Abstract
Introduction
We previously conducted a phase I/Ib study (NCT03712943) with regorafenib and nivolumab in patients with refractory metastatic mismatch repair proficient (pMMR) colorectal cancer (CRC). This study aimed to investigate the role of Xerna™ TME Panel in predicting the treatment response.
Methods
Twenty-two archival pretreatment tumor samples were subjected to the Xerna™ TME Panel, a machine learning-based RNA-sequencing biomarker assay. The Xerna tumor microenvironment (TME) subtypes were evaluated for correlation with overall survival (OS), progression-free survival (PFS), disease control rate (DCR), and other biomarkers including KRAS, PD-L1, CD8 expression, and Treg cells in TME.
Results
Based on Xerna™ TME Panel, 4 patients with immune-active (IA) subtype and 6 patients with immune-suppressed subtype were classified as biomarker-positive, and five with angiogenic (A) subtype and seven with immune desert subtype were biomarker-negative. While not reaching statistical significance, Xerna TME biomarker-positive patients seemed to have longer median PFS (7.9 vs. 4.1 months, p = 0.254), median OS (15.75 vs. 11.9 months, p = 0.378), and higher DCR (70% vs. 58%, p = 0.675). The IA subtype in our cohort had higher levels of CD4+ FOXP3+ Treg cells, whereas the A subtype showed lower levels of Treg cells.
Conclusion
Xerna™ TME Panel analysis in patients with refractory metastatic pMMR CRC who were treated with regorafenib plus nivolumab might be of value for predictive clinical benefit. Further studies are needed to evaluate the predictive role of Xerna™ TME Panel analysis in patients with refractory metastatic pMMR CRC.
Keywords: Colorectal cancer, Regorafenib, Nivolumab, Biomarker, Tumor microenvironment
Introduction
Immune checkpoint inhibitors (ICIs) have shown potent antitumor activity in patients with mismatch repair deficient/microsatellite instability-high cancers but limited efficacy in mismatch repair proficient (pMMR)/microsatellite stable (MSS) colorectal cancer (CRC), which are characterized by a lower expression of immune checkpoint proteins and tumor mutational burden, and an immune desert tumor microenvironment (TME) [1]. The multi-kinase inhibitor regorafenib has been approved for the treatment of patients with metastatic CRC refractory to chemotherapy. The main therapeutic effects of regorafenib are anti-angiogenesis and remodeling of TME through various mechanisms [2]. In murine MSS colon cancer models, regorafenib combined with an anti-programmed cell death-1 (PD-1) antibody significantly improved antitumor activity compared to single agent, suggesting the synergistic immunomodulatory effects of combination therapy in mediating a sustained inhibition of cancer regrowth [3].
The combination of regorafenib with PD-1 targeting for patients with refractory CRC has been evaluated in both prospective and retrospective settings [4–15]. The treatment efficacy in different studies among different patient populations varies greatly. The REGONIVO study reported a promising objective response rate (ORR) of 33% with regorafenib and nivolumab in 24 patients with refractory MSS CRC from Japan [4]. However, subsequent trials in North America only demonstrated very limited efficacy of this combination [5, 15].
Discovering potential predictive biomarkers that can identify patients who may benefit from the combination therapy with regorafenib and PD-1 inhibitors is important for implementation of this strategy in the clinical setting. We have previously evaluated the safety and efficacy of regorafenib and nivolumab in patients with refractory metastatic pMMR CRC in an open-label, single-arm phase I/Ib study (NCT03712943) which demonstrated limited anticancer activity [5].
Xerna™ TME Panel is a pan-tumor, RNA expression-based classifier that identifies the dominant biology of the TME and assigns into therapeutically actionable tumor subtypes defined by angiogenesis and immune gene expression [16]. It has demonstrated ability to predict response to multiple TME-targeted therapies, including immunotherapies and anti-angiogenic (A) agents in gastric cancer, ovarian cancer, and melanoma. In addition, the panel has a potential as a prognostic marker in CRC [16–19]. It was hypothesized that patients with a high immune score based on Xerna™ TME Panel would experience the most clinical benefit from ICIs or combination immune therapies [16, 19]. Based on these data, the predictive role of Xerna™ TME Panel was evaluated in CRC patients who received regorafenib and nivolumab. This study aimed to investigate the role of Xerna™ TME Panel, an RNA-sequencing biomarker assay, in predicting the treatment response.
Methods
Patients
We collected tumor samples and clinical data of patients with refractory pMMR metastatic colorectal adenocarcinoma who were enrolled in the phase I/Ib study of regorafenib plus nivolumab (NCT03712943) [5]. This study was reviewed and approved by the Institutional Review Board at Moffitt Cancer Center. All patients received at least one dose of regorafenib and nivolumab.
Xerna™ TME Panel
Twenty-two archival pretreatment tumor samples were subjected to the Xerna™ TME Panel. The Xerna™ TME Panel uses formalin-fixed paraffin-embedded tissue-derived RNA gene expression data based on 124 genes to classify patients into dominant biologic processes of the TME. The input gene signature represents A and immunogenic properties of stromal biology, and the machine learning neural network that comprises the Xerna™ TME Panel algorithm has learned interactions between these critical processes. The Xerna™ TME Panel can be used to classify patient tumor samples into one of four TME phenotypes based on gene expression signatures along an immune and A axis: A, immune active (IA), immune desert (ID), and immune suppressed (IS) (shown in Fig. 1) [16, 17, 19]. Xerna TME A subtype is characterized by angiogenesis gene and protein expression profile and is histologically marked by dense, dysfunctional vessels. IA subtype is characterized by expression of genes for inflammatory response and immune activation and histologically shows tumor infiltration by immune cells. ID subtype lacks the immune or angiogenesis gene signatures and histologically demonstrates low vessel density and low immune cell infiltration. IS subtype is characterized by gene expression profile of angiogenesis, inflammation, and M2 macrophage biology and is histologically marked by dense, pathological vessels as well as infiltration of immune and Treg cells [19].
Fig. 1.
Illustration of the four Xerna TME subtypes.
Biomarkers
Additional biomarkers include KRAS (wild type vs. mutant), programmed death-ligand 1 (PD-L1, negative vs. positive), CD8 expression (low vs. high), and CD4+ FOXP3+ regulatory T cells (Treg, low vs. high) in TME. The archival pretreatment tumor samples were incubated with anti-PD-L1 (E1L3N; Cell Signaling, Danvers, MA, USA), anti-CD8 (SP57; Ventana, Oro Valley, AZ, USA), anti-CD4 (SP35; Cell Marque, Rocklin, CA, USA), and anti-FOXP3 antibody (236A/E7; Abcam, Cambridge, MA, USA). Samples with PD-L1 membranous staining in ≥1% tumor cells were considered positive. Samples with any lymphocytes exhibiting membranous staining for CD4 and CD8 and exhibiting intracellular staining for FOXP3 were considered positive.
Statistical Analysis
Statistical significance between groups was analyzed using the χ2 tests or Fisher’s exact test for categorical variables and the Kruskal-Wallis or Mann-Whitney U non-parametric test for continuous variables. The estimated overall survival (OS) and progression-free survival (PFS) were derived using the Kaplan-Meier method and compared by the Mantel-Cox log-rank test. The statistical analyses were performed using IBM SPSS Statistics for Windows, version 28.0 (IBM Corp, Armonk, NY, USA). Kaplan-Meier survival curves were generated in R (version 4.2.1; www.r-project.org), using the “survminer” package and the “ggsurvplot” function. All reported p values were two-sided. The level of significance was set at p < 0.05.
Results
Patient Characteristics and Xerna TME
Baseline characteristics and Xerna™ TME Panel result of 22 patients who received regorafenib plus nivolumab are summarized in Table 1. Median age was 57.5 years old (range: 38–78). There were 10 female and 12 male patients. Gene expression level of 22 pretreatment tumor samples was analyzed by the Xerna™ TME Panel algorithm. Five patients were classified as A, four as IA, seven as ID, and six as IS subtype. IA and IS subtypes are considered biomarker-positive with high immune score and are hypothesized to respond best to immune-based therapies, and A and ID subtypes are biomarker-negative based on previous studies [16, 19]. Ten (45.5%) patients’ samples showed high immune score (IA and IS subtypes), while 12 (54.5%) showed low immune score (A and ID subtypes). Patients’ characters are summarized in Table 1. No significant correlation was observed between the Xerna TME subtypes and gender, age, race, the Eastern Cooperative Oncology Group (ECOG) Performance Status, side of primary tumor (left vs. right), metastatic site (i.e., liver, lung), or lines of previous chemotherapy.
Table 1.
Patient characteristics
| Baseline characteristics | n = 22 | Xerna TME classification | P1 | P2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| IA (n = 4) | IS (n = 6) | A (n = 5) | ID (n = 7) | IA + IS (n = 10) | A + ID (n = 12) | ||||
| Gender, n (%) | 0.841 | 0.691 | |||||||
| Female | 10 (45) | 2 (50) | 2 (33) | 2 (40) | 4 (57) | 4 (40) | 6 (50) | ||
| Male | 12 (55) | 2 (50) | 4 (67) | 3 (60) | 3 (43) | 6 (60) | 6 (50) | ||
| Age, median (range), years | 57.5 (38–78) | 53.5 (50–71) | 61.5 (47–75) | 60 (53–78) | 58 (38–70) | 56.5 (47–75) | 59 (38–78) | 0.609 | 1.000 |
| ECOG Performance Status at screening, n (%) | 0.841 | 0.691 | |||||||
| 0 | 12 (55) | 2 (50) | 4 (67) | 3 (60) | 3 (43) | 6 (60) | 6 (50) | ||
| 1 | 10 (45) | 2 (50) | 2 (33) | 2 (40) | 4 (57) | 4 (40) | 6 (50) | ||
| Race, n (%) | 0.523 | 1.000 | |||||||
| White | 21 (95) | 4 (100) | 6 (100) | 5 (100) | 6 (86) | 10 (100) | 11 (92) | ||
| Hispanic | 1 (5) | 0 (0) | 0 (0) | 0 (0) | 1 (14) | 0 (0) | 1 (8) | ||
| Side of Primary Tumor, n (%) | 0.723 | 0.415 | |||||||
| Left | 13 (59) | 3 (75) | 4 (67) | 2 (40) | 4 (57) | 7 (70) | 6 (50) | ||
| Right | 9 (41) | 1 (25) | 2 (33) | 3 (60) | 3 (43) | 3 (30) | 6 (50) | ||
| Metastatic site, n (%) | |||||||||
| Liver | 16 (73) | 4 (100) | 3 (50) | 4 (80) | 5 (71) | 7 (70) | 9 (75) | 0.362 | 1.000 |
| Lung | 15 (68) | 2 (50) | 4 (67) | 4 (80) | 5 (71) | 6 (60) | 9 (75) | 0.808 | 0.652 |
| Previous Chemotherapy, n (%) | 0.303 | 0.204 | |||||||
| 2nd line | 14 (63.6) | 4 (100) | 4 (67) | 2 (40) | 4 (57) | 8 (80) | 6 (50) | ||
| 3rd line and beyond | 8 (36.4) | 0 (0) | 2 33) | 3 (60) | 3 (43) | 2 (20) | 6 (50) | ||
ECOG, Eastern Cooperative Oncology Group. Xerna TME subtypes: angiogenic (A), immune active (IA), immune desert (ID), and immune suppressed (IS).
P1: comparison among TME subtypes.
P2: comparison between high immune score (IA + IS) and low immune score (A + ID).
Xerna TME and Corresponding Clinical Outcome
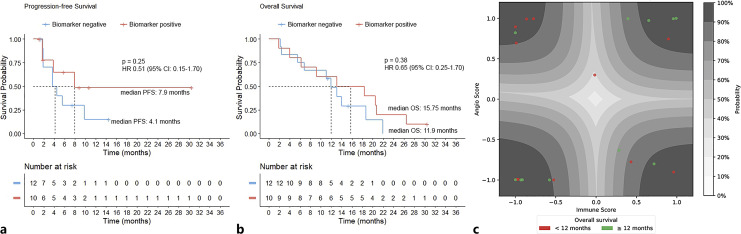
Among Xerna TME biomarker-positive patients, 2 achieved partial response and 5 had stable disease with a disease control rate (DCR) of 70%; four of them did not have disease progression for over 6 months. Among the biomarker-negative patients, 7 had stable disease with a DCR of 58% (p = 0.675; Table 2); 2 of them remained progression free for over 6 months. While not reaching statistical significance, Xerna TME biomarker-positive patients showed trends for higher median PFS (7.9 months vs. 4.1 months, p = 0.254) and median OS (15.75 months vs. 11.9 months, p = 0.378) compared to biomarker-negative patients (Table 2; shown in Fig. 2).
Table 2.
Clinical outcome and Xerna TME
| Outcome | Xerna TME classification | P1 | P2 | |||||
|---|---|---|---|---|---|---|---|---|
| IA (n = 4) | IS (n = 6) | A (n = 5) | ID (n = 7) | IA + IS (n = 10) | A + ID (n = 12) | |||
| 0.835 | 0.439 | |||||||
| PR (n = 2) | 1 | 1 | 0 | 0 | 2 | 0 | ||
| SD (n = 12) | 1 | 4 | 3 | 4 | 5 | 7 | ||
| PD (n = 5) | 1 | 1 | 1 | 2 | 2 | 3 | ||
| Median PFS, months | 7.9 | Not reached | 3.7 | 4.5 | 7.9 | 4.1 | 0.639 | 0.254 |
| Median OS, months | 9.1 | 13.1 | 11.0 | 13.1 | 15.75 | 11.9 | 0.701 | 0.378 |
OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease. Xerna TME subtypes: angiogenic (A), immune active (IA), immune desert (ID), and immune suppressed (IS).
P1: comparison among TME subtypes.
P2: comparison between high immune score (IA + IS) and low immune score (A + ID).
Fig. 2.
Xerna™ TME panel analysis. a, b Kaplan-Meier curves of PFS (a) and OS (b) of all patients (n = 22) stratified by the Xerna TME biomarker. c Latent space plot of Xerna TME calls for all samples. Glyphs are color-coded according to their OS. Contours represent different levels of probabilities for the Xerna TME calls. Xerna TME biomarker-negative subtypes include A and ID subtypes, and biomarker-positive subtypes include IA and IS ones.
Xerna TME and Other Markers
Previously, we reported low frequency of Tregs and extra-hepatic metastases might be potential biomarkers to predict response in patients with refractory pMMR CRC receiving regorafenib plus nivolumab [5]. We evaluated correlation between these markers and Xerna TME.
Three patients with IA subtype whose samples were also tested for CD4 and FOXP3 expression had high Treg cells in the TME. All 5 patients with A subtype had low Treg cells (Table 3). No significant correlation was observed between the Xerna TME subtypes and KRAS mutation, PD-L1 or CD8 expression (Table 3).
Table 3.
Correlation between Xerna TME subtypes and other biomarkers
| Variable | Xerna TME classification | P1 | P2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IA (n = 4) | IS (n = 6) | A (n = 5) | ID (n = 7) | IA + IS (n = 10) | A + ID (n = 12) | ||||
| KRAS status, n (%) | 0.848 | 1.000 | |||||||
| Wild type | 7 (32) | 1 (25) | 2 (33) | 1 (20) | 3 (43) | 3 (30) | 4 (33) | ||
| Mutant | 15 (68) | 3 (75) | 4 (67) | 4 (80) | 4 (57) | 7 (70) | 8 (67) | ||
| PD-L1, n (%) | 0.730 | 1.000 | |||||||
| Negative | 10 (48) | 2 (67) | 2 (33) | 3 (60) | 3 (43) | 4 (44) | 6 (50) | ||
| Positive | 11 (52) | 1 (33) | 4 (67) | 2 (40) | 4 (57) | 5 (56) | 6 (50) | ||
| CD8 expression, n (%) | 0.326 | 0.231 | |||||||
| Low | 10 (45) | 1 (25) | 2 (33) | 4 (80) | 3 (43) | 3 (30) | 7 (58) | ||
| High | 12 (55) | 3 (75) | 4 (67) | 1 (20) | 4 (57) | 7 (70) | 5 (42) | ||
| Treg, n (%) | 0.049 | 0.087 | |||||||
| Low | 12 (57) | 0 (0) | 3 (50) | 5 (100) | 4 (57) | 3 (33) | 9 (75) | ||
| High | 9 (43) | 3 (100) | 3 (50) | 0 (0) | 3 (43) | 6 (67) | 3 (25) | ||
Xerna TME subtypes: angiogenic (A), immune active (IA), immune desert (ID), and immune suppressed (IS).
P1: comparison among TME subtypes.
P2: comparison between high immune score (IA + IS) and low immune score (A + ID).
Discussion
Regorafenib targets multiple receptor tyrosine kinases that are involved in tumor angiogenesis and metastasis (e.g., vascular endothelial growth factor receptors 1–3, tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains 2, fibroblast growth factor receptors 1–2, and platelet-derived growth factor receptor), proliferation (e.g., KIT, RAF, RET), and the TME. Regorafenib also disrupts tumor immunity by inhibiting colony-stimulating factor-1 receptor and causes a reduction in tumor-associated macrophages that promote tumor cell occurrence, development, and migration [20]. Despite the enthusiasm that combination of regorafenib with ICI might exhibit synergistic effects with the potential of regorafenib in reversing the inherent resistance of pMMR/MSS CRCs to ICIs, the efficacy is marginal across various studies. Identification of biomarkers that predict treatment benefit may assist in selecting patients for this strategy. Previous studies have linked liver metastasis, cytotoxic T cells, Tregs, tumor-associated macrophages, plasma levels of biomarkers of vascular biology, circulating tumor DNA, neutrophil-to-lymphocyte ratio, gut microbiome, etc., with treatment response [6, 9, 10, 12–15]. However, predictive biomarkers for anti-angiogenesis and immunotherapies have been challenged by the limited studies and the inconsistent results.
The Xerna™ TME Panel is a novel machine learning-based RNA-sequencing biomarker assay that was developed to guide patient selection for TME-targeted therapies across multiple tumor types [16]. TME subtypes have been shown to have prognostic value for determining survival and disease recurrence risk across tumor types and are predictive for response to anti-A and immune targeting therapies [16–19]. In a gastric cancer cohort treated with either pembrolizumab or nivolumab monotherapy, the PD-L1-positive immune-high subtypes (IA and IS) achieved an ORR of 44%, compared to an ORR of 0% in the PD-L1-positive immune-low subtypes (A and ID) [16]. In our study, the IA subtype (high immune score tumors) in our cohort seemed to have higher levels of CD8+ cytotoxic T cells, whereas the A subtype (low immune score tumors) showed lower levels of CD8+ expression on the immunohistochemical staining suggesting the Xerna™ panel may reflect TME of CRC, accurately. Xerna™ TME biomarker-positive patients with high immune score tumors (IA and IS) seemed to have higher DCR and median PFS and OS compared to those with low immune score subtypes (A and ID), although the difference was not statistically significant. This may be attributed to relatively small sample size. Not all patients enrolled in the trial had available pretreatment samples; therefore, the low percentage of tests performed in our study limited our ability to further evaluate these potential biomarkers.
In conclusion, our study showed potential predictive clinical benefit of Xerna™ TME Panel analysis in patients with refractory metastatic pMMR CRC who were treated with regorafenib plus nivolumab. Experimental demonstration of molecular alterations after treatment with regorafenib and nivolumab in xenograft mouse models to validate the biomarkers will be of interest. Prospective and larger cohort studies are needed to better define predictive biomarkers for this combination in the future.
Acknowledgment
The authors would like to thank Mr. Bence Kovari for his assistance in data acquisition and analysis.
Statement of Ethics
This study was reviewed and approved by the Institutional Review Board at Moffitt Cancer Center, approval number MCC #19581. All patients provided written informed consent.
Conflict of Interest Statement
Richard Kim received honorarium from Lilly, BMS, and Bayer. Seema Iyer, Mark Uhlik, and Laura Benjamin are employees of and have financial interests in OncXerna Therapeutics Inc. The other authors report no conflicts of interest for this work.
Funding Sources
This work was supported by Bristol Myers Squibb (BMS). The funder had no role in the design, data collection, data analysis, and reporting of this study.
Author Contributions
Dr. Ruoyu Miao, Dr. Dae Won Kim, and Dr. Richard Kim contributed to the concept, design, and drafting of the article. Dr. Ruoyu Miao contributed to statistical analysis. Ms. Seema Iyer, Mr. Mark Uhlik, and Ms. Laura Benjamin contributed to RNA expression analysis. All authors (Dr. Ruoyu Miao, Dr. Dae Won Kim, Dr. James Yu, Dr. Mokenge Malafa, Dr. Rutika Mehta, Dr. Jonathan Strosberg, Dr. Iman Imanirad, Ms. Seema Iyer, Mr. Mark Uhlik, Ms. Laura Benjamin, and Dr. Richard Kim) contributed to acquisition, analysis, or interpretation of data; critical revision of the manuscript for important intellectual content; and administrative, technical, or material support. Dr. Richard Kim obtained funding.
Funding Statement
This work was supported by Bristol Myers Squibb (BMS). The funder had no role in the design, data collection, data analysis, and reporting of this study.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. Further inquiries can be directed to the corresponding author.
References
- 1. Akin Telli T, Bregni G, Vanhooren M, Saude Conde R, Hendlisz A, Sclafani F. Regorafenib in combination with immune checkpoint inhibitors for mismatch repair proficient (pMMR)/microsatellite stable (MSS) colorectal cancer. Cancer Treat Rev. 2022;110:102460. [DOI] [PubMed] [Google Scholar]
- 2. Arai H, Battaglin F, Wang J, Lo JH, Soni S, Zhang W, et al. Molecular insight of regorafenib treatment for colorectal cancer. Cancer Treat Rev. 2019;81:101912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Doleschel D, Hoff S, Koletnik S, Rix A, Zopf D, Kiessling F, et al. Regorafenib enhances anti-PD1 immunotherapy efficacy in murine colorectal cancers and their combination prevents tumor regrowth. J Exp Clin Cancer Res. 2021;40(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38(18):2053–61. [DOI] [PubMed] [Google Scholar]
- 5. Wang C, Chevalier D, Saluja J, Sandhu J, Lau C, Fakih M. Regorafenib and nivolumab or pembrolizumab combination and circulating tumor DNA response assessment in refractory microsatellite stable colorectal cancer. Oncologist. 2020;25(8):e1188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cousin S, Cantarel C, Guegan JP, Gomez-Roca C, Metges JP, Adenis A, et al. Regorafenib-avelumab combination in patients with microsatellite stable colorectal cancer (REGOMUNE): a single-arm, open-label, phase II trial. Clin Cancer Res. 2021;27(8):2139–47. [DOI] [PubMed] [Google Scholar]
- 7. Wang F, He MM, Yao YC, Zhao X, Wang ZQ, Jin Y, et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med. 2021;2(9):100383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barzi A, Azad NS, Yang Y, Tsao-Wei D, Rehman R, Fakih M, et al. Phase I/II study of regorafenib (rego) and pembrolizumab (pembro) in refractory microsatellite stable colorectal cancer (MSSCRC). J Clin Oncol. 2022;40(4 Suppl):15–. [Google Scholar]
- 9. Chen B, Zhao H, Huang J, Lv H, Xu W, Nie C, et al. Efficacy of regorafenib combined with PD-1 inhibitors in elderly patients with advanced metastatic colorectal cancer. BMC Geriatr. 2022;22(1):987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He WZ, Wang L, Yin CX, Yi JH, Jin YN, Jiang C, et al. Regorafenib with or without a programmed cell death protein 1 antibody as third-line treatment for microsatellite stable metastatic colorectal cancer. Cancer Med. 2023;12(6):6488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim RD, Kovari BP, Martinez M, Xie H, Sahin IH, Mehta R, et al. A phase I/Ib study of regorafenib and nivolumab in mismatch repair proficient advanced refractory colorectal cancer. Eur J Cancer. 2022;169:93–102. [DOI] [PubMed] [Google Scholar]
- 12. Wang H, Liu W, Zhao Y, Hu H, Zhang B, Yang S. Real-world effectiveness of regorafenib in the treatment of patients with metastatic colorectal cancer: a retrospective, observational study. Asia Pac J Clin Oncol. 2023;19(5):e291–9. [DOI] [PubMed] [Google Scholar]
- 13. Xu YJ, Zhang P, Hu JL, Liang H, Zhu YY, Cui Y, et al. Regorafenib combined with programmed cell death-1 inhibitor against refractory colorectal cancer and the platelet-to-lymphocyte ratio’s prediction on effectiveness. World J Gastrointest Oncol. 2022;14(4):920–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang K, Han L, Wu S, Qu X, Li Q, Zhao C, et al. Real-world outcomes of regorafenib combined with immune checkpoint inhibitors in patients with advanced or metastatic microsatellite stable colorectal cancer: a multicenter study. Cancer Immunol Immunother. 2022;71(6):1443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fakih M, Raghav KPS, Chang DZ, Larson T, Cohn AL, Huyck TK, et al. Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: a single-arm, open-label, multicentre phase 2 study. EClinicalMedicine. 2023;58:101917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uhlik M, Pointing D, Iyer S, Ausec L, Štajdohar M, Cvitkovič R, et al. Xerna™ TME panel is a machine learning-based transcriptomic biomarker designed to predict therapeutic response in multiple cancers. Front Oncol. 2023;13:1158345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fu S, Corr BR, Culm-Merdek K, Mockbee C, Youssoufian H, Stagg R, et al. Phase ib study of navicixizumab plus paclitaxel in patients with platinum-resistant ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2022;40(23):2568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iyer S, Ausec L, Pointing D, Zganec M, Cvitkovic R, Stajdohar M, et al. Abstract 1232: Xerna TME Panel: a pan-cancer RNA-based investigational assay designed to predict patient responses to angiogenic and immune targeted therapies. Cancer Res. 2022;82(12 Suppl):1232–2. [Google Scholar]
- 19. Uhlik M, Fong C. Predicting patient response to immune-targeted therapies via interrogation of the tumor microenvironment. Immuno Oncol Insights. 2022;3(9):513–25. [Google Scholar]
- 20. Grothey A, Blay JY, Pavlakis N, Yoshino T, Bruix J. Evolving role of regorafenib for the treatment of advanced cancers. Cancer Treat Rev. 2020;86:101993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. Further inquiries can be directed to the corresponding author.