Abstract
Introduction:
Coronary artery calcium (CAC) scans contain useful information beyond the Agatston CAC score that is not currently reported. We recently reported that artificial intelligence (AI)-enabled cardiac chambers volumetry in CAC scans (AI-CAC™) predicted incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis (MESA). In this study, we investigated the performance of AI-CAC cardiac chambers for prediction of incident heart failure (HF).
Methods:
We applied AI-CAC to 5750 CAC scans of asymptomatic individuals (52% female, White 40%, Black 26%, Hispanic 22% Chinese 12%) free of known cardiovascular disease at the MESA baseline examination (2000–2002). We used the 15-year outcomes data and compared the time-dependent area under the curve (AUC) of AI-CAC volumetry versus NT-proBNP, Agatston score, and 9 known clinical risk factors (age, gender, diabetes, current smoking, hypertension medication, systolic and diastolic blood pressure, LDL, HDL for predicting incident HF over 15 years.
Results:
Over 15 years of follow-up, 256 HF events accrued. The time-dependent AUC [95% CI] at 15 years for predicting HF with AI-CAC all chambers volumetry (0.86 [0.82,0.91]) was significantly higher than NT-proBNP (0.74 [0.69, 0.77]) and Agatston score (0.71 [0.68, 0.78]) (p < 0.0001), and comparable to clinical risk factors (0.85, p = 0.4141). Category-free Net Reclassification Index (NRI) [95% CI] adding AI-CAC LV significantly improved on clinical risk factors (0.32 [0.16,0.41]), NT-proBNP (0.46 [0.33,0.58]), and Agatston score (0.71 [0.57,0.81]) for HF prediction at 15 years (p < 0.0001).
Conclusion:
AI-CAC volumetry significantly outperformed NT-proBNP and the Agatston CAC score, and significantly improved the AUC and category-free NRI of clinical risk factors for incident HF prediction.
Keywords: Left ventricular volume, Coronary artery calcium, Artificial intelligence, Heart failure, NT-proBNP
1. Introduction
Coronary artery calcium (CAC) scoring is a strong predictor of coronary heart disease (CHD) events in asymptomatic individuals.1 However, it is a weak predictor of heart failure (HF).2 HF is a significant healthcare burden due to large and rising numbers of HF hospitalizations and rehospitalizations.3 Approximately 2% of the total US healthcare budget is spent on HF, and half of that is attributable to late diagnosis leading to inpatient admissions.4 Numerous studies have highlighted the importance of early detection of asymptomatic high-risk patients to prevent progression to overt clinical HF. These high-risk patients include those with asymptomatic left ventricular dysfunction (ALVD), left ventricular hypertrophy (LVH), cardiomegaly, and enlarged cardiac chambers that if not detected and treated will lead to HF. They can be detected with imaging modalities such as echocardiography, cardiac computed tomography, and cardiac magnetic resonance imaging. Similarly, measurement of B-type natriuretic peptide (BNP) or the biologically inactive amino-terminal of BNP (NT-proBNP) that are circulating biomarkers of cardiac volume overload is associated with subclinical HF5,6,7,8. However, screening the asymptomatic population for detection of subclinical HF by any diagnostic means is not part of current guidelines due to cost and feasibility limitations.9
CAC scans contain insightful information beyond a CAC score that is not currently reported.10 We recently reported that artificial intelligence (AI)-enabled volumetry of left atrium in CAC scans enabled prediction of incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis (MESA) occurring within one year.11 MESA is comprised of 6814 asymptomatic individuals free of known cardiovascular disease at baseline who were recruited for prospective evaluation in 2000–2002. In this study, we investigated the predictive value of AI-enabled cardiac chambers volumetry in CAC scans (AI-CAC) for prediction of incident symptomatic heart failure (HF). We further compared the predictive performance of AI-CAC with the best clinical model comprising 99 known risk factors (age, gender, diabetes, current smoking, hypertension medication, systolic and diastolic blood pressure, LDL, HDL plus the addition of NT-proBNP or the Agatston CAC score.
2. Methods
2.1. Study population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study that began in July 2000 to investigate the prevalence, correlates, and progression of subclinical cardiovascular disease (CVD) in individuals without known CVD at baseline. The cohort included 6814 women and men aged 45–84 years old recruited from 6 US communities (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; northern Manhattan, NY; and St. Paul, MN). Individuals with a history of physician-diagnosed MI, angina, heart failure, stroke, or transient ischemic attack, or who had undergone an invasive procedure for CVD (coronary artery bypass graft, angioplasty, valve replacement, pacemaker placement or other vascular surgeries) were excluded.12
For our study, we removed data from 771 MESA participants who did not consent to use of their data by commercial entities leaving 6043 participants at baseline. Among the remaining participants, after removing cases with missing data including 125 cases with missing slices in CAC scans and 168 cases with missing NT-proBNP values, the total number of cases available for analysis was 5750. The 125 cases with missing slices were 49.8% male and 50.2% females with age 60.8 ± 10.1. None of these cases had a diagnosis of HF, and our investigations did not reveal any association with dependent or independent variables in our study.
2.2. Outcomes
Participants were contacted by telephone every 9–12 months during follow-up and asked to report all new cardiovascular diagnoses. International Classification of Disease (ICD) codes were obtained for hospitalizations. Reviewers classified HF as definite, probable, or absent. Definite or probable HF required heart failure symptoms, such as shortness of breath or edema. Asymptomatic HF was not a MESA endpoint, therefore could not be investigated. In addition to symptoms, probable HF required HF diagnosed by a physician and patient receiving medical treatment for HF. Definite HF required one or more other criteria, such as pulmonary edema/congestion by chest X-ray; dilated ventricle or poor LV function by echocardiography or ventriculography; or evidence of left ventricular diastolic dysfunction. The criteria for possible HF are a physician diagnosis of HF and receiving medical treatment for HF. A total of 74 cases were identified with pre-baseline atrial fibrillation (AF) and were included as one of the known clinical risk factors for this study.
2.3. AI-CAC
The AI-enabled automated cardiac chambers volumetry tool in AI-CAC™ is called AutoChamber™ (HeartLung.AI, Houston, TX), a deep learning model that used TotalSegmentator13 as the base input and was further developed to segment not only each of the four cardiac chambers; left atrium (LA), left ventricle (LV), right atrium (RA), and right ventricle (RV) but also ascending aorta, aortic root and valves, pulmonary arteries, and several other components which are not presented here. In this manuscript, the AI-estimated LA, LV, RA, RV volumes and LV Mass are referred to as AI-CAC all cardiac chambers or AI cardiac chambers volumetry, interchangeably.
Fig. 1 shows the AutoChamber segmentations of enlarged LV along with other cardiac chambers in two cases who were otherwise classified as low risk based on Agatston CAC score of 0 and ASCVD pooled cohorts equation (PCE) < 5%. The base architecture of the TotalSegmentator model was trained on 1139 cases obtained from University of Basel with 447 cases of coronary CT angiography (CCTA) using nnU-Net, a self-configuring method for deep learning-based biomedical image segmentation.14 The initial input training data were paired non-contrast and contrast-enhanced ECG-gated cardiac CT scans of the same individuals with 1.5 mm slice thickness. Because the images were taken from the same patients in the same session, registration was accomplished with good alignment. Following this transfer of segmentations, a nnU-Net deep learning tool was used for training the model.
Fig. 1.
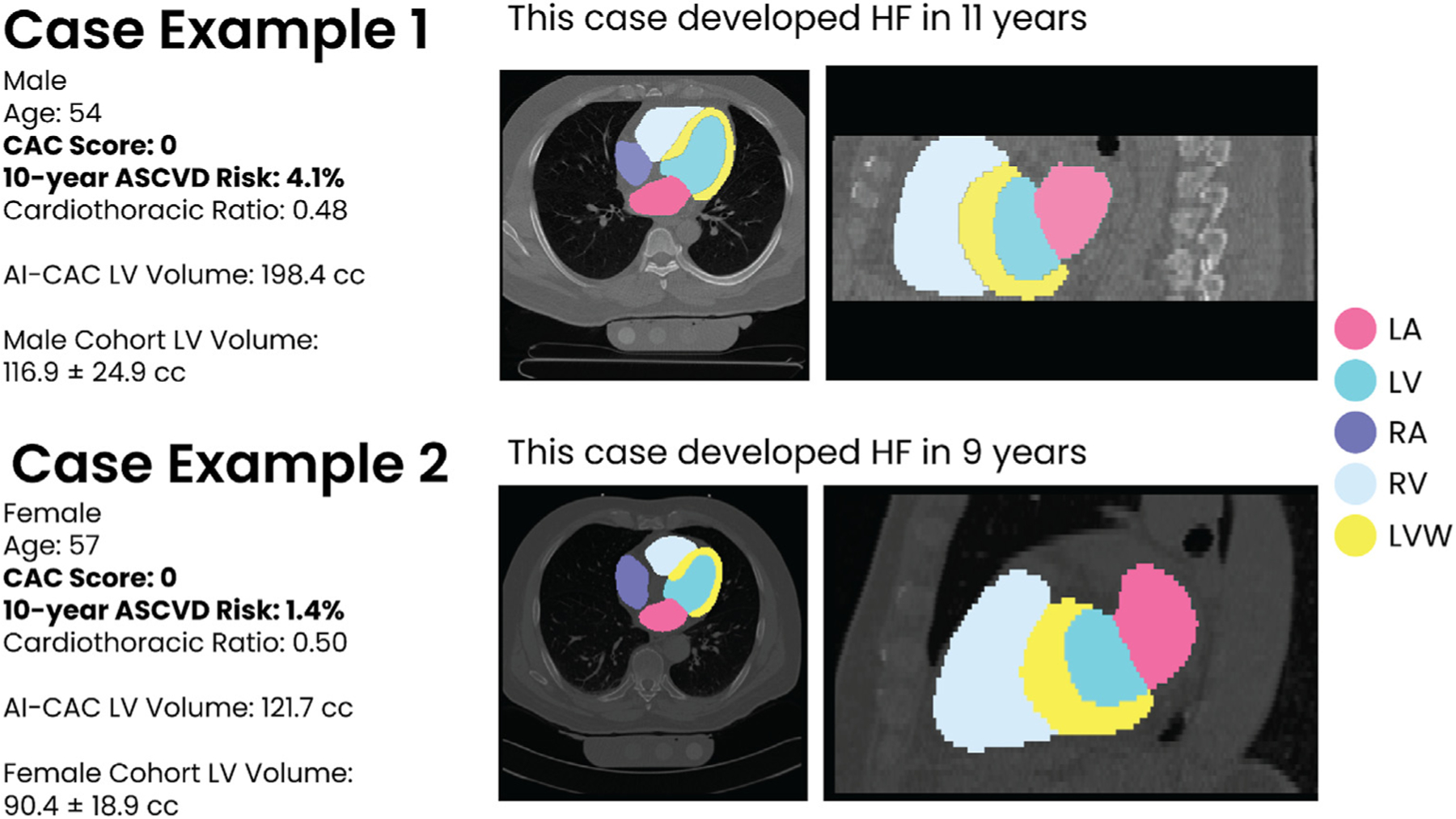
Examples of two asymptomatic cases who were classified as low risk by Coronary Artery Calcium (CAC) zero and ASCVD Pooled Cohorts Equation (PCE) less than five percent but were flagged by AI-CAC™ with enlarged left ventricle (LV) volume.
Additionally, iterative training was implemented whereby human supervisors corrected errors made by the model, and the corrected data were used to further train the model, leading to improved accuracy. AI-CAC was run on 6043 non-contrast CAC scans that consented to commercial data usage out of the 6814 scans available in MESA exam 1. Expert rules built in AI-CAC excluded 125 cases due to missing slices in image reconstruction created by some of the electron beam CT scanners used in MESA baseline. Each AI-CAC chambers volumetry took on average of 20 s per scan.
2.4. NT-proBNP
NT-proBNP data were obtained from MESA core laboratory for MESA exam 1 participants. A detailed study design for MESA has been published elsewhere.12 Details on NT-proBNP assays used in MESA have been reported.15 N-terminal proBNP is more reproducible than NT-proBNP at the lower end of the distribution range, and more stable at room temperature. However, both BNP and N-terminal proBNP are clinically available. Intra-assay and inter-assay coefficients of variation at various concentrations of NT-proBNP have been previously reported.16,17 The analytical measurement range for NT-proBNP in exam 1 was 4.9–11699 pg/ml. The lower limit of detection for the NT-proBNP assay is 5 pg/mL, thus cases above 0 and below 4.99 were treated as 4.99 pg/mL. Numerous studies have established NT-proBNP as a predictor of incident HF, with a relatively high negative predictive value (94%–98%), but a significantly lower positive predictive value (44%–57% for non-acute heart failure, 66%–67% for acute heart failure).18,19,20
2.5. Statistical analysis
We used SAS (SAS Institute Inc., Cary, NC) and R-4.3.3 software for our statistical analyses. All values are reported as means ± SD except for Agatston CAC Score and NT-proBNP which did not show normal distribution and is presented in median and interquartile range (IQR). All tests of significance were two tailed, and significance was defined at the p <0.05 level. Confidence intervals are presented at the 95% level.
Discrimination for HF prediction was performed over 5, 10, and 15 years follow up. The time-dependent ROC (receiver operator curve) AUC (area under the curve) was calculated based on survival probabilities using the inverse probability of censoring weighting estimator to measure the true positive rate and false positive rate for each threshold value. Pointwise confidence intervals were computed from the asymptotic normality of the time-dependent AUC estimator using 2000 bootstrapped samples. Standard errors and confidence intervals were estimated from the independent and identically distributed (iid)-representation of the estimator. Significance in AUC difference was determined using the DeLong test. Due to the limited number of HF events, internal validation was not used when evaluating model performance. The Cochran-Mantel-Haenszel test of trends was used to determine general association in proportions between AI-CAC LV volume Quartiles and Agatston CAC Score categories.
Category-free (continuous) net reclassification index (NRI) was calculated using the sum of the differences between the proportions of upward reclassifications and downward reclassifications for HF events and HF non-events, respectively. NRI was developed as a statistical measure to evaluate the improvement in risk prediction models when additional variables are incorporated into a base model.21
In our study, the base models are 9 known clinical risk factors (defined as age, gender, diabetes, current smoking, hypertension medication, systolic and diastolic blood pressure, LDL, and HDL), NT-proBNP, and Agatston CAC score. For the clinical model, diabetes and smoking status are dichotomized as yes/no. All other variables were modeled continuously. The AI enabled cardiac chambers volumetry (AI-CAC) model integrates all cardiac chambers (LA, LV, RA, RV) and LV wall mass within the same model. LV and LV Mass are modeled as a composite variable to reduce multicollinearity. All variables were modeled continuously. NT-proBNP and Agatston CAC Score values were right skewed, therefore natural log transformed and modeled continuously. Composite model analysis included 9 clinical risk factors, Agatston CAC Score, and AI-CAC all cardiac chambers.
3. Results
In this cohort, ages ranged from 45 to 84, 53.3% of the participants were female, 38% were White, 28% were Black, 22% were Hispanic, and 12% were Chinese. Baseline characteristics of MESA participants who were diagnosed with incident HF versus those who were not over the period of 15 years follow up were calculated. At 5-, 10-, and 15-years follow-up 92, 188, and 256 HF cases were identified respectively. In univariate comparisons, incident HF cases were older, more likely male, and more likely White. The incident HF cases had higher cardiac chamber volumes for LA, LV, RA, RV, LV Mass, and NT-proBNP levels versus those without incident HF (all comparisons p < 0.001) (Table 1).
Table 1.
Baseline characteristics of the Multi-Ethnic Study of Atherosclerosis (MESA) participants including cases with and without heart failure (HF) at 5-,10-, and 15-years.
| Overall | No HF accrued at | HF accrued at | No HF accrued at | HF accrued at | No HF accrued at | HF accrued at | |
|---|---|---|---|---|---|---|---|
| (N = 5750) | 5yrs (N = 5685) | 5yrs (N = 92) | 10yrs (N = 5562) | 10yrs (N = 188) | 15yrs (N = 5494) | 15yrs (N = 256) | |
| Age (per 10 years) | |||||||
| Age 45–54 | 28% | 30% | 6% | 32% | 5% | 30% | 6% |
| Age 55–64 | 27% | 28% | 19% | 29% | 22% | 27% | 22% |
| Age 65–74 | 29% | 28% | 34% | 28% | 38% | 28% | 39% |
| Age 75–84 | 14% | 12% | 39% | 10% | 33% | 13% | 32% |
| Female sex (%) | 52.% | 52% | 38% | 54% | 38% | 52% | 38% |
| Body Surface Area | 1.90 ± 0.24 | 1.90 ± 0.24 | 1.98 ± 0.25 | 1.90 ± 0.24 | 1.98 ± 0.24 | 1.90 ± 0.24 | 1.98 ± 0.24 |
| Race/Ethnicity | |||||||
| White (%) | 39% | 39% | 35% | 39% | 42% | 39% | 42% |
| Chinese (%) | 12% | 12% | 4% | 12% | 6% | 12% | 7% |
| Black (%) | 26% | 25% | 38% | 25% | 29% | 26% | 29% |
| Hispanic (%) | 22% | 22% | 21% | 22% | 21% | 22% | 21% |
| AI-CAC Cardiac Chambers a | |||||||
| LV volume (cc) | 102.5 ± 25.4 | 101.8 ± 24.5 | 124.9 ± 38.2 | 101.6 ± 24.3 | 118.1 ± 33.4 | 101.7 ± 24.6 | 119.1 ± 33.9 |
| LA volume (cc) | 61.4 ± 16.1 | 60.7 ± 15.6 | 75.4 ± 19.2 | 60.1 ± 15.3 | 74.5 ± 19.3 | 60.7 ± 15.6 | 74.6 ± 19.6 |
| RV volume (cc) | 134.3 ± 34.4 | 134.1 ± 34.3 | 147.0 ± 41.7 | 134.2 ± 34.2 | 143.5 ± 39.3 | 133.7 ± 34.1 | 144.2 ± 40.5 |
| RA volume (cc) | 77.0 ± 18.9 | 76.6 ± 18.7 | 86.2 ± 21.9 | 76.5 ± 18.5 | 85.5 ± 22.8 | 76.5 ± 18.5 | 86.6 ± 24.1 |
| LV Mass volume (g) | 107.8 ± 26.4 | 107.1 ± 25.7 | 130.4 ± 36.2 | 106.7 ± 25.5 | 125.0 ± 32.5 | 106.9 ± 25.7 | 125.7 ± 33.3 |
| Total Heart volume (cc) | 482.3 ± 108.7 | 480.3 ± 107.5 | 564.0 ± 142.1 | 479.1 ± 106.9 | 546.6 ± 130.7 | 478.1 ± 105.8 | 541.1 ± 132.3 |
| 10-yr ASCVD Risk Score (GOFF ET AL 2013) | 14% ± 13% | 13% ± 13% | 28% ± 16% | 12% ± 12% | 25% ± 16% | 10% ± 10% | 25% ± 16% |
| NT-proBNP (pg/mL) (Median – IQR) | 52.07 (23.75–106.1) |
50.38 (22.76–100.45) |
163.10 (83.06–306.05) |
47.64 (21.81–94.04) |
125.5 (63.16–240.70) |
44.75 (20.26–87.53) |
111.8 (58.63–225.4) |
| Agatston score (Median - IQR) | 0.93 (0–90.66) | 0 (0–73.40) | 127.23 (2.10–451.14) | 0 (0–58.02) | 126.17 (0–463.99) | 0 (0–41.25) | 128.03 (0–471.28) |
| Agatston score (mean) | 151.0 ± 426.0 | 126.3 ± 359.7 | 479.8 ± 903.1 | 107.1 ± 310.7 | 472.2 ± 878.8 | 134.1 ± 382.9 | 472.2 ± 860.4 |
| Risk Factors | |||||||
| Diabetes | 12% | 11% | 32% | 11% | 30% | 11% | 28% |
| Hypertension | 44% | 43% | 75% | 41% | 70% | 43% | 70% |
| Current Smoking | 13% | 12% | 15% | 12% | 11% | 13% | 14% |
| Alcohol Usage | 68% | 69% | 57% | 70% | 60% | 71% | 59% |
| Blood Pressure Lowering Rx | 37% | 35% | 65% | 34% | 59% | 36% | 59% |
| Lipid Lowering Rx | 16% | 16% | 20% | 16% | 21% | 16% | 20% |
| LDL Cholesterol (mg/dL) | 117.2 ± 31 | 117.4 + 31.3 | 115.6 ± 30.4 | 117.7 ± 31.3 | 114.4 ± 31.0 | 118.1 ± 31.0 | 113.7 ± 32.3 |
| HDL Cholesterol (mg/dL) | 51.0 ± 14 | 51.0 ± 15.0 | 48.3 ± 14.1 | 51.1 ± 15.0 | 48.1 ± 14.1 | 51.2 ± 15.0 | 48.6 ± 14.1 |
| Total Cholesterol (mg/dL) | 194.2 ± 35 | 194.6 ± 35.6 | 190.1 ± 33.8 | 194.9 ± 35.4 | 190.2 ± 34.0 | 195.4 ± 35.3 | 189.7 ± 35.6 |
| High sensitivity C-reactive protein (mg/dL) | 3.72 ± 5.66 | 3.6 ± 5.4 | 5.5 ± 8.2 | 3.6 ± 5.2 | 4.7 ± 6.5 | 3.54 ± 5.03 | 4.52 ± 6.32 |
| Creatinine (mg/dL) | 0.96 ± 0.29 | 0.9 ± 0.2 | 1.0 ± 0.3 | 0.9 ± 0.2 | 1.0 ± 0.3 | 0.93 ± 0.20 | 1.07 ± 0.59 |
Left atrium (LA), left ventricle (LV), right atrium (RA), right ventricle (RV), left ventricular wall (LV Mass).
The time-dependent AUC [95% CI] for AI-CAC all cardiac chambers adjusted by age, gender, and BSA was comparable to the 9 clinical risk factors model at 5-, 10-, and 15 years (0.83 [0.79, 0.87] vs. 0.79 [0.77, 0.85], p = 0.58) (0.82 [0.79, 0.86] vs. 0.80 [0.76, 0.85], p = 0.09) (0.86 [0.82, 0.91] vs. 0.85 [0.82, 0.90], p = 0.41), respectively. The AUC for AI-CAC all cardiac chambers was comparable to NT-proBNP for 5 years (0.83 [0.79, 0.87]vs. 0.79, p = 0.21) and significantly higher than NT-proBNP for 10- and 15-year prediction of HF (0.82 [0.79, 0.86] vs. 0.75 [0.71, 0.78], p < 00.0001 and 0.86 [0.82, 0.91] vs. 0.74 [0.69, 0.77], p < 00.0001, respectively). The AUC for AI-CAC all cardiac chambers versus the Agatston CAC score was 0.83 [0.79, 0.87]vs. 0.69 [0.65, 0.76] for 5 years (p < 0001); 0.82 [0.79, 0.86] vs. 0.71 [0.66,0.75] for 10 years (p < 00.0001); and 0.86 [0.82, 0.91] vs. 0.71 [0.68, 0.78] for 15 years (p < 00.0001). (Table 2). The AUC for AI-CAC LV volume and mass alone predicted HF similarly to all cardiac chambers despite slightly lower AUC at 5-, 10-, and 15- years was 0.82 [0.78, 0.86], 0.79, [0.75, 0.85] and 0.85 [0.82, 0.89], respectively.
Table 2.
Time Dependent Area under curve (AUC) of AI-CAC All Cardiac Chambers Volumetry vs. N-terminal pro-brain natriuretic peptide (NT-proBNP), Agatston CAC score, and 9 Clinical Risk Factors for 5-,10-, and 15-year prediction of heart failure (HF).
| 5 Years |
10 Years |
15 Years |
||||
|---|---|---|---|---|---|---|
| HF Events accrued over follow-up |
92 |
188 |
256 |
|||
| AI-CAC Model Comparison | AUC (95% CI) | P value | AUC (95% CI) | P value | AUC (95% CI) | P value |
| AI-CAC (AI-enabled All Cardiac Chambers Volumetry)a | 0.83 (0.79, 0.87) | – | 0.82 (0.79, 0.86) | – | 0.86 (0.82, 0.91) | – |
| Agatston CAC Score | 0.69 (0.65, 0.76) | 0.0004 | 0.71 (0.66, 0.75) | <0.0001 | 0.71 (0.68, 0.78) | <0.0001 |
| NT-proBNP | 0.79 (0.75, 0.84) | 0.2640 | 0.75 (0.71, 0.78) | <0.0001 | 0.74 (0.69, 0.77) | <0.0001 |
| Clinical Risk Factors Modelb | 0.79 (0.77, 0.85) | 0.5809 | 0.80 (0.76, 0.85) | 0.0857 | 0.85 (0.82, 0.90) | 0.4141 |
| Composite Model (Clinical Risk Factors + Agatston CAC Score + AI-CAC) Comparison | ||||||
| Composite Model | 0.86 (0.82, 0.92) | – | 0.85 (0.81, 0.90) | – | 0.89 (0.86, 0.94) | – |
| AI-CAC (AI-enabled All Cardiac Chambers Volumetry) | 0.83 (0.79, 0.87) | 0.0042 | 0.82 (0.79, 0.86) | 0.0004 | 0.86 (0.82, 0.91) | <0.0001 |
| Agatston CAC Score | 0.69 (0.65, 0.76) | <0.0001 | 0.71 (0.66, 0.75) | <0.0001 | 0.71 (0.68, 0.78) | <0.0001 |
| NT-proBNP | 0.80 (0.75, 0.84) | 0.0035 | 0.75 (0.71, 0.78) | <0.0001 | 0.74 (0.69, 0.77) | <0.0001 |
| Clinical Risk Factors Modelb | 0.82 (0.77, 0.87) | 0.0013 | 0.80 (0.76, 0.85) | <0.0001 | 0.82 (0.79, 0.85) | <0.0001 |
Adjusted by age, gender, body surface area (BSA). P-values are determined by the DeLong test in comparison to the AI-CAC all chambers model.
9 known risk factors: Age, gender, diabetes, current smoking, hypertension medication, systolic blood pressure, diastolic blood pressure, LDL cholesterol, HDL cholesterol.
The addition of Agatston CAC Score and AI-CAC cardiac chambers volumetry to the 9 clinical risk factor model was highly significant (Fig. 2a and b). The continuous NRI for 5-, 10-, and 15-years prediction of HF when AI-CAC was added to the Agatston CAC score as the only predictor in the base model was highly significant (0.81 [0.48, 0.88], 0.67 [0.57, 0.85], 0.71 [0.57, 0.81], respectively p < 0.0001). The NRI [95% CI] for AI-CAC over 5, 10, and 15 years when added to base model NT-proBNP was significant (0.49 [0.29, 0.69], 0.47 [0.32, 0.61], 0.46 [0.33, 0.58], respectively, p for all <0.0001). The NRI for AI-CAC over 5,10, and 15 years when added to the clinical risk factors model was 0.43 [0.22, 0.63], 0.35 [0.19, 0.48], 0.32 [0.16, 0.41], respectively, p for all <00.0001. (Table 3).
Fig. 2.
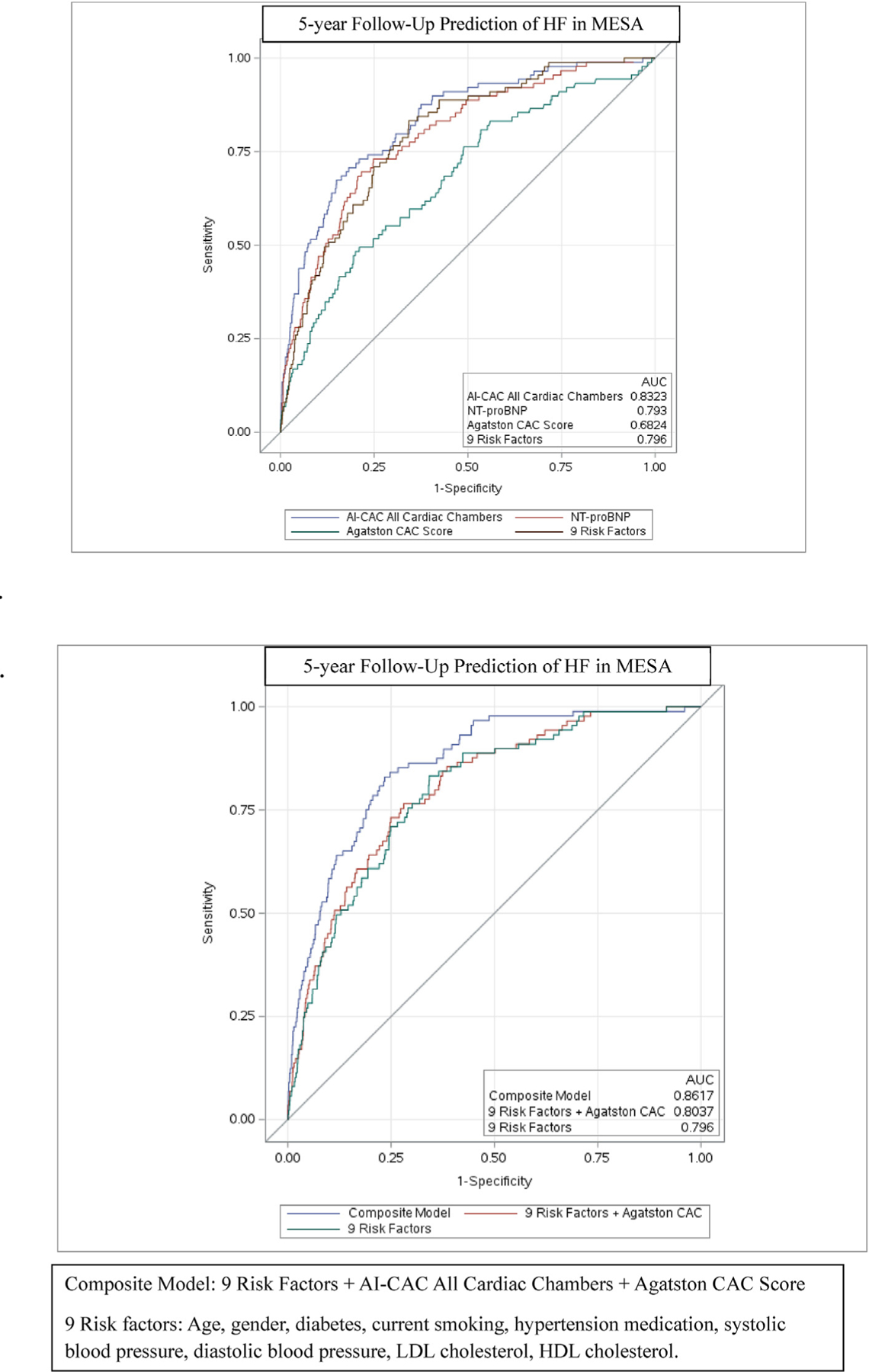
a-b. Comparing Receiver Operating Curve (ROC) Area under Curve (AUC) between AI-enabled cardiac chamber volumetry (AI-CAC) vs NT-proBNP, Agatston Score, and Clinical risk model over 5 years for incident heart failure (HF).
Table 3.
Net Reclassification Index (NRI) of (AI)-enabled Cardiac Chambers (AI-CAC) LV Volumetry added to N-terminal pro-brain natriuretic peptide (NT-proBNP), Agatston CAC score, and Clinical Risk Factors for 5-,10-, and 15-year prediction of heart failure (HF).
| Adding AI-CAC LV | NRI (95% CI) | NRI Standard Error | Z-Value for NRI | NRI P-Value | % of Events correctly reclassified | Event P-Value | % of Nonevents correctly reclassified | Non-Event P-Value |
|---|---|---|---|---|---|---|---|---|
| Category-Free NRI for Base Model CAC Score | ||||||||
| Year 5 | 0.81 (0.48,0.88) | 0.10140 | 6.53119 | <0.0001 | 26% | 0.0123 | 43% | <0.0001 |
| Year 10 | 0.67 (0.57,0.85) | 0.070585 | 9.59314 | <0.0001 | 31% | <0.0001 | 40% | <0.0001 |
| Year 15 | 0.71 (0.57,0.81) | 0.060764 | 10.9591 | <0.0001 | 30% | <0.0001 | 40% | <0.0001 |
| Category-Free NRI for Base Model NT-proBNP | ||||||||
| Year 5 | 0.49 (0.29,0.69) | 0.10377 | 4.67412 | <0.0001 | 19% | 0.0747 | 31% | <0.0001 |
| Year 10 | 0.47 (0.32,0.61) | 0.073624 | 6.23003 | <0.0001 | 17% | 0.0227 | 30% | <0.0001 |
| Year 15 | 0.46 (0.33,0.58) | 0.062706 | 7.24196 | <0.0001 | 18% | 0.0034 | 28% | <0.0001 |
| Category-Free NRI for Base Model Clinical (9 known risk factors)a | ||||||||
| Year 5 | 0.43 (0.22,0.63) | 0.10601 | 3.21928 | 0.0013 | 12% | 0.2436 | 22% | <0.0001 |
| Year 10 | 0.35 (0.19,0.48) | 0.074690 | 4.21359 | <0.0001 | 13% | 0.0752 | 19% | <0.0001 |
| Year 15 | 0.32 (0.16,0.41) | 0.063964 | 4.10521 | <0.0001 | 11% | 0.0896 | 16% | <0.0001 |
9 known Clinical risk factors: Age, gender, diabetes, current smoking, hypertension medication, systolic blood pressure, diastolic blood pressure, LDL cholesterol, HDL cholesterol.
AI-CAC LV volumetry demonstrated high discrimination for detection of asymptomatic left ventricular dysfunction (ALVD) as defined by LV ejection fraction <50%. The AUC [95% CI] for AI-CAC LV vs. NT-proBNP was 0.84 [0.73, 0.92] vs 0.60 [0.52, 0.71], (p < 0.0001) respectively (Fig. 4).
Fig. 4.
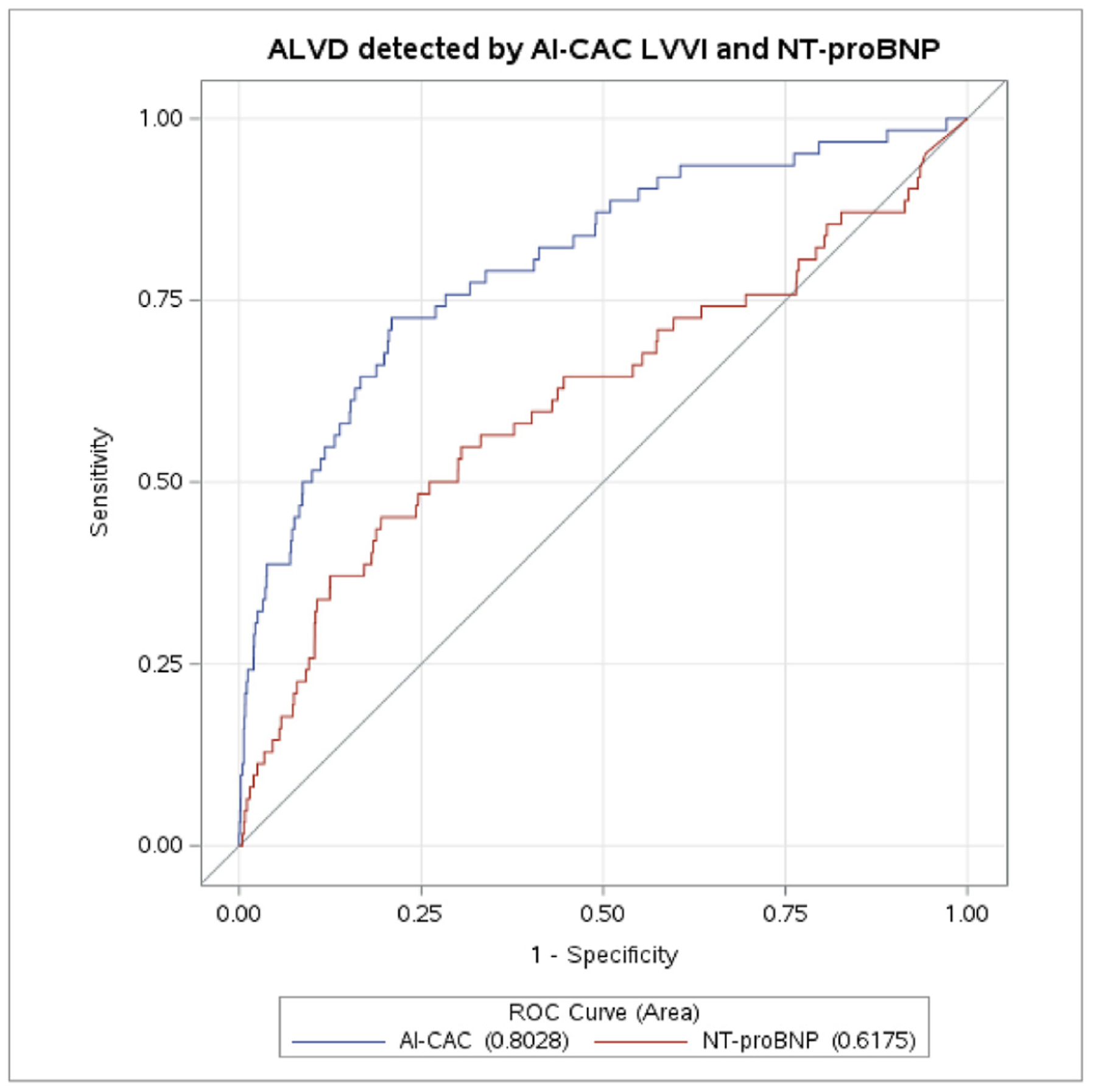
a-b. Detection of asymptomatic left ventricular dsyfunction (ALVD) defined by left ventricular (LV) ejection fraction less than 55% by AI-CAC LV Volume and NT-proBNP.
Despite the correlation (p < 0.0001), a significant number of low-risk categorized participants by CAC score and ASCVD Pooled Cohorts Equation have enlarged cardiac chambers (Fig. 3a and b). Over 15 years, 18 (6.2%) of HF events classified as low-risk by ASCVD Pooled Cohorts Equation (PCE) risk estimate <5% and 71 (24.3%) classified as low-risk by CAC 0 were flagged by AI-CAC top quartile (Table 4).
Fig. 3.
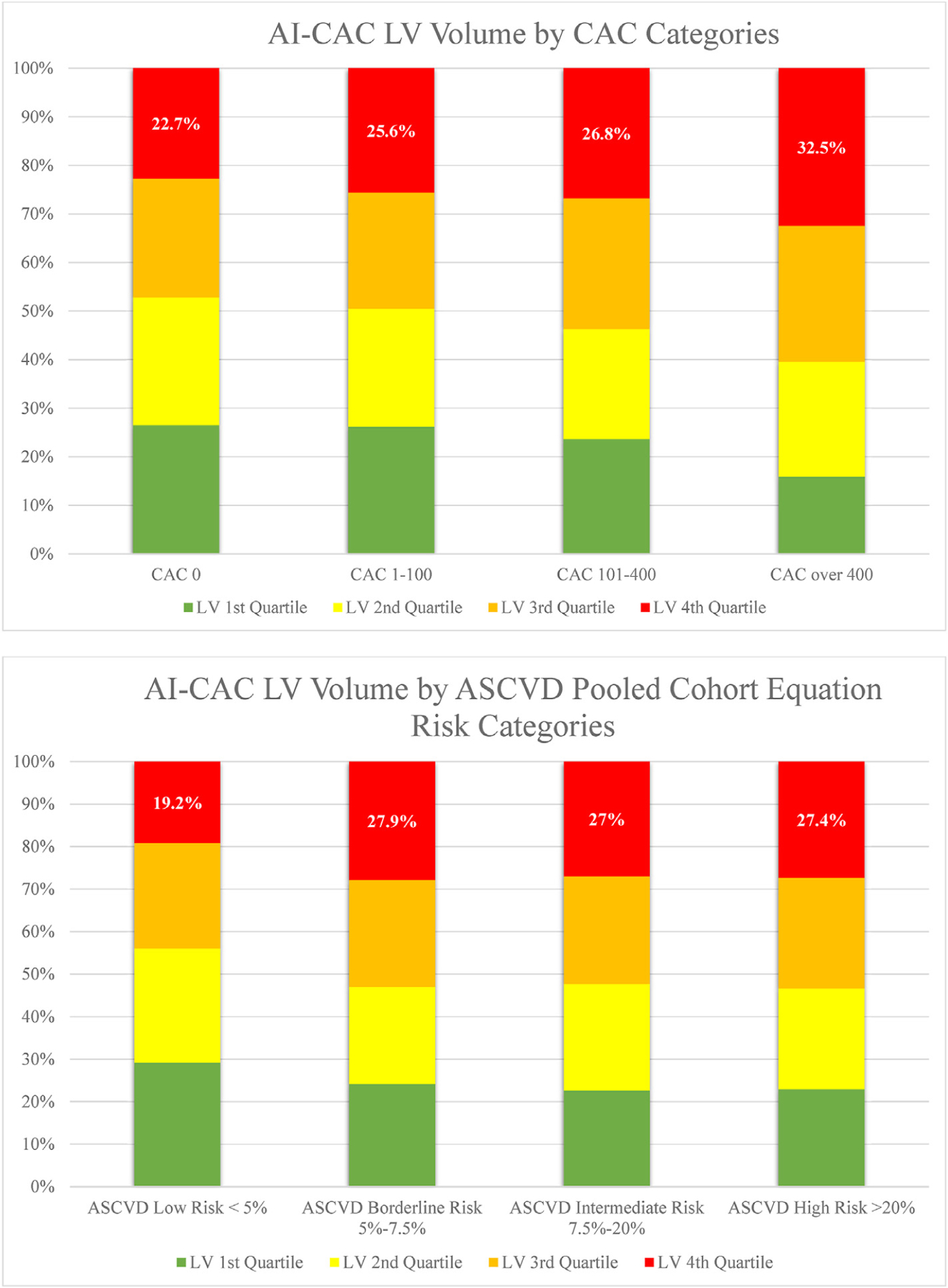
a-b) Quartiles of AI-CAC Left Ventricle (LV) Volume by coronary artery calcium (CAC) and ASCVD Pooled Cohorts Equation Categories.
Table 4.
Low risk reclassification for Cardiovascular Events and heart failure (HF) by AI-CAC enlarged left atrium (LA) and left ventricle (LV) in participants with Agatston coronary artery calcium (CAC) score of 0 or ASCVD Pooled Cohorts Equation (PCE) 10-year risk estimate below 5%.
| ASCVD PCE Risk Estimate <5% & Top Quartile LA or LV | CAC 0 & Top Quartile LA or LV | |
|---|---|---|
| 15-year follow-up All Cardiovascular Events* (n = 1732) |
197 (11.3%) | 501 (28.9%) |
| HF (n = 292) | 18 (6.2%) | 71 (24.3%) |
|
10-year follow-up All Cardiovascular Events* (n = 674) |
72 (10.7%) | 259 (38.4%) |
| HF (n = 212) | 13 (6.1%) | 50 (23.6%) |
|
5-year follow-up All Cardiovascular Events* (n = 311) |
32 (10.3%) | 116 (37.3%) |
| HF (n = 106) | 5 (4.7%) | 24 (22.6%) |
All Cardiovascular Events: stroke, myocardial infarction, angina, resuscitated cardiac arrest, all cardiovascular disease related deaths, HF, and atrial fibrillation.
Examples of two asymptomatic cases who were classified as low risk by CAC 0 and ASCVD PCE but were flagged by AI-CAC with enlarged LA and LV volume are shown in Fig. 1. Both cases went on to develop HF over subsequent years.
4. Discussion
To our knowledge this is the first report of an AI-enabled automated cardiac chamber volumetry in non-contrast cardiac CT scans obtained for CAC scoring that predicted future HF in a multi-ethnic asymptomatic population. Our study has shown that the AI-CAC technique non-significantly outperformed NT-proBNP for HF prediction at 5 years, and significantly outperformed NT-proBNP at 10 and 15 years. Additionally, it significantly outperformed the Agatston CAC Score for HF prediction at all years and provided a sizable NRI on top of both NT-proBNP and the Agatston Score. We recently reported that the same AI-CAC technique in CAC scans enabled prediction of future AF and outperformed both CHARGE-AF and NT-proBNP for predicting AF at 5, 10, and 15 years.11 Our study is not the first to raise awareness on the potential utility of non-coronary findings in CAC scans as they have been reported previously using manual 2D measurements of LV22–25 and LA sizes26,27,28,29. Furthermore, our study is not the first to suggest using AI for cardiac segmentation in non-contrast CT scans.30,31 However, we provide the first evidence on the predictive power of such an AI tool for incident HF as well as its practical feasibility that is operator independent and fast versus tedious manual measurements that are time consuming and poorly reproducible.
NT-proBNP is a serum biomarker of cardiac volume overload particularly and has been studied extensively in various cardiovascular diseases, in particular heart failure32,33,.34 Elevated levels of NT-proBNP are known to be associated with the presence of heart failure and reduced ejection fraction.35 Our study shows that AI enabled all cardiac chambers volumetry non-significantly outperformed NT-proBNP for 5-year HF prediction and significantly outperformed NT-proBNP for 10- and 15-year prediction. The small sample size of HF at 5 years may have resulted in the non-significant difference in prediction despite higher AUC for AI vs NT-proBNP (0.83 [0.79, 0.87] vs. 0.79, p = 0.21). Additionally, AI-CAC LV improved NRI of NT-proBNP at all years. It is possible that AI is outperforming NT-proBNP because NT-proBNP is not specific to LV volume overload, whereas AI cardiac chambers volumetry in CAC scans directly measures LV volume. AI-CAC LV and LV Mass alone similarly predicted HF over 5, 10, and 15 years to all cardiac chambers, despite slightly lower AUC. AI-CAC LA and RV volumes showed modest but significantly lower discrimination for incident HF over 15 years. AI-CAC RA volume alone was not significant in predicting incident HF.
Recent studies have suggested another opportunistic screening tool using AI-enabled deep learning ECG signals for detection of prevalent ALVD.36,37 These AI ECG models using a standard 10-s 12-lead ECG data in asymptomatic populations as well as patient populations in clinical datasets have shown to predict future AF38 and HF39 with comparable accuracy to the HF risk calculators from the ARIC study and the Framingham Heart Study.39 In another study by Yao et al.,40 ECGs were obtained as part of routine care from a total of 22,641 adults without prior HF. The primary outcome was a new diagnosis of low EF (≤50%) within 90 days of the ECG. The results indicated that use of AI-ECG enabled the early diagnosis of low EF in patients in the setting of routine primary care.40 We do not have paired data to compare the performance of our AI-enabled cardiac chambers volumetry in CT scans with these AI-enabled deep learning ECG tools for detection of incident AF and HF. However, it is important to note that AI-ECGs are often referred to as “Black-Box AI” meaning the AI output does not show clinicians what features or segments of ECG signals were used for LVH and ALVD detection, whereas in our approach the AI colorfully segments the cardiac chambers in non-contrast CT images where the volumetry measurements are done and enlarged cardiac chambers are detected.
4.1. Non-coronary findings in CAC scans
As stated above, our study is not the first to raise awareness on the potential utility of non-coronary findings in CAC scans as they have been reported previously using manual 2D measurements of LV22–25 and LA sizes26,27,28,29. However, despite multiple automated CAC scoring tools available, currently, there is no clinically available tool for clinicians to enable automated cardiac chambers volumetry in CAC scans. Such measurements are only possible on contrast-enhanced CT scans which require more radiation plus injection of an X-ray contrast agent that is burdensome,41 whereas our AI can be applied to any new or existing non-contrast CAC scans for automated cardiac chambers volumetry reports. Other imaging modalities including contrast-enhanced cardiac MRI and echocardiography are not suitable as an opportunistic add-on screening tool to non-contrast CT scans such as ECG-gated CAC and non-gated lung scans. Therefore, non-contrast chest CT scans are prime candidates for opportunistic AI-enabled cardiac chambers volumetry for detection of high-risk future AF11 and HF. The AI approach can enable automatic screening of over 10 million chest CT scans done each year in the US alone.42 Such an AI tool can run in the background of radiology PACS and alert providers to cases with enlarged cardiac chambers. Unfortunately, many high-risk patients with enlarged cardiac chambers, LVH and ALVD are currently undetected, therefore untreated. Early detection of these cases can allow for close monitoring of progression to clinical AF for stroke prevention and intensive medical treatment for clinical HF prevention.
4.2. Limitations
Our study has some limitations. The MESA Exam 1 baseline CT scans, performed between 2000 and 2002, were predominantly conducted using electron-beam computed tomography (EBCT) scanners. This technology is no longer the commonly used method of CAC scanning. Since our AI training was done completely outside of MESA and used a modern multi-detector (256 slice) scanner, we do not anticipate this to affect the generalizability of our findings. Because asymptomatic HF was not a MESA endpoint, we could not investigate the relationship between cardiac chambers volume and asymptomatic HF.
5. Conclusion
In this multi-ethnic population study, AI-powered automated cardiac chambers volumetry enabled prediction of incident HF in new or existing CAC scans and improved on the HF predictive value of clinical risk factors, NT-proBNP and the Agatston Score.
Clinical perspectives.
The potential value of non-coronary findings in coronary calcium scans is significant. AI-enabled cardiac chambers volumetry can detect patients with enlarged LV and alert providers to take preventive actions. The clinical utility of this opportunistic add-on AI to CAC scans warrants further validation in other longitudinal cohorts.
Acknowledgements
This research was supported by 2R42AR070713 and R01HL146666 and MESA was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding and acknowledgement
This research was supported by 2R42AR070713 and R01HL146666 and MESA was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). Preparation of the manuscript was supported by funding from HeartLung.AI.
Footnotes
Conflict of interest
Several members of the writing group are inventors of the AI tool mentioned in this paper. Dr. Naghavi is the founder of HeartLung.AI. Dr. Reeves, Dr. Atlas, Dr. Yankelevitz, Dr, Wong, and Dr. Li are consultants for HeartLung.AI. Chenyu Zhang is a software developer for HeartLung.AI. Kyle Atlas is a graduate research associate of HeartLung.AI. The remaining authors have nothing to disclose.
References
- 1.Greenland P, Lloyd-Jones DM. Role of coronary artery calcium testing for risk assessment in primary prevention of atherosclerotic cardiovascular disease: a review. JAMA Cardiol. 2022;7(2):219–224. 10.1001/jamacardio.2021.3948. [DOI] [PubMed] [Google Scholar]
- 2.Kälsch H, Lehmann N, Möhlenkamp S, et al. Association of coronary artery calcium and congestive heart failure in the general population: results of the Heinz Nixdorf Recall study. Clin Res Cardiol. 2010;99(3):175–182. 10.1007/s00392-009-0104-3. [DOI] [PubMed] [Google Scholar]
- 3.Osenenko KM, Kuti E, Deighton AM, Pimple P, Szabo SM. Burden of hospitalization for heart failure in the United States: a systematic literature review. JMCP. 2022; 28(2):157–167. 10.18553/jmcp.2022.28.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7–11. 10.15420/cfr.2016:25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108(8):977–982. 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 6.Roger VL. Asymptomatic left ventricular dysfunction. JACC (J Am Coll Cardiol): Heart Fail. 2016;4(4):249–251. 10.1016/j.jchf.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Echouffo-Tcheugui JB, Erqou S, Butler J, Yancy CW, Fonarow GC. Assessing the risk of progression from asymptomatic left ventricular dysfunction to overt heart failure. JACC (J Am Coll Cardiol): Heart Fail. 2016;4(4):237–248. 10.1016/j.jchf.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Sara JD, Toya T, Taher R, Lerman A, Gersh B, Anavekar NS. Asymptomatic left ventricle systolic dysfunction. Eur Cardiol. 2020;15:e13. 10.15420/ecr.2019.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Writing Committee Members, ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Card Fail. 2022;28(5): e1–e167. 10.1016/j.cardfail.2022.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Woo P, Mao S, Wang S, Detrano RC. Left ventricular size determined by electron beam computed tomography predicts significant coronary artery disease and events. Am J Cardiol. 1997;79(9):1236–1238. 10.1016/s0002-9149(97)00088-x. [DOI] [PubMed] [Google Scholar]
- 11.Reeves A, Naghavi M, Yankelevitz D, et al. Artificial Intelligence-Enabled Automated Left Atrial Volumetry in Coronary Calcium Scans Predicts Atrial Fibrillation as Early as One Year: Multi-Ethnic Study of Atherosclerosis Society of Cardiovascular Computed Tomography 17 (2023) S1–S96. Published online July 28, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 13.Wasserthal J, Meyer M, Breit HC, Cyriac J, Yang S, Segeroth M. TotalSegmentator: Robust Segmentation of 104 Anatomical Structures in CT Images. 2022. 10.48550/arXiv.2208.05868. Published online August 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isensee F, Jaeger PF, Kohl SAA, Petersen J, Maier-Hein KH. nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation. Nat Methods. 2021;18(2):203–211. 10.1038/s41592-020-01008-z. [DOI] [PubMed] [Google Scholar]
- 15.Patton KK, Heckbert SR, Alonso A, et al. N-terminal pro-B-type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis: the effects of age, sex and ethnicity. Heart. 2013;99(24):1832–1836. 10.1136/heartjnl-2013-304724. [DOI] [PubMed] [Google Scholar]
- 16.N-terminal pro-B-type natriuretic peptide, left ventricular mass, and incident heart failure: multi-Ethnic Study of Atherosclerosis - PubMed. https://pubmed.ncbi.nlm.nih.gov/23032197/. Accessed May 16, 2023. [DOI] [PMC free article] [PubMed]
- 17.Elecsys® NT-proBNP. Diagnostics. Accessed May 16, 2023. https://diagnostics.roche.com/us/en/products/params/elecsys-nt-probnp.html.
- 18.Zaphiriou A, Robb S, Murray-Thomas T, et al. The diagnostic accuracy of plasma BNP and NTproBNP in patients referred from primary care with suspected heart failure: results of the UK natriuretic peptide study. Eur J Heart Fail. 2005;7(4):537–541. 10.1016/j.ejheart.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Kelder JC, Cramer MJ, Verweij WM, Grobbee DE, Hoes AW. Clinical utility of three B-type natriuretic peptide assays for the initial diagnostic assessment of new slow-onset heart failure. J Card Fail. 2011;17(9):729–734. 10.1016/j.cardfail.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 20.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021;42(36):3599–3726. 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 21.Pencina MJ, D’Agostino RB, D’Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. 10.1002/sim.2929. ; discussion 207–212. [DOI] [PubMed] [Google Scholar]
- 22.Daniel KR, Bertoni AG, Ding J, et al. Comparison of methods to measure heart size using noncontrast-enhanced computed tomography: correlation with left ventricular mass. J Comput Assist Tomogr. 2008;32(6):934–941. 10.1097/RCT.0b013e318159a49e. [DOI] [PubMed] [Google Scholar]
- 23.Bittencourt MS, Blankstein R, Mao S, et al. Left ventricular area on non-contrast cardiac computed tomography as a predictor of incident heart failure - the Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr. 2016;10(6):500–506. 10.1016/j.jcct.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qureshi WT, Nasir K, Hacioglu Y, et al. Determination and distribution of left ventricular size as measured by noncontrast CT in the Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr. 2015;9(2):113–119. 10.1016/j.jcct.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Dykun I, Mahabadi AA, Lehmann N, et al. Left ventricle size quantification using non-contrast-enhanced cardiac computed tomography–association with cardiovascular risk factors and coronary artery calcium score in the general population: the Heinz Nixdorf Recall Study. Acta Radiol. 2015;56(8):933–942. 10.1177/0284185114542996. [DOI] [PubMed] [Google Scholar]
- 26.Mahabadi AA, Lehmann N, Sonneck NC, et al. Left atrial size quantification using non-contrast-enhanced cardiac computed tomography - association with cardiovascular risk factors and gender-specific distribution in the general population: the Heinz Nixdorf Recall study. Acta Radiol. 2014;55(8):917–925. 10.1177/0284185113507446. [DOI] [PubMed] [Google Scholar]
- 27.Mahabadi AA, Lehmann N, Kälsch H, et al. Association of epicardial adipose tissue and left atrial size on non-contrast CT with atrial fibrillation: the Heinz Nixdorf Recall Study. Eur Heart J Cardiovasc Imaging. 2014;15(8):863–869. 10.1093/ehjci/jeu006. [DOI] [PubMed] [Google Scholar]
- 28.Mahabadi AA, Geisel MH, Lehmann N, et al. Association of computed tomography-derived left atrial size with major cardiovascular events in the general population: the Heinz Nixdorf Recall Study. Int J Cardiol. 2014;174(2):318–323. 10.1016/j.ijcard.2014.04.068. [DOI] [PubMed] [Google Scholar]
- 29.Mahabadi AA, Lehmann N, Möhlenkamp S, et al. Noncoronary measures enhance the predictive value of cardiac CT above traditional risk factors and CAC score in the general population. JACC Cardiovasc Imaging. 2016;9(10):1177–1185. 10.1016/j.jcmg.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Bruns S, Wolterink JM, Takx RAP, et al. Deep learning from dual-energy information for whole-heart segmentation in dual-energy and single-energy non-contrast-enhanced cardiac CT. Med Phys. 2020;47(10):5048–5060. 10.1002/mp.14451. [DOI] [PubMed] [Google Scholar]
- 31.Jacob AJ, Abdelkarim O, Zook S, et al. AI-based, automated chamber volumetry from gated, non-contrast CT. J Cardiovasc Comput Tomogr. 2023;17(5):336–340. 10.1016/j.jcct.2023.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol. 2002;90(3):254–258. 10.1016/s0002-9149(02)02464-5. [DOI] [PubMed] [Google Scholar]
- 33.Shibazaki K, Kimura K, Okada Y, Iguchi Y, Terasawa Y, Aoki J. Heart failure may be associated with the onset of ischemic stroke with atrial fibrillation: a brain natriuretic peptide study. J Neurol Sci. 2009;281(1–2):55–57. 10.1016/j.jns.2009.02.374. [DOI] [PubMed] [Google Scholar]
- 34.Vasan RS, Benjamin EJ, Larson MG, et al. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. JAMA. 2002;288(10):1252–1259. 10.1001/jama.288.10.1252. [DOI] [PubMed] [Google Scholar]
- 35.Rørth R, Jhund PS, Yilmaz MB, et al. Comparison of BNP and NT-proBNP in patients with heart failure and reduced ejection fraction. Circulation: Heart Fail. 2020;13(2): e006541. 10.1161/CIRCHEARTFAILURE.119.006541. [DOI] [PubMed] [Google Scholar]
- 36.Attia ZI, Dugan J, Rideout A, et al. Automated detection of low ejection fraction from a one-lead electrocardiogram: application of an AI algorithm to an electrocardiogram-enabled Digital Stethoscope. Eur Heart J Digit Health. 2022;3(3): 373–379. 10.1093/ehjdh/ztac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kashou AH, Medina-Inojosa JR, Noseworthy PA, et al. Artificial intelligence-augmented electrocardiogram detection of left ventricular systolic dysfunction in the general population. Mayo Clin Proc. 2021;96(10):2576–2586. 10.1016/j.mayocp.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Attia ZI, Noseworthy PA, Lopez-Jimenez F, et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019;394(10201): 861–867. 10.1016/S0140-6736(19)31721-0. [DOI] [PubMed] [Google Scholar]
- 39.Akbilgic O, Butler L, Karabayir I, et al. ECG-AI: electrocardiographic artificial intelligence model for prediction of heart failure. European Heart Journal Digital Health. 2021;2(4):626. 10.1093/ehjdh/ztab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao X, Rushlow DR, Inselman JW, et al. Artificial intelligence-enabled electrocardiograms for identification of patients with low ejection fraction: a pragmatic, randomized clinical trial. Nat Med. 2021;27(5):815–819. 10.1038/s41591-021-01335-4. [DOI] [PubMed] [Google Scholar]
- 41.Power SP, Moloney F, Twomey M, James K, O’Connor OJ, Maher MM. Computed tomography and patient risk: facts, perceptions and uncertainties. World J Radiol. 2016;8(12):902–915. 10.4329/wjr.v8.i12.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahesh M, Ansari AJ, Mettler FA. Patient exposure from radiologic and nuclear medicine procedures in the United States and worldwide: 2009–2018. Radiology. 2023;307(1):e221263. 10.1148/radiol.221263. [DOI] [PMC free article] [PubMed] [Google Scholar]


