Acute variceal bleeding (AVB) is a decompensating event in patients with cirrhosis and is associated with high mortality. Varices commonly develop in the esophageal and gastrofundal regions are sites for AVB. Varices outside the gastroesophageal region or ectopic varices are uncommon and account for <5% of AVB. This review focuses on the management of AVB from esophageal and gastrofundal varices.
AVB occurs with rising portal pressure that causes the tension on the variceal wall to exceed its elastic limit. As portal hypertension is the main pathophysiological cause for AVB, treatment should be directed at lowering portal pressure and not correcting hemostatic abnormalities.1 The overall goal of the management of AVB is to control bleeding, prevent early rebleeding (within 5 d), and prevent 6-week mortality, the latter being the main outcome of interest.1–3 HVPG ≥20 mm Hg is a strong predictor of early rebleeding and 6-week mortality in AVB but is typically not measured in clinical practice.4 Commonly used scores for determining the severity of cirrhosis, like the Child-Turcotte-Pugh (CTP) score and Model for End-Stage Liver Disease, can be used for prognostication in AVB.5
INITIAL MANAGEMENT OF PATIENTS WITH SUSPECTED AVB
Patients with suspected AVB should be admitted to the intensive care unit. Intravenous access with large-bore peripheral i.v. catheters or a central line should be established. Patients should be resuscitated to maintain hemodynamic stability and tissue perfusion. In the absence of a massive exsanguinating bleed or concomitant active cardiovascular disease, a restrictive transfusion strategy for packed red blood cells by maintaining hemoglobin around 7 –9 g/dL is recommended.6 Transfusion of fresh frozen plasma, recombinant factor VIIa, and tranexamic acid are not recommended as they are unlikely to correct coagulopathy. In addition, fresh frozen plasma may lead to volume overload and worsening of portal hypertension.7 Platelet transfusion and products to replace fibrinogen should be considered on a case-by-case basis. Patients with altered mental status or those at high risk for aspiration should be intubated with the goal of extubating after successful endoscopic therapy.
As patients with cirrhosis and gastrointestinal bleeding are at high risk of developing infections, antibiotics should be started as soon as possible and continued for a maximum of 7 days. Antibiotics must provide gram-negative coverage and should be determined based on local antibiotic policy and resistance patterns. In practice, a third-generation cephalosporin such as i.v. ceftriaxone (1 g every 24 hours) is commonly used.
Vasoactive drugs should be started as soon as AVB is suspected. The use of vasoactive drugs is associated with a lower risk of 7-day mortality and decreased transfusion requirements.8 The vasoactive drugs used in AVB are somatostatin, octreotide, vasopressin, and terlipressin. They are comparable in achieving control of AVB in 5 days.9 However, terlipressin and vasopressin are associated with a higher incidence of adverse events.10 Octreotide has the most favorable safety profile and is thus the drug of choice in AVB.11 It is administered as a 50 µg i.v. bolus followed by a 50 µg/h infusion for 2–5 days. The bolus can be repeated in the first hour if bleeding is not controlled.
ENDOSCOPY
Endoscopic variceal ligation (EVL) is the mainstay of the management of AVB from esophageal varices. Unlike esophageal varices, which are superficial, gastric varices are deeper and are typically not amenable to EVL. Sclerotherapy, ie, endoscopic therapy with a tissue adhesive like cyanoacrylate, can be performed for both bleeding esophageal and gastric varices, where available. An upper endoscopy should be performed as soon as possible and ideally within 12 hours. For patients without QTc prolongation or significant drug-drug interactions, a prokinetic agent such as erythromycin (250 mg i.v. over 20–30 min) can be given at least 20 minutes prior to endoscopy to improve gastric visualization by clearing blood and clots. During endoscopy, active bleeding from varices or a “white nipple sign” that denotes a recently ruptured varix may be noted. However, if the only lesion found is varices in a patient with suspected AVB, AVB should be inferred, and EVL should be performed. The evidence for endoscopic ultrasound (EUS)–based therapies for gastric varices (EUS-tissue adhesive, EUS-coil, EUS–thrombin, and EUS with combined therapy) is evolving and is dependent on the availability of expertise.
TRANSJUGULAR INTRAHEPATIC PORTOSYSTEMIC SHUNT
A TIPS is an endovascular shunt, created under real-time fluoroscopic and ultrasound guidance, connecting the portal circulation with the systemic circulation with the goal of reducing, portosystemic pressure gradient (PSPG). After the portal vein is accessed and an intrahepatic parenchymal tract is created, an expandable polytetrafluoroethylene-covered stent is used to line the tract from the portal vein entry to the hepatic vein ostium. It is dilated to the minimum diameter needed to achieve a PSPG <12 mm Hg.
Pre-TIPS workup should include cross-sectional imaging for delineation of portal vascular anatomy and an echocardiogram to assess for the presence of systolic and diastolic dysfunction and pulmonary hypertension. TIPS is contraindicated in heart failure (ejection fraction <50%), severe pulmonary hypertension (>45 mm Hg), severe HE, systemic infection, and sepsis. In patients with CTP score ≥14, Model for End-Stage Liver Disease score >30, or lactate >12 mmol/L, TIPS may be futile and should be used only as a bridge to liver transplantation. Procedural complications are rare (<5%) but can include i.p. bleeding, arterial injury, liver infarct, hepatic capsular puncture, hemobilia, immediate TIPS thrombosis, and sepsis. Long-term complications include HE, deterioration in liver function, and complications related to cardiac overload. The risk factors for HE are previous HE, older age, advanced liver dysfunction, kidney dysfunction, hyponatremia, sarcopenia, and a low PSPG. TIPS diameter of 8 mm (as compared to 10 mm) may be adequate to prevent rebleeding while reducing the risk of encephalopathy.12
The indications for the use of TIPS in AVB are listed below:
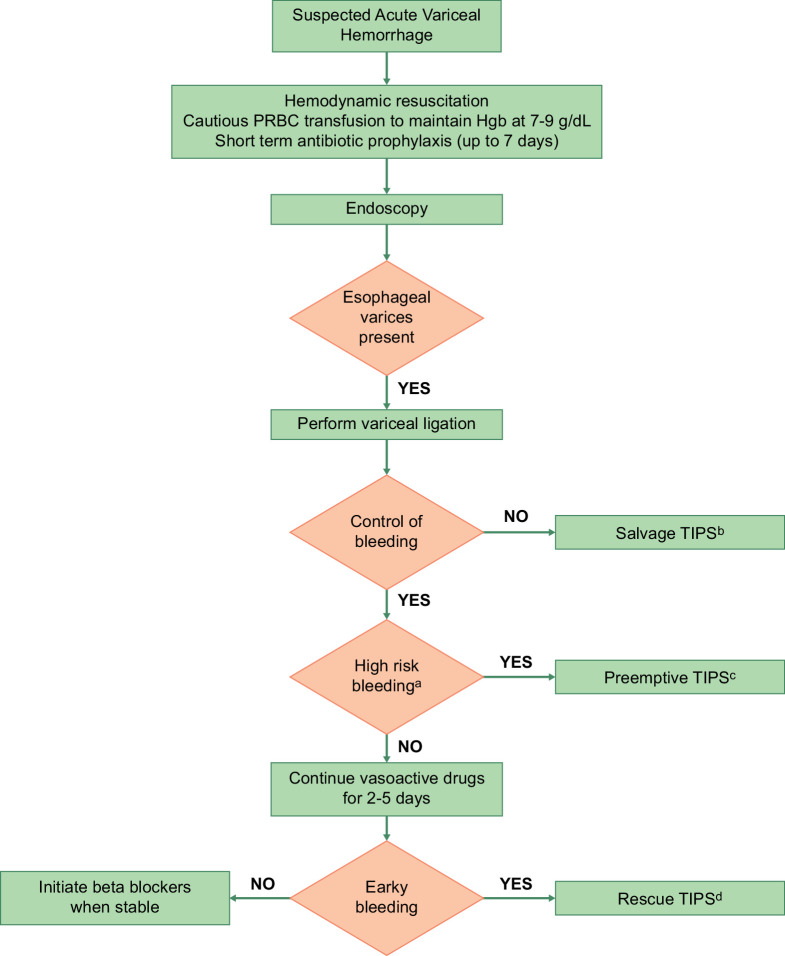
Pre-emptive TIPS: Despite initial management and endoscopic therapy, 10%–20% of patients with AVB will have early rebleeding. In selected patients who are high risk of early rebleeding, a pre-emptive TIPS (placed within 72 hours of endoscopy) is associated with improved 6-week survival.13,14 A pre-emptive TIPS should be considered in patients who are CTP class C 10–13 points or CTP class B 8–9 points with active bleeding.12 In patients who have CTP class C scores ≥14, TIPS can lead to higher rates of acute-on-chronic liver failure and 6-week mortality. Therefore, the risks versus benefits of pre-emptive TIPS need to be considered carefully.
Salvage and rescue TIPS: TIPS can be an option as “salvage” therapy for patients with uncontrolled bleeding despite medical and endoscopic therapy. In patients who have early rebleeding (within 5 d), TIPS can be used for “rescue.”
TIPS as first-line therapy: TIPS can be used as first-line therapy in patients with gastric or ectopic variceal bleeding but not esophageal variceal bleeding. On a case-by-case basis, TIPS can be complemented with variceal embolization to reduce the risk of rebleeding and H.
TIPS as second-line therapy: TIPS should be considered in patients with 1 (or more) prior episodes of AVB who present with rebleeding (independent of pre-emptive TIPS candidacy).
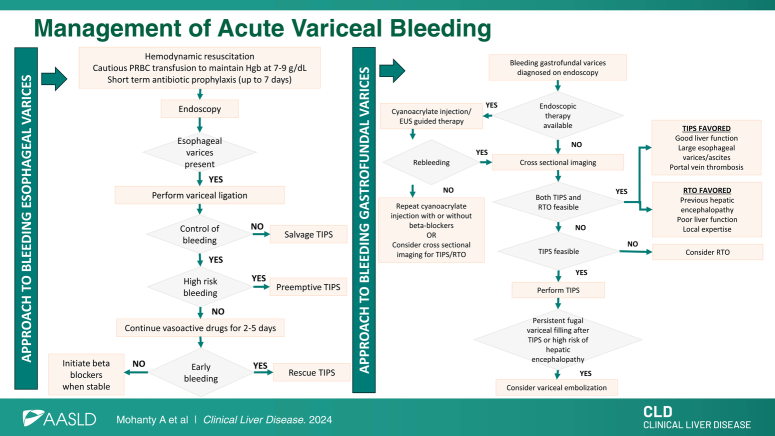
Figure 1 summarizes the management of AVB.
FIGURE 1.
Management of acute variceal bleeding. (A) Patients with Child-Turcotte-Pugh class C 10–13 points or Child-Turcotte-Pugh class B 8–9 points with active bleeding are considered to be at high risk for the failure of standard therapy. (B) Salvage TIPS: TIPS placed in patients with uncontrolled (ongoing) bleeding despite medical and endoscopic therapy. (C) Pre-emptive TIPS: TIPS placed pre-emptively within 72 hours of the hemorrhage, in patients with hemorrhage controlled with medical/endoscopic therapy and at high risk for failure of standard therapy. (D) Rescue TIPS: TIPS placed for early (within 5 d) recurrence of variceal hemorrhage. Abbreviation: PRBC, packed red blood cell.
RETROGRADE TRANSVENOUS OBLITERATION
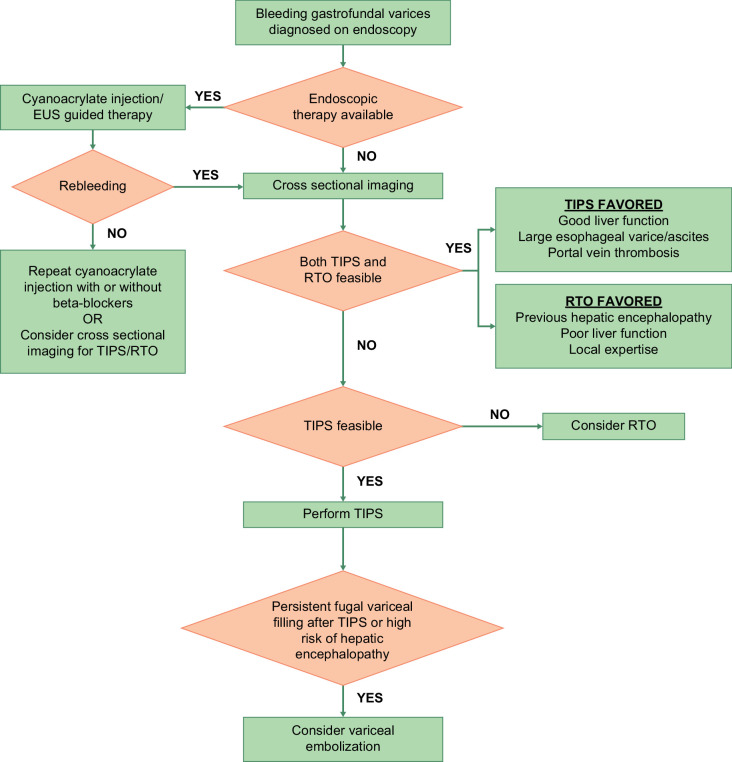
Retrograde transvenous obliteration (RTO) is a type of variceal embolization technique mainly performed for gastrofundal varices, where varices are accessed from the systemic circulation via a gastrorenal or a gastrocaval shunt. RTO can be considered as a first-line therapy for patients with AVB from gastrofundal varices as an alternative or as complementary to TIPS. RTO does not reduce portal pressure and can redirect blood to the liver, thereby increasing portal pressure but potentially improving liver function. While treating gastrofundal varices, the location of the varices, anatomy of efferent and afferent vessels, presence of shunts, flow dynamics of circuit, presence of splenic vein thrombosis, and liver function should be considered. RTO is favored in patients with gastrofundal varices who are not good candidates for TIPS, such as those with poor liver function or cardiopulmonary function. TIPS is favored in patients with significant ascites or large PVT.
Figure 2 shows an approach to the management of gastric varices.
FIGURE 2.
An approach to the management of gastrofundal varices. In patients with acute variceal bleeding from gastrofundal varices, endoscopic therapy (cyanoacrylate injection or endoscopic ultrasound–guided therapy), TIPS, or RTO can be used as first-line therapy. Endoscopic therapy for gastrofundal varices is available in select centers. Choice between TIPS and/or RTO depends on multiple factors, including vascular anatomy, liver function, and availability of local expertise. Patients with rebleeding after endoscopic therapy should undergo TIPS. Abbreviations: EUS, endoscopic ultrasound; RTO, retrograde transvenous obliteration.
MANAGEMENT OF AVB AFTER ENDOSCOPY AND/OR ENDOVASCULAR THERAPY
Beta-blockers
In patients who have recovered from an episode of AVB with EVL or had a TIPS placed with PSPG ≥12 mm Hg, beta-blockers should be started. They should also be considered in patients who have undergone successful RTO. A combination of EVL and beta-blockers are superior to either strategy alone in reducing the risk of rebleeding.15
Proton pump inhibitors
Proton pump inhibitors, if initiated, should be discontinued after variceal bleeding is conformed endoscopically as they are associated with increased risk of encephalopathy and infections.16,17
Enteral feeding
Enteral feeding should be initiated after the control of AVB. Variceal banding is not a contraindication to the placement of nasogastric feeding tubes.3
Endoscopic variceal ligation
In patients who have undergone EVL (without TIPS), EVL should be repeated in 2 weeks after hospital discharge and every 2 weeks thereafter till complete eradication of varices.
Post-TIPS management
TIPS thrombosis or stenosis can occur, resulting in TIPS dysfunction. Doppler ultrasound of the TIPS at 1 week, 3 months, 6 months, and 6 months thereafter is recommended. TIPS should be interrogated and revised if the mean maximum flow velocity at the portal vein is <28 cm/s flow when the flow is hepatofugal and <39 cm/s when the flow is hepatopetal or if there are recurrent signs of portal hypertension.12
Post RTO management
Complete obliteration of variceal complex after RTO should be confirmed using contrast-enhanced tomography or EU, as partial obliteration may lead to catastrophic bleeding. Additional endoscopic or endovascular procedures may be needed on a case-by-case basis to achieve complete obliteration. As there is a risk of an increase in PSPG after successful RTO, an endoscopy should be performed 1 to 2 months after RTO to assess for the development or progression of esophageal varices.12
Acknowledgments
CONFLICTS OF INTEREST
Arpan Mohanty has advised and received grants from Gilead. She received grants from Kinetix Group. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: AVB, acute variceal bleeding; CTP, Child-Turcotte-Pugh; EUS, endoscopic ultrasound; EVL, endoscopic variceal ligation; PSPG, portosystemic pressure gradient; RTO, retrograde transvenous obliteration.
Contributor Information
Arpan Mohanty, Email: amohanty@bu.edu.
Guadalupe Garcia-Tsao, Email: guadalupe.garcia-tsao@yale.edu.
REFERENCES
- 1. de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Faculty BV, et al. Renewing consensus in portal hypertension. J Hepatol. 2022;76:959–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–35. [DOI] [PubMed] [Google Scholar]
- 3. Kaplan DE, Ripoll C, Thiele M, Fortune BE, Simonetto DA, Garcia-Tsao G, et al. AASLD practice guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2023. doi: 10.1097/HEP.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 4. Abraldes JG, Villanueva C, Bañares R, Aracil C, Catalina MV, García-Pagán JC, et al. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol. 2008;48:229–36. [DOI] [PubMed] [Google Scholar]
- 5. Buckholz A, Wong R, Curry MP, Baffy G, Chak E, Rustagi T, et al. MELD, MELD 3.0, versus Child score to predict mortality after acute variceal hemorrhage: A multicenter US cohort. Hepatol Commun. 2023;7. doi: 10.1097/HC9.0000000000000258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. [DOI] [PubMed] [Google Scholar]
- 7. Mohanty A, Kapuria D, Canakis A, Lin H, Amat MJ, Rangel Paniz G, et al. Fresh Frozen Plasma transfusion in acute variceal hemorrhage: Results from a multicenter cohort study. Liver Int. 2021;41. doi: 10.1111/liv.14936 [DOI] [PubMed] [Google Scholar]
- 8. Wells M, Chande N, Adams P, Beaton M, Levstik M, Boyce E, et al. Meta-analysis: vasoactive medications for the management of acute variceal bleeds. Aliment Pharmacol Ther. 2012;35:1267–78. [DOI] [PubMed] [Google Scholar]
- 9. Seo YS, Park SY, Kim MY, Kim JH, Park JY, Yim HJ, et al. Lack of difference among terlipressin, somatostatin, and octreotide in the control of acute gastroesophageal variceal hemorrhage. Hepatology. 2014;60:954–63. [DOI] [PubMed] [Google Scholar]
- 10. Zhou X, Tripathi D, Song T, Shao L, Han B, Zhu J, et al. Terlipressin for the treatment of acute variceal bleeding: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2018;97:e13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia-Tsao G, Abraldes JG, Rich NE, Wong VW. AGA clinical practice update on the use of vasoactive drugs and intravenous albumin in cirrhosis: Expert review. Gastroenterology. 2024;166:202–10. doi: 10.1053/j.gastro.2023.10.016 [DOI] [PubMed] [Google Scholar]
- 12. Lee EW, Eghtesad B, Garcia-Tsao G, Haskal ZJ, Hernandez-Gea V, Jalaeian H, et al. AASLD Practice Guidance on the use of TIPS, variceal embolization, and retrograde transvenous obliteration in the management of variceal hemorrhage. Hepatology. 2023;79. doi: 10.1097/HEP.0000000000000530 [DOI] [PubMed] [Google Scholar]
- 13. García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370–9. [DOI] [PubMed] [Google Scholar]
- 14. Nicoară-Farcău O, Han G, Rudler M, Angrisani D, Monescillo A, Torres F, et al. Effects of early placement of transjugular portosystemic shunts in patients with high-risk acute variceal bleeding: A meta-analysis of individual patient data. Gastroenterology. 2021;160:193–205.e10. [DOI] [PubMed] [Google Scholar]
- 15. Puente A, Hernández‐Gea V, Graupera I, Roque M, Colomo A, Poca M, et al. Drugs plus ligation to prevent rebleeding in cirrhosis: An updated systematic review. Liver Int. 2014;34:823–33. [DOI] [PubMed] [Google Scholar]
- 16. Thomson MJ, Lok ASF, Tapper EB. Appropriate and potentially inappropriate medication use in decompensated cirrhosis. Hepatology. 2021;73:2429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Leary JG, Reddy KR, Wong F, Kamath PS, Patton HM, Biggins SW, et al. Long-term use of antibiotics and proton pump inhibitors predict development of infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:753–9.e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]