A27, and NA5A31 (Table 1; Fig. 6). This effect could be explained due to disruptions of the interaction of the residues at the COOH terminus of TMD of NA with the exoplasmic lipid bilayer. (ii) However, the highly conserved residues at the extreme NH2 terminus of NA TMD were also critical for TX-100 resistance (Fig. 6). This is evident from the behavior of a number of mutants like NA(gs)TR, NA4A7, NA5A11, and other chimeras containing the G7S8 mutation (Table 1). Therefore, we think that although the contact of residues with the exoplasmic lipid leaflet is critical for raft association, the residues at the cytoplasmic end may affect the stability of NA TMD interaction with lipid raft. Furthermore, removal of the cytoplasmic tail of HA or NA considerably reduces the TX-100 resistance of these proteins (R. Lamb, personal communication; D. P. Nayak, unpublished data). Increased TX-100 resistance of TRΔ57 (19%) compared to that of TR (8%) also supports the idea that not only the TR TMD sequence but also the cytoplasmic tail of TR affected the interaction of TMD with the lipid raft. Therefore, the data presented here support the idea that, in addition to the sequences of TMD interacting with the exoplasmic lipid leaflet, the highly conserved cytoplasmic tail and the adjacent amino acid sequences may aid in stabilizing the interaction of TMD with lipid raft. Our analysis of the NA TMD for apical signal show that the apical signal is not a single discrete sequence consisting of a few residues; rather it is dispersed throughout the NA TMD. The varying degree of apical transport of different mutants and chimeras also support this concept of the involvement of NA TMD over an extended region in apical transport. Our results show that the NH2-terminal three-fourths of the NA TMD (aa 9 to 27) is sufficient to provide the apical transport signal (no. 8) (Table 1). Amino acid residues 14 to 18 and 27 to 31 have the least effect on apical transport. On the other hand, three discrete regions, aa 11 to 13, 23 to 26, and 32 to 35 are most critical for apical transport (Table 1; Fig. 6).
FIG. 6.
The effect of alteration of NA TMD sequences on apical sorting and raft association. The increasing height or depth of boxes indicates the increasing effects of those amino acids.
Our data show that mutations and chimeras exhibited variations in both the degree of apical transport and the degree of TX-100 resistance but that these variations were not tightly coupled, i.e., the degree of apical transport did not match the degree of TX-100 resistance. However, we did not observe any randomly sorted mutants exhibiting a high degree of TX-100 resistance, suggesting that all missorted proteins were excluded from rafts. Similarly, none of the proteins which were completely excluded from raft association as shown from their low TX-100 insolubility (no. 5 and 15) (Table 1) sorted apically, showing that some degree of raft association was necessary for apical sorting of these proteins. Also, proteins exhibiting an intermediate level (about 60%) of apical transport (e.g., no. 6, 7, and 12 [Table 1]) also exhibited intermediate TX-100 resistance (25 to 35%). However, proteins exhibiting the wild-type apical transport did not always exhibit the wild-type level of TX-100 resistance. Rather these apical proteins exhibited a great deal of variation in TX-100 resistance. Some proteins (3/4NA1/4TR and NA4A7) exhibiting the wild-type level of apical transport were only half as TX-100 resistant as NATR containing the wild type NA TMD. Furthermore, one mutant (NA5A27) exhibiting predominantly apical phenotype was highly TX-100 soluble (Table 1). These results show that although both the apical sorting signal and the sequences for raft association reside in the NA TMD, they are not identical and vary independently. Furthermore, our results also support the idea that the apical sorting signal(s) in the TMD can be recognized by the apical sorting machineries outside the raft and that a high degree of raft-association is not an obligatory requirement for apical sorting, as seen for some NA TMD mutants.
Overall the data presented here support the involvement of multiple steps in lipid raft association as well as interaction with apical sorting machineries, as has been proposed recently for MDCK cells (18). Although it is clear that apical and basolateral sorting take place in the TGN, forming separate vesicles destined for apical or basolateral surface delivery, different apical proteins take divergent routes to reach the apical plasma membrane. The majority of the membrane-anchored apical proteins use cholesterol glycosphingolipid-enriched lipid rafts as a platform for apical transport. However, some proteins need not interact with these lipid rafts but can still be transported to the apical surface. For these proteins, the apical sorting machineries are proposed to interact with the glycans present in the ectodomain of these proteins, as has been shown for bovine enteropeptidase (39). However, there appears to be variation among the apical proteins, which interact with lipid rafts. Both transmembrane and GPI-anchored proteins have been shown to interact with lipid rafts and apically transported. For GPI-anchored proteins lacking the transmembrane peptide, high-affinity interaction with cholesterol-enriched lipid rafts and efficient partitioning in these lipid rafts may be sufficient for sorting and the cargo protein may be carried along with the lipid rafts to the apical membrane. These proteins may not require any other apical signals. However, for either type I (influenza HA) or type II (influenza NA) transmembrane proteins, interaction with lipid rafts alone may not be sufficient for apical transport and interaction with apical sorting machinery may further facilitate apical transport. It was shown that some HA mutants exhibited a wild-type level of TX-100 resistance but were not transported apically (18). However, we did not observe any such mutants with NA TMD. The data on TX-100 resistance and apical transport of NA TMD mutants presented here suggest that tight association and stable interaction of all apical proteins with cholesterol-enriched lipid raft are not necessary for apical transport. A similar conclusion can be derived from the apical transport of influenza virus HA in cholesterol-depleted cells (18). Taken together, these results would support that apical sorting machinery outside the lipid raft may interact with apical signals present in the TMD of HA and NA in transporting the protein to the apical membrane. This would support the presence of different apical sorting machineries including the existence of apical sorting machinery outside the lipid raft.
The observation that the mutants NA5A11, NA4A23, and NA5A31 were transported predominantly to the basolateral surface of MDCK cells could be explained either by exclusion from apical transport pathways, forcing into the basolateral pathway, or by creation of a positive basolateral signal that allowed active basolateral sorting. There has been no evidence presented for either a cryptic basolateral sorting signals in NA or for the existence of basolateral sorting signals in TMD. However, there is evidence that efficient basolateral sorting of Na+/K+ ATPase (23) and HA 2A520 mutant (18) requires an intact apical pathway containing detergent-insoluble glycolipid- and cholesterol-rich complexes, which presumably excludes and forces those proteins into the basolateral pathway. Similar pathways may be involved in the basolateral sorting of these NA TMD mutants. This could also explain why NA5A31 (no. 15) (Table 1), which contains the NA TMD region capable of apical sorting (no. 8) (Table 1), was transported basolaterally. Alanine substitutions in this mutant drastically disrupted the raft association (only 8% TX-100 insoluble), thereby driving the protein to the basolateral membrane.
In conclusion, studies reported here show that NA TMD does not contain a single discrete apical signal; rather, multiple regions in the NA TMD function for apical transport (Fig. 6). Although raft association may play an important role in apical transport, raft association and apical transport are not tightly coupled and NA TMD can interact with apical sorting machinery outside the lipid raft. Finally, these studies were done using a reporter protein for reasons stated earlier. The effect of these TMD mutations on the wild-type viral NA possessing the apical signal(s) in its ectodomain as well as on the life cycle of influenza virus remains to seen. Such studies are under way.
ACKNOWLEDGMENTS
These studies were partially supported by NIAID/NIH grants AI-16348 and AI-41681.
We thank Eleanor Berlin for typing the manuscript.
REFERENCES
- 1.Avalos R T, Yu Z, Nayak D P. Association of influenza virus NP and M1 proteins with cellular cytoskeletal elements in influenza virus-infected cells. J Virol. 1997;71:2947–2958. doi: 10.1128/jvi.71.4.2947-2958.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartles J R, Feracci H M, Stieger B, Hubbard A L. Biogenesis of the rat hepatocyte plasma membrane in vivo: comparison of the pathways taken by apical and basolateral proteins using subcellular fractionation. J Cell Biol. 1987;105:1241–1251. doi: 10.1083/jcb.105.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benting J H, Rietveld A G, Simons K. N-Glycans mediate the apical sorting of a GPI-anchored, raft-associated protein in Madin-Darby canine kidney cells. J Cell Biol. 1999;146:313–320. doi: 10.1083/jcb.146.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewer C B, Roth M G. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutin. J Cell Biol. 1991;114:413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown D A, Rose J K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 6.Casanova J E, Apodaca G, Mostov K E. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell. 1991;66:65–75. doi: 10.1016/0092-8674(91)90139-p. [DOI] [PubMed] [Google Scholar]
- 7.Casey P I. Protein lipidation in cell signaling. Science. 1995;268:221–224. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- 8.Cheong K H, Zacchetti D, Schneeberger E E, Simons K. VIP17/MAL, a lipid raft-associated protein, is involved in apical transport in MDCK cells. Proc Natl Acad Sci USA. 1999;96:6241–6248. doi: 10.1073/pnas.96.11.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fölsch H, Ohno H, Bonifacino J S, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- 10.Huang X F, Compans R W, Chen S, Lamb R A, Arvan P. Polarized apical targeting directed by the signal/anchor region of simian virus 5 hemagglutinin-neuraminidase. J Biol Chem. 1997;272:27598–27604. doi: 10.1074/jbc.272.44.27598. [DOI] [PubMed] [Google Scholar]
- 11.Hunziker W, Harter C, Matter K, Mellman I. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell. 1991;66:907–920. doi: 10.1016/0092-8674(91)90437-4. [DOI] [PubMed] [Google Scholar]
- 12.Ikonen E, Tagaya M, Ullrich O, Montecucco C, Simons K. Different requirements for NSF, SNAP, and Rab proteins in apical and basolateral transport in MDCK cells. Cell. 1995;81:571–580. doi: 10.1016/0092-8674(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 13.Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kundu A, Jabbar M A, Nayak D P. Cell surface transport, oligomerization, and endocytosis of chimeric type II glycoproteins: role of cytoplasmic and anchor domains. Mol Cell Biol. 1991;11:2675–2685. doi: 10.1128/mcb.11.5.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kundu A, Nayak D P. Analysis of the signals for polarized transport of influenza virus (A/WSN/33) neuraminidase and human transferrin receptor, type II transmembrane proteins. J Virol. 1994;68:1812–1818. doi: 10.1128/jvi.68.3.1812-1818.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kundu A, Avalos R T, Sanderson C M, Nayak D P. Transmembrane domain of influenza virus neuraminidase, a type II protein, possesses an apical sorting signal in polarized MDCK cells. J Virol. 1996;70:6508–6515. doi: 10.1128/jvi.70.9.6508-6515.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Bivic A, Quaroni A, Nichols B, Rodriguez-Boulan E. Biogenetic pathways of plasma membrane proteins in Caco-2, a human intestinal epithelial cell line. J Cell Biol. 1990;111:1351–1361. doi: 10.1083/jcb.111.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin S, Naim H Y, Rodrigues A C, Roth M G. Mutations in the middle of the transmembrane domain reverse the polarity of transport of the influenza virus hemagglutinin in MDCK epithelial cells. J Cell Biol. 1998;142:51–57. doi: 10.1083/jcb.142.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marzolo M P, Bull P, Gonzalez A. Apical sorting of hepatitis B surface antigen (HBsAg) is independent of N-glycosylation and glycosylphosphatidylinositol-anchored protein segregation. Proc Natl Acad Sci USA. 1997;94:1834–1839. doi: 10.1073/pnas.94.5.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matlin K S, Simons K. Sorting of an apical plasma membrane glycoprotein occurs before it reaches the cell surface in cultured epithelial cells. J Cell Biol. 1984;99:2131–2139. doi: 10.1083/jcb.99.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matter K, Brauchbar M, Bucher K, Hauri H P. Sorting of endogenous plasma membrane proteins occurs from two sites in cultured human intestinal epithelial cells (Caco-2) Cell. 1990;60:429–437. doi: 10.1016/0092-8674(90)90594-5. [DOI] [PubMed] [Google Scholar]
- 22.Matter K, Hunziker W, Mellman I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992;71:741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- 23.Mays R W, Siemers K A, Fritz B A, Lowe A W, van Meer G, Nelson W J. Hierarchy of mechanisms involved in generating Na/K-ATPase polarity in MDCK epithelial cells. J Cell Biol. 1995;130:1105–1115. doi: 10.1083/jcb.130.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miguel A A, Fan L, Alarcón B. Multiple sorting signals determine apical localization of a nonglycosylated integral membrane protein. J Biol Chem. 1997;272:30748–30752. doi: 10.1074/jbc.272.49.30748. [DOI] [PubMed] [Google Scholar]
- 25.Morrison T G, McGinnes L J. Cytochalasin D accelerates the release of newcastle disease virus from infected cells. Virus Res. 1985;4:93–106. doi: 10.1016/0168-1702(85)90023-1. [DOI] [PubMed] [Google Scholar]
- 26.Mostov K, Apodaca G, Aroeti B, Okamoto C. Plasma membrane protein sorting in polarized epithelial cells. J Cell Biol. 1992;116:577–583. doi: 10.1083/jcb.116.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naim H Y, Joberty G, Alfalah M, Jacob R. Temporal association of the N- and O-linked glycosylation events and their implication in the polarized sorting of intestinal brush border sucrase-isomaltase, aminopeptidase N, and dipeptidyl peptidase IV. J Biol Chem. 1999;274:17961–17967. doi: 10.1074/jbc.274.25.17961. [DOI] [PubMed] [Google Scholar]
- 28.Odorizzi G, Trowbridge I S. Structural requirements for basolateral sorting of the human transferrin receptor in the biosynthetic and endocytic pathways of Madin-Darby canine kidney cells. J Cell Biol. 1997;137:1255–1264. doi: 10.1083/jcb.137.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prill V, Lehmann L, von Figura K, Peters C. The cytoplasmic tail of lysosomal acid phosphatase contains overlapping but distinct signals for basolateral sorting and rapid internalization in polarized MDCK cells. EMBO J. 1993;12:2181–2193. doi: 10.1002/j.1460-2075.1993.tb05866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rindler M J, Ivanov I E, Plesken H, Rodriguez-Boulan E, Sabatini D D. Viral glycoproteins destined for apical or basolateral plasma membrane domains traverse the same Golgi apparatus during their intracellular transport in doubly infected Madin-Darby canine kidney cells. J Cell Biol. 1984;98:1304–1319. doi: 10.1083/jcb.98.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodgers W, Rose J K. Signals determining protein tyrosine kinase and glycosyl-phosphatidyl-anchored protein targeting to a glycoipid-enriched membrane fraction. Mol Cell Biol. 1994;14:5384–5391. doi: 10.1128/mcb.14.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Boulan E, Nelson W J. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;254:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- 33.Sarkar G, Sommer S S. The “megaprimer” method of site-directed mutagenesis. BioTechniques. 1993;8:404–407. [PubMed] [Google Scholar]
- 34.Scheiffele P, Peränen J, Simons K. N-glycans as apical sorting signals in epithelial cells. Nature (London) 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- 35.Scheiffele P, Roth M G, Simons K. Interaction of influenza virus hemagglutinin with sphingolipid-cholesterol membrane via transmembrane domain. EMBO J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon K, Virta H. Growing Madin-Darby canine kidney cells for studying epithelial cell biology. In: Celis J E, editor. Cell biology: a laboratory handbook. New York, N.Y: Academic Press, Inc.; 1994. pp. 225–231. [Google Scholar]
- 37.Simons K, Wandingerness A. Polarized sorting in epithelia. Cell. 1990;62:207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- 38.Simons K, Ikonen E. Functional rafts in cell membranes. Nature (London) 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 39.Zheng X, Lu D, Sadler J E. Apical sorting of bovine enteropeptidase does not involve detergent-resistant association with sphingolipid-cholesterol rafts. J Biol Chem. 1999;274:1596–1605. doi: 10.1074/jbc.274.3.1596. [DOI] [PubMed] [Google Scholar]
- 40.Zurzolo C, Le Bivic A, Rodriguez-Boulan E. Cell surface biotinylation techniques. In: Celis J E, editor. Cell biology: a laboratory handbook. New York, N.Y: Academic Press, Inc.; 1994. pp. 185–192. [Google Scholar]