Abstract
Background
Focal segmental glomerulosclerosis (FSGS) is a histological pattern of glomerular damage that includes idiopathic conditions as well as genetic and non-genetic forms. Among these various etiologies, different phenotypes within the spectrum of congenital anomalies of the kidney and urinary tract (CAKUT) have been associated with FSGS.
Summary
Until recently, the main pathomechanism of how congenital kidney and urinary tract defects lead to FSGS was attributed to a reduced number of nephrons, resulting in biomechanical stress on the remaining glomeruli, detachment of podocytes, and subsequent inability to maintain normal glomerular architecture. The discovery of deleterious single-nucleotide variants in PAX2, a transcription factor crucial in normal kidney development and a known cause of papillorenal syndrome, in individuals with adult-onset FSGS without congenital kidney defects has shed new light on developmental defects that become evident during podocyte injury.
Key Message
In this mini-review, we challenge the assumption that FSGS in CAKUT is caused by glomerular hyperfiltration alone and hypothesize a multifactorial pathogenesis that includes overlapping cellular mechanisms that are activated in both damaged podocytes as well as nephron progenitor cells.
Keywords: Podocyte injury, Congenital anomalies of the kidney and urinary tract, Genetics, Phenotypic expansion
Introduction
Focal segmental glomerulosclerosis (FSGS) describes a histological pattern of glomerular impairment consequential to podocyte injury and detachment [1]. FSGS is clinically characterized by (nephrotic range) proteinuria and loss of glomerular filtration rate due to progressive sclerosis in a subset of glomeruli [2], which reflects the inability of damaged podocytes to maintain the complex glomerular architecture. Podocytes are postmitotic cells with the ability to replicate DNA, but incapable of completing cytokinesis [3]. In case of podocyte loss, a subset of parietal epithelial cells positioned along Bowman’s capsule, known as podocyte progenitors, can produce new podocytes [4]. Nevertheless, the process of podocyte regeneration often proves to be inefficient and may contribute to the development of FSGS. As such, podocytopathies are among the most frequent causes of kidney failure in adults and children.
Historically, FSGS lesions are classified according to their various etiologies [5]. It is hypothesized that permeability factor-mediated forms of FSGS are caused by an auto-immune response due to (an) unknown circulating factor(s) (for a recent review, see [6]). In addition, a variety of direct (toxic) causes of podocyte injury such as viral infections (e.g., HIV1 virus, hepatitis C virus, Epstein-Barr virus, cytomegalovirus, parvovirus B19), the use of nephrotoxic agents (e.g., lithium, heroin, anabolic steroids), or hyperfiltration-mediated injury due to obesity, congenital heart disease, or sickle cell anemia have been associated with FSGS. In addition, the advent of genomic technologies has now uncovered pathogenic single-nucleotide variants (SNVs) in more than 80 podocyte-associated genes that cause genetic FSGS. Most familial forms of FSGS follow a Mendelian pattern of inheritance. However, also non-Mendelian forms have been described, which include high-risk APOL1 genotypes in people who identify as black, African American, Afro-Caribbean, and/or Latina/Latino. As is expected from the pathogenesis of FSGS, the vast majority of implicated genes are expressed in podocytes and play a role in their cell function, including maintaining foot process interdigitation with those from neighboring podocytes, forming the glomerular slit membrane and stabilization of the actin cytoskeleton. An extensive discussion of all identified genetic factors that play a role in podocytopathies is beyond the scope of this manuscript, but has been elegantly presented in [4].
Of note, the clinical implementation of genetic testing in podocytopathies has additionally resulted in phenotypic expansion of several genes that were previously attributed to other genetic kidney diseases, such as Alport syndrome (COL4A3-5), Dent’s disease (CLCN5, OCRL), and autosomal dominant tubulointerstitial disease (UMOD) [7–10]. In this perspective, the recent observations that pathogenic SNVs in PAX2, a gene historically associated with syndromic congenital anomalies of the kidney and urinary tract (CAKUT) [11, 12], cause isolated forms of genetic FSGS were surprising as its functions were not directly linked to podocyte biology. Animal experiments on kidney mass reduction, however, have demonstrated that adaptive changes as a consequence to excessive nephron workload result in podocyte injury, detachment and ultimately loss, and, as such, hyperfiltration-mediated FSGS forms [13, 14]. The fact that FSGS lesions have been found in individuals with CAKUT phenotypes such as vesicoureteral reflux (VUR), unilateral kidney agenesis, and congenital obstructive uropathy [15–17], which all may have a reduced nephron number, suggests such a pathogenesis. However, most individuals who developed FSGS due to pathogenic SNVs in PAX2 did not exhibit a CAKUT phenotype and had normal kidneys on ultrasound, indicating other potential cellular mechanisms, and phenotypic expansion of disease.
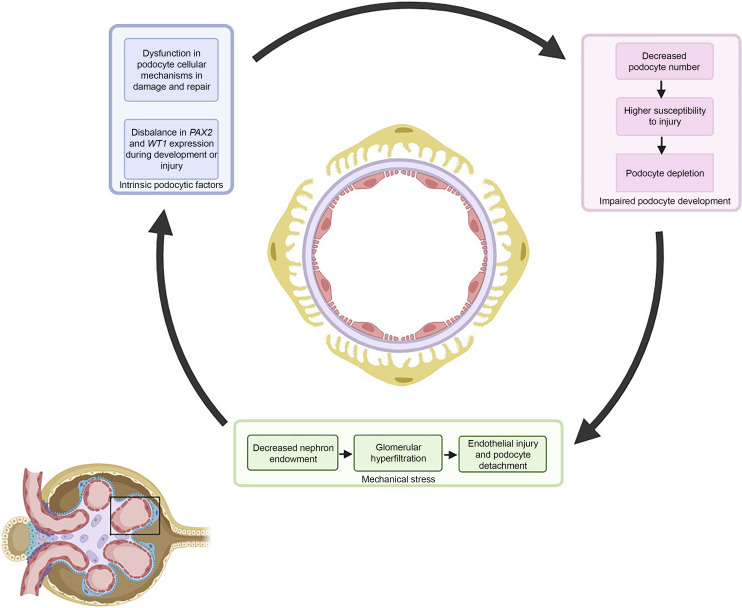
In this mini-review, we set out to describe an overview of genetic FSGS due to pathogenic SNVs in developmental kidney genes as well as the potential mechanisms of how their dysfunction leads to podocytopathy. We challenge the assumption that FSGS in CAKUT is caused by biomechanical stress on the podocyte alone, and suggest a more complex pathophysiology that includes overlapping molecular responses of injured podocytes in FSGS and nephron progenitor cells during kidney development (Fig. 1).
Fig. 1.
Potential pathomechanisms of how pathogenic variants in genes involved in kidney development contribute to FSGS. The multifactorial pathogenesis of FSGS caused by deleterious SNVs in nephrogenesis genes consists of intrinsic podocytic factors, impaired podocyte endowment, and biomechanical stress on the glomerular environment. Displayed is a cross section of the glomerular capillary loop (inlay) with podocytes (yellow), glomerular basement membrane (purple), and endothelial cells (red).
FSGS in CAKUT
CAKUT are among the most frequent human developmental defects with a combined prevalence of 1 in ∼300 live births [18, 19]. The broad spectrum of CAKUT encompasses isolated defects of kidney tissue (kidney agenesis, kidney hypodysplasia, multicystic kidney dysplasia, duplex kidneys or ectopic kidney tissue), upper urinary tract defects such as ureteropelvic junction obstruction (UPJO), VUR and ureterovesical junction obstruction, and lower urinary tract malformations that mainly include posterior urethral valves (PUVs) [20]. All of these conditions have their own individual clinical and epidemiological characteristics, but collectively make up the predominant cause of kidney replacement therapy in children (30–50% of pediatric cases with kidney failure [21]). The main reason to group these different structural anomalies together is the frequent co-occurrence of multiple CAKUT phenotypes within one affected individual [22], as well as the fact that identical pathogenic SNVs within genes attributed to CAKUT result in different phenotypes between individuals and even within families (i.e., variable expressivity) [20]. These clinical observations strongly point toward a shared molecular etiology of disease. However, despite the fact that nowadays more than 60 genes have been identified in the pathogenesis of nonsyndromic and more than 100 genomic disorders are associated with CAKUT phenotypes, a molecular diagnosis can only be made in ∼10% of patients. The previously mentioned extreme heterogeneity of disease, relatively high percentage of incomplete penetrance, and variable expressivity within families, and the fact that relatively milder phenotypes such as low-grade VUR or antenatal hydronephrosis may completely resolve over childhood, still significantly hamper genetic discovery in CAKUT.
Experiments in animal models with 80–90% kidney mass reduction have led to the glomerular hyperfiltration hypothesis in the 1980s, which postulates that increased glomerular flow/pressure in the reduced number of remnant nephrons results not only in an increased single nephron glomerular filtration rate but also in endothelial cell injury with subsequent platelet activation as well as permeability changes of the glomerulus (Fig. 1). This downward spiral of multifactorial glomerular injury damages the podocyte and leads to FSGS in rats, especially when they were fed a high-protein diet to accelerate glomerular hyperfiltration [13]. The kidney mass reduction model differs from the development of human FSGS, as the latter is better characterized by a slower and chronic onset [23]. Acute glomerular hemodynamic changes alone may therefore be insufficient to underlie FSGS lesions in humans [24]. Nevertheless, unbiased stereological methods have indeed identified an association between a low podocyte number, density, and increased cell volume per glomerulus and the development of glomerulosclerosis, persistent proteinuria, and kidney failure [25]. This “podocyte depletion hypothesis” is based on the low regeneration capacity of human podocytes [26] and may explain why a subset of individuals with kidney disease, obesity, or a very low birth weight develop chronic kidney disease later in life, while others do not.
FSGS lesions have also been observed in children with a solitary functioning kidney due to unilateral kidney agenesis [16] and in healthy kidney transplantation donors after donation [27]. As nephrogenesis exclusively takes place during gestation, both conditions could be considered as a clinical model for nephron number reduction and subsequent podocyte depletion. Because all CAKUT phenotypes may lead to a low nephron/podocyte endowment to some extent, the landmark observations that FSGS occurs in different CAKUT phenotypes date back to almost 50 years ago, when Kincaid-Smith identified FSGS lesions in pediatric nephrectomized kidneys from young patients with VUR and/or atrophic pyelonephritis [15]. This observation has since then been confirmed by others, and is relatively common in cohorts of individuals with kidney failure due to VUR. Hinchcliffe et al. [28] performed a histological review of 86 pediatric nephrectomy specimens from VUR patients and found FSGS in 18 (21%) kidneys, 9 (10%) of patients who were less than 5 years old. On the contrary, no FSGS lesions were found in 18 nephrectomized kidneys affected by hypodysplasia without VUR. The authors therefore speculate that other factors than biomechanical stress on podocytes induced by glomerular hyperfiltration may drive the development of FSGS and kidney failure due to VUR [28]. In addition, Steinhardt et al. described a cohort of pediatric patients with different CAKUT phenotypes (UPJO, duplex kidneys, and PUV) and found FSGS lesions in a large number of nephrectomized specimens throughout the spectrum of obstructive uropathy (UPJO 14/20 [70%], duplex kidney 10/12 [83%], PUV 4/5 [80%]) [17]. Interestingly, FSGS was only identified in the co-occurrence of profound tubule-interstitial and peri-glomerular inflammation, indicating potential lead points for unraveling its exact pathomechanisms [17]. As a kidney biopsy or nephrectomy is generally not performed in children with CAKUT, one has to take into account that the reported proportions of FSGS in CAKUT have been calculated in severely affected kidneys with impaired function from highly selected populations.
WT1: A Genetic Link between Kidney Development and FSGS
The WT1 gene on Chr.11p13 encodes a transcription factor with a fundamental role during early kidney development. Cells from the intermediate mesoderm that express WT1 regulate the formation and sustaining of the metanephric mesenchyme (MM) as well as its interaction with the outgrowing ureteric bud. This process will give rise to the mammalian kidney. In addition, WT1 is required for nephron progenitor cells from the MM to epithelialize into embryonic podocytes [29]. WT1 also functions as a tumor suppressor gene, and constitutional and somatic variants lead to mixed clusters of nephrogenic rests within the kidney that predispose to nephroblastoma [30]. Given all these critical roles of WT1, it is not surprising that many different isoforms exist in concert during development, and that (post-)transcriptional regulation and epigenetic mechanisms (e.g., chromatin remodeling, activation of miRNA expression) further determine activating or repressing WT1 in a temporo-spatial manner. The pluripotency of WT1 is also highlighted by the many different genomic disorders that are attributed to SNVs and structural variations involving this gene. For example, heterozygous microdeletions of Chr.11p3 lead to Wilms’ tumor, aniridia, genitourinary abnormalities and mental retardation syndrome due to the co-occurring heterozygous deletion of PAX6 at this locus, whereas autosomal dominant missense mutations in WT1 can cause Denys-Drash syndrome, a rare genetic disorder characterized by glomerular dysplasia, diffuse mesangial sclerosis, Wilms’ tumor, and ambiguous genitalia, and heterozygous intronic splicing mutations cause Frasier syndrome, a syndrome that involves genital dysgenesis in XY males and streak gonads and primary amenorrhea in a subset of XX females. Individuals with Frasier syndrome develop progressive diffuse mesangial sclerosis or FSGS during childhood or young adulthood. Compared to Wilms’ tumor, aniridia, genitourinary abnormalities and mental retardation and Denys-Drash syndrome, Frasier syndrome more frequently includes malignancies of the internal genitalia and has a lower prevalence of Wilms’ tumor. Besides these well-described genomic disorders, autosomal dominant mutations in exons 8 and 9 have been identified in children with nonsyndromic nephrotic proteinuria or congenital nephrotic syndrome, which may be due to variable expressivity of disease. An analysis of patients with pathogenic SNVs in WT1 demonstrated that missense variants affecting DNA-binding residues more often demonstrated with diffuse mesangial sclerosis (74%) and early steroid-resistant nephrotic syndrome onset, while truncating mutations were more frequently associated with the development of Wilms’ tumor (78%) and typically later-onset steroid-resistant nephrotic syndrome [31]. As can be derived from the abovementioned genomic disorders, WT1 plays a pivotal role in kidney development but less than 10% of individuals carrying pathogenic SNVs in WT1 have a phenotype that belongs to the clinical spectrum of CAKUT, such as duplex kidney horseshoe, VUR, and UPJO [31, 32]. This proportion is much lower than the reported proportions of structural abnormalities in the genitourinary tract of patients. Following this observation, it remains unclear if the development of FSGS or diffuse mesangial sclerosis is the result from WT1 dysfunction during kidney development and subsequent lower podocyte endowment, or comes from the direct functions of WT1 on the mature podocytes of patients (Fig. 1).
PAX2: A Kidney Development Gene Attributed to Cause Isolated Familial FSGS
PAX2 is a transcription factor that contains a DNA-binding paired box domain, and heterozygous deleterious SNVs in this gene cause autosomal dominant papillorenal syndrome. The kidneys in papillorenal syndrome usually show hypodysplasia and contain less but larger nephrons [33], which is consistent with the expression of PAX2 in mesenchymal progenitor cells during kidney development. Other CAKUT phenotypes such as obstructive uropathy have also been associated with pathogenic PAX2 variants [34]. Ocular abnormalities include, among others, optic disk dysplasia and retinal coloboma, but also hearing, neurological, and skeletomuscular abnormalities can be identified in affected individuals. Nevertheless, in accordance with a hallmark of CAKUT etiology, pathogenic SNVs in PAX2 may result in extremely variable phenotypes that include isolated congenital kidney defects but also phenotypes without any involvement of the kidney and urinary tract. For a detailed overview of the clinical implications of papillorenal syndrome, see [33].
By performing exome-sequencing and stringent variant selection in an index family of autosomal dominant FSGS, Barua et al. [11] identified previously unreported disease-segregating SNVs in a highly conserved domain of PAX2, which were predicted to be deleterious. Additional sequencing in affected individuals of a familial FSGS cohort identified another seven extremely rare and predicted to be deleterious SNVs in PAX2, which consisted of 4% of the total familial FSGS cohort. Only one of the carriers with FSGS had a co-existing CAKUT phenotype. A follow-up study in a pediatric cohort of steroid-resistant nephrotic syndrome and FSGS presented additional evidence of phenotypic expansion of extremely rare pathogenic PAX2 SNVs with the identification of heterozygous missense SNVs in 1.3% of cases [12]. Since the publication of these landmark papers, others, too, have reported on the incidence of PAX2 mutations in nonsyndromic FSGS (see Table 1) [35–42].
Table 1.
Reported pathogenic PAX2 SNVs in familial FSGS
| Ref | Self-reported ethnicity | Familial or individual case | DNA change | Amino acid change | Age at presentation, years | Diagnosis | Biopsy-proven | CAKUT |
|---|---|---|---|---|---|---|---|---|
| Barua [11] (2014) | White | F | c.491C>A | p.Thr164Asn | 8 | FSGS | Yes | None |
| Barua [11] (2014) | African American | F | c.239C>T | p.Pro80Leu | 7–11 | Proteinuria | Unknown | None |
| Barua [11] (2014) | European | F | c.565G>A | p.Gly189Arg | 17–68 | FSGS | Yes | Bilateral pelvis |
| Barua [11] (2014) | Unknown | F | c.398C>T | p.Ser133Phe | Unknown | Proteinuria | Yes | None |
| Barua [11] (2014) | Middle Eastern | F | c.167G>A | p.Arg56Gln | 36 | FSGS | Unknown | None |
| Barua [11] (2014) | East Indian | F | c.448A>G | p.Thr150Ala | 31–32 | FSGS | Yes | None |
| Barua [11] (2014) | European America | F | c.310C>T | p.Arg104* | 15–24 | FSGS | Yes | Papillorenal syndrome |
| Vivante [12] (2019) | Arab | F | c.69-70InsG | p.Val26Glyfs*28 | 1 | FSGS | Yes | Coloboma, cardiomyopathy, microcephaly |
| Vivante [12] (2019) | Egypt | F | c.254G>T | p.Gly85Val | 10–35 | FSGS | Yes (2/3) | None |
| Vivante [12] (2019) | European | F | c.862-1G>A | Splice variant | 20 | FSGS | Yes (1/2) | None |
| Vivante [12] (2019) | European | F | c.275C>T | p.Thr92Met | 18 | FSGS | Yes | None |
| Xiong [35] (2022) | Chinese | I | c.754C>T | p.Arg115* | 8 | Proteinuria | Yes | Bilateral kidney hypodysplasia |
| Longaretti [36] (2021) | Unknown | I | c.565G>A | p.Gly189Arg | Adult-onset | FSGS | Unknown | Unknown |
| Hu [37] (2021) | Chinese | F | c.76_77insG | p.Val26Glyfs*28 | 15 | FSGS | Yes | Bilateral kidney hypodysplasia |
| Ammar [38] (2021) | Tunisian | F | c.232A>C | p.Ile78Leu | Unknown | FSGS | Unknown | Unknown |
| Bito [39] (2020) | Caucasian | I | c.430C>T | p.Gln144* | 16 | FSGS | Yes | None |
| Rachwani Anil [40] (2019) | Unknown | I | c.418C>T | p.Arg140Ile | 14 | FSGS | Yes | Bilateral kidney hypodysplasia |
| Saida [41] (2020) | Unknown | I | c.70-72delinsA | p.Gly24Argfs*29 | 6 | FSGS | Yes | Coloboma, normal-sized kidneys |
| Okumura [42] (2015) | Unknown | I | c.1023C>A | p.Tyr341* | 28 | FSGS | Yes | Papillorenal syndrome |
| Okumura [42] (2015) | Unknown | I | c.57–58 ins GTGAACC | p.Gln22Argfs*34 | 14 | FSGS | Yes | Coloboma, normal kidneys |
| Okumura [42] (2015) | Unknown | I | c.224-225insAC | p.Gly76Profs*8 | 38 | FSGS | Yes | Papillorenal syndrome |
CAKUT, congenital anomalies of the kidney and urinary tract; F, familial case; FSGS, focal segmental glomerular sclerosis; I, individual case; SNV, single-nucleotide variant.
Potential Mechanisms on How PAX2 Dysfunction Leads to FSGS
The identification of pathogenic SNVs in PAX2 in individuals with isolated and non-isolated genetic forms of FSGS has fueled the debate on potential pathomechanisms of such variants leading to podocyte injury, detachment, and loss. In human kidney and urinary tract development, PAX2 plays a significant role by orchestrating the interaction between the protruding ureteric bud into the MM, which is the source of nephron progenitor cells ultimately differentiating into nephron cells such as podocytes. As such, deleterious SNVs in PAX2 may contribute to less epithelialization of nephron progenitor cells and thus a lower podocyte number and density. As the current resolution of clinical imaging methods is insufficient to identify this podocyte depletion, kidneys of affected individuals will not appear as abnormal. Additional “hits” to podocyte function such as high salt intake, glomerular hypertension, infection, medication, and others may even further set off FSGS development in individuals with a pathogenic SNV in PAX2. In this context, the differences in podocyte endowment as well as in exposure to environmental factors may underlie the variable expressivity and incomplete penetrance that is observed between these individuals with PAX2-driven FSGS (Table 1) [11].
Another potential explanation can be found in the shared cellular pathway of PAX2 and WT1, which plays an abovementioned role in kidney development as well as in podocyte biology. In kidney and urinary tract development, PAX2 and WT1 expression overlaps both spatially and temporarily and their regulation is reciprocally arranged; i.e., PAX2 represses WT1 and vice versa. Disturbances in this tightly regulated interaction result in impaired podocyte function due to podocyte foot process effacement and underdeveloped endothelial fenestrae in mice [43].
By the generation of podocytes using induced pluripotent stem cells (iPSCs) from an individual who developed FSGS during adulthood due to an ultra-rare heterozygous missense mutation in PAX2 (c.565G>A, p.G189R), Longaretti et al. [36] provided a dual perspective on the pathogenesis of podocyte injury in FSGS caused by deleterious PAX2 SNVs. First, in a series of in vitro experiments, patient iPSCs developed into podocytes with normal structure and function, which challenges the concept that podocyte architecture is primarily affected by this missense SNV in PAX2. In addition, the authors showed that G189R-PAX2 iPSCs showed a marked reduction in normal ureteric bud morphogenesis compared to iPSCs from healthy controls, whereas correction of this variant by gene-editing restored normal ureteric budding in tubule-like structures. Both findings indicate a pivotal role of abnormal nephrogenesis and lower podocyte endowment in the development of FSGS caused by PAX2 dysfunction [36]. Second, the importance of podocyte endowment is further highlighted by the observation that patient-derived podocytes exposed to the nephrotoxic agent puromycin aminonucleoside were less viable and more susceptible to additional cell injury than exposed podocytes from healthy controls. Transcriptomic analysis of exposed patient podocytes showed marked changes compared to healthy exposed podocytes in the regulation of focal adhesion pathways, which are critical for podocyte function in their cell interaction with the glomerular basement membrane. From this study, one could hypothesize that the dual vulnerability of a lower podocyte endowment and the higher susceptibility of affected podocytes to injury, which are both induced by pathogenic PAX2 SNVs, predispose to the development of genetic FSGS (Fig. 1). Naturally, this hypothesis will require additional validation by in vivo experiments before definitive conclusions can be drawn on the role of PAX2 dysfunction in FSGS.
Reactivation of Kidney Developmental Genes Orchestrates Repair during Kidney Injury
Table 1 shows an overview of clinical characteristics of all reported individuals with pathogenic PAX2 variants in FSGS. A subset of patients demonstrated additional phenotypes that are compatible with the spectrum of papillorenal syndrome (n = 10), whereas others had nonsyndromic (isolated) FSGS (n = 9) or the information about additional phenotypes was incomplete (n = 2). Interestingly, the majority of identified pathogenic PAX2 SNVs in nonsyndromic FSGS contained missense mutations (7/9, 78%), while truncating SNVs were much more frequently observed in individuals with papillorenal syndrome (8/10, 80%) (Table 1). Both patients with an unknown phenotype had a PAX2 missense SNVs. This finding is relevant as it may indicate different pathomechanisms: while shortening or absence of PAX2 due to truncating variants increases the probability of a reduced number of nephrons and podocytes due to a CAKUT phenotype, less deleterious PAX2 missense SNVs in nonsyndromic FSGS are more likely to lead to normal shaped kidneys on ultrasound. However, additional triggers or hits caused by environmental factors during childhood or early adulthood may induce reactivation of developmental pathways, which ultimately lead to the development of FSGS. A similar mechanism has been identified in kidney tubular cells from rodents as well as humans that indeed re-express developmental genes and proteins that include PAX2, LHX1, SOX9, GATA3 after injury [44–48]. Reactivation of these developmental pathways leads to expression of epidermal growth factor, insulin-like growth factor, transforming growth factor-β, and the canonical Wnt signaling pathway, which all are pivotal in cell fate determination and cell migration [49]. It is therefore not unlikely to suspect the upregulation of similar developmental pathways during podocyte injury, and that individuals carrying pathogenic missense SNVs in PAX2 or other developmental kidney genes progress to FSGS due to maladaptive podocytic repair responses. In-depth gene*time interaction testing of single-cell RNA-sequencing data (scRNA-seq) derived from early stage podocytopathies is required to identify groups of kidney developmental genes that are downregulated during maturation and re-expressed during podocytopathy and repair. Fortunately, the large Nephrotic Syndrome Study Network (NEPTUNE) research framework has provided open access to glomerular scRNA-seq via GEO Accession viewer (nih.gov) to test this hypothesis in the upcoming years.
Future Directions
The advent of genomic methodologies has uncovered novel pathomechanisms in podocyte injury and FSGS development. Although nephrogenesis has been completed before birth, developmental pathomechanisms seem to play a pivotal role in the cellular response of podocytopathies. To better understand how developmental genes contribute to FSGS, it is highly necessary to determine podocyte numbers in a clinical setting, so that clinicians can identify patients at increased risk to develop FSGS due to lower podocyte endowment. Current technologies that combine a kidney biopsy with imaging modalities such as MRI are ready for prime time in glomerulopathies [50], but may be less suitable for CAKUT since this is a relative contraindication for kidney biopsy. When tissue is available from such patients, the recent improvement in the power and resolution of spatially resolved transcriptional techniques will most likely give important new insights in the activated mechanisms in podocyte injury and repair. These techniques allow for the distinct identification, localization, and transcriptional profiling of independent podocytes if applied in an early stage of disease [48]. Using a spatially resolved transcriptional technique in FSGS biopsies therefore may potentially dissect the activation of expressed development genes and other relevant pathophysiological pathways in podocytopathies. Another potential technology that would not require a kidney biopsy would be scRNA-seq of urine-derived podocytes from CAKUT patients or individuals with developmental kidney gene defects. These so-called liquid kidney biopsies use the principle that kidney cells, including podocytes and proximal tubular cells, are increasingly excreted in urine of individuals affected by kidney diseases (such as cystinosis, FSGS, acute kidney injury) than in healthy individuals. Limitations of this method that still need to be overcome are the relatively low yield of podocytes compared to a kidney biopsy and the lack of spatial orientation. Nevertheless, scRNA-seq of urine-derived podocytes could still provide a noninvasive and patient-friendly method to identify the pathomechanisms crucial in podocytopathy and repair. Additional integration with data from other multi-omics technologies and functional validation studies using model systems such as kidney organoids would ultimately provide a powerful approach to identify molecular targets for the development of tailored therapies for patients such as RNA interference and stem cell therapies to support or restore normal glomerular architecture. The effects of these targeted therapies are now studied in different functional models of kidney disease and transplantation with one of the main challenges being its delivery into the kidney [44, 51].
Conclusion
In summary, genes that play a role during kidney and urinary tract development have also been attributed to the etiology of FSGS. Although the exact pathogenesis has not been elucidated, their role seems multifactorial and includes intrinsic podocyte characteristics, a lower total podocyte number, and biomechanical stress within the podocyte environment. Our hope for the next decade is that high-resolution translational techniques will further dissect podocyte biology and set the stage for precision medicine strategies that will lead to better outcomes for individuals with genetic forms of FSGS.
Acknowledgments
We thank Mrs Dr. F. Oliveira Arcolino for her valuable input on our manuscript. Figure 1 is created using Biorender.com.
Conflict of Interest Statement
R.W. is supported by a consortium grant from the Dutch Kidney Foundation (20OC002). R.W. is a board member of the working group on CAKUT, urinary tract infection, and bladder dysfunction of the European Society of Pediatric Nephrology (ESPN). E.L. is supported by the ERC Consolidator grant 101045467 – NEOGRAFT. E.L. is a board member of the working group on inherited kidney disorders of the ESPN. R.W. and E.L. are members of the NL research consortium Kidnie, which is supported by the Dutch Kidney Foundation. R.W. and E.L. are working in the reference center of the European Reference Kidney Network (ERKNet).
Funding Sources
No funding was received.
Author Contributions
Conceptualization: R.W. and L.S.K. Writing and editing of the manuscript, and final review: R.W., L.S.K., and E.L.
Funding Statement
No funding was received.
References
- 1. Rosenberg AZ, Kopp JB. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12(3):502–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shabaka A, Tato Ribera A, Fernandez-Juarez G. Focal segmental glomerulosclerosis: state-of-the-art and clinical perspective. Nephron. 2020;144(9):413–27. [DOI] [PubMed] [Google Scholar]
- 3. Lasagni L, Lazzeri E, Shankland SJ, Anders HJ, Romagnani P. Podocyte mitosis: a catastrophe. Curr Mol Med. 2013;13(1):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kopp JB, Anders HJ, Susztak K, Podestà MA, Remuzzi G, Hildebrandt F, et al. Nat Rev Dis Primers. 2020;6(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahn W, Bomback AS. Approach to diagnosis and management of primary glomerular diseases due to podocytopathies in adults: core curriculum 2020. Am J Kidney Dis. 2020;75(6):955–64. [DOI] [PubMed] [Google Scholar]
- 6. Salfi G, Casiraghi F, Remuzzi G. Current understanding of the molecular mechanisms of circulating permeability factor in focal segmental glomerulosclerosis. Front Immunol. 2023;14:1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dahl NK, Bloom MS, Chebib FT, Clark D, Westemeyer M, Jandeska S, et al. The clinical utility of genetic testing in the diagnosis and management of adults with chronic kidney disease. J Am Soc Nephrol. 2023;34(12):2039–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitrotti A, Giliberti M, Di Leo V, di Bari I, Pontrelli P, Gesualdo L. Hidden genetics behind glomerular scars: an opportunity to understand the heterogeneity of focal segmental glomerulosclerosis? Pediatr Nephrol. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Solanki AK, Arif E, Morinelli T, Wilson RC, Hardiman G, Deng P, et al. A novel CLCN5 mutation associated with focal segmental glomerulosclerosis and podocyte injury. Kidney Int Rep. 2018;3(6):1443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Preston R, Naylor RW, Stewart G, Bierzynska A, Saleem MA, Lowe M, et al. A role for OCRL in glomerular function and disease. Pediatr Nephrol. 2020;35(4):641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barua M, Stellacci E, Stella L, Weins A, Genovese G, Muto V, et al. Mutations in PAX2 associate with adult-onset FSGS. J Am Soc Nephrol. 2014;25(9):1942–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vivante A, Chacham OS, Shril S, Schreiber R, Mane SM, Pode-Shakked B, et al. Dominant PAX2 mutations may cause steroid-resistant nephrotic syndrome and FSGS in children. Pediatr Nephrol. 2019;34(9):1607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brenner BM. Nephron adaptation to renal injury or ablation. Am J Physiol. 1985;249(3 Pt 2):F324–37. [DOI] [PubMed] [Google Scholar]
- 14. Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981;241(1):F85–93. [DOI] [PubMed] [Google Scholar]
- 15. Kincaid-Smith P. Glomerular and vascular lesions in chronic atrophic pyelonephritis and reflux nephropathy. Adv Nephrol Necker Hosp. 1975;5:3–17. [PubMed] [Google Scholar]
- 16. Kiprov DD, Colvin RB, McCluskey RT. Focal and segmental glomerulosclerosis and porteinuria associated with unilateral renal agenesis. Lab Invest. 1982;46(3):275–81. [PubMed] [Google Scholar]
- 17. Steinhardt GF, Ramon G, Salinas-Madrigal L. Glomerulosclerosis in obstructive uropathy. J Urol. 1988;140(5 Pt 2):1316–8. [DOI] [PubMed] [Google Scholar]
- 18. Garne E, Dolk H, Loane M, Boyd PA; EUROCAT . EUROCAT website data on prenatal detection rates of congenital anomalies. J Med Screen. 2010;17(2):97–8. [DOI] [PubMed] [Google Scholar]
- 19. Birth defects monitoring program (BDMP)/McDonnell douglas health information system (MDHIS) surveillance data, 1985-1988. Teratology. 1993;48(6):676–94. [DOI] [PubMed] [Google Scholar]
- 20. Westland R, Renkema KY, Knoers N. Clinical integration of genome diagnostics for congenital anomalies of the kidney and urinary tract. Clin J Am Soc Nephrol. 2020;16(1):128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kramer A, Boenink R, Noordzij M, Bosdriesz JR, Stel VS, Beltrán P, et al. The ERA-EDTA registry annual report 2017: a summary. Clin Kidney J. 2020;13(4):693–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knoers N. The term CAKUT has outlived its usefulness: the case for the defense. Pediatr Nephrol. 2022;37(11):2793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Mik SM, Hoogduijn MJ, de Bruin RW, Dor FJ. Pathophysiology and treatment of focal segmental glomerulosclerosis: the role of animal models. BMC Nephrol. 2013;14:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwartz MM. The role of podocyte injury in the pathogenesis of focal segmental glomerulosclerosis. Ren Fail. 2000;22(6):663–84. [DOI] [PubMed] [Google Scholar]
- 25. Haruhara K, Kanzaki G, Tsuboi N. Nephrons, podocytes and chronic kidney disease: strategic antihypertensive therapy for renoprotection. Hypertens Res. 2023;46(2):299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71(12):1205–14. [DOI] [PubMed] [Google Scholar]
- 27. Murata M, Takeda A, Ootsuka Y, Shinjo H, Ito C, Watanabe Y, et al. Study of glomerulopathy in donors after kidney transplantation. Nephron. 2020;144(Suppl 1):86–90. [DOI] [PubMed] [Google Scholar]
- 28. Hinchliffe SA, Kreczy A, Ciftci AO, Chan YF, Judd BA, van Velzen D. Focal and segmental glomerulosclerosis in children with reflux nephropathy. Pediatr Pathol. 1994;14(2):327–38. [DOI] [PubMed] [Google Scholar]
- 29. Torban E, Goodyer P. Wilms’ tumor gene 1: lessons from the interface between kidney development and cancer. Am J Physiol Renal Physiol. 2024;326(1):F3–19. [DOI] [PubMed] [Google Scholar]
- 30. Nordenskjold A, Friedman E, Sandstedt B, Söderhäll S, Anvret M. Constitutional and somatic mutations in the WT1 gene in Wilms’ tumor patients. Int J Cancer. 1995;63(4):516–22. [DOI] [PubMed] [Google Scholar]
- 31. Lipska BS, Ranchin B, Iatropoulos P, Gellermann J, Melk A, Ozaltin F, et al. Genotype-phenotype associations in WT1 glomerulopathy. Kidney Int. 2014;85(5):1169–78. [DOI] [PubMed] [Google Scholar]
- 32. Lehnhardt A, Karnatz C, Ahlenstiel-Grunow T, Benz K, Benz MR, Budde K, et al. Clinical and molecular characterization of patients with heterozygous mutations in wilms tumor suppressor gene 1. Clin J Am Soc Nephrol. 2015;10(5):825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schimmenti LA. Renal coloboma syndrome. Eur J Hum Genet. 2011;19(12):1207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ahram DF, Lim TY, Ke J, Jin G, Verbitsky M, Bodria M, et al. Rare single nucleotide and copy number variants and the etiology of congenital obstructive uropathy: implications for genetic diagnosis. J Am Soc Nephrol. 2023;34(6):1105–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xiong HY, Shi YQ, Zhong C, Yang Q, Zhang G, Yang H, et al. Detection of de novo PAX2 variants and phenotypes in Chinese population: a single-center study. Front Genet. 2022;13:799562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Longaretti L, Trionfini P, Brizi V, Xinaris C, Mele C, Breno M, et al. Unravelling the role of PAX2 mutation in human focal segmental glomerulosclerosis. Biomedicines. 2021;9(12):1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hu R, Wen Y, Ye W, Zhang L, Si N, Zheng K. FSGS in Chinese twins with a de novo PAX2 mutation: a case report and review of the literature. J Nephrol. 2021;34(6):2155–8. [DOI] [PubMed] [Google Scholar]
- 38. Ammar S, Kanoun H, Kammoun K, Domingo-Gallego A, Ruiz P, Lorente-Grandoso L, et al. Next-generation sequencing in patients with familial FSGS: first report of collagen gene mutations in Tunisian patients. J Hum Genet. 2021;66(8):795–803. [DOI] [PubMed] [Google Scholar]
- 39. Bito L, Kalmár T, Maróti Z, Turkevi-Nagy S, Bereczki C, Iványi B. PAX2 mutation-related oligomeganephronia in a young adult patient. Case Rep Nephrol Dial. 2020;10(3):163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rachwani Anil R, Rocha-de-Lossada C, Ayala CH, Contreras ME. A new mutation in the PAX2 gene in a Papillorenal Syndrome patient. Am J Ophthalmol Case Rep. 2019;16:100563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saida K, Kamei K, Morisada N, Ogura M, Ogata K, Matsuoka K, et al. A novel truncating PAX2 mutation in a boy with renal coloboma syndrome with focal segmental glomerulosclerosis causing rapid progression to end-stage kidney disease. CEN Case Rep. 2020;9(1):19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Okumura T, Furuichi K, Higashide T, Sakurai M, Hashimoto SI, Shinozaki Y, et al. Association of PAX2 and other gene mutations with the clinical manifestations of renal coloboma syndrome. PLoS One. 2015;10(11):e0142843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dressler GR, Wilkinson JE, Rothenpieler UW, Patterson LT, Williams-Simons L, Westphal H. Deregulation of Pax-2 expression in transgenic mice generates severe kidney abnormalities. Nature. 1993;362(6415):65–7. [DOI] [PubMed] [Google Scholar]
- 44. Slaats GG, Chen J, Levtchenko E, Verhaar MC, Arcolino FO. Advances and potential of regenerative medicine in pediatric nephrology. Pediatr Nephrol. 2024;39(2):383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kumar S, Liu J, Pang P, Krautzberger AM, Reginensi A, Akiyama H, et al. Sox9 activation highlights a cellular pathway of renal repair in the acutely injured mammalian kidney. Cell Rep. 2015;12(8):1325–38. [DOI] [PubMed] [Google Scholar]
- 46. Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD. Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci U S A. 2014;111(4):1527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smeets B, Boor P, Dijkman H, Sharma SV, Jirak P, Mooren F, et al. Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol. 2013;229(5):645–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lake BB, Menon R, Winfree S, Hu Q, Melo Ferreira R, Kalhor K, et al. An atlas of healthy and injured cell states and niches in the human kidney. Nature. 2023;619(7970):585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Villanueva S, Cespedes C, Gonzalez A, Vio CP. bFGF induces an earlier expression of nephrogenic proteins after ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2006;291(6):R1677–87. [DOI] [PubMed] [Google Scholar]
- 50. Tsuboi N, Sasaki T, Okabayashi Y, Haruhara K, Kanzaki G, Yokoo T. Assessment of nephron number and single-nephron glomerular filtration rate in a clinical setting. Hypertens Res. 2021;44(6):605–17. [DOI] [PubMed] [Google Scholar]
- 51. Bondue T, van den Heuvel L, Levtchenko E, Brock R. The potential of RNA-based therapy for kidney diseases. Pediatr Nephrol. 2023;38(2):327–44. [DOI] [PMC free article] [PubMed] [Google Scholar]