Abstract
DISEASE BURDEN IN EUROPE
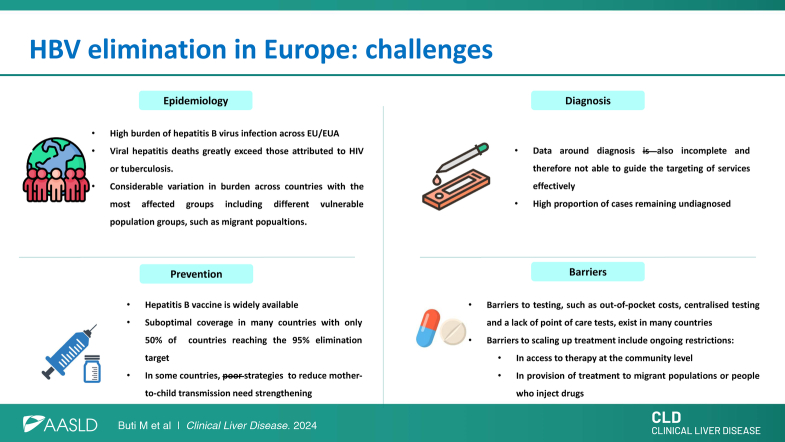
In 2016, an estimated 3.6 million people were living with chronic HBV infection in the 30 countries of the European Union (EU) and European Economic Area (EEA). Together with HCV infection, HBV is responsible for ~55% of all deaths related to liver cancer and 45% of deaths due to cirrhosis and other chronic liver conditions. Notably, deaths from HCC continue to increase, with hepatitis-specific deaths in the EU exceeding those attributed to either tuberculosis or HIV.1,2
The epidemiological status of HBV infection in EU/EEA countries exhibits marked geographical differences and varying burdens of disease among key affected groups, such as men who have sex with men, people who inject drugs (PWID), and some migrant populations.3 The prevalence of HBV is low in most EU/EEA countries with the virus affecting <0.1% of the population, especially among countries in the North West part of the region where a high proportion of cases are reported among migrants from countries with a higher endemicity and incomplete vaccination programs.3 Nonetheless, a recent systematic review of HBV prevalence across EU/EEA countries found studies with prevalence estimates up to 8% among key risk groups such as specific migrant populations (eg, refugees) and people in prison. However, prevalence among most population groups has actually decreased in line with changes in prevalence among the general population, which reflects the impact of highly effective vaccination programs.3,4
CURRENT HBV ELIMINATION PROGRESS
In 2016, the WHO launched a proposal to eliminate viral hepatitis, with a focus on 5 key interventions: vaccinating infants, preventing perinatal transmission, ensuring blood and injection safety, promoting harm reduction measures to prevent transmission through injecting drug use, and increasing access to testing and effective treatment.5 We possess all the necessary tools to achieve HBV elimination, such as widespread access to hepatitis B vaccination and accurate diagnostic tests, measures to ensure blood safety, and highly effective antiviral treatment with nucleos(t)ide analogs, which can suppress viral replication, prevent progression to cirrhosis, and reduce the risk of developing liver cancer.
The global hepatitis strategy, endorsed by all WHO member states, aims to achieve a 90% reduction in new chronic hepatitis infections and a 65% decrease in related deaths by 2030.6 In tandem, the 2020 Global Health Sector Strategy (GHSS) has requested that approaches to eradicate AIDS, viral hepatitis epidemics, and sexually transmitted infections include collaborative efforts in overlapping areas while respecting the specific characteristics of each disease.5 These objectives align with the goals of the 2030 Agenda for Sustainable Development and WHO’s General Program of Work.7
The WHO European Action Plan set the following interim targets for 2020 concerning HBV infection: a 50% diagnosis rate among individuals living with chronic HBV; treatment initiation in 75% of eligible diagnosed patients; and viral suppression in 90% of those receiving long-term treatment.6 However, to determine the progress toward these targets, considerable investment is required in most countries to improve data collection systems across clinical and public health domains to enable the collection of high-quality data.7
In 2017, the European Center for Disease Prevention and Control (ECDC) began monitoring the progress of EU/EEA Member States toward achieving the hepatitis elimination targets. This effort involves the collection, collation, and analysis of data on the epidemiological situation, prevention strategies, and testing and treatment indicators. The first results, published in 2019, highlighted major gaps in the available national data to monitor advances, especially those related to testing and treatment.8 Additional challenges included incomplete or unreliable reporting, with many data sources outdated or of suboptimal quality. In the second data collection in 2021, data were less complete with only 12 countries reporting data on the number of individuals who were HBV-infected, 9 data on diagnosed cases, and 8 on treated patients.9 This decline may be related to the effect of the COVID-19 pandemic in the region which was reported to have a huge impact on the work of health professionals and clinical activity in 2020 and 2021,10,11 but it also highlights the weaknesses and lack of maturity of existing data collection systems.
The decline in reporting highlights the need to enhance and complement existing national systems with data from alternative epidemiological sources and surveillance schemes. One solution is through the implementation of sentinel surveillance. The pilot project for sentinel surveillance performed in Croatia, Romania, and Spain successfully collected high-quality clinical and laboratory data on HBV and HCV cases, including treatment outcomes.12 Rollout plans for this system, in conjunction with support for developing comprehensive case registries and undertaking data linkage across various collection schemes, are now underway. The data collected in the second data collection while incomplete indicated that regarding the 50% diagnosis target for 2020, 4 of the 8 countries reporting data met the target, with countries reporting a proportion diagnosed ranging from 17.5% to 66.7%.10 The other targets for HBV, for linkage to care, treatment, and viral suppression, had very limited data that did not allow for an estimate of progress. Another method to estimate progress in the cascade of care is with mathematical models. Recent estimations for assessing progress toward the elimination targets from modeling undertaken by the Polaris group, predict that none of the countries worldwide will eliminate hepatitis B by 2030 based on current strategies.13
The 2020 ECDC report on prevention highlighted that in terms of progress around HBV vaccination, an important element for disease prevention, only 50% of EU/EEA countries with universal childhood vaccination had reached the target of 95% vaccination coverage despite most countries having established vaccination programs in place for well over 20 years.13 Countries use various approaches to help prevent mother-to-child HBV transmission, including antenatal screening and birth doses of the HBV vaccine. Of the 13 countries with data on antenatal screening, 10 (77%) achieved the target of 90% coverage. Only 6 countries had data on birth dose vaccine coverage in infants born to mothers who are HBsAg-positive, with reported rates of 82%–100%, highlighting a need for more complete reporting and for further scaling up of programs in some countries.14
Sterile syringe distribution and opioid substitution therapy are effective approaches to reduce the risks of blood-borne virus transmission among PWID. Recent guidance from ECDC and the European Monitoring Centre for Drugs and Drug Addiction focused on the prevention and control of infectious diseases among PWID and recommended the provision of sterile injecting equipment and drug dependence treatment as key interventions to reduce the risk of individuals contracting and transmitting infectious diseases, including viral hepatitis, through injecting drug use.15 However, only 2 of the 14 EU/EEA countries with available data on coverage of both programs reported in 2021 had reached both the syringe distribution and opioid substitution therapy target in this regard, highlighting the need to improve reporting and strengthen harm reduction programs for PWID.15
Costs associated with HBV testing and therapy are barriers to reducing the undiagnosed population and ensuring infected individuals in need of treatment actually receive it. In some countries, point-of-care testing is unavailable and the cost of laboratory testing remains high, with some individuals forced to pay out-of-pocket costs for testing and therapy.16 HBV therapy is sometimes only approved in the hospital setting16 and, based on information reported to ECDC, there remain restrictions in access to treatment for some migrant populations in 8 countries and for PWID in 3 countries.16,17 These factors, together with the stigma faced by individuals living with hepatitis B, constitute additional obstacles to eradication. Besides, in the case of migrants, language and cultural barriers also have to be taken into consideration. These challenges can be partially solved through the implementation of national plans against hepatitis B with dedicated funding for care, and communication campaigns to increase awareness of the disease among patients, health care providers, and policymakers. However, it remains of concern that the majority of countries in the region lack HBV screening guidelines or elimination plans.
POTENTIAL SOLUTIONS
A critical factor to address in tackling the epidemic of chronic HBV is for local communities to gain a clear understanding of their epidemiology. This will enable targeted actions, especially in relation to efforts to reduce the undiagnosed population to ensure that infected individuals receive a prompt diagnosis and linkage to care, thereby minimizing associated morbidity and mortality. To achieve this, it is essential to reinforce screening programs, particularly in at-risk populations and across age-specific cohorts of the population in countries where the epidemiological context justifies such an approach.9 However, a scaling up of testing alone is not the solution; linkage to care and assessment of eligibility for antiviral therapy are of utmost importance. The current linkage model in many European countries is based on testing for viral hepatitis and initial diagnosis of liver disease in primary care centers and subsequent specialist referral to complete the diagnosis and provide therapy, if indicated. However, this system is not centered on the patient and can be bureaucratic and can result in a loss to follow-up of patients. A study that evaluated the proportion of patients diagnosed with hepatitis B and linked to care patients found that 20% of the total were not linked to care.18 Thus, a simplified community-centered approach should be implemented to facilitate a timely diagnosis and transition to care, particularly for vulnerable populations.
Certain best practice examples in Europe show that scaling up testing among key populations is possible. The VH-COMSAVAC program in Barcelona was designed to screen for HBV infection in migrants from sub-Saharan Africa.19 Individuals with no history of HBV infection and no previous immunity are vaccinated, and infected individuals are evaluated for therapy. Another example is hospital emergency room screening, which has shown a higher prevalence of hepatitis B infection than in the general population and facilitates seamless linkage to care.20 Again, important steps in this line are the generation of robust epidemiological data to guide HBV elimination strategies and a strong political commitment to provide specific funding.
HBV is more prevalent in vulnerable and unserved populations more affected by social determinants of health.21,22 This underscores the importance of developing policies focused on equity and providing targeted interventions among marginalized populations. Given previous analyses highlighting the disproportionate burden of HBV among migrant populations in the region and dynamic demographic changes over time, it is critical that countries have up-to-date and accurate data on their local epidemic that takes into account, where relevant, migrant populations from high-endemicity countries. Such data are critical to developing a comprehensive response to HBV that ensures equitable access to services across the whole population and improves health outcomes. Table 1 shows the key final points. In summary, within the EU/EEA region, critical barriers remain to the effective scaling up of diagnosis and access to treatment and the coverage of some key prevention programs require further expansion. The lack of robust recent data on the HBV continuum of care is an obstacle to effective monitoring and evaluation of progress toward achieving the WHO elimination targets. Public health and clinical organizations, such as ECDC and EASL, have critical roles to play in supporting countries in their elimination efforts through the provision of guidance, sharing of good practices, and collaborations to help develop sustainable systems that provide reliable information for action. Continued cooperation between the organizations is important to bridge the gap between public health and clinical groups and help synergize elimination efforts across the region. The forthcoming WHO hepatitis B global guidelines will be an opportunity to map new directions for HBV elimination based on simplicity and integrated services and will provide an opportunity to refocus and reinvigorate local efforts.
TABLE 1.
HBV elimination in Europe: Key points
| Estimated burden of HBV infection across the EU/EEA is high with viral hepatitis–specific deaths exceeding those attributed to HIV or tuberculosis |
| Considerable variation in burden across countries and between different risk groups |
| Effective prevention against hepatitis B through vaccination is widely available, but suboptimal coverage in many countries with only half of all countries reaching the 95% elimination target |
| Data relating to strategies to reduce mother-to-child transmission also highlight a need for more complete reporting with gaps in the data and for further scaling up in some countries |
| Data around diagnosis is also incomplete but indicate that a high proportion of cases remain undiagnosed and barriers to testing, such as out-of-pocket cost and a lack of point-of-care tests, still exist |
| Barriers around scaling up treatment also remain with treatment often only available in hospital settings and restrictions in access to treatment for migrant populations or people who inject drugs in some countries |
Acknowledgments
CONFLICTS OF INTEREST
Maria Buti advises Gilead, GSK and Janssen. The remaining author has no conflicts to report.
Footnotes
Abbreviations: ECDC, European Center for Disease Prevention and Control; EEA, European Economic Area; EU, European Union; GHSS, Global Health Sector Strategy; PWID, people who inject drugs.
REFERENCES
- 1. Hofstraat SHI, Falla AM, Duffell EF, Hahné SJM, Amato-Gauci AJ, Veldhuijzen IK, et al. Current prevalence of chronic hepatitis B and C virus infection in the general population, blood donors and pregnant women in the EU/EEA: A systematic review. Epidemiol Infect. 2017;145:2873–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mårdh O, Quinten C, Amato-Gauci AJ, Duffel E. Mortality from liver diseases attributable to hepatitis B and C in the EU/EEA—Descriptive analysis and estimation of 2015 baseline. Infect Dis. 2020;52:625–637. [DOI] [PubMed] [Google Scholar]
- 3. Bivegete S, McNaughton AL, Trickey A, Thornton Z, Scanlan B, Lim AG, et al. Estimates of hepatitis B virus prevalence among general population and key risk groups in EU/EEA/UK countries: A systematic review. Euro Surveill. 2023;28:2200738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. High-level resource mobilization conference to eliminate viral hepatitis. 2023. https://www.who.int/news/item/17-05-2023-high-level-resource-mobilization-conference-to-eliminate-viralhepatitis
- 5. World Health Organization. Combating Hepatitis B and C to reach elimination by 2030. World Health Organization (WHO); 2016. [Google Scholar]
- 6. Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022–2030. 2022. Accessed June 9, 2024. https://www.who.int/teams/global-hiv-hepatitisandstisprogrammes
- 7. WHO . Transforming our world: The 2030 agenda for sustainable development. 2015. https://sustainabledevelopment.un.org
- 8. European Centre for Disease Prevention and Control . Prevention of Hepatitis B and C in the EU/EEA and the UK November 2020. ECDC; 2022. https://www.ecdc.Europe.eu/sites [Google Scholar]
- 9. Sharrock KC, Noori T, Axelsson M, Buti M, Diaz A, Fursa O, et al. Monitoring progress towards elimination of hepatitis B and C in the EU/EEA. PLoS Glob Public Health. 2022;7:e000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. European Centre for Disease Prevention and Control . Monitoring of Responses to the Hepatitis B and C Epidemics in EU/EEA Countries—2020 data. ECDC; 2022. https://www.ecdc.europa.eu/sites/default/files/documents/Monitoring-responses-to-hepatitis-B-and-C-epidemics-2020-data.pdf [Google Scholar]
- 11. Kondili LA, Buti M, Riveiro-Barciela M, Maticic M, Negro F, Berg T, et al. Impact of the COVID-19 pandemic on hepatitis B and C elimination: An EASL survey. JHEP Rep. 2022;9:100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nardone A, Nerlander L, Duffell E, Valenciano M, Buti M, Marcos-Fosch C, et al. A pilot sentinel surveillance system to monitor treatment and treatment outcomes of chronic hepatitis B and C infections in clinical centres in three European countries, 2019. Euro Surveill. 2023;28:2200184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Polaris Observatory Collaborators . Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: A modelling study. Lancet Gastroenterol Hepatol. 2023;8:879–907. [DOI] [PubMed] [Google Scholar]
- 14. European Centre for Disease Prevention and Control . Prevention of Hepatitis B and C in the EU/EEA. ECDC; 2022. https://www.ecdc.europa.eu/sites/default/files/documents/hepatitis-B-and-C-prevention-eu-december-2022.pdf [Google Scholar]
- 15. European Centre for Disease Prevention and Control (ECDC) and European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) . Prevention and Control of Infectious Diseases Among People Who Inject Drugs: 2023 Update. ECDC; 2023. https://www.ecdc.europa.eu/sites/default/files/documents/Guidance-prevention-control-PWID-6-November.pdf [Google Scholar]
- 16. Karlsen TH, Sheron N, Zelber-Sagi S, Carrieri P, Dusheiko G, Bugianesi E, et al. The EASL-Lancet Liver Commission: Protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399:61–116. [DOI] [PubMed] [Google Scholar]
- 17. Ahmad AA, Falla AM, Duffell E, Noori T, Bechini A, Reintjes R, et al. Estimating the scale of chronic hepatitis B virus infection among migrants in EU/EEA countries. BMC Infect Dis. 2018;18:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feliu A, Barreira-Diaz A, Rando A, Vargas-Accarino E, Palom A, Vico-Romero J, et al. Assessing the rate of non-linkage to care and identifying barriers in individuals living with hepatitis B. Results of the LINK-B Study. Liver Int. 2024;44:706–714. [DOI] [PubMed] [Google Scholar]
- 19. Picchio CA, Nomah DK, Araujo SG, Rando-Segura A, Fernández E, Buti M, et al. A novel model of care for simplified testing of HBV in African communities during the COVID-19 pandemic in Spain. Sci Rep. 2021;11:17063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Llaneras J, Ruiz-Cobo JC, Rando A, Barreira-Diaz A, Domínguez-Hernández R, Rodríguez-Frías F, et al. Integrated screening of hepatitis B, C and D in the emergency department of a tertiary care hospital. JHEP Rep. 2023;6:100932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zampino R, Boemio A, Sagnelli C, Alessio L, Adinolfi LE, Sagnelli E, et al. Hepatitis B virus burden in developing countries. World J Gastroenterol. 2015;42:11941–11953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kondili LA, Lazarus JV, Jepsen P, Murray F, Schattenberg JM, Korenjak M, et al. Inequities in primary liver cancer in Europe: The state of play. J Hepatol. 2024;80:645–660. [DOI] [PubMed] [Google Scholar]