Abstract
OBJECTIVES:
To derive systematic-review informed, modified Delphi consensus regarding anticoagulation monitoring assays and target levels in pediatric extracorporeal membrane oxygenation (ECMO) for the Pediatric ECMO Anticoagulation CollaborativE.
DATA SOURCES:
A structured literature search was performed using PubMed, EMBASE, and Cochrane Library (CENTRAL) databases from January 1988 to May 2021.
STUDY SELECTION:
Anticoagulation monitoring of pediatric patients on ECMO.
DATA EXTRACTION:
Two authors reviewed all citations independently, with a third independent reviewer resolving any conflicts. Evidence tables were constructed using a standardized data extraction form.
DATA SYNTHESIS:
Risk of bias was assessed using the Quality in Prognosis Studies tool or the revised Cochrane risk of bias for randomized trials, as appropriate and the evidence was evaluated using the Grading of Recommendations Assessment, Development and Evaluation system. Forty-eight experts met over 2 years to develop evidence-based recommendations and, when evidence was lacking, expert-based consensus statements for clinical recommendations focused on anticoagulation monitoring and targets, using a web-based modified Delphi process to build consensus (defined as > 80% agreement). One weak recommendation, two consensus statements, and three good practice statements were developed and, in all, agreement greater than 80% was reached. We also derived some resources for anticoagulation monitoring for ECMO clinician use at the bedside.
CONCLUSIONS:
There is insufficient evidence to formulate optimal anticoagulation monitoring during pediatric ECMO, but we propose one recommendation, two consensus and three good practice statements. Overall, the available pediatric evidence is poor and significant gaps exist in the literature.
Keywords: anticoagulant, extracorporeal membrane oxygenation, hematologic assays, pediatrics
During extracorporeal membrane oxygenation (ECMO), blood is in contact with the foreign surface of the ECMO circuit leading to activation of hemostasis, which can lead to patient thrombosis and/or ECMO circuit thrombosis and failure. Systemic anticoagulation mitigates this response but may contribute to life-threatening bleeding in the patient, especially when combined with the alterations in the hemostatic system associated with critical illness, platelet and clotting factor consumption due to the ECMO circuit and/or underlying critical illness, and age-related differences in hemostatic regulation across the pediatric age range (1–3). Because of the risk of bleeding, it is important to closely monitor systemic anticoagulation while on ECMO. However, small numbers of patients and variability in both clinical pathology and physical circuit component combinations make it difficult to design studies to determine best monitoring practices, and practice variation is high (4, 5). The objective of this subgroup of the Pediatric ECMO Anticoagulation CollaborativE (PEACE) was to derive systematic-review informed, modified Delphi consensus recommendations on anticoagulation monitoring in pediatric ECMO patients.
MATERIALS AND METHODS
Detailed methods and definitions of clinically relevant bleeding are described in the PEACE executive summary (6). Briefly, a structured literature search was performed using PubMed, EMBASE, and Cochrane Library (CENTRAL) databases from January 1988 to May 2020, with an update in May 2021, using a combination of medical subject heading terms and text words to evaluate anticoagulation monitoring (Supplemental Methods 1, http://links.lww.com/PCC/C496). Two authors reviewed all citations independently, with a third independent reviewer resolving any conflicts. Evidence tables were constructed using a standardized data extraction form (6). Risk of bias was assessed using the Quality in Prognosis Studies (QUIPS) tool or the revised Cochrane risk of bias for randomized trials, as appropriate (7–9), and the evidence was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (10, 11). A panel of 48 experts met over the course of 2 years to develop evidence-based recommendations and, when evidence was lacking, expert-based consensus statements or good practice statements for anticoagulation monitoring. The supporting literature was reviewed, and statements were developed using the Evidence to Decision framework, emphasizing the panel’s assessment of risks versus benefits of each proposed statement and a prioritized list of patient outcomes that had been created by a web-based survey of expert panel members (12–14). A web-based modified Delphi process was used to build consensus via the Research and Development/University of California Appropriateness Method. Consensus was defined as greater than 80% agreement. Additional references, not included in the structured literature search, were included in rationale statements to provide context but were not used to derive recommendations, consensus or good practice statements.
RESULTS
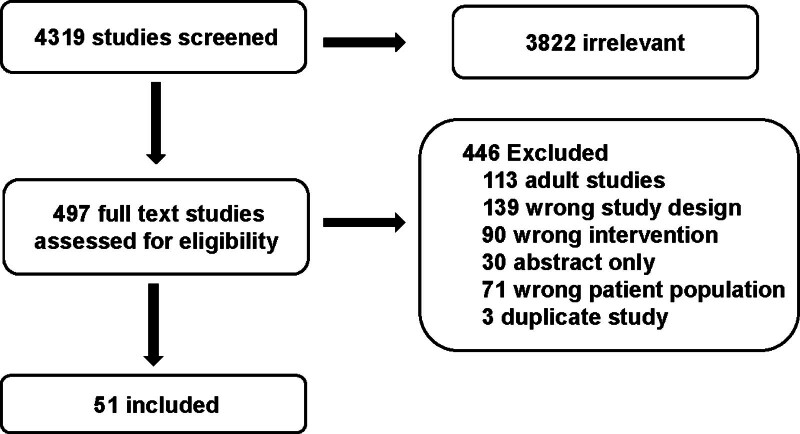
The structured literature search identified 4319 abstracts. Of these, 3822 references were excluded based on the abstract. An additional 446 references were excluded based on full article review, leaving 51 references that were used for consensus statement creation (Fig. 1). The included references are detailed in Supplemental Table 1 (http://links.lww.com/PCC/C496). A summary of risk of bias assessments are presented in Supplemental Figure 1 (http://links.lww.com/PCC/C496). One weak recommendation, two consensus statements, and three good practice statements met preset criteria for agreement (i.e., > 80%) were developed and are presented here.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram of studies screened and included in the anticoagulation monitoring subgroup.
Assays to Monitor UFH Anticoagulation
Clinical Recommendation
3.1 When monitoring unfractionated heparin-based anticoagulation, it is reasonable to consider a combination of anticoagulation monitoring assays including one or more “time to clot” assays (activated clotting time [ACT], activated partial thromboplastin time [aPTT], and/or viscoelastic tests) in combination with anti-factor Xa (anti-Xa) assay, where available. Consensus panel expertise with weak agreement, 89% agreement (n = 44), median 8, interquartile range [IQR] 7–9.
Good Practice Statements.
3.2 A thorough understanding of anticoagulation assays is necessary for management of ECMO and includes: 1) Obtaining manufacturer package insert information, 2) using institutional experts in hemostasis to educate ECMO clinicians. 98% agreement (n = 44), median 8, IQR 7–9.
3.3 Use a multidisciplinary approach, which may include input from critical care, surgery, transfusion medicine, hematology and pharmacy, to develop an institutional anticoagulation protocol; also consider consulting these experts in ECMO hemostasis in cases not easily managed with the institutional protocol. 82% agreement (n = 44), median 8, IQR 7–9.
3.4 Investigate promptly any discrepancies in results of anticoagulation assays in ECMO to identify underlying causes for the discrepancy. 85% agreement (n = 46), median 9, IQR 7–9.
Summary of the evidence:
Heparin monitoring assays include measurements of time to conversion of fibrin to fibrinogen (colloquially “time to clot assays”) such as the ACT, the aPTT, thromboelastogram, rotational thromboelastometry (ROTEM), and the anti-Xa assay, a measure of heparin dose efficacy on exogenous factor Xa (15). aPTT has been considered more predictive of coagulation status than ACT both within and between neonatal ECMO patients (16). aPTT monitoring of heparin therapy for ECMO patients has been shown to be associated with decreased hemorrhagic complications when compared with ACT (17). Of note, however, there are many different aPTT reagents available, each with different sensitivities to age-related hemostatic changes, different linearity, and response to heparin dosing. ECMO clinicians need to understand the properties of their local laboratory test to interpret and manage patient care.
Assessment of coagulation status by viscoelastic assays such as thromboelastogram or ROTEM has become more common (5, 18). In a retrospective comparison of neonatal ECMO patients who experienced hemorrhage (> 2 mL/kg/ECMO hr) to those who did not, significant differences between thromboelastogram results were noted over time (19). Another study noted a reduction in hemothoraces in neonatal congenital diaphragmatic hernia patients on ECMO when thromboelastogram monitoring was added to their anticoagulation management algorithm (20). Although these studies suggest the assays may be useful decision support for prediction and choice of therapies for bleeding and clotting, other studies of neonatal ECMO patients have been less convincing (21). Although some studies have reported normative thromboelastogram and ROTEM data for well children, there is insufficient information to standardize the measurements of heparin effect in children or the critically ill (22). Indeed, in a retrospective single center study of children on ECMO, the proportion of variation in coagulation test results explained by heparin dose was 13.3% for anti-Xa, 11.9% for ratio thromboelastogram R time, and 9.9% for delta thromboelastogram R time, compared with less than 1% for ACT and aPTT (23).
Several studies have found poor correlation between ACT and aPTT and the anti-Xa assay in ECMO patients likely due to ongoing factor and platelet consumption (18, 24, 25). The anti-Xa assay more closely correlates to heparin dosing than the time to clot assays (26, 27). Studies comparing pediatric ECMO patients managed with anti-Xa monitoring strategy with prior practice noted fewer heparin boluses, less frequent blood draws and changes in heparin infusion dosing, fewer circuit changes and hemorrhagic complications, and improved survival (28, 29). Unfortunately, studies are confounded by technology changes in addition to changes in anticoagulation monitoring strategy and management. One small study appeared to suggest that anti-Xa was more closely associated with circuit thrombotic complications, with decreased anti-Xa measurements associated with increased odds of subsequent circuit change, while there was no difference in ACT measurement between the groups (30).
There are several studies that support using multiple anticoagulation monitoring assays in combination (31–35). These approaches have been associated with decreased blood product transfusion, decreased bleeding complications, and increased circuit duration (34, 35). Frequency of anticoagulation monitoring was assessed in an observational cohort study which compared anticoagulation monitoring using a “simple” strategy (every 2-hr ACT measurement; daily anti-Xa and aPTT) at one center versus an “intensive” strategy (every 2 hr ACT; every 12 hr aPTT, international normalized ratio [INR], anti-Xa, and daily antithrombin) at another center and found no difference in outcomes including bleeding and thrombotic complications and survival (36). There is, additionally, no evidence that pediatric ECMO patients anticoagulated using a lower compared with higher anticoagulation targets have improved survival or decreased bleeding and thrombotic events. In one study of 604 patients supported with venoarterial ECMO for cardiac and respiratory indications, higher heparin dose, but not ACT level, was associated with improved survival (37). Finally, there is no current evidence to support that time to target therapeutic anticoagulation test or longer duration in the therapeutic range is associated with improved survival or lower bleeding and thrombotic events in children supported with ECMO (38, 39). In one study, inability to achieve anticoagulation target and frequent heparin dose changes were associated with increased intracranial events (40). Finally, several studies have concluded that current laboratory assays may not be sufficient to predict bleeding and clotting complications in pediatric patients managed with ECMO, despite different combinations of assays (41, 42).
Balance of benefits versus harms:
We suggest that a “time to clot” assay (ACT, aPTT, prothrombin time [PT]/INR, and/or thromboelastogram/ROTEM) is used to assess patient coagulation status in combination with the anti-Xa assay to assess heparin efficacy. Additional assays that may be helpful include platelet and fibrinogen levels, platelet function assay, and antithrombin activity assay. Anticoagulation targets should be adjusted considering patient and circuit related factors and perceived risk of bleeding versus clotting. Table 1 includes the characteristics of commonly used anticoagulation monitoring assays and Table 2 outlines factors associated with variability in results. A suggested approach to interpreting discrepancies in unfractionated heparin (UFH) monitoring assays (i.e., scenarios in which simultaneous results of different assays would direct different clinician actions) is in Supplemental Table 2 (http://links.lww.com/PCC/C496).
TABLE 1.
Commonly Used Anticoagulation Monitoring Assays for Unfractionated Heparin and Bivalirudin
| Test | Activated Clotting Time | Activated Partial Thromboplastin Time | Anti-Factor Xaa | Viscoelastic Testing (Thromboelastogram and ROTEM) | Dilute Thrombin Time | Prothrombin Time/International Normalized Ratiob |
|---|---|---|---|---|---|---|
| Agent monitored | Any | Unfractionated heparin and bivalirudin | Unfractionated heparin | Any | Unfractionated heparin and bivalirudin | Bivalirudin |
| Sample | Whole blood | Citrated plasma | Citrated plasma | Whole blood | Citrated plasma | Citrated plasma |
| Measures | Time to clot | Time to clot | Chromogenic | Time to clot | Time to clot | Time to clot |
| Clot detection method | Optical or electromechanical | Optical or electromechanical | Not applicable | Electromechanical | Optical or electromechanical | Optical or electromechanical |
| Testing location | POC | POC and laboratory | Laboratory | POC and laboratory | Laboratory | Laboratory |
| Therapeutic range | Varies by analyzer | Varies by analzer and reagent but should correlate to anti-factor Xa of 0.35–0.7 or protamine titration of 0.2–0.4 U/mL for heparin. Usually 60–90 s for bivalirudin | 0.35–0.7 U/mLa | Varies by thromboelastogram/ROTEM | Varies by analyzer but 60–90 s commonly used | Varies by indication for treatment |
| Therapeutic ranges validation | Cardiopulmonary bypass | Treatment of venous thrombosis | Treatment of venous thrombosis | Not validated | Not validated | Not validated |
| Established pediatric ranges | No | No | No | No | No | No |
POC = point of care, ROTEM = rotational thromboelastometry.
Multiple assay configurations (exogenous antithrombin/dextran sulphate [DS] stabilizer/neither antithrombin nor DS) will all lead to markedly different results on same sample.
International normalized ratio traditionally used to monitor vitamin K antagonists, however, this table only refers to its role in the monitoring of bivalirudin.
“Time to clot” assays measure formation of a fibrin “clot” as determined by a threshold mass of fibrin.
TABLE 2.
Commonly Used Anticoagulation Monitoring Assays and Factors Associated With Variability in Results
| Influences on Coagulation Monitoring | Activated Clotting Time | Anti-Factor Xaa | aPTT When monitoring UFH | aPTT When Monitoring Bivalirudin | Thromboelastogram R-time or ROTEM INTEM Clotting Time | Thromboelastogram Maximum Amplitude or ROTEM INTEM Maximum Clot Firmness | Prothrombin Time/International Normalized Ratio When Monitoring Bivalirudin | Dilute Thrombin Time When Monitoring Bivalirudin |
|---|---|---|---|---|---|---|---|---|
| Sample factors | ||||||||
| Heparin contamination | ↑ | ↑ | ↑ | ↑ | ↑ | ↔/↓ | ↔/↑ | ↑ |
| Dilution | ↑ | ↓ | ↑ | ↑ | ↑ | ↔/↓ | ↑ | ↑ |
| Under/over-filled sample tubes | Sample must be redrawn because results will be uninterpretable | |||||||
| Delay in sample analysis (beyond 2 hr) | Sample must be redrawn because results will be uninterpretable (optimal time to sampling for bivalirudin is unknown. Some current protocols restrict to 1 hr) | |||||||
| Gross hemolysis due to sampling | Sample must be redrawn because results will be uninterpretable | |||||||
| Patient factors | ||||||||
| Hypothermia | ↑ | ↔a | ↔a | ↔a | ↔a | ↔a | ↔a | ↔a |
| Elevated levels of inflammatory markers including: fibrinogen and factor VIII | ↓ | ↔ | ↓ | ↓ | ↓ | ↔/↑ | ↔ | ↓ |
| Increased heparin binding proteins in the presence of systemic inflammation, infection, malignancies | ↓ | ↓ | ↓ | ↔ | ↓ | ↔/↑ | ↔ | ↔ |
| Thrombocytopenia (generally < 50 × 109) | ↑ | ↔ | ↔ | ↔ | ↔/↑ | ↓ | ↔ | ↔ |
| Antithrombin deficiency | ↓ | ↓b | ↓ | ↓ | ↔ | |||
| Consumptive coagulopathy (i.e., disseminated intravascular coagulation) | ↑ | ↔/↓b | ↑ | ↑ | ↓ | |||
| Hepatic dysfunction (decreased coagulation factor production) | ↑ | ↔/↓b | ↑ | ↑ | ↓ | |||
| Procoagulant factor deficiency(s) | ↑ | ↔ | ↑ | ↑ | ↑ | ↔/↓ | ↑ | ↔ |
| Presence of a lupus anticoagulant | ↑ | ↔ | ↑ | ↑ | ↑ | ↔ | ↔ | ↔ |
| Assay factors | ||||||||
| Elevated triglyceride level | ↔ | ↓c | ↔/↑c | ↔/↑c | ↔ | ↔ | ↔/↑c | ↔ |
| Elevated total bilirubin level | ↔ | ↓c | ↔/↑c | ↔/↑c | ↔ | ↔ | ↔/↑c | ↔ |
| Elevated plasma-free hemoglobin | ↔ | ↓c | ↔/↑c | ↔/↑c | ↔ | ↔ | ↔/↑c | ↔ |
| Drug factors | ||||||||
| Loss of linearity outside specific dose ranges | Low dose | N/A | Very high dose | High dose | Low and high dose | N/A | Therapeutic range | N/A |
| Impaired renal function (decreased UFH or bivalirudin elimination) | ↑ | ↑ | ↑ | ↑ | ↑ | ↔/↓ | ↑ | ↑ |
aPTT = activated partial thromboplastin time, INTEM = intrinsically activated thromboelastometry, N/A = not applicable, ROTEM = rotational thromboelastometry, UFH = unfractionated heparin.
↑ = increase in laboratory result, ↓ = decrease in laboratory result, ↔ = no change in laboratory result status.
Hypothermia does not alter results because test is performed at standard 37°C. Whether test result accurately reflects biological activity inpatient is unknown.
For anti-factor Xa assay without added exogenous antithrombin.
There is a threshold above which triglycerides, bilirubin, or hemoglobin will interfere with optical detection systems that is specific for each analyzer. See manufacturers notes for details.
Interference in Anticoagulation Monitoring Assays
Consensus Statement
3.5 In each center, we consider that ECMO clinicians and their laboratory define thresholds of bilirubin, plasma free hemoglobin and triglycerides above which chromogenic or optical clot detection-based anticoagulation monitoring assays should be considered unreliable. Consensus panel expertise with strong agreement, 98% agreement (n = 43), median 8, IQR 7–9.
Summary of the evidence:
Tests to assess coagulation may be affected by patient factors and specific aspects of laboratory tests used. Thus, the provider titrating anticoagulation must understand intrinsic test result variance and external factors that affect test results. We collated characteristics of commonly used anticoagulation monitoring assays and factors associated with variability in results (Tables 1 and 2).
Hemolysis and hyperbilirubinemia, commonly seen in ECMO patients may affect the results of the anti-Xa assay. Elevated levels of substances such as plasma-free hemoglobin and bilirubin may lead to an underestimation of anti-Xa activity. Optical assays of anti-Xa, aPTT, and PT may variably be impacted (43, 44). Thus, heparin titration in the presence of elevated plasma-free hemoglobin and serum bilirubin may result in erroneous evaluation of anticoagulation status and may lead increased risk of bleeding or clotting.
Monitoring Anticoagulation With Direct Thrombin Inhibitors
Clinical Recommendation
3.6 There is insufficient evidence to recommend a specific assay or therapeutic range for monitoring direct thrombin inhibitors (DTIs) in pediatric ECMO. Recommendation (no level), very low-quality pediatric evidence, 83% agreement (n = 46), median 7, IQR 7–9.
Summary of the evidence.
Analytic response of monitoring assays for direct thrombin inhibitors (DTIs):
The analytic response of aPTT, plasma dilute thrombin time, ecarin clotting time, and PT/INR hemostasis assays have been evaluated for use in monitoring anticoagulation with bivalirudin. aPTT is the most commonly reported assay used to monitor bivalirudin during ECMO (45–50) and shows a curvilinear increase in clotting time as a function of bivalirudin concentration (51). Because the aPTT cannot be diluted, it can be difficult to assess the bivalirudin when levels are supratherapeutic. The aPTT is prolonged by low coagulation factor levels, sample contamination with heparin, and lupus inhibitors, which are common in hospitalized patients (33, 43, 52, 53) and does not correlate well with other tests used to monitor direct thrombin inhibitors (DTIs) or with DTI levels in many patient (51, 54–58).
Two different approaches to dilution of the thrombin time assay have been reported, including either dilution of the patient plasma sample (diluted thrombin time) (59) or the use of a standardized lower thrombin dose (i.e., 5 National Institutes of Health units in the dilute thrombin time) (55, 56). Both modified thrombin times have resulted in improved sensitivity in DTI monitoring. No studies have currently reported use of the plasma dilute thrombin time to monitor bivalirudin during pediatric ECMO, but its improved analytic response and reduced interference warrant further study.
The ecarin clotting time is a highly specific test for DTIs that correlates well with plasma diluted thrombin times (51, 54, 55). Its response is almost linear with DTI level and can be calibrated to report the level of drug (54). It has good sensitivity and specificity for DTIs, but availability is limited.
The PT/INR is also prolonged by DTIs, correlation between PT and DTI levels is similar to aPTT and ACT (54). Prolongation of the PT was weak compared with other assays, it was difficult to distinguish therapeutic doses of bivalirudin from over or under dosage (60). Further studies of PT/INR as a bivalirudin monitoring tool would be needed due to the small size of current studies.
Clinical monitoring of DTIs during ECMO:
Only limited data are available on the use of DTIs, mainly bivalirudin and to a lesser extent, argatroban, during ECMO (45–50). No validated therapeutic ranges are available. Four studies reported use of bivalirudin in pediatric patients on ECMO using aPTT monitoring (45, 47, 48, 50).
Recent studies evaluating aPTT to monitor bivalirudin have reported similar rates of thrombosis, bleeding, transfusion and mortality between pediatric ECMO patients anticoagulated with bivalirudin and heparin, while other studies have reported decreased circuit interventions and decreased transfusion, time to reach therapeutic targets and cost using bivalirudin versus heparin (45, 47, 48, 50). None of these studies were randomized trials.
Balance of benefits versus harms:
At this time, there is insufficient evidence to recommend a specific assay or therapeutic range for monitoring DTIs used in pediatric patients supported with ECMO. Multicenter quality improvement bundles and clinical studies using DTIs to prevent thrombosis in pediatric patients with ventricular assist devices typically use the aPTT to monitor DTIs, but there is no evidence that the aPTT is the optimal test or that the target range being used is the optimal therapeutic range during ECMO support (50). Adjunctive markers of optimal therapeutic effect for DTIs such as ecarin clotting time and dilute thrombin time warrant further investigation.
CONCLUSIONS
Data to guide frequency of monitoring and use of specific anticoagulation assay(s) during anticoagulation with UFH or DTIs in pediatric ECMO are scarce and, at times, contradictory. We suggest that an appropriate panel of assays combined with interpretation by experts in anticoagulation, ECMO, and pediatric intensive care medicine, may result in superior bleeding and clotting outcomes; however, research in this area is desperately needed. It is crucial for clinicians caring for ECMO patients to have a thorough understanding of the anticoagulation monitoring assays used in their institutions to effectively interpret assay results.
ACKNOWLEDGMENTS
The authors thank all members of Pediatric Extracorporeal Membrane Oxygenation (ECMO) Anticoagulation CollaborativE (PEACE) for their support, especially during the COVID-19 pandemic. The authors acknowledge the important contributions of Dr. M. Patricia Massicotte to the design and execution of the PEACE project. In addition, they thank AABB, the American Society of Extracorporeal Therapists, the American Pediatric Surgical Association, the Children’s Hospital Neonatal Consortium, the Collaborative Pediatric Critical Care Research Network, the European Society for Pediatric and Neonatal Intensive Care, the International Society of Blood Transfusion, Pediatric Cardiac Critical Care Consortium, Pediatric Cardiac Intensive Care Society, the Society for Critical Care Medicine (Pediatric Section and Clinical Pharmacy and Pharmacology Section), and the Society of Thoracic Surgeons for contribution through representation on the PEACE Expert Panel.
Supplementary Material
Footnotes
Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE (PEACE) members are listed in Appendix 1 (http://links.lww.com/PCC/C496).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
The Executive Committee (Dr. Alexander, Dr. Muszynski, Dr. Bembea, Dr. Cheifetz, Dr. Steiner, and Dr. Barbaro) served as arbitrators for conflict-of-interest management. Dr. Alexander’s institution received funding from Novartis (Prospective Trial to Assess the Angiotensin Receptor Blocker Neprilysin Inhibitor LCZ696 Versus Angiotensin-Converting Enzyme Inhibitor for the Medical Treatment of Pediatric HF [PANORAMA-HF]). Dr. Ozment received funding from Kaufman & Canoles Law Firm, Social Cascade, and Wiseman Ashworth Law Group. Drs. Ozment and Monagle disclosed the off-label product use of anticoagulants (heparin and bivalirudin) in neonatal and pediatric Extracorporeal Membrane Oxygenation (ECMO) patients. Drs. Alexander and Muszynski’s institutions received funding from the National Institutes of Health (NIH). Dr. Alexander’s institution received funding from the Extracorporeal Life Support Organization (ELSO) and Novartis. Drs. Alexander and Muszynski received support for article research from the NIH. Dr. Emani’s institution received funding from Cellvie Bio; he received funding from Cheisi Pharma. Dr. Hyslop disclosed that on a volunteer basis he is the Co-Chair of ELSO Registry Database Development Committee and Coordinator Liaison to ELSO Steering Committee. Dr. Alexander and Thiagarajan’s institution received funding from the US Department of Defense Clinical Trials Award, Trial of Indication-Based Transfusion of RBCs in ECMO trial (W81XWH2210301). Dr. Thiagarajan received funding from ELSO and the Society of Critical Care Medicine. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Contributor Information
Collaborators: Peta M.A. Alexander, Melania M. Bembea, Katherine Cashen, Ira M. Cheifetz, Heidi J. Dalton, Adam S. Himebauch, Oliver Karam, Katie M. Moynihan, Marianne E. Nellis, Caroline Ozment, Lakshmi Raman, Natalie E. Rintoul, Ahmed S. Said, Arun Saini, Marie E. Steiner, Ravi R. Thiagarajan, Kevin Watt, Ariane Willems, Nicole D. Zantek, Ryan P. Barbaro, Katherine Steffen, Adam M. Vogel, Christopher Almond, Marc M. Anders, Gail M. Annich, Leonardo R. Brandão, Wayne Chandler, Megan Delaney, Robert DiGeronimo, Sitaram Emani, Samir K. Gadepalli, Alejandro V. Garcia, Bereketeab Haileselassie, Adam S. Himebauch, Robert Hyslop, Martin C. J. Kneyber, Lisa Baumann Kreuziger, Jennifer Le, Laura Loftis, Ali B.V. McMichael, D. Michael McMullan, Paul Monagle, Kathleen Nicol, Matthew L. Paden, Jason Patregnani, John R. Priest, Leslie Raffini, Lindsay M. Ryerson, Steven R. Sloan, Jun Teruya, Andrew R. Yates, Alison Gehred, Elizabeth Lyman, MLIS, and Jennifer A. Muszynski
REFERENCES
- 1.O’Halloran CP, Andren KG, Mecklosky J, et al. : Mortality and factors associated with hemorrhage during pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2020; 21:75–81 [DOI] [PubMed] [Google Scholar]
- 2.Muszynski JA, Reeder RW, Hall MW, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network (CPCCRN): RBC transfusion practice in pediatric extracorporeal membrane oxygenation support. Crit Care Med. 2018; 46:e552–e559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalton HJ, Reeder R, Garcia-Filion P, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network: Factors associated with bleeding and thrombosis in children receiving extracorporeal membrane oxygenation. Am J Respir Crit Care Med. 2017; 196:762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bembea MM, Hoskote A, Guerguerian AM: Pediatric ECMO research: The case for collaboration. Front Pediatr. 2018; 6:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozment CP, Scott BL, Bembea MM, et al. : Anticoagulation and transfusion management during neonatal and pediatric extracorporeal membrane oxygenation: A survey of medical directors in the United States. Pediatr Crit Care Med. 2021; 22:530–541 [DOI] [PubMed] [Google Scholar]
- 6.Alexander PMA, Bembea M, Cashen K, et al. ; Pediatric Extracorporeal Membrane Oxygenation (ECMO) Anticoagulation CollaborativE (PEACE), in collaboration with the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network, the Pediatric Critical Care Blood Research Network (BloodNet), and the Pediatric ECMO subgroup of PALISI and the Extracorporeal Life Support Organization (PediECMO): Executive summary: The Pediatric Extracorporeal Membrane Oxygenation Anticoagulation CollaborativE Consensus Conference. Pediatr Crit Care Med. 2024; 25:643-675 [Google Scholar]
- 7.Sterne JAC, Savovic J, Page MJ, et al. : RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366:l4898. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JPT, Sterne JAC, Savović J, et al. : A revised tool for assessing risk of bias in randomized trials. In: Cochrane Methods. Chandler J, McKenzie J, Boutron I, et al. (Eds). Cochrane Database of Systematic Reviews 2016; 10 (Suppl 1) [Google Scholar]
- 9.Hayden JA, van der Windt DA, Cartwright JL, et al. : Assessing bias in studies of prognostic factors. Ann Intern Med. 2013; 158:280–286 [DOI] [PubMed] [Google Scholar]
- 10.Balshem H, Helfand M, Schunemann HJ, et al. : GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011; 64:401–406 [DOI] [PubMed] [Google Scholar]
- 11.Neumann I, Santesso N, Akl EA, et al. : A guide for health professionals to interpret and use recommendations in guidelines developed with the GRADE approach. J Clin Epidemiol. 2016; 72:45–55 [DOI] [PubMed] [Google Scholar]
- 12.Alonso-Coello P, Oxman AD, Moberg J, et al. ; GRADE Working Group: GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016; 353:i2089. [DOI] [PubMed] [Google Scholar]
- 13.Neumann I, Brignardello-Petersen R, Wiercioch W, et al. : The GRADE evidence-to-decision framework: A report of its testing and application in 15 international guideline panels. Implement Sci. 2016; 11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alonso-Coello P, Schunemann HJ, Moberg J, et al. ; GRADE Working Group: GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016; 353:i2016. [DOI] [PubMed] [Google Scholar]
- 15.Parker RI: Anticoagulation in extracorporeal membrane oxygenation: A dilemma wrapped in an enigma. Pediatr Crit Care Med. 2021; 22:584–588 [DOI] [PubMed] [Google Scholar]
- 16.Sulkowski JP, Preston TJ, Cooper JN, et al. : Comparison of routine laboratory measures of heparin anticoagulation for neonates on extracorporeal membrane oxygenation. J Extra Corpor Technol. 2014; 46:69–76 [PMC free article] [PubMed] [Google Scholar]
- 17.Maul TM, Wolff EL, Kuch BA, et al. : Activated partial thromboplastin time is a better trending tool in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2012; 13:e363–e371 [DOI] [PubMed] [Google Scholar]
- 18.Bembea MM, Schwartz JM, Shah N, et al. : Anticoagulation monitoring during pediatric extracorporeal membrane oxygenation. ASAIO J. 2013; 59:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zavadil DP, Stammers AH, Willett LD, et al. : Hematological abnormalities in neonatal patients treated with extracorporeal membrane oxygenation (ECMO). J Extra Corpor Technol. 1998; 30:83–90 [PubMed] [Google Scholar]
- 20.Phillips RC, Shahi N, Leopold D, et al. : Thromboelastography-guided management of coagulopathy in neonates with congenital diaphragmatic hernia supported by extracorporeal membrane oxygenation. Pediatr Surg Int. 2020; 36:1027–1033 [DOI] [PubMed] [Google Scholar]
- 21.Stammers AH, Willett L, Fristoe L, et al. : Coagulation monitoring during extracorporeal membrane oxygenation: The role of thrombelastography. J Extra Corpor Technol. 1995; 27:137–145 [PubMed] [Google Scholar]
- 22.Moynihan KM, Johnson K, Rane M, et al. : Pediatric thromboelastograph 6s and laboratory coagulation reference values. Arch Pathol Lab Med. 2021; 145:1413–1423 [DOI] [PubMed] [Google Scholar]
- 23.Moynihan K, Johnson K, Straney L, et al. : Coagulation monitoring correlation with heparin dose in pediatric extracorporeal life support. Perfusion. 2017; 32:675–685 [DOI] [PubMed] [Google Scholar]
- 24.McMichael ABV, Hornik CP, Hupp SR, et al. : Correlation among antifactor Xa, activated partial thromboplastin time, and heparin dose and association with pediatric extracorporeal membrane oxygenation complications. ASAIO J. 2020; 66:307–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khaja WA, Bilen O, Lukner RB, et al. : Evaluation of heparin assay for coagulation management in newborns undergoing ECMO. Am J Clin Pathol. 2010; 134:950–954 [DOI] [PubMed] [Google Scholar]
- 26.Liveris A, Bello RA, Friedmann P, et al. : Anti-factor Xa assay is a superior correlate of heparin dose than activated partial thromboplastin time or activated clotting time in pediatric extracorporeal membrane oxygenation*. Pediatr Crit Care Med. 2014; 15:e72–e79 [DOI] [PubMed] [Google Scholar]
- 27.Nankervis CA, Preston TJ, Dysart KC, et al. : Assessing heparin dosing in neonates on venoarterial extracorporeal membrane oxygenation. ASAIO J. 2007; 53:111–114 [DOI] [PubMed] [Google Scholar]
- 28.Niebler RA, Parker H, Hoffman GM: Impact of anticoagulation and circuit technology on complications during extracorporeal membrane oxygenation. ASAIO J. 2019; 65:270–276 [DOI] [PubMed] [Google Scholar]
- 29.O’Meara LC, Alten JA, Goldberg KG, et al. : Anti-Xa directed protocol for anticoagulation management in children supported with extracorporeal membrane oxygenation. ASAIO J. 2015; 61:339–344 [DOI] [PubMed] [Google Scholar]
- 30.Irby K, Swearingen C, Byrnes J, et al. : Unfractionated heparin activity measured by anti-factor Xa levels is associated with the need for extracorporeal membrane oxygenation circuit/membrane oxygenator change: A retrospective pediatric study. Pediatr Crit Care Med. 2014; 15:e175–e182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kessel AD, Kline M, Zinger M, et al. : The impact and statistical analysis of a multifaceted anticoagulation strategy in children supported on ECMO: Performance and pitfalls. J Intensive Care Med. 2017; 32:59–67 [DOI] [PubMed] [Google Scholar]
- 32.Bingham KR, Riley JB, Schears GJ: Anticoagulation management during first five days of infant-pediatric extracorporeal life support. J Extra Corpor Technol. 2018; 50:30–37 [PMC free article] [PubMed] [Google Scholar]
- 33.Saifee NH, Brogan TV, McMullan DM, et al. : Monitoring hemostasis during extracorporeal life support. ASAIO J. 2020; 66:230–237 [DOI] [PubMed] [Google Scholar]
- 34.Northrop MS, Sidonio RF, Phillips SE, et al. : The use of an extracorporeal membrane oxygenation anticoagulation laboratory protocol is associated with decreased blood product use, decreased hemorrhagic complications, and increased circuit life. Pediatr Crit Care Med. 2015; 16:66–74 [DOI] [PubMed] [Google Scholar]
- 35.Henderson N, Sullivan JE, Myers J, et al. : Use of thromboelastography to predict thrombotic complications in pediatric and neonatal extracorporeal membranous oxygenation. J Extra Corpor Technol. 2018; 50:149–154 [PMC free article] [PubMed] [Google Scholar]
- 36.Yu JS, Barbaro RP, Granoski DA, et al. : Prospective side by side comparison of outcomes and complications with a simple versus intensive anticoagulation monitoring strategy in pediatric extracorporeal life support patients. Pediatr Crit Care Med. 2017; 18:1055–1062 [DOI] [PubMed] [Google Scholar]
- 37.Baird CW, Zurakowski D, Robinson B, et al. : Anticoagulation and pediatric extracorporeal membrane oxygenation: Impact of activated clotting time and heparin dose on survival. Ann Thorac Surg. 2007; 83:912–9; discussion 919 [DOI] [PubMed] [Google Scholar]
- 38.Ankola AA, Bailly DK, Reeder RW, et al. : Risk factors associated with bleeding in children with cardiac disease receiving extracorporeal membrane oxygenation: A multi-center data linkage analysis. Front Cardiovasc Med. 2021; 8:812881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailly DK, Reeder RW, Muszynski JA, et al. : Anticoagulation practices associated with bleeding and thrombosis in pediatric extracorporeal membrane oxygenation: A multi-center secondary analysis. Perfusion. 2023; 38:363–372 [DOI] [PubMed] [Google Scholar]
- 40.Hirthler MA, Blackwell E, Abbe D, et al. : Coagulation parameter instability as an early predictor of intracranial hemorrhage during extracorporeal membrane oxygenation. J Pediatr Surg. 1992; 27:40–43 [DOI] [PubMed] [Google Scholar]
- 41.Reed RC, Rutledge JC: Laboratory and clinical predictors of thrombosis and hemorrhage in 29 pediatric extracorporeal membrane oxygenation nonsurvivors. Pediatr Dev Pathol. 2010; 13:385–392 [DOI] [PubMed] [Google Scholar]
- 42.Anton-Martin P, Journeycake J, Modem V, et al. : Coagulation profile is not a predictor of acute cerebrovascular events in pediatric extracorporeal membrane oxygenation patients. ASAIO J. 2017; 63:793–801 [DOI] [PubMed] [Google Scholar]
- 43.Khan J, Chandler WL: Discordant partial thromboplastin time (PTT) vs anti-Xa heparin activity. Am J Clin Pathol. 2019; 151:424–432 [DOI] [PubMed] [Google Scholar]
- 44.Kostousov V, Nguyen K, Hundalani SG, et al. : The influence of free hemoglobin and bilirubin on heparin monitoring by activated partial thromboplastin time and anti-Xa assay. Arch Pathol Lab Med. 2014; 138:1503–1506 [DOI] [PubMed] [Google Scholar]
- 45.Ryerson LM, Balutis KR, Granoski DA, et al. : Prospective exploratory experience with bivalirudin anticoagulation in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2020; 21:975–985 [DOI] [PubMed] [Google Scholar]
- 46.Machado DS, Garvan C, Philip J, et al. : Bivalirudin may reduce the need for red blood cell transfusion in pediatric cardiac patients on extracorporeal membrane oxygenation. ASAIO J. 2021; 67:688–696 [DOI] [PubMed] [Google Scholar]
- 47.Hamzah M, Jarden AM, Ezetendu C, et al. : Evaluation of bivalirudin as an alternative to heparin for systemic anticoagulation in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2020; 21:827–834 [DOI] [PubMed] [Google Scholar]
- 48.Schill MR, Douds MT, Burns EL, et al. : Is anticoagulation with bivalirudin comparable to heparin for pediatric extracorporeal life support? Results from a high-volume center. Artif Organs. 2021; 45:15–21 [DOI] [PubMed] [Google Scholar]
- 49.Nagle EL, Dager WE, Duby JJ, et al. : Bivalirudin in pediatric patients maintained on extracorporeal life support. Pediatr Crit Care Med. 2013; 14:e182–e188 [DOI] [PubMed] [Google Scholar]
- 50.Campbell CT, Diaz L, Kelly B: Description of bivalirudin use for anticoagulation in pediatric patients on mechanical circulatory support. Ann Pharmacother. 2021; 55:59–64 [DOI] [PubMed] [Google Scholar]
- 51.Hafner G, Roser M, Nauck M: Methods for the monitoring of direct thrombin inhibitors. Semin Thromb Hemost. 2002; 28:425–430 [DOI] [PubMed] [Google Scholar]
- 52.Smythe MA, Forsyth LL, Warkentin TE, et al. : Progressive, fatal thrombosis associated with heparin-induced thrombocytopenia after cardiac surgery despite “therapeutic” anticoagulation with argatroban: Potential role for PTT and ACT confounding. J Cardiothorac Vasc Anesth. 2015; 29:1319–1321 [DOI] [PubMed] [Google Scholar]
- 53.Wenzel C, Stoiser B, Locker GJ, et al. : Frequent development of lupus anticoagulants in critically ill patients treated under intensive care conditions. Crit Care Med. 2002; 30:763–770 [DOI] [PubMed] [Google Scholar]
- 54.Seidel H, Kolde HJ: Monitoring of argatroban and lepirudin: What is the input of laboratory values in “real life?”. Clin Appl Thromb Hemost. 2018; 24:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lind SE, Boyle ME, Fisher S, et al. : Comparison of the aPTT with alternative tests for monitoring direct thrombin inhibitors in patient samples. Am J Clin Pathol. 2014; 141:665–674 [DOI] [PubMed] [Google Scholar]
- 56.Guy S, Kitchen S, Maclean R, et al. : Limitation of the activated partial thromboplastin time as a monitoring method of the direct thrombin inhibitor argatroban. Int J Lab Hematol. 2015; 37:834–843 [DOI] [PubMed] [Google Scholar]
- 57.Beyer JT, Lind SE, Fisher S, et al. : Evaluation of intravenous direct thrombin inhibitor monitoring tests: Correlation with plasma concentrations and clinical outcomes in hospitalized patients. J Thromb Thrombolysis. 2020; 49:259–267 [DOI] [PubMed] [Google Scholar]
- 58.Beiderlinden M, Werner P, Bahlmann A, et al. : Monitoring of argatroban and lepirudin anticoagulation in critically ill patients by conventional laboratory parameters and rotational—a prospectively controlled randomized double-blind clinical trial. BMC Anesthesiol. 2018; 18:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Love JE, Ferrell C, Chandler WL: Monitoring direct thrombin inhibitors with a plasma diluted thrombin time. Thromb Haemost. 2007; 98:234–242 [PubMed] [Google Scholar]
- 60.Curvers J, van de Kerkhof D, Stroobants AK, et al. : Measuring direct thrombin inhibitors with routine and dedicated coagulation assays: Which assay is helpful? Am J Clin Pathol. 2012; 138:551–558 [DOI] [PubMed] [Google Scholar]