Abstract
OBJECTIVES:
To derive systematic-review informed, modified Delphi consensus regarding the management of bleeding and thrombotic complications during pediatric extracorporeal membrane oxygenation (ECMO) for the Pediatric ECMO Anticoagulation CollaborativE Consensus Conference.
DATA SOURCES:
A structured literature search was performed using PubMed, EMBASE, and Cochrane Library (CENTRAL) databases from January 1988 to May 2021.
STUDY SELECTION:
The management of bleeding and thrombotic complications of ECMO.
DATA EXTRACTION:
Two authors reviewed all citations independently, with a third independent reviewer resolving conflicts. Twelve references were used for data extraction and informed recommendations. Evidence tables were constructed using a standardized data extraction form.
DATA SYNTHESIS:
Risk of bias was assessed using the Quality in Prognosis Studies tool. The evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation system. Forty-eight experts met over 2 years to develop evidence-based recommendations and, when evidence was lacking, expert-based consensus statements for the management of bleeding and thrombotic complications in pediatric ECMO patients. A web-based modified Delphi process was used to build consensus via the Research And Development/University of California Appropriateness Method. Consensus was defined as greater than 80% agreement. Two good practice statements, 5 weak recommendations, and 18 consensus statements are presented.
CONCLUSIONS:
Although bleeding and thrombotic complications during pediatric ECMO remain common, limited definitive data exist to support an evidence-based approach to treating these complications. Research is needed to improve hemostatic management of children supported with ECMO.
Keywords: anticoagulation, complications, extracorporeal membrane oxygenation, hemorrhage, pediatrics, thrombosis
Despite ongoing improvements in extracorporeal membrane oxygenation (ECMO) technology, bleeding and thrombosis remain significant complications associated with high rates of morbidity and mortality (1–6). Little evidence exists to guide management of bleeding and thrombosis in pediatric ECMO patients resulting in wide practice variability (7).
The objective of this subgroup of the Pediatric ECMO Anticoagulation CollaborativE (PEACE) was to derive systematic-review informed, modified Delphi consensus regarding the management of bleeding and thrombotic complications during pediatric ECMO support. Routine anticoagulation and blood product transfusion management for pediatric ECMO patients not experiencing bleeding or thrombosis are covered in other sections of the PEACE supplement (8–13).
MATERIALS AND METHODS
Detailed methods, including population(s) for whom these guidelines are intended and definitions of clinically relevant bleeding, are described in the PEACE executive summary (8). Briefly, a structured literature search was performed using PubMed, EMBASE, and Cochrane Library (CENTRAL) databases from January 1988 to May 2020, with an update in May 2021, guided by subgroup-specific population, intervention, comparator, and outcome questions (Supplemental Methods 1, http://links.lww.com/PCC/C499). Two authors reviewed each citation independently with a third reviewer resolving conflicts. Evidence tables were constructed using a standardized data extraction form (8). Risk of bias was assessed using the Quality in Prognosis Studies (QUIPS) tool or the revised Cochrane risk of bias for randomized trials, as appropriate (14–16), and the evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation system (17, 18).
A panel of six experts developed evidence-based and, when evidence was insufficient, expert-based statements for bleeding and thrombotic complications for children supported with ECMO. These statements were reviewed and ratified by the 48 PEACE experts, who met over two years. Statements were developed using the Evidence to Decision framework, emphasizing the panel’s assessment of the benefits versus harms of each proposed statement and a prioritized list of patient outcomes (19–21). A web-based modified Delphi process was used to build consensus via the Research and Development/University of California Appropriateness Method. Consensus was defined a priori as greater than 80% agreement (22, 23). Additional references, not included in the structured literature search, were included in rationale statements to provide context but were not used to derive recommendations or consensus statements.
RESULTS
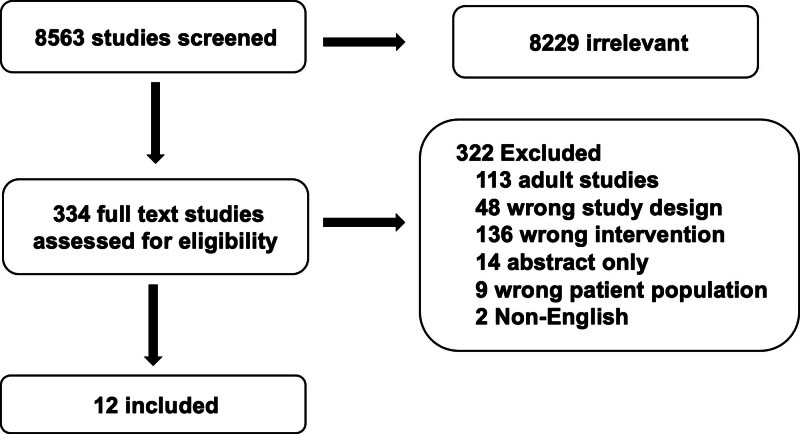
The structured literature search identified 8563 abstracts. Of these, 8229 references were excluded based on abstract review. An additional 322 references were excluded during full article review, leaving 12 references that were used for recommendation and consensus statement creation (Fig. 1). The included references are detailed in Supplemental Table 1 (http://links.lww.com/PCC/C499). A summary of risk of bias assessments is in Supplemental Figure 1 (http://links.lww.com/PCC/C499). The relevant consensus recommendations for patient management of bleeding and thrombosis are displayed in Figures 2 and 3, respectively.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram of the systematic review.
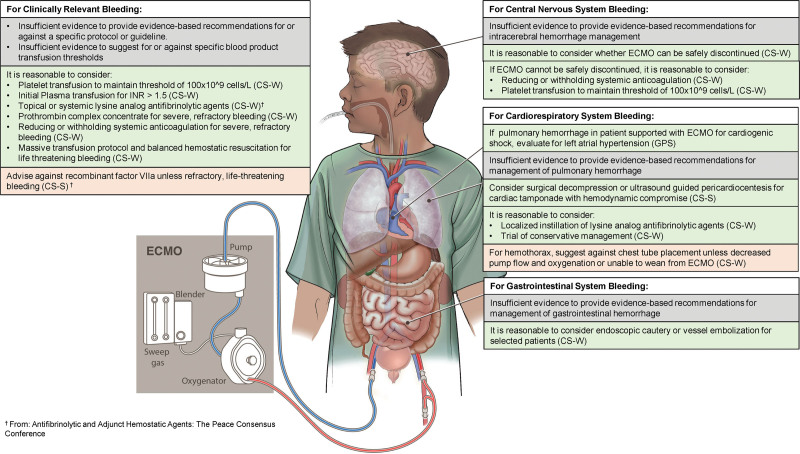
Figure 2.
Recommendations for the management of clinically significant bleeding. CS-S = consensus statement with strong agreement, CS-W = consensus statement with weak agreement, ECMO = extracorporeal membrane oxygenation, GPS = good practice statement, INR = international normalized ratio.
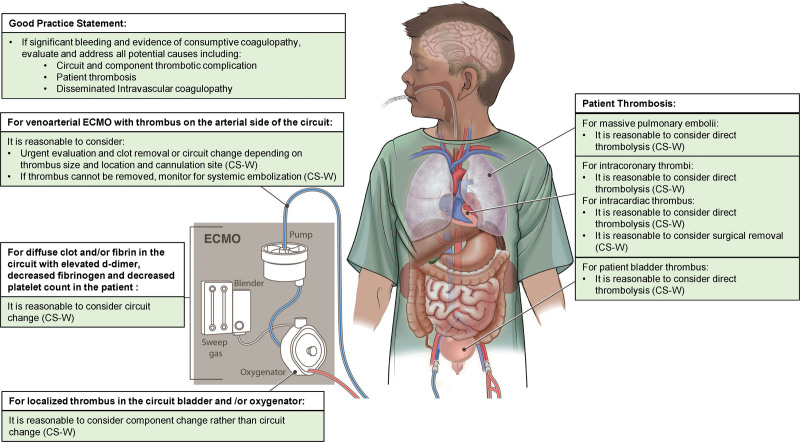
Figure 3.
Recommendations for the management of clinically significant thrombosis. CS-W = consensus statement with weak agreement, ECMO = extracorporeal membrane oxygenation.
Management of Bleeding and Thrombotic Complications
Recommendation
8.1 There is insufficient evidence to provide evidence-based recommendations for or against a specific protocol or guideline to manage bleeding or thrombotic complications in ECMO patients. Weak recommendation, very low-quality pediatric evidence, 91% agreement (n = 46), median 8 (interquartile range [IQR] 7–9).
Although bleeding and thrombosis are major complications of ECMO, insufficient data exist to formulate evidence-based recommendations for their prevention or management. Reports in the neonatal and pediatric ECMO population are largely small case series which are insufficient to lend credence to any provided protocol. In a single study, institution of a bleeding protocol with or without aminocaproic acid was not associated with shorter circuit times in pediatric ECMO patients, suggesting that circuit thrombosis may not be a major concern when anticoagulation and hemostatic agents are used judiciously (24). However, little data exist to create a specific, evidence-based bleeding protocol.
Management of Bleeding
Recommendation
8.2 There is insufficient evidence to suggest for or against specific blood product transfusion thresholds to manage bleeding or thrombotic complications in ECMO patients. Weak recommendation, very low-quality pediatric evidence, 83% agreement (n = 46), median 7.5 (IQR 7–9).
Consensus Statements
8.3 For clinically relevant bleeding (definitions of clinically relevant bleeding are provided in the Executive Summary of the PEACE Consensus Conference (8)) in ECMO patients, it is reasonable to consider platelet transfusion to maintain a threshold of at least 100 × 109 cells/L. Higher thresholds may be considered for patients in whom platelet dysfunction is suspected. Consensus panel expertise with weak agreement, 81% agreement (n = 43), median 8 (IQR 7–9).
8.4 For clinically relevant bleeding in ECMO patients, it is reasonable to consider an initial plasma transfusion for international normalized ratio (INR) > 1.5. Repeated transfusions for the sole purpose of correcting the INR may not improve outcomes. Consensus panel expertise with weak agreement, 88% agreement (n = 43), median 7 (IQR 7–9).
Summary of the evidence:
No included studies prospectively evaluated thresholds for platelet transfusion in bleeding pediatric patients on ECMO. Multiple studies associate platelet transfusion volume with adverse outcomes in both bleeding and nonbleeding patients (25–27). However, these studies are confounded by indication bias, whereby patients with greater bleeding receive greater transfusion volumes. Platelet dysfunction has been identified in pediatric ECMO patients suggesting that platelet transfusion may be helpful in the bleeding patient regardless of platelet count (28). However, the extent to which platelet dysfunction contributes to bleeding and the threshold of dysfunction that should prompt treatment are unknown. Likewise, it is unclear whether platelet transfusion reverses platelet dysfunction and would treat bleeding in this setting (29).
Optimum thresholds for plasma transfusion in bleeding pediatric ECMO patients are currently unknown. No included studies prospectively evaluated these thresholds. It is also unknown whether prothrombin time or INR are optimal laboratory values to target transfusion therapy in bleeding pediatric ECMO patients. In a multicenter point prevalence study of critically ill children transfused with plasma, the median change in INR from preimplementation to posttransfusion was –0.1 (IQR –0.3 to 1) in children with pretransfusion INR less than 2.5, whereas the change in INR was –1.1 (IQR –2 to –4) in children with pretransfusion INR greater than 2.5, suggesting that plasma transfusion to target an INR value of less than 2.5 may not be efficacious (30). These data were similar to adult studies that suggest plasma transfusion may not be efficacious in correcting INR values of less than 2.0 (31–33).
Balance of benefit versus harm:
Decisions to transfuse platelet or plasma products must balance the potential benefits of hemostatic transfusion with potential risks of transfusion. Potential benefits of platelet and plasma transfusion in clinically significant bleeding can be inferred from the trauma setting in which hemostatic resuscitation strategies that include higher plasma or platelet to RBC ratios confer bleeding-related survival benefits (34). However, thresholds at which transfusion benefit outweighs harm for bleeding pediatric ECMO patients are unknown. In the absence of more definitive data, the expert panel agreed that for clinically relevant bleeding, it may be reasonable to transfuse platelets to a threshold of 100 × 109 cells/L, and/or to transfuse fresh frozen plasma for an INR greater than 1.5. The panel acknowledged a lack of evidence to guide these particular thresholds and the critical need for further research to define evidence-based thresholds (35). Although multiple methods exist to evaluate platelet function, including platelet aggregometry, platelet function analyzer, and viscoelastic testing, data to guide definitions of platelet dysfunction associated with bleeding risk on ECMO are lacking, and the panel was unable to derive a consensus-based definition. Although a specific definition is lacking, it was felt that transfusing platelets to a threshold greater than 100 × 109 cells/L may be reasonable with active bleeding in populations with evidence of or at risk of platelet dysfunction, such as those on ECMO following cardiopulmonary bypass (36). In the expert panel’s opinion, repeat plasma transfusion for the sole purpose of correcting the INR likely carries more risk than benefit. In the setting of ongoing bleeding and persistent INR elevation despite plasma transfusion, additional evaluation including viscoelastic testing might help guide further hemostatic resuscitation.
Consensus Statement
8.5 It is reasonable to consider the use of prothrombin complex concentrates (PCC) when there is severe bleeding refractory to hemostatic blood product transfusion, antifibrinolytic therapy, decreased/discontinued anticoagulation, and/or consideration for surgical intervention as clinically applicable. Consensus panel expertise with weak agreement, 93% agreement (n = 43), median 8 (IQR 7–9).
Summary of the evidence:
PCC are lyophilized plasma-derived vitamin K-dependent coagulation factors (II, VII, IX, and X). They are categorized as three-factor or four-factor PCC based on whether they contain therapeutic concentrations of factor VII. No included studies evaluated the use of PCC in pediatric ECMO patients, and the added benefit of either three-factor or four-factor PCC over plasma transfusion in bleeding ECMO patients remains uncertain. There is a case report of pump thrombosis after PCC in an adult ECMO patient, raising a potential concern for thrombotic risk (37). However, this report was confounded by the coadministration of activated factor VII. In a meta-analysis of 17 observational studies of adults with bleeding due to trauma, cardiac surgery, or liver surgery, PCC use was associated with lower blood product utilization overall, and with lower bleeding volumes in cardiac surgery patients (38). Overall, mortality was not significantly different in PCC groups versus comparison groups, although PCC used with plasma was associated with lower mortality in trauma patients. PCC use was not associated with an increase in thromboembolic events. Included studies used either three-factor or four-factor PCC, without comparison between the two. In a propensity-matched retrospective study of adult patients with trauma-induced coagulopathy, four-factor PCC was associated with a faster time to correct INR and fewer RBC transfusions compared with three-factor PCC (39). In a recent RCT of 100 adults with bleeding and an INR greater than 1.6 during cardiopulmonary bypass surgery, four-factor PCC compared with plasma resulted in fewer intraoperative RBC transfusions and greater reductions in INR with no significant differences in bleeding volume, postoperative transfusions, thromboembolic events, or mortality (40).
Balance of benefit versus harm:
Although data suggest that PCC may have benefit to reverse coagulopathy in bleeding patients without significant increase in thrombotic risk, PCC use in ECMO patients has not been thoroughly evaluated. Acknowledging a paucity of data, the panel felt that for bleeding refractory to plasma transfusion and/or other measures, PCC may be preferred due to higher concentrations of specific clotting factors and/or smaller volumes compared with repeated plasma transfusions. Given available evidence, four-factor PCC may be preferred over three-factor PCC, though this question has not been addressed in ECMO patients.
Consensus Statement
8.6 In ECMO patients with severe bleeding refractory to other measures, it is reasonable to consider reducing or withholding systemic anticoagulation with frequent reassessment of bleeding and clotting to guide resumption of full systemic anticoagulation. Consensus panel expertise with weak agreement, 87% agreement (n = 46), median 8 (IQR 7–9).
Summary of the evidence:
No studies have evaluated stopping systemic anticoagulation to treat refractory bleeding in pediatric ECMO patients.
Balance of benefit versus harm:
Decisions to decrease or withhold systemic anticoagulation in the bleeding pediatric ECMO patient must balance risks of ongoing bleeding with risks of circuit or patient thrombosis. In the setting of life-threatening hemorrhage, the benefit of decreasing or withholding systemic anticoagulation likely outweighs the risk for most patients. Optimal indications to decrease or withhold anticoagulation in patients with clinically relevant but not life-threatening bleeding are less clear and require an individualized risk assessment. Risk assessment should consider the consequences of bleeding as well as the higher clotting risk for younger patients with smaller circulating blood volume relative to circuit volume and/or the risks of brief cessation of ECMO flow if circuit thrombosis requires a circuit change. Risks of embolic stroke, which may be greater in patients supported with venoarterial (VA) ECMO versus venovenous (VV) ECMO, should also be considered.
Consensus Statement
8.7 In ECMO patients with life-threatening bleeding, it is reasonable to consider activating a massive transfusion protocol and utilizing balanced hemostatic resuscitation. Consensus panel expertise with weak agreement, 90% agreement (n = 42), median 8 (IQR 7–9).
Summary of the evidence:
No prospective studies have evaluated massive transfusion protocols in pediatric ECMO patients. Randomized controlled trials in adult trauma patients support a balanced resuscitation strategy with plasma:RBC:platelet ratios of 1:1:1 to 1:1:2 for life-threatening hemorrhage (34). In a recent multicenter observational study of 191 children with life-threatening hemorrhage after traumatic injury, higher plasma to RBC ratio, lower plasma deficit (defined as mL/kg RBC transfusion minus mL/kg plasma transfusion), and lower platelet deficit were independently associated with lower mortality (41). In a single-center study of pediatric trauma patients, institution of a massive transfusion protocol was associated with shorter time to first plasma transfusion and higher plasma:RBC ratio (42). While differences in clinical outcomes in the preimplementation versus postimplementation eras were not demonstrated, the study was likely underpowered to detect these differences.
Balance of benefit versus harm:
Current guidelines for blood product transfusion in critically ill children support the use of a balanced transfusion strategy for life-threatening hemorrhage (43). Although their utility in pediatric ECMO patients has not been firmly established, massive transfusion protocols that promote a balanced resuscitation strategy are associated with benefits in adult and pediatric trauma patients. That said, life-threatening hemorrhage in an anticoagulated patient with high risk of underlying coagulopathy may require even higher plasma:platelet:RBC transfusion ratios to correct coagulopathy compared with a trauma patient. As such, although massive transfusion protocols are helpful to ensure efficient delivery of blood products to the bedside, augmenting the massive transfusion protocol with hemostatic testing and adjusting hemostatic resuscitation accordingly is likely beneficial (44).
CNS Bleeding
Recommendation
8.8 There is insufficient evidence to provide an evidence-based recommendation for intracerebral hemorrhage management for pediatric ECMO. Weak recommendation, very low-quality pediatric evidence, 91% agreement (n = 46), median 7.5 (IQR 7–9).
Consensus Statements
8.9 It is reasonable to consider whether ECMO can be safely discontinued when intracranial hemorrhage (ICH) is diagnosed. Consensus panel expertise with weak agreement, 91% agreement (n = 46), median 8 (IQR 7–9).
8.10 In patients with ICH for whom ECMO cannot be safely discontinued, it is reasonable to consider decreasing or stopping systemic anticoagulation with frequent reassessment of bleeding and clotting to guide the duration of decreased or no anticoagulation. Consensus panel expertise with weak agreement, 87% agreement (n = 46), median 8 (IQR 7–9).
8.11 In patients with ICH for whom ECMO cannot be safely discontinued, it is reasonable to consider transfusing platelets to at least a platelet count of 100 × 109 cells/L, or higher in the setting of platelet dysfunction. Consensus panel expertise with weak agreement, 88% agreement (n = 42), median 7.5 (IQR 7–9).
Summary of the evidence:
No included studies evaluated management strategies for pediatric ECMO patients with ICH. Fina et al (45) reported a series of six adult patients supported with ECMO in whom systemic anticoagulation was held for 10 ± 4 hours without clot formation. Anecdotal and laboratory reports indicate the ability to hold heparin in adolescent and adult patients for longer periods of time without complication (46, 47).
Balance of benefit versus harm:
For an individual patient supported with ECMO in whom ICH is diagnosed, the clinical care team must weigh the advantages and disadvantages of discontinuing ECMO support. Consideration must be given to the likelihood of ICH expansion (partially based on the degree of anticoagulation required) in comparison to the likelihood of survival if ECMO was discontinued. Decisions should incorporate the wishes of the parents/guardians with respect to their child’s overall prognosis.
The duration for which systemic anticoagulation can be held for pediatric ECMO patients, who have lower flow rates compared with adults, remains unknown. Variables include the patient’s underlying coagulation cascade balance, type of ECMO system, duration of ECMO run, and presence of thrombus in the system. Increasing ECMO flow rates when systemic anticoagulation is held or decreased is an intriguing approach; however, there is a paucity of clinical date to guide this decision. Limited in vitro data suggest that both low and high ECMO flow rates may contribute to clot formation (48), thus empirically increasing the flow rate to prevent thrombus should be viewed cautiously.
Cardiorespiratory System Bleeding:
Good Practice Statement
8.12 In patients supported with ECMO for cardiogenic shock, the presence of pulmonary hemorrhage should prompt evaluation for left atrial hypertension and consideration of left heart decompression. 100% agreement (n = 46), median 8 (IQR 8–9).
Pulmonary hemorrhage is an established complication of left atrial hypertension in cardiogenic shock (49, 50). This risk is magnified in the anticoagulated ECMO patient. Thus, the occurrence of pulmonary hemorrhage in a patient supported with ECMO for cardiogenic shock should promptly lead to diagnostic evaluation of left atrial hypertension and consideration of left heart decompression as indicated.
Recommendation
8.13 There is insufficient evidence to provide an evidence-based recommendation for management of pulmonary hemorrhage for pediatric ECMO patients. Weak recommendation, very low-qualty pediatric evidence, 91% agreement (n = 46), median 7 (IQR 7–8.25).
Consensus Statements
8.14 In ECMO patients with pulmonary hemorrhage, it is reasonable to consider localized instillation of lysine analog antifibrinolytic agents as part of a multimodal approach to bleeding control. Consensus panel expertise with weak agreement, 91% agreement (n = 46), median 7 (IQR 7–8.25).
8.15 In ECMO patients with thoracic hemorrhage/hemothorax, it is reasonable to consider a trial of conservative management with blood product replacement and/or withholding anticoagulation. Consensus panel expertise with weak agreement, 91% agreement (n = 46), median 7.5 (IQR 7–8.25).
8.16 In ECMO patients with hemothorax, due to the risk of additional bleeding, we suggest against chest tube placement except in the setting of decreased pump flow and oxygenation, or if unable to wean from ECMO. It is reasonable to consider surgical intervention if no improvement with conservative measures. Consensus panel expertise with weak agreement, 85% agreement (n = 46), median 7.5 (IQR 7–9).
8.17 In pediatric ECMO patients with hemodynamic compromise due to cardiac tamponade, consider surgical decompression or ultrasound-guided pericardiocentesis with or without placement of a pericardial drain, dependent on patient. Consensus panel expertise with strong agreement, 98% agreement (n = 42), median 8 (IQR 7–9).
Summary of the evidence:
Management of perioperative thoracic bleeding is discussed in another article of this supplement (35). Outside of the perioperative period, no pediatric literature exists to guide management of thoracic bleeding in pediatric ECMO patients. A single case series describes nebulized tranexamic acid via endotracheal tube in three adults with pulmonary hemorrhage while on VV ECMO (51). Each patient had control of bleeding and was ultimately decannulated from ECMO successfully.
Balance of benefit versus harm:
Although bleeding from lung parenchyma and intercostal vessels is often difficult to control, aggressive interventional approaches may exacerbate hemorrhage rather than control it. Our expert consensus approach is to trial a conservative management strategy before invasive strategies to achieve hemostasis (52, 53). This management strategy may include a trial of nebulized lysine analog antifibrinolytic agents, although data to guide dosing, frequency, or optimal instillation techniques are lacking. The decision to place a chest tube in the setting of hemothorax must weigh the potential benefit to cardiopulmonary function with the potential risk of further bleeding from chest tube placement itself. There is no clear evidence to support a superior method of chest tube placement, including whether the Seldinger technique versus surgical chest tube placement is preferred (54).
Although clinicians must always balance the benefit:risk ratio of any procedure, especially when performed on an anticoagulated patient, cardiac tamponade with hemodynamic compromise can be life-threatening even with full ECMO support. Tamponade in the ECMO-supported patient can lead to failure of venous return and lack of cardiac output (from patient or ECMO pump) as well as compromised myocardial perfusion and subsequent progressive myocardial injury (55). In this emergent situation, surgical or ultrasound-guided decompression is essential. The technical approach should be tailored to the expertise available.
Gastrointestinal System Bleeding
Recommendation
8.18 There is insufficient evidence to provide an evidence-based recommendation for management of gastrointestinal hemorrhage for pediatric ECMO. Weak recommendation, very low-quality pediatric evidence, 87% agreement (n = 46), median 7 (IQR 7–9).
Consensus Statement
8.19 It is reasonable to consider endoscopic cautery or vessel embolization to control bleeding for selected pediatric patients with gastrointestinal hemorrhage on ECMO. Consensus panel expertise with weak agreement, 89% agreement (n = 46), median 7.5 (IQR 7–8).
Summary of the evidence:
Although data are limited, the use of endoscopic cautery has been described in a case report of an adult managed with ECMO who experienced gastrointestinal bleeding from a small bowel arteriovenous malformation (56).
Balance of benefit versus harm:
Although no pediatric data are available, the expert panel agreed that considering endoscopic cautery or vessel embolization is reasonable for the pediatric ECMO patient with gastrointestinal bleeding originating from a focal source and refractory to conservative measures, balancing the severity of bleeding with the risks of bleeding from the procedure itself.
Patient Thrombosis
Consensus Statements
8.20 In ECMO patients with massive pulmonary emboli, intracardiac or intracoronary thrombi, or patient bladder thrombus, it is reasonable to consider direct thrombolysis. Consensus panel expertise with weak agreement, 82% agreement (n = 46), median 7 (IQR 7–8).
8.21 In ECMO patients with intracardiac thrombus, it is reasonable to consider surgical removal of the thrombus. Consensus panel expertise with weak agreement, 87% agreement (n = 46), median 8 (IQR 7–8).
Summary of the evidence:
A single case report describes successful use of direct thrombolysis in a newborn with intra-atrial thrombus while on ECMO (57). Successful surgical removal of intracardiac thrombus in a patient with biventricular ventricular mechanical circulatory support has also been reported (58).
Balance of benefit versus harm:
In the absence of definitive data, relative risks versus benefits of either direct thrombolysis or thrombectomy for thoracic thromboses in the pediatric ECMO population remain uncertain. For individual patients, decisions are best made by a multidisciplinary team considering the pathophysiology and underlying hemostasis of the child, risks of the thrombus itself, and risks of each procedure. Decisions must consider the availability of required equipment, resources, and institutional expertise.
Circuit and Circuit Component Thrombosis
Good Practice Statement
8.22 In ECMO patients with significant bleeding and evidence of consumptive coagulopathy, evaluate and address all potential causes, including the circuit and components, patient thrombosis, and diagnoses associated with disseminated intravascular coagulopathy (DIC). 100% agreement (n = 43), median 8 (IQR 7–9).
Signs and symptoms of consumptive coagulopathy can arise from a variety of etiologies in pediatric ECMO patients, including patient thrombus, circuit thrombus, or DIC. Each of these etiologies requires a different set of diagnostic and management approaches. Specifically, if thrombus is not identified, consumptive coagulopathy may be a sign of a new or worsening inflammatory process, including nosocomial sepsis, and investigation for new infection or for alternate sources of systemic inflammation is warranted. Worsening thrombocytopenia, including increased use of platelet transfusions to maintain a threshold platelet count, should prompt evaluation for thrombocytopenia-associated multiple organ failure, an inflammatory multiple organ dysfunction phenotype characterized by low ADAMTS13 activity, accumulation of ultra-large von Willebrand factor multimers and thrombotic microangiopathy (59, 60).
Consensus Statements
8.23 In venoarterial ECMO patients with thrombus identified on the arterial side of the circuit, it is reasonable to consider urgent evaluation and consideration of options for clot removal or circuit change depending on cannulation site, thrombus size, and thrombus location. If the clot cannot be removed, careful neurologic monitoring should occur as patient is at high risk for systemic embolization, including stroke. Consensus panel expertise with weak agreement, 90% agreement (n = 42), median 8 (IQR 7–9).
8.24 It is reasonable to consider component change rather than entire circuit change for localized thrombus in the bladder and/or oxygenator during ECMO. Consensus panel expertise with weak agreement, 87% agreement (n = 46), median 8 (IQR 7–9).
8.25 In ECMO patients, it is reasonable to consider circuit change for diffuse clot and/or fibrin deposition with associated decrease in patient fibrinogen and platelet count and increase in d-dimer. Consensus panel expertise with weak agreement, 89% agreement (n = 46), median 7 (IQR 7–9).
Summary of the evidence:
No included studies evaluated the optimal approach in the pediatric population to remove clots from the arterial side of the ECMO circuit or optimal timing/indications for a circuit change.
Balance of benefit versus harm:
When thrombosis occurs in components of the ECMO system, the clinical team must decide whether to replace the affected components or change the entire pump system. Although no definitive data exist to support either approach, one must consider the multiple variables including institutional expertise, estimated time off pump to change the component/system, expense, component/system availability, possibility of the clot reforming at the same site, and anticipated risk to the patient.
CONCLUSIONS
Although bleeding and thrombotic complications during pediatric ECMO remain common and adversely affect patient outcomes, limited definitive data exist to support an evidence-based approach to preventing and treating these complications. Further research is needed to improve hemostatic management of children supported with ECMO and to prevent and manage life-affecting and life-threatening complications.
ACKNOWLEDGMENTS
We thank all members of the Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE (PEACE) for their support, especially during the COVID-19 pandemic. The authors acknowledge the important contributions of Dr. M. Patricia Massicotte to the design and execution of the PEACE project. In addition, we thank AABB, the American Society of Extracorporeal Therapists, the American Pediatric Surgical Association, the Children’s Hospital Neonatal Consortium, the Collaborative Pediatric Critical Care Research Network, the European Society for Pediatric and Neonatal Intensive Care, the International Society of Blood Transfusion, Pediatric Cardiac Critical Care Consortium, Pediatric Cardiac Intensive Care Society, the Society for Critical Care Medicine (Pediatric Section and Clinical Pharmacy and Pharmacology Section), and the Society of Thoracic Surgeons for contribution through representation on the PEACE Expert Panel. Finally, the authors thank Anthony Baker, CMI, Lead Medical Illustrator and Senior Graphic Designer at the Ohio State University Health Sciences Library, for assistance with figure preparation.
Supplementary Material
Footnotes
Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE (PEACE) members are listed in Appendix 1 (http://links.lww.com/PCC/C499).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
This work was supported by funding from the National Institutes of Health/the National Institute of Child Health and Human Development (R13HD104432 Pediatric Extracorporeal Membrane Oxygenation [ECMO] Anticoagulation CollaborativE); an unrestricted grant from the Extracorporeal Life Support Organization; and contributions from the Abigail Wexner Research Institute at Nationwide Children’s Hospital, Department of ECMO and Advanced Technologies, Children’s Healthcare of Atlanta, and Department of Cardiology, Boston Children’s Hospital.
The Executive Committee (Drs. Alexander, Muszynski, Bembea, Cheifetz, Steiner, and Barbaro) served as arbitrators for conflict-of-interest management. Dr. Alexander’s institution received funding from Novartis (Prospective Trial to Assess the Angiotensin Receptor Blocker Neprilysin Inhibitor LCZ696 Versus Angiotensin-Converting Enzyme Inhibitor for the Medical Treatment of Pediatric HF [PANORAMA-HF]). Dr. Patregnani discloses consultation payments from MNK Pharmaceuticals and Pfizer. Dr. Bembea’s institution received funding from the National Institute of Neurologic Disorder and Stroke (R01NS106292), the Grifols Investigator Sponsored Research Grant, and the Department of Defense. Drs. Bembea, Alexander, and Muszynski received support for article research from the National Institutes of Health (NIH). Dr. Patregnani received funding from Mallinckrodt Pharmaceutical. Drs. Alexander and Muszynski’s institutions received funding from the NIH. Dr. Alexander’s institution received funding from the Extracorporeal Life Support Organization and Novartis. Dr. Cheifetz received funding from UptoDate. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Collaborators: Peta M.A. Alexander, Melania M. Bembea, Katherine Cashen, Ira M. Cheifetz, Heidi J. Dalton, Adam S. Himebauch, Oliver Karam, Katie M. Moynihan, Marianne E. Nellis, Caroline Ozment, Lakshmi Raman, Natalie E. Rintoul, Ahmed S. Said, Arun Saini, Marie E. Steiner, Ravi R. Thiagarajan, Kevin Watt, Ariane Willems, Nicole D. Zantek, Ryan P. Barbaro, Katherine Steffen, Adam M. Vogel, Christopher Almond, Marc M. Anders, Gail Annich, Leonardo R. Brandão, Wayne Chandler, Megan Delaney, Robert DiGeronimo, Sitaram Emani, Samir K. Gadepalli, Alejandro V. Garcia, Bereketeab Haileselassie, Adam S. Himebauch, Robert Hyslop, Martin C. J. Kneyber, Lisa Baumann Kreuziger, Jennifer Le, Laura Loftis, Ali B.V. McMichael, D. Michael McMullan, Paul Monagle, Kathleen Nico, Matthew L. Paden, Jason Patregnani, John R. Priest, Leslie Raffini, Lindsay M. Ryerson, Steven R. Sloan, Jun Teruya, Andrew R. Yates, Alison Gehred, Elizabeth Lyman, and Jennifer A. Muszynski
REFERENCES
- 1.Werho DK, Pasquali SK, Yu S, et al. ; Extracorporeal Life Support Organization Member Centers: Hemorrhagic complications in pediatric cardiac patients on extracorporeal membrane oxygenation: An analysis of the Extracorporeal Life Support Organization Registry. Pediatr Crit Care Med. 2015; 16:276–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalton HJ, Reeder R, Garcia-Filion P, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network: Factors associated with bleeding and thrombosis in children receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 2017; 196:762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annich GM: Extracorporeal life support: The precarious balance of hemostasis. J Thromb Haemost. 2015; 13:S336–S342 [DOI] [PubMed] [Google Scholar]
- 4.O’Halloran CP, Andren KG, Mecklosky J, et al. : Mortality and factors associated with hemorrhage during pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2020; 21:75–81 [DOI] [PubMed] [Google Scholar]
- 5.Barbaro RP, Paden ML, Guner YS, et al. ; ELSO member centers: Pediatric Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017; 63:456–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Extracorporeal Life Support Organization (ELSO): International Summary—ECLS Registry Report July 30, 2023. Available at: https://www.elso.org/registry/internationalsummaryandreports/internationalsummary.aspx. Accessed January 10, 2024
- 7.Ozment CP, Scott BL, Bembea MM, et al. ; Pediatric ECMO (PediECMO) subgroup of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network and the Extracorporeal Life Support Organization (ELSO): Anticoagulation and transfusion management during neonatal and pediatric extracorporeal membrane oxygenation: A survey of medical directors in the United States. Pediatr Crit Care Med. 2021; 22:530–541 [DOI] [PubMed] [Google Scholar]
- 8.Alexander PMA, Bembea M, Cashen K, et al. ; Pediatric Extracorporeal Membrane Oxygenation (ECMO) Anticoagulation CollaborativE (PEACE), in collaboration with the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, the Pediatric Critical Care Blood Research Network (BloodNet), and the Pediatric ECMO subgroup of PALISI and the Extracorporeal Life Support Organization (PediECMO): Executive summary: The Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE Consensus Conference. Pediatr Crit Care Med. 2024; 25:643-675 [Google Scholar]
- 9.Cashen K, Saini A, Brandão L, et al. ; Pediatric Extracorporeal Membrane Oxygenation (ECMO) Anticoagulation CollaborativE (PEACE), in collaboration with the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, the Pediatric Critical Care Blood Research Network (BloodNet), and the Pediatric ECMO subgroup of PALISI and the Extracorporeal Life Support Organization (PediECMO): Anticoagulant medications: The Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE Consensus Conference. Pediatr Crit Care Med. 2024; 25 (Suppl 1):e7-e13 [Google Scholar]
- 10.Ozment C, Alexander PMA, Chandler W, et al. ; Pediatric Extracorporeal Membrane Oxygenation (ECMO) Anticoagulation CollaborativE (PEACE), in collaboration with the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, the Pediatric Critical Care Blood Research Network (BloodNet), and the Pediatric ECMO subgroup of PALISI and the Extracorporeal Life Support Organization (PediECMO): Anticoagulation monitoring and targets: The Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE Consensus Conference. Pediatr Crit Care Med. 2024; 25 (Suppl 1):e14-e24 [Google Scholar]
- 11.Nellis ME, Moynihan KM, Sloan SR, et al. ; Pediatric Extracorporeal Membrane Oxygenation (ECMO) Anticoagulation CollaborativE (PEACE), in collaboration with the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, the Pediatric Critical Care Blood Research Network (BloodNet), and the Pediatric ECMO subgroup of PALISI and the Extracorporeal Life Support Organization (PediECMO): Prophylactic transfusions strategies in children supported by extracorporeal membrane oxygenation: The Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE Consensus Conference. Pediatr Crit Care Med. 2024; 25 (Suppl 1):e25-e34 [Google Scholar]
- 12.Zantek ND, Steiner ME, Teruya J, et al. ; Pediatric Extracorporeal Membrane Oxygenation (ECMO) Anticoagulation CollaborativE (PEACE), in collaboration with the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, the Pediatric Critical Care Blood Research Network (BloodNet), and the Pediatric ECMO subgroup of PALISI and the Extracorporeal Life Support Organization (PediECMO): Recommendations on monitoring and replacement of antithrombin, fibrinogen and von Willebrand factor in pediatric patients on extracorporeal membrane oxygenation: The Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE Consensus Conference. Pediatr Crit Care Med. 2024; 25 (Suppl 1):e35-e43 [Google Scholar]
- 13.Moynihan KM, Ryerson L, Le J, et al. ; Pediatric Extracorporeal Membrane Oxygenation (ECMO) Anticoagulation CollaborativE (PEACE), in collaboration with the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, the Pediatric Critical Care Blood Research Network (BloodNet), and the Pediatric ECMO subgroup of PALISI and the Extracorporeal Life Support Organization (PediECMO): Antifibrinolytic and adjunct hemostatic agents: The Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE Consensus Conference. Pediatr Crit Care Med. 2024; 25 (Suppl 1):e44-e52 [Google Scholar]
- 14.Balshem H, Helfand M, Schunemann HJ, et al. : GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011; 64:401–406 [DOI] [PubMed] [Google Scholar]
- 15.Neumann I, Santesso N, Akl EA, et al. : A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016; 72:45–55 [DOI] [PubMed] [Google Scholar]
- 16.Sterne JAC, Savovic J, Page MJ, et al. : RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366:l4898. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Sterne JAC, Savovic J, et al. : A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev. 2016; 10:29–31 [Google Scholar]
- 18.Hayden JA, van der Windt DA, Cartwright JL, et al. : Assessing bias in studies of prognostic factors. Ann Intern Med. 2013; 158:280–286 [DOI] [PubMed] [Google Scholar]
- 19.Alonso-Coello P, Oxman AD, Moberg J, et al. ; GRADE Working Group: GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016; 353:i2089. [DOI] [PubMed] [Google Scholar]
- 20.Alonso-Coello P, Schunemann HJ, Moberg J, et al. ; GRADE Working Group: GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016; 353:i2016. [DOI] [PubMed] [Google Scholar]
- 21.Neumann I, Brignardello-Petersen R, Wiercioch W, et al. : The GRADE evidence-to-decision framework: A report of its testing and application in 15 international guideline panels. Implement Sci. 2016; 11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR, Lazaro P, et al. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: RAND Corporation; 2001. [Google Scholar]
- 23.Diamond IR, Grant RC, Feldman BM, et al. : Defining consensus: A systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014; 67:401–409 [DOI] [PubMed] [Google Scholar]
- 24.Muensterer OJ, Laney D, Georgeson KE: Survival time of ECMO circuits on and off bleeding protocol: Is there a higher risk of circuit clotting? Eur J Pediatr Surg. 2011; 21:30–32 [DOI] [PubMed] [Google Scholar]
- 25.Cashen K, Dalton H, Reeder RW, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN): Platelet transfusion practice and related outcomes in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2020; 21:178–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karam O, Goel R, Dalton H, et al. : Epidemiology of hemostatic transfusions in children supported by extracorporeal membrane oxygenation. Crit Care Med. 2020; 48:e698–e705 [DOI] [PubMed] [Google Scholar]
- 27.Keene SD, Patel RM, Stansfield BK, et al. : Blood product transfusion and mortality in neonatal extracorporeal membrane oxygenation. Transfusion. 2020; 60:262–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saini A, Hartman ME, Gage BF, et al. : Incidence of platelet dysfunction by thromboelastography-platelet mapping in children supported with ECMO: A pilot retrospective study. Front Pediatr. 2015; 3:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvikas J, Zenati M, Campwala I, et al. : Rapid detection of platelet inhibition and dysfunction in traumatic brain injury: A prospective observational study. J Trauma Acute Care Surg. 2022; 92:167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karam O, Demaret P, Shefler A, et al. ; Canadian Critical Care Trials Group (CCCTG): Indications and effects of plasma transfusions in critically ill children. Am J Respir Crit Care Med. 2015; 191:1395–1402 [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Wahab OI, Healy B, Dzik WH: Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion. 2006; 46:1279–1285 [DOI] [PubMed] [Google Scholar]
- 32.Holland LL, Brooks JP: Toward rational fresh frozen plasma transfusion: The effect of plasma transfusion on coagulation test results. Am J Clin Pathol. 2006; 126:133–139 [DOI] [PubMed] [Google Scholar]
- 33.Stanworth SJ, Grant-Casey J, Lowe D, et al. : The use of fresh-frozen plasma in England: High levels of inappropriate use in adults and children. Transfusion. 2011; 51:62–70 [DOI] [PubMed] [Google Scholar]
- 34.Holcomb JB, Tilley BC, Baraniuk S, et al. ; PROPPR Study Group: Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: The PROPPR randomized clinical trial. JAMA. 2015; 313:471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willems A, Anders MM, Garcia A, et al. ; Pediatric Extracorporeal Membrane Oxygenation (ECMO) Anticoagulation CollaborativE (PEACE), in collaboration with the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, the Pediatric Critical Care Blood Research Network (BloodNet), and the Pediatric ECMO subgroup of PALISI and the Extracorporeal Life Support Organization (PediECMO): Management of extracorporeal membrane oxygenation anticoagulation in the perioperative period: The Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE consensus conference. Pediatr Crit Care Med. 2024; 25 (Suppl 1):e53–e65 [Google Scholar]
- 36.ElMahrouk AF, Ismail MF, Hamouda T, et al. : Extracorporeal membrane oxygenation in postcardiotomy pediatric patients-15 years of experience outside Europe and North America. Thorac Cardiovasc Surg. 2019; 67:28–36 [DOI] [PubMed] [Google Scholar]
- 37.Bui JD, Despotis GD, Trulock EP, et al. : Fatal thrombosis after administration of activated prothrombin complex concentrates in a patient supported by extracorporeal membrane oxygenation who had received activated recombinant factor VII. J Thorac Cardiovasc Surg. 2002; 124:852–854 [DOI] [PubMed] [Google Scholar]
- 38.van den Brink DP, Wirtz MR, Neto AS, et al. : Effectiveness of prothrombin complex concentrate for the treatment of bleeding: A systematic review and meta-analysis. J Thromb Haemost. 2020; 18:2457–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeeshan M, Hamidi M, Kulvatunyou N, et al. : 3-factor versus 4-factor PCC in coagulopathy of trauma: four is better than three. Shock. 2019; 52:23–28 [DOI] [PubMed] [Google Scholar]
- 40.Smith MM, Schroeder DR, Nelson JA, et al. : Prothrombin complex concentrate vs plasma for post-cardiopulmonary bypass coagulopathy and bleeding: A randomized clinical trial. JAMA Surg. 2022; 157:757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spinella PC, Leonard JC, Marshall C, et al. ; Massive Transfusion In Children (MATIC) Investigators and BloodNet: Transfusion ratios and deficits in injured children with life-threatening bleeding. Pediatr Crit Care Med. 2022; 23:235–244 [DOI] [PubMed] [Google Scholar]
- 42.Hwu RS, Spinella PC, Keller MS, et al. : The effect of massive transfusion protocol implementation on pediatric trauma care. Transfusion. 2016; 56:2712–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karam O, Russell RT, Stricker P, et al. ; Pediatric Critical Care Transfusion and Anemia Expertise Initiative (TAXI): Recommendations on RBC transfusion in critically ill children with nonlife-threatening bleeding or hemorrhagic shock from the pediatric critical care transfusion and anemia expertise initiative. Pediatr Crit Care Med. 2018; 19(9S Suppl 1):S127–S132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakayama Y, Nakajima Y, Tanaka KA, et al. : Thromboelastometry-guided intraoperative haemostatic management reduces bleeding and red cell transfusion after paediatric cardiac surgery. Br J Anaesth. 2015; 114:91–102 [DOI] [PubMed] [Google Scholar]
- 45.Fina D, Matteucci M, Jiritano F, et al. : Extracorporeal membrane oxygenation without systemic anticoagulation: A case-series in challenging conditions. J Thorac Dis. 2020; 12:2113–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whittlesey GC, Drucker DE, Salley SO, et al. : ECMO without heparin: Laboratory and clinical experience. J Pediatr Surg. 1991; 26:320–324; discussion 324 [DOI] [PubMed] [Google Scholar]
- 47.Wen PH, Chan WH, Chen YC, et al. : Non-heparinized ECMO serves a rescue method in a multitrauma patient combining pulmonary contusion and nonoperative internal bleeding: A case report and literature review. World J Emerg Surg. 2015; 10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer AD, Rishmawi AR, Kamucheka R, et al. : Effect of blood flow on platelets, leukocytes, and extracellular vesicles in thrombosis of simulated neonatal extracorporeal circulation. J Thromb Haemost. 2020; 18:399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zampi JD, Alghanem F, Yu S, et al. : Relationship between time to left atrial decompression and outcomes in patients receiving venoarterial extracorporeal membrane oxygenation support: a multicenter pediatric interventional cardiology early-career society study. Pediatr Crit Care Med. 2019; 20:728–736 [DOI] [PubMed] [Google Scholar]
- 50.Coleman RD, Chartan CA, Qureshi AM, et al. : Left atrial decompression on venoarterial extracorporeal membrane oxygenation: Getting to the heart of the matter. Pediatr Crit Care Med. 2019; 20:780–781 [DOI] [PubMed] [Google Scholar]
- 51.Cabanilla MG, Villalobos NE, Ahmed S: A role for nebulized tranexamic acid in veno-venous ECMO patients. J Clin Pharm Ther. 2022; 47:125–128 [DOI] [PubMed] [Google Scholar]
- 52.Herbert DG, Buscher H, Nair P: Prolonged venovenous extracorporeal membrane oxygenation without anticoagulation: A case of Goodpasture syndrome-related pulmonary haemorrhage. Crit Care Resusc. 2014; 16:69–72 [PubMed] [Google Scholar]
- 53.Ried M, Sommerauer L, Lubnow M, et al. : Thoracic bleeding complications in patients with venovenous extracorporeal membrane oxygenation. Ann Thorac Surg. 2018; 106:1668–1674 [DOI] [PubMed] [Google Scholar]
- 54.Jackson HT, Longshore S, Feldman J, et al. : Chest tube placement in children during extracorporeal membrane oxygenation (ECMO). J Pediatr Surg. 2014; 49:51–3; discussion 53 [DOI] [PubMed] [Google Scholar]
- 55.Kurian MS, Reynolds ER, Humes RA, et al. : Cardiac tamponade caused by serous pericardial effusion in patients on extracorporeal membrane oxygenation. J Pediatr Surg. 1999; 34:1311–1314 [DOI] [PubMed] [Google Scholar]
- 56.Sarosiek K, Hirose H, Pitcher HT, et al. : Adult extracorporeal membrane oxygenation and gastrointestinal bleeding from small bowel arteriovenous malformations: A novel treatment using spiral enteroscopy. J Thorac Cardiovasc Surg. 2012; 143:1221–1222 [DOI] [PubMed] [Google Scholar]
- 57.Garcia A, Gander JW, Gross ER, et al. : The use of recombinant tissue-type plasminogen activator in a newborn with an intracardiac thrombus developed during extracorporeal membrane oxygenation. J Pediatr Surg. 2011; 46:2021–2024 [DOI] [PubMed] [Google Scholar]
- 58.Mejia EJ, O’Connor MJ, Samelson-Jones BJ, et al. : Successful treatment of intracardiac thrombosis in the presence of fulminant myocarditis requiring ECMO associated with COVID-19. J Heart Lung Transplant. 2022; 41:849–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chong M, Lopez-Magallon AJ, Saenz L, et al. : Use of therapeutic plasma exchange during extracorporeal life support in critically ill cardiac children with thrombocytopenia-associated multi-organ failure. Front Pediatr. 2017; 5:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fortenberry JD, Nguyen T, Grunwell JR, et al. ; Thrombocytopenia-Associated Multiple Organ Failure (TAMOF) Network Study Group: Therapeutic plasma exchange in children with thrombocytopenia-associated multiple organ failure: The thrombocytopenia-associated multiple organ failure network prospective experience. Crit Care Med. 2019; 47:e173–e181 [DOI] [PMC free article] [PubMed] [Google Scholar]