Abstract
Background
Autosomal dominant polycystic kidney disease (ADPKD) is a common inherited condition; however, its relationship with renal cell carcinoma (RCC) remains unclear. This paper aims to establish the prevalence of RCC and its subtypes amongst ADPKD patients.
Methods
A database search was conducted to retrieve studies reporting RCC occurrence within ADPKD patients until July 2023. Key outcomes included number and subtype of RCC cases, and number of RCCs presenting incidentally. A random-effects meta-analysis was performed.
Results
Our search yielded 569 articles, 16 met the inclusion criteria. Nephrectomy specimens from 1,147 ADPKD patients were identified. Of studies reporting per-kidney results (n = 13), 73 RCCs were detected amongst 1,493 kidneys, equating to a per-kidney prevalence of 4.3% (95% CI, 3.1–5.7, I2 = 15.7%). 75 ADPKD patients were found to have RCC (75/1,147), resulting in a per-person prevalence of 5.7% (95% CI, 3.7–7.9, I2 = 40.3%) (n = 16). As 7 patients had bilateral disease, 82 RCCs were detected in total. Of these, 39 were clear cell RCC, 35 were papillary and 8 were other. As such, papillary RCCs made up 41.1% (95% CI, 25.9–56.9, I2 = 18.1%) of detected cancers. The majority of RCCs were detected incidentally (72.5% [95% CI, 43.7–95.1, I2 = 66.9%]).
Conclusion
ADPKD appears to be associated with the papillary RCC subtype. The clinical implications of these findings are unclear, however, may become apparent as outcomes and life expectancy amongst APDKD patients improve.
Keywords: Autosomal dominant polycystic kidney disease, Renal cell carcinoma, Papillary, Prevalence, Meta-analysis
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is a common inherited condition, characterised by the development and growth of numerous fluid-filled renal cysts. It is estimated to affect approximately 1 in 1,000 live births [1] and is a common cause of chronic kidney disease, accounting for 5–10% of cases of end-stage renal disease (ESRD) [2]. There are pathogenic factors that support an association between ADPKD and renal cell carcinoma (RCC). Firstly, ADPKD displays characteristics of neoplasia, including dysregulated proliferative signalling, altered patterns of apoptosis and increased angiogenesis [3]. Additionally, these conditions share pathogenic similarities, including the role of the mTOR pathway in promoting cyst growth [4] and the β-catenin pathway being implicated in aiding the transition from ADPKD to RCC [5].
Despite this, the association between RCC and ADPKD remains unclear [6]. RCC can be difficult to diagnose in the setting of ADPKD due to similar clinical presentations, and the challenges in detecting RCC on imaging given the distorted architecture of ADPKD kidneys [7]. However, certain features on computed tomography imaging can increase RCC suspicion within ADPKD kidneys, such as high-density lesions (as measured in Hounsfield units), as well as cysts with atypical features [8]. Additionally, studies analysing the relationship between these conditions often include ADPKD patients with ESRD, many of which are dialysis recipients. Both ESRD and dialysis associated acquired cystic disease are established risk factors for RCC making it challenging to distinguish the exact contribution of ADPKD to RCC development in their presence [9].
In addition to questions pertaining to the prevalence of RCC in ADPKD, some literature has shown that RCCs in ADPKD are associated with an increased likelihood to present bilaterally and/or multifocally, display sarcomatoid dedifferentiation [7] and to be of the papillary (pRCC) subtype of RCC [6, 10, 11]. There are important distinctions as different variants have different prognoses and management strategies [12].
This systematic review and meta-analysis aims to establish the prevalence of RCC amongst ADPKD patients. Additionally, we aim to explore the relationship between ADPKD and different RCC subtypes. This evidence may help determine potential pathogenic mechanisms linking these conditions and assist to inform clinical decision-making in this patient cohort.
Materials and Methods
Study Selection
This systematic review and meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was prospectively registered with PROSPERO (CRD42023415177). The inclusion criteria required studies to: (1) outline the occurrence of RCCs in patients with ADPKD; (2) confirm the diagnosis of RCC histologically; and (3) specify the histological subtype. Case reports and review articles were excluded.
Search Strategy and Screening
A comprehensive literature search was conducted in July 2023 across databases including Ovid MEDLINE, PubMed, and Google Scholar. A formalised search strategy was developed to encompass terms relating to kidney neoplasms, ADPKD, and prevalence. Articles published from January 1980 to July 2023 were eligible for inclusion. The full search strategy can be found in online supplementary Appendix A (for all online suppl. material, see https://doi.org/10.1159/000536245). Two independent authors (A.D. and K.Q.) screened studies using the Covidence platform (Covidence, Melbourne, Australia). Following title and abstract screening, articles underwent full-text review to determine final eligibility. Reference and citation checking of included articles was performed to ensure completeness of search. If RCC subtype was not disclosed in the full-text, authors were contacted to obtain this information. Disagreements were resolved through discussion with a third author (J.P.).
Quality Assessment and Risk of Bias
The most appropriate critical appraisal tool [13] to assess for risk of bias amongst included studies was the Joanna Briggs Institute (JBI) critical appraisal checklist for studies reporting prevalence data [14]. This was a nine-question checklist with a particular focus on data collection methods, the quality of data gathered, and coverage and classification bias [14]. Quality assessment was performed by two authors (A.D. and K.Q.), and disagreements were resolved through discussion with a third author (J.P.).
Data Extraction and Outcome Measures
Key data points included the total number of ADPKD patients and ADPKD kidneys analysed, the number of RCCs detected per patient and per kidney, the subtype of each RCC, and whether RCCs were detected incidentally or following clinical suspicion. Multifocal tumours (in a single kidney) were considered a single tumour. Additionally, if multiple RCC subtypes were present within a single kidney, this was classified as “mixed.” Incidental RCCs referred to those detected without prior clinical suspicion, that is, following a nephrectomy performed for an alternate indication (e.g., symptomatic disease, facilitation of renal transplant). Demographic details, including age and biological sex, in addition to prior history of ESRD and dialysis, were obtained if available.
The prevalence of RCC was determined on both a per-person and per-kidney basis to account for bilateral RCC presentations. For example, a patient with bilateral RCC was considered a single case on a per-person basis but 2 cases on a per-kidney basis. The proportion of total RCCs presenting incidentally was calculated, in addition to the prevalence of each RCC subtype. RCCs and their respective subtypes were defined according to the World Health Organisation’s Classification of Urinary and Male Genital Tumours (5th Edition) [15].
Statistical Analysis
Statistical analysis was performed using StataBE v18.0 (StataCorp, TX, USA). Meta-analysis of prevalence was performed using the meta function which computes the Freeman-Tukey double-arcsine-transformed proportion for each study. A random-effects model (restricted maximum likelihood) was employed throughout as this was deemed most appropriate for our sample. Study heterogeneity was assessed using the I2 statistic, I2 >50% was considered highly heterogeneous.
Results
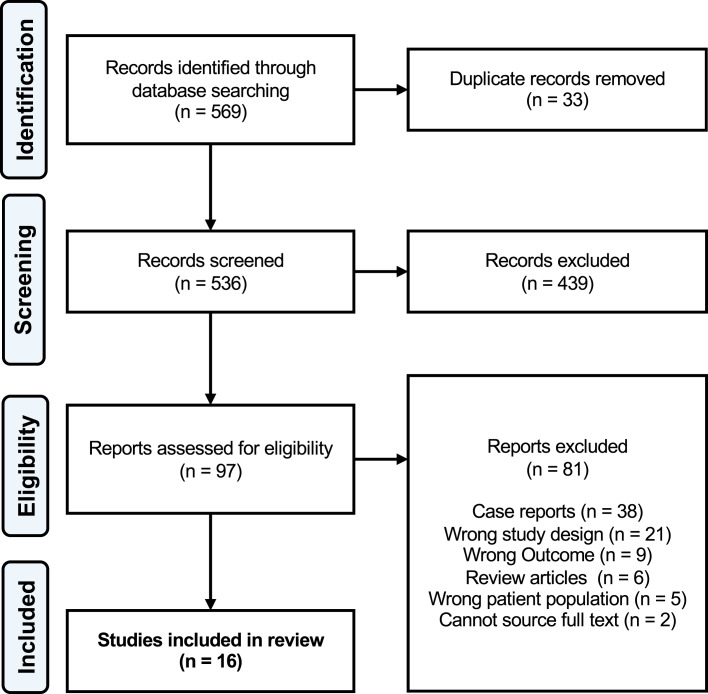
Our search yielded 569 articles of which 33 were duplicates. Following title and abstract screening, 439 studies were deemed irrelevant and 97 proceeded to full-text screening. Of these, 16 studies were included in the final analysis (PRISMA Flow Diagram is shown in Fig. 1).
Fig. 1.
PRISMA flow diagram.
A further 81 articles were excluded during full-text screening. The primary reason for exclusion was publication as a case report/series or review article. Wrong study design referred to articles that either did not document histological assessment of nephrectomy specimens or did not explicitly state that RCC was confirmed through histological analysis. Wrong outcome related to papers which performed histological analysis of nephrectomy specimens but did not assess for RCC or specify RCC subtype. Corresponding authors in the former group were contacted for further information; however, only one was able to respond by providing the subtypes of RCCs in their study, allowing for their inclusion.
Study Characteristics
All 16 studies were retrospective analyses of an exclusively ADPKD cohort, and utilised histological examination of native nephrectomy specimens to assess for the presence of RCC [6, 10, 11, 16–28]. Studies were published between 1987 and 2022 and examined nephrectomy specimens from 1,147 ADPKD patients. Studies were from Australia [23], France [11], Germany [6, 26], Italy [16, 27], the Netherlands [18], Russia [22], the United Kingdom [17, 21, 25], and the United States of America [10, 19, 20, 24, 28].
Six studies specifically assessed the relationship between RCC and ADPKD [6, 10, 11, 19, 20, 23], whilst the remaining identified RCC as part of routine histological analysis of ADPKD nephrectomies. Indications for nephrectomy were symptomatic cystic disease, peri-renal transplant, and suspected renal malignancy.
Gregoire et al. [19] analysed ADPKD kidneys collected via nephrectomy and autopsy; autopsy figures were excluded from data extraction to maintain consistency across studies. Furthermore, 2 cases of RCC in Gupta et al. [10] were excluded as they were not detected during their original registry sampling [10]. A summary of included studies is presented in Table 1.
Table 1.
Study characteristics
| Study | Country | Characteristics of total ADPKD cohort | ADPKD patients with RCC, n | ADPKD kidneys with RCC, n | Incidental RCCs, n (%)** | RCC subtypes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| patients, n | proportion with ESRD, % | kidneys analysed, n | proportion male, % | mean age, years | clear cell, n (%)** | papillary, n (%)** | other, n (%)** | |||||
| Asimakopoulos et al. [16] (2015) | Italy | 19 | 100 | 19 | 42 | 53 | 0 | 0 | – | – | – | – |
| Bellini et al. [17] (2019) | UK | 33 | 100 | 66 | 55 | – | 1 | 1 | 1 (100) | 0 (0) | 1 (100) | 0 (0) |
| Casteleijn et al. [18] (2022)* | The Netherlands | 134 | – | – | 66 | 53.5 | 4 | 4 | 4 (100) | 3 (75) | 1 (25) | 0 (0) |
| Gregoire et al. [19] (1987) | USA | 38 | 100 | 100 | 55 | 46 | 0 | – | – | – | – | – |
| Gupta et al. [10] (2022) | USA | 231 | 100 | 422 | – | – | 21 | 23 | – | 10 (43) | 9 (39) | 4 (17) |
| Hajj et al. [11] (2009) | France | 79 | 100 | 89 | 53 | 50 | 10 | 11 | 7 (64) | 6 (55) | 4 (36) | 1 (9) |
| Hansen et al. [20] (2015) | USA | 16 | – | – | 55 | 56 | 4 | – | 5 (100) | 4 (80) | 0 (0) | 1 (20) |
| Jilg et al. [6] (2013) | Germany | 240 | 100 | 301 | – | 54 | 11 | 12 | 7 (58) | 4 (33) | 7 (58) | 1 (8) |
| Kirkman et al. [21] (2011) | UK | 35 | 100 | 58 | 46 | – | 3 | 3 | 3 (100) | 2 (67) | 1 (33) | 0 (0) |
| Lubennikov et al. [22] (2021) | Russia | 108 | 100 | 216 | 56 | 55 | 7 | 7 | 7 (100) | 5 (71) | 2 (29) | 0 (0) |
| Mansbridge et al. [23] (2021) | Australia | 28 | – | 32 | 50 | 56 | 4 | 4 | 1 (25) | 1 (25) | 3 (75) | 0 (0) |
| Martin et al. [24] (2012) | USA | 37 | 100 | 74 | 51 | – | 3 | 3 | 1 (33) | 0 (0) | 2 (67) | 1 (33) |
| Patel et al. [25] (2011) | UK | 31 | 100 | 59 | 61 | 49 | 2 | 3 | 0 (0) | 1 (33) | 2 (67) | 0 (0) |
| Pfister et al. [26] (2010) | Germany | 40 | 100 | 51 | 65 | 59 | 3 | 3 | 0 (0) | 3 (100) | 0 (0) | 0 (0) |
| Veroux et al. [27] (2016) | Italy | 40 | 100 | 40 | 65 | 51 | 1 | 1 | 1 (100) | 0 (0) | 1 (100) | 0 (0) |
| Wagner et al. [28] (2007) | USA | 32 | 100 | 64 | 53 | 53 | 1 | 2 | 2 (100) | 0 (0) | 2 (100) | 0 (0) |
ADPKD, autosomal dominant polycystic kidney disease; ESRD, end-stage renal disease; n, number; RRC, renal cell carcinoma; UK, United Kingdom; USA, United States of America; –, not mentioned.
*Author contacted to obtain information regarding RCC subtype.
**Calculated as a percentage of total RCC’s detected in respective study.
Quality Assessment and Risk of Bias
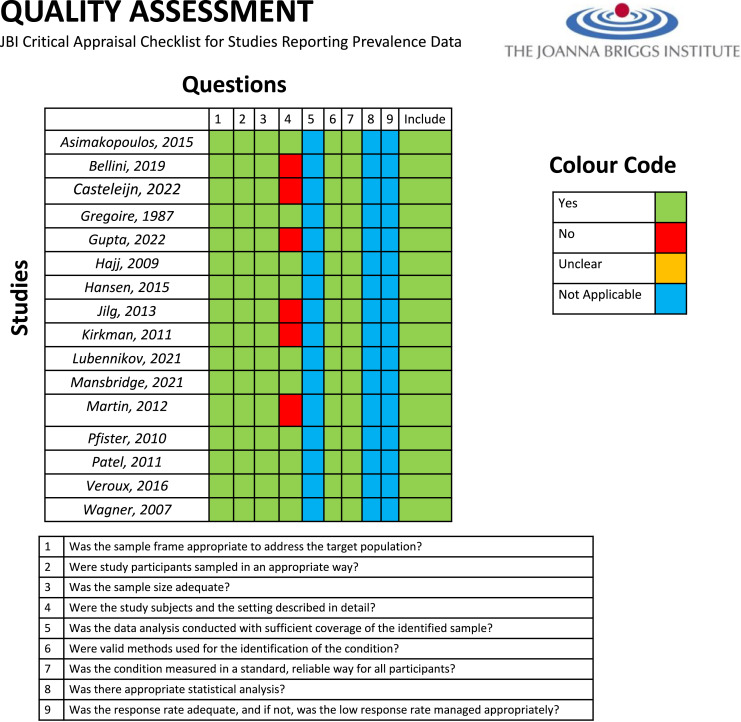
Study quality was assessed using the JBI critical appraisal checklist for studies reporting prevalence data (shown in Fig. 2) [14]. All studies appropriately addressed questions 1, 2, 3, 6, and 7 as they included study cohorts of exclusively ADPKD patients, and assessed for the presence of RCC within nephrectomy specimens utilising histopathology. Six studies did not adequately describe subjects in detail [7, 10, 17, 18, 21, 24]; however, this did not affect result calculation as analysis of demographic data was not an objective of this paper. Questions 5 and 8 were not applicable as we did not require papers to perform statistical analysis, and question 9 was not applicable for retrospective study designs.
Fig. 2.
JBI critical appraisal checklist for studies reporting prevalence data.
Prevalence of RCC
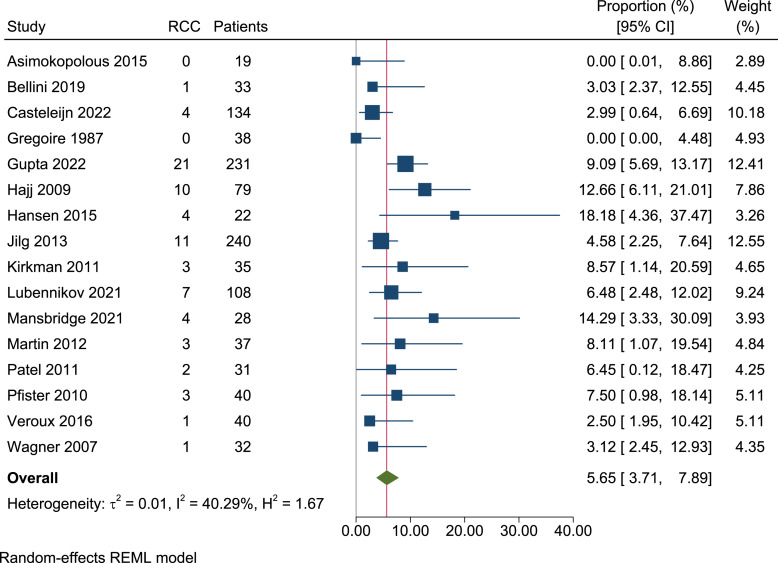
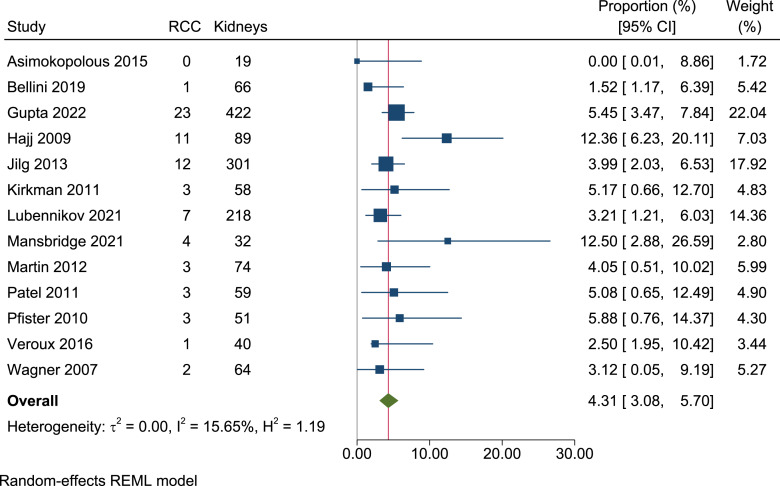
In total, we identified 1,147 ADPKD patients, of which 75 had RCC (crude prevalence 6.5%). Following meta-analysis of prevalence, the overall per-person prevalence of RCC amongst ADPKD patients was 5.7% (95% CI, 3.7–7.9, I2 = 40.3%) (shown in Fig. 3). Bilateral RCCs were detected in 7 patients (9.3% [7/75]). As such, 82 RCCs were detected in total. Thirteen papers stated the total number of kidneys analysed, reporting 73 RCCs amongst 1,493 kidneys [6, 10, 11, 16, 17, 21–28]. Following meta-analysis, the per-kidney prevalence was 4.3% (95% CI, 3.1–5.7, I2 = 15.7%) (shown in Fig. 4).
Fig. 3.
Forest plot of the person prevalence of renal cell carcinoma in ADPKD patients (1,147 patients, 16 papers). RCC, renal cell carcinoma.
Fig. 4.
Forest plot of the per-kidney prevalence of renal cell carcinoma in ADPKD patients (1,493 kidneys, 13 papers). RCC, renal cell carcinoma.
Incidental RCC
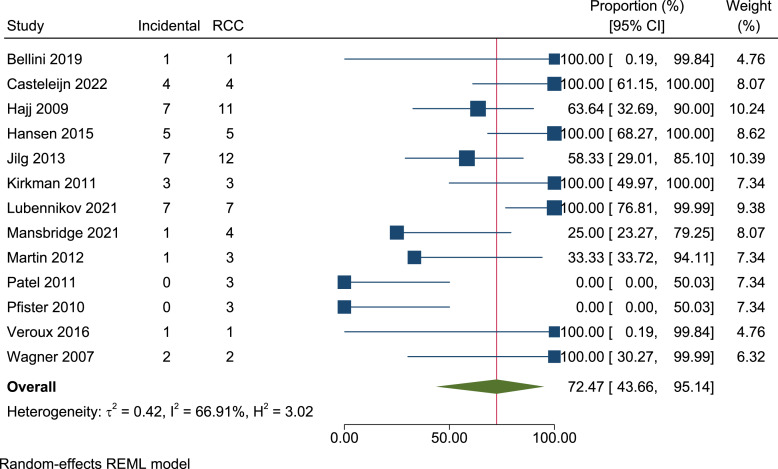
Thirteen articles included the indications for nephrectomy. The main indications for nephrectomy amongst ADPKD patients were to increase space prior to, or following kidney transplant, or in cases of persistent symptomatic disease. A less frequent indication for nephrectomy was tumour suspicion. As such, 66% (39/59) of RCCs were detected incidentally, following a nephrectomy performed for an alternate indication to tumour suspicion. Following meta-analysis, the percentage of RCC presenting incidentally was 72.5% (95% CI, 43.7–95.1, I2 = 66.9%) (shown in Fig. 5).
Fig. 5.
Forest plot of the proportion of ADPKD patients with renal cell carcinomas detected incidentally (59 renal cell carcinomas, 13 papers). RCC, renal cell carcinoma.
RCC Subtypes
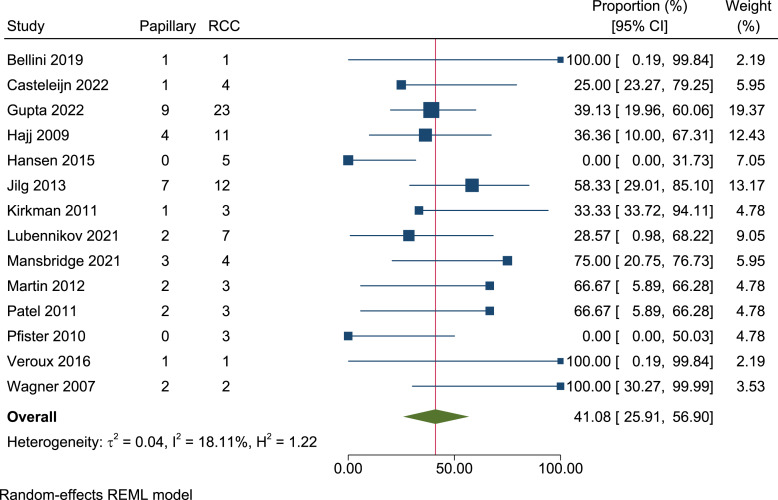
Of the 82 RCCs identified, 39 were clear cell RCC and 35 were pRCC. The remaining cases were 4 chromophobes, 1 acquired cystic disease-associated RCC, 1 mixed papillary, clear cell and sarcomatoid, and 2 mixed clear cell and papillary. Following meta-analysis of proportion, the prevalence of pRCCs across the 82 RCCs present was 41.1% (95% CI, 25.9–56.9, I2 = 18.1%) (shown in Fig. 6). Amongst the 82 cases, two were noted to have aggressive sarcomatoid features [6, 20].
Fig. 6.
Forest plot of the proportion of renal cell carcinomas identified as papillary renal cell carcinomas (82 renal cell carcinomas, 14 papers). RCC, renal cell carcinoma.
Discussion
ADPKD is a common inherited condition characterised by the growth of numerous fluid-filled renal cysts and progression to ESRD. Whilst ADPKD shares pathogenic similarities with that of neoplasia, it remains unclear whether it increases one’s risk of RCC [6, 29]. Our review established the prevalence of RCC amongst ADPKD patients to be 5.7%. This figure is greater than both the estimated lifetime risk of RCC across Europe and North America (1.3–1.8%), and the estimated global lifetime risk of developing RCC (0.7% in men and 0.4% in women) [30, 31]. This suggests an increased risk of RCC within the native kidneys of those with ADPKD. When considering per-kidney prevalence, RCC was detected in 4.3% of ADPKD kidneys. Additionally, we identified a higher proportion of concurrent bilateral RCC presentations (9.9%) in ADPKD, in comparison to the general population (1–5%) [32]. This is consistent with findings by Keith et al. [7] who found 12% of RCC cases to present bilaterally amongst their ADPKD case series. Our figure is also likely an underestimate, as not all papers preformed bilateral nephrectomies. The high prevalence of both RCC and bilateral RCC amongst those with ADPKD is suggestive of an association between these conditions.
The majority of RCCs (72.5%) detected in this study were discovered incidentally. This is an expected finding, as most nephrectomies performed were for reasons other than suspected renal malignancy. In line with this, most RCCs were detected at a low stage and size, which would have made preoperative identification challenging. The implications of this finding are somewhat unclear. This is primarily because we cannot predict the natural history of incidental small RCCs given their natural oncological course was disrupted – some may have progressed to metastatic disease, whereas others may have remained indolent. However, it does hold relevance when considering the increasing life span of ADPKD patients, which is mainly attributed to the greater accessibility of renal replacement therapy (RRT) [33]. For example, within Australia and New Zealand, the age-adjusted 5-year survival for ADPKD patients with ESRD has improved from 33% in 1963 to 89% in 2014 [34]. Therefore, a particular cohort in which this finding may hold clinical significance is ADPKD patients on RRT with their native kidneys in situ. As the theoretical risk of RCC increases the longer the native kidneys are in situ, there is a greater potential for the development of clinically significant RCCs. As such, consideration of RCC screening in this cohort may be appropriate. Whilst RCC is not currently a common cause of death (the leading causes of death being cardiovascular disease and infection) for ADPKD patients with ESRD [35], the high rate of incidental RCCs may affect clinical practice moving forward.
It should be noted that most ADPKD patients included in this systematic review had a history of ESRD, and in some cases, dialysis. As both ESRD and dialysis are known risk factors for RCC, it is difficult to ascertain the exact contribution of ADPKD to RCC development in their presence [9]. We were unable to adjust for these confounding variables as there was a lack of data outlining the extent of ESRD within all study cohorts. In particular, only five studies explicitly stated whether those with RCC had previously undergone dialysis. Of interest, a large cohort study has found an increased risk of RCC amongst pre-ESRD patients with ADPKD [36, 37]. Therefore, it is possible ADPKD independently increases RCC risk, separate from its relationship to ESRD and dialysis, and that this risk is exacerbated when it coexists with these factors. To confidently understand the relationship between ADPKD and RCC, a large population study investigating and comparing the individual RCC risk of ADPKD, ESRD and acquired cystic disease due to dialysis is necessary.
Among cases of RCCs, 45% were classified as pRCC. Notably, pRCC only accounts for 10% of RCCs diagnosed within the general population [31]. Whilst clear cell RCC was the most common subtype detected, this high prevalence of pRCC suggests a predisposition of ADPKD kidneys to develop this particular subtype. It is hypothesised that the increased prevalence of pRCC in ADPKD may be related to the presence of epithelial hyperplasia and papillary adenomas within ADPKD kidneys [19, 38]. Both findings share histological similarities with pRCC [39]; however, a clear link is yet to be established. The association between ADPKD and pRCC warrants further investigation into the pathogenic mechanism underpinning this relationship. ADPKD has also been associated with an increased rate of sarcomatoid dedifferentiation, a highly aggressive RCC variant [7, 40]. Our results did not support this relationship; however, sarcomatoid dedifferentiation is likely rare in incidental disease [7].
Limitations
Our study must be considered in the context of the following limitations. Firstly, the prevalence of ADPKD differs amongst ethnicities [41]. As almost all studies were performed in western countries our results are unlikely to be applicable globally. Secondly, we were unable to control for the contribution of ESRD and acquired cystic disease to overall RCC risk due to a lack of limited patient characteristics presented in included studies. This also limited our ability to understand the relationship between these factors and ADPKD in contributing to RCC risk, which would be a valuable focus for future research when further data are available. Thirdly, it is possible that the utilisation of nephrectomy specimens may underestimate RCC risk amongst ADPKD patients as it misses patients who did not require a nephrectomy. This is a particular concern given that the majority of RCC cases in ADPKD were detected incidentally. It is also possible that reliance on nephrectomy specimens could overestimate RCC prevalence, as patients requiring nephrectomy are more likely to be symptomatic and have more advanced chronic kidney disease. Lastly the high proportion of incidental RCCs impacts out ability to understand the true clinical impact of these findings.
Conclusion
RCCs, particularly of the papillary subtype, are common amongst ADPKD patients and occur at a greater frequency in comparison with general population. As such, treating clinicians should be aware of the risk of RCC amongst this cohort, particularly in those with ESRD and a history of RRT with native kidneys in situ. The high proportion of incidental RCCs also warrants clinical consideration as the life expectancy of ADPKD patients improves. To complete our understanding of the inherent risk of RCC in ADPKD, increased epidemiological data are required.
Acknowledgments
We would like thank Bendigo Health Librarian Angela Johns-Hayden for her assistance in developing our search strategy.
Statement of Ethics
Ethics approval was not required as this article was a systematic review and meta-analysis of published papers.
Conflict of Interest Statement
The authors report no conflict of interest.
Funding Sources
No funding was applicable.
Author Contributions
Anna Drake contributed to creating the methodology, data curation, and manuscript writing. Jessica Paynter contributed to creating the methodology and manuscript writing. Jake Tempo assisted in formal data analysis and manuscript review and editing. Arthur Yim assisted in formal data analysis and manuscript review and editing. Todd Manning contributed to project conceptualisation and assisted in project supervision. Janelle Brennan assisted in project supervision and manuscript review and editing. Kirby Qin contributed to project conceptualisation, creating the methodology, formal data analysis, and manuscript writing.
Funding Statement
No funding was applicable.
Data Availability Statement
Pertinent data are included in manuscript tables. Any further details can be requested via contact with the corresponding author.
Supplementary Material
References
- 1. Lanktree MB, Haghighi A, Guiard E, Iliuta IA, Song X, Harris PC, et al. Prevalence estimates of polycystic kidney and liver disease by population sequencing. J Am Soc Nephrol. 2018;29(10):2593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luciano RL, Dahl NK. Extra-renal manifestations of autosomal dominant polycystic kidney disease (ADPKD): considerations for routine screening and management. Nephrol Dial Transplant. 2014;29(2):247–54. [DOI] [PubMed] [Google Scholar]
- 3. Seeger-Nukpezah T, Geynisman DM, Nikonova AS, Benzing T, Golemis EA. The hallmarks of cancer: relevance to the pathogenesis of polycystic kidney disease. Nat Rev Nephrol. 2015;11(9):515–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gui Y, Dai C. mTOR signaling in kidney diseases. Kidney360. 2020;1(11):1319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rao H, Li X, Liu M, Liu J, Feng W, Tang H, et al. Multilevel regulation of β-catenin activity by SETD2 suppresses the transition from polycystic kidney disease to clear cell renal cell carcinoma. Cancer Res. 2021;81(13):3554–67. [DOI] [PubMed] [Google Scholar]
- 6. Jilg CA, Drendel V, Bacher J, Pisarski P, Neeff H, Drognitz O, et al. Autosomal dominant polycystic kidney disease: prevalence of renal neoplasias in surgical kidney specimens. Nephron Clin Pract. 2013;123(1–2):13–21. [DOI] [PubMed] [Google Scholar]
- 7. Keith DS, Torres VE, King BF, Zincki H, Farrow GM. Renal cell carcinoma in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1994;4(9):1661–9. [DOI] [PubMed] [Google Scholar]
- 8. Walker JB, Loloi J, Birk A, Raman JD. Computed tomography imaging characteristics of histologically confirmed papillary renal cell carcinoma-implications for ancillary imaging. J Kidney Cancer VHL. 2019;6(2):10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Åkerlund J, Holmberg E, Lindblad P, Stendahl M, Ljungberg B, Thorstenson A, et al. Increased risk for renal cell carcinoma in end stage renal disease: a population-based case-control study. Scand J Urol. 2021;55(3):209–14. [DOI] [PubMed] [Google Scholar]
- 10. Gupta S, Lohse CM, Rowsey R, McCarthy MR, Shen W, Herrera-Hernandez L, et al. Renal neoplasia in polycystic kidney disease: an assessment of tuberous sclerosis complex-associated renal neoplasia and PKD1/TSC2 contiguous gene deletion syndrome. Eur Urol. 2022;81(3):229–33. [DOI] [PubMed] [Google Scholar]
- 11. Hajj P, Ferlicot S, Massoud W, Awad A, Hammoudi Y, Charpentier B, et al. Prevalence of renal cell carcinoma in patients with autosomal dominant polycystic kidney disease and chronic renal failure. Urology. 2009;74(3):631–4. [DOI] [PubMed] [Google Scholar]
- 12. Muglia VF, Prando A. Renal cell carcinoma: histological classification and correlation with imaging findings. Radiol Bras. 2015;48(3):166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Chapter 5: systematic reviews of prevalence and incidence. In: Aromataris E, Munn Z, editors. JBI manual for evidence synthesis JBI; 2020. [Google Scholar]
- 15. Urinary and Male genital Tumours: World Health Organization; 2022. [Google Scholar]
- 16. Asimakopoulos AD, Gaston R, Miano R, Annino F, Mugnier C, Dutto L, et al. Laparoscopic pretransplant nephrectomy with morcellation in autosomic-dominant polycystic kidney disease patients with end-stage renal disease. Surg Endosc. 2015;29(1):236–44. [DOI] [PubMed] [Google Scholar]
- 17. Bellini MI, Charalmpidis S, Brookes P, Hill P, Dor F, Papalois V. Bilateral nephrectomy for adult polycystic kidney disease does not affect the graft function of transplant patients and does not result in sensitisation. Biomed Res Int. 2019;2019:7423158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Casteleijn NF, Geertsema P, Koorevaar IW, Inkelaar FDJ, Jansen MR, Lohuis SJ, et al. The need for routine native nephrectomy in the workup for kidney transplantation in autosomal dominant polycystic kidney disease patients. Urol Int. 2023;107(2):148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gregoire JR, Torres VE, Holley KE, Farrow GM. Renal epithelial hyperplastic and neoplastic proliferation in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1987;9(1):27–38. [DOI] [PubMed] [Google Scholar]
- 20. Hansen CC, Derrick M, Warriach I, Cammack JT, Cammack JT, Riese W. The association between autosomal dominant polycystic kidney disease and renal cell carcinoma. Open J Urol. 2015;5:84–90. [Google Scholar]
- 21. Kirkman MA, van Dellen D, Mehra S, Campbell BA, Tavakoli A, Pararajasingam R, et al. Native nephrectomy for autosomal dominant polycystic kidney disease: before or after kidney transplantation? BJU Int. 2011;108(4):590–4. [DOI] [PubMed] [Google Scholar]
- 22. Lubennikov AE, Petrovskii NV, Krupinov GE, Shilov EM, Trushkin RN, Kotenko ON, et al. Bilateral nephrectomy in patients with autosomal dominant polycystic kidney disease and end-stage chronic renal failure. Nephron. 2021;145(2):164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mansbridge M, Lawson M, Preston J, Griffin A, Wood S, Rhee H. Renal cell carcinoma in native nephrectomy specimens of autosomal dominant polycystic kidney disease (ADPKD) patients with end-stage renal disease: findings from an Australian transplant center. J Clin Urol. 2021;16(2):121–5. [Google Scholar]
- 24. Martin AD, Mekeel KL, Castle EP, Vaish SS, Martin GL, Moss AA, et al. Laparoscopic bilateral native nephrectomies with simultaneous kidney transplantation. BJU Int. 2012;110(11 Pt C):E1003–7. [DOI] [PubMed] [Google Scholar]
- 25. Patel P, Horsfield C, Compton F, Taylor J, Koffman G, Olsburgh J. Native nephrectomy in transplant patients with autosomal dominant polycystic kidney disease. Ann R Coll Surg Engl. 2011;93(5):391–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pfister D, Thuer D, Heidenreich A. [Pitfalls and outcome of nephrectomy for patients with polycystic kidney disease: peri- and postoperative results]. Urologe. 2010;49(9):1156–62. [DOI] [PubMed] [Google Scholar]
- 27. Veroux M, Zerbo D, Basile G, Gozzo C, Sinagra N, Giaquinta A, et al. Simultaneous native nephrectomy and kidney transplantation in patients with autosomal dominant polycystic kidney disease. PLoS One. 2016;11(6):e0155481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wagner MD, Prather JC, Barry JM. Selective, concurrent bilateral nephrectomies at renal transplantation for autosomal dominant polycystic kidney disease. J Urol. 2007;177(6):2250–4. [DOI] [PubMed] [Google Scholar]
- 29. Wetmore JB, Calvet JP, Yu AS, Lynch CF, Wang CJ, Kasiske BL, et al. Polycystic kidney disease and cancer after renal transplantation. J Am Soc Nephrol. 2014;25(10):2335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J, et al. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75(1):74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Padala SA, Barsouk A, Thandra KC, Saginala K, Mohammed A, Vakiti A, et al. Epidemiology of renal cell carcinoma. World J Oncol. 2020;11(3):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin W, Watts KL, Davuluri M, Aboumohamed A. Non-familial synchronous bilateral renal cell carcinoma with bilateral synchronous renal vein extension and inferior vena cava thrombus. Curr Urol. 2019;13(1):51–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Orskov B, Rømming Sørensen V, Feldt-Rasmussen B, Strandgaard S. Improved prognosis in patients with autosomal dominant polycystic kidney disease in Denmark. Clin J Am Soc Nephrol. 2010;5(11):2034–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fernando MR, Dent H, McDonald SP, Rangan GK. Incidence and survival of end-stage kidney disease due to polycystic kidney disease in Australia and New Zealand (1963–2014). Popul Health Metr. 2017;15(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Orskov B, Sørensen VR, Feldt-Rasmussen B, Strandgaard S. Changes in causes of death and risk of cancer in Danish patients with autosomal dominant polycystic kidney disease and end-stage renal disease. Nephrol Dial Transplant. 2012;27(4):1607–13. [DOI] [PubMed] [Google Scholar]
- 36. Denton MD, Magee CC, Ovuworie C, Mauiyyedi S, Pascual M, Colvin RB, et al. Prevalence of renal cell carcinoma in patients with ESRD pre-transplantation: a pathologic analysis. Kidney Int. 2002;61(6):2201–9. [DOI] [PubMed] [Google Scholar]
- 37. Yu TM, Chuang YW, Yu MC, Chen CH, Yang CK, Huang ST, et al. Risk of cancer in patients with polycystic kidney disease: a propensity-score matched analysis of a nationwide, population-based cohort study. Lancet Oncol. 2016;17(10):1419–25. [DOI] [PubMed] [Google Scholar]
- 38. Truong LD, Choi YJ, Shen SS, Ayala G, Amato R, Krishnan B. Renal cystic neoplasms and renal neoplasms associated with cystic renal diseases: pathogenetic and molecular links. Adv Anat Pathol. 2003;10(3):135–59. [DOI] [PubMed] [Google Scholar]
- 39. Wang KL, Weinrach DM, Luan C, Han M, Lin F, Teh BT, et al. Renal papillary adenoma: a putative precursor of papillary renal cell carcinoma. Hum Pathol. 2007;38(2):239–46. [DOI] [PubMed] [Google Scholar]
- 40. Pichler R, Compérat E, Klatte T, Pichler M, Loidl W, Lusuardi L, et al. Renal cell carcinoma with sarcomatoid features: finally New therapeutic hope? Cancers. 2019;11(3):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aung TT, Bhandari SK, Chen Q, Malik FT, Willey CJ, Reynolds K, et al. Autosomal dominant polycystic kidney disease prevalence among a racially diverse United States population, 2002 through 2018. Kidney360. 2021;2(12):2010–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Pertinent data are included in manuscript tables. Any further details can be requested via contact with the corresponding author.