Abstract
Objectives:
Neighborhood contextual factors are associated with liver transplant outcomes. We analyzed associations between neighborhood-level socioeconomic status and healthcare utilization for pediatric liver transplant recipients.
Methods:
We merged the PHIS and SRTR databases and included liver transplant recipients ≤21 years hospitalized between 1/1/2004-5/31/2022. Outcomes were annual inpatient bed-days, risk of hospitalizations, and risk of liver biopsies. The primary exposure was zip code-based neighborhood income at transplant. We applied causal inference for variable selection in multivariable analysis. We modeled annual inpatient bed days with mixed-effect zero-inflated Poisson regression. We modeled rates of hospitalization and liver biopsy with a Cox-type proportional rate model.
Results:
We included 1,006 participants from 29 institutions. Children from low-income neighborhoods were more likely to be publicly insured (67% vs 46%), Black (20% vs 12%), Hispanic (30% vs 17%) and have higher MELD/PELD scores at transplant (17 vs 13) than the remaining cohort. We found no differences in inpatient bed-days or rates of hospitalization across neighborhood groups. In univariable analysis, low-income neighborhoods were associated with increased rates of liver biopsy (RR 1.57, 95% CI: 1.04, 2.34, p=0.03). These findings persisted after adjusting for insurance, race, and ethnicity (RR 1.86, 95% CI: 1.23, 2.83, p<0.01).
Conclusions:
This is the first study to describe neighborhood-level differences in healthcare utilization in pediatric liver transplant. Children from low-income neighborhoods undergo more liver biopsies than other children. These procedures are invasive and potentially preventable. In addition to improving outcomes, interventions to mitigate health inequities in liver transplant may reduce resource utilization.
Keywords: neighborhood income, liver biopsy, graft rejection, medication adherence
Introduction:
Pediatric liver transplantation outcomes have improved considerably in recent decades, with 95% survival at 1 year1. However, long-term allograft and patient health remain suboptimal. Indeed, at 10 years post-transplant, less than a third of pediatric liver transplant recipients are morbidity free2,3.
Neighborhood-level factors impact outcomes after liver transplantation4,5. We have demonstrated that neighborhood-level measures of socioeconomic status and primary care access are associated with graft failure and death after liver transplant5,6. Further, children living in socioeconomically deprived neighborhoods are more likely to be poorly adherent to immune suppressant medications4.
There is limited knowledge of whether children after liver transplant from low socioeconomic status neighborhoods have increased healthcare utilization. We aimed to characterize healthcare utilization by neighborhood income levels. We hypothesized that children from low-income neighborhoods would have higher resource utilization. To test this hypothesis, we merged the Scientific Registry of Transplant Recipients Database (SRTR) with the Pediatric Health Information System (PHIS), the largest administrative dataset of pediatric healthcare utilization in the U.S.
Methods:
Data Sources
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidate, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. For each transplant recipient, the SRTR contains date of transplant, demographic data, and if applicable, data on graft failure and death.
The Pediatric Health Information System (PHIS) is an administrative database developed by the Children’s Hospital Association. It includes encounter-level clinical and financial data from 52 tertiary care free-standing children’s hospitals in the United States7,8. For each encounter, PHIS includes International Classification of Disease (ICD) diagnostic and procedural codes, costs of services rendered, and patient demographic data. Patients in the PHIS database are given unique patient identifiers and can be followed longitudinally across encounters.
Study Participants & Data Merging
We included inpatient and observation hospitalizations for patients age ≤21 years at time of transplant. We isolated patients by extracting PHIS records from January 2004 to December 2022 for patients with an ICD-9/10 diagnostic code consistent with history of liver transplant and at least one hospitalization with an ICD-9/10 procedure code for liver or organ transplant. Eligible ICD codes are listed in Figure, Supplemental Digital Content 1. We applied administrative censoring of the PHIS dataset on 5/31/2022 as that marked the endpoint of the SRTR dataset, with no further records available after that date.
We performed a merge of the PHIS and SRTR databases at the patient level, merging records of patients with matching hospitals, hospitalization dates for liver transplant, patient age, and sex. We merged 1,006 of 1,214 (82.9%) available patient records with this approach, and excluded patients whose records were unable to be merged from our analysis. Because we sought to evaluate post-transplant hospitalizations, we excluded hospitalizations occurring prior to liver transplantation from our analysis and we excluded patients that died during their initial transplant hospitalization. A flow chart describing our inclusion criteria, exclusion criteria, and merging protocol is shown in Figure, Supplemental Digital Content 1. A comparison of merged and unmerged patients is displayed in Table, Supplemental Digital Content 2. Compared to excluded patients, included patients were younger at time of transplant, less likely to live in the Southern United States, and more likely to live in the Western United States.
This study was deemed exempt by the University of California, San Francisco Institutional Review Board.
Outcomes
Our primary outcome was healthcare utilization, measured as total annual inpatient bed days since transplant, risk of hospitalizations, and risk of liver biopsies. Total annual inpatient bed days were calculated on a yearly basis starting from day of transplant. Hospitalizations were defined as all-cause admissions, either inpatient or observation.
These outcome measures were selected to capture broad aspects of healthcare utilization that impose a substantial burden on the healthcare system and patients. We also selected these outcome measures to enable characterization of utilization that may be influenced by provider bias (e.g., decision to perform liver biopsy) or patient challenges with self-management (e.g., poor adherence raising concern for T-cell mediated rejection), and thus may be modifiable with socially informed interventions. We did not directly capture rates of T-cell mediated rejection (TCMR) as centers have variable practices for diagnosing and treating TCMR, and we were concerned about the interpretability of billing codes for TCMR reported within PHIS. However, we conceptualized that providers may have different thresholds for seeking a liver biopsy based on their perceptions of a patient’s social risk factors, and therefore, the metric of liver biopsy episodes may effectively capture both provider bias and differential risks of TCMR.
Our prior work has shown an association between disadvantaged neighborhoods and medication non-adherence and graft failure4,5. We therefore hypothesized that low neighborhood income would be associated with an increased risk of liver biopsy, particularly after the first year of transplant, as late complications of liver transplant are more likely to reflect challenges with self-management9.
Primary Exposure
Our primary exposure was neighborhood income, derived from the median household income (HHI) of the patient’s zip code according to 2010 U.S. Census data. This measure has been previously used as a surrogate marker for neighborhood socioeconomic status10,11. We applied HHI data from the 2010 U.S. Census as this data was readily available in PHIS and it most closely reflected the midpoint of the observation period (2004-2022). We categorized patients into four HHI groups, using previously described household income cut-offs10 based on U.S. federal poverty guidelines12. HHI-1 (<$33,075) represents low-income neighborhoods, HHI-2 ($33,075 to $44,099) and HHI-3 ($44,100 to 66,149) represent middle-income neighborhoods, and HHI-4 (≥$66,150) represents high-income neighborhoods.
Statistical Analysis
We constructed a directed acyclic graph to identify potential confounders for the relationship between neighborhood income and total annual inpatient bed days, cumulative rate of hospitalizations, and cumulative rate of liver biopsies (Figure, Supplemental Digital Content 3). We adjusted for these possible confounders in our multivariable models. Participant race was classified into the following categories: “Black”, “White”, and “Other”, and participant ethnicity was classified as “Hispanic” or “not Hispanic”. We recognize race and ethnicity as social constructs that measure structural inequities experienced by marginalized groups, and included these variables in our multivariable models accordingly. To assess variations across racial and ethnic groups, we selected “White” and “not Hispanic” as reference groups as these groups had the largest sample sizes in this cohort. We recognize that these groups should not be considered the default reference groups. We classified insurance status as “Private”, “Public” and “Other”. We considered insurance type as a proxy for individual socioeconomic status and selected “Private” as the reference group for insurance type.
We evaluated the association between neighborhood income and total annual inpatient bed days with a mixed-effects zero-inflated Poisson regression model13. We selected this model as it enabled us to account for within-patient variation in inpatient bed days across different years. Additionally, it allowed us to account for the prevalence of excessive zero inpatient bed days in consecutive years post-transplant. The model comprises two components: the zero-inflation component, which estimates the probability of observing positive inpatient bed days, and the Poisson component, which models the positive bed days in a Poisson regression. A subject-specific random intercept is included in both components to accommodate individual variability.
To assess the association between neighborhood income and hospitalizations, we performed a recurrent event analysis of hospitalizations that occurred after liver transplant. The observation of hospitalizations was terminated at the end of the study period (5/31/2022), on the patient's 22nd birthday, or on the date of death, whichever occurred first. We recognize the terminal event may potentially inform the risk of experiencing repeat hospitalization. Thus, we selected a Cox-type proportional rate model that does not require a noninformative censoring assumption14. We evaluated the association between neighborhood income and liver biopsies with the same approach. We also performed landmark analyses to assess risk of liver biopsy within one year of transplant and more than one year after transplant.
Statistical analyses were performed using R Core Team (2023)15. The mixed-effects zero-inflated Poisson regression model was conducted using the glmmTMB package13, and recurrent event analyses were performed with the reReg package16.
Results
Patient Characteristics
Our final cohort included 1,006 liver transplant recipients with 6,396 hospitalizations at 29 freestanding children’s hospitals. Approximately 14% of the sample identified as Black, 20% identified as Hispanic, and 52% had public insurance. Descriptive statistics of the study sample are displayed in Table 1. The most common causes for hospitalization were liver transplant complication (15%), liver transplant rejection (5%), fever (4%), and dehydration (3%). The median neighborhood income for the cohort was $41,178 (interquartile range [IQR] $32,547, $52,030) and approximately 27% of the sample lived in a low-income neighborhood, hereafter referred to as “HHI-1”. Compared to other neighborhoods, children from HHI-1 had higher model for end stage liver disease (MELD) or pediatric end stage liver disease model (PELD) scores at time of transplant (median score 17 vs. 13). Children from HHI-1 were also more likely to have public insurance (67% vs. 46%), more likely to be Black (20% vs. 12%), and more likely to be Hispanic (30% vs. 17%).
Table 1.
Baseline characteristics by neighborhood income.
| Total cohort | Neighborhood income | ||||
|---|---|---|---|---|---|
| N(%) or median (IQR) | |||||
| Total cohort n (%) |
HHI-1 <$33,075 n(%) |
HHI-2-4 ≥$33,075 n (%) |
P-value | ||
| Total patients | 1006 | 269 | 737 | ||
| Age at transplant (years) | 2.6 (1.0, 10.2) | 2.3 (1.0, 9.5) | 2.7 (0.9, 10.4) | 0.16 | |
| Median (Q1, Q3) | |||||
| Sex | 504 (50%) | 145 (54%) | 359 (49%) | 0.15 | |
| Female | |||||
| Region of United States | 0.77 | ||||
| Midwest | 266 (26%) | 71 (26%) | 195 (27%) | ||
| Northeast | 313 (31%) | 84 (31%) | 229 (31%) | ||
| South | 199 (20%) | 58 (22%) | 141 (19%) | ||
| West | 228 (23%) | 56 (21%) | 172 (23%) | ||
| Race | <0.01 | ||||
| White | 787 (78%) | 203 (76%) | 584 (79%) | ||
| Black | 141 (14%) | 55 (20%) | 86 (12%) | ||
| Asian | 50 (5%) | 6 (2%) | 44 (6%) | ||
| Multiracial | 17 (2%) | 4 (2%) | 13 (2%) | ||
| American Indian | 6 (1%) | 1 (0%) | 5 (1%) | ||
| Pacific Islander | 5 (1%) | 0 (0%) | 5 (1%) | ||
| Ethnicity | <0.001 | ||||
| Non-Hispanic | 800 (80%) | 189 (70%) | 611 (83%) | ||
| Hispanic | 206 (21%) | 80 (30%) | 126 (17%) | ||
| Insurance | < 0.001 | ||||
| Public | 522 (52%) | 180 (67%) | 342 (46%) | ||
| Private | 445 (44%) | 71 (26%) | 374 (51%) | ||
| Other | 17 (2%) | 12 (5%) | 5 (1%) | ||
| None | 22 (2%) | 6 (2%) | 16 (2%) | ||
| Recipient diagnosis | 0.30 | ||||
| Biliary atresia (BA) | 337 (34%) | 95 (35%) | 242 (33%) | ||
| Cholestatic disease (non-BA) | 166 (17%) | 47 (18%) | 119 (16%) | ||
| Acute liver failure | 90 (9%) | 27 (10%) | 63 (9%) | ||
| Metabolic | 129 (13%) | 23 (9%) | 106 (14%) | ||
| Oncologic (tumor) | 67 (7%) | 18 (7%) | 49 (7%) | ||
| Autoimmune Hepatitis | 39 (4%) | 13 (5%) | 26 (4%) | ||
| Other | 174 (17%) | 45 (17%) | 129 (18%) | ||
| Missing | 4 (0%) | 1 (0%) | 3 (0%) | ||
| Laboratory MELD/PELD at transplant | 14 (4, 24) | 17 (6, 25) | 13 (4, 24) | 0.04 | |
| Median (Q1, Q3) | |||||
| Status 1a/1b | 284 (28%) | 72 (27%) | 212 (29%) | 0.53 | |
| Yes | |||||
| Living donor transplant | 139 (14%) | 39 (15%) | 100 (14%) | 0.71 | |
| Yes | |||||
IQR: interquartile range; MELD: model for end-stage liver disease; PELD: pediatric end stage liver disease.
Insurance categories include public (in-state Medicaid, out-of-state Medicaid, Tricare, Medicare, or other government insurance), private (commercial HMO, commercial PPO, or other commercial insurance), and other (self-pay, charity, other payer, or hospital chose not to bill).
Total Annual Inpatient Bed Days
In univariable analysis, there were no differences in total annual inpatient bed days by neighborhood income group (HHI-1: regression coefficient [β] 0.10, 95% confidence interval [CI] −0.09, 0.29, p=0.30). After adjusting for insurance, race, and ethnicity, we still found no difference in total annual inpatient bed days by neighborhood income group (HHI-1: β 0.11, 95% CI −0.08, 0.31, p=0.26) (Table 2).
Table 2.
Univariable and multivariable analysis depicting total annual inpatient bed days per year and cumulative rate of hospitalizations (N=1,006).
| Total annual inpatient bed days | Cumulative rate of hospitalizations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||||||
| Variable | β | 95% CI | P-value | β | 95% CI | P-value | RR | 95% CI | P-value | RR | 95% CI | P-value |
| Neighborhood income | ||||||||||||
| HHI-4 | REF | REF | REF | REF | ||||||||
| HHI-3 | 0.09 | −0.10, 0.27 | 0.37 | 0.09 | −0.09, 0.28 | 0.32 | 1.08 | 0.77, 1.54 | 0.63 | 1.12 | 0.82, 1.52 | 0.49 |
| HHI-2 | 0.08 | −0.10, 0.27 | 0.39 | 0.09 | −0.10, 0.28 | 0.35 | 0.97 | 0.72, 1.31 | 0.83 | 1.00 | 0.73, 1.38 | >0.99 |
| HHI-1 | 0.10 | −0.09, 0.29 | 0.30 | 0.11 | −0.08, 0.31 | 0.26 | 1.05 | 0.77, 1.43 | 0.77 | 1.11 | 0.79, 1.57 | 0.56 |
| Insurance | ||||||||||||
| Private | REF | REF | REF | REF | ||||||||
| Public | 0.00 | −0.13, 0.13 | 0.99 | −0.03 | −0.16, 0.11 | 0.68 | 0.96 | 0.76, 1.23 | 0.77 | 0.96 | 0.69, 1.35 | 0.83 |
| Other | −0.01 | −0.14, 0.12 | 0.88 | −0.03 | −0.16, 0.11 | 0.71 | 0.57 | 0.38, 0.85 | <0.01 | 0.57 | 0.37, 0.88 | 0.01 |
| Race | ||||||||||||
| White | REF | REF | REF | REF | ||||||||
| Black | −0.06 | −0.21, 0.09 | 0.45 | −0.04 | −0.20, 0.11 | 0.59 | 1.11 | 0.72, 1.68 | 0.66 | 1.08 | 0.68, 1.72 | 0.74 |
| Other | 0.08 | −0.12, 0.27 | 0.44 | 0.10 | −0.09, 0.29 | 0.32 | 1.17 | 0.88, 1.55 | 0.28 | 1.16 | 0.87, 1.57 | 0.31 |
| Ethnicity | ||||||||||||
| Not Hispanic | REF | REF | REF | REF | ||||||||
| Hispanic | 0.08 | −0.05, 0.20 | 0.23 | 0.08 | −0.06, 0.21 | 0.27 | 0.94 | 0.78, 1.13 | 0.50 | 0.96 | 0.73, 1.27 | 0.79 |
β: regression coefficient; RR: rate ratio; CI: confidence interval; HHI: median annual household income of patient zip code.
HHI-1: <$33,075; HHI-2 $33,075 to $44,099; HHI-3 $44,100 to $66,149; HHI-4 ≥$66,150.
Risk of Hospitalizations
In univariable analysis, we found no difference in the cumulative rate of hospitalizations by neighborhood income group (HHI-1: RR 1.05, 95% CI 0.77, 1.43, p=0.77). There were still no differences in cumulative rate of hospitalization by neighborhood income group after adjusting for insurance, race, and ethnicity (HHI-1: RR 1.11, 95% CI 0.79, 1.57, p=0.56) (Table 2).
Risk of Liver Biopsies
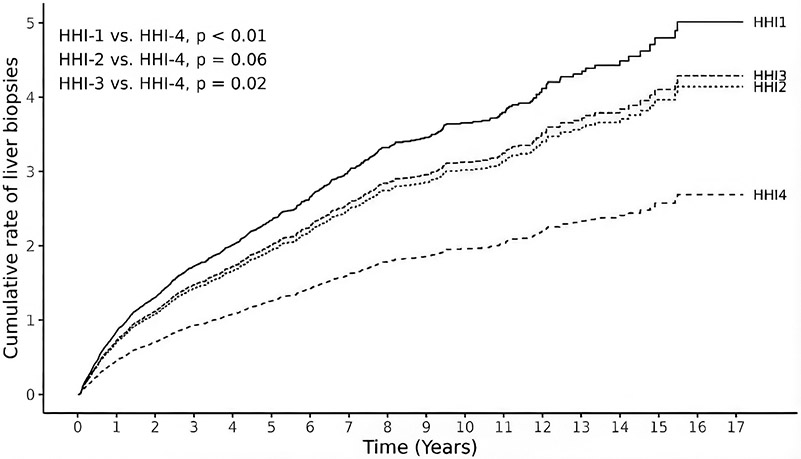
Of 1,006 participants, 463 (46%) underwent at least one liver biopsy during the study period. In univariable analysis, children from HHI-1 had a 57% higher cumulative rate of liver biopsies compared to HHI-4 (RR 1.57, 95% CI 1.04, 2.34, p=0.03) (Table 3). These findings persisted after adjusting for insurance, race, and ethnicity, with children from HHI-1 experiencing an 86% higher cumulative rate of liver biopsies compared to children from HHI-4 (RR 1.86, 95% CI 1.23, 2.83, p<0.01). Figure 1 depicts the cumulative rate of liver biopsies by neighborhood income group over the total study period, within one year of transplant, and after the first year of transplant.
Table 3.
Univariable and multivariable recurrent event analysis depicting the cumulative rate of liver biopsy during first year post-transplant, one or more years after transplant, and over total study period (N=1,006).
| Cumulative rate of liver biopsies | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total study period (N=1,006) | ≤1 year post transplant (N=1,006) | >1 year post transplant (N=614) | ||||||||||||||||
| Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | |||||||||||||
| Variable | RR | 95% CI | P-value | RR | 95% CI | P-value | RR | 95% CI | P-value | RR | 95% CI | P-value | RR | 95% CI | P-value | RR | 95% CI | P-value |
| HHI | ||||||||||||||||||
| HHI-4 | REF | REF | REF | REF | REF | REF | ||||||||||||
| HHI-3 | 1.45 | 0.94, 2.20 | 0.09 | 1.60 | 1.07, 2.36 | 0.02 | 1.65 | 1.07, 2.56 | 0.02 | 1.86 | 1.19, 2.92 | 0.01 | 0.48 | 0.15, 1.52 | 0.21 | 0.51 | 0.17, 1.57 | 0.24 |
| HHI-2 | 1.34 | 0.85, 2.10 | 0.21 | 1.54 | 0.98, 2.44 | 0.06 | 1.39 | 0.89, 2.18 | 0.15 | 1.65 | 1.03, 2.66 | 0.04 | 0.50 | 0.17, 1.48 | 0.21 | 0.57 | 0.19, 1.70 | 0.32 |
| HHI-1 | 1.57 | 1.04, 2.34 | 0.03 | 1.86 | 1.23, 2.83 | <0.01 | 1.60 | 1.03, 2.46 | 0.04 | 1.99 | 1.24, 3.22 | <0.01 | 1.45 | 0.34, 6.17 | 0.61 | 1.90 | 0.34, 10.38 | 0.47 |
| Insurance | ||||||||||||||||||
| Private | REF | REF | REF | REF | REF | REF | ||||||||||||
| Public | 0.76 | 0.59, 0.97 | 0.03 | 0.65 | 0.50, 0.86 | <0.01 | 0.73 | 0.57, 0.94 | 0.02 | 0.64 | 0.49, 0.85 | <0.01 | 0.56 | 0.24, 1.32 | 0.18 | 0.59 | 0.22, 1.57 | 0.29 |
| Other | 0.91 | 0.44, 1.92 | 0.81 | 0.83 | 0.39, 1.75 | 0.62 | 0.78 | 0.43, 1.42 | 0.42 | 0.71 | 0.30, 1.68 | 0.44 | 2.08 | 0.23, 19.11 | 0.52 | 1.55 | 0.18, 13.07 | 0.69 |
| Race | ||||||||||||||||||
| White | REF | REF | REF | REF | REF | REF | ||||||||||||
| Black | 0.88 | 0.66, 1.17 | 0.37 | 1.02 | 0.74, 1.40 | 0.91 | 0.88 | 0.63, 1.22 | 0.44 | 1.00 | 0.71, 1.40 | 0.99 | 0.63 | 0.30, 0.76 | 0.22 | 0.48 | 0.20, 1.14 | 0.09 |
| Other | 1.43 | 0.97, 2.12 | 0.07 | 1.65 | 1.12, 2.44 | 0.01 | 1.65 | 1.11, 2.48 | 0.02 | 1.86 | 1.24, 2.80 | <0.01 | 0.64 | 0.25, 1.62 | 0.34 | 0.68 | 0.31, 1.48 | 0.33 |
| Ethnicity | ||||||||||||||||||
| Not Hispanic | REF | REF | REF | REF | REF | REF | ||||||||||||
| Hispanic | 1.32 | 1.00, 1.73 | 0.05 | 1.49 | 1.08, 2.05 | 0.01 | 1.21 | 0.90, 1.62 | 0.21 | 1.39 | 1.01, 1.92 | 0.04 | 0.58 | 0.28, 1.22 | 0.15 | 0.51 | 0.24, 1.09 | 0.08 |
RR: rate ratio; CI: confidence interval; HHI: median annual household income of patient zip code. HHI-1: <$33,075; HHI-2 $33,075 to $44,099; HHI-3 $44,100 to $66,149; HHI-4 ≥$66,150.
Figure 1.
Multivariable recurrent event analysis for cumulative rate of liver biopsies over the total study period by neighborhood income group (N=1,006).
HHI represents median annual household income of patient zip code.
HHI-1: <$33,075; HHI-2 $33,075 to $44,099; HHI-3 $44,100 to $66,149; HHI-4 ≥$66,150.
Discussion
This is the first study to evaluate the association between neighborhood income and healthcare utilization in pediatric liver transplant recipients, accomplished through a unique merge of the PHIS and SRTR databases. We found no evidence that children from low-income neighborhoods spend more time in the hospital or have a higher risk of hospitalizations after liver transplantation. Yet, we found that children living in low-income neighborhoods undergo nearly double the rates of liver biopsy compared to children from wealthy neighborhoods, experiencing approximately four additional liver biopsies in the first 10 years post-transplant. Children from middle-income neighborhoods also undergo more liver biopsies relative to those from wealthy neighborhoods. These findings suggest that neighborhood wealth is a protective factor against liver biopsies among transplant recipients. Indeed, strategies to address disparate resource utilization may be beneficial for a large proportion of liver transplant recipients, benefiting those from both low- and middle-income neighborhoods.
Liver biopsies come at a cost to patients, including the risks associated with an invasive procedure, financial costs, and missed days from school or work17-20. Moreover, while a relatively safe procedure, liver biopsies are resource intensive procedures—they usually require anesthesia, proceduralist involvement, and an overnight stay19,20. Thus, addressing this disparity may improve both caregiver experience of hassle and hospital churn and capacity. It is possible that the observed increase in liver biopsies among those from lower income neighborhoods reflects poorer allograft health, provider bias, or heightened concern for poor adherence. It is also possible that children from the wealthiest neighborhoods may experience certain advantages that promote optimal allograft function and reduced need for biopsy, such as strong support networks and less competing demands in the household that facilitate medication adherence. Characterization of assets available to those with greater neighborhood wealth may be one strategy to develop interventions that will benefit children from less advantaged neighborhoods.
We have previously shown that pediatric liver transplant recipients from disadvantaged neighborhoods have higher rates of medication non-adherence and allograft injury4,5. Combined with our findings of increased liver biopsy rates, this represents a potential cascade in which challenges with self-management increase risk for allograft injury, and increased allograft injury results in a higher rate of liver biopsies. To confirm this cascade, future research should assess the relationship between neighborhood income and episodes of T-cell mediated rejection using a more robust data source (e.g., the Society for Pediatric Liver Transplantation registry). Consideration of neighborhood context when evaluating transplant recipients may be one avenue to risk stratify patients and mitigate disparate resource utilization.
We hypothesized that neighborhood-level differences in liver biopsy would emerge only after the first year of transplant, as later complications more often reflect challenges with self-management5,9. Contrary to this hypothesis, neighborhood-level differences in risk of liver biopsy were most prominent in the first year and attenuated after one year post-transplant. One potential explanation is that clinicians may have a lower threshold for performing biopsies on patients who are deemed socially at risk, due to concerns for medication non-adherence or potential loss to follow up. Such risk stratification may be beneficial for patients, as early recognition of graft rejection is important for achieving optimal outcomes. However, another possibility is that clinician bias leads to these higher rates of liver biopsy. This study lays the groundwork for future studies to characterize the reasons for the disparate rates of liver biopsy.
We found no differences in annual inpatient bed days by neighborhood income group. This contrasts other pediatric conditions, in which low neighborhood income has been associated with longer hospitalizations21,22. We also found no difference in the risk of hospitalizations by neighborhood income group. This was unexpected, as neighborhood deprivation is associated with increased morbidity and mortality5,23. Perhaps our data reflect a difference in healthcare-seeking behaviors across neighborhood groups, with a delayed presentation to care by individuals from less advantaged neighborhoods. Latency in seeking medical care could result in more severe initial hospital presentation, potentially explaining the increased rates of liver biopsy we observed among patients from less affluent neighborhoods. Future work should characterize disease severity of hospitalized liver transplant recipients by neighborhood income to elucidate whether patients from low- and middle-income neighborhoods are underutilizing the healthcare system relative to their medical needs.
Consistent with our prior research, children from disadvantaged neighborhoods had higher MELD/PELD scores at time of transplant5,24. This finding adds to the growing body of research suggesting neighborhood-level disparities exist prior to transplantation5,24. Notably, high MELD/PELD scores at transplant have been associated with an increased risk of adverse outcomes25, including graft failure or death. Thus, strategies to narrow disparities in the pre-transplant period may have the potential to mitigate adverse health outcomes after liver transplantation.
We acknowledge the following limitations. First, we only assessed hospitalizations within the PHIS network. Therefore, hospitalizations that occurred outside of a PHIS center were not accounted for in our analyses. However, we confirmed that all participants in our cohort received their liver transplant at a PHIS participating hospital, enabling us to capture all hospitalizations that occurred at a child’s primary transplant center. Second, neighborhood income was derived from patient’s zip code. We recognize there may be variability of neighborhood characteristics within zip codes, including safety, wealth, and social support. However, zip-code level income is easily accessible through U.S. Census Bureau Data and has been used as a measure of neighborhood socioeconomic status in other pediatric diseases10,11,21. Third, we did not directly assess hospitalizations due to T-cell mediated rejection, an important source of healthcare utilization in liver transplant recipients. The PHIS database relies on billing codes, and we were concerned about the heterogenous nature of the diagnostic codes for TCMR. Nevertheless, our evaluation of cumulative hospitalizations and the risk of liver biopsy enabled us to indirectly gauge the impacts of neighborhood income on healthcare utilization in pediatric liver transplantation, which may reflect the influence of neighborhood income on self-management challenges and provider biases. Finally, we acknowledge that with the sole use of the SRTR and PHIS databases, it is impossible to confirm a 100% patient-level match across databases. However, to address this, we based our approach to merging patient records on a previously validated method for merging patient records across the two databases26. Further, our merge of the SRTR and PHIS enabled us to capture more robust demographic information than could have been obtained from either database alone, which greatly enhanced our analyses in a way that may be useful for future studies.
Our findings add to the growing body of research showing neighborhood context as an important predictor of liver transplant outcomes4,5,23, demonstrating that low neighborhood income is associated with an increased risk of undergoing liver biopsy, and that overall, children from wealthy neighborhoods undergo less liver biopsies relative to other groups. To deliver more high-value care, future studies should uncover the underlying reasons for this disparity in healthcare utilization.
Supplementary Material
Table, Supplemental Digital Content 2. Demographic characteristics for merged patients included in the analysis and unmerged patients excluded from analysis.
Demographic data derived from the PHIS as SRTR data not available.
Insurance categories include public (in-state Medicaid, out-of-state Medicaid, Tricare, Medicare, or other government insurance), private (commercial HMO, commercial PPO, or other commercial insurance), and other (self-pay, charity, other payer, or hospital chose not to bill).
Figure, Supplemental Digital Content 3. Directed acyclic graph for the hypothesized impact of neighborhood income on healthcare utilization, including total annual inpatient bed days, cumulative rate of hospitalizations, and cumulative rate of liver biopsies. Bolded borders represent the primary exposure, neighborhood income, and the primary outcome, healthcare utilization. Solid borders denote variables accessible within PHIS or SRTR databases. Dashed borders represent variables not included within PHIS or SRTR databases.
Figure, Supplemental Digital Content 1: Flow chart of SRTR-PHIS patient-level merge.
What is known:
Social determinants of health, including a child’s neighborhood context, are closely tied to long-term outcomes in pediatric liver transplant.
Children living in socioeconomically disadvantaged neighborhoods have increased rates of medication non-adherence, graft failure, and death.
What is new:
Children from low-income neighborhoods experience higher rates of liver biopsy post-transplant. These procedures are invasive and potentially preventable.
In addition to improving pediatric outcomes, interventions to mitigate health inequities in liver transplant may reduce resource utilization.
ACKNOWLEDGEMENTS:
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
FUNDING:
Research reported in this publication was supported by the National Institutes of Health under Award Numbers K23DK132454 (S.I.W.), K24AG080021 (J.C.L.), 5R01AG059183-04 (J.C.L.), UCSF Liver Center P30 DK026743 (support for S.I.W., S.C., J.C.L.), T32DK007762-45S1 (S.A.G.) The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABREVIATIONS USED IN THIS PAPER:
- PHIS
Pediatric Health Information System
- SRTR
Scientific Registry of Transplant Recipients
- RR
rate ratio
- CI
confidence interval
- OPTN
Organ Procurement and Transplantation Network
- HRSA
Health Resources and Services Administration
- ICD
International Classification of Disease
- HHI
median household income of patient zip code
- MELD
model for end stage liver disease
- PELD
pediatric end stage liver disease model
Footnotes
DATA TRANSPARENCY STATEMENT: The data for this study were extracted from the Pediatric Health Information System database and the Scientific Registry of Transplant Recipients database. Due to data sharing policies, these data are not shared.
CONFLICTS OF INTEREST AND SOURCE OF FUNDING: The authors have no relevant conflicts of interests to report.
References
- 1.Kwong AJ, Kim WR, Lake JR, et al. OPTN/SRTR 2019 Annual Data Report: Liver. Am J Transplant. 2021;21(S2):208–315. doi: 10.1111/ajt.16494 [DOI] [PubMed] [Google Scholar]
- 2.Ng VL, Alonso EM, Bucuvalas JC, et al. Health status of children alive 10 years after pediatric liver transplantation performed in the US and Canada: report of the studies of pediatric liver transplantation experience. J Pediatr. 2012;160(5):820–826.e3. doi: 10.1016/j.jpeds.2011.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lund LK, Grabhorn EF, Rüther D, et al. Long-term Outcome of Pediatric Liver Transplant Recipients Who Have Reached Adulthood: A Single-center Experience. Transplantation. Published online February 23, 2023:e004556. doi: 10.1097/TP.0000000000004556 [DOI] [PubMed] [Google Scholar]
- 4.Wadhwani SI, Bucuvalas JC, Brokamp C, et al. Association Between Neighborhood-level Socioeconomic Deprivation and the Medication Level Variability Index for Children Following Liver Transplantation. Transplantation. 2020;104(11):2346–2353. doi: 10.1097/TP.0000000000003157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wadhwani SI, Beck AF, Bucuvalas J, Gottlieb L, Kotagal U, Lai JC. Neighborhood socioeconomic deprivation is associated with worse patient and graft survival following pediatric liver transplantation. Am J Transplant. 2020;20(6):1597–1605. doi: 10.1111/ajt.15786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shifman HP, Rasnick E, Huang CY, et al. Association of Primary Care Shortage Areas with Adverse Outcomes after Pediatric Liver Transplant. J Pediatr. 2022;246:103–109.e2. doi: 10.1016/j.jpeds.2022.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narus SP, Srivastava R, Gouripeddi R, et al. Federating Clinical Data from Six Pediatric Hospitals: Process and Initial Results from the PHIS+ Consortium. AMIA Annu Symp Proc. 2011;2011:994–1003. Accessed June 5, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3243196/ [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington Y, Rauch DA, Leary JC, Andrews TR. How Generalizable Is Freestanding Children’s Hospital Data Such as PHIS (Pediatric Health Information System)? Pediatrics. 2021;147(3_MeetingAbstract):567–569. doi: 10.1542/peds.147.3MA6.567 [DOI] [Google Scholar]
- 9.Modi AC, Pai AL, Hommel KA, et al. Pediatric self-management: a framework for research, practice, and policy. Pediatrics. 2012;129(2):e473–485. doi: 10.1542/peds.2011-1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fieldston ES, Zaniletti I, Hall M, et al. Community Household Income and Resource Utilization for Common Inpatient Pediatric Conditions. Pediatr Evanst. 2013;132(6):e1592–e1601. doi: 10.1542/peds.2013-0619 [DOI] [PubMed] [Google Scholar]
- 11.Zonfrillo MR, Zaniletti I, Hall M, et al. Socioeconomic Status and Hospitalization Costs for Children with Brain and Spinal Cord Injury. J Pediatr. 2016;169:250–255. doi: 10.1016/j.jpeds.2015.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.2010 Poverty Guidelines, Federal Register Notice. ASPE. Accessed August 21, 2023. https://aspe.hhs.gov/2010-poverty-guidelines-federal-register-notice [Google Scholar]
- 13.Brooks ME, Kristensen K, van Benthem KJ, et al. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017;9(2):378–400. Accessed September 28, 2023. https://journal.r-project.org/archive/2017/RJ-2017-066/index.html [Google Scholar]
- 14.Wang MC, Qin J, Chiang CT. Analyzing Recurrent Event Data With Informative Censoring. J Am Stat Assoc. 2001;96(455): 10.1198/016214501753209031. doi: 10.1198/016214501753209031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.R: The R Project for Statistical Computing. Accessed September 28, 2023. https://www.r-project.org/
- 16.Chiou SH, Xu G, Yan J, Huang CY. Regression Modeling for Recurrent Events Possibly with an Informative Terminal Event Using R Package reReg. J Stat Softw. 2023;105:1–34. doi: 10.18637/jss.v105.i05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wadhwani SI, Barrera AG, Shifman HP, et al. Caregiver perspectives on the everyday medical and social needs of long-term pediatric liver transplant patients. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2022;28(11):1735–1746. doi: 10.1002/lt.26498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang LV, Shah AN, Hoefgen ER, et al. Lost Earnings and Nonmedical Expenses of Pediatric Hospitalizations. Pediatrics. 2018;142(3):e20180195. doi: 10.1542/peds.2018-0195 [DOI] [PubMed] [Google Scholar]
- 19.Smayra K, Miangul S, Yap N, et al. Technical Success, Sample Adequacy, and Complications of Pediatric Transjugular Liver Biopsy: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2023;68(10):3846–3856. doi: 10.1007/s10620-023-08071-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanada Y, Sakuma Y, Onishi Y, et al. Ultrasonographically guided percutaneous transhepatic liver biopsy after pediatric liver transplantation. Pediatr Transplant. 2021;25(4):e13997. doi: 10.1111/petr.13997 [DOI] [PubMed] [Google Scholar]
- 21.Anderson BR, Fieldston ES, Newburger JW, Bacha EA, Glied SA. Disparities in Outcomes and Resource Use After Hospitalization for Cardiac Surgery by Neighborhood Income. Pediatrics. 2018;141(3):e20172432. doi: 10.1542/peds.2017-2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narla S, Hsu DY, Thyssen JP, Silverberg JI. Predictors of Hospitalization, Length of Stay, and Costs of Care Among Adult and Pediatric Inpatients With Atopic Dermatitis in the United States. Dermat Contact Atopic Occup Drug. 2018;29(1):22–31. doi: 10.1097/DER.0000000000000323 [DOI] [PubMed] [Google Scholar]
- 23.Wadhwani SI, Huang CY, Gottlieb L, et al. Center variation in long-term outcomes for socioeconomically deprived children. Am J Transplant. 2021;21(9):3123–3132. doi: 10.1111/ajt.16529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wadhwani SI, Ge J, Gottlieb L, et al. Racial/ethnic disparities in wait-list outcomes are only partly explained by socioeconomic deprivation among children awaiting liver transplantation. Hepatology. 2022;75(1):115–124. doi: 10.1002/hep.32106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yalung JE, Shifman HP, Manning ER, et al. Ambient air pollution is associated with graft failure/death in pediatric liver transplant recipients. Am J Transplant. Published online October 28, 2023. doi: 10.1016/j.ajt.2023.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godown J, Hall M, Thompson B, et al. Expanding analytic possibilities in pediatric solid organ transplantation through linkage of administrative and clinical registry databases. Pediatr Transplant. 2019;23(3):e13379. doi: 10.1111/petr.13379 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table, Supplemental Digital Content 2. Demographic characteristics for merged patients included in the analysis and unmerged patients excluded from analysis.
Demographic data derived from the PHIS as SRTR data not available.
Insurance categories include public (in-state Medicaid, out-of-state Medicaid, Tricare, Medicare, or other government insurance), private (commercial HMO, commercial PPO, or other commercial insurance), and other (self-pay, charity, other payer, or hospital chose not to bill).
Figure, Supplemental Digital Content 3. Directed acyclic graph for the hypothesized impact of neighborhood income on healthcare utilization, including total annual inpatient bed days, cumulative rate of hospitalizations, and cumulative rate of liver biopsies. Bolded borders represent the primary exposure, neighborhood income, and the primary outcome, healthcare utilization. Solid borders denote variables accessible within PHIS or SRTR databases. Dashed borders represent variables not included within PHIS or SRTR databases.
Figure, Supplemental Digital Content 1: Flow chart of SRTR-PHIS patient-level merge.