Abstract
Eating behaviors in response to acute stressors are highly variable: whereas many individuals eat more following stressors, others eat less or show no change in food consumption. Understanding factors that predict individual differences in eating behaviors may help elucidate the psychosocial mechanisms underlying obesity, yet few experimental studies on this topic have been conducted to date. To address this issue, we conducted the present pre-registered study, where we investigated how lifetime stressor exposure moderates the extent to which eating expectancies enhance the learned association between stress-induced negative affect and snack intake. Participants were 44 women (30% non-White) between 18 and 50 years old (M = 27.9), with a mean body mass index of 25.6, who completed assessments of lifetime stressor exposure, eating behaviors, and eating expectancies (eating helps manage negative affect); in a subsequent visit, they were given snacks after an acute social stress task (TSST). The moderated moderation model (PROCESS model 3) yielded a significant three-way interaction. When eating expectancies were high, acute social stress-induced negative affect predicted greater M&M intake for women with very high total lifetime stressor exposure but less M&M intake for women with fewer lifetime stressors. These data thus highlight how lifetime stressor exposure interacts with eating expectancies and acute stress-induced negative affect to predict eating behavior. Replications in larger samples may help explain variability in stress-eating as well as how lifetime stressors contribute to obesity.
Keywords: Stress, Chronic stress, Eating, Food, Mood, Reward, Eating expectancies
1. Introduction
Acute life stressors are common and often prompt changes in eating behaviors (Adam & Epel, 2007; Chao et al., 2017; Epel et al., 2012; Sinha, 2018). Preferences tend to shift toward highly palatable foods following stressor exposure (Chao et al., 2020; Tryon et al., 2013; Zellner et al., 2006), yet stress-related eating behaviors are highly variable. Whereas many individuals increase their food intake under stress, others decrease intake or show no change (Adam & Epel, 2007; Epel et al., 2012; Hill et al., 2021). Understanding the causes of this variability in stress-eating is becoming increasingly important given the high rates of stress and obesity in the United States, and the association between stress and a wide variety of obesity-related health issues (Chao et al., 2017; Tomiyama, 2019).
Individual difference models propose that heterogeneity in vulnerability factors such as negative affect contribute to variability in stress-eating (Habhab et al., 2009). However, these data are not consistent with respect to how negative affect impacts food intake following stressors. Increases in negative affect are associated with greater palatable food intake under stress (Fay & Finlayson, 2011; Fong et al., 2019) as well as decreased intake or are unrelated to stress-eating (Evers et al., 2018; Macht, 2008). These inconsistencies in the literature suggest that the association between negative affect and stress-eating may be moderated by other vulnerability factors, such as life stressor exposure. Acute social stress-induced negative affect is a stronger predictor of snacking for women with higher perceived life stress (Klatzkin et al., 2019), and Kazmierski et al. (2022) found that negative affect was associated with more obesogenic eating for those with high, but not low, adversity exposure. Individual differences in life stressor exposure may impact the strength of stress-induced negative affect as a trigger for eating; yet, no study to date has investigated the mechanisms underlying this moderation. In addition, we know of no studies that have investigated how stressors occurring over the entire life course are related to eating behavior, even though cumulative lifetime stressor exposure has been found to predict a variety of behavioral and clinical outcomes (Slavich et al., 2019; Sturmbauer et al., 2019).
1.1. Reinforcement learning
One possible mechanism by which life stress strengthens the hyperphagic effects of acute social stress-induced negative affect may be via heightened reinforcement learning. Comfort eating increases pleasure and decreases anxiety by dampening hypothalamic pituitary adrenal-axis reactivity and increasing dopamine release in brain reward pathways (Epel et al., 2012; Finch & Tomiyama, 2014). Both laboratory and naturalistic studies report short-term reductions in negative affect following consumption of highly palatable foods (i.e., negative reinforcement; Finch & Tomiyama, 2014; Macht & Mueller, 2007; Wouters et al., 2018). Furthermore, affect regulation theory proposes that heightened negative affect triggers binge eating to regulate emotions, and when negative affect is reduced by binge eating, this leads to the reinforcement of binge eating behavior (Hawkins & Clement, 1984). However, results from naturalistic studies have been mixed and indicate that loss-of-control-eating may not be reinforced by a reduction in negative affect (Haedt-Matt & Keel, 2011; Mikhail, 2021). This inconsistency suggests that individual difference factors may be moderating the relation between post-ingestive reductions in negative affect and reinforcement learning in this context.
Reinforcement learning may be enhanced for women with greater life stressor exposure (Dallman et al., 2003; Epel et al., 2012; Tomiyama et al., 2011). Higher perceived life stress over the past three months has been associated with greater decreases in negative affect following post-stress snacking (Klatzkin et al., 2019). Additionally, chronic stress increases basal levels of dopamine receptors in the nucleus accumbens and this reward system dysregulation may prime the brain for negative reinforcement learning (Wei et al., 2019). Furthermore, chronic cortisol elevation in individuals with greater chronic stress increases the rewarding value of pleasurable activities and may increase the likelihood of negative reinforcement from stress-eating to cause more eating under stress as a form of self-medication (Adam & Epel, 2007; Dallman et al., 2003). This may explain why chronic stress is associated with increased vulnerability to addiction, increased escalation of drug self-administration, and changes in dopaminergic responses to acute stress (Sinha, 2018).
1.2. Emotional eating cycle
According to the emotional eating cycle (Klatzkin et al., 2021, pp. 871–906), greater reinforcement learning in women with greater lifetime stressor exposure would strengthen the learned association between negative emotions (Box A) and food intake (Box B) via negative reinforcement from decreased negative affect (Box C) and ultimately enhance the emotional eating cycle in a feed-forward manner to promote obesity (Box D). The present study tested the emotional eating cycle (Fig. 1) by examining if greater reinforcement learning strengthens negative affect (Box A) as a trigger for stress-eating (Box B) for women with greater stressor exposure across the life course.
Fig. 1.
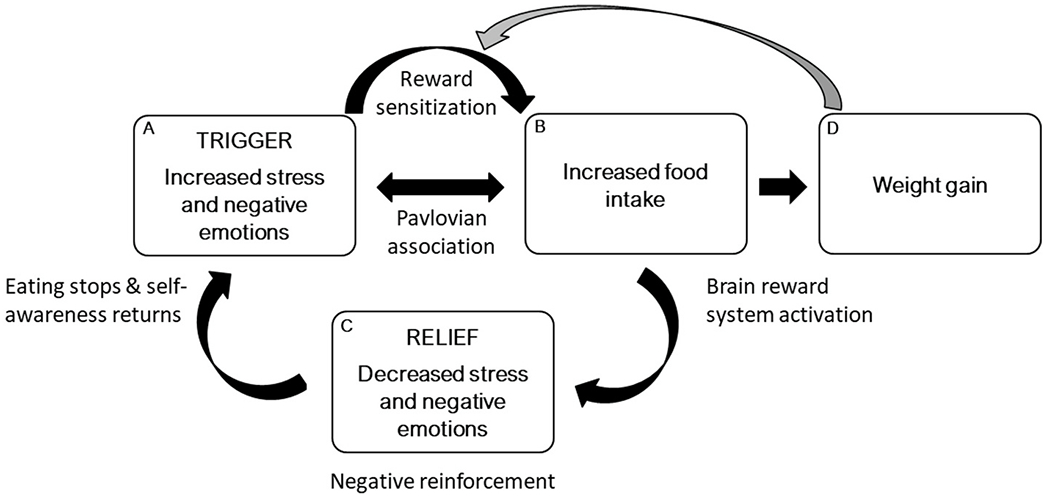
An emotion regulation model is presented in which emotional eating is part of a feed-forward cycle. Greater stress and negative emotions (i.e., trigger; box A) sensitize the brain reward system (pathway) and lead to more food intake (box B) and weight gain (box D). Greater food intake (box B) causes further activation of the brain reward system and leads to less stress and negative emotions (i.e., relief; box C). However, this short-term emotional relief (i.e., negative reinforcement) is not sustained, as stress and negative emotions (box A) return upon the cessation of eating. Over time, greater exposure to stressors and negative emotions (box A) is more likely to trigger food intake because of positive feedback from factors such as conditioning, brain reward processes, enhanced emotion regulation motives, and weight gain. The gray arrow indicates that weight gain (box D) enhances reward sensitization, which creates a positive feedback loop. Reproduced from Klatzkin et al. (2021, pp. 871–906).
1.3. Eating expectancies
Greater reinforcement learning in the context of stress-eating is likely to increase eating expectancies (eating helps manage negative affect) for individuals with greater lifetime stressor exposure. Expectancy theory proposes that individuals make decisions based on previously learned associations between behaviors and outcome (Behan, 1953). Therefore, increased eating expectancies can result from enhanced negative reinforcement learning (Smith et al., 2018) and are predictive of eating behaviors such as binge eating (Fischer et al., 2018) and the development and maintenance of bulimic symptoms (Bohon et al., 2009; Hayaki, 2009). Therefore, we use self-reported eating expectancies to assess reinforcement learning in the present study.
We propose a model in which greater lifetime stressor exposure strengthens the extent to which eating expectancies moderate the relationship between acute social stress-induced negative affect and snack intake (Fig. 2). Specifically, our pre-registered confirmatory hypothesis (https://osf.io/kyrv4) was that higher eating expectancies would enhance the salience of acute social stress-induced negative affect as a predictor of snack intake, and that this moderation effect would be more pronounced for women who have experienced more lifetime stressors.
Fig. 2.
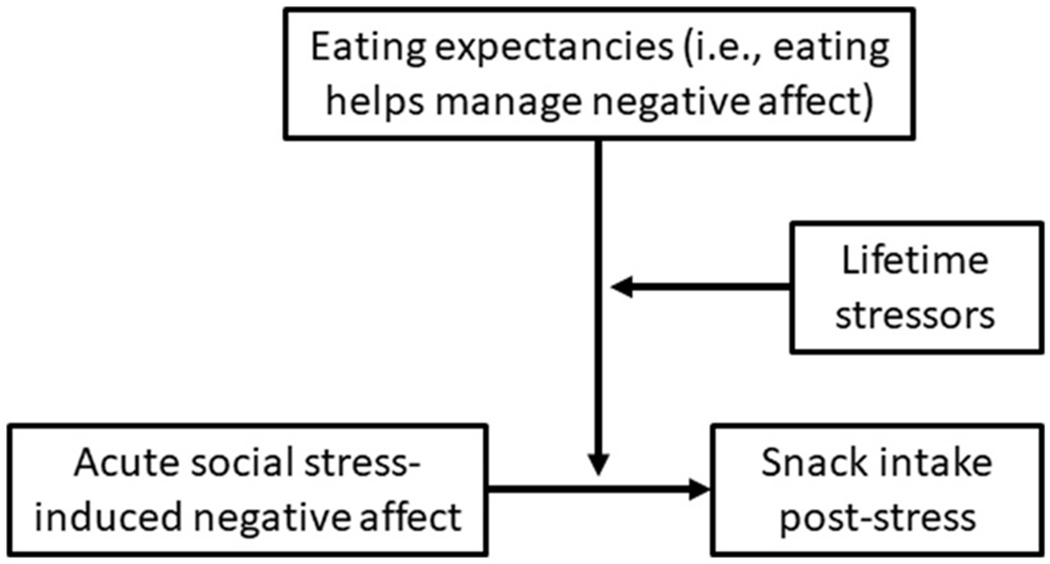
PROCESS theoretical model 3: moderated moderation.
1.4. Open practices statement
The data for this study are publicly accessible at https://osf.io/ajyhv/files/osfstorage. The study preregistration can be found at https://osf.io/kyrv4. We use the terminology ‘confirmatory’ and ‘exploratory’ in line with usage by the Center for Open Science (see https://www.cos.io/initiatives/prereg).The materials used in this study are widely available; however, requests for any materials can be sent to the corresponding author.
2. Method
2.1. Participants
Participants were 44 women (median household income = $75,000) between 18 and 50 years old (M = 27.9, SD = 7.3), with a mean body mass index of 25.6 (SD = 5.8), who responded to advertisements for a study investigating the effects of stress physiology on taste experiences. The majority of participants identified as non-Hispanic white (70%) and the remaining 30% identified as Black, African, or African American (11%), Native American (2%), Asian (13%), and Hispanic/Latinx (4%). We recruited women in Memphis, Tennessee via a partnership with a local community center as well as from Introduction to Psychological Science courses at Rhodes College. Women tend to eat greater amounts of food in response to stress and show a greater association between stress and obesity than men (Konttinen et al., 2010; Udo et al., 2014). Therefore, only women were recruited and included in this study.
Participants were excluded if they self-reported current or prior cardiovascular disease, diabetes, or blood pressure above 160/95 mmHg; were currently taking blood pressure, stimulant, or psychoactive medications; were in current treatment for eating or weight problems; were regular smokers; or were pregnant, lactating, or menopausal. The research was approved by the Institutional Review Board at Rhodes College. Participants provided written informed consent and were either paid for their time (Memphis-area women) or earned course credit (undergraduate women). The hypotheses were pre-registered with the open science framework after data collection had commenced but prior to data analysis https://osf.io/kyrv4.
2.2. Procedure
Women responding to the advertisements completed preliminary screening questions aimed at assessing the exclusionary criteria described above. They also answered questions assessing perceived life stress, lifetime stressor exposure, depressive symptoms, uncontrolled eating, emotional eating, cognitive restraint, trait impulsiveness, eating concerns, eating habits, and eating expectancies (Bekhbat & Neigh, 2017, de Wit et al., 2010; Meule, 2013; Yau & Potenza, 2013). A total of 62 women completed the preliminary screening.
Each laboratory testing session began between the hours of 3:00 p.m. and 5:30 p.m. (see Fig. 3). The order of rest and stress laboratory sessions was counterbalanced between participants. The rest and stress days were the same with the exception that on the rest day, stress testing was replaced with a rest period of the same length during which participants listened to classical music and had the option to read popular science magazines. On the day of the study, participants did not wake from sleep less than 2 h prior to the testing session, take any antihistamines, psychotropic medications, or neural stimulants, exercise strenuously (i.e., cardiovascular exercise for more than a few minutes), drink more than a single caffeinated beverage, eat or drink (except water) 2 h prior to the study, or consume any alcohol 12 h prior to the study. Participants were also asked to arrive “not too hungry, but not too full” and to “make sure to eat some food at 2 h before the study visit to avoid excess hunger.” Research assistants confirmed compliance with study requirements upon arrival to the laboratory; else, participants were rescheduled.
Fig. 3.
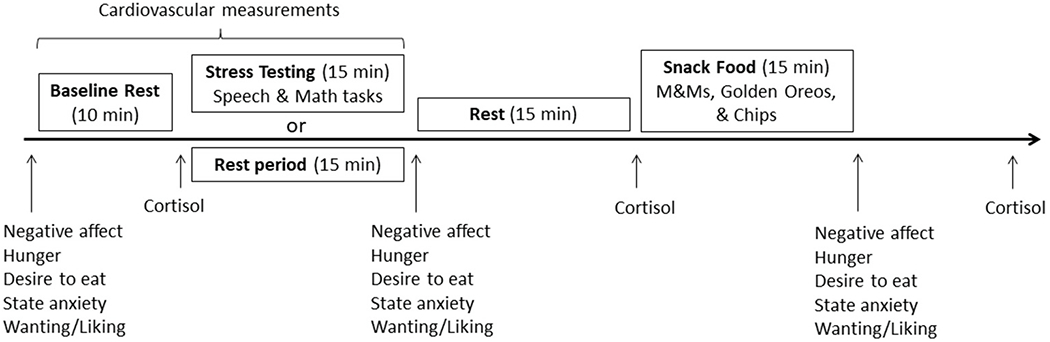
Laboratory protocol for stress and rest days.
From September 2019 to March 2020, 27 participants completed rest day testing and 32 completed stress day testing before data collection was paused due to COVID-19. Given the strict eligibility criteria, COVID-19 safety concerns that delayed resuming testing until January 2022, and the timeline for study completion (undergraduate research assistants depart campus in May 2022), only 12 additional participants underwent the full laboratory stress testing protocol following the COVID-related interruption to data collection. Therefore, a total of 44 women who completed the stress testing session comprise the present report. Fourteen Memphis-area women who successfully completed the preliminary screening did not complete the stress day visit. Seven of these fourteen Memphis-area women did not complete the scheduled stress testing visit due to the outbreak of COVID-19 in March 2020. Of these community participants, one completed the rest day visit prior to the cessation of data collection. Only three college students who successfully completed the preliminary screening did not complete subsequent stress testing.
The later sample of college students (n = 12) did not differ from the earlier sample of community members (n = 32) in eating expectancies, F (1, 42) = 0.14, p = 0.71, M&M intake, F(1, 42) = 0.43, p = 0.52, acute social stress-induced anxiety ratings, F(1, 42) = 1.4, p = 0.24, acute social stress-induced SBP, F(1, 42) = 0.49, p = 0.48, or acute social stress-induced negative affect ratings, F(1, 42) = 0.94, p = 0.34. Controlling for age, life stressor count did not significantly differ between college students and community members, F(1, 41) = 18.06, p = 0.71.
For context, our full sample of 44 women reported comparable total life stressors count (M = 18, SD = 11.8) than a recent sample of 28 community women between 18 and 29 years old (M = 22.9, SD = 17.5) (Slavich et al., 2019) and greater expectations that eating helps manage negative affect (M = 64.4, SD = 23.0) than two separate samples of undergraduate women (Sample 1: n = 121, M = 51.4, SD = 21.3; Sample 2: n = 249, M = 51.20, SD = 22.29; Brosof et al., 2019; Hayaki, 2009).
2.3. Psychological measures—preliminary screening
2.3.1. Lifetime stressor exposure
The Stress and Adversity Inventory (Slavich & Shields, 2018) was used to assess participants’ exposure to acute and chronic stressors occurring over the entire life course (see http://www.strainsetup.com). The STRAIN is a National Institutes of Mental Health-recommended instrument that assesses a person’s cumulative exposure to 55 different major life events (e.g., deaths of relatives, job losses, negative health events, etc.) and chronic difficulties (e.g., ongoing health problems, work problems, relationship problems, financial problems, etc.). Included in this list are 26 pre-defined acute life events and 29 pre-defined chronic difficulties that are known to impact health (e.g., have you ever experienced exclusion or unfair treatment at a job - for example, because of your gender, sexual orientation, race, or ethnicity?). The STRAIN has excellent test-rest reliability, construct validity, discriminate validity, and has been shown to predict a variety of biological, clinical, and behavioral outcomes including impulsivity, coping and risky behaviors (e.g., Cazassa et al., 2020; Lam et al., 2019; McMullin et al., 2021; Murphy et al., 2022; Olvera Alvarez et al., 2019; Slavich & Shields, 2018). In the present study, we first used the STRAIN’s severity of chronic difficulties scores to test our pre-registered hypothesis and then used the total count of lifetime stressors (including both acute and chronic lifetime stressors) to test our pre-registered exploratory hypothesis. Higher scores indicate greater severity and number of stressors experienced.
2.3.2. Subjective eating measures
The Three Factor Eating Questionnaire (TFEQ-R18; Karlsson et al., 2000) is a revised and shortened version of the original 51-item TFEQ (Stunkard & Messick, 1985). The TFEQ-R18 has three subscales: uncontrolled eating (the tendency to overeat, with the feeling of being out of control; range 3–12), emotional eating (the tendency to eat in response to negative emotions; range 9–36), and restrained eating (tendency to restrict eating to control weight; range 6–24). Greater scores indicate greater uncontrolled, emotional, or restrained eating. Cronbach’s alpha for the 9 items on the uncontrolled eating subscale (e. g., Sometimes when I start eating, I just can’t seem to stop; α = 0.85), the 3 items on the emotional eating subscale (e.g., When I feel anxious, I find myself eating; α = 0.86), and the 6 items on the restrained eating subscale (e.g., I deliberately take small helpings as a means of controlling my weight; α = 0.77) of the TFEQ were satisfactory.
2.3.3. Depressive symptoms
Depressive symptoms were assessed using the Beck Depression Inventory (BDI; Beck & Beamesderfer, 1974). The BDI assesses self-reported cognitive, affective, overt behavioral, somatic, and interpersonal symptoms of depression. Each of the 21 forced-choice items (e.g., sadness, self-dislike, guilty feelings) has at least four answer choices which increase in severity from 0 to 3 (e.g., “I do not feel sad” to “I am so sad or unhappy that I can’t stand it”). Cronbach’s alpha for the BDI was very good (α = 0.90).
2.3.4. Eating expectancies
The Eating Expectancy Inventory (Hohlstein et al., 1998) is a validated self-report inventory measuring participants’ beliefs and attitudes about food. Participants completed two subscales measuring whether they believe that eating: (1) helps manage negative affect (range 18–126); and (2) is pleasurable and useful as a reward (range 6–42). Greater scores indicate greater endorsement of each attitude. Cronbach’s alpha for 18 items on the negative affect subscale (e.g., When I am feeling anxious or tense, eating helps me relax; α = 0.95) and the 6 items on the reward subscale (e.g., When I do something good, eating is a way to reward myself; α = 0.84) were satisfactory. The present study used only the negative affect subscale in analyses because it directly relates to our hypothesis regarding reductions in acute social stress-induced negative affect following eating. We did not include subscales 3, 4, or 5 (eating leads to feeling out of control, eating enhances cognitive competence, and eating alleviates boredom) because they do not serve to test our pre-registered hypothesis specifically focused on expectancies related to negative affect.
2.3.5. Trait impulsiveness
The Barratt Impulsiveness Scale (BIS-11; Patton et al., 1995) assessed attentional (range 8–32), motor (range 11–14), and non-planning impulsiveness (range 11–14), with greater scores indicating greater impulsiveness. Cronbach’s alpha for 8 items on the attentional subscale (e.g., I don’t pay attention; α = 0.75) and the 11 items on the non-planning impulsiveness subscale (e.g., I do things without thinking; α = 0.74) were satisfactory. However, Cronbach’s alpha for the 11 items on the motor subscale (e.g., I squirm at plays or lectures, α = 0.52) was not acceptable. We used the total of all three subscales to control for impulsivity in our exploratory analyses, but given the low Cronbach’s alpha for the motor subscale, we reran our analysis controlling for the total score of only the non-planning and attentional subscales. The moderated moderation model was still significant, F(16,27) = 2.10, p = 0.043; R2 = 0.55, as was the conditional three-way interaction effect on M&M intake (b = 0.032g, SE = 0.013, p = 0.021; 95% CI: [0.005–0.069]) and the increase in R2 attributable to the three-way interaction (0.10, F(1,27) = 6.02, p = 0.021). Thus, the results of the exploratory analysis reported below include the total score of all three subscales as a covariate.
2.4. Laboratory protocol
2.4.1. Baseline rest
Researchers placed an automated blood pressure cuff on the nondominant arm of the participant. Participants then completed questionnaires that assessed state anxiety, positive and negative affect, hunger, and desire to eat, as well as how much they liked the snack foods and wanted to eat the snack foods. We then assessed cardiovascular measures of systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR).
2.4.2. Trier Social Stress Test (TSST)
The researcher informed the participants that they would be undergoing a mental stress test (i.e., the TSST) that includes giving a speech and performing serial subtraction while being audio- and visually recorded. The TSST reliably induces large and consistent cardiovascular responses (Kirschbaum et al., 1993). The researcher then asked participants to take 5 min to prepare their speech that should describe why they would be the best candidate for their ideal job. Immediately following the preparation period, the selection committee returned to the testing room and asked the participants to deliver their speech for 5 min. Finally, the researcher asked the participants to perform mental math for 5 min by serially subtracting 7 from 2000 aloud as quickly and accurately as possible. Cardiovascular and cortisol reactivity were assessed throughout the TSST. For more detail regarding the TSST procedure, see Klatzkin et al. (2019).
Following the TSST, participants were told that the recordings of their performance would be analyzed while they completed questionnaires assessing state anxiety, positive and negative affect, hunger, and desire to eat, as well as how much they liked the snack foods and wanted to eat the snack foods. Following questionnaire completion, the researcher returned to inform the participant that “there has been a problem with the recording, and it may be necessary to redo the task”. This information was given to prolong the stressor until 15 min after the end of the TSST when cortisol levels peak post-stress. Following saliva collection, the researcher informed the participant that the problem with the recording had been fixed and that they would not be required to redo the stress tasks.
2.4.3. Snack food
Participants were given three clear bowls filled with either M&Ms (250g, 9 servings, 1250 calories), mini golden Oreos (150g, 5.2 servings, 724 calories), or potato chips (100g, 3.6 servings, 570 calories). The researcher told the participant the following, “We are interested in how stress affects the perceived taste and texture of snack foods. When we return, we will ask you to rate each of these foods across various tastes and textures. Please sample each snack so that you will be able to provide these ratings. Feel free to eat as much as you would like, and to ask for more if you want it. We’ll be back in 15 min with more questionnaires and to collect your ratings.” Participants were then left alone for 15 min to consume the snacks while free to move about the private testing room. Researchers weighed each bowl before and after food consumption to determine food intake.
2.4.4. Post-snack
Following the snack period, participants again completed assessments measuring state anxiety, positive and negative affect, hunger, and desire to eat. Participants also rated the degree to which they found each snack food to be salty, sweet, crunchy, and enjoyable. Finally, a researcher assessed height (cm) and weight (kg) to calculate BMI (kg/m2) using a Seca 769 digital column scale and stadiometer and waist circumference with an anthropometric tape measure. We chose to measure weight at the conclusion of all study visits to ensure that the priming knowledge of one’s weight would not influence eating behaviors.
2.5. Physiological measures
The Oscar 2 oscillometric ambulatory blood pressure monitor (SunTech Medical Instruments, Inc., Raleigh, NC) provided automated measurement of systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate (HR) while participants were in a comfortable seated position. Blood pressure and HR measures were taken at minutes 0, 5, and 10 of baseline and minutes 0, 2, and 4 of both the speech and serial subtraction periods. The cardiovascular data recorded at minute 10 of baseline constituted the baseline values of SBP, DBP, and HR. The peak value of SBP, DBP, and HR for each participant during each stress task constituted the speech and math stress values.
Saliva was collected in 1.5 mL Eppendorf tubes at the end of the baseline rest period, and 15 and 45 min following the end of the TSST or rest period (Fig. 3). Participants passively drooled into the tube for a maximum of 2 min per sample. Saliva samples were frozen within 30 min of collection at −20 °C until assayed. The mean intra-assay coefficient of variation was 9.14% and the inter-assay coefficient was 4.83%.
2.6. Subjective psychological measures—baseline, post-stress/rest, and post-snack
2.6.1. Positive and negative affect
Affect was quantified with the Positive and Negative Affect Schedule (PANAS), a 20-item multiple-choice survey validated in a university population (Watson et al., 1988). Participants choose from 1 (Very Slightly or Not At All) to 5 (Extremely) for each word describing a different feeling or emotion felt at the present moment (e.g. distressed, hostile, nervous). The positive subscale consisted of 10 words and a possible range from 10 to 50, with higher scores indicating more positive affect. The negative subscale consisted of 10 words and a possible range from 10 to 50, with higher scores indicating more negative affect. Cronbach’s alpha for the 10 items on the positive affect subscale (α = 0.90) and the 10 items on the negative affect subscale (α = 0.75) of the PANAS were very high and satisfactory, respectively. To measure the independent variable in our model, acute social stress-induced negative affect, we used the difference between negative affect ratings at baseline and stress to test our pre-registered hypothesis and negative affect ratings post-stress to test our exploratory hypothesis.
2.6.2. Drive to eat
Current hunger and desire to eat were measured on separate Likert scales from 0 (None) to 10 (Most imaginable) in response to the prompt, “Please rate your hunger on the scale below.”
2.6.3. State anxiety
The State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983) is a 20-item self-report questionnaire assessing current anxiety (e.g., I feel nervous and restless). The STAI-State ranges from 20 to 80, with higher scores indicating greater anxiety. Cronbach’s alpha for STAI was very good, α = 0.89.
2.6.4. Wanting of snack foods
Visual analogue scales were used for participants to rate how much they currently wanted to eat chips, M&Ms, and golden Oreos on separate sliding scales with non-numerical anchors not at all and most imaginable. The scales were accompanied by the following text: “If you were offered the following foods right now, how much would you want to eat them? Please answer in terms of how you feel right now, at this moment.”
2.6.5. Liking of snack foods
Visual analogue scales were used for participants to rate how much they currently liked chips, M&Ms, and golden Oreos on separate sliding scales with non-numerical anchors not at all and most imaginable. The scales were accompanied by the following text: “How much do you like the following foods, not considering if you want to eat them right now?”
3. Data analysis
In accordance with recommendations from the Center for Open Science (https://www.cos.io), we performed our analyses in two phases. The first phase consisted of confirmatory analyses that directly tested our pre-registered hypotheses. In the second phase of data analysis, we tested selected pre-registered exploratory analyses that were informed by the results of our confirmatory analysis.
All data were analyzed using IBM SPSS (version 23), and each model was tested with moderated moderation analyses using PROCESS model 3 (version 3.5.3; Hayes, 2018). Significant interactions were probed by use of the Johnson-Neyman test, which enabled us to determine where in the distribution of lifetime stressors the interaction of acute social stress-induced negative affect and eating expectancies was statistically significant.
3.1. Confirmatory analysis
PROCESS model 3 was used to examine whether the moderation of the association between acute social stress-induced negative affect (change from baseline to stress) and total food intake by eating expectancies was itself moderated by chronic stress. As such, we tested a three-way interaction effect of acute social stress-induced negative affect, eating expectancies, and chronic lifetime stressor severity on the total amount of food consumed. The following variables were included as covariates: TFEQ-R18 total score, age, changes in cortisol and state anxiety from baseline to stress, baseline SBP, and change in negative affect ratings from stress to post-snacking.
3.2. Exploratory analysis
Our exploratory analyses tested the same model as our confirmatory analyses yet defined the variables in different ways. As stated in our preregistration, we wanted to investigate if the moderator variables have distinct effects on different snack foods. Therefore, our exploratory analysis predicted M&M intake only rather than total snack food intake. Our pre-registration also stated that we would explore different cumulative lifetime stressor exposure summary scores from the STRAIN. Therefore, in contrast to our confirmatory analysis that used chronic lifetime stressor severity as a moderator, our exploratory analysis used total lifetime stressors count. We also proposed in our pre-registration that acute social stress-induced negative affect may be more appropriately measured using negative affect ratings post-stress, controlling for baseline ratings. Therefore, negative affect ratings post-stress was the dependent variable predicting M&M intake in the exploratory analysis. We specifically used M&M intake in the exploratory analysis due to data suggesting that sweet foods are preferred over salty foods under stress (Habhab et al., 2009; Zellner et al., 2006) and that eating chocolate following negative mood induction led to greater decreases in negative mood as compared to eating unpalatable chocolate or eating nothing (Macht & Mueller, 2007). Our moderator of eating expectancies remained the same from the confirmatory to the exploratory analyses.
Our exploratory analysis used PROCESS model 3 to examine whether the moderation of the association between acute social stress-induced negative affect and M&M intake by eating expectancies was itself moderated by total lifetime stressors; that is, the three-way interaction effect of acute social stress-induced negative affect, eating expectancies, and total lifetime stressors on M&M intake (see Fig. 2).
We included the following variables as covariates in this analysis: restrained eating sub-score from the TFEQ-R18, age, trait impulsiveness, baseline negative affect and hunger ratings, changes in SBP and state anxiety ratings from baseline to stress, and changes in state anxiety and negative affect ratings from stress to post-snacking. We used restrained eating scores on the TFEQ as covariates in our model because of their positive correlation with over-eating behaviors such as emotional eating (Vainik et al., 2015). Because the STRAIN assesses stressors over the entire life course, we included age as a covariate in the model. High impulsiveness is associated with various measures of overeating (for a review, see Meule, 2013); therefore, we controlled for trait impulsiveness as measured by the Barratt Impulsiveness Scale. Given that our model tested the influence of acute social stress-induced negative affect on eating, we controlled for negative affect and hunger ratings at baseline as well as the change in SBP and state anxiety from baseline to stress. Finally, we included the changes in state anxiety and negative affect ratings from stress to post-snacking as covariates because the degree of emotional relief from stress by eating is associated with negative reinforcement learning and increased eating expectancies (Behan, 1953; Smith et al., 2018).
4. Results
4.1. Manipulation check
The social stress task induced significant increases from baseline rest in subjective ratings of hunger, F(1,43) = 5.84, p = 0.020, state anxiety, F(1,43) = 54.1, p < 0.001, and negative affect, F(1,43) = 39.6, p < 0.001. In addition, as expected, the social stress task also induced significant increases in cortisol, F(1,43) = 7.94, p = 0.007, SBP, F(1,43) = 237.0, p < 0.001, DBP, F(1,43) = 413.7, p < 0.001, and HR, F(1,43) = 155.9, p < 0.001.
4.2. Confirmatory analysis
Our confirmatory analysis did not support our theoretical model (Fig. 2). Contrary to our pre-registered hypothesis, the confirmatory analysis yielded non-significant results for the moderated moderation model, F(13,29) = 0.92, p = 0.54; R2 = 0.29, the conditional three-way interaction effect on total food intake, (b = −0.024g, SE = 0.026, p = 0.36; 95% CI: [−0.047 - 0.029]), and the increase in R2 attributable to the three-way interaction (R2 = 0.021), F(1,29) = 0.85, p = 0.36.
4.3. Exploratory analysis
Results from our pre-registered exploratory analysis supported our theoretical model (Fig. 2); greater total lifetime stressor exposure strengthened the extent to which eating expectancies moderated the association between acute social stress-induced negative affect and M&M intake (Fig. 4). The moderated moderation model was significant, F(16,27) = 2.11, p = 0.042; R2 = 0.75, as was the conditional three-way interaction effect on M&M intake (b = 0.034g, SE = 0.013, p = 0.016; 95% CI: [0.007–0.061]) and the increase in R2 attributable to the three-way interaction (0.11, F(1,27) = 6.56, p = 0.016).
Fig. 4.
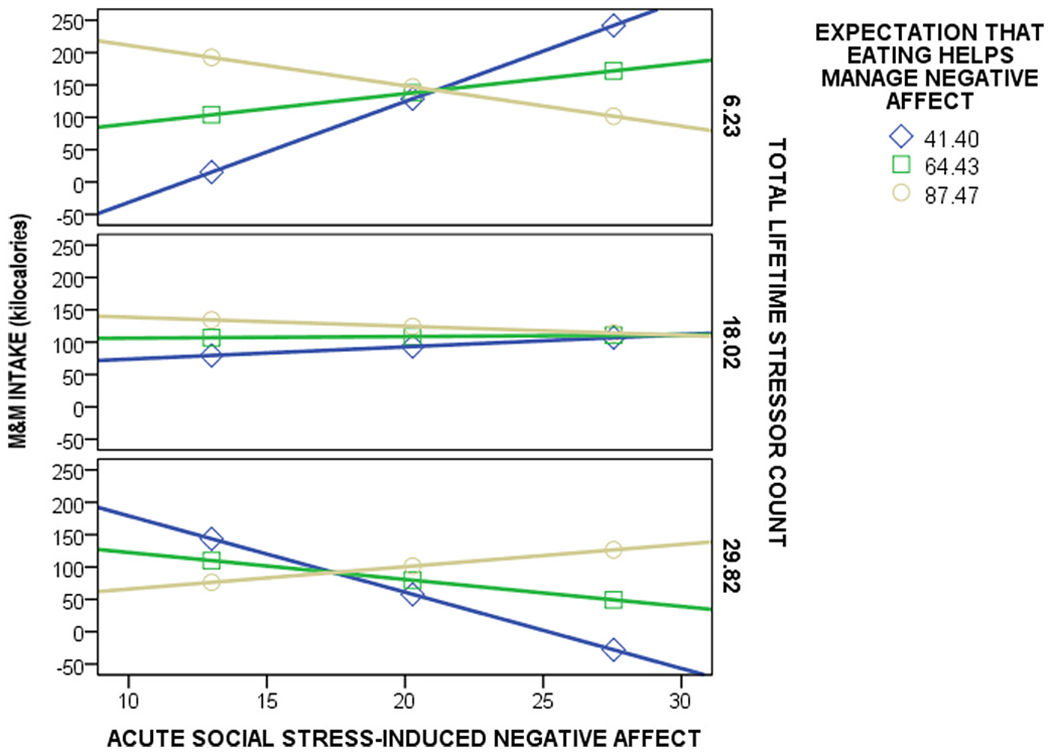
Total lifetime stressor count strengthened the moderating effect of eating expectancies on the relation between acute social stress-induced negative affect and greater M&M intake post-stress. The moderated moderation (PROCESS model 3) was significant, F(16,27) = 2.11, p = 0.042; R2 = 0.75, indicating that there was a significant conditional three-way interaction effect on M&M intake; when eating expectancies were high, acute social stress-induced negative affect predicted more M&M intake for women with very high total lifetime stressor exposure and less M&M intake for women with lower total lifetime stressor exposure (b = 0.034g, SE = 0.013, p = 0.016; 95% CI: [0.007–0.061]). High and low values for total lifetime stressor exposure and eating expectancies were determined based on 1 standard deviation above and below the mean.
Probing the interaction between acute social stress-induced negative affect and eating expectancies on M&M intake revealed that the interaction was significant at one SD below the mean of total lifetime stressors (b = −0.47g, F(1,27) = 11.41, p = 0.002), but not at the mean (b = −0.07g, F(1,27) = 0.58, p = 0.45) or at the mean plus 1 SD (b = 0.33, F(1,27) = 2.31, p = 0.14). The Johnson-Neyman test further revealed that for those who experienced 15.1 or less total lifetime stressors, greater acute social stress-induced negative affect predicted greater M&M intake for those with lower eating expectancies; 50.0% of total lifetime stressors were less than 15.1. Therefore, when total lifetime stressor exposure was lower, acute social stress-induced negative affect predicted greater M&M intake for women with lower eating expectancies.
The Johnson-Neyman test also showed that for those who experienced 44.5 or more total lifetime stressors (i.e., above +1 SD of the mean), greater acute social stress-induced negative affect predicted greater M&M intake for those with very high eating expectancies; 2.3% of total lifetime stressors were greater than 44.5. Therefore, when total lifetime stressor exposure was very high, acute social stress-induced negative affect predicted greater M&M intake for women with higher eating expectancies (Fig. 4).
Total lifetime stressor exposure (b = 48.18g, SE = 21.79, p = 0.035; 95% CI: 3.48–92.89), eating expectancies (b = 14.17g, SE = 4.63, p = 0 0.005; 95% CI: [4.67–23.67]), and acute social stress-induced negative affect (b = 51.22g, SE = 16.49, p = 0.004; 95% CI: [17.38–85.05]) significantly predicted M&M intake. Finally, the interactions between acute social stress-induced negative affect and eating expectancies (b = −0.69g, SE = 0.21, p = 0.003; 95% CI: [−1.12 to −0.25]), acute social stress-induced negative affect and total lifetime stressor exposure (b = −2.57g, SE = 1.06, p = 0.022; 95%CI: [−4.75 to −0.40]), and eating expectancies and total lifetime stressor exposure (b = −0.67g, SE = 0.29, p = 0.029; 95%CI: [−1.26 to −0.73]) on M&M intake were significant.
5. Discussion
The present pre-registered study investigated variability in stress-related eating behavior by examining how lifetime stressor exposure and acute social stress-induced negative affect interact to increase snack intake. Based on our theoretical model, we hypothesized that greater lifetime stressors would increase the extent to which eating expectancies (eating helps manage negative affect) strengthen acute social stress-induced negative affect as a predictor of snack intake (Fig. 2). The data supported our a priori theoretical model. When eating expectancies were high, acute social stress-induced negative affect was related to eating more M&Ms for women with very high lifetime stressor exposure and less M&Ms for women with lower lifetime stressor exposure.
Despite the need for cautious interpretation of this three-way interaction given the small sample size, these results are consistent with the emotional eating cycle (Klatzkin et al., 2021, pp. 871–906), which posits that greater negative reinforcement in response to stress-related eating strengthens the association between negative affect and food intake in a positive feedback loop to increase the likelihood of future stress-related eating via reinforcement learning (Fig. 1). As enhanced negative reinforcement learning increases eating expectancies (Behan, 1953; Smith et al., 2018), our findings that greater eating expectancies enhance the association between higher acute social stress-induced negative affect and M&M intake for women with greater lifetime stressors supports the emotional eating cycle and provides evidence that the cycle may be strengthened for women who have experienced more lifetime stressors.
Greater reinforcement learning, stress-eating, and obesity in women with more chronic stressors may increase the ability to more accurately predict eating in response to stress and negative emotions (Dallman et al., 2003; Epel et al., 2012; Tomiyama et al., 2011). More learning opportunities to determine how effective stress-eating is at reducing negative affect may lead to more accurate eating expectancies. Consequently, women with very high lifetime stressor exposure may eat more snack foods in the presence of high negative affect when eating expectancies are high. In contrast, women with lower lifetime stressor exposure may have less opportunities to gauge the effectiveness of eating as an emotion regulation strategy and consequently, high eating expectancies do not accurately reflect eating behaviors (i.e., less eating with greater negative affect). Additional research is needed to investigate other psychosocial and biological factors that may influence the reinforcing properties of food such as a history of trauma, as early life adversity may alter brain regions associated with reward and emotion regulation in women, and lead to greater obesity in adulthood (Hemmingsson et al., 2014; Osadchiy et al., 2019).
5.1. Strengths and limitations
Several strengths of this study should be noted. First, although exploratory in nature, we pre-registered this study and the analyses, and tested predictions derived from a well-developed theoretical model of stress-related eating behavior. Second, we used a well-validated, laboratory-based acute social stress task (i.e., the TSST) and confirmed stress induction via multiple physiological and self-reported manipulation checks. Third, we used a valid measure of food intake (i.e., the bogus taste test; Robinson et al., 2017). Finally, we examined the moderating effects of lifetime stressor exposure, which was assessed using a well-validated instrument for measuring all the acute and chronic stressors that individuals have experienced over the life course (i.e., the STRAIN).
Several limitations should also be noted. First, participants in this relatively small study were all women with a mean body mass index of 25 (i.e., overweight, but not obese). Additional research using larger samples is essential to examine the generalizability of these results across the weight spectrum and gender. Second, although responses to our measure of eating expectancies were likely informed by participants’ prior experiences of negative reinforcement learning (Behan, 1953; Smith et al., 2018), we did not directly test reinforcement learning in this study. Therefore, we were unable to provide direct evidence supporting the component of the emotional eating cycle (Fig. 1) in which greater reductions in negative affect following stress-related eating (i.e., negative reinforcement, Box C) enhance negative affect (Box A) as a trigger for food intake (Box B). To test this model more effectively, future studies should measure reductions in negative affect from stress-eating on a first laboratory visit and acute social stress-induced negative affect and food intake on a subsequent visit. Thirdly, although our model significantly predicted M&M intake, it did not significantly predict total food intake or consumption of golden oreos or chips as proposed in our pre-registration. This may be due to lack of power to detect such an effect given our small sample size. However, prior studies have reported similar food-specific results, and these results may help to explain why eating chocolate may be a preferred emotion regulation strategy compared to salty foods. Indeed, Zellner et al. (2006) found that participants self-reported eating sweet foods over salty foods when stressed and, following a stress manipulation, ate more M&Ms than peanuts and chips. Moreover, Habhab et al. (2009) reported that participants ate more sweet food (i.e., M&Ms and graham crackers) than salty food (i.e., chips and pretzels) under high stress conditions but showed no preference under low stress conditions. Chocolate may also provide greater negative reinforcement following stress or negative mood. Macht and Mueller (2007) showed that eating chocolate in response to a negative mood induction led to increased ratings of joy and improvements in negative mood as compared to eating unpalatable chocolate or eating nothing. Moreover, Wirtz et al. (2014) found that dark chocolate buffered the endocrine stress response in men to a greater degree than placebo chocolate. Therefore, it is possible that the food-specific result obtained here for M&Ms is a limitation, but it is also possible that this pattern of results is revealing a unique and consistent effect of stress exposure on eating preferences that should be investigated in the future. Finally, it was not possible to interpret group comparisons between individuals who successfully completed the preliminary screening yet did not complete the stress study visit and those who completed both the preliminary screening and stress testing due to small samples and COVID-19 complications.
5.2. Conclusion
In conclusion, the present findings help to explain variability in stress-related eating by elucidating a mechanism by which individual differences in stress-related vulnerability factors influence snack intake. Results of this pre-registered study support the emotional eating cycle (Fig. 1; Klatzkin et al., 2021, pp. 871–906) as well as Sinha (2018) who stated that women experiencing greater chronic stress may have distinct mechanisms underlying obesity with a need for specific interventions. Replications in larger and more diverse samples may inform eating- and obesity-related treatments for women that include life stress assessments and focus on helping individuals develop coping behaviors that target negative mood and reward-based cognitive processing (Valderhaug & Slavich, 2020).
Acknowledgements
We would like to thank Caroline Ferrell, Cleo Nikodem, Barrett Leonhard, Olivia Street, Dot Perkins, Bella Lallo, Erica Mosby, Hadiyah Qureshi, and Alli Hagler for helping with data collection.
Funding
G.M.S. was supported by grant #OPR21101 from the California Governor’s Office of Planning and Research/California Initiative to Advance Precision Medicine. These organizations had no role in designing or planning this study; in collecting, analyzing, or interpreting the data; in writing the article; or in deciding to submit this article for publication.
Footnotes
Declaration of competing interest
We have no conflicts of interest to declare.
Data availability
Data will be made available on request.
References
- Adam TC, & Epel ES (2007). Stress, eating and the reward system. Physiology & Behavior, 91(4), 449–458. 10.1016/j.physbeh.2007.04.011 [DOI] [PubMed] [Google Scholar]
- Beck AT, & Beamesderfer A (1974). Assessment of depression: The depression inventory, 0. Modern Problems of Pharmacopsychiatry, 7, 151–169. [DOI] [PubMed] [Google Scholar]
- Behan RA (1953). Expectancies and hullian theory. Psychological Review, 60(4), 252–256. 10.1037/h0059102 [DOI] [PubMed] [Google Scholar]
- Bekhbat M, & Neigh GN (2017). Sex differences in the neuro-immune consequences of stress: Focus on depression and anxiety. Brain, Behavior, and Immunity. 10.1016/j.bbi.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohon C, Stice E, & Burton E (2009). Maintenance factors for persistence of bulimic pathology: A prospective natural history study. International Journal of Eating Disorders, 42(2), 173–178. 10.1002/eat.20600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosof LC, Munn-Chernoff MA, Bulik CM, & Baker JH (2019). Associations between eating expectancies and Eating disorder symptoms in men and women. Appetite, 141, Article 104309. 10.1016/j.appet.2019.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazassa MJ, Oliveira M, da S, Spahr CM, Shields GS, & Slavich GM (2020). The stress and adversity inventory for adults (adult STRAIN) in Brazilian Portuguese: Initial validation and links with executive function, sleep, and mental and physical health. Frontiers in Psychology, 10. https://www.frontiersin.org/articles/10.3389/fpsyg.2019.03083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao AM, Fogelman N, Hart R, Grilo CM, & Sinha R (2020). A laboratory-based study of the priming effects of food cues and stress on hunger and food intake in individuals with obesity. Obesity, 28(11), 2090–2097. 10.1002/oby.22952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao AM, Jastreboff AM, White MA, Grilo CM, & Sinha R (2017). Stress, cortisol, and other appetite-related hormones: Prospective prediction of 6-month changes in food cravings and weight. Obesity, 25(4), 713–720. 10.1002/oby.21790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, la Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, & Manalo S (2003). Chronic stress and obesity: A new view of “comfort food. Proceedings of the National Academy of Sciences of the United States of America, 100(20), 11696–11701. 10.1073/pnas.1934666100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Tomiyama AJ, & Dallman MF (2012). Stress and reward: Neural networks, eating, and obesity. In Food and addiction: A comprehensive handbook (pp. 266–272). Oxford University Press. 10.1093/med:psych/9780199738168.003.0040. [DOI] [Google Scholar]
- Evers C, Dingemans A, Junghans AF, & Boevé A (2018). Feeling bad or feeling good, does emotion affect your consumption of food? A meta-analysis of the experimental evidence. Neuroscience & Biobehavioral Reviews, 92, 195–208. 10.1016/j.neubiorev.2018.05.028 [DOI] [PubMed] [Google Scholar]
- Fay SH, & Finlayson G (2011). Negative affect-induced food intake in non-dieting women is reward driven and associated with restrained-disinhibited eating subtype. Appetite, 56(3), 682–688. 10.1016/j.appet.2011.02.004 [DOI] [PubMed] [Google Scholar]
- Finch LE, & Tomiyama AJ (2014). Stress-induced eating dampens physiological and behavioral stress responses. 10.1016/B978-0-12-407869-7.00018-0 [DOI] [Google Scholar]
- Fischer S, Wonderlich J, Breithaupt L, Byrne C, & Engel S (2018). Negative urgency and expectancies increase vulnerability to binge eating in bulimia nervosa. Eating Disorders, 26(1), 39–51. 10.1080/10640266.2018.1418253 [DOI] [PubMed] [Google Scholar]
- Fong M, Li A, Hill AJ, Cunich M, Skilton MR, Madigan CD, & Caterson ID (2019). Mood and appetite: Their relationship with discretionary and total daily energy intake. Physiology & Behavior, 207, 122–131. 10.1016/j.physbeh.2019.05.011 [DOI] [PubMed] [Google Scholar]
- Habhab S, Sheldon JP, & Loeb RC (2009). The relationship between stress, dietary restraint, and food preferences in women. Appetite, 52(2), 437–444. 10.1016/j.appet.2008.12.006 [DOI] [PubMed] [Google Scholar]
- Haedt-Matt AA, & Keel PK (2011). Revisiting the affect regulation model of binge eating: A meta-analysis of studies using ecological momentary assessment. Psychological Bulletin, 137(4), 660–681. 10.1037/a0023660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RC, & Clement PF (1984). Binge eating: Measurement problems and a conceptual model. In The binge purge syndrome: Diagnosis, treatment, and research (pp. 229–251). Springer. [Google Scholar]
- Hayaki J (2009). Negative reinforcement eating expectancies, emotion dysregulation, and symptoms of bulimia nervosa. International Journal of Eating Disorders, 42(6), 552–556. 10.1002/eat.20646 [DOI] [PubMed] [Google Scholar]
- Hemmingsson E, Johansson K, & Reynisdottir S (2014). Effects of childhood abuse on adult obesity: A systematic review and meta-analysis. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity, 15(11), 882–893. 10.1111/obr.12216 [DOI] [PubMed] [Google Scholar]
- Hill D, Conner M, Clancy F, Moss R, Wilding S, Bristow M, & O’Connor DB (2021). Stress and eating behaviours in healthy adults: A systematic review and meta-analysis, 0(0) Health Psychology Review, 1–25. 10.1080/17437199.2021.1923406. [DOI] [PubMed] [Google Scholar]
- Hohlstein LA, Smith GT, & Atlas JG (1998). An application of expectancy theory to eating disorders: Development and validation of measures of eating and dieting expectancies. Psychological Assessment, 10(1), 49–58. 10.1037/1040-3590.10.1.49 [DOI] [Google Scholar]
- Karlsson J, Persson LO, Sjöström L, & Sullivan M (2000). Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity, 24(12), 1715–1725. 10.1038/sj.ijo.0801442 [DOI] [PubMed] [Google Scholar]
- Kazmierski KFM, Borelli JL, & Rao U (2022). Negative affect, childhood adversity, and adolescents’ eating following stress. Appetite, 168, Article 105766. 10.1016/j.appet.2021.105766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, & Hellhammer DH (1993). The ‘trier social stress test’ – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Dasani R, Warren M, Cattaneo C, Nadel T, Nikodem C, &Kissileff HR (2019). Negative affect is associated with increased stress-eating for women with high perceived life stress. Physiology & Behavior, 210, Article 112639. 10.1016/j.physbeh.2019.112639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzkin R, Nolan L, Chaudhry R, Geliebter A, & Kissileff H (2021). Measures of emotions as influences on eating and weight control (pp. 871–906). 10.1016/B978-0-12-821124-3.00027-2 [DOI] [Google Scholar]
- Konttinen H, Männistö S, Sarlio-Lähteenkorva S, Silventoinen K, & Haukkala A (2010). Emotional eating, depressive symptoms and self-reported food consumption. A population-based study. Appetite, 54(3), 473–479. 10.1016/j.appet.2010.01.014 [DOI] [PubMed] [Google Scholar]
- Lam JCW, Shields GS, Trainor BC, Slavich GM, & Yonelinas AP (2019). Greater lifetime stress exposure predicts blunted cortisol but heightened DHEA responses to acute stress. Stress and Health, 35(1), 15–26. 10.1002/smi.2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macht M (2008). How emotions affect eating: A five-way model. Appetite, 50(1), 1–11. 10.1016/j.appet.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Macht M, & Mueller J (2007). Immediate effects of chocolate on experimentally induced mood states. Appetite, 49(3), 667–674. 10.1016/j.appet.2007.05.004 [DOI] [PubMed] [Google Scholar]
- McMullin SD, Shields GS, Slavich GM, & Buchanan TW (2021). Cumulative lifetime stress exposure predicts greater impulsivity and addictive behaviors. Journal of Health Psychology, 26(14), 2921–2936. 10.1177/1359105320937055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meule A (2013). Impulsivity and overeating: A closer look at the subscales of the barratt impulsiveness scale, 0. Frontiers in Psychology. 10.3389/fpsyg.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhail ME (2021). Affect dysregulation in context: Implications and future directions of experience sampling research on affect regulation models of loss of control eating. Frontiers in Psychiatry, 12, Article 747854. 10.3389/fpsyt.2021.747854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MLM, Sichko S, Bui TQ, Libowitz MR, Shields GS, & Slavich GM (2022). Intergenerational transmission of lifetime stressor exposure in adolescent girls at differential maternal risk for depression. Journal of Clinical Psychology. 10.1002/jclp.23417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvera Alvarez HA, Provencio-Vasquez E, Slavich GM, Laurent JGC, Browning M, McKee-Lopez G, Robbins L, & Spengler JD (2019). Stress and health in nursing students: The nurse engagement and wellness study. Nursing Research, 68(6), 453–463. 10.1097/NNR.0000000000000383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osadchiy V, Mayer EA, Bhatt R, Labus JS, Gao L, Kilpatrick LA, Liu C, Tillisch K, Naliboff B, Chang L, & Gupta A (2019). History of early life adversity is associated with increased food addiction and sex-specific alterations in reward network connectivity in obesity. Obesity Science & Practice, 5(5), 416–436. 10.1002/osp4.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, & Barratt ES (1995). Factor structure of the barratt impulsiveness scale. Journal of Clinical Psychology, 51(6), 768–774. , 2-1. [DOI] [PubMed] [Google Scholar]
- Robinson E, Haynes A, Hardman CA, Kemps E, Higgs S, & Jones A (2017). The bogus taste test: Validity as a measure of laboratory food intake. Appetite, 116, 223–231. 10.1016/j.appet.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R (2018). Role of addiction and stress neurobiology on food intake and obesity. Biological Psychology, 131, 5–13. 10.1016/j.biopsycho.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, & Shields GS (2018). Assessing lifetime stress exposure using the stress and adversity inventory for adults (adult STRAIN): An overview and initial validation. Psychosomatic Medicine, 80(1), 17–27. 10.1097/PSY.0000000000000534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Stewart JG, Esposito EC, Shields GS, & Auerbach RP (2019). The Stress and Adversity Inventory for Adolescents (Adolescent STRAIN): Associations with mental and physical health, risky behaviors, and psychiatric diagnoses in youth seeking treatment. Journal of Child Psychology and Psychiatry, 60(9), 998–1009. 10.1111/jcpp.13038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Mason TB, Peterson CB, & Pearson CM (2018). Relationships between eating disorder-specific and transdiagnostic risk factors for binge eating: An integrative moderated mediation model of emotion regulation, anticipatory reward, and expectancy. Eating Behaviors, 31, 131–136. 10.1016/j.eatbeh.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R, Vagg P, & Jacobs G (1983). Manual for the state-trait anxiety inventory (form Y1 – Y2) (Vol. IV). Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Stunkard AJ, & Messick S (1985). The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research, 29(1), 71–83. [DOI] [PubMed] [Google Scholar]
- Sturmbauer SC, Shields GS, Hetzel E-L, Rohleder N, & Slavich GM (2019). The stress and adversity inventory for adults (adult STRAIN) in German: An overview and initial validation. PLoS One, 14(5), Article e0216419. 10.1371/journal.pone.0216419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama AJ (2019). Stress and obesity. Annual Review of Psychology, 70(1), 703–718. 10.1146/annurev-psych-010418-102936 [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, Dallman MF, & Epel ES (2011). Comfort food is comforting to those most stressed: Evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology, 36(10), 1513–1519. 10.1016/j.psyneuen.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryon MS, DeCant R, & Laugero KD (2013). Having your cake and eating it too: A habit of comfort food may link chronic social stress exposure and acute stress-induced cortisol hyporesponsiveness. Physiology & Behavior, 114(115), 32–37. 10.1016/j.physbeh.2013.02.018 [DOI] [PubMed] [Google Scholar]
- Udo T, Grilo CM, & McKee SA (2014). Gender differences in the impact of stressful life events on changes in body mass index. Preventive Medicine, 69, 49–53. 10.1016/j.ypmed.2014.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainik U, Neseliler S, Konstabel K, Fellows LK, & Dagher A (2015). Eating traits questionnaires as a continuum of a single concept. Uncontrolled eating. Appetite, 90, 229–239. 10.1016/j.appet.2015.03.004 [DOI] [PubMed] [Google Scholar]
- Valderhaug TG, & Slavich GM (2020). Assessing life stress: A critical priority in obesity research and treatment. Obesity, 28(9), 1571–1573. 10.1002/oby.22911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. [DOI] [PubMed] [Google Scholar]
- Wei N-L, Quan Z-F, Zhao T, Yu X-D, Xie Q, Zeng J, Ma F-K, Wang F, Tang Q-S, Wu H, & Zhu J-H (2019). p>Chronic stress increases susceptibility to food addiction by increasing the levels of DR2 and MOR in the nucleus accumbens</p>. Neuropsychiatric Disease and Treatment, 15, 1211–1229. 10.2147/NDT.S204818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz PH, von Känel R, Meister RE, Arpagaus A, Treichler S, Kuebler U, Huber S, & Ehlert U (2014). Dark chocolate intake buffers stress reactivity in humans. Journal of the American College of Cardiology, 63(21), 2297–2299. 10.1016/j.jacc.2014.02.580 [DOI] [PubMed] [Google Scholar]
- de Wit LM, Fokkema M, van Straten A, Lamers F, Cuijpers P, & Penninx BWJH (2010). Depressive and anxiety disorders and the association with obesity, physical, and social activities. Depression and Anxiety, 27(11), 1057–1065. 10.1002/da.20738 [DOI] [PubMed] [Google Scholar]
- Wouters S, Jacobs N, Duif M, Lechner L, & Thewissen V (2018). Negative affective stress reactivity: The dampening effect of snacking. Stress and Health, 34(2), 286–295. 10.1002/smi.2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau YHC, & Potenza MN (2013). Stress and eating behaviors. Minerva Endocrinologica, 38(3), 255–267. [PMC free article] [PubMed] [Google Scholar]
- Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, & Wolf A (2006). Food selection changes under stress. Physiology & Behavior, 87(4), 789–793. 10.1016/j.physbeh.2006.01.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


