Abstract
OBJECTIVES:
To derive systematic-review informed, modified Delphi consensus regarding prophylactic transfusions in neonates and children supported with extracorporeal membrane oxygenation (ECMO) from the Pediatric ECMO Anticoagulation CollaborativE.
DATA SOURCES:
A structured literature search was performed using PubMed, EMBASE, and Cochrane Library (CENTRAL) databases from January 1988 to May 2020, with an update in May 2021.
STUDY SELECTION:
Included studies assessed use of prophylactic blood product transfusion in pediatric ECMO.
DATA EXTRACTION:
Two authors reviewed all citations independently, with a third independent reviewer resolving conflicts. Thirty-three references were used for data extraction and informed recommendations. Evidence tables were constructed using a standardized data extraction form.
MEASUREMENTS AND MAIN RESULTS:
The evidence was evaluated using the Grading of Recommendations Assessment, Development and Evaluation system. Forty-eight experts met over 2 years to develop evidence-informed recommendations and, when evidence was lacking, expert-based consensus statements or good practice statements for prophylactic transfusion strategies for children supported with ECMO. A web-based modified Delphi process was used to build consensus via the Research And Development/University of California Appropriateness Method. Consensus was based on a modified Delphi process with agreement defined as greater than 80%. We developed two good practice statements, 4 weak recommendations, and three expert consensus statements.
CONCLUSIONS:
Despite the frequency with which pediatric ECMO patients are transfused, there is insufficient evidence to formulate evidence-based prophylactic transfusion strategies.
Keywords: blood transfusion, extracorporeal membrane oxygenation, pediatrics, plasma, platelet transfusion
Children supported by extracorporeal membrane oxygenation (ECMO) are at significant risk of bleeding because of numerous factors including hemodilution, platelet dysfunction, and need for anticoagulation (1–5). Bleeding in these children is independently associated with mortality (3, 5), and therefore clinicians prescribe blood components to either prevent bleeding or to treat blood loss. Children supported by ECMO are exposed to large quantities of blood products (6–8); in one cohort of 514 children, 80% of subjects received greater than 40 mL/kg blood products on at least one study day (4).
Despite potential therapeutic benefit, blood product transfusions in pediatric ECMO patients have been independently associated with mortality, bleeding, and thrombosis in observational studies (3, 9, 10). Clinicians do not have evidence-based guidance to direct transfusion of blood components in this vulnerable patient population. Although approximately 80% of recently surveyed pediatric ECMO centers have transfusion protocols (11), protocols vary widely and are often based on expert opinion alone. Given significant morbidities and mortality associated with ECMO and blood product transfusion in both this population and other critically ill children (12–14), the objective of this subgroup of the Pediatric ECMO Anticoagulation Collaborative (PEACE) group was to derive systematic-review informed, modified Delphi consensus for prophylactic transfusion strategies.
MATERIALS AND METHODS
Detailed methods and definitions of clinically relevant bleeding are described in the PEACE executive summary (15). Briefly, a structured literature search was performed using PubMed, EMBASE, and Cochrane Library (CENTRAL) databases from January 1988 to May 2020, with an update in May 2021, using a combination of medical subject heading terms and text words based on subgroup-specific population, intervention, comparator, and outcome questions (Supplemental Methods 1, http://links.lww.com/PCC/C497). Two authors reviewed all citations independently, with a third independent reviewer resolving any conflicts. Evidence tables were constructed using a standardized data extraction form (15). Risk of bias was assessed using the Quality in Prognosis Studies (QUIPS) tool or the revised Cochrane risk of bias for randomized trials, as appropriate (16–18) and the evidence was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (19, 20). A panel of 48 experts met over the course of 2 years to develop evidence-based recommendations and, when evidence was lacking, expert-based consensus statements for prophylactic transfusion strategies for pediatric ECMO. Prophylactic transfusion was defined as transfusion of any blood product to nonbleeding pediatric ECMO patients who are not undergoing invasive procedures. Blood product transfusion for perioperative ECMO patients with or without bleeding and for bleeding patients outside of a perioperative period are addressed in other articles within the PEACE supplement (21, 22). The supporting literature was reviewed, and statements were developed using the Evidence to Decision framework, emphasizing the panel’s assessment of risks versus benefits of each proposed statement and a prioritized list of patient outcomes that had been created by a web-based survey of expert panel members (23, 24). A web-based modified Delphi process was used to build consensus via the Research And Development/University of California Appropriateness Method. Consensus was defined as greater than 80% agreement (25, 26). Additional references, not included in the structured literature search, were included in rationale statements to provide context but were not used to derive recommendations or consensus statements, or good practice statements.
RESULTS
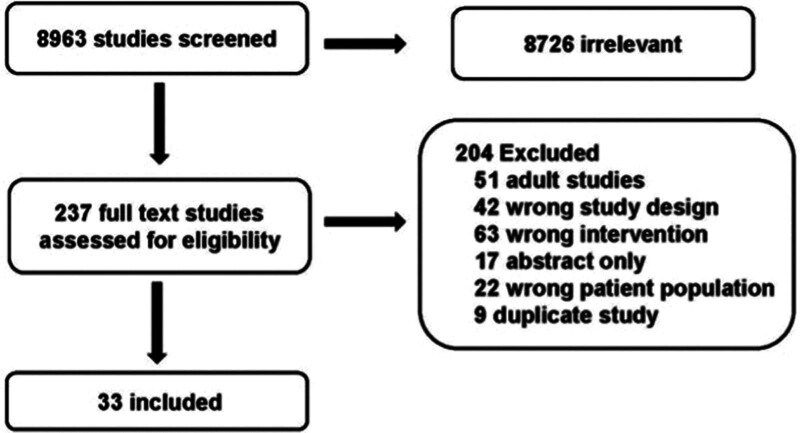
The structured literature search identified 8963 abstracts. Of these, 8726 references were excluded based on the abstract. An additional 204 references were excluded based on full article review, leaving 33 references that were used for recommendation and consensus statement creation (Fig. 1). The included references are detailed in Supplemental Table 1 (http://links.lww.com/PCC/C497).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram of studies screened and included in the prophylactic transfusion strategies in children supported by extracorporeal membrane oxygenation.
References included 2 randomized trials and 31 observational studies, the majority of which (n = 20) were retrospective studies or retrospective secondary analyses of prospective observational data. A summary of risk of bias assessments are in Supplemental Figure 1 (http://links.lww.com/PCC/C497). The following statements were developed and reached agreement (> 80%).
Good Practice Recommendations.
4.1 In ECMO, measures should be taken to minimize the overall transfusion volume. 93% agreement (n = 46), median 9 (interquartile range [IQR] 7–9).
Blood product transfusions are potentially life-saving interventions that can support oxygenation and coagulation during pediatric ECMO. However, they are also associated with morbidity and mortality. Specific complications attributable to blood products include transfusion-associated circulatory overload, transfusion-related acute lung injury, transfusion-related immunomodulation, graft-versus-host disease, hemolysis, and infection (27). Considering these risks, a judicious approach to blood product transfusion with additional measures to minimize overall transfusion volume is justified. Although evidence-based prophylactic transfusion targets remain undefined, thoughtful decision-making weighing risks and benefits for each patient is recommended, including justification beyond a laboratory value (28, 29). Additional consideration should be given to blood conservation strategies that may eliminate or mitigate transfusion requirements as part of a multimodal blood management strategy including surgical and medical interventions to address bleeding, decreased blood sampling, and blood/cell salvage techniques (30–32). Although reducing unnecessary transfusion is likely beneficial, strategies must also balance potential risks of not transfusing when necessary.
Good Practice Recommendations.
4.2 When deciding to transfuse plasma or platelets during pediatric ECMO, not only monitor hemostasis (such as coagulation system dysfunction and the platelet count), but also consider the patient’s perceived risk of bleeding and the benefits and alternatives to plasma and platelet transfusion. 93% agreement (n = 46), median 8 IQR 7–9).
Most plasma and platelets transfused to pediatric ECMO patients are administered prophylactically. Decisions to transfuse are commonly based on laboratory parameters: prothrombin, activated partial thromboplastin time, international normalized ratio (INR), and/or platelet count (33). However, these laboratory assays have not consistently correlated with risk of bleeding (6, 34, 35). Similarly, viscoelastic testing has varied results in its ability to predict bleeding in adults supported by ECMO (36, 37). Clinical characteristics of the patient, such as the indication for ECMO, weight of child, and need for hemodialysis, have all been independently associated with bleeding and would likely influence bleeding risk and predict potential benefits of prophylactic transfusion of hemostatic blood products beyond laboratory data alone (3, 5, 38). Therefore, the decision to transfuse should not be based on laboratory assays alone but must include the clinical scenario and overall bleeding risks for individual patients.
RBC Transfusion
Recommendations.
4.3 In pediatric ECMO, there is insufficient evidence to make a recommendation regarding specific indications for red blood cell (RBC) transfusion. Weak Recommendation, very low-quality pediatric evidence, 91% agreement (n = 46), median 8 (IQR 7–9).
4.4 In ECMO, we consider that the decision to transfuse RBCs should be based on the clinical scenario and global assessment of the adequacy of oxygen delivery and oxygen consumption, and not hemoglobin alone. Weak Recommendation, low-quality pediatric evidence, 100% agreement (n = 46), median 9 (IQR 8–9).
Summary of Evidence
In five observational studies, enrolling over 1200 patients, higher RBC transfusion volume was associated with adverse clinical outcomes (3, 4, 39–41). These associations are held when statistically accounting for measured confounders. However, because these studies are limited by residual confounding because of indication bias, they cannot confidently estimate risk versus benefit of RBC transfusion or identify specific transfusion indications. In a small interventional trial that randomized 20 neonates to a threshold hematocrit of 45% versus 35% to guide RBC transfusion, the lower hematocrit threshold was associated with fewer clots in the ECMO circuit components, although other clinical outcomes were not evaluated (42). In a pre-study/post-study of 72 neonates on ECMO, a change in threshold hematocrit from 40% to 35% was associated with a significant decrease in RBC exposure without a difference in clinical outcomes, suggesting that the more restrictive threshold was safe. Interventional studies outside of the neonatal population or using alternate thresholds or transfusion indications have not been reported.
Balance of Benefits Versus Harms
The decision to transfuse RBCs centers on the relative risk of transfusion compared with the risk of not transfusing and allowing permissive anemia. RBCs are often transfused with the goal of improving tissue oxygen delivery. However, the physiologic impact of transfused RBCs in a patient supported by ECMO is not well understood. In randomized trials of critically ill adults and children, RBC transfusion based on restrictive hemoglobin thresholds has not resulted in differences in clinical outcomes, suggesting that permissive anemia is safe in these populations (43–45). Each of these studies excluded ECMO patients, and the effects of restrictive transfusion strategies in pediatric ECMO are largely unknown. In observational studies of neonatal, pediatric, and adult ECMO patients, increased RBC transfusion is associated with poorer outcomes suggesting transfusion-related harm; however, these studies are impacted by indication bias (4, 39, 41, 46). A meta-analysis that included 10 retrospective and 3 prospective observational studies with 1070 adult patients found that a lower transfusion threshold was associated with lower mortality risk and acute kidney injury, particularly in those on venovenous ECMO (47). However, the studies were noted to have publication bias and poor methodological quality. It is also likely that hemoglobin alone is not the best strategy to decide when to transfuse pediatric ECMO patients due to wide variation in ECMO indications, underlying pathophysiology, and the extent to which the ECMO circuit fully supports cardiopulmonary function. It is therefore our opinion that the best approach to RBC transfusion is to determine RBC transfusion indications for each individual patient based on a global assessment of oxygen delivery, rather than based on a pre-defined hemoglobin or hematocrit threshold.
Recommendations.
4.5 In ECMO, there is insufficient evidence to make a recommendation for or against the benefit of a specific storage duration of RBC units to either prime the circuit or transfuse to the patient. Weak Recommendation, very low-quality pediatric evidence, 84% agreement (n = 44), median 8 (IQR 7–9).
Summary of Evidence
Interventional trials evaluating RBC storage duration have not included pediatric ECMO patients (48). Two included observational studies failed to identify associations between RBC storage duration and changes in measures of the adequacy of oxygen delivery posttransfusion in pediatric ECMO patients (29, 49). However, in each of these studies, most RBC transfusions were given in response to mild anemia and in the absence of inadequate oxygen delivery, and clinical outcomes related to RBC storage duration were not reported.
Balance of Benefits Versus Harms
Many ECMO centers prioritize the transfusion of fresh RBC units to ECMO patients to avoid the effects of the RBC storage lesion. The RBC storage lesion consists of oxidative damage, metabolic impairments, and loss of RBC membrane integrity leading to the release of biologically active lipid mediators, cell-free hemoglobin, and microvesicles (50). Although effects of RBC storage would be expected to impact both the safety and efficacy of RBC transfusion, randomized controlled trials of critically ill children (excluding those on ECMO), hospitalized adults, and cardiovascular surgery patients failed to show a difference in outcomes between those transfused with fresher versus older RBC units (48, 51–55). Applicability of these studies to pediatric ECMO patients is not definitive since none of these studies focused on patients receiving large volume transfusions such as routinely occur with pediatric ECMO. Hence, there is insufficient evidence to make a recommendation on the specific storage duration of RBC units to transfuse.
Prophylactic Platelet and Plasma Transfusion
Recommendations.
4.6 In ECMO there is insufficient evidence to recommend specific thresholds for prophylactic plasma and/or platelet transfusions. Weak Recommendation, very low-quality pediatric evidence, 89% agreement (n = 46), median 8 (IQR 7–9).
Summary of Evidence
Our literature search identified three studies evaluating associations between platelet and/or plasma transfusion and clinical outcomes in pediatric ECMO patients. Although both platelet and plasma transfusion volumes were associated with adverse outcomes, low platelet count and coagulopathy are also associated with adverse outcomes (3, 6, 10, 39, 56–60). The extent to which the transfusions themselves or the indications for transfusion contribute to adverse outcomes remains unclear. Interventional studies of plasma and/or platelet transfusion strategies in pediatric ECMO patients have not been reported.
Balance of Benefits Versus Harms
Like RBC transfusion, the decision to transfuse plasma or platelets rests on the assessment of the relative risks of transfusing versus the risks of not transfusing and allowing permissive thrombocytopenia and/or coagulopathy. In the absence of interventional studies, the degree to which treating thrombocytopenia or coagulopathy with transfusion and the optimum thresholds to determine when the benefits of transfusion would outweigh the risks are unknown, and evidence-based thresholds for transfusion cannot be provided.
Consensus Statement.
4.7 In ECMO, we consider that prophylactic platelet transfusions administered when the platelet count is > 100 × 109 cells/L are unlikely to benefit the patient and may cause harm. Consensus panel expertise with strong agreement, 100% agreement (n = 44), median 8 (IQR 7–9).
Summary of Evidence
In three included observational studies, platelet transfusion volume was independently associated with adverse outcomes including bleeding events and mortality (6, 10, 39). At the same time, in six included observational studies, thrombocytopenia was associated with adverse outcomes (3, 56–60). Because of heterogeneity in study design, including patient populations and outcomes evaluated, threshold platelet counts to predict bleeding or other adverse outcomes are unclear. It is also unclear whether transfusing to correct any identified threshold would affect bleeding risk.
Balance of Benefits Versus Harms
Children supported by ECMO are exposed to a high volume of platelet transfusions and are estimated to receive platelet transfusions on nearly 70% of ECMO days (6). Extracorporeal Life Support Organization (ELSO) guidelines recommend transfusing platelets to maintain a total platelet count of 80–100 × 109 cells/L (61, 62) to prevent bleeding, based on expert opinion. No trials have randomized pediatric ECMO patients to different platelet thresholds, and there is no rigorous scientific evidence identifying a threshold for platelet transfusion that is associated with a reduced prevalence of bleeding complications. Observational data suggest that platelet transfusion volume may be associated with increased bleeding risk in pediatric ECMO patients (6). Although these data are likely confounded by indication bias, it is notable that in a recent randomized trial of platelet transfusion thresholds in neonates, excluding those on ECMO, the composite outcome of death or major bleeding episodes occurred more frequently in patients randomized to a platelet threshold of 50 versus 25 × 109 cells/L, suggesting that platelet transfusion to correct moderate thrombocytopenia may not be efficacious (63). In a secondary analysis of a multicenter observational study of 511 pediatric ECMO patients, a linear association between lower platelet count and higher mortality was seen up to a platelet count of 115 × 109/L, above which relationships between platelet count and mortality were less evident (10). These data suggest that mild thrombocytopenia may not contribute to bleeding or mortality risk, although data were limited by a small number of patients with platelet counts greater than 115 × 109/L and a resultant high degree of uncertainty. In the same analyses, when adjusted for covariates, platelet count was not independently associated with mortality although platelet transfusion volume was. In addition to correcting platelet counts, platelet transfusion is sometimes given to pediatric ECMO patients based on measures of platelet dysfunction. Platelet dysfunction due to interaction with the ECMO circuit has been well documented; however, platelet transfusion may not improve platelet dysfunction and whether transfusing platelets in response to platelet dysfunction decreases bleeding risk is unknown (59, 64).
Although there is insufficient evidence to determine optimum platelet transfusion thresholds for pediatric ECMO patients, given the known risks of platelet transfusion and associations with adverse outcomes, it is reasonable to refrain from transfusing platelets in nonbleeding children when the platelet count is greater than or equal to 100 × 109 cells/L.
Consensus Statement.
4.8 In pediatric ECMO, we consider that prophylactic plasma transfusions administered to correct an International Normalized Ratio (INR) when the INR is < 1.5 are unlikely to benefit the patient and may cause harm. Consensus panel expertise with strong agreement, 95% agreement (n = 44), median 8 (IQR 7.25–9).
Summary of Evidence
Our literature search identified a single observational study evaluating associations between plasma transfusion and outcomes in pediatric ECMO patients. In a secondary analysis of a multicenter observational study, higher average daily plasma transfusion volume was associated with higher chest tube output and bleeding requiring RBC transfusion (6). In a secondary analysis of an international point prevalence study, plasma transfusions given to pediatric ECMO patients when the pretransfusion INR was less than or equal to 2.0, resulted in a nonsignificant reduction in INR of 0.1 (33).
Balance of Benefits Versus Harms
Plasma is frequently transfused to children on ECMO. In a large observational study of 514 critically ill children supported by ECMO, plasma was transfused on 34% of the ECMO days (6). The median daily plasma transfusion dose was 16.4 mL/kg and the median overall dose was 52 mL/kg. Although it is unclear when plasma should be given to pediatric ECMO patients, several studies suggest that transfusing plasma to correct an INR when the INR is less than 1.5 is not efficacious. For instance, in 442 critically ill children, plasma transfusion had no effect on INR when the baseline INR was less than 1.5 (65). Plasma transfusion is not without risk, and plasma transfusion has been independently associated with increased organ failure, increased nosocomial infections, increased length of stay in critically ill children (13), and increased mortality in children with respiratory failure (66). Therefore, considering the absence of a measurable effect of plasma transfusion when the INR is low and the independent association with worse clinical outcomes, it is reasonable to refrain from transfusing plasma to nonbleeding children on ECMO if the INR is less than 1.5.
Consensus Statement.
4.9 In pediatric ECMO, in patients with low fibrinogen levels, to prevent bleeding, fibrinogen concentrate or cryoprecipitate, when available, may be considered instead of plasma transfusion. Consensus panel expertise with weak agreement, 87% agreement (n = 46), median 8 (IQR 7–9).
Summary of Evidence
No included studies evaluated plasma transfusion compared with cryoprecipitate or fibrinogen concentrate (FC) to correct hypofibrinogenemia.
Balance of Benefits Versus Harms
Plasma, cryoprecipitate (cryo), and FC are frequently given to children to prevent bleeding associated with hypofibrinogenemia (67). Although plasma is widely used, it has a relatively low fibrinogen content. Cryo is produced by thawing frozen plasma and precipitates to achieve a higher fibrinogen content compared with plasma (300–3000 mg/dL) (67, 68). FC is derived from pooled plasma but is pathogen-reduced and purified, thereby decreasing infection risk and immunologic and allergic reactions associated with allogeneic blood product transfusion (69). FC also provides a standardized fibrinogen content of 2000 mg/dL allowing for more accurate dosing and is stored at room temperature and thereby readily available. Fibrinogen is typically the first coagulation factor to decrease during bleeding and repletion of fibrinogen helps restore hemostasis; however, there remains a lack of evidence regarding optimal timing and dosing of fibrinogen to prevent bleeding (70, 71). On ECMO, fibrinogen loss primarily occurs secondary to hemodilution as well as adhesion of fibrin to nonendothelial surfaces and hyperfibrinolysis (72). Recent literature comparing FC to cryo, including several studies of children undergoing cardiac surgery, has demonstrated similar efficacy and safety without clear advantages between products (68, 73–75). Despite a lack of specific outcome data in neonates and children, it seems reasonable to avoid hypofibrinogenemia by transfusing preferentially with either cryo or FC to prevent bleeding complications during ECMO (76).
CONCLUSIONS
Observational studies have documented significant associations between the prophylactic transfusion of blood components and increased mortality and morbidity in children supported by ECMO, thereby suggesting the use of restrictive transfusion strategies. However, there are no interventional data to support recommendations for specific transfusion thresholds.
ACKNOWLEDGMENTS
We thank all members of the Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE (PEACE) for their support, especially during the COVID-19 pandemic. The authors acknowledge the important contributions of Dr. M. Patricia Massicotte to the design and execution of the PEACE project. In addition, we thank AABB, the American Society of Extracorporeal Therapists, the American Pediatric Surgical Association, the Children’s Hospital Neonatal Consortium, the Collaborative Pediatric Critical Care Research Network, the European Society for Pediatric and Neonatal Intensive Care, the International Society of Blood Transfusion, Pediatric Cardiac Critical Care Consortium, Pediatric Cardiac Intensive Care Society, the Society for Critical Care Medicine (Pediatric Section and Clinical Pharmacy and Pharmacology Section), and the Society of Thoracic Surgeons.
Supplementary Material
Footnotes
Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE (PEACE) members are listed in Appendix 1 (http://links.lww.com/PCC/C497).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
This work was supported by funding from the National Institutes of Health (NIH)/the National Institute of Child Health and Human Development (R13HD104432-01 Pediatric Extracorporeal Membrane Oxygenation [ECMO] Anticoagulation CollaborativE); an unrestricted grant from the Extracorporeal Life Support Organization; and contributions from the Abigail Wexner Research Institute at Nationwide Children’s Hospital, Department of ECMO and Advanced Technologies, Children’s Healthcare of Atlanta, and Department of Cardiology, Boston Children’s Hospital.
The Executive Committee (Drs. Alexander, Muszynski, Bembea, Cheifetz, Steiner, and Barbaro) served as arbitrators for conflict of interest management. Dr. Alexander’s institution received funding from Novartis (consultant on the end-point adjudication committee for Prospective Trial to Assess the Angiotensin Receptor Blocker Neprilysin Inhibitor LCZ696 Versus Angiotensin-Converting Enzyme Inhibitor for the Medical Treatment of Pediatric HF [PANORAMA-HF] clinical trial). Dr. Sloan commenced employment with CSL Behring after the consensus process was complete. Dr. Kneyber received funding from Metran. Drs. Alexander and Muszynski’s institutions received funding from the National Institutes of Health (NIH); they received support for article research from the NIH. Dr. Alexander’s institution received funding from the Extracorporeal Life Support Organization and Novartis. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Besser MW, Klein AA: The coagulopathy of cardiopulmonary bypass. Crit Rev Clin Lab Sci. 2010; 47:197–212 [DOI] [PubMed] [Google Scholar]
- 2.Reynolds MM, Annich GM: The artificial endothelium. Organogenesis. 2011; 7:42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Halloran CP, Andren KG, Mecklosky J, et al. : Mortality and factors associated with hemorrhage during pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2020; 21:75–81 [DOI] [PubMed] [Google Scholar]
- 4.Muszynski JA, Reeder RW, Hall MW, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN): RBC transfusion practice in pediatric extracorporeal membrane oxygenation support. Crit Care Med. 2018; 46:e552–e559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalton HJ, Reeder R, Garcia-Filion P, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network: Factors associated with bleeding and thrombosis in children receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 2017; 196:762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karam O, Goel R, Dalton H, et al. : Epidemiology of hemostatic transfusions in children supported by extracorporeal membrane oxygenation. Crit Care Med. 2020; 48:e698–e705 [DOI] [PubMed] [Google Scholar]
- 7.Nellis ME, Vasovic LV, Goel R, et al. : Epidemiology of the use of hemostatic agents in children supported by extracorporeal membrane oxygenation: A pediatric health information system database study. Front Pediatr. 2021; 9:673613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Halloran CP, Alexander PMA, Andren KG, et al. : RBC exposure in pediatric extracorporeal membrane oxygenation: Epidemiology and factors associated with large blood transfusion volume. Pediatr Crit Care Med. 2018; 19:767–774 [DOI] [PubMed] [Google Scholar]
- 9.Muszynski JA, Spinella PC, Cholette JM, et al. ; Pediatric Critical Care Blood Research Network (Blood Net): Transfusion-related immunomodulation: Review of the literature and implications for pediatric critical illness. Transfusion. 2017; 57:195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cashen K, Dalton H, Reeder RW, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN): Platelet transfusion practice and related outcomes in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2020; 21:178–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozment CP, Scott BL, Bembea MM, et al. ; Pediatric ECMO (PediECMO) subgroup of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network and the Extracorporeal Life Support Organization (ELSO): Anticoagulation and transfusion management during neonatal and pediatric extracorporeal membrane oxygenation: A survey of medical directors in the United States. Pediatr Crit Care Med. 2021; 22:530–541 [DOI] [PubMed] [Google Scholar]
- 12.Bateman ST, Lacroix J, Boven K, et al. ; Pediatric Acute Lung Injury and Sepsis Investigators Network: Anemia, blood loss, and blood transfusions in North American children in the intensive care unit. Am J Respir Crit Care Med. 2008; 178:26–33 [DOI] [PubMed] [Google Scholar]
- 13.Karam O, Lacroix J, Robitaille N, et al. : Association between plasma transfusions and clinical outcome in critically ill children: A prospective observational study. Vox Sang. 2013; 104:342–349 [DOI] [PubMed] [Google Scholar]
- 14.Nellis ME, Karam O, Mauer E, et al. ; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) network, Pediatric Critical Care Blood Research Network (BloodNet), and the P3T Investigators: Platelet transfusion practices in critically ill children. Crit Care Med. 2018; 46:1309–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander PMA, Bembea M, Cashen K, et al. ; Pediatric Extracorporeal Membrane Oxygenation (ECMO) Anticoagulation CollaborativE (PEACE), in collaboration with the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, the Pediatric Critical Care Blood Research Network (BloodNet), and the Pediatric ECMO subgroup of PALISI and the Extracorporeal Life Support Organization (PediECMO): Executive summary: The Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE Consensus Conference. Pediatr Crit Care Med. 2024; 25:643–675 [Google Scholar]
- 16.Sterne JAC, Savovic J, Page MJ, et al. : RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366:l4898. [DOI] [PubMed] [Google Scholar]
- 17.Hayden JA, van der Windt DA, Cartwright JL, et al. : Assessing bias in studies of prognostic factors. Ann Intern Med. 2013; 158:280–286 [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Sterne JAC, Savovic J, et al. : A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev. 2016; 10:29–31 [Google Scholar]
- 19.Balshem H, Helfand M, Schunemann HJ, et al. : GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011; 64:401–406 [DOI] [PubMed] [Google Scholar]
- 20.Neumann I, Santesso N, Akl EA, et al. : A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016; 72:45–55 [DOI] [PubMed] [Google Scholar]
- 21.Willems A, Anders MM, Garcia A, et al. ; Pediatric Extracorporeal Membrane Oxygenation (ECMO) Anticoagulation CollaborativE (PEACE), in collaboration with the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, the Pediatric Critical Care Blood Research Network (BloodNet), and the Pediatric ECMO subgroup of PALISI and the Extracorporeal Life Support Organization (PediECMO): Management of extracorporeal membrane oxygenation anticoagulation in the perioperative period: The Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE Consensus Conference. Pediatr Crit Care Med. 2024; 25 (Suppl 1):e53–65 [Google Scholar]
- 22.Rintoul NE, McMichael ABV, Bembea MM, et al. ; Pediatric Extracorporeal Membrane Oxygenation (ECMO) Anticoagulation CollaborativE (PEACE), in collaboration with the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, the Pediatric Critical Care Blood Research Network (BloodNet), and the Pediatric ECMO subgroup of PALISI and the Extracorporeal Life Support Organization (PediECMO): Management of bleeding and thrombotic complications during pediatric extracorporeal membrane oxygenation: The Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE Consensus Conference. Pediatr Crit Care Med. 2024; 25 (Suppl 1):e66–e77 [Google Scholar]
- 23.Alonso-Coello P, Oxman AD, Moberg J, et al. ; GRADE Working Group: GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016; 353:i2089. [DOI] [PubMed] [Google Scholar]
- 24.Neumann I, Brignardello-Petersen R, Wiercioch W, et al. : The GRADE evidence-to-decision framework: A report of its testing and application in 15 international guideline panels. Implement Sci. 2016; 11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diamond IR, Grant RC, Feldman BM, et al. : Defining consensus: A systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014; 67:401–409 [DOI] [PubMed] [Google Scholar]
- 26.Fitch K, Bernstein SJ, Aguilar MD, et al. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA, RAND, 2001 [Google Scholar]
- 27.Friedman T, Javidroozi M, Lobel G, et al. : Complications of allogeneic blood product administration, with emphasis on transfusion-related acute lung injury and transfusion-associated circulatory overload. Adv Anesth. 2017; 35:159–173 [DOI] [PubMed] [Google Scholar]
- 28.Bembea MM, Cheifetz IM, Fortenberry JD, et al. ; Pediatric Critical Care Transfusion and Anemia Expertise Initiative (TAXI): Recommendations on the indications for RBC transfusion for the critically ill child receiving support from extracorporeal membrane oxygenation, ventricular assist, and renal replacement therapy devices from the pediatric critical care transfusion and anemia expertise initiative. Pediatr Crit Care Med. 2018; 19(9S Suppl 1):S157–S162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiser RT, Irby K, Ward RM, et al. : RBC transfusion in pediatric patients supported with extracorporeal membrane oxygenation: Is there an impact on tissue oxygenation? Pediatr Crit Care Med. 2014; 15:806–813 [DOI] [PubMed] [Google Scholar]
- 30.Flores CJ, Lakkundi A, McIntosh J, et al. : Embedding best transfusion practice and blood management in neonatal intensive care. BMJ Open Qual. 2020; 9:e000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mueller MM, Van Remoortel H, Meybohm P, et al. ; ICC PBM Frankfurt 2018 Group: Patient blood management: Recommendations from the 2018 Frankfurt Consensus Conference. BMJ Open Qual. 2020; 9:e000694 [Google Scholar]
- 32.Faraoni D, Meier J, New HV, et al. : Patient blood management for neonates and children undergoing cardiac surgery: 2019 NATA guidelines. J Cardiothorac Vasc Anesth. 2019; 33:3249–3263 [DOI] [PubMed] [Google Scholar]
- 33.Nellis ME, Saini A, Spinella PC, et al. ; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, Pediatric Critical Care Blood Research Network (BloodNet), and the PlasmaTV Investigators and the P3T Investigators: Pediatric plasma and platelet transfusions on extracorporeal membrane oxygenation: A subgroup analysis of two large international point-prevalence studies and the role of local guidelines. Pediatr Crit Care Med. 2020; 21:267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uhl L, Assmann SF, Hamza TH, et al. : Laboratory predictors of bleeding and the effect of platelet and RBC transfusions on bleeding outcomes in the PLADO trial. Blood. 2017; 130:1247–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMichael ABV, Hornik CP, Hupp SR, et al. : Correlation among antifactor Xa, activated partial thromboplastin time, and heparin dose and association with pediatric extracorporeal membrane oxygenation complications. ASAIO J. 2020; 66:307–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laine A, Niemi T, Suojaranta-Ylinen R, et al. : Decreased maximum clot firmness in rotational thromboelastometry (ROTEM®) is associated with bleeding during extracorporeal mechanical circulatory support. Perfusion. 2016; 31:625–633 [DOI] [PubMed] [Google Scholar]
- 37.Hellmann C, Schmutz A, Kalbhenn J: Bleeding during veno-venous ECMO cannot reliably be predicted by rotational thrombelastometry (ROTEM™). Perfusion. 2018; 33:289–296 [DOI] [PubMed] [Google Scholar]
- 38.Nellis ME, Dalton H, Karam O; PediECMO Investigators: Quantifiable bleeding in children supported by extracorporeal membrane oxygenation and outcome. Crit Care Med. 2019; 47:e886–e892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keene SD, Patel RM, Stansfield BK, et al. : Blood product transfusion and mortality in neonatal extracorporeal membrane oxygenation. Transfusion. 2020; 60:262–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar TK, Zurakowski D, Dalton H, et al. : Extracorporeal membrane oxygenation in postcardiotomy patients: Factors influencing outcome. J Thorac Cardiovasc Surg. 2010; 140:330–336.e2 [DOI] [PubMed] [Google Scholar]
- 41.Smith A, Hardison D, Bridges B, et al. : Red blood cell transfusion volume and mortality among patients receiving extracorporeal membrane oxygenation. Perfusion. 2013; 28:54–60 [DOI] [PubMed] [Google Scholar]
- 42.Griffin MP, Minifee PK, Daeschner CW, 3rd, et al. : Benefits of a lower hematocrit during extracorporeal membrane oxygenation? Am J Dis Child. 1992; 146:373–374 [DOI] [PubMed] [Google Scholar]
- 43.Hébert PC, Wells G, Blajchman MA, et al. : A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion requirements in critical care investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999; 340:409–417 [DOI] [PubMed] [Google Scholar]
- 44.Lacroix J, Hébert PC, Hutchison JS, et al. ; TRIPICU Investigators: Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007; 356:1609–1619 [DOI] [PubMed] [Google Scholar]
- 45.Holst LB, Haase N, Wetterslev J, et al. ; TRISS Trial Group: Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014; 371:1381–1391 [DOI] [PubMed] [Google Scholar]
- 46.Lo Pinto H, Allyn J, Persichini R, et al. : Predictors of red blood cell transfusion and its association with prognosis in patients undergoing extracorporeal membrane oxygenation. Int J Artif Organs. 2018; 41:644–652 [DOI] [PubMed] [Google Scholar]
- 47.Abbasciano RG, Yusuff H, Vlaar APJ, et al. : Blood transfusion threshold in patients receiving extracorporeal membrane oxygenation support for cardiac and respiratory failure—a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2021; 35:1192–1202 [DOI] [PubMed] [Google Scholar]
- 48.Spinella PC, Tucci M, Fergusson DA, et al. ; ABC-PICU Investigators, the Canadian Critical Care Trials Group, the Pediatric Acute Lung Injury and Sepsis Investigators Network, the BloodNet Pediatric Critical Care Blood Research Network, and the Groupe Francophone de Réanimation et Urgences P: Effect of fresh vs standard-issue red blood cell transfusions on multiple organ dysfunction syndrome in critically ill pediatric patients: A randomized clinical trial. JAMA. 2019; 322:2179–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Datta S, Chang S, Jackson NJ, et al. : Impact of age of packed RBC transfusion on oxygenation in patients receiving extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2019; 20:841–846 [DOI] [PubMed] [Google Scholar]
- 50.Yoshida T, Prudent M, D’Alessandro A: Red blood cell storage lesion: Causes and potential clinical consequences. Blood Transfus. 2019; 17:27–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cooper DJ, McQuilten ZK, Nichol A, et al. ; TRANSFUSE Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group: Age of red cells for transfusion and outcomes in critically ill adults. N Engl J Med. 2017; 377:1858–1867 [DOI] [PubMed] [Google Scholar]
- 52.Heddle NM, Cook RJ, Arnold DM, et al. : Effect of short-term vs. long-term blood storage on mortality after transfusion. N Engl J Med. 2016; 375:1937–1945 [DOI] [PubMed] [Google Scholar]
- 53.Lacroix J, Hebert PC, Fergusson DA, et al. ; ABLE Investigators: Age of transfused blood in critically ill adults. N Engl J Med. 2015; 372:1410–1418 [DOI] [PubMed] [Google Scholar]
- 54.Steiner ME, Ness PM, Assmann SF, et al. : Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med. 2015; 372:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fergusson DA, Hebert P, Hogan DL, et al. : Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: The ARIPI randomized trial. JAMA. 2012; 308:1443–1451 [DOI] [PubMed] [Google Scholar]
- 56.Dela Cruz TV, Stewart DL, Winston SJ, et al. : Risk factors for intracranial hemorrhage in the extracorporeal membrane oxygenation patient. J Perinatol. 1997; 17:18–23 [PubMed] [Google Scholar]
- 57.Doymaz S, Zinger M, Sweberg T: Risk factors associated with intracranial hemorrhage in neonates with persistent pulmonary hypertension on ECMO. J Intensive Care. 2015; 3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nardell K, Annich GM, Hirsch JC, et al. : Risk factors for bleeding in pediatric post-cardiotomy patients requiring ECLS. Perfusion. 2009; 24:191–197 [DOI] [PubMed] [Google Scholar]
- 59.Saini A, Hartman ME, Gage BF, et al. : Incidence of platelet dysfunction by thromboelastography-platelet mapping in children supported with ECMO: A pilot retrospective study. Front Pediatr. 2015; 3:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stallion A, Cofer BR, Rafferty JA, et al. : The significant relationship between platelet count and haemorrhagic complications on ECMO. Perfusion. 1994; 9:265–269 [DOI] [PubMed] [Google Scholar]
- 61.Wild KT, Rintoul N, Kattan J, et al. : Extracorporeal Life Support Organization (ELSO): Guidelines for neonatal respiratory failure. ASAIO J. 2020; 66:463–470 [DOI] [PubMed] [Google Scholar]
- 62.Brown G, Moynihan KM, Deatrick KB, et al. : Extracorporeal Life Support Organization (ELSO): Guidelines for pediatric cardiac failure. ASAIO J. 2021; 67:463–475 [DOI] [PubMed] [Google Scholar]
- 63.Curley A, Stanworth SJ, Willoughby K, et al. ; PlaNeT2 MATISSE Collaborators: Randomized trial of platelet-transfusion thresholds in neonates. N Engl J Med. 2019; 380:242–251 [DOI] [PubMed] [Google Scholar]
- 64.Robinson TM, Kickler TS, Walker LK, et al. : Effect of extracorporeal membrane oxygenation on platelets in newborns. Crit Care Med. 1993; 21:1029–1034 [DOI] [PubMed] [Google Scholar]
- 65.Karam O, Demaret P, Shefler A, et al. ; Canadian Critical Care Trials Group (CCCTG): Indications and effects of plasma transfusions in critically ill children. Am J Respir Crit Care Med. 2015; 191:1395–1402 [DOI] [PubMed] [Google Scholar]
- 66.Church GD, Matthay MA, Liu K, et al. : Blood product transfusions and clinical outcomes in pediatric patients with acute lung injury. Pediatr Crit Care Med. 2009; 10:297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nascimento B, Levy JH, Tien H, et al. : Cryoprecipitate transfusion in bleeding patients. Cjem. 2020; 22:S4–S11 [DOI] [PubMed] [Google Scholar]
- 68.Downey LA, Andrews J, Hedlin H, et al. : Fibrinogen concentrate as an alternative to cryoprecipitate in a postcardiopulmonary transfusion algorithm in infants undergoing cardiac surgery: A prospective randomized controlled trial. Anesth Analg. 2020; 130:740–751 [DOI] [PubMed] [Google Scholar]
- 69.Solomon C, Gröner A, Ye J, et al. : Safety of fibrinogen concentrate: Analysis of more than 27 years of pharmacovigilance data. Thromb Haemost. 2015; 113:759–771 [DOI] [PubMed] [Google Scholar]
- 70.Haas T, Spielmann N, Restin T, et al. : Higher fibrinogen concentrations for reduction of transfusion requirements during major paediatric surgery: A prospective randomised controlled trial. Br J Anaesth. 2015; 115:234–243 [DOI] [PubMed] [Google Scholar]
- 71.Faraoni D, Willems A, Savan V, et al. : Plasma fibrinogen concentration is correlated with postoperative blood loss in children undergoing cardiac surgery. A retrospective review. Eur J Anaesthesiol. 2014; 31:317–326 [DOI] [PubMed] [Google Scholar]
- 72.Fang ZA, Navaei AH, Hensch L, et al. : Hemostatic management of extracorporeal circuits including cardiopulmonary bypass and extracorporeal membrane oxygenation. Semin Thromb Hemost. 2020; 46:62–72 [DOI] [PubMed] [Google Scholar]
- 73.Galas FR, de Almeida JP, Fukushima JT, et al. : Hemostatic effects of fibrinogen concentrate compared with cryoprecipitate in children after cardiac surgery: A randomized pilot trial. J Thorac Cardiovasc Surg. 2014; 148:1647–1655 [DOI] [PubMed] [Google Scholar]
- 74.Callum J, Farkouh ME, Scales DC, et al. ; FIBRES Research Group: Effect of fibrinogen concentrate vs cryoprecipitate on blood component transfusion after cardiac surgery: The FIBRES randomized clinical trial. JAMA. 2019; 322:1966–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li JY, Gong J, Zhu F, et al. : Fibrinogen concentrate in cardiovascular surgery: A meta-analysis of randomized controlled trials. Anesth Analg. 2018; 127:612–621 [DOI] [PubMed] [Google Scholar]
- 76.Muszynski JA, Bembea MM, Gehred A, et al. ; Pediatric Extracorporeal Membrane Oxygenation (ECMO) Anticoagulation Collaborative (PEACE), in collaboration with the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, the Pediatric Critical Care Blood Research Network (BloodNet), and the Pediatric ECMO subgroup of PALISI and the Extracorporeal Life Support Organization (PediECMO): Priorities for clinical research in pediatric extracorporeal membrane oxygenation anticoagulation from the Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE Consensus Conference. Pediatr Crit Care Med. 2024; 25 (Suppl 1):e78–e89 [Google Scholar]