Abstract
Introduction
Melanoma is the most aggressive skin cancer, with an increasing occurrence. Despite the recent important improvements due to novel immunotherapy approaches, when late diagnosed, melanoma prognosis is poor due to the metastatic progression and drug-resistance onset. Therefore, there is an urgent need to identify additional therapeutic targets. Melanoma invasive behavior is related to the activity of metalloproteases, able to degrade extracellular matrix leading to tumor dissemination. A recent study suggested that the most potent proteases inhibitor alpha-2-macroglobulin (A2MG) from plasma of hibernating fishes exerts potent antiproliferative effects. Our previous studies showed a significant reduction of A2MG in sera from mice/human melanoma models.
Methods
Gene and protein expression studies have been performed by using platforms and databases available online containing expression data from thousands of patients and healthy controls.
Results
We carried out an extensive bioinformatics analysis to evaluate the A2MG gene/protein expression on a large cohort of patients affected by many different cancer types, compared to healthy control subjects, and we found a highly significant difference of A2MG expression in 20 out of 31 cancer types (including melanoma) compared to healthy controls. Similar results were also confirmed at the proteomic level using another platform available online. Further, we found that higher A2MG expression is significantly related to overall survival in different cancers including melanoma.
Conclusion
Our results strongly suggest A2MG as a novel molecular target in melanoma therapy, as well as in other cancer types.
Keywords: Alpha-2-macroglobulin, Antitumor, Biomarker, Melanoma, Proteases
Introduction
Within the last decade, the clinical evolution of melanoma has been significantly improved thanks to the use of novel drugs such as the immune-checkpoint inhibitors. Nevertheless, primary or secondary resistance occurs in a significant proportion of patients, making it necessary additional clinical approaches. Amini et al.’s study [1] describes a significant antimelanoma activity of proteins from hibernating common carpa-derived plasma (HCCP). Other studies indicate alpha-2-macroglobulin (A2MG) as responsible of HCCP anticancer effects [2, 3] likely due to the potent inhibitory activity on proteases, necessary for the metastatic progression [4]. Such data are in line with our previous study, showing that sera from melanoma mice and from melanoma patients contain significantly lower A2MG levels than controls [5]. We obtained those results by applying a multidenaturation protocol able to emphasize structural differences among serum/plasma proteins and to reveal metabolic pathways expressed by melanoma cells when drug resistance develops [6]. The lower expression of A2MG in sera from cancer models suggested a potential anticancer activity. Indeed, recently A2MG serum levels were reported among the best classifiers to evaluate the risk of breast cancer progression [7]. Prompted by these evidences, we investigated A2MG expression in cancer patients and its correlation with overall survival.
Methods
We carried out gene and protein expression studies on GEPIA2 (http://gepia2.cancer-pku.cn/) and on UALCAN (https://ualcan.path.uab.edu/analysis-prot.html), respectively, and exploited the statistical analyses implemented within these platforms.
Results
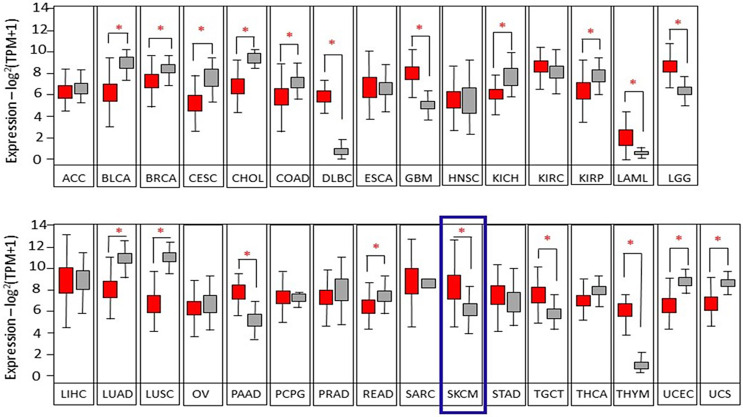
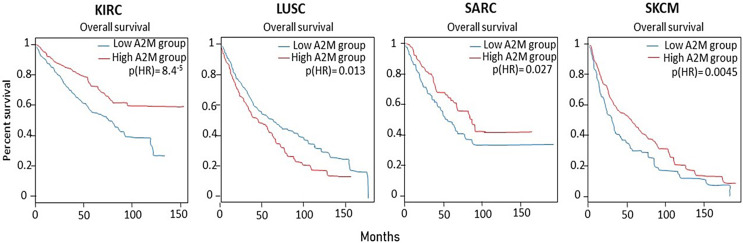
To date, the evidence indicating the role of A2MG gene or protein expression in a large number of cancer patients, and the relation to overall survival, is lacking. We performed gene expression analysis on GEPIA2 portal [8] examining transcriptomic datasets of 15,000 individuals from 31 cancer types including skin cancer melanoma (SKCM) and controls. Figure 1 shows gene expression in cancer biopsies compared to healthy controls. The cancer types are named according to TCGA acronyms and are reported in Table 1. Interestingly, A2MG gene expression is significantly changed compared to controls in 20 out of 31 cancer types, including melanoma (SKCM) (highlighted with a blue frame in Fig. 1). Namely, in most cancer types (i.e., 12 out of 20), A2MG levels are significantly reduced in cancer compared to control biopsies, while in 8 out of 20 cancers, A2MG levels are significantly increased. The asterisks indicate highly significant differences (p < 0.0001). Melanoma data refer to 461 patients compared to 558 controls. Figure 2 shows that in 4 cancer types, A2MG gene expression is significantly related to overall survival: in kidney carcinoma, sarcoma, and melanoma (SKCM), high A2MG levels are significantly related to better survival (p[HR] = 0.000084, 0.027, and 0.0045, respectively). On the contrary, in lung carcinoma, low levels of A2MG are related to better survival (p[HR] = 0.013).
Fig. 1.
Box-plot indicating gene expression levels in cancer biopsies (red boxes) compared to corresponding controls (gray boxes). Significant differences are indicated by the asterisk (*p < 0.0001). The cancer types are named according to TCGA acronyms.
Table 1.
Acronyms reported according to the TCGA cancer classification
| ACC | Adrenocortical carcinoma |
| BLCA | Bladder urothelial carcinoma |
| BRCA | Breast invasive carcinoma |
| CESC | Cervical squamous cell carcinoma and endocervical adenocarcinoma |
| CHOL | Cholangiocarcinoma |
| COAD | Colon adenocarcinoma |
| DLBC | Lymphoid neoplasm diffuse large B-cell lymphoma |
| ESCA | Esophageal carcinoma |
| GBM | Glioblastoma multiforme |
| HNSC | Head and neck squamous cell carcinoma |
| KICH | Kidney chromophobe |
| KIRC | Kidney renal clear cell carcinoma |
| KIRP | Kidney renal papillary cell carcinoma |
| LAML | Acute myeloid leukemia |
| LGG | Lower grade glioma |
| LIHC | Liver hepatocellular carcinoma |
| LUAD | Lung adenocarcinoma |
| LUSC | Lung squamous cell carcinoma |
| OV | Ovarian serous cystadenocarcinoma |
| PAAD | Pancreatic adenocarcinoma |
| PCPG | Pheochromocytoma and paraganglioma |
| PRAD | Prostate adenocarcinoma |
| READ | Rectum adenocarcinoma |
| SARC | Sarcoma |
| SKCM | Skin cutaneous melanoma |
| STAD | Stomach adenocarcinoma |
| TGCT | Testicular germ cell tumors |
| THCA | Thyroid carcinoma |
| THYM | Thymoma |
| UCEC | Uterine corpus endometrial carcinoma |
| UCS | Uterine carcinosarcoma |
Fig. 2.
Overall survival in high (red line) and low A2MG expression (blue line) patients is significantly different in KIRC, LUSC, SARC, and SKCM cancer types.
Proteomic data indicate that A2MG protein expression is significantly reduced in breast, ovarian, colon cancer, uterine corpus endometrial carcinoma, lung adenocarcinoma, lung squamous cell, head and neck squamous cell carcinoma, and hepatocellular carcinoma (data not shown). Conversely, A2MG protein level is significantly increased in clear cell renal carcinoma and glioblastoma multiforme patients (data not shown). Comparing results from gene expression (shown in Fig. 1) to protein expression, in most cases the change is toward the same direction, namely, in breast, colon, uterine corpus endometrial, lung, lung squamous cell, and glioblastoma multiforme cancers (data not shown), indicating a similar change at both gene and protein levels. Unfortunately, UALCAN platform does not yet include melanoma specimens.
Discussion
The gene/protein expression in cancer compared to healthy controls suggests that A2MG may play a key role in tumor. In some cancer types, A2MG tissue expression is reduced compared to controls, while in others it is increased, likely due to the several biological functions requiring A2MG action, e.g., inhibition of almost all known proteases and binding to important cytokines interfering with their activities [9]. In particular, it has to be emphasized the central role played by matrix metalloproteases in tumor development and metastatic progression also taking into account their immunomodulatory role [10]. Therefore, A2MG may exert different functions on cancer cells depending on specific tumor microenvironments, as shown by different roles played by some metalloproteases in cancers [11]. Further, it is noteworthy that cold treatment is crucial for HCCP preparation [1–3], and similarly, cooling down is among the denaturation treatments required to highlight structural alterations in serum proteins from melanoma mice or melanoma human patients [5]. Interestingly, A2MG-binding activities were reported to be temperature dependent [12].
In conclusion, the presented results, obtained from large patient-derived datasets, for the first time show that: (i) A2MG expression is significantly different in patients of several cancer types including melanoma; (ii) different gene expression is confirmed at the protein level, with very high statistical significance; and (iii) strikingly, increased A2MG expression levels are significantly related to the survival rate in several cancer types including melanoma, and a more characterized stratification of patients in larger clinical studies may improve the relevance of the observed effects. These considerations open an innovative scenario where plasma proteins such as A2MG may act as a novel therapeutic option against cancer.
Acknowledgment
The technological support from Complex Protein Mixture (CPM) facility at Department of OMM (ISS) is kindly acknowledged.
Statement of Ethics
Ethical approval and consent were not required as this study was based on publicly available data.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was partially supported by Contributo Liberale from Banca d’Italia to F.F. and by Italian Ministry of Health (RC2023, 3.3) to A.F.
Author Contributions
F.F.: conception and design, acquisition, analysis, and interpretation of data for the work and fund raising. D.D.: acquisition, analysis, and interpretation of data for the work. A.F.: design, acquisition, analysis, and interpretation of data for the work and fund raising.
Funding Statement
This work was partially supported by Contributo Liberale from Banca d’Italia to F.F. and by Italian Ministry of Health (RC2023, 3.3) to A.F.
Data Availability Statement
Gene and protein expression data are available online. Further inquiries can be directed to the corresponding author.
References
- 1. Amini E, Rahgozar S, Golpich M, Kefayat A, Fesharaki M. Potential anti-cancer effects of hibernating common carp (Cyprinus carpio) plasma on B16-F10 murine melanoma: in vitro and in vivo studies. Int J Biol Macromol. 2023;238:124058. [DOI] [PubMed] [Google Scholar]
- 2. Sieckmann DG, Jaffe H, Golech S, Cai D, Hallenbeck JM, McCarron RM. Anti-lymphoproliferative activity of alpha-2-macroglobulin in the plasma of hibernating 13-lined ground squirrels and woodchucks. Vet Immunol Immunopathol. 2014;161(1–2):1–11. [DOI] [PubMed] [Google Scholar]
- 3. Golpich M, Amini E, Kefayat A, Fesharaki M, Moshtaghian J. In vitro and in vivo anti-cancer effects of hibernating common carp (Cyprinus carpio) plasma on metastatic triple-negative breast cancer. Sci Rep. 2022;12(1):2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147(5):992–1009. [DOI] [PubMed] [Google Scholar]
- 5. Verdoliva V, Senatore C, Polci ML, Rossi S, Cordella M, Carlucci G, et al. Differential denaturation of serum proteome reveals a significant amount of hidden information in complex mixtures of proteins. PLoS One. 2013;8(3):e57104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tabolacci C, Giordano D, Rossi S, Cordella M, D’Arcangelo D, Moschella F, et al. Identification of dihydrolipoamide dehydrogenase as potential target of vemurafenib-resistant melanoma cells. Molecules. 2022;27(22):7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Starodubtseva NL, Tokareva AO, Rodionov VV, Brzhozovskiy AG, Bugrova AE, Chagovets VV, et al. Integrating proteomics and lipidomics for evaluating the risk of breast cancer progression: a pilot study. Biomedicines. 2023;11(7):1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47(W1):W556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rehman AA, Ahsan H, Khan FH. α-2-Macroglobulin: a physiological guardian. J Cell Physiol. 2013;228(8):1665–75. [DOI] [PubMed] [Google Scholar]
- 10. Wang Q, Wang K, Tan X, Li Z, Wang H. Immunomodulatory role of metalloproteases in cancers: current progress and future trends. Front Immunol. 2022;13:1064033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gobin E, Bagwell K, Wagner J, Mysona D, Sandirasegarane S, Smith N, et al. A pan-cancer perspective of matrix metalloproteases (MMP) gene expression profile and their diagnostic/prognostic potential. BMC Cancer. 2019;19(1):581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rajagopalan S, Gonias SL, Pizzo SV. The temperature-dependent reaction between alpha 2-macroglobulin and streptokinase-plasmin (ogen) complex. J Biol Chem. 1987;262(8):3660–4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Gene and protein expression data are available online. Further inquiries can be directed to the corresponding author.