The recent recognition of the global importance of mental disorders has put psychiatry firmly on the international health agenda. The World Health Organization has estimated that neuropsychiatric disorders and suicide account for 12.7% of the global burden of disease.1 Major depression, schizophrenia, bipolar affective disorder, alcohol misuse, and obsessive compulsive disorder account for five of the 10 leading causes of disability in low and middle income countries. In high income countries, dementia is the third most common neuropsychiatric disorder. We examine social, therapeutic, and aetiological developments in the specialty and discuss how they are influencing clinical practice.
Methods
The recent advances selected are based on our clinical and academic interests. We identified the information presented from mainstream and specialist journals.
Combating the stigma of mental illness
Psychiatric stigmatisation is the inappropriate and erroneous association of mental illness with something disgraceful or shameful. Stigma generates a hidden burden and results in barriers to mental health care, reluctance to seek appropriate care, delay in return to wellbeing, and discrimination in allocation of resources.2 Stigmatising attitudes are found universally and can be deeply entrenched. For example, in some African countries traditional supernatural belief systems may lead to negative and hazardous responses to mental illness.3
Two factors are important in reducing the burden of stigma: personal awareness of mental illness and availability of effective treatments. Now that we have effective treatments for the major psychiatric disorders, the key to changing attitudes is education. Before embarking on large scale public campaigns, it is essential to establish baseline attitudes and knowledge to determine what exactly needs to be changed and how this can be most effectively achieved and monitored. For example, the Royal College of Psychiatrists conducted a baseline survey of UK public attitudes as part of its campaign to combat stigmatisation, Changing Minds: Every Family in the Land. The survey ( 1737 respondents, 65% response rate) looked at attitudes to seven common psychiatric disorders and found that people with mental illness were commonly reported as “hard to talk with” (33-65%), “feeling different” from others (40-60%), and “unpredictable” (29-77%).4 It also found that different mental illnesses attracted different types of prejudice, indicating that the general public has a more sophisticated view of mental illness than simply categorising mentally ill people as one homogeneous group.
Recent advances
Stigmatisation of people with mental disorders is being tackled by national and international campaigns based on research of current public opinions
Neurophysiological abnormalities are being identified for post-traumatic stress disorder that should help differentiate it from other conditions and lead to new treatments
Repetitive transcranial magnetic stimulation is a promising new treatment for depression
Studies are under way to establish the cost effectiveness of atypical antipsychotic drugs
Identification and characterisation of the enzymes responsible for cleaving β amyloid from amyloid precursor protein has created the possibility of rational treatments for Alzheimer's disease
Another current initiative to combat stigma is the World Psychiatric Association's Open the Doors campaign (http://openthedoors.com), which aims to counter the problems of stigma and discrimination associated with schizophrenia worldwide. The WHO is also continuing to highlight the global importance of mental illness; the World Health Report 2001 (www.who.int/whr) is dedicated to a worldwide account of mental illness that should help lead the way to developing culturally sensitive schemes to challenge stigma.
Healthcare professionals need to challenge their own stigmatising attitudes—for instance, by separating the individual from the illness and speaking about a “person with schizophrenia” rather than a “schizophrenic.” Additionally, psychiatrists are in a unique position to actively challenge stigmatising media representations of mental illness.5
Neurobiological aspects of post-traumatic stress disorder
The trigger for post-traumatic stress disorder is a traumatic stress that is sufficiently threatening to physical integrity to cause pervasive distress in almost anyone. Such stresses are now recognised to include a variety of personal threats—for example, combat, sexual assault, car crash, or intensive cancer treatment. The disorder arises because of incomplete emotional processing of the trauma, resulting in a delayed and protracted response. Post-traumatic stress disorder is characterised by three broad symptom clusters: hyperarousal (hypervigilance, increased startle reaction, and insomnia), traumatic re-experiencing (intrusive nightmares and memory “flashbacks”), and avoidance of anything reminiscent of the trauma. Extreme trauma (such as torture) can lead to chronic post-traumatic stress disorder and even enduring personality change, characterised by mistrust, hostility, and social withdrawal.
Evidence of a distinct pathophysiology for post-traumatic stress disorder is emerging. Like depression, post-traumatic stress disorder is associated with increased secretion of corticotropin releasing factor. Unlike depression, however, the increased secretion is associated with hypocortisolaemia,6 indicating a grossly exaggerated negative feedback inhibition of the hypothalamic-pituitary-adrenal axis that is possibly secondary to up-regulation of glucocorticoid receptors.7 Functional neuroimaging studies in post-traumatic stress disorder have shown activation abnormalities in several cerebral structures that function in memory, fear responses, and visuospatial processing; these include the hippocampus, amygdala, anterior cingulate cortex, and various regions of the prefrontal cortex.8 Although such changes could be induced by comorbid depression or substance misuse or be adaptive responses to chronic stress, they may differentiate post-traumatic stress disorder from other disorders and facilitate the development of specific treatments.
It is becoming increasingly apparent that drugs have a first line role in managing post-traumatic stress disorder alongside psychological treatments. A recent meta-analysis of randomised placebo controlled trials of selective serotonin reuptake inhibitors has shown substantial benefit on core symptoms, with odds ratios for response ranging from 2.2 to 5.6.9 Selective serotonin reuptake inhibitors can also alleviate comorbid depression and anxiety.
Repetitive transcranial magnetic stimulation: new treatment for depression
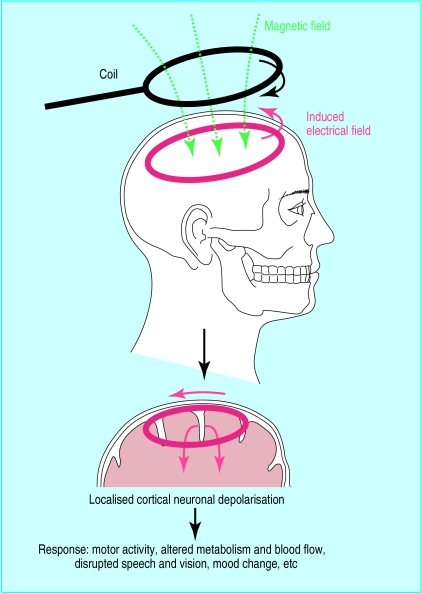
Transcranial magnetic stimulation is an established method to non-invasively stimulate the cerebral cortex and investigate cortical function (fig 1). Use of transcranial magnetic stimulation to treat neuropsychiatric disorders, especially depression, has attracted much research interest.10 The procedure is straightforward, and a typical treatment course involves brief (about 20 minutes) daily outpatient sessions over two to three weeks. Unlike electroconvulsive therapy, with which it is often compared, repetitive transcranial magnetic stimulation does not require any anaesthesia or induction of seizures. The most important safety concern is risk of seizures with prolonged use of fast, repetitive transcranial magnetic stimulation. However, no seizures have been reported since the introduction of guidelines for safe use of the technique.11
Figure 1.
Transcranial magnetic stimulation is based on the principle that an electric current passing through a flat handheld insulated coil placed tangentially on the scalp generates a magnetic field. This can be targeted to a selected scalp area (∼cm2) and induces a secondary electrical field in the underlying cortex, causing neuronal depolarisation. Patients can be given a single brief magnetic pulse or a series of pulses (repetitive transcranial magnetic stimulation). Excitability of neurones is enhanced by fast repetitive transcranial magnetic stimulation (usually 5-20 Hz) but inhibited by slow repetitive transcranial magnetic stimulation (<1 Hz). The effect depends on the site stimulated
Brain imaging studies have implicated hypofunction of the left dorsolateral prefrontal cortex in depression. Initial open trials of fast—that is, activating—repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex suggested that it might be beneficial in up to 50% of patients with treatment resistant depression. Subsequent larger randomised controlled trials have shown more modest but significant responses to two weeks' treatment.10,12,13 The frontal lobe imbalance model of depression suggests that suppression of the contralateral prefrontal cortex might also be therapeutic. Indeed, in the largest randomised controlled study reported, slow repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex has been found to be more effective than sham treatment.14 Initial reports also suggest that repetitive transcranial magnetic stimulation might be as effective as electroconvulsive therapy in non-psychotic depression.15
There are several methodological problems with published trials of repetitive transcranial magnetic stimulation: most patients have treatment resistant depression, the placebo “sham” coil treatments do exert some effect,16 and, because of the nature of the treatment, the trials are not double blinded. Nevertheless, an antidepressant effect is apparent, although optimal treatment levels, maintenance of therapeutic effects, and long term outcome remain to be established. It is also unclear which clinical characteristics are predictive of response. Further international research is under way in academic research centres. However, if it does prove to be an effective, acceptable, and economically viable alternative to current treatments, repetitive transcranial magnetic stimulation treatment centres could readily be set up in district general hospitals.
Cost effectiveness of atypical antipsychotics
The generic term atypical antipsychotics refers to the newer generation of drugs that produce fewer extrapyramidal side effects than conventional antipsychotics such as chlorpromazine.17 Examples include clozapine, risperidone, olanzapine, amisulpride, and quetiapine. Atypical antipsychotic drugs have similar efficacy to conventional antipsychotics, although clozapine has been shown to be more effective in treatment resistant schizophrenia.18 Because atypical antipsychotic drugs are better tolerated, it is often suggested that they should become first line treatment for schizophrenia. However, as the newer drugs can be up to 30 times more expensive than the older drugs, such a change has major cost implications.19
If atypical drugs are truly associated with greater patient benefits then it is reasonable to expect them to be cost effective. Unfortunately, economic evaluations often fail to take full account of direct and indirect costs (for example, what is the cost equivalent of not experiencing akathisia or parkinsonism?), are retrospective, and exclude subjects who discontinue treatment. Indeed, one recent review was unable to identify any double blind, randomised, controlled, and long term pharmacoeconomic trial of atypical drugs.19
Trials of the cost effectiveness of clozapine show potential savings, usually due to a reduction in hospital stay. However, methodological limitations mean that no firm conclusions can be drawn.20,21 The available data for other atypical antipsychotics are even more equivocal.20 Better conducted trials that take appropriate measures of quality of life into account are still required to justify endorsement of atypical antipsychotics on grounds of cost alone. In the United Kingdom, the National Institute for Clinical Excellence is due to provide clinical guidelines on prescribing atypical antipsychotics and their cost effectiveness in December 2001, and the NHS funded randomised multicentre CUtLASS (cost utility of the latest antipsychotics in severe schizophrenia) study will hopefully provide a definitive economic verdict.
Identification of β-secretase and γ-secretase in Alzheimer's disease
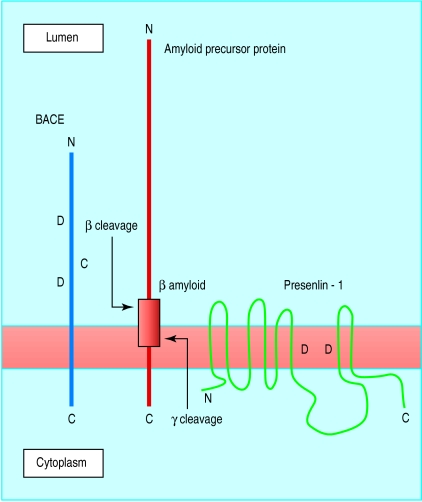
Aberrant processing of amyloid precursor protein, leading to increased production and aggregation of β amyloid peptide in the brain, is believed to be central to the pathogenesis of Alzheimer's disease. β Amyloid peptide is cleaved from within amyloid precursor protein by two enzymes known as β-secretase and γ-secretase (fig 2). One possible treatment strategy for Alzheimer's disease would be to decrease production of β amyloid by inhibiting these secretases.
Figure 2.
Proteolytic cleavage of amyloid precursor protein by β site amyloid precursor protein cleavage enzyme (BACE) and presenilin-1, a candidate protein for γ-secretase. Inhibition of β or γ cleavage reduces production of β amyloid, which has been linked to Alzheimer's disease. D denotes conserved aspartic acid residues that are candidate active sites for cleavage
The enzyme β-secretase was recently identified by several different groups and named BACE (β site amyloid precursor protein cleaving enzyme).22 BACE is a brain enriched transmembrane protein found mainly in the Golgi apparatus within the cell body. There are two aspartic acid residues in its luminal domain that help form the active site and that can access the β cleavage site in the luminal domain of amyloid precursor protein (fig 2). Overexpression of BACE increases cleavage of amyloid precursor protein at the β-secretase site, whereas inhibition decreases cleavage.23,24
The presenilin proteins (PS1 and PS2), mutations in which cause early onset familial Alzheimer's disease, have been shown to regulate γ-secretase activity and may actually be γ-secretase.22 For example, cultured neurones from PS1 knockout mice have reduced γ-secretase activity, which results in a fivefold decrease in production of β amyloid.25 Knocking out both PS1 and PS2 abolishes γ-secretase cleavage of amyloid precursor protein,26 and mutation of putative active aspartic acid sites within PS1 reduces γ-secretase cleavage of amyloid precursor protein.27 It may, however, be difficult to produce drugs to inhibit the activity of BACE and γ-secretase or presenilins. BACE is relatively inaccessible because of it intracellular location, and inhibiting γ-secretase may have adverse consequences for non-neuronal systems.22 Nevertheless, these findings open up new avenues for research into treatment for Alzheimer's disease.
Additional educational resources
Stigma and mental illness
Royal College of Psychiatrists campaign Changing Minds: Every Family in the Land
WHO World Health Report
World Psychiatric Association anti-stigma website
Jorm AF. Mental health literacy: public knowledge and beliefs about mental disorders. Br J Psychiatry 2000;177:396-401
Post-traumatic stress disorder
National Center for Post-Traumatic Stress Disorder
www.sover.net/∼schwcof/ptsd.html
Post-traumatic stress disorder bibliography
Transcranial magnetic stimulation
International Society for Transcranial Stimulation
Hoffman RE, Boutros NN, Hu S, Berman RM, Krystal JH, Charney DS. Transcranial magnetic stimulation and auditory hallucinations in schizophrenia. Lancet 2000;355:1073-5.
Atypical antipsychotics and cost effectiveness
www.iop.kcl.ac.uk/Extras/Cutlass/index.html
Website for the cost utility of the latest antipsychotics in severe schizophrenia (CUtLASS) study
National Institute for Clinical Excellence
Alzheimer's disease
Alzheimer Research Forum
Alzheimer's Society
Footnotes
Competing interests: None declared.
References
- 1.World Health Organization. The world health report 2000—health systems: improving performance. Geneva: WHO; 2000. [Google Scholar]
- 2.Rosenfield S. Labelling mental illness: the effects of received services and perceived stigma on life satisfaction. American Sociological Review. 1997;62:660–672. [Google Scholar]
- 3.Gureje O, Alem A. Mental health policy development in Africa. Bull World Health Org. 2000;78:475–482. [PMC free article] [PubMed] [Google Scholar]
- 4.Crisp AH, Gelder MG, Rix S, Meltzer HI, Rowlands OJ. Stigmatisation of people with mental illnesses. Br J Psychiatry. 2000;177:4–7. doi: 10.1192/bjp.177.1.4. [DOI] [PubMed] [Google Scholar]
- 5.Byrne P. Stigma of mental illness and ways of diminishing it. Adv Psychiat Treat. 2000;6:65–72. [Google Scholar]
- 6.Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- 7.Liberzon I, Lopez JF, Flagel SB, Vazquez DM, Young EA. Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: relevance to post-traumatic stress disorder. J Neuroendocrinol. 1999;11:11–17. doi: 10.1046/j.1365-2826.1999.00288.x. [DOI] [PubMed] [Google Scholar]
- 8.Newport DJ, Nemeroff CB. Neurobiology of posttraumatic stress disorder. Curr Opin Neurobiol. 2000;10:211–218. doi: 10.1016/s0959-4388(00)00080-5. [DOI] [PubMed] [Google Scholar]
- 9.Stein DJ, Seedat S, van der Linden GJH, Zungu-Dirwayi N. Selective serotonin reuptake inhibitors in the treatment of post-traumatic stress disorder: a metaanalysis of randomized controlled trials. Int Clin Psychopharmacol. 2000;15:S31–S39. doi: 10.1097/00004850-200008002-00006. [DOI] [PubMed] [Google Scholar]
- 10.George MS, Lisanby SH, Sackeim HA. Transcranial magnetic stimulation—applications in neuropsychiatry. Arch Gen Psychiatry. 1999;56:300–311. doi: 10.1001/archpsyc.56.4.300. [DOI] [PubMed] [Google Scholar]
- 11.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the international workshop on the safety of repetitive transcranial magnetic stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 12.Berman RM, Narasimhan M, Sanacora G, Miano AP, Hoffman RE, Hu XS, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry. 2000;47:332–337. doi: 10.1016/s0006-3223(99)00243-7. [DOI] [PubMed] [Google Scholar]
- 13.George MS, Nahas Z, Molloy M, Speer AM, Oliver NC, Li XB, et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry. 2000;48:962–970. doi: 10.1016/s0006-3223(00)01048-9. [DOI] [PubMed] [Google Scholar]
- 14.Klein E, Kreinin I, Christyakov A, Koren D, Mecz L, Marmur S, et al. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression—a double-blind controlled study. Arch Gen Psychiatry. 1999;56:315–320. doi: 10.1001/archpsyc.56.4.315. [DOI] [PubMed] [Google Scholar]
- 15.Grunhaus L, Dannon PN, Schreiber S, Dolberg OH, Amiaz R, Ziv R, et al. Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder. Biol Psychiatry. 2000;47:314–324. doi: 10.1016/s0006-3223(99)00254-1. [DOI] [PubMed] [Google Scholar]
- 16.Loo CK, Taylor JL, Gandevia SC, McDarmont BN, Mitchell PB, Sachdev PS. Transcranial magnetic stimulation (TMS) in controlled treatment studies: are some “sham” forms active? Biol Psychiatry. 2000;47:325–331. doi: 10.1016/s0006-3223(99)00285-1. [DOI] [PubMed] [Google Scholar]
- 17.Voruganti L, Cortese L, Oyewumi L, Cernovsky Z, Zirul S, Awad A. Comparative evaluation of conventional and novel antipsychotic drugs with reference to their subjective tolerability, side-effect profile and impact on quality of life. Schizophr Res. 2000;43:135–145. doi: 10.1016/s0920-9964(99)00154-1. [DOI] [PubMed] [Google Scholar]
- 18.Wahlbeck K, Cheine M, Essali A, Adams C. Evidence of clozapine's effectiveness in schizophrenia: a systematic review and meta-analysis of randomized trials. Am J Psychiatry. 1999;156:990–999. doi: 10.1176/ajp.156.7.990. [DOI] [PubMed] [Google Scholar]
- 19.Taylor D, Aitchison K. The pharmaco-economics of atypical antipsychotics. Int J Psychiatr Clin Pract. 1999;3:237–248. doi: 10.3109/13651509909068390. [DOI] [PubMed] [Google Scholar]
- 20.Revicki DA. Pharmacoeconomic studies of atypical antipsychotic drugs for the treatment of schizophrenia. Schizophr Res. 1999;35:S101–S109. doi: 10.1016/s0920-9964(98)00168-6. [DOI] [PubMed] [Google Scholar]
- 21.Essock SM, Frisman LK, Covell NH, Hargreaves WA. Cost-effectiveness of clozapine compared with conventional antipsychotic medication for patients in state hospitals. Arch Gen Psychiatry. 2000;57:987–994. doi: 10.1001/archpsyc.57.10.987. [DOI] [PubMed] [Google Scholar]
- 22.Vassar R, Citron M. Aβ-generating enzymes: recent advances in β- and γ-secretase research. Neuron. 2000;27:419–422. doi: 10.1016/s0896-6273(00)00051-9. [DOI] [PubMed] [Google Scholar]
- 23.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. β-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 24.Yan R, Bienkowski MJ, Shuck ME, Miao HY, Tory MC, Pauley AM, et al. Membrane-anchored aspartyl protease with Alzheimer's disease β-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 25.De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 26.Zhang ZH, Nadeau P, Song WH, Donoviel D, Yuan ML, Bernstein A, et al. Presenilins are required for γ-secretase cleavage of β-APP and transmembrane cleavage of Notch-1. Nat Cell Biol. 2000;2:463–465. doi: 10.1038/35017108. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe MS, Xia WM, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]