Abstract
Aquareovirus, a member of the family Reoviridae, is a large virus with multiple capsid layers surrounding a genome composed of 11 segments of double-stranded RNA. Biochemical studies have shown that treatment with the proteolytic agent trypsin significantly alters the infectivity of the virus. The most infectious stage of the virus is produced by a 5-min treatment with trypsin. However, prolonged trypsin treatment almost completely abolishes the infectivity. We have used three-dimensional electron cryomicroscopy to gain insight into the structural basis of protease-induced alterations in infectivity by examining the structural changes in the virion at various time intervals of trypsin treatment. Our data show that after 5 min of trypsinization, projection-like spikes made of VP7 (35 kDa), associated with the underlying trimeric subunits, are completely removed. Concurrent with the removal of VP7, conformational changes are observed in the trimeric subunit composed of putative VP5 (71 kDa). The removal of VP7 and the accompanied structural changes may expose regions in the putative VP5 important for cell entry processes. Prolonged trypsinization not only entirely removes the outer capsid layer, producing the poorly infectious core particle, but also causes significant conformational changes in the turret protein. These changes result in shortening of the turret and narrowing of its central channel. The turret, as in orthoreoviruses, is likely to play a major role in the capping and translocation of mRNA during transcription, and the observed conformational flexibility in the turret protein may have implications in rendering the particle transcriptionally active or inactive.
Aquareovirus, a member of the family Reoviridae, is a double-stranded RNA (dsRNA) virus. The Reoviridae includes pathogens that can infect a wide variety of organisms including vertebrates, invertebrates, and plants. Several of these viruses have large and complex icosahedral structures with diameters around 1,000 Å. Most have multiple layers of protein enclosing multiple segments of dsRNA. The inner protein layers are involved in facilitating the endogenous transcriptional activity, whereas the outer capsid layers are important for facilitating cell entry. While significant structural and biochemical information is known about several members of the family such as Rotavirus (25), Orthoreovirus (21, 26), and Orbivirus (9, 27), the Aquareovirus genus is not as well characterized. In common with rotaviruses, aquareoviruses have 11 segments of dsRNA (30). However, earlier structural studies have shown that architecturally, aquareoviruses more strongly resemble the orthoreoviruses (29).
In contrast to rotaviruses and orthoreoviruses, which infect mammals including humans, aquareoviruses cause infection in aquatic organisms like bony fish, shellfish, and crustaceans. Currently, more than 50 aquareoviruses have been isolated worldwide. These viruses have been isolated from fish with obvious diseases, such as hemorrhagic disease, hepatitis, and pancreatitis, but the majority have been isolated during routine examination of seemingly healthy fish and shellfish (18).
Biochemical studies have shown that the aquareovirions contain seven structural proteins: VP1 (∼130 kDa), VP2 (∼127 kDa), VP3 (∼126 kDa), VP4 (∼73 kDa), VP5 (∼71 kDa), VP6 (∼46 kDa), and VP7 (∼35 kDa) (30). Structural studies using electron cryomicroscopy (cryo-EM) and computer image-processing techniques of the intact virion have revealed that each particle is made of multiple capsid layers with an overall diameter of ∼800 Å (29). The structural organization of the outer capsid layers, like other members of the Reoviridae, is based on a T=13 icosahedral lattice with a left-handed skew. In aquareovirus, the lattice is an incomplete T=13, since it is punctuated at each fivefold axis by a large turret as seen in orthoreoviruses. In electron micrographs of aquareovirus particles, the outer layers are often seen clearly separated from the innermost layer, which is ∼600 Å in diameter, by an electron-lucent ring. One of the interesting differences between aquareoviruses and orthoreoviruses is that aquareovirus particles lack the slender spikes at the fivefold vertices seen in orthoreovirus particles. In orthoreoviruses, these spikes are made of ς1 protein, which mediates virus binding to the host cell receptor(s) (15). Another noticeable difference is in the innermost T=1 capsid layer. Aquareovirus particles have 120 nodules instead of 150 as seen in orthoreovirus particles.
The major focus of the present paper is to investigate trypsin-induced structural changes in aquareoviruses and to correlate them with changes in infectivity. These studies may have an in vivo relevance, since trypsin is present in the pancreatic tissues, which are susceptible to aquareovirus infection. Protease-induced enhancement of infectivity is well documented in two of the members of the Reoviridae, Rotavirus and Bluetongue virus (BTV), although the precise molecular mechanism is not known. In rotavirus, trypsin cleaves the spike protein VP4 and this cleavage increases the infectivity significantly (7). In BTV, after proteolytic treatment, the particles, called intermediate subviral particles (ISVPs), have a selective enhanced infectivity for insect cells but not for mammalian cells (20). In contrast, treatment of orthoreoviruses with proteases is not known to result in enhanced infectivity (11). In fact, ISVPs of the T3D strain are less infectious than are native virions. Although these particles lack an outer capsid protein (ς3) and have a cleaved spike protein (ς1), the loss of viral infectivity is associated with cleavage of the ς1 protein (22). Prolonged protease treatment of orthoreovirus removes the outer protein layers completely, and the resulting core particles become transcriptionally active. The complete removal of the outer protein shell and the subsequent opening of a channel at the fivefold axis support the hypothesis that mRNA molecules exit through the channels of the λ2 turrets (5).
Biochemical studies have shown that proteolytic treatment of aquareoviruses for short (5-min) periods with either trypsin or chymotrypsin serves to activate the virus and results in significantly enhanced infectivity for susceptible cells. Trypsin results in a greater increase and so has been the focus of previous studies (19) as well as this study. Prolonged periods of trypsinization, greater than 20 min, result in a level of infectivity significantly lower than that of native virions. In this study, using cryo-EM techniques, we have observed the trypsin-induced structural changes at different times and have interpreted these changes in the light of the biochemical data. Although the protease-induced structural changes in aquareoviruses are generally similar to those seen in orthoreoviruses, there are significant and intriguing differences, particularly in the turrets. We have compared and contrasted our structural results with the published data on the chymotrypsin treatment of orthoreoviruses (5).
MATERIALS AND METHODS
Native virion preparation.
The SBR strain of aquareovirus was originally obtained from diseased striped bass (Morone saxatilis) (1). The aquareovirus virions were propagated and purified in the same way as previously reported, with a particle-to-PFU ratio of 100:1 (19).
Subviral-particle preparation.
Purified native aquareovirus virions (100 μg) were treated with 10 μg of trypsin (Worthington Biochemical Corp., Lakewood, N.J.) per ml in 1 ml of phosphate-buffered saline and incubated for 5 min, 15 min, or 2 h at 37°C with gentle agitation. The incubation was terminated by placing the particles on ice. Digested proteins were then immediately separated from the particles, and the particles were concentrated by gently pelleting the core particles at 12,400 × g overnight. The pellet was resuspended in phosphate-buffered saline for the 5-min and 15-min trypsinized preparations, but the 2-h preparation was resuspended in core buffer (1 M NaCl, 100 mM MgCl2, 25 mM HEPES [pH 8.0]) (5). This buffer resulted in far better distribution of the particles, as confirmed by cryo-EM. The protein composition of the particles before and after trypsinization was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis immediately prior to structural analysis. The protein profiles of the particles at various times during trypsinization were identical to those reported earlier (19) and hence are not shown.
Cryo-EM.
Specimen preparation for cryo-EM was carried out using standard procedures (6). The specimen suspension, native aquareovirus or trypsin-treated aquareovirus, at a concentration of ∼1.3 mg/ml (4-μl aliquot), was applied to one side of a holey carbon grid. This grid was then blotted and plunged into a bath of liquid ethane (−180°C). The frozen-hydrated sample was transferred to a precooled GATAN cryoholder and observed in a JEOL 1200 transmission electron microscope operated at 100 kV and maintained at a specimen temperature of −163°C. Regions of interest were imaged at ×30,000 magnification with an electron dose of 5 electrons/Å2. From each region, a focal pair was recorded with intended defocus values of 1.2 and 2.4 μm. The electron images were recorded with 1-s exposure on Kodak SO-163 films. These films were developed in Kodak D-19 developer for 12 min at 21°C and fixed in Kodak fixer for 10 min.
Three-dimensional structural analysis.
Micrographs were selected based on particle concentration, quality of ice, and appropriate defocus. The images were digitized on a Zeiss SCAI microdensitometer (Carl Zeiss, Inc., Englewood, Colo.), using a 7-μm step size. The pixels were then averaged to give a 14-μm step size that corresponded to 4.67 Å/pixel in the object. Intact particles were boxed with a pixel area of 256 by 256, and core particles were boxed with a box size of 192 by 192. The determination of the orientational parameters, their refinement, and the three-dimensional reconstructions were carried out using the ICOS Toolkit software suite (13). Orientations of the particles were determined using the common-lines approach (2), and their refinement was carried out using the cross-common-lines method (8). Three-dimensional reconstruction, from a set of particles (native full virions [NV], 105 particles; naturally degraded [ND], 322 particles; 5-min trypsinized [5MT], 61 particles; 15-min trypsinized [15MT], 24 particles; and 2-h trypsinized, 109 particles) which adequately represented the icosahedral asymmetric unit, was computed using cylindrical expansion methods (2). The further-from-focus micrograph in each focal pair was processed first to obtain a low-resolution reconstruction, and this was then used to assist in finding correct orientations for the particles imaged in the corresponding closer-to-focus micrograph.
The reconstructions of the various particles were computed to a resolution within the first zero of the contrast transfer function (CTF) of the corresponding micrograph. The defocus values were determined from CTF ring positions in the sum of particle Fourier transforms. The defocus values of various specimens in the closer-to-focus micrographs ranged from 1.2 to 1.48 μm. The reconstructions were corrected for the effects of the CTF using procedures described earlier (37). The final resolution for each reconstruction was determined by Fourier ring correlation analysis (31). Since the resolution of the reconstruction of 5-min-trypsinized particles was 23 Å, all the other reconstructions used for comparative analysis, such as native and other trypsinized particles, were recomputed to this resolution. The structure of the empty particles, from 17 particles, was computed to a nominal resolution of 26 Å. Contour levels in each reconstruction were chosen to represent equal volume between the radii of ∼234 and ∼294 Å (which contains mass common to all the reconstructions) and represented all core proteins in their assumed quantities except for the turret protein. Any magnification differences between different images were taken into account by using rotavirus double-layered particles (DLPs) as an internal standard. Rotavirus DLPs were mixed with either native aquareovirus virions or the cores just before cryofreezing of the specimen for microscopy. The peaks of density for the VP2 and VP6 layers of the DLPs were found to be invariant among the micrographs of the two preparations. The reconstructions were viewed on a Silicon Graphics Workstation using IRIS Explorer v3.5 (Numerical Algorithms Group, Inc.).
RESULTS
Cryo-EM.
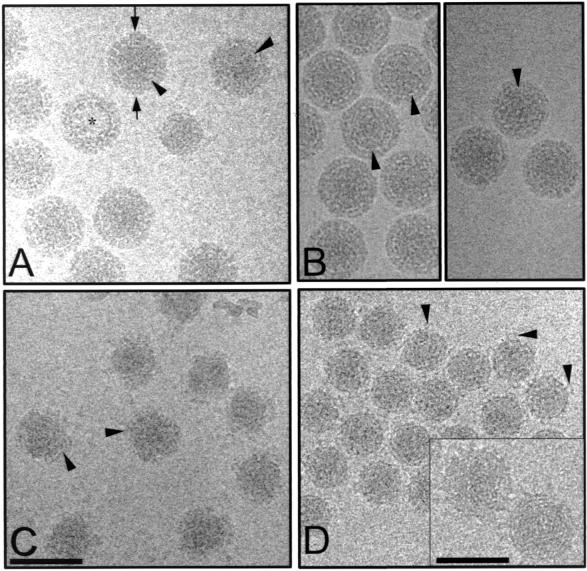
Trypsinization of aquareovirus over time resulted in the removal of proteins in a specific and reproducible manner. Micrographs of unstained, frozen hydrated aquareovirus at different stages of trypsinization, along with the images of NV, are given in Fig. 1. Included in these are NV which were kept at 4°C for approximately 14 days before being prepared for cryo-EM. These are ND particles, since reconstructions of such particles clearly showed some degradation with respect to virions that had been prepared for microscopy immediately after purification. Although NV (Fig. 1A), ND, and 5MT particles (Fig. 1B) appear similar to each other, a close examination of the NV particles indicates the presence of small additional density at the periphery (Fig. 1A). NV, ND, and 5MT particles all have a diameter of ∼800 Å and show a well-defined electron lucent boundary between the inner and outer layers. The 15MT virions were in the process of being uncoated (Fig. 1C). These particles have a nonuniform morphology with disorganized and fuzzy outer layers. The diameters of these partially uncoated particles ranged from ∼600 to ∼800 Å. After 2 h of incubation with trypsin, the particles were all of a similar size (Fig. 1D). These particles, referred to hereafter as cores, are ∼600 Å in diameter and exhibit distinct looped features that are attached to the particle. In the close-to-focus images, whorl-like patterns are observed; we attribute these to dsRNA (Fig. 1D, inset). In each preparation, empty particles that did not contain genomic RNA were also observed.
FIG. 1.
Images of frozen-hydrated aquareovirus (SBR strain) particles examined by cryo-EM. (A) Native aquareovirus (NV). The asterisk indicates particles devoid of dsRNA (empty particles). The arrows indicate the additional mass density at the periphery of the NV particles. (B) The left panel shows ND aquareovirus particles, and the right panel shows 5MT aquareovirus particles. Arrowheads in panels A and B indicate the electron-lucent boundary. (C) 15MT aquareovirus particles. Arrowheads indicate the disordered outer capsid layer. (D) Cores. Arrowheads indicate pentonal turrets. In the inset, close-to-focus images of the cores show whorl-like patterns representing the genome. All images were taken at an electron dose of 5 Å/electron2 and at a magnification of ×30,000, except for the inset in panel D, which was taken with an electron dose of 5 Å/electron2 and a magnification of ×40,000. Bars, 1,000 Å, except for the inset (600 Å).
Image processing.
The digitized micrographs for each preparation were used for image reconstructions. We found that 95% of the inverse eigenvalues were below 0.1, indicating that there was adequate sampling for the computed resolutions. To take into consideration any magnification differences that may have been present during microscopy of various specimens, rotavirus DLPs were used as an internal calibration standard. Rotavirus DLPs were mixed with samples of NV and cores. Independent reconstructions of the DLPs from the two preparations gave identical radial density profiles. The radial density profiles for NV and for the core structure from these two preparations were compared. The NV had a major peak at a radius of 257 Å, corresponding to the inner capsid layer, that was directly superimposable onto the radial density profile for the core (Fig. 2). The radial density profiles for the NV, ND, and 5MT structures, which were determined in the absence of rotavirus DLPs, used in this study have been radially scaled between 93 and 410 Å with respect to the NV, using rotavirus DLP as an internal control. Likewise, the core structure was radially scaled with respect to the core structure imaged in the presence of rotavirus DLP.
FIG. 2.
Radial density profiles computed from NV. ND, 5MT, and core reconstructions. A horizontal line represents averaged radial density of zero. Possible locations of aquareovirus structural proteins with respect to radius are indicated.
Figure 2 shows the density distribution profiles for the four reconstructions of aquareovirus. The inner capsid shell in all cases lies between 240 and 290 Å in radius. A second capsid shell is observed in NV, ND, and 5MT structures. The radial density profile reveals this shell as having two peaks of density, one at ∼315 Å and one at ∼380 Å. Immediately inside the inner capsid shell of all the particle types is a series of regularly spaced peaks that are 26 Å apart.
Structures of NV, ND, and 5MT particles.
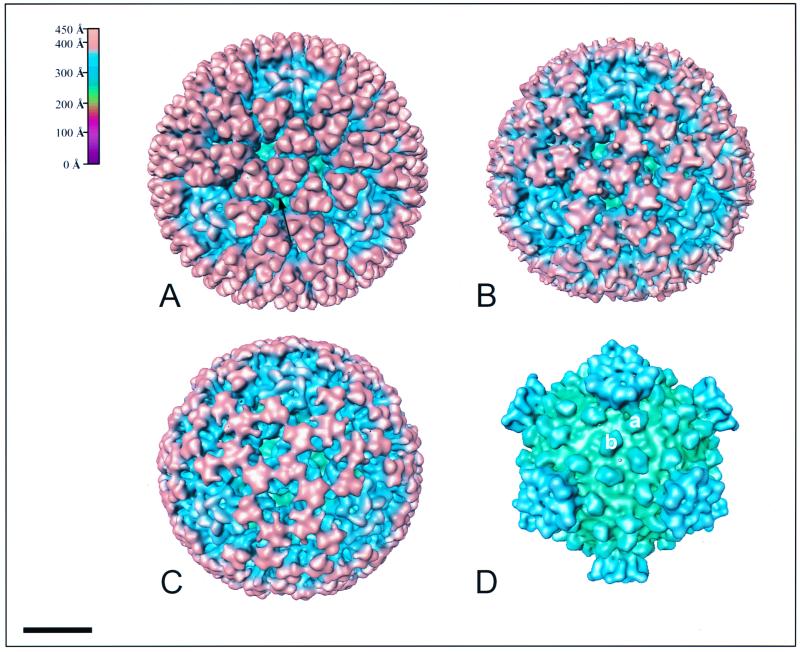
The NV, ND, and 5MT structures have many similar characteristics (Fig. 3A to C). All three structures are composed of two capsid shells, as seen in the radial density profiles computed from the respective three-dimensional reconstructions (Fig. 2A to C). These radial density profiles (Fig. 2) indicate that NV, with a diameter of ∼820 Å, is slightly larger than ND and 5MT, which have diameters of ∼800 and ∼785 Å, respectively. The outer capsid is arranged on an incomplete T=13 lattice with a left-handed configuration. The fivefold axis of aquareoviruses at all stages of proteolytic cleavage contains a turret. In NV, ND, and 5MT, this structure is recessed inward compared with the outer shell of the particle. The radius at the fivefold axis is ∼383 Å. A more detailed analysis of the turret structure is given below.
FIG. 3.
Surface-shaded representations of the NV (A), ND (B), 5MT (C), and core (D) structures viewed along the icosahedral threefold axis. The capsids are radially depth-cued as shown in the color chart (top left of figure). The same coloring scheme is used in other figures unless mentioned otherwise. (A) Finger-like projections protrude from the surface of the virion. Three projections attach to each of the underlying trimeric subunits. At the fivefold axes is a depression, in which sits a turret. At the top of each turret is a petal-like structure surrounding the central channel. (B) The ND particle shows the remnants of the finger-like projections. (C) The triangular subunits onto which the projections attach are more visible in the 5MT structure. The turrets retain the same basic morphology. (D) The removal of the outer capsid reveals a core, which possesses 120 nodules closely associated with the underlying capsid layer and turrets at all the fivefold axes. Each icosahedral asymmetric unit contains two nodules, labeled a and b. The a nodules surround the fivefold axis with mass extending up the turret, whereas the b nodules surround the threefold axis. Bar, 200 Å.
Loss of projection densities.
In NV, 600 knobby protrusions were observed attached to the outer capsid (Fig. 3A). A major difference between the NV and 5MT structures was apparent, since these protrusions were completely absent in the 5MT structure (Fig. 3C). SDS-PAGE of 5MT particles has shown that these particles do not contain VP7 (35 kDa) (19). In NV, these densities protruded above the surface of the particle, resulting in a particle diameter of ∼820 Å at the threefold axes (Fig. 3A). A difference map between the NV and 5MT structures clearly showed the locations and morphology of the extra density. The volume occupied by these densities was sufficient to accommodate 600 copies of a 35-kDa protein, indicating that these densities are due to VP7. Each protrusion was roughly cylindrical and is referred to below as a projection. Figure 4A and C clearly show that three projections clamp onto the three sides of a trimeric subunit in the outer capsid. Each projection is composed of a bulbous projection head (Ph in Fig. 4C) that protrudes ∼17 Å above the capsid surface. The body of the projection (Pb in Fig. 4C) interacts with the side of the trimeric subunit.
FIG. 4.
Trypsin-induced structural changes in the trimeric subunit. (A and B) The trimeric subunit at the threefold axis is excised from NV (A) and 5MT (B) maps. The arrow in panel A indicates the position of the threefold axis. (C) The native map is colored red and is superimposed onto the 5MT subunit. Note the cylindrical morphology of the projection. The projection head (Ph) and projection body (Pb) are labeled. (D) The 5MT subunit is colored green and is superimposed onto the native map. The asterisk denotes the lateral “horn-like” extension.
The ND particle was formed without the addition of exogenous trypsin and appears to be an intermediate form between the NV and 5MT structures (Fig. 3B). Small pieces of density were observed in the place of the projections. By SDS-PAGE, the presence of a band at 35 kDa was observed in purified, pelleted ND particles. It is possible that these ND particles still have VP7 attached in some positions but in the process of being removed. The major portion of VP7 is likely to be oriented differently within the particle and between the particles and hence is averaged out. The pieces of density that we see may represent a small portion of VP7 attached to the ND virion that is coherently contributing to the reconstruction.
In NV, at the base of some projections, weak connections are made with the projections from neighboring subunits related by local twofold axes (arrow in Fig. 3A). As the projections are removed, these connections are lost and are absent in both ND and 5MT structures. Concurrent with the removal of the projections, structural changes are observed in the upper part of the trimeric subunit (Fig. 4).
Structural changes in trimeric subunits.
The projections attach to underlying trimeric subunits. These subunits span a radius from ∼294 to ∼392 Å. The upper part of the trimeric subunit between radii of ∼369 and ∼392 Å forms triangular proteins. These are most easily observed in the 5MT structure (Fig. 3C). The lower part of the trimeric subunit (between a radius of ∼294 and ∼369 Å) forms a network of proteins, as previously seen (29). Simultaneous with the removal of the projections, additional structural changes are made in the upper layer of the trimeric subunit. “Horn-like” mass densities extend laterally from the side of the subunits to interact with neighboring subunits. Figure 4B and D show the location of these additional features in 5MT compared with the NV. These ∼24-Å bridges of mass connect neighboring subunits across the local twofold axes surrounding the strict threefold axes (Fig. 3C).
The trimeric subunits appeared to be completely disordered after a 15-min trypsinization. A reconstruction of the 15MT particles was attempted, although the orientational parameters of only a few particles could be determined. The structure showed a disordered outer layer, but the features of the core were well preserved.
Structure of inner core.
Prolonged trypsinization of the virion (2 hour) resulted in the complete removal of the outer protein layers (Fig. 3D). These particles strongly resemble the orthoreovirus cores produced by proteolytic cleavage (5). Two features that are common to both orthoreovirus and aquareovirus cores are the turrets found at the icosahedral vertices and the nodules present on the inner capsid layer surrounding the threefold and fivefold axes. The turrets extend out to a radius of ∼365 Å. The nodules surrounding the fivefold axes are connected to the turrets by extensions that continue upward from the nodule and appear to support the turret on the surface of the core like the legs of a five-legged stool. The nodules are ∼19 Å high and ∼63 Å long by ∼42 Å wide. They are very similar in size to the nodules on the surface of the orthoreovirus core. However, while 150 nodules are present in orthoreoviruses, only 120 are found in aquareoviruses. Nodules are absent from all twofold axes in aquareoviruses. In both viruses, the upper surface of the nodules makes weak connections with the protein layer above.
Conformational changes in the turret.
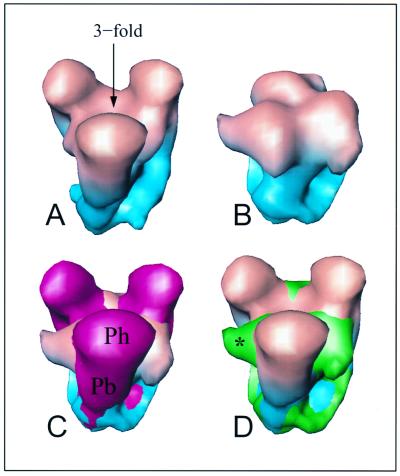
A view down the fivefold axis of NV, 5MT, and core particles shows the top of the turret to have two concentric layers of protein surrounding a central channel (Figs. 5A to C). In NV, the lower ring (Fig. 5A), at a radius of 365 Å, is composed of five bilobed structures that are connected to one another. Five petal-like features arranged with a right-handed skew form the upper ring, at a radius of 383 Å (Fig. 5A). These petals, ∼66 Å long, form a lid narrowing the opening of the central channel to 34 Å wide. They not only are attached to one another but also are attached to one piece of the bilobed density of the lower ring. Although the overall structure of the turret remains the same, significant structural changes are observed in the top portion of the turret, particularly after prolonged trypsinization. In the core, the opening of the central channel is reduced to ∼20 Å (Fig. 5C). The point of contact between the petals and the bilobed features has been shifted to a more central position, making the petals in the core appear shorter. A comparison of thin sections through the turrets of NV and the core (bottom panel of Fig. 5) shows that the petals undergo a large movement during trypsinization. The appearance of a change in the volume of the sections in this figure is because these sections do not incorporate equivalent volumes as a result of the movement. Mass volume calculations of the entire turret portions of the NV and the core structures in fact show no significant change in volume. The “lid” of the turret is positioned at a higher radius in the NV than in the core structures (Fig. 5D to F). Petals that project toward the center of the channel appear to be able to pivot on a hinge upward and outward to give the large ∼34-Å channel observed in the native virion. After 2 h of trypsinization, the petals apparently rotate inward and downward, decreasing the channel opening to ∼20 Å and shortening the height of the turret by ∼18 Å. In contrast to large-scale structural changes seen between the turrets of NV and the core, subtle differences along the same lines are seen between the turrets of NV, ND, and the 5MT structures. Both the ND and the 5MT structures have turrets that are slightly shorter than that of the NV but taller than that of the core.
FIG. 5.
Structural differences between the turret of native and trypsin-treated particles. (A to C) View of the turret, along the fivefold axis, in NV (A), 5MT (B), and core (C). In panel A, petal-like features are indicated by an asterisk, the bilobed pieces of mass are denoted by a solid diamond, and the “link” joining the bilobed pieces of density is shown by the arrow. A dashed line indicates the location of the section extracted for panels D to F. The channel at the center of the fivefold axis decreases considerably in size from the NV to the core (A to C). (D to F) A thin slice, as indicated in panel A, across the turret perpendicular to the fivefold axis extracted from NV (D) and core (E) structures. A superimposition of NV (shown as a transparent surface) and core slices is shown (F). The downward movement of the petals in the core is clearly demonstrated. An asterisk marks the possible location of a pivot point about which the petal can rotate.
Internal features.
Fingerprint-like whorls of density were observed in some of the close-to-focus negatives (Fig. 1D, inset). The inner protein-dsRNA interface of the virus was previously determined to be at a 235-Å radius (29). Examination of the interior of the NV, ND, 5MT, and core structures showed concentric layers of mass separated by ∼26-Å intervals directly beneath the inner capsid layer. The internal features of the NV are shown in Fig. 6A. This was in contrast to the “empty” particles, in which no concentric rings of mass were observed, suggesting that these most probably are dsRNA. Similarly arranged shells of mass are seen in other members of the same family, including Rotavirus (24), Orthoreovirus (5), BTV (9), and Cytoplasmic polyhedrosis virus (CPV) (10, 36).
FIG. 6.
Internal features of the virus. (A) A 23-Å thick equatorial slice of the NV perpendicular to the fivefold axis, showing the concentric layers of mass ∼26 Å apart beneath the inner capsid layer. (B) A conical cutaway of the mass density at the fivefold axis, showing a large flower-shaped structure (pink) directly beneath the turret.
In empty NV particles (i.e., those that do not contain a dsRNA genome), a flower-shaped feature was observed. This feature is attached to the inside of the inner capsid layer directly beneath the fivefold axis (Fig. 6B). A similar feature is also present in the particles containing dsRNA, but due to the surrounding density it was not easy to identify. This flower-shaped structure is very similar to a feature seen along the fivefold axes of a recombinant rotavirus DLP (24) and was proposed to be the transcription complex composed of the RNA polymerase and/or the guanylyl transferase. Similar features have also been observed in Orthoreovirus (4) and CPV (36).
DISCUSSION
It is established that at least two members of the Reoviridae family, Rotavirus and BTV, require proteolytic treatment in order to achieve optimal infectivity. However, it is less clear how the enhancement of infectivity is caused by proteolysis. For aquareoviruses, it has been shown that trypsinization causes a significant increase in infectivity (19). We have used three-dimensional cryo-EM to investigate the effect of trypsin on the structure of aquareoviruses, to gain insight into the molecular mechanism of protease-enhanced infectivity. The observed capsid undressing caused by proteolysis as a function of time, combined with published biochemical analysis, has also allowed us to infer topographical locations of the various proteins in the aquareovirus structure. The overall architecture of Aquareovirus is clearly more similar to orthoreoviruses than to any other member in the Reoviridae. The proteolytic degradation of the aquareovirus structure presented here also parallels that of the orthoreoviruses in several ways and yet there are also interesting differences, particularly in the turret regions. It is possible that, as with orthoreoviruses (21), the observed disassembly stages in aquareoviruses may mimic the process that occurs during the initial stages of infection, and the structural information presented here may have relevance for understanding the process of aquareovirus infection. Unlike orthoreoviruses, detailed functional studies of the aquareovirus proteins are still lacking. However, a careful comparison between the two viruses, particularly with respect to their protease-induced structural changes, has allowed us to propose functional and structural equivalents between aquareoviruses and orthoreoviruses (Table 1).
TABLE 1.
Possible equivalents between orthoreovirusa and aquareovirus structural proteins
| Orthoreovirus protein | No. of copies | Mass (kDa) | Function of orthoreovirus proteins and likely functions of analogous aquareovirus proteins | Putative aquareovirus protein | Likely no. of copies | Mass (kDa) |
|---|---|---|---|---|---|---|
| ς3 | 600 | 41 | Major outer capsid protein - projections; hydrophilic; metalloprotein with Zn finger motif | VP7 | 600 | 35 |
| ς1 | 36–48 | 51 | Cell attachment protein; viral hemagglutinin; neutralizing antigen; compact and extended conformation | Not present | ||
| μ1 | 600 | 76 | Major outer capsid protein; undergoes a series of proteolytic cleavages; myristoylated amino terminus | VP5 | 600 | 71 |
| μ2 | 12 | 83 | Minor core protein - putative cofactor for the viral polymerase | No equivalent | ||
| λ1 | 120 | 137 | Major core protein; binds genomic dsRNA; has Zn finger motif; contains NTP binding motif | VP3 | 120 | 126 |
| ς2 | 150 | 47 | Major core protein; forms nodules; binds genomic dsRNA | VP6 | 120 | 46 |
| λ2 | 60 | 144 | Pentameric core spike; guanylyl transferase activity | VP1 | 60 | 130 |
| λ3 | 12 | 142 | Minor core protein; putative catalytic subunit of viral polymerase | VP2 | 12 | 127 |
| No equivalent | Present in outer capsid of aquareovirus | VP4 | Unknown | 73 |
Information taken from reference 5.
Effect of trypsin on the outer-layer proteins.
Comparative SDS-PAGE analysis of native and trypsin-treated aquareoviruses clearly indicates that VP7 (35 kDa), VP4 (73 kDa), and VP5 (71 kDa) are present in the outer coat of the virion (19). All three proteins are removed upon prolonged and complete trypsinization, resulting in the core particles. However, biochemical studies indicate that VP7 (35 kDa) is the most external protein, since it is the first protein to be removed during trypsinization and also since it is glycosylated (28, 30).
A major structural difference between the NV and the 5MT structure is the loss of projections in the 5MT structure. The NV has a full complement of 600 projections extending from the trimeric subunits, whereas the 5MT structure lacks these projections and thus has a smoother surface. The volume calculations, assuming a density of 1.30 g/cm3, indicate that each of these projections can accommodate a 35-kDa protein. Therefore, the projections seen in the native structure are most probably composed of VP7. In orthoreoviruses, a similar phenomenon occurs with 1-h chymotrypsin treatment. In the native orthoreoviruses, 600 copies of ς3 (41 kDa) form projection-like spikes on the outer surface of the particle. After protease treatment, ς3 is removed from the particle, leaving a smoother surfaced virion known as an ISVP. It therefore appears that aquareovirus VP7 is structurally analogous to orthoreovirus ς3, although there is no amino acid sequence similarity between the two proteins (17).
It is to be noted that the previously published aquareovirus structure (29) does not show the projections that we have seen in the NV. This earlier structure is very similar to the ND or the 5MT structure presented here. We reckon that in the structure published by Shaw et al. (29), projections may have fallen off, perhaps because of residual proteases present in the virus preparations. In the present studies we have been careful to freeze the NV samples for cryo-EM studies immediately after virus purification. These studies clearly indicate that VP7 is highly sensitive to proteases and can easily become disassociated from the virion. In line with this suggestion are the conclusions from very early biochemical studies on aquareoviruses, suggesting that VP5 was the outermost protein; none of the aquareovirus proteins were observed to be glycosylated in that study (28). It is quite likely that the virus particles used in the earlier studies had already lost VP7.
The other major structural feature of the outer layer, apart from the projections, is the trimeric subunit. One of two currently unassigned outer layer proteins, VP5 or VP4, which have very similar molecular weights, should account for this density. Two pieces of evidence suggest that VP5 is the better candidate. Biochemical studies have shown that VP5 is trypsinized before VP4, suggesting that it is the next most accessible protein after VP7 (19, 28). Also, the intensity of the protein band for VP5 is noticeably greater than that of the band for VP4, suggesting that VP5 is present in greater number in each virion. Assignment of VP5 to the trimeric subunits requires that this protein be present in 600 copies per virion, and therefore such a protein would show up as an intense band in the gel. We hypothesize that VP5 constitutes the trimeric subunits in the protein layer that lies between the projections and the core. Comparison of the NV and 5MT structures shows a noticeable structural change in the trimeric subunits (Fig. 4). This observation correlates with the biochemical studies which show that the putative VP5 protein becomes cleaved after 5 min of trypsin treatment, resulting in a 52-kDa fragment which stays associated with the particle (19). The remaining fragment has not been visualized by SDS-PAGE, and so the size of the fragment is unknown, but the fragment is presumably no longer attached to the particle. If VP5 is assigned to the trimeric subunit, a relevant question is where VP4 is located in the virion structure. Further structural studies using monoclonal antibodies against VP4 and/or VP5 are necessary to address this question.
Removal of VP7 and the concurrent cleavage of the putative VP5 correlates with increased infectivity in aquareoviruses. The infectivity of the 5MT particles is 4.4 × 109 PFU compared with 1.8 × 107 PFU for the native virion (19). It is likely that the 52-kDa cleavage product has relevance for cell entry, possibly in the same way that μ1 of orthoreoviruses has been implicated in membrane insertion (23). Orthoreovirus μ1 is located beneath the ς3 projection, which is analogous to the location of VP5 beneath the VP7 projection in aquareoviruses. It is also possible that the removal of the VP7 in aquareoviruses may expose some regions of the putative VP5 critical for efficient entry into cells. It is not clear if the removal of VP7 is necessary for the cleavage of VP5.
More than 5 min of trypsinization results in particles that have a significant decrease in infectivity (19). We have shown that after a 15-min incubation, the outer layer of the majority of particles is clearly being disordered and/or disassembled (Fig. 1C). At this time point, the infectivity is low (between 1.5 × 106 and 2.9 × 108 PFU), indicating that the structural integrity of the trimeric subunit is essential for optimal infectivity.
Lack of a ς1 equivalent in aquareoviruses.
Treatment of orthoreoviruses with chymotrypsin also causes ς1, a spike-like protein emanating from the fivefold axes, to adopt a more extended conformation. This protein mediates the capacity of the virions to agglutinate red blood cells (3) and is also the receptor recognition protein (15). We have not seen any evidence of an equivalent structural feature at the fivefold axes, either in the native or in the trypsin-treated aquareovirus. It is likely that aquareovirus has no ς1-equivalent protein, in agreement with biochemical studies indicating that aquareoviruses do not exhibit hemagglutinating activity (33).
Effect of prolonged trypsinization.
A 2-h treatment of aquareoviruses with trypsin produces core particles, which retain the turrets at the fivefold axes. The four distinct structural features of the core are the turrets, the nodules, a shell of density beneath the nodules (Fig. 3D), which encloses the genome, and the inwardly protruding flower-shaped feature at the fivefold axis (Fig. 6B). Except for the large conformational changes in the turret regions (Fig. 5), the structure of the core particles derived from prolonged trypsinization remains essentially similar to that of the core region in the native particles. The interactions between the core and the outer-layer particles were described earlier (29). SDS-PAGE of the core particles shows that these particles are composed of four proteins, VP1, VP2, VP3, and VP6 (19). It is possible that each of these proteins can be associated with one of the four distinct structural features of the core. Treatment of orthoreoviruses with chymotrypsin for 3 h produces architecturally similar core particles (5). However, in orthoreoviruses, these particles are formed from five proteins. The major distinction between the cores of these two viruses is that aquareoviruses have only 120 nodules, in contrast to 150 nodules in the orthoreovirus cores. The nodules at the icosahedral twofold axes are absent in the aquareovirus structure. In combination with the observed structural features of the core particles and the relative intensities of the core proteins in SDS-PAGE, we have ascribed possible locations to the aquareovirus proteins that compose the core.
Core proteins and their locations.
On SDS-PAGE (12% polyacrylamide), the three largest aquareovirus proteins, VP1, VP2, and VP3 migrate very close together; however, they are separated on a 6% polyacrylamide gel with 4% bisacrylamide cross-linkage (19). As judged by the intensities of the bands in SDS-PAGE, VP3 (∼126 kDa) and VP6 (46 kDa) are more abundant than VP1 and VP2. VP1 is present in larger copy numbers than VP2. Based on their molecular weights and the volume calculations from the structure, VP6 and VP3 would be suitable candidates for the nodules and the spherical shell of the core, respectively. In orthoreoviruses, the capsid shell of the core is composed of two proteins, ς2 (47 kDa) and λ1 (137 kDa), having molecular masses similar to those of VP6 and VP3, respectively. The structural organization of 150 ς2 molecules, which form the nodules, and 120 λ1 molecules, which constitute the shell in the core of orthoreoviruses is now known from recent X-ray crystallographic studies (26). It is likely that the molecular interactions between the proposed VP6 and VP3 molecules in the aquareovirus core are very similar to those seen between ς2 and λ1 in orthoreoviruses.
The structural organization of the innermost shell, with 120 subunits, appears to be a common theme among the members of the Reoviridae. Such a unique organization, perhaps necessitated by the requirement to transcribe the dsRNA segments endogenously, is seen in BTV (9), Rotavirus (14), and Rice dwarf virus (RDV) (16), in addition to Orthoreovirus (4), and CPV (10, 36). The core structures of Orthoreovirus, CPV, and Aquareovirus, which may be called the “turreted viruses,” are distinguished from those of Rotavirus, BTV, and RDV by having additional features such as turrets and nodules. The innermost layers of Rotavirus, BTV, and RDV, in contrast, have a smooth appearance.
Turret structure.
The turret is a large structure present at the fivefold axes and, as in orthoreoviruses, may play an important role during the transcription. Cryo-EM reconstruction of the actively transcribing orthoreovirus cores has shown that the transcripts are extruded through the central channel of the turret (34). Even in Rotavirus, and very probably in BTV, nascent transcripts exit through a channel at the fivefold axis (12). In Aquareovirus, between VP1 and VP2, which are the remaining proteins, VP1 is the most likely candidate for the turret structure, since it is present in greater abundance than VP2. We propose that each turret is composed of 5 copies of a protein, yielding 60 copies of the protein per virion. Such an assignment is well supported by volume calculations from the structure. In Orthoreovirus, the turret is composed of 60 copies of λ2 (144 kDa). It is very likely that the function of VP1 in Aquareovirus is analogous to λ2 in Orthoreovirus, which, as a part of the transcriptase apparatus, mediates guanylyl transferase activity (32).
The remaining protein, VP2, with a molecular mass of ∼127 kDa, has the band of weakest intensity on the 6% PAGE and so is likely to have relatively few copies per virion. We propose that the flower-shaped density directly beneath the fivefold axis is composed of VP2. In orthoreoviruses, a similar structural feature has been ascribed to λ3 (142 kDa) (4) and is thought to be a part of the transcriptase complex. Possibly like λ3 in orthoreoviruses, VP2 is present in very small amounts (12 copies) per virion and functions as a viral polymerase. It appears that aquareoviruses lack the equivalent of λ2 (83 kDa), a minor core protein in orthoreoviruses, which has been suggested to be a cofactor for the viral polymerase (35).
Conformational change in turret structure.
Treatment of aquareovirus virions with trypsin produces a large conformational change in the turret structure. In the native virion, the turret is at its tallest and the channel is at its widest. After trypsinization, the turret protein (putative VP1) rearranges, resulting in a shorter turret with a smaller channel aperture. In orthoreoviruses, treatment with chymotrypsin produces the opposite effect. A conformational change in the turret converts the short turret with a closed channel in the virion to an extended turret with an open channel in the core. The conformational differences in the turret observed between the orthoreovirus and aquareovirus cores may be due to the higher salt content of the buffer used with the aquareovirus cores. Such a buffer was required to retain well-dispersed particles in ice.
In the aquareovirus NV, interactions were observed between the two rings of density in the petal-like feature at the top of the turret. These perhaps serve to give some stability, enabling the petals to remain in an “open” channel configuration. After a 2-h trypsinization, this interaction appears to be relaxed somewhat, so that the petals are no longer as strongly held outward and have moved inward toward one another, resulting in a much smaller channel. The ability of this turret protein to rearrange under various conditions may have implications in the transcription of the virus. Although the transcriptional activity of the virions, ISVP, or core of aquareovirus has yet to be established, the observed narrowing of the channel, in contrast to the widening seen in orthoreovirus, appears to be rather counterintuitive. It is possible that a different set of conditions is required to establish transcriptional activity in aquareovirus. All trypsinization experiments shown here were conducted at 37°C, as in previous biochemical studies (19). However, the aquatic animals that become infected by aquareoviruses live at ∼20°C, and it is possible that this temperature would reveal a different conformation of the turret proteins. Nevertheless, this study has revealed a labile domain in the turret protein. Its conformation is most probably the determining factor in whether the particle is able to release the nascent transcripts.
It is remarkable that despite differences in host specificity and in the number of dsRNA segments, aquareoviruses and orthoreoviruses exhibit such extensive similarity. These similarities may suggest that these viruses, while belonging to distinct genera in the family Reoviridae, may indeed have evolved from a common ancestor. Despite similar protease-induced disassembly profiles, the orthoreoviruses do not exhibit any increased infectivity like aquareoviruses. Protease-enhanced infectivity in aquareoviruses may have resulted from their necessity to survive in protease-rich regions of the host organisms.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI36040 to B.V.V.P.
We thank J. A. Lawton for helpful discussions and critical reading of the manuscript.
REFERENCES
- 1.Baya A, Toranzo A E, Nunez S, Barja J L, Hetrick F M. Association of a Moraxella sp. and reo-like virus with mortalities of striped bass, Morone saxatilis. In: Perkins F, Cheng T, editors. Pathology in marine science. New York, N.Y: Academic Press, Inc.; 1990. pp. 91–99. [Google Scholar]
- 2.Crowther R A. Procedures for three-dimensional reconstruction of spherical viruses by Fourier synthesis from electron micrographs. Philos Trans R Soc London Ser B. 1971;261:221–230. doi: 10.1098/rstb.1971.0054. [DOI] [PubMed] [Google Scholar]
- 3.Dermody T S, Nibert M L, Bassel-Duby R, Fields B N. A sigma 1 region important for hemagglutination by serotype 3 reovirus strains. J Virol. 1990;64:5173–5176. doi: 10.1128/jvi.64.10.5173-5176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dryden K A, Farsetta D L, Wang G, Keegan J M, Fields B N, Baker T S, Nibert M L. Internal/structures containing transcriptase-related proteins in top component particles of mammalian orthoreovirus. Virology. 1998;245:33–46. doi: 10.1006/viro.1998.9146. [DOI] [PubMed] [Google Scholar]
- 5.Dryden K A, Wang G, Yeager M, Nibert M L, Coombs K M, Furlong D B, Fields B N, Baker T S. Early steps in reovirus infection are associated with dramatic changes in supramolecular structure and protein conformation: analysis of virions and subviral particles by cryoelectron microscopy and image reconstruction. J Cell Biol. 1993;122:1023–1041. doi: 10.1083/jcb.122.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubochet J, Adrian M, Chang J J, Homo J C, Lepault J, McDowall A W, Schultz P. Cryo-electron microscopy of vitrified specimens. Q Rev Biophys. 1988;21:129–228. doi: 10.1017/s0033583500004297. [DOI] [PubMed] [Google Scholar]
- 7.Estes M K, Graham D Y, Mason B B. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J Virol. 1981;39:879–888. doi: 10.1128/jvi.39.3.879-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuller S D. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987;27:923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- 9.Grimes J M, Burroughs J N, Gouet P, Diprose J M, Malby R, Zientara S, Mertens P P, Stuart D I. The atomic structure of the bluetongue virus core. Nature. 1998;395:470–478. doi: 10.1038/26694. [DOI] [PubMed] [Google Scholar]
- 10.Hill C L, Booth T F, Prasad B V, Grimes J M, Mertens P P, Sutton G C, Stuart D I. The structure of a cypovirus and the functional organization of dsRNA viruses. Nat Struct Biol. 1999;6:565–568. doi: 10.1038/9347. [DOI] [PubMed] [Google Scholar]
- 11.Joklik W K. Studies on the effect of chymotrypsin on reovirions. Virology. 1972;49:700–715. doi: 10.1016/0042-6822(72)90527-2. [DOI] [PubMed] [Google Scholar]
- 12.Lawton J A, Estes M K, Prasad B V. Three-dimensional visualization of mRNA release from actively transcribing rotavirus particles. Nat Struct Biol. 1997;4:118–121. doi: 10.1038/nsb0297-118. [DOI] [PubMed] [Google Scholar]
- 13.Lawton J A, Venkataram Prasad B V. Automated software package for icosahedral virus reconstruction. J Struct Biol. 1996;116:209–215. doi: 10.1006/jsbi.1996.0032. [DOI] [PubMed] [Google Scholar]
- 14.Lawton J A, Zeng C Q, Mukherjee S K, Cohen J, Estes M K, Prasad B V. Three-dimensional structural analysis of recombinant rotavirus-like particles with intact and amino-terminal-deleted VP2: implications for the architecture of the VP2 capsid layer. J Virol. 1997;71:7353–7360. doi: 10.1128/jvi.71.10.7353-7360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee P W K, Hayes E C, Joklik W K. Protein sigma 1 is the reovirus cell attachment protein. Virology. 1981;108:156–163. doi: 10.1016/0042-6822(81)90535-3. [DOI] [PubMed] [Google Scholar]
- 16.Lu G, Zhou Z H, Baker M L, Jakana J, Cai D, Wei X, Chen S, Gu X, Chiu W. Structure of double-shelled rice dwarf virus. J Virol. 1998;72:8541–8549. doi: 10.1128/jvi.72.11.8541-8549.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lupiani B, Reddy S M, Subramanian K, Samal S K. Cloning, sequence analysis and expression of the major outer capsid protein gene of an aquareovirus. J Gen Virol. 1997;78:1379–1383. doi: 10.1099/0022-1317-78-6-1379. [DOI] [PubMed] [Google Scholar]
- 18.Lupiani B, Subramanian K, Samal S. Aquareoviruses. Annu Rev Fish Dis. 1995;5:175–208. [Google Scholar]
- 19.McPhillips T H, Dinan D, Subramanian K, Samal S K. Enhancement of aquareovirus infectivity by treatment with proteases: mechanism of action. J Virol. 1998;72:3387–3389. doi: 10.1128/jvi.72.4.3387-3389.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mertens P P C, Burroughs J N, Walton A, Wellby M P, Fu H, O'Hara R S, Brookes S M, Mellor P S. Enhanced infectivity of modified bluetongue virus particles for two insect cell lines and for two Culicoides vector species. Virology. 1996;217:582–593. doi: 10.1006/viro.1996.0153. [DOI] [PubMed] [Google Scholar]
- 21.Nibert M L. Structure of mammalian orthoreovirus particles. In: Tyler K, Oldstone M, editors. Structure, proteins, and genetics. Vol. 1. Berlin, Germany: Springer-Verlag KG; 1998. pp. 1–30. [DOI] [PubMed] [Google Scholar]
- 22.Nibert M L, Chappell J D, Dermody T S. Infectious subvirion particles of reovirus type 3 Dearing exhibit a loss in infectivity and contain a cleaved sigma 1 protein. J Virol. 1995;69:5057–5067. doi: 10.1128/jvi.69.8.5057-5067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nibert M L, Fields B N. A carboxy-terminal fragment of protein mu 1/mu 1C is present in infectious subvirion particles of mammalian reoviruses and is proposed to have a role in penetration. J Virol. 1992;66:6408–6418. doi: 10.1128/jvi.66.11.6408-6418.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasad B V, Rothnagel R, Zeng C Q, Jakana J, Lawton J A, Chiu W, Estes M K. Visualization of ordered genomic RNA and localization of transcriptional complexes in rotavirus. Nature. 1996;382:471–473. doi: 10.1038/382471a0. [DOI] [PubMed] [Google Scholar]
- 25.Prasad B V V, Estes M K. Molecular basis of rotavirus replication. In: Chiu W, Burnett R, Garcea R, editors. Structural biology of viruses. New York, N.Y: Oxford University Press; 1997. pp. 239–268. [Google Scholar]
- 26.Reinisch K, Nibert M L, Harrison S. Structure of the reovirus core at 3.6 Å resolution. Nature. 2000;404:960–967. doi: 10.1038/35010041. [DOI] [PubMed] [Google Scholar]
- 27.Roy P. Orbiviruses and their replication. In: Fields B, Knipe D, Howley P, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1709–1766. [Google Scholar]
- 28.Samal S K, Dopazo C P, McPhillips T H, Baya A, Mohanty S B, Hetrick F M. Molecular characterization of a rotaviruslike virus isolated from striped bass (Morone saxatilis) J Virol. 1990;64:5235–5240. doi: 10.1128/jvi.64.11.5235-5240.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw A L, Samal S K, Subramanian K, Prasad B V. The structure of aquareovirus shows how the different geometries of the two layers of the capsid are reconciled to provide symmetrical interactions and stabilization. Structure. 1996;4:957–967. doi: 10.1016/s0969-2126(96)00102-5. [DOI] [PubMed] [Google Scholar]
- 30.Subramanian K, McPhillips T H, Samal S K. Characterization of the polypeptides and determination of genome coding assignments of an aquareovirus. Virology. 1994;205:75–81. doi: 10.1006/viro.1994.1621. [DOI] [PubMed] [Google Scholar]
- 31.van Heel M. Similarity measures between images. Ultramicroscopy. 1987;21:95–99. [Google Scholar]
- 32.White C K, Zweerink H J. Studies on the structure of reovirus cores: selective removal of polypeptide lambda 2. Virology. 1976;70:171–180. doi: 10.1016/0042-6822(76)90247-6. [DOI] [PubMed] [Google Scholar]
- 33.Winton J R, Lannan C N, Fryer J L, Kimura T. Isolation of a new reovirus from chum salmon in Japan. Fish Pathol. 1981;15:155–162. [Google Scholar]
- 34.Yeager M, Weiner S, Coombs K M. Transcriptionally active reovirus core particles visualized by electron cryo-microscopy and image reconstruction. Biophys J. 1996;70:A116. [Google Scholar]
- 35.Yin P, Cheang M, Coombs K M. The M1 gene is associated with differences in the temperature optimum of the transcriptase activity in reovirus core particles. J Virol. 1996;70:1223–1227. doi: 10.1128/jvi.70.2.1223-1227.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Zhang J, Yu X, Lu X, Zhang Q, Jakana J, Chen D H, Zhang X, Zhou Z H. Visualization of protein-RNA interactions in cytoplasmic polyhedrosis virus. J Virol. 1999;73:1624–1629. doi: 10.1128/jvi.73.2.1624-1629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Z H, Prasad B V V, Jakana J, Rixon F J, Chiu W. Protein subunit structures in the herpes simplex virus A-capsid determined from 400 kV spot-scan electron cryomicroscopy. J Mol Biol. 1994;242:456–469. doi: 10.1006/jmbi.1994.1594. [DOI] [PubMed] [Google Scholar]