Abstract
Purpose
Varicocele has been associated with high seminal oxidative stress (OS), impaired semen quality, and reduced male fertility potential. However, the exact mechanism(s) underlying the development of varicocele-mediated infertility and the cause-effect relationship between varicocele and testicular dysfunction are not fully understood. The aim of this systematic review and meta-analysis (SRMA) is to investigate the impact of varicocele on testicular OS markers and sperm parameters in experimental animals with varicocele as compared to animals without varicocele.
Materials and Methods
A literature search was performed using the Scopus and PubMed databases on studies that investigated testicular OS markers and sperm parameters in animals with varicocele. The primary outcomes included malondialdehyde (MDA) (nmol/mg) levels whereas the secondary outcomes included total sperm count (×106), sperm vitality (%), total sperm motility (%), and sperm DNA fragmentation (SDF) (%). Standardized mean difference (SMD) (95% confidence interval [CI]) was chosen to express the effect size. The quality of the included studies was evaluated using the Cambridge Quality Checklist.
Results
Out of 76 identified articles, 6 studies on rats were included in the meta-analysis. The analysis showed a significant increase of MDA (SMD: 15.61 [1.93, 29.29]; p=0.03) in rats with varicocele vs. controls. We also observed a significant decrease in total sperm count (SMD: -17.45 [-28.97, -5.93]; p<0.01), sperm vitality (SMD: -16.41 [-26.30, -6.52]; p<0.01), total sperm motility (SMD: -17.67 [-24.90, -10.44]; p<0.01), and a significant increase of SDF (SMD: 7.41 [1.23, 13.59]; p=0.02), in rats with varicocele vs. controls. The quality of the included studies was ranked as high.
Conclusions
This SRMA indicates a significant increase in levels of testicular MDA and SDF and a reduction of sperm quality in experimental animals with varicocele. These findings support the potential role of testicular OS in the development of varicocele-induced testicular damage.
Keywords: Infertility, Oxidative stress, Reactive oxygen species, Spermatozoa, Varicocele
INTRODUCTION
Varicocele is a common medical condition characterized by dilatation of the veins within the pampiniform plexus in the spermatic cord. It affects approximately 10% to 15% of men, with a higher incidence observed in infertile men [1].
One of the consequences of varicocele is the increased production of reactive oxygen species (ROS) in the testes, leading to an increased oxidative stress (OS) [2]. The ROS are highly reactive molecules that can cause damage to cellular structures, including proteins, lipids, and deoxyribonucleic acid (DNA) [3]. Testicular tissues are vulnerable to OS due to their high oxygen consumption [4].
The increased levels of OS in men with varicoceles can result in detrimental effects on testicular function, including impairing sperm motility, morphology, and vitality, reducing sperm count, and increasing sperm DNA fragmentation (SDF) [5]. Both spermatogenesis and Leydig cell steroidogenesis are vulnerable to OS, and the alteration in the oxygen tension and presence of ROS can disrupt the blood-testis barrier, which is critical for maintaining the microenvironment of the testes and protecting developing germ cells from harmful substances [6].
Previous studies in animals have investigated the relationship between varicocele and OS in the testes, demonstrating that varicocele induces ROS production [7]. Karna et al [8] found that varicocele caused a significant increase in ROS production and lipid peroxidation in the testes of mice, leading to a reduction in sperm count and motility. Nevertheless, the cause-effect relationship between varicocele and testicular dysfunction is not fully understood. Factors such as heat stress, alterations in the testicular environment and testicular hypoxia may play a role in the pathogenesis of OS and consequently the development of infertility secondary to varicocele [9].
The aim of this systematic review and meta-analysis (SRMA) was to investigate the impact of varicocele on testicular OS markers and sperm parameters in experimental animals with varicocele, comparing them to animals without varicocele. By synthesizing existing research on animal models, we aim to enhance our understanding of the relationship between varicocele and OS, shedding light on the mechanisms involved in varicocele-induced testicular damage.
MATERIALS AND METHODS
1. Search strategy
A systematic search was conducted on Scopus and PubMed databases, using a combination of Medical Subject Heading (MeSH) terms and free words. An initial keyword string was created on Scopus using the following string: (TITLE-ABS-KEY (varicocele* AND sperm) AND (oxidative stress OR heat stress OR reactive oxygen species) AND (KEY animal) AND (LIMIT-TO (DOCTYPE, "ar"))). A Pubmed search with the following string: "varicocele*"[All Fields] AND ("sperm"[All Fields] OR "spermatozoa"[MeSH Terms] OR "spermatozoa"[All Fields]) AND ("heat stress"[All Fields] OR "oxidative stress"[All Fields] OR “reactive oxygen species” [All Fields]) was also performed. Relevant publications were searched from 1906 until the end of August 2022 and references were checked to implement the retrieved articles. English and non-English articles have been included in the search.
2. Selection criteria
A SRMA of animal studies has been conducted following the PECOS (Population, Exposure, Comparator, Outcomes, Study design) approach as previously described (Supplement Table 1) [10]. This analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [11].
This SRMA included animal studies (cohort studies) investigating testicular OS markers and sperm parameters in experimental animals with varicocele as compared to animals without varicocele. Human studies, abstracts, conference papers, reviews, and book chapters were excluded. Initial manual screening of the retrieved abstracts was conducted by two independent researchers (G.I.R. and F.B.) and duplicates were removed. The eligibility of the identified abstracts was considered based on the inclusion and exclusion criteria of this review and following the PECOS model [12]. Discrepancies in the inclusion between the two investigators were resolved by discussion or further consultation with a third author (D.D.).
3. Outcome measures
The primary aim of this study was to investigate the differences in levels of testicular malondialdehyde (MDA) levels (nmol/mg), ROS, and total antioxidant capacity (TAC) between experimental animals with varicoceles and animals without varicoceles. Secondary outcomes included the sperm concentration (×106/mL), total sperm count (×106), sperm vitality (%), total sperm motility (%), and SDF (%).
4. Data extraction
The data were extracted from eligible articles for which the full text was available. The following information was extracted: first author’s name, year of publication, journal, study design, animal species, testicular OS markers (MDA [nmol/mg], ROS and TAC [nmol/mg]), and sperm parameters (sperm concentration [×106/mL], total sperm count [×106/mL], sperm vitality [%], total sperm motility [%], and SDF [%]).
To ensure the accuracy of the extracted data and to reduce the potential errors due to manual search of data, screening for eligibility and data extraction were performed in duplicate and cross-checked within researchers. In cases of discrepancy between two researchers, the disagreements were resolved through further discussions or the results in dispute were verified by the senior authors of the team, and a final decision was made in each case.
5. Quality assessment
The quality of the included studies was assessed using the Cambridge Quality Checklist [13].
6. Statistical analysis
The statistical analysis was performed using the R programming language version 4, 12. The standardized mean difference (SMD) was chosen as the effect size for statistical comparison between cases and controls to allow comparison between studies that measured the outcome using different instruments [14]. We also reported the mean difference (MD). Heterogeneity across pooled studies was assessed using Cochran’s Q test and heterogeneity index (I2). The fixed effect model was adopted for calculating the pooled effect size in cases of low/non-significant heterogeneity, while the random effect model was used for studies with high/significant heterogeneity. The sensitivity analysis was performed by repeating the meta-analysis after excluding one study at a time (leave-one-out method) and observing the changes in the pooled effect size when removed [15].
We assessed publication bias by using a funnel plot, however, the p-value could not be calculated because the number of included studies were limited. Asymmetrical funnel plots qualitatively indicated the presence of publication bias, suggested by the missing studies from one side of the graph.
RESULTS
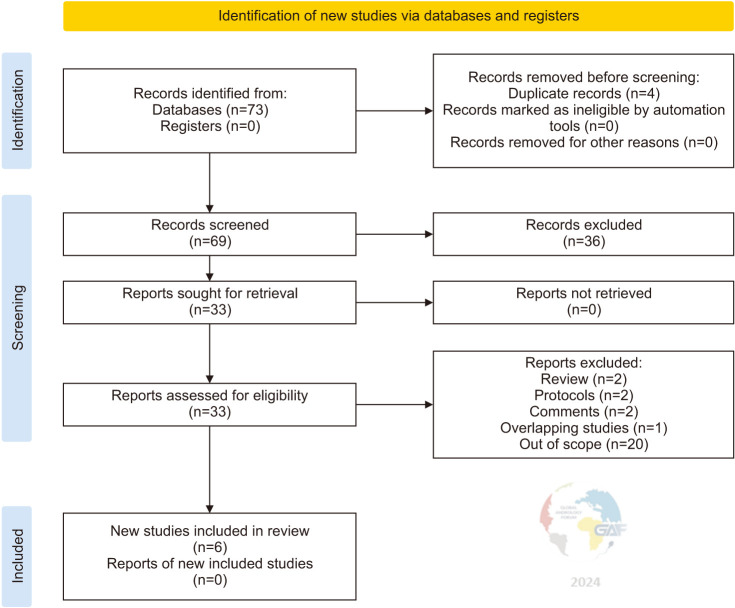
Out of 76 identified articles, 6 articles were included in the quantitative analysis (Table 1) [7,16,17,18,19,20].
Table 1. Description of included studies.
| Author | Year | Type of study | Type of animal | Technique of varicocele induction | Follow-up |
|---|---|---|---|---|---|
| Gur et al [20] | 2021 | Cohort | Wistar rats | Left renal vein ligation (partial) | 2 mo |
| Sadeghi et al [7] | 2020 | Cohort | Wistar rats | Left renal vein ligation | 2 mo |
| Taghizadeh et al [18] | 2017 | Cohort | Wistar rats | Left renal vein ligation (partial) | 4 mo |
| Khosravanian et al [17] | 2014 | Cohort | Wistar rats | Left renal vein ligation | 8 wk |
| Moshtaghion et al [16] | 2013 | Cohort | Wistar rats | Left renal vein ligation | 42 wk |
| Razi et al [19] | 2011 | Cohort | Wistar rats | Left renal vein ligation | 8 mo |
Fig. 1 shows the flowchart of the study according to guidelines [11]. Table 2 shows the risk of bias evaluation for all studies. Results of outcomes such as sperm concentration (×106/mL), TAC, and ROS are not reported since the number of studies was insufficient for generating forest plots.
Fig. 1. PRISMA flow diagram summarizing the identification, screening, eligibility, and inclusion of articles.
Table 2. Quality of evidence of the included studies using the Cambridge Quality Checklist.
| Author | Checklist for correlates (0–5) | Checklist for risk factors (1–3) | Checklist for causal risk factor (1–7) | Total score (2–15) |
|---|---|---|---|---|
| Gur et al [20] | 4 | 3 | 6 | 13 |
| Sadeghi et al [7] | 4 | 3 | 6 | 13 |
| Taghizadeh et al [18] | 4 | 3 | 6 | 13 |
| Khosravanian et al [17] | 4 | 3 | 6 | 13 |
| Moshtaghion et al [16] | 4 | 3 | 6 | 13 |
| Razi et al [19] | 4 | 3 | 6 | 13 |
1. Testicular oxidative stress markers
1) Malondialdehyde
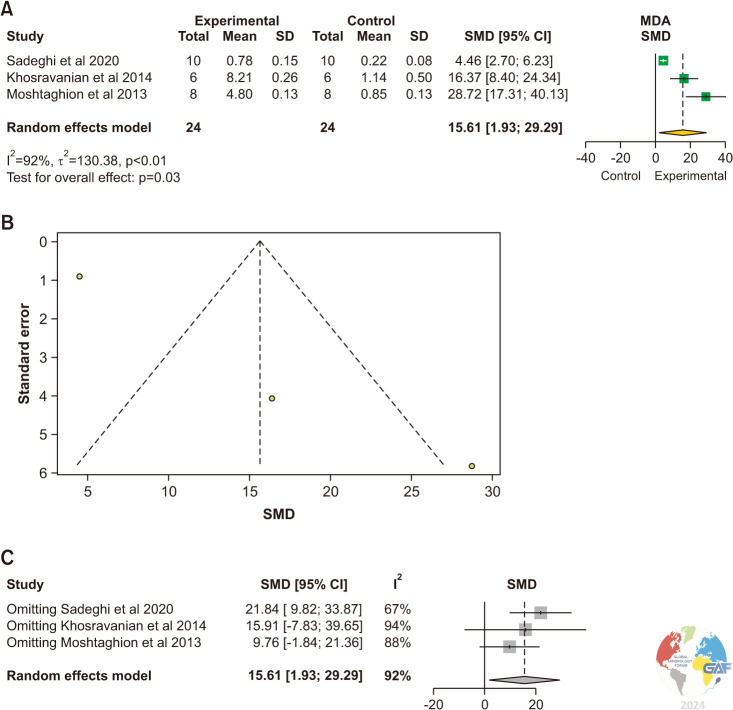
MDA levels were evaluated in 3 studies [7,16,17]. The levels of MDA were significantly elevated in the testicular tissue of experimental animals with varicocele compared to control animals without varicocele (SMD: 15.61 [1.93, 29.29]; p=0.03) (Fig. 2). Significant inter-study heterogeneity was observed (I2=92%; χ2 p<0.01). The asymmetry of the funnel plot denoted publication bias.
Fig. 2. (A) Forest plot of the MDA in animals with varicocele compared to controls. (B) Funnel plot of the MDA in animals with varicocele compared to controls. (C) Sensitivity analysis of the MDA in animals with varicocele compared to controls. MDA: malondialdehyde, SD: standard deviation, SMD: standardized mean difference.
The sensitivity analysis demonstrated no significant changes (Fig. 2C).
The pooled estimate was significantly greater in the experimental group (MD: 3.85, 95% CI: 0.17, 7.54; p=0.04) (Supplement Fig. 1).
2. Sperm parameters
1) Total sperm count
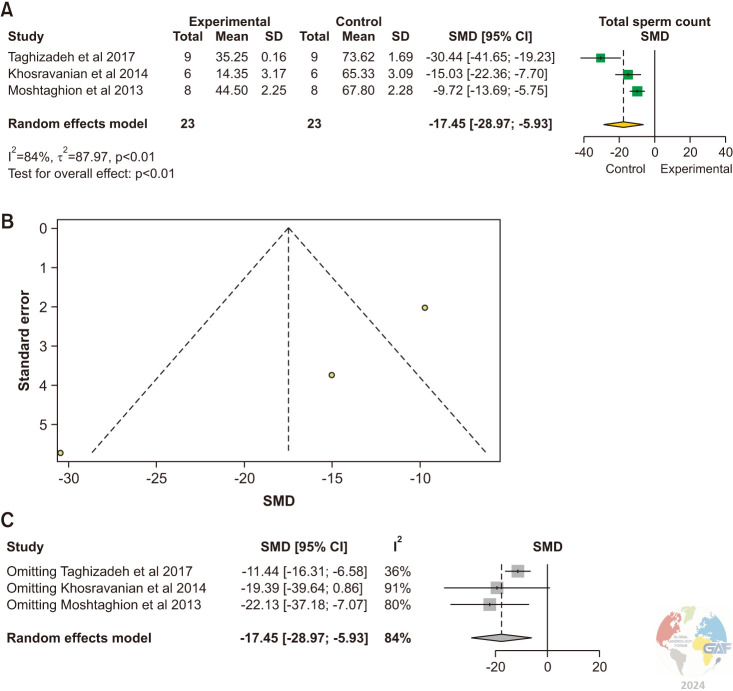
The total sperm count was evaluated in 3 studies [16,17,18]. There was a statistically significant reduction in total sperm counts in experimental animals with varicoceles in comparison to control animals without varicoceles (SMD: -17.45 [-28.97, 5.93]; p<0.01) (Fig. 3). Significant inter-study heterogeneity was observed (I2=84%; χ2 p<0.01).
Fig. 3. (A) Forest plot of the total sperm count in animals with varicocele compared to controls. (B) Funnel plot of the total sperm count in animals with varicocele compared to controls. (C) Sensitivity analysis of total sperm count in animals with varicocele compared to controls. SD: standard deviation, SMD: standardized mean difference.
There was asymmetrical study distribution in the funnel plot indicating publication bias. The study by Khosravanian et al [17] was found sensitive to change the pooled estimate with no significant difference between groups when removed.
The pooled estimate was significantly lower in the experimental group (MD: -37.51, 95% CI: -53.14, -21.87; p<0.01) (Supplement Fig. 2).
2) Sperm vitality
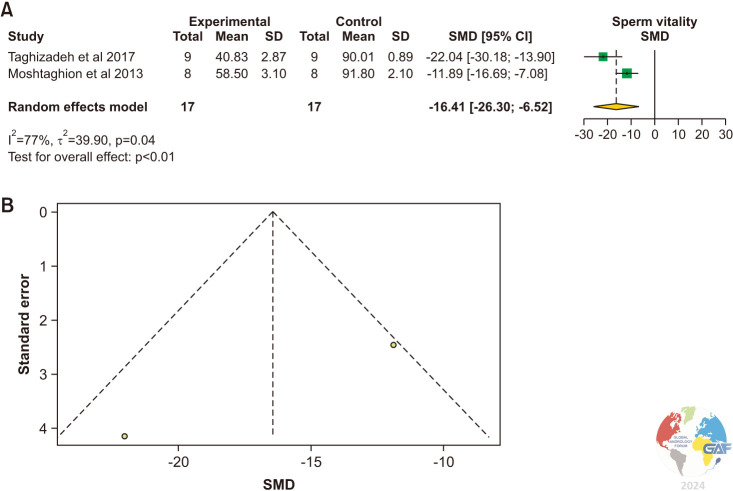
Sperm vitality was evaluated in two studies using Eosin-Nigrosin staining [16,18]. There was a statistically significant reduction of sperm vitality in experimental animals with varicocele versus control animals (SMD: -16.41 [-26.30, -6.52]; p<0.01) (Fig. 4). Significant interstudy heterogeneity was observed (I2=77%; χ2 p=0.04).
Fig. 4. (A) Forest plot of the sperm vitality in animals with varicocele compared to controls. (B) Funnel plot of the sperm vitality in animals with varicocele compared to controls. SD: standard deviation, SMD: standardized mean difference.
The pooled estimate was significantly lower in the experimental group (MD: -41.26, 95% CI: -56.83, -25.70; p<0.01). (Supplement Fig. 3).
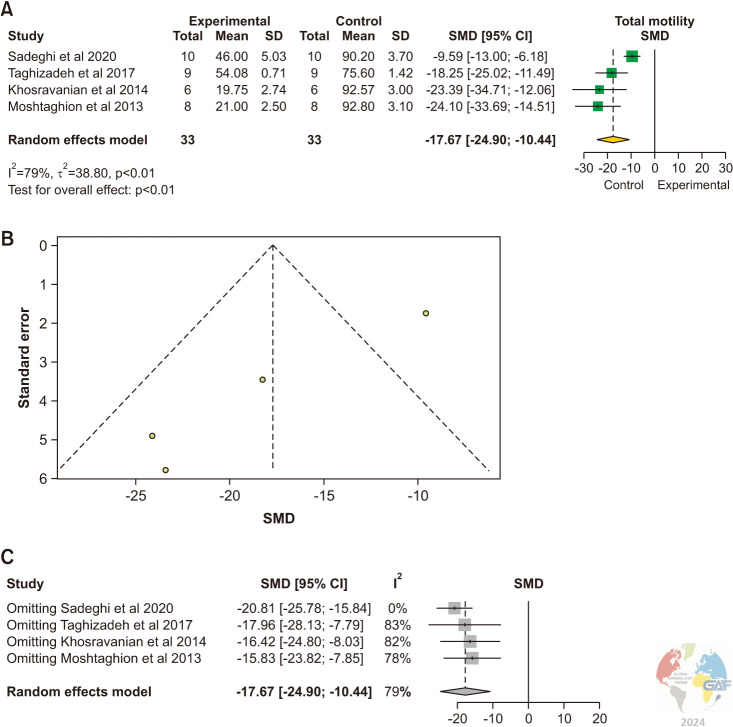
3) Total sperm motility
Sperm motility was evaluated in 4 studies [7,16,17,18]. There was a statistically significant reduction of total sperm motility in experimental animals with varicocele versus control animals (SMD: -17.67 [-24.90, -10.44]; p<0.01) (Fig. 5).
Fig. 5. (A) Forest plot of the total sperm motility in animals with varicocele compared to controls. (B) Funnel plot of the total sperm motility in animals with varicocele compared to controls. (C) Sensitivity analysis of the total sperm count in animals with varicocele compared to controls. SD: standard deviation, SMD: standardized mean difference.
Significant inter-study heterogeneity was observed (I2=79%; χ2 p<0.01). The asymmetry of the funnel plot highlighted the presence of publication bias. Sensitivity analysis showed no studies influencing the pooled estimate when removed.
The pooled estimate was significantly lower in the experimental group (MD: -52.56, 95% CI: -76.70, -28.43; p<0.01) (Supplement Fig. 4).
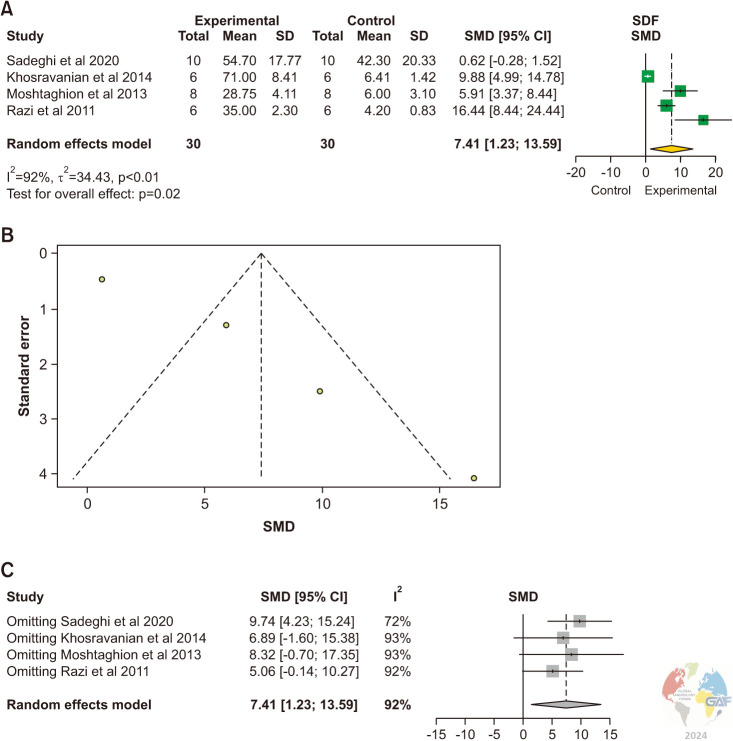
4) Sperm DNA fragmentation
SDF levels were evaluated in 4 studies [7,16,17,19]. Acridine orange staining kit was used in all studies. The pooled estimate was significantly higher in the experimental group (SMD: 7.41, 95% CI [1.23, 13.59], p=0.02). Publication bias is demonstrated in the funnel plot (Fig. 6). Sensitivity analysis showed 3 studies which changed the pooled estimate when removed [16,17,19].
Fig. 6. (A) Forest plot of the SDF in animals with varicocele compared to controls. (B) Funnel plot of the SDF in animals with varicocele compared to controls. (C) Sensitivity analysis of the SDF in animals with varicocele compared to controls. SDF: sperm DNA fragmentation, SD: standard deviation, SMD: standardized mean difference.
Significant inter-study heterogeneity was observed (χ2 p<0.01). Studies are unequally distributed across the funnel plot.
The pooled estimate was significantly higher in the experimental group (MD: 33.1, 95% CI [11.2, 55.1], p-value 0.003). Publication bias is demonstrated in the funnel plot. Sensitivity analysis showed no studies influenced the pooled estimate when removed (Supplement Fig. 5).
3. Quality check of included studies
Table 2 shows the results of the quality of all studies, demonstrating overall high quality.
DISCUSSION
The results of this SRMA indicate a significant increase in OS as evidenced by increased levels of testicular tissue MDA in experimental animals with varicocele as compared to those without varicocele. MDA is the byproduct of lipid peroxidation caused by imbalance of ROS, and serves as a marker of OS [5,21], Interestingly, Jang et al [22] reported increased levels of ROS in testicular tissue of rats with varicoceles by quantifying the levels of 8-hydroxy-20-deoxyguanosine (8-OHdG) as a product of oxidatively modified DNA. The results of the latter study are in line with our findings of high OS in testicular tissue of experimental animals with varicoceles. Another study by Sadraei et al [23] demonstrated increased percentage of intracytoplasmic ROS in sperm suspensions obtained from the caudal epididymis of rat models with varicocele. However, the use of two different samples (testicular tissue and epididymal sperm suspension), in the latter studies, prevented us from conducting a quantitative analysis of ROS as an outcome in experimental animals with varicoceles.
The exact mechanism of pathogenesis of OS in experimental animals with varicocele is not fully understood despite excess heat exposure and hypoxia have been postulated. Varicocele is thought to increase testicular temperature, secondary to the increase in heat shock proteins (HSPs) and their impact on sperm protein denaturation, apoptosis, and male infertility [24]. Additionally, these detrimental effects consequently lead to OS, hypoxia, damage to the germ cells and Leydig cells and reduced sperm production [25,26].
HSPs are a family of proteins that are expressed constitutively in cells and play a role in regulating various cellular pathways, including transport, translation, transcription, and signal transduction. These proteins are also involved in responding to stimuli such as heat stress. The inability to produce adequate concentrations of functional HSP may lead to increased sperm protein denaturation, apoptosis, and male infertility in varicocele patients [27]. HSPA2, a member of the HSP family, is expressed in male germ cells and is essential for spermatogenesis. HSPA2 mRNA and protein expression levels were lower in oligozoospermic men with varicocele, while HSPA2 protein activity increased after varicocele repair [27]. Heat exposure can induce OS in tissues, increase sperm protein denaturation, and apoptosis in varicocele patients [28].
Varicocele-induced altered intratesticular vascular perfusion and pressure patterns together with local hypoxia are considered important factors of male infertility [9]. Hypoxia in patients with varicocele leads to a decrease in oxygen partial pressure in testis tissue, resulting in a metabolic disorder [29]. The presence of hypoxia in the testicular microenvironment triggers changes in the expression of various hypoxia-related factors and genes, subsequently impacting the testicular microenvironment. The hypoxia-inducible factor-1 (HIF-1) is generated in response to tissue hypoxia and is expressed in germ cells. HIF-1 binds to vascular endothelial growth factor (VEGF) and plays a crucial role in mitigating the damage caused by tissue hypoxia. Zhang et al [30] showed that hypoxia can increase the testicular expression of HIF-1α in rats with varicocele, resulting in a significant increase in spermatogenic cell apoptosis. Following the onset of hypoxia, the degradation pathway of HIF-1α is obstructed, leading to the accumulation and nuclear translocation of HIF-1α.
It has also been demonstrated that active HIF-1 binds to various hypoxia-sensitive genes, including erythropoietin and VEGF, and promotes the transcription of these target genes, resulting in further cellular damage including dysregulation of endothelial cell proliferation, angiogenesis, and vascular permeability. A recent study showed that intratesticular injection of VEGF can enhance spermatogenesis and decrease apoptosis in rats with varicoceles [31]. Research has demonstrated that the application of the CRISPR/Cas9 gene editing technique to silence the HIF-1α gene in rat testes with varicoceles has an impact on the regulation of spermatogenesis in varicocele rats [32].
Our results indicate a significant reduction in basic sperm parameters (total count, total motility, and vitality) in experimental animals with varicocele as compared to those without varicocele. A reduction in sperm vitality and total motility has been attributed to the OS status caused by varicocele [33]. Furthermore, the current meta-analysis indicates a significant increase in sperm SDF in experimental animals with varicocele when compared to those without varicocele. Increased SDF levels observed in the experimental animals with varicoceles may also be related, at least in part, to varicocele-mediated OS. High levels of SDF have been correlated with decreased human fertility and increased risk of miscarriage [34].
Despite sufficient evidence in animal studies suggesting varicocele-associated detrimental impact on sperm function and thus fertility, the relationship between varicocele and infertility in humans has been a subject of ongoing debate. While many studies suggest an association between varicocele and impaired male fertility, there are some criticisms and challenges surrounding this relationship and arguments among clinicians on the benefits of varicocele repair [35].
Nonetheless, a recent meta-analysis conducted on 16 studies comprising of 2,420 infertile men with clinical varicocele showed significant improvement in postoperative semen parameters, including sperm concentration, total sperm count, progressive sperm motility, total sperm motility, and normal sperm morphology [36].
The results of the current study suggest that varicocele-induced testicular damage is mediated by OS. Therefore, the positive effects of varicocele repair on sperm parameters could be mediated through a reduction of testicular/seminal OS.
The Strengths, Weaknesses, Opportunities, and Threats (SWOT) analysis for the present SRMA is shown in Fig. 7. Despite the interesting findings of this study in relation to the impact of varicocele on sperm parameters and OS in animal studies, a few limitations are noted. First, we analyzed data of one biomarker of OS, namely MDA in experimental animals with and without varicocele. Future studies are warranted to investigate other OS parameters such as ROS and free radical scavenging capacity of individual antioxidants, such as superoxide dismutase, glutathione peroxidase, catalase and TAC. Second, we did not assess the relationship between the duration of varicocele induction and the deterioration of semen parameters or development of OS status. Although the results obtained from induced varicoceles in experimental animals cannot be directly applied to humans, understanding the speed at which the negative effects on spermatogenesis and sperm parameters occur in animals with varicocele could be important. Third, the included studies neither reported pregnancy rates nor live birth rates in animals with varicocele versus controls. This aspect could be crucial as varicocele not only impairs semen parameters and sperm DNA but may also lead to lower pregnancy and live birth rates. Despite the above-mentioned limitations, this SRMA provides convincing evidence of the adverse effects of varicocele on the fertility potential.
Fig. 7. Strengths, Weaknesses, Opportunities, and Threats (SWOT) analysis.
CONCLUSIONS
The results of this meta-analysis provide evidence that varicocele results in a significant increase in levels of testicular OS in animal models. Additionally, the results of this meta-analysis revealed that varicocele has a detrimental impact on total sperm count, sperm vitality, and total sperm motility. In addition, varicocele in experimental animals is associated with higher SDF levels. These findings support the potential role of OS in the pathogenesis of varicocele-induced testicular damage.
Acknowledgements
None.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: None.
- Conceptualization: GAF.
- Methodology: GAF, R Saleh.
- Data curation: GIR, FF, ARJ, DD, OK, ES, IS, RBV, FB.
- Supervision: R Saleh, R Shah, AA.
- Validation: all authors.
- Writing - Original draft preparation: GIR, FF, ARJ, DD, OK, ES, IS, RBV, FB.
- Writing - Reviewing and Editing: GIR, DD, OK, ES, FB, R Saleh, AMH, R Shah, AA.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.230260.
PECOS (Population, Exposure, Comparator, Outcomes, Study design) of this systematic review
Forest plot for MD of the MDA in animals with varicocele compared to controls. SD: standard deviation, MD: mean difference, MDA: malondialdehyde.
Forest plot for MD of the TAC in animals with varicocele compared to controls. SD: standard deviation, MD: mean difference, TAC: total antioxidant capacity.
Forest plot for MD of the sperm vitality in animals with varicocele compared to controls. SD: standard deviation, MD: mean difference.
Forest plot for MD of the total motility in animals with varicocele compared to controls. SD: standard deviation, MD: mean difference.
(A) Forest plot for MD of the SDF in animals with varicocele compared to controls. (B) Sensitivity analysis of the SDF in animals with varicocele compared to controls. SD: standard deviation, MD: mean difference, SDF: sperm DNA fragmentation.
References
- 1.Alsaikhan B, Alrabeeah K, Delouya G, Zini A. Epidemiology of varicocele. Asian J Androl. 2016;18:179–181. doi: 10.4103/1008-682X.172640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood GJA, Cardoso JPG, Paluello DV, Nunes TF, Cocuzza M. Varicocele-associated infertility and the role of oxidative stress on sperm DNA fragmentation. Front Reprod Health. 2021;3:695992. doi: 10.3389/frph.2021.695992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E. The chemistry of reactive oxygen species (ROS) Revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int J Mol Sci. 2021;22:4642. doi: 10.3390/ijms22094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008;1:15–24. doi: 10.4161/oxim.1.1.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finelli R, Leisegang K, Kandil H, Agarwal A. Oxidative stress: a comprehensive review of biochemical, molecular, and genetic aspects in the pathogenesis and management of varicocele. World J Mens Health. 2022;40:87–103. doi: 10.5534/wjmh.210153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal A, Hamada A, Esteves SC. Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol. 2012;9:678–690. doi: 10.1038/nrurol.2012.197. [DOI] [PubMed] [Google Scholar]
- 7.Sadeghi N, Erfani-Majd N, Tavalaee M, Tabandeh MR, Drevet JR, Nasr-Esfahani MH. Signs of ROS-associated autophagy in testis and sperm in a rat model of varicocele. Oxid Med Cell Longev. 2020;2020:5140383. doi: 10.1155/2020/5140383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karna KK, Choi BR, You JH, Shin YS, Cui WS, Lee SW, et al. The ameliorative effect of monotropein, astragalin, and spiraeoside on oxidative stress, endoplasmic reticulum stress, and mitochondrial signaling pathway in varicocelized rats. BMC Complement Altern Med. 2019;19:333. doi: 10.1186/s12906-019-2736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Sun Y, Wang L, Xu C, Yang Q, Liu B, et al. Hypoxia-induced apoptosis in the bilateral testes of rats with left-sided varicocele: a new way to think about the varicocele. J Androl. 2010;31:299–305. doi: 10.2164/jandrol.108.007153. [DOI] [PubMed] [Google Scholar]
- 10.Morgan RL, Whaley P, Thayer KA, Schünemann HJ. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. 2018;121(Pt 1):1027–1031. doi: 10.1016/j.envint.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 13.Murray J, Farrington DP, Eisner MP. Drawing conclusions about causes from systematic reviews of risk factors: the Cambridge quality checklists. J Exp Criminol. 2009;5:1–23. [Google Scholar]
- 14.Andrade C. Mean difference, standardized mean difference (SMD), and their use in meta-analysis: as simple as it gets. J Clin Psychiatry. 2020;81:20f13681. doi: 10.4088/JCP.20f13681. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moshtaghion SM, Malekinejad H, Razi M, Shafie-Irannejad V. Silymarin protects from varicocele-induced damages in testis and improves sperm quality: evidence for E2f1 involvement. Syst Biol Reprod Med. 2013;59:270–280. doi: 10.3109/19396368.2013.794253. [DOI] [PubMed] [Google Scholar]
- 17.Khosravanian N, Razi M, Farokhi F, Khosravanian H. Testosterone and vitamin E administration up-regulated varicocele-reduced Hsp70-2 protein expression and ameliorated biochemical alterations. J Assist Reprod Genet. 2014;31:341–354. doi: 10.1007/s10815-013-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taghizadeh L, Eidi A, Mortazavi P, Rohani AH. Effect of selenium on testicular damage induced by varicocele in adult male Wistar rats. J Trace Elem Med Biol. 2017;44:177–185. doi: 10.1016/j.jtemb.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Razi M, Sadrkhanloo RA, Malekinejad H, Sarafzadeh-Rezaei F. Varicocele time-dependently affects DNA integrity of sperm cells: evidence for lower in vitro fertilization rate in varicocele-positive rats. Int J Fertil Steril. 2011;5:174–185. [PMC free article] [PubMed] [Google Scholar]
- 20.Gur FM, Timurkaan S, Taskin E, Guven C, Gur HE, Senturk M, et al. Thymoquinone improves testicular damage and sperm quality in experimentally varicocele-induced adolescent rats. Andrologia. 2021;53:e14033. doi: 10.1111/and.14033. [DOI] [PubMed] [Google Scholar]
- 21.Tafuri S, Ciani F, Iorio EL, Esposito L, Cocchia N. In: New discoveries in embryology. Wu B, editor. IntechOpen; 2015. Reactive oxygen species (ROS) and male fertility. [Google Scholar]
- 22.Jang H, Kim SJ, Yuk SM, Han DS, Ha US, Hong SH, et al. Effects of anthocyanin extracted from black soybean seed coat on spermatogenesis in a rat varicocele-induced model. Reprod Fertil Dev. 2012;24:649–655. doi: 10.1071/RD11174. [DOI] [PubMed] [Google Scholar]
- 23.Sadraei MR, Tavalaee M, Forouzanfar M, Nasr-Esfahani MH. Effect of curcumin, and nano-curcumin on sperm function in varicocele rat model. Andrologia. 2022;54:e14282. doi: 10.1111/and.14282. [DOI] [PubMed] [Google Scholar]
- 24.Chan CC, Sun GH, Shui HA, Wu GJ. Differential spermatozoal protein expression profiles in men with varicocele compared to control subjects: upregulation of heat shock proteins 70 and 90 in varicocele. Urology. 2013;81:1379.e1–1379.e8. doi: 10.1016/j.urology.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 25.Bellastella G, Carotenuto R, Caiazzo F, Longo M, Cirillo P, Scappaticcio L, et al. Varicocele: an endocrinological perspective. Front Reprod Health. 2022;4:863695. doi: 10.3389/frph.2022.863695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afiyani AA, Deemeh MR, Tavalaee M, Razi M, Bahadorani M, Shokrollahi B, et al. Evaluation of heat-shock protein A2 (HSPA2) in male rats before and after varicocele induction. Mol Reprod Dev. 2014;81:766–776. doi: 10.1002/mrd.22345. [DOI] [PubMed] [Google Scholar]
- 28.Mikhael NW, El-Refaie AM, Sabry JH, Akl EM, Habashy AY, Mostafa T. Assessment of seminal granulysin in infertile men with varicocele. Andrologia. 2018;50:e13066. doi: 10.1111/and.13066. [DOI] [PubMed] [Google Scholar]
- 29.Gat Y, Gornish M, Chakraborty J, Perlow A, Levinger U, Pasqualotto F. Azoospermia and maturation arrest: malfunction of valves in erect poster of humans leads to hypoxia in sperm production site. Andrologia. 2010;42:389–394. doi: 10.1111/j.1439-0272.2010.01083.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang K, Wang Z, Wang H, Fu Q, Zhang H, Cao Q. Hypoxia-induced apoptosis and mechanism of epididymal dysfunction in rats with left-side varicocele. Andrologia. 2016;48:318–324. doi: 10.1111/and.12449. [DOI] [PubMed] [Google Scholar]
- 31.Tek M, Cayan S, Yilmaz N, Oğuz I, Erdem E, Akbay E. The effect of vascular endothelial growth factor on spermatogenesis and apoptosis in experimentally varicocele-induced adolescent rats. Fertil Steril. 2009;91(5 Suppl):2247–2252. doi: 10.1016/j.fertnstert.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Wang HQ, Wang T, Gao F, Ren WZ. Application of CRISPR/Cas technology in spermatogenesis research and male infertility treatment. Genes (Basel) 2022;13:1000. doi: 10.3390/genes13061000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang K, Gao Y, Wang C, Liang M, Liao Y, Hu K. Role of oxidative stress in varicocele. Front Genet. 2022;13:850114. doi: 10.3389/fgene.2022.850114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal A, Farkouh A, Parekh N, Zini A, Arafa M, Kandil H, et al. Sperm DNA fragmentation: a critical assessment of clinical practice guidelines. World J Mens Health. 2022;40:30–37. doi: 10.5534/wjmh.210056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah R, Agarwal A, Kavoussi P, Rambhatla A, Saleh R, Cannarella R, et al. Global Andrology Forum. Consensus and diversity in the management of varicocele for male infertility: results of a global practice survey and comparison with guidelines and recommendations. World J Mens Health. 2023;41:164–197. doi: 10.5534/wjmh.220048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal A, Cannarella R, Saleh R, Boitrelle F, Gül M, Toprak T, et al. Impact of varicocele repair on semen parameters in infertile men: a systematic review and meta-analysis. World J Mens Health. 2023;41:289–310. doi: 10.5534/wjmh.220142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PECOS (Population, Exposure, Comparator, Outcomes, Study design) of this systematic review
Forest plot for MD of the MDA in animals with varicocele compared to controls. SD: standard deviation, MD: mean difference, MDA: malondialdehyde.
Forest plot for MD of the TAC in animals with varicocele compared to controls. SD: standard deviation, MD: mean difference, TAC: total antioxidant capacity.
Forest plot for MD of the sperm vitality in animals with varicocele compared to controls. SD: standard deviation, MD: mean difference.
Forest plot for MD of the total motility in animals with varicocele compared to controls. SD: standard deviation, MD: mean difference.
(A) Forest plot for MD of the SDF in animals with varicocele compared to controls. (B) Sensitivity analysis of the SDF in animals with varicocele compared to controls. SD: standard deviation, MD: mean difference, SDF: sperm DNA fragmentation.