Abstract
Introduction
The inflammatory burden index (IBI) serves as a prognostic marker for several cancers. Here, we evaluated the predictive value of preoperative IBI associated with the surgical and oncological outcomes of patients with esophageal cancer (EC).
Methods
The IBI was formulated as C-reactive protein × neutrophil/lymphocyte. We retrospectively analyzed preoperative IBI of 147 EC patients receiving esophagectomy between 2008 and 2018. Cox proportional hazards models and multivariable logistic regression were employed to identify independent risk factors of surgical site infection and prognosis.
Results
Increased preoperative IBI significantly correlated with higher tumor stage. Patients with high IBI experienced shorter overall survival (p = 0.0002) and disease-free survival (p = 0.002) compared with those with low IBI. In the adjusted Cox proportional hazards regression models, increased IBI served as an independent prognostic factor for overall survival (hazard ratio, 3.56; 95% confidence interval, 1.79–7.34; p = 0.0003) and disease-free survival (hazard ratio, 3.03; 95% confidence interval, 1.60–5.92; p = 0.007). Multivariable analysis identified preoperative high IBI which served as an independent risk factor for overall surgical site infection (odds ratio, 2.53; 95% confidence interval, 1.00–6.38; p = 0.049).
Conclusion
Preoperative IBI may serve as a useful predictor of prognosis and surgical site infection of patients with EC after esophagectomy.
Keywords: Inflammatory burden index, Esophageal cancer, Prognosis, Surgical site infection
Introduction
Esophageal cancer (EC) is the seventh most frequently diagnosed cancer and the sixth leading cause of cancer death worldwide. In 2020, 604,100 new cases, with an estimated 544,100 deaths, were reported [1, 2]. Unfortunately, survival of patients undergoing potentially curative resection remains poor despite advances in clinical oncology [3]. Surgical site infection (SSI), which is defined as postoperative infection, is categorized into organ-space SSI, and superficial and deep incisional SSI. SSI represents the most common postoperative complication. In the gastrointestinal surgery field in Japan, the incidence of SSI associated with esophageal surgery is highest at 19% [4]. Therefore, the identification of a biomarker associated with long- and short-term outcomes may contribute to better patient stratification to achieve improved responses to treatment response [5, 6]. Such clinical markers for patients with EC, which are more effective and easier to use, will likely help optimize clinical decision-making.
Evidence indicates that tumor-promoting inflammation represents a hallmark of cancer [7, 8]. There is growing evidence that cancer-related inflammation is deeply involved in tumor progression [9, 10]. Furthermore, a large body of evidence also suggests that biomarkers of preoperative systemic inflammation, which comprise components present in the peripheral blood circulation, are independently associated with the prognosis of patients with EC [11–13]. For example, in 2022, Xie and colleagues [14] reported a new index called the inflammatory burden index (IBI) for malignancies, which comprises neutrophil and lymphocyte counts combined with C-reactive protein (CRP) levels. Neutrophils and lymphocytes represent the most abundant immune cell types present in peripheral blood [15]. CRP levels, a typical biomarker used to characterize systemic inflammation, indicate active inflammation [16]. The analysis of Xie et al. [14] of a large cohort comprising multiple solid tumor types revealed that the IBI achieved higher accuracy in predicting patients’ survival compared with other systemic inflammatory biomarkers. Furthermore, the IBI serves as a prognostic indicator of cancer types, including locally advanced gastric cancer, hepatocellular carcinoma, and non-small cell lung cancer [17–19]. Here, we evaluated the clinical significance of the preoperative IBI in patients with EC who undergo surgical resection to clarify the predictive value of this index for prognosis and postoperative SSI in patients with EC.
Methods
Patients and Methods
The present observational study enrolled consecutive 157 patients who underwent surgery for primary EC at Mie University Hospital between 2008 and 2018. The diagnoses of all patients were confirmed by postoperative pathology. Ten patients were excluded because of incomplete clinical data. We retrospectively evaluated clinical information, pathological findings, and prognosis obtained from hospital records. Fifty-three patients (36.1%) received neoadjuvant therapy (NAT) (weekly chemotherapy for two cycles or three cycles combined with radiotherapy [30 Gy delivered in 15 fractions]). SSI is defined as an infection occurring within 30 days after surgery in the region of the body affected by that surgery that includes superficial incisional, deep incisional, and organ/space.
Follow-up visits were performed every 3–4 months for at least 1 year using our standard protocol. Data collected from inpatient and outpatient records included age, sex, preoperative treatment, preoperative blood test data (CRP levels, neutrophil and lymphocyte counts), postoperative tumor-specific data (histology, T stage, lymphatic vessel invasion, venous invasion, lymph node metastasis, and distant metastasis). The TNM staging was based on the postoperative pathological examination. Survival data included overall survival (OS) and disease-free survival (DFS). OS was measured from the date the patient underwent surgery until the date of death resulting from any cause or the last known follow-up for patients that were still alive. DFS analysis was measured from the date the patient underwent curative surgery to the date of disease recurrence, death from any cause (i.e., noncancer deaths were not censored), or until the last contact with the patient. In subgroup analysis based on albumin (ALB), ALB was categorized as having a low preoperative ALB (<4.0 g/dL) versus having a normal preoperative ALB (≥4.0 g/dL). This study complied with the Declaration of Helsinki and was approved by the Clinical Research Ethics Review Committee of Mie University Hospital (H2023-146).
Laboratory Measurements
Patients’ blood samples were obtained within 1 day before the resection of the primary tumor. The IBI was calculated as follows: CRP (mg/dL) × neutrophil count (number/µL)/lymphocyte count (number/µL).
Statistical Methods
The Shapiro-Wilk test showed non-normal distribution of IBI levels, and therefore, the results are expressed as the median (interquartile range). The significance of differences between groups was calculated using the Mann-Whitney U test. Comparisons between ordered groups were tested using the Jonckheere-Terpstra test for trends. For time-to-event analyses, survival estimates were calculated using the Kaplan-Meier method, and groups were compared using the log-rank test. Receiver operating characteristic curves with Youden’s index were generated to determine the optimum cutoff values for analyzing prognosis.
OS was measured from the date when the patient underwent surgery until the date of death from any cause or the last known follow-up for living patients. DFS was measured from the date when the patient underwent curative surgery to the date of disease recurrence, death from any cause, or until the last contact with the patient. Cox proportional hazards models were used to identify independent risk factors for recurrence and survival. Variables with p < 0.05 in univariate analysis were selected for inclusion in the Cox proportional hazards regression model. Binary logistic regression was used to identify independent risk factors. Logistic regression analysis included variables with p < 0.2 in univariate analysis. All p values are 2-sided, and p < 0.05 indicates statistical significance. All calculations were performed using JMP software version 11 (SAS Institute Inc., Cary, NC, USA) and MedCalc Statistical Software version 15.2.2 (MedCalc Software Bvba, Ostend, Belgium).
Results
Relationship between IBI and Clinicopathological Characteristics of Patients with EC
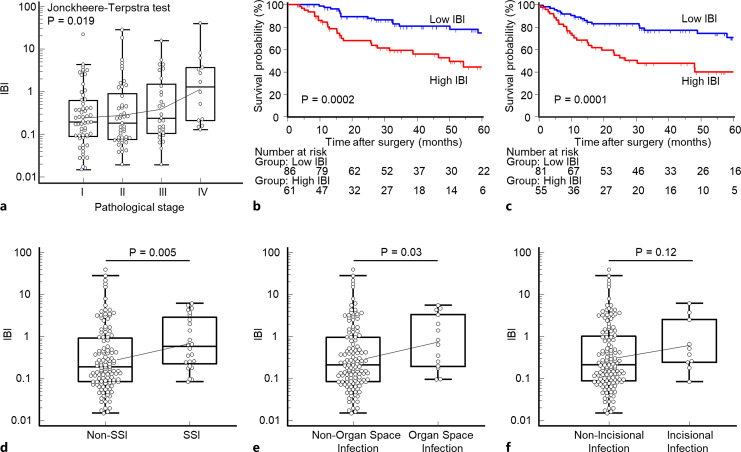
The patient population comprised 124 males (84.4%) and 23 females (15.6%), median age 69 years, range 35–90. We evaluated the correlation between clinicopathological factors and the preoperative IBI of these patients. Table 1 shows the relationship between patients’ preoperative IBI and clinicopathological characteristics. An increased level of preoperative IBI significantly correlated with advanced T stage (p = 0.048) and lymphatic vessel invasion (p = 0.02). There was a trend for an elevated IBI in patients with lymph node metastasis (p = 0.08), which was not statistically significant. In patients receiving NAT, the IBI was significantly higher compared with patients not administered NAT (p = 0.01). Moreover, a significant increasing trend was observed in the preoperative IBI along with tumor-stage progression (p = 0.02) (shown in Fig. 1a).
Table 1.
Association between clinicopathological variables and preoperative IBI in EC patients
| Variable | N = 147 | Preoperative IBI, | p value |
|---|---|---|---|
| median (IQR) | |||
| Gender | |||
| Male | 124 | 0.24 (0.09–1.28) | 0.34 |
| Female | 23 | 0.21 (0.08–0.58) | |
| Age | |||
| <69a years | 73 | 0.17 (0.08–0.51) | 0.02* |
| ≥69 years | 74 | 0.29 (0.10–2.47) | |
| NAT | |||
| Yes | 53 | 0.44 (0.13–3.23) | 0.01* |
| No | 94 | 0.18 (0.08–0.55) | |
| Histological type | |||
| Squamous | 132 | 0.24 (0.09–1.34) | 0.61 |
| Other | 15 | 0.17 (0.10–0.58) | |
| Differentiation | |||
| Poor | 25 | 0.26 (0.09–1.71) | 0.71 |
| Other | 122 | 0.23 (0.09–1.13) | |
| Pathological T category | |||
| pT0/T1/2 | 101 | 0.19 (0.08–0.74) | 0.05* |
| pT3/4 | 46 | 0.37 (0.13–2.33) | |
| Venous invasion | |||
| Present | 50 | 0.32 (0.10–2.63) | 0.12 |
| Absent | 97 | 0.20 (0.09–0.83) | |
| Lymphatic vessel invasion | |||
| Present | 65 | 0.29 (0.11–1.90) | 0.02* |
| Absent | 82 | 0.18 (0.08–0.50) | |
| Lymph node metastasis | |||
| Present | 72 | 0.24 (0.11–1.76) | 0.08 |
| Absent | 75 | 0.20 (0.08–0.62) | |
| Distant metastasis | |||
| Present | 9 | 0.23 (0.15–7.14) | 0.15 |
| Absent | 138 | 0.24 (0.09–1.00) | |
IBI, inflammatory burden index; IQR, interquartile range.
*p < 0.05.
aThe median age at surgery is 69 years in this cohort.
Fig. 1.
Association analysis of the preoperative IBI and tumor stage, prognosis, or postoperative SSI. a Increased preoperative IBI significantly correlated with advanced pathological tumor stage (p = 0.02, Jonckheere-Terpstra test). b, c Prognostic significance of the preoperative IBI for OS and DFS. The high preoperative IBI group associated with significantly poorer prognosis for OS and DFS compared with the low IBI group (OS, p = 0.0002, log-rank test; DFS, p = 0.002, log-rank test). d, e The preoperative IBI was significantly higher in patients with overall SSI (p = 0.005) or organ-space SSI (p = 0.03) compared with those without SSI. f There was no significant difference between patients with or without incisional infection (p = 0.12). The mean ± SEM was computed after log transformation and plotted after back transformation. SSI, surgical site infection; IBI, inflammatory burden index.
A Low IBI Serves as a Negative Predictive Factor for Long-Term Outcomes of OS and DFS of Patients with EC
To investigate the prognostic value between the preoperative IBI and oncological outcomes, we conducted Kaplan-Meier survival analysis according to preoperative IBI status. The optimal cutoff value of the IBI was 0.325 according to the receiver operating characteristic by maximizing the Youden’s index for survival. Patients with a high IBI experienced significantly shorter survival compared with the low IBI group as functions of OS (log-rank test, p = 0.0002) (shown in Fig. 1b) and DFS (log-rank test, p = 0.0001) (shown in Fig. 1c). Multivariate Cox regression analysis revealed that in addition to venous invasion and lymphatic vessel invasion, the preoperative IBI served as an independent prognostic factor for OS (hazard ratio = 3.56, 95% confidence interval [CI]: 1.79–7.34, p = 0.0003) (Table 2). In addition, high IBI status was also one of the independent prognostic factors for poor DFS in multivariate analysis (hazard ratio = 3.03, 95% CI: 1.60–5.92, p = 0.007) (Table 3). We performed a subgroup analysis for OS and DFS based on pathological stage. The subgroup survival analysis of OS indicated a significant difference (p < 0.05) or tendency (p < 0.1) between high IBI patients and low IBI patients among stage III and stage IV patients. Meanwhile, patients with high IBI had worse outcomes than those with low IBI at all pathological stages in subgroup survival analysis of DFS (shown in online suppl. Fig. 1; for all online suppl. material, see https://doi.org/10.1159/000535727). We further conducted other subgroup analyses by the patients’ preoperative factors to identify a population with a significantly modified risk. The results indicated that the prognosis of patients with high IBI was significantly worse than patients with low IBI in EC patients with normal ALB levels only (≥4.0 g/dL) (shown in online suppl. Fig. 2).
Table 2.
Multivariate analysis for predictors of OS
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Gender (male) | 0.99 | 0.48–2.30 | 0.97 | |||
| Age (≧69 years)a | 1.25 | 0.68–2.33 | 0.47 | |||
| NAT (yes) | 2.53 | 1.37–4.71 | 0.003* | 1.20 | 0.60–2.44 | 0.61 |
| Histological type (SCC) | 2.33 | 0.71–14.3 | 0.18 | |||
| Differentiation (poor) | 1.61 | 0.75–3.15 | 0.21 | |||
| Pathological T category (pT3/4) | 2.94 | 1.59–5.43 | <0.001* | 1.11 | 0.53–2.26 | 0.77 |
| Venous invasion (present) | 4.56 | 2.45–8.67 | <0.001* | 3.15 | 1.55–6.57 | 0.001* |
| Lymphatic vessel invasion (present) | 7.05 | 3.48–15.8 | <0.001* | 5.10 | 2.19–13.0 | <0.001* |
| Lymph node metastasis (present) | 5.17 | 1.73–12.6 | 0.006* | 1.20 | 0.49–3.16 | 0.69 |
| Distant metastasis | 9.64 | 3.18–24.1 | <0.001* | 3.13 | 0.91–9.50 | 0.07 |
| Preoperative IBI (≥0.325)b | 3.05 | 1.65–5.82 | <0.001* | 3.56 | 1.79–7.34 | 0.0003 |
IBI, inflammatory burden index; SCC, squamous cell carcinoma; HR, hazard ratio; CI, confidence interval; EC, esophageal cancer; ROC, receiver operating characteristic.
*p < 0.05.
aThe median age at surgery is 69 years in EC patients.
bCutoff threshold of IBI is determined by ROC analysis with Youden’s index for OS in EC patients.
Table 3.
Multivariate analysis for predictors of DFS
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Gender (male) | 1.39 | 0.63–3.66 | 0.44 | |||
| Age (≧69 years)a | 1.43 | 0.80–2.62 | 0.23 | |||
| NAT (yes) | 1.99 | 1.09–3.59 | 0.03* | 1.06 | 0.54–2.06 | 0.85 |
| Histological type (SCC) | 1.81 | 0.66–7.46 | 0.28 | |||
| Differentiation (poor) | 0.94 | 0.38–1.98 | 0.88 | |||
| Pathological T category (pT3/4) | 2.37 | 1.28–4.28 | 0.007* | 1.26 | 0.65–2.38 | 0.48 |
| Venous invasion (present) | 3.65 | 2.02–6.65 | <0.001* | 2.49 | 1.32–4.79 | 0.005* |
| Lymphatic vessel invasion (present) | 3.98 | 2.17–7.64 | <0.001* | 2.51 | 1.25–5.23 | 0.009* |
| Lymph node metastasis (present) | 4.41 | 2.33–8.93 | <0.001* | 1.86 | 0.84–4.36 | 0.13 |
| Preoperative IBI (≥0.325)b | 3.08 | 1.70–5.77 | 0.0002* | 3.03 | 1.60–5.92 | 0.007* |
HR, hazard ratio.
*p < 0.05.
aThe median age at surgery is 69 years in EC patients. bCutoff threshold of IBI is determined by ROC analysis with Youden’s index for OS in EC patients.
The Preoperative IBI Serves as an Independent Predictive Factor for SSI
We found that the preoperative IBI of patients with EC with overall SSI was significantly higher than that of patients without SSI (p = 0.005) (shown in Fig. 1d). Moreover, the preoperative IBI was significantly increased in patients with EC with organ-space SSI compared with those without (p = 0.03) (shown in Fig. 1e). There was no significant difference in the preoperative IBI between patients with EC with or without incision infection (p = 0.12) (shown in Fig. 1f). Multivariate logistic analysis revealed that a high preoperative IBI was the only independent predictive factor for perioperative SSI in patients with EC (OR = 2.53, 95% CI: 1.00–6.38, p = 0.049) (Table 4).
Table 4.
Multivariate analysis for predictors of SSI
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Gender (male) | 0.91 | 0.28–2.97 | 0.88 | |||
| Age (≧69 years)a | 1.20 | 0.50–2.89 | 0.68 | |||
| Oncological factors | ||||||
| NAT (yes) | 0.75 | 0.31–1.84 | 0.53 | |||
| Histological type (SCC) | 0.34 | 0.04–2.70 | 0.31 | |||
| Differentiation (poor) | 1.82 | 0.64–5.19 | 0.26 | |||
| Pathological T category (T3/T4) | 0.89 | 0.34–2.31 | 0.81 | |||
| Venous invasion (present) | 0.60 | 0.22–1.61 | 0.31 | |||
| Lymphatic vessel invasion (present) | 1.97 | 0.81–4.80 | 0.13 | 1.66 | 0.66–4.13 | 0.28 |
| Lymph node metastasis (present) | 0.78 | 0.32–1.88 | 0.58 | |||
| Distant metastasis (present) | 2.15 | 0.39–11.8 | 0.38 | |||
| Preoperative blood test | ||||||
| Preoperative IBI (≥0.325)b | 2.79 | 1.13–6.89 | 0.03* | 2.53 | 1.00–6.38 | 0.049* |
| Surgical factors | ||||||
| Operation time (>median) | 1.87 | 0.76–4.59 | 0.17 | 1.76 | 0.70–4.44 | 0.23 |
| Blood loss (>median) | 1.81 | 0.74–4.44 | 0.20 | 1.44 | 0.57–3.67 | 0.44 |
SSI, surgical site infection; IBI, inflammatory burden index; SCC, squamous cell carcinoma; EC, esophageal cancer; HR, hazard ratio; CI, confidence interval; ROC, receiver operating characteristic.
*p < 0.05.
aThe median age at surgery is 69 years in EC patients.
bCutoff threshold of IBI is determined by ROC analysis with Youden’s index for OS in EC patients.
Discussion
Here, we investigated the significance of the preoperative IBI as a marker of oncological and surgical outcomes of patients with EC. Our results show that the preoperative IBI significantly correlated with advanced tumor stage and SSI. Moreover, preoperative high IBI status served as an independent risk factor for survival and postoperative SSI. We also determined a suitable cutoff value of the IBI for predicting survival and SSI, which will make this index easier to use in clinical practice.
Cancer-associated inflammation caused by host-tumor interactions is closely associated with the development of cancer [20, 21]. Increasing evidence supports the conclusion that the systemic inflammatory response and nutritional status influence the prognosis of patients with cancer [22, 23]. CRP levels and the neutrophil count are the most representative inflammation markers and are therefore widely used to evaluate systemic immune responses in clinical practice. Furthermore, preoperative serum CRP levels predict survival prognosis in patients who undergo curative resection for EC [24].
The lymphocyte count serves as an indicator of nutritional status, which is included in established nutritional assessment and screening tools [25–27], and reflects the immune response. Evidence shows that the lymphocyte count in peripheral blood positively correlates with the level of CD8+ tumor-infiltrating lymphocytes in EC [28]. Furthermore, well-known indices such as the prognostic nutritional index and the Glasgow prognostic score incorporate lymphocyte counts or CRP levels, which contribute prognostic value for EC [29, 30]. The neutrophil-to-lymphocyte ratio, based on neutrophil and lymphocyte counts, was proposed as a prognostic indicator of the systemic inflammatory response in various cancers, including EC [11, 31, 32]. Our present study indicates that a high IBI is related to clinicopathological features and poorer prognosis of EC, which is consistent with the evidence cited above. In the further subgroup analyses by the pathological stage, patients with high IBI had worse outcomes than those with low IBI at late stages. This result may be because surgical removal of the tumor was highly effective in treating early-stage tumors. Meanwhile, we observed that patients with high IBI were more likely to experience tumor relapse at all pathological stages. This suggests that preoperative IBI has broad applicability for clinical decision-making. Other research indicates an interaction between the inflammatory response and malnutrition. The systemic inflammatory response may cause malnutrition via accelerating protein catabolism and muscle wasting [33]. In contrast, malnutrition which is closely associated with immune suppression may lead to immune function deficiency and stimulation of the inflammatory response [34]. In our present study, subgroup analyses by the preoperative ALB showed that preoperative IBI had better prognostic value in EC patients with normal ALB levels only. Although ALB is the most commonly used index of nutritional status, it is difficult to assess nutritional status by a single indicator. Moreover, the normal nutritional status on screening with some nutritional assessment scales does not mean that the patients do not need nutritional intervention [35]. The result reminded the complex relationship between nutrition, inflammation, and cancer. Considering these conditions together, the IBI may reflect the combined effects of inflammation and malnutrition and may therefore serve as a more reliable prognostic marker of long-term oncological outcomes than the neutrophil-to-lymphocyte ratio or serum CRP levels alone. Therefore, the IBI may help identify patients at high risk of EC who may require more proactive preoperative nutritional support and postoperative treatment.
In the present study, univariate analysis showed that patients receiving NAT were characterized by worse prognosis, likely because NAT is closely related to tumor stage, and patients with a higher pathological stage were administered a higher proportion of NAT (data not shown). Multivariate analysis adjusting for TNM staging and other clinicopathological characteristics did not indicate a significant association of prognosis with preoperative NAT.
Here, we found that the preoperative IBI is significantly associated with postoperative SSI, particularly with organ-space infection. Preoperative biomarkers that predict SSI after esophagectomy are limited. SSI is one of the most serious adverse effects occurring around the perioperative period. For example, SSI after esophagectomy significantly increases healthcare costs and affects morbidity [5, 36]. Therefore, the search for preoperative risk factors associated with SSI is worthwhile. While identifying such preoperative factors that predict postoperative SSI is difficult, certain preoperative factors are consistently associated with SSI, such as nutritional status [37, 38]. For example, our previous studies demonstrate that potential peripheral blood-based biomarkers predict perioperative SSI in patients with gastrointestinal tumors [39–42]. Furthermore, a recent multicenter cohort study found that postoperative SSI serves as an independent predictive factor for poor prognosis of patients with EC [6].
Although some studies show that the postoperative [43, 44] rather than the preoperative CRP predicts postoperative anastomotic leakage (AL) after esophagectomy, there is also a study showing preoperative CRP combined with a nutritional indicator predicts AL in patients with EC: Sugimoto et al. reported that the preoperative CRP-to-ALB ratio serves as an independent predictive factor for AL. Neither preoperative CRP nor ALB alone may predict AL [45]. In our present study, multivariable analysis revealed that a high preoperative IBI was the only risk factor that remained significant and significantly associated with overall SSI. An increased IBI reflects an enhanced systemic inflammatory response and poor nutritional status in patients with EC. The above evidence indicates that the preoperative IBI may provide a useful predictor of SSI in patients with EC after esophagectomy.
Here, we determined a suitable cutoff value of the IBI for predicting survival and SSI in EC. Xie et al. [14] proved the prognostic value of IBI by survival analysis in a prospective cohort, which had 6,359 patients with 379 EC. The prognostic ability of the IBI was also validated through randomized internal validation. However, a uniform cutoff value (value = 16) was used to analyze more than 10 tumor types. We think that research results are likely to vary due to different cutoff values, and the cutoff values also vary in distinct cancer types. An inappropriate cutoff value may generate unreproducible or conflicting results. Previous study revealed that lung cancer exhibits a high inflammatory burden, while those of EC and hepatobiliary tumors are moderate [14]. This may explain that the cutoff value (value = 0.325) applied in our present study is close to that employed to analyze hepatocellular carcinoma (cutoff value = 0.72) [17], but very different from the cutoff value (value = 1.7) applied to a study of lung cancer [19]. Therefore, our EC cohort may serve the role of an independent external validation cohort.
The present study has several limitations beyond the common limitations of a retrospective study performed at a single institution in Asia. First, although we established a suitable IBI cutoff value to predict the prognosis and SSI of patients with EC, the reliability of this cutoff value requires validation when applied to an independent cohort. Especially when considering a large difference (i.e., risk factors, histology type, and prognosis) between Western countries and Asia, external validation should be conducted when its usage is extended to a more general population. Second, certain well-recognized risk factors for SSI were not recorded, such as perioperative hyperglycemia and intraoperative blood transfusion. Third, the systemic inflammatory response and nutritional status are subject to change before and after treatment. In our present study, we found that patients administered NAT had an elevated IBI. However, we were limited by existing data and therefore did not compare the change in the IBI of patients administered preoperative NAT, which may serve as an interesting direction in our future research.
In conclusion, our study highlights the clinical significance of the preoperative IBI for prognosis and SSI in patients with EC undergoing an esophagectomy. The IBI may therefore help surgeons and oncologists determine more effective perioperative strategies for treating patients with EC.
Acknowledgment
We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Statement of Ethics
This study protocol was reviewed and approved by the Institutional Review Board of Mie University Hospital, approval number [H2023-146]. Opt-out informed consent protocol was used for use of participant data for research purposes. This consent procedure was reviewed and approved by Institutional Review Boards of our institution (Mie University Hospital), approval number [H2023-146], date of decision [31 July 2023].
Conflict of Interest Statement
None of the authors have conflicts of interest to disclose for this study.
Funding Sources
This study was not supported by any sponsor or funder.
Author Contributions
C.Y., Y.T., and YOku conceptualized and designed the study, performed statistical analysis, and drafted the manuscript. T.K., T.S., M.K., S.Y., YOki, and M.O. provided the samples and clinical information. C.Y., Y.T., YOki, YOku, T.K., T.S., R.M., M.K., S.Y., and M.O. analyzed and interpreted the data. All authors revised the manuscript for important intellectual content. C.Y. and Y.T. confirmed the authenticity of all the raw data. All authors read and approved the final manuscript.
Funding Statement
This study was not supported by any sponsor or funder.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary Material
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2. Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163(3):649–58.e2. [DOI] [PubMed] [Google Scholar]
- 3. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ministry of Health, Labour and Welfare . Japan nosocomial infections surveillance; 2016. https://janismhlwgojp/english/indexasp [accessed 24 August 2023]. [Google Scholar]
- 5. Lerut T, Moons J, Coosemans W, Van Raemdonck D, De Leyn P, Decaluwé H, et al. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg. 2009;250(5):798–807. [DOI] [PubMed] [Google Scholar]
- 6. Matsuda A, Maruyama H, Akagi S, Inoue T, Uemura K, Kobayashi M, et al. Survival impact of surgical site infection in esophageal cancer surgery: a multicenter retrospective cohort study. Ann Gastroenterol Surg. 2023;7(4):603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309–24. [DOI] [PubMed] [Google Scholar]
- 8. Khandia R, Munjal A. Interplay between inflammation and cancer. Adv Protein Chem Struct Biol. 2020;119:199–245. [DOI] [PubMed] [Google Scholar]
- 9. Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371(26):2499–509. [DOI] [PubMed] [Google Scholar]
- 10. Munn LL. Cancer and inflammation. WIREs Mech Dis. 2017;9(2). [Google Scholar]
- 11. Pirozzolo G, Gisbertz SS, Castoro C, van Berge Henegouwen MI, Scarpa M. Neutrophil-to-lymphocyte ratio as prognostic marker in esophageal cancer: a systematic review and meta-analysis. J Thorac Dis. 2019;11(7):3136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishibashi Y, Tsujimoto H, Yaguchi Y, Kishi Y, Ueno H. Prognostic significance of systemic inflammatory markers in esophageal cancer: systematic review and meta-analysis. Ann Gastroenterol Surg. 2020;4(1):56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamamoto A, Toiyama Y, Okugawa Y, Ichikawa T, Imaoka H, Yasuda H, et al. Clinical implications of the preoperative lymphocyte C-reactive protein ratio in esophageal cancer patients. Surg Today. 2021;51(5):745–55. [DOI] [PubMed] [Google Scholar]
- 14. Xie H, Ruan G, Ge Y, Zhang Q, Zhang H, Lin S, et al. Inflammatory burden as a prognostic biomarker for cancer. Clin Nutr. 2022;41(6):1236–43. [DOI] [PubMed] [Google Scholar]
- 15. Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–89. [DOI] [PubMed] [Google Scholar]
- 16. Black S, Kushner I, Samols D. C-Reactive protein. J Biol Chem. 2004;279(47):48487–90. [DOI] [PubMed] [Google Scholar]
- 17. Song R, Ni H, Huang J, Yang C, Qin S, Wei H, et al. Prognostic value of inflammation-immunity-nutrition score and inflammatory burden index for hepatocellular carcinoma patients after hepatectomy. J Inflamm Res. 2022;15:6463–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ding P, Wu H, Liu P, Sun C, Yang P, Tian Y, et al. The inflammatory burden index: a promising prognostic predictor in patients with locally advanced gastric cancer. Clin Nutr. 2023;42(2):247–8. [DOI] [PubMed] [Google Scholar]
- 19. Xie H, Ruan G, Wei L, Deng L, Zhang Q, Ge Y, et al. The inflammatory burden index is a superior systemic inflammation biomarker for the prognosis of non-small cell lung cancer. J Cachexia Sarcopenia Muscle. 2023;14(2):869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. [DOI] [PubMed] [Google Scholar]
- 21. Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12(10):584–96. [DOI] [PubMed] [Google Scholar]
- 22. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. [DOI] [PubMed] [Google Scholar]
- 23. Anandavadivelan P, Lagergren P. Cachexia in patients with oesophageal cancer. Nat Rev Clin Oncol. 2016;13(3):185–98. [DOI] [PubMed] [Google Scholar]
- 24. Huang W, Wu L, Liu X, Long H, Rong T, Ma G. Preoperative serum C-reactive protein levels and postoperative survival in patients with esophageal squamous cell carcinoma: a propensity score matching analysis. J Cardiothorac Surg. 2019;14(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seltzer MH, Bastidas JA, Cooper DM, Engler P, Slocum B, Fletcher HS. Instant nutritional assessment. JPEN J Parenter Enteral Nutr. 1979;3(3):157–9. [DOI] [PubMed] [Google Scholar]
- 26. Elmore MF, Wagner DR, Knoll DM, Eizember L, Oswalt MA, Glowinski EA, et al. Developing an effective adult nutrition screening tool for a community hospital. J Am Diet Assoc. 1994;94(10):1113–8, 1121; quiz 1119-20. [DOI] [PubMed] [Google Scholar]
- 27. González Madroño A, Mancha A, Rodríguez FJ, de Ulibarri JI, Culebras J. The use of biochemical and immunological parameters in nutritional screening and assessment. Nutr Hosp. 2011;26(3):594–601. [DOI] [PubMed] [Google Scholar]
- 28. Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. 2020;271(4):693–700. [DOI] [PubMed] [Google Scholar]
- 29. Vashist YK, Loos J, Dedow J, Tachezy M, Uzunoglu G, Kutup A, et al. Glasgow Prognostic Score is a predictor of perioperative and long-term outcome in patients with only surgically treated esophageal cancer. Ann Surg Oncol. 2011;18(4):1130–8. [DOI] [PubMed] [Google Scholar]
- 30. Qi Q, Song Q, Cheng Y, Wang N. Prognostic significance of preoperative prognostic nutritional index for overall survival and postoperative complications in esophageal cancer patients. Cancer Manag Res. 2021;13:8585–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–30. [DOI] [PubMed] [Google Scholar]
- 32. Yodying H, Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Yamada M, et al. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2016;23(2):646–54. [DOI] [PubMed] [Google Scholar]
- 33. Stumpf F, Keller B, Gressies C, Schuetz P. Inflammation and nutrition: friend or foe? Nutrients. 2023;15(5):1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ibrahim MK, Zambruni M, Melby CL, Melby PC. Impact of childhood malnutrition on host defense and infection. Clin Microbiol Rev. 2017;30(4):919–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kurban M, Zeng N, Wang M, Liu H, Wu JR, Feng G, et al. Role of human body composition analysis and malnutrition risk questionnaire in the assessment of nutritional status of patients with initially diagnosed crohn’s disease. Front Med. 2020;7:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caccialanza R, Pedrazzoli P, Cereda E, Gavazzi C, Pinto C, Paccagnella A, et al. Nutritional support in cancer patients: a position paper from the Italian society of medical oncology (AIOM) and the Italian society of artificial nutrition and metabolism (SINPE). J Cancer. 2016;7(2):131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yin LX, Chen BM, Zhao GF, Yuan QF, Xue Q, Xu K. Scoring system to predict the risk of surgical site infection in patients with esophageal cancer after esophagectomy with cervical anastomosis. Surg Infect. 2018;19(7):696–703. [DOI] [PubMed] [Google Scholar]
- 39. Toiyama Y, Shimura T, Yasuda H, Fujikawa H, Okita Y, Kobayashi M, et al. Clinical burden of C-reactive protein/albumin ratio before curative surgery for patients with gastric cancer. Anticancer Res. 2016;36(12):6491–8. [DOI] [PubMed] [Google Scholar]
- 40. Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Ichikawa T, Yin C, et al. Lymphocyte-to-C-reactive protein ratio and score are clinically feasible nutrition-inflammation markers of outcome in patients with gastric cancer. Clin Nutr. 2020;39(4):1209–17. [DOI] [PubMed] [Google Scholar]
- 41. Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Ide S, Kitajima T, et al. Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann Surg. 2020;272(2):342–51. [DOI] [PubMed] [Google Scholar]
- 42. Yin C, Toiyama Y, Okugawa Y, Omura Y, Kusunoki Y, Kusunoki K, et al. Clinical significance of advanced lung cancer inflammation index, a nutritional and inflammation index, in gastric cancer patients after surgical resection: a propensity score matching analysis. Clin Nutr. 2021;40(3):1130–6. [DOI] [PubMed] [Google Scholar]
- 43. Barbaro A, Eldredge TA, Shenfine J. Diagnosing anastomotic leak post-esophagectomy: a systematic review. Dis Esophagus. 2021;34(2):doaa076. [DOI] [PubMed] [Google Scholar]
- 44. Hagens ERC, Feenstra ML, Lam WC, Eshuis WJ, Lameris W, van Berge Henegouwen MI, et al. C-reactive protein as a negative predictive marker for anastomotic leakage after minimally invasive esophageal surgery. World J Surg. 2023;47(8):1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sugimoto A, Toyokawa T, Miki Y, Yoshii M, Tamura T, Sakurai K, et al. Preoperative C-reactive protein to albumin ratio predicts anastomotic leakage after esophagectomy for thoracic esophageal cancer: a single-center retrospective cohort study. BMC Surg. 2021;21(1):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.