Abstract
OBJECTIVES:
To derive systematic-review informed, modified Delphi consensus regarding the influence of extracorporeal membrane oxygenation (ECMO) circuit components on anticoagulation practices for pediatric ECMO for the Pediatric ECMO Anticoagulation CollaborativE.
DATA SOURCES:
A structured literature search was performed using PubMed, EMBASE, and Cochrane Library (CENTRAL) databases from January 1988 to May 2021.
STUDY SELECTION:
Management of ECMO anticoagulation in the setting of different ECMO circuit components.
DATA EXTRACTION:
Two authors reviewed all citations independently, with a third independent reviewer resolving conflicts. Twenty-nine references were used for data extraction and informed recommendations, evidence-based consensus statements, and good practice statements. Evidence tables were constructed using a standardized data extraction form.
DATA SYNTHESIS:
Risk of bias was assessed using the Quality in Prognosis Studies tool. The evidence was evaluated using the Grading of Recommendations Assessment, Development and Evaluation system. Forty-eight experts met over 2 years to develop evidence-based recommendations and, when evidence was lacking, expert-based consensus statements or good practice statements for the influence of ECMO circuit and components on anticoagulation management. A web-based modified Delphi process was used to build consensus via the Research And Development/University of California Appropriateness Method. Consensus was defined as greater than 80% agreement. One good practice statement, 2 weak recommendations, and 2 consensus statements are presented.
CONCLUSIONS:
The incorporation of new component technologies into clinical practice has outpaced clinical investigations of anticoagulation strategies for pediatric ECMO. Future investigations should leverage academic and industrial collaborations, translational platforms, and modern biostatistical methods to improve patient outcomes.
Keywords: anticoagulation, extracorporeal membrane oxygenation, hemolysis, oxygenators, pediatrics
Over the past three decades, the increased use of pediatric extracorporeal membrane oxygenation (ECMO) has been paralleled with ongoing evolution in ECMO circuit components, configuration, and technology. ECMO cannula structure and dimensions, pump type (centrifugal or roller), tubing characteristics (size, connectors, biocompatible coating), membrane oxygenator characteristics (design, composition, material), and adjuvant devices (ultrafiltration, dialysis, plasmapheresis, etc.) may influence anticoagulation management. However, studies describing differences in anticoagulation management or hemorrhagic or thrombotic complications with the use of specific ECMO circuit components or configurations are limited (1). The objective of this subgroup of the Pediatric ECMO Anticoagulation CollaborativE (PEACE) was to derive systematic-review informed, modified Delphi consensus regarding the influence of circuit components on anticoagulation management during pediatric ECMO support intended to help guide bedside clinicians.
MATERIALS AND METHODS
Detailed methods and definitions of clinically relevant bleeding are described in the PEACE executive summary (2). Briefly, a structured literature search was performed using PubMed, EMBASE, and Cochrane Library (CENTRAL) databases from January 1988 to May 2020, with an update in May 2021, using a combination of medical subject heading terms and text words to investigate in pediatric patients supported on ECMO (population), does use of alternate circuit components (intervention/comparator) influence anticoagulation practice or outcomes (Supplemental Methods 1, http://links.lww.com/PCC/C492). Two authors reviewed all citations independently, with a third independent reviewer resolving any conflicts. Evidence tables were constructed using a standardized data extraction form (2). Risk of bias (RoB) was assessed using the Quality in Prognosis Studies (QUIPS) tool or the revised Cochrane RoB for randomized controlled trials, as appropriate (3–5), and the evidence was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (6, 7). A panel of 48 experts met over the course of 2 years to develop evidence-based recommendations and, when evidence was lacking, expert-based consensus statements or good practice statements for the influence of ECMO circuit and components on anticoagulation management. The supporting literature was reviewed and statements were developed using the evidence-to-decision framework, emphasizing the panel’s assessment of risks versus benefits of each proposed statement and a prioritized list of patient outcomes that had been created by a web-based survey of expert panel members (8–10). A web-based modified Delphi process was used to build consensus via the Research And Development/University of California Appropriateness Method. Consensus was defined as greater than 80% agreement (11, 12). Additional references, not included in the structured literature search, were included in rationale statements to provide context but were not used to derive recommendations, consensus statements, or good practice statements.
RESULTS
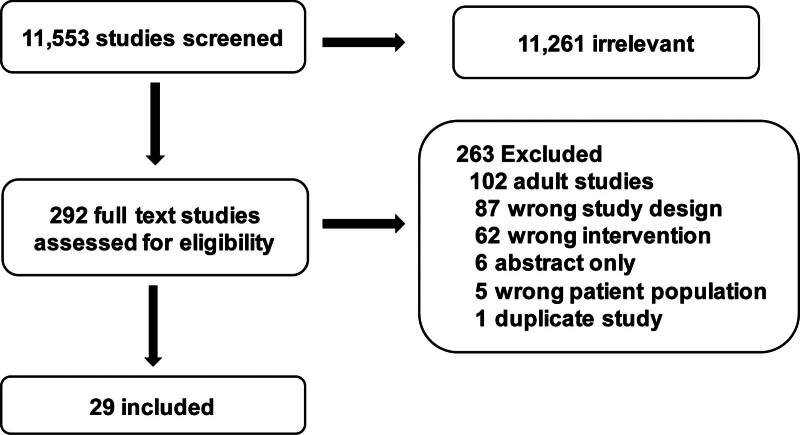
The structured literature search identified 11,553 abstracts. Of these, 11,261 references were excluded based on the abstract. An additional 263 references were excluded based on full article review, leaving 29 references that were used for recommendation and consensus statement creation (Fig. 1). The included references are detailed in Supplemental Table S1 (http://links.lww.com/PCC/C492). A summary of RoB assessments is in Supplemental Figure S1 (http://links.lww.com/PCC/C492). Two recommendations, one good practice statement, and two consensus statements were developed and, in all, agreement greater than 80% was reached.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram of studies screened and included in the pediatric extracorporeal membrane oxygenation circuit components subgroup.
Good Practice Statement
1.1 Use policies informed by national and international guidelines to maintain local multidisciplinary groups of ECMO practitioners with expertise in up-to-date circuit technologies and good practices to optimize patient outcomes. 98% agreement (n = 47), median 9, interquartile range (IQR) 8–9.
Recommendations
1.2 There is insufficient evidence to recommend a specific pump technology, circuit configuration or cannulation technique to improve mortality or morbidity for pediatric ECMO. Weak recommendation, very low-quality pediatric evidence, 93% agreement (n = 47), median 8, IQR 7–8.
1.3 There is insufficient evidence to recommend specific changes to anticoagulation strategy based on pump technology for pediatric ECMO. Weak recommendation, very low-quality pediatric evidence, 96% agreement (n = 47), median 8, IQR 7–9.
Summary of the Evidence:
Fifteen of the 29 studies that met inclusion criteria for data extraction reported data on a mixture of centrifugal pump and roller pump use (13–23), with 11 studies focused on comparing centrifugal and roller pump technologies (14–17, 19, 20, 23–28). Study designs included case–control, prospective cohort, retrospective cohort, registry-based, and propensity-matched studies. There was heterogeneity in the reported patient outcomes in studies comparing centrifugal versus roller pumps including mortality, survival, thrombotic, and hemorrhagic complications. In multiple studies, associations were seen between the use of centrifugal pump technology and greater odds of hemolysis, although associations with patient-centered clinical outcomes were not reported (14–16, 24, 25, 27). There was an overall decrease in the prevalence of hemolysis in the recent era, regardless of pump technology (27). A single-center retrospective study showed decreased rate of hemolysis associated with transition from roller to centrifugal pump technology (26). In a propensity-matched retrospective cohort study of infants less than 10 kg from the Extracorporeal Life Support Organization (ELSO) Registry, centrifugal pump use was associated with a lower odds of survival to hospital discharge (odds ratio 0.91; 95% CI, 0.83–0.99), with mediation analysis supporting hemolysis as a mediator of the association (25). Another single-center, retrospective study found increased prevalence of hemorrhagic complications when using centrifugal pump compared with using roller pump, but this observation was not associated with a difference in intracranial hemorrhage, overall mortality, or mortality secondary to coagulopathy (28).
These findings are important to interpret in the context of two major limitations. First, there was an important change in ECMO technology in 2009, with subsequent increased centrifugal pump use; the widespread adoption of this technology cannot be delineated from the published studies. Second, the majority of the reviewed studies were single-center studies, mostly in the United States, where adoption of centrifugal pump technology was later than in other countries. Future research studies should account for the inter- and intra-institutional differences in circuit component and configuration practices (29). Additionally, new studies are needed that focus on practices and outcomes with the growing experience of using centrifugal pump technology.
Consensus Statement
1.4 It is reasonable to consider minimizing the number of circuit connections for pediatric ECMO. Consensus panel expertise with weak agreement, 93% agreement (n = 47), median 8, IQR 7–9.
Summary of the Evidence:
There was a paucity of evidence from clinical research studies on the influence of circuit connectors on anticoagulation practices. Ex vivo studies support the concept of increased thrombogenicity at points of circuit connectors (19, 30).
Balance of Benefits Versus Harms:
We suggest weighing the benefits of each additional circuit connector with the potential risk of increased thrombotic burden. More clinical research is needed on the impact of the number and types of circuit connectors on bleeding, thrombosis, and morbidity of patients supported with ECMO.
Consensus Statement
1.5 Consider monitoring for hemolysis during pediatric ECMO as a marker for circuit-related red cell damage with different circuit technologies, flow rates, and thrombosis. Consensus panel expertise with strong agreement, 95% agreement (n = 44), median 8, IQR 7–9.
Summary of the Evidence:
Of the 29 informing studies (Supplemental Table S1, http://links.lww.com/PCC/C492), 15 reported plasma-free hemoglobin as a measure of hemolysis (13, 15–18, 20, 21, 24, 25, 27, 31–35). There was heterogeneity in the methods of measuring and reporting plasma-free hemoglobin. Cutoff values used for clinically significant hemolysis also varied with the most common being greater than 50 mg/dL, which is the definition used in the ELSO Registry (36). There was a lack of consistent association between reported plasma-free hemoglobin levels and patient outcomes. The interpretation of plasma-free hemoglobin should be considered primarily as a marker of RBC trauma and hemolysis that could be secondary to increased thrombotic load in addition to other patient and circuit factors.
Balance of Benefits Versus Harms:
We suggest using plasma-free hemoglobin as one of the screening tools for hemolysis because it is correlated with other markers of hemolysis (e.g., lactate dehydrogenase, haptoglobin, etc.). Because multiple modalities are used for monitoring hemolysis, it is reasonable to consider developing institutional standardized laboratory collection and processing practices of markers of hemolysis during pediatric ECMO. We suggest considering the potential influence of additional circuit components and partial or total circuit exchange when interpreting plasma-free hemoglobin values. There should be transparency of methods when reporting markers of hemolysis and prioritizing clinical research studies to investigate the impact of different circuit components on hemolysis, the association of hemolysis with different clinically significant outcomes, and the different cutoff levels of hemolysis associated with these outcomes (29).
Other Evidence to Decision Considerations:
There was paucity of evidence on the impact of: 1) additional circuit components, such as in-line hemofilters or renal replacement therapy (31), 2) alternative cannulation strategies, 3) ventricular assist device technologies (31, 37–39), or 4) extracorporeal carbon dioxide removal technologies on bleeding and thrombosis in neonates, infants, children, and adolescents supported by ECMO. One retrospective, single-center cohort study included in the PEACE review showed higher peak and peak percent change in plasma-free hemoglobin in congenital heart disease patients managed with ECMO and continuous renal replacement therapy (31). However, a thorough evaluation of the impact of adjuvant devices on anticoagulation practices and on clinically relevant outcomes was beyond the scope of the PEACE systematic review and deserves future consideration.
CONCLUSIONS
The existing clinical research investigating the influence of ECMO circuit components on anticoagulation practices is limited, with only low-quality evidence to inform recommendations, good practice statements, and consensus statements. The evolution and incorporation of new circuit component technologies into clinical practice have outpaced clinical investigations with respect to the effect of these technologies on anticoagulation strategies for pediatric ECMO and consequent patient outcomes. Despite the challenges with the broad applicability of published clinical research data and institutional variability in practice, there is real opportunity to use translational platforms, multicenter collaborations, partnerships with industry, electronic data gathering methods, standardization of reporting transparency, and modern causal inference-focused biostatistical methods to increase the data quality for more successful bedside application to improve patient outcomes (29).
ACKNOWLEDGMENTS
The authors thank all members of the Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE (PEACE) for their support, especially during the COVID-19 pandemic. They acknowledge the important contributions of Dr. M. Patricia Massicotte to the design and execution of the PEACE project. In addition, the authors thank AABB, the American Society of Extracorporeal Therapists, the American Pediatric Surgical Association, the Children’s Hospital Neonatal Consortium, the Collaborative Pediatric Critical Care Research Network, the European Society for Pediatric and Neonatal Intensive Care, the International Society of Blood Transfusion, Pediatric Cardiac Critical Care Consortium, Pediatric Cardiac Intensive Care Society, the Society for Critical Care Medicine (Pediatric Section and Clinical Pharmacy and Pharmacology Section), and the Society of Thoracic Surgeons for contributing expertise into the development of PEACE consensus.
Supplementary Material
Footnotes
Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE (PEACE) members are listed in Appendix 1 (http://links.lww.com/PCC/C492).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
Dr. Himebauch receives support from the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) under award number K23HL153759. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Said acknowledges research support from the Children’s Discovery Institute Faculty Development Award at Washington University in St. Louis. Dr. Turner is used by the American Board of Pediatrics, and the content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the American Board of Pediatrics. Drs. Muszynski, and Alexander received support for article research from the NIH. Dr. Alexander’s institution received funding from the National Institute of Child Health and Human Development (1R13HD104432), Extracorporeal Life Support Organization (ELSO), and Novartis. Dr. Paden received funding from ELSO, he disclosed that he is past president and board member of ELSO, and he disclosed the off-label product use of extracorporeal membrane oxygenation. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Collaborators: Peta M.A. Alexander, Melania M. Bembea, Katherine Cashen, Ira M. Cheifetz, Heidi J. Dalton, Adam S. Himebauch, Oliver Karam, Katie M. Moynihan, Marianne E. Nellis, Caroline Ozment, Lakshmi Raman, Natalie E. Rintoul, Ahmed S. Said, Arun Saini, Marie E. Steiner, Ravi R. Thiagarajan, Kevin Watt, Ariane Willems, Nicole D. Zantek, Ryan P. Barbaro, Katherine Steffen, Adam M. Vogel, Christopher Almond, Marc M. Anders, Gail M. Annich, Leonardo R. Brandão, Wayne Chandler, Megan Delaney, Robert DiGeronimo, Sitaram Emani, Samir K. Gadepalli, Alejandro V. Garcia, Bereketeab Haileselassie, Adam S. Himebauch, Robert Hyslop, Martin C. J. Kneyber, Lisa Baumann Kreuziger, Jennifer Le, Laura Loftis, Ali B.V. McMichael, D. Michael McMullan, Paul Monagle, Kathleen Nicol, Matthew L. Paden, Jason Patregnani, John R. Priest, Leslie Raffini, Lindsay M. Ryerson, Steven R. Sloan, Jun Teruya, Andrew R. Yates, Alison Gehred, Elizabeth Lyman, and Jennifer A. Muszynski
REFERENCES
- 1.Niebler RA, Parker H, Hoffman GM: Impact of anticoagulation and circuit technology on complications during extracorporeal membrane oxygenation. ASAIO J. 2019; 65:270–276 [DOI] [PubMed] [Google Scholar]
- 2.Alexander PMA, Bembea M, Cashen K, et al. ; Pediatric Extracorporeal Membrane Oxygenation (ECMO) Anticoagulation CollaborativE (PEACE), in collaboration with the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, the Pediatric Critical Care Blood Research Network (BloodNet), and the Pediatric ECMO subgroup of PALISI and the Extracorporeal Life Support Organization (PediECMO): Executive summary: Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE Consensus Conference. Pediatr Crit Care Med. 2024; 25:643-675 [Google Scholar]
- 3.Sterne JAC, Savović J, Page MJ, et al. : RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366:l4898. [DOI] [PubMed] [Google Scholar]
- 4.Higgins JPT, Sterne JAC, J S, et al. : A revised tool for assessing risk of bias in randomized trials. Cochrane Methods. Chandler J, McKenzie J, Boutron I, et al. (Eds). Cochrane Database of Systematic Reviews. 2016; 10(Suppl 1) [Google Scholar]
- 5.Hayden JA, van der Windt DA, Cartwright JL, et al. : Assessing bias in studies of prognostic factors. Ann Intern Med. 2013; 158:280–286 [DOI] [PubMed] [Google Scholar]
- 6.Balshem H, Helfand M, Schunemann HJ, et al. : GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011; 64:401–406 [DOI] [PubMed] [Google Scholar]
- 7.Neumann I, Santesso N, Akl EA, et al. : A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016; 72:45–55 [DOI] [PubMed] [Google Scholar]
- 8.Alonso-Coello P, Oxman AD, Moberg J, et al. ; GRADE Working Group: GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016; 353:i2089. [DOI] [PubMed] [Google Scholar]
- 9.Neumann I, Brignardello-Petersen R, Wiercioch W, et al. : The GRADE evidence-to-decision framework: A report of its testing and application in 15 international guideline panels. Implement Sci. 2016; 11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso-Coello P, Schunemann HJ, Moberg J, et al. ; GRADE Working Group: GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016; 353:i2016. [DOI] [PubMed] [Google Scholar]
- 11.Fitch K, Bernstein SJ, Aguilar MD, et al. : The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA, RAND, 2001 [Google Scholar]
- 12.Diamond IR, Grant RC, Feldman BM, et al. : Defining consensus: A systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014; 67:401–409 [DOI] [PubMed] [Google Scholar]
- 13.Dalton HJ, Reeder R, Garcia-Filion P, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network: Factors associated with bleeding and thrombosis in children receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 2017; 196:762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett CS, Jaggers JJ, Cook EF, et al. : Outcomes of neonates undergoing extracorporeal membrane oxygenation support using centrifugal versus roller blood pumps. Ann Thorac Surg. 2012; 94:1635–1641 [DOI] [PubMed] [Google Scholar]
- 15.Barrett CS, Jaggers JJ, Cook EF, et al. : Pediatric ECMO outcomes: Comparison of centrifugal versus roller blood pumps using propensity score matching. ASAIO J. 2013; 59:145–151 [DOI] [PubMed] [Google Scholar]
- 16.Byrnes J, McKamie W, Swearingen C, et al. : Hemolysis during cardiac extracorporeal membrane oxygenation: A case-control comparison of roller pumps and centrifugal pumps in a pediatric population. ASAIO J. 2011; 57:456–461 [DOI] [PubMed] [Google Scholar]
- 17.Cornelius AM, Riley JB, Schears GJ, et al. : Plasma-free hemoglobin levels in advanced vs. conventional infant and pediatric extracorporeal life support circuits. J Extra Corpor Technol. 2013; 45:21–25 [PMC free article] [PubMed] [Google Scholar]
- 18.Dalton HJ, Cashen K, Reeder RW, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN): Hemolysis during pediatric extracorporeal membrane oxygenation: Associations with circuitry, complications, and mortality. Pediatr Crit Care Med. 2018; 19:1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hastings SM, Ku DN, Wagoner S, et al. : Sources of circuit thrombosis in pediatric extracorporeal membrane oxygenation. ASAIO J. 2017; 63:86–92 [DOI] [PubMed] [Google Scholar]
- 20.Jenks CL, Zia A, Venkataraman R, et al. : High hemoglobin is an independent risk factor for the development of hemolysis during pediatric extracorporeal life support. J Intensive Care Med. 2019; 34:259–264 [DOI] [PubMed] [Google Scholar]
- 21.Masalunga C, Cruz M, Porter B, et al. : Increased hemolysis from saline pre-washing RBCs or centrifugal pumps in neonatal ECMO. J Perinatol. 2007; 27:380–384 [DOI] [PubMed] [Google Scholar]
- 22.Maul TM, Aspenleiter M, Palmer D, et al. : Impact of circuit size on coagulation and hemolysis complications in pediatric extracorporeal membrane oxygenation. ASAIO J. 2020; 66:1048–1053 [DOI] [PubMed] [Google Scholar]
- 23.McMullan DM, Emmert JA, Permut LC, et al. : Minimizing bleeding associated with mechanical circulatory support following pediatric heart surgery. Eur J Cardiothorac Surg. 2011; 39:392–397 [DOI] [PubMed] [Google Scholar]
- 24.O’Brien C, Monteagudo J, Schad C, et al. : Centrifugal pumps and hemolysis in pediatric extracorporeal membrane oxygenation (ECMO) patients: An analysis of Extracorporeal Life Support Organization (ELSO) Registry data. J Pediatr Surg. 2017; 52:975–978 [DOI] [PubMed] [Google Scholar]
- 25.O’Halloran CP, Thiagarajan RR, Yarlagadda VV, et al. : Outcomes of infants supported with extracorporeal membrane oxygenation using centrifugal versus roller pumps: An analysis from the extracorporeal life support organization registry. Pediatr Crit Care Med. 2019; 20:1177–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson KN, Carr B, Mychaliska GB, et al. : Switching to centrifugal pumps may decrease hemolysis rates among pediatric ECMO patients. Perfusion. 2022; 37:123–127 [DOI] [PubMed] [Google Scholar]
- 27.Guner YS, Delaplain PT, Schomberg J, et al. ; ELSO CDH Interest Group: Risk factors for hemolysis during extracorporeal life support for congenital diaphragmatic hernia. J Surg Res. 2021; 263:14–23 [DOI] [PubMed] [Google Scholar]
- 28.Erdem O, Kuiper JW, Houmes RJ, et al. : Coagulation complications after conversion from roller to centrifugal pump in neonatal and pediatric extracorporeal membrane oxygenation. J Pediatr Surg. 2021; 56:1378–1385 [DOI] [PubMed] [Google Scholar]
- 29.Muszynski JA, Bembea MM, Gehred A, et al. ; Pediatric Extracorporeal Membrane Oxygenation (ECMO) Anticoagulation CollaborativE (PEACE), in collaboration with the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, the Pediatric Critical Care Blood Research Network (BloodNet), and the Pediatric ECMO subgroup of PALISI and the Extracorporeal Life Support Organization (PediECMO): Priorities for clinical research in pediatric extracorporeal membrane oxygenation anticoagulation from the Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE Consensus Conference. Pediatr Crit Care Med. 2024; 25 (Suppl 1):e78–e89 [Google Scholar]
- 30.Hastings SM, Deshpande SR, Wagoner S, et al. : Thrombosis in centrifugal pumps: Location and composition in clinical and in vitro circuits. Int J Artif Organs. 2016; 39:200–204 [DOI] [PubMed] [Google Scholar]
- 31.Betrus C, Remenapp R, Charpie J, et al. : Enhanced hemolysis in pediatric patients requiring extracorporeal membrane oxygenation and continuous renal replacement therapy. Ann Thorac Cardiovasc Surg. 2007; 13:378–383 [PubMed] [Google Scholar]
- 32.Granegger M, Thamsen B, Schloglhofer T, et al. : Blood trauma potential of the HeartWare Ventricular Assist Device in pediatric patients. J Thorac Cardiovasc Surg. 2020; 159:1519–1527.e1 [DOI] [PubMed] [Google Scholar]
- 33.McDonald JV, Green TP, Steinhorn RH: The role of the centrifugal pump in hemolysis during neonatal extracorporeal support. ASAIO J. 1997; 43:35–38 [PubMed] [Google Scholar]
- 34.Thiara AP, Hoel TN, Kristiansen F, et al. : Evaluation of oxygenators and centrifugal pumps for long-term pediatric extracorporeal membrane oxygenation. Perfusion. 2007; 22:323–326 [DOI] [PubMed] [Google Scholar]
- 35.Yu K, Long C, Hei F, et al. : Clinical evaluation of two different extracorporeal membrane oxygenation systems: A single center report. Artif Organs. 2011; 35:733–737 [DOI] [PubMed] [Google Scholar]
- 36.Extracorporeal Life Support Organization: ELSO Registry Data Definitions. Ann Arbor, MI, Extracorporeal Life Support Organization; 2022. Available at: https://www.elso.org/Portals/0/Files/PDF/ELSO%20Registry%20Data%20Definitions%2005_17_22.pdf. Accessed January 10, 2024
- 37.Stiller B, Lemmer J, Merkle F, et al. : Consumption of blood products during mechanical circulatory support in children: Comparison between ECMO and a pulsatile ventricular assist device. Intensive Care Med. 2004; 30:1814–1820 [DOI] [PubMed] [Google Scholar]
- 38.Monge MC, Kulat BT, Eltayeb O, et al. : Novel modifications of a ventricular assist device for infants and children. Ann Thorac Surg. 2016; 102:147–153 [DOI] [PubMed] [Google Scholar]
- 39.Copeland H, Nolan PE, Covington D, et al. : A method for anticoagulation of children on mechanical circulatory support. Artif Organs. 2011; 35:1018–1023 [DOI] [PubMed] [Google Scholar]