To the Editor:
The standard-of-care treatment for patients with acute myeloid leukemia (AML) is being challenged by new classes of targeted therapies, including FLT3, IDH and BCL2 inhibitors [1]. Contemporaneously, measurable residual disease (MRD) testing is being considered as a surrogate for traditional clinical outcomes in the clinical trials of these new therapies [2]. To successfully integrate MRD assays into clinical trials, testing must be standardized between laboratories to ensure results are comparable in multicentre MRD assessment studies. Thus, results from different clinical trials can be meaningfully compared and, ultimately, clinical thresholds around common MRD levels applicable to all AML patients globally can be developed for these new treatments.
To this end, the European LeukemiaNet (ELN) group published recommendations in 2018 [3], updated in 2021, that standardized the technical and reporting aspects of AML MRD testing for reverse transcription PCR (RT-PCR) and flow cytometry based approaches, with the 2021 update extending this to next generation sequencing (NGS) analysis and the use of MRD as a surrogate marker in clinical trials. Further to this, external quality assessment (EQA) programs have been established to assess the quality of testing [4], For molecular MRD testing, EQA data has shown that interlaboratory variation for molecular AML MRD testing was substantially greater than that seen for BCR::ABL1 testing in chronic myeloid leukemia (CML), despite both tests being reverse transcription quantitative PCR-based (Fig. 1) [5, 6]. However, BCR::ABL1 MRD testing has been subject to many years of standardization, including the development of the BCR::ABL1 international scale (IS) [7].
Fig. 1.
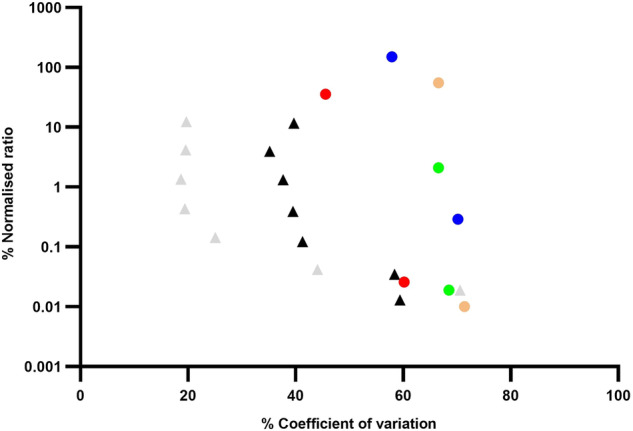
Comparison of interlaboratory variation for standardized BCR::ABL1IS RT-dPCR (gray triangle markers) and RT-qPCR (black triangle markers) [5] testing with unstandardized RUNX1::RUNX1T1 (blue circle markers), CBFB::MYH11 (red circle markers), PML::RARA (green circle markers) and NPM1 (orange circle markers) RT-qPCR testing [6].
An expert advisory board was established through the NHS Chief Scientific Officer’s Knowledge Transfer Partnership program to assess how molecular AML MRD testing could be further standardized [8]. This letter represents the findings of the board. The advisory board was composed of experts, including clinical scientists working in the establishment of UK AML MRD testing and those leading the standardization of BCR::ABL1 testing in CML, clinicians experienced in managing patients with AML-based on MRD testing results, measurement science (metrology) experts and reference material producers. Representatives from commercial providers involved in manufacturing in vitro diagnostic (IVD) kits, reference standards and instrumentations were also present. Finally, representatives were present from the ELN David group. The Foundation for the National Institute of Health (FNIH) Biomarkers Consortium were consulted outside of the meeting. ELN David and the FNIH Biomarkers Consortium are both active in AML MRD standardization and were approached to ensure that any standardization recommendations were complementary to the activities of these groups.
The stated aims of the expert advisory board were to answer the following questions:
Would further standardization of molecular AML MRD be beneficial?
What markers should be standardized?
How should the standardization of these markers be prioritized?
What would be the best approaches to standardizing these markers?
To expedite the workings of the board, several surveys were performed in advance of the meeting: one for the experts involved in the meeting, one for the laboratories currently performing molecular AML MRD testing and another for commercial providers of IVDs, standards and platforms (Supplementary Tables 1–6).
The recommendations of the board and pre-board survey have been outlined below:
All board members felt further standardization of molecular AML MRD testing would be beneficial to the field.
The main blocks to standardization cited by EQA participants were lack of time and money (Supplementary Table 1), therefore, any standardization efforts should be easy to implement and inexpensive (Supplementary Table 2).
Standardization of RUNX1::RUNX1T1, CBFB::MYH11 type A and NPM1 type A testing by RT-PCR-based approaches was deemed to be the priority (Supplementary Table 3). These markers are some of the most prevalent somatic changes found in patients with AML. Furthermore, there are commercially available cell lines facilitating standardization projects. Despite the high prevalence of the PML::RARA rearrangement in AML patients, PML::RARA MRD testing is primarily interpreted qualitatively thus limiting the impact of any standardization projects [3].
RT-PCR-based testing of NPM1 type B and D and FLT3 internal tandem duplications (ITD) should be considered for future standardization projects. NPM1 type B and D are only found in around 10% and 8% of NPM1 positive patients with AML, respectively, compared to 70% who have the NPM1 type A variant [9]; however, this is still a substantial number of patients and standardization should be considered if stable cell lines can be produced. Cell lines for NPM1 type B and D should be developed to facilitate future standardization projects. Despite recent publications showing the prognostic importance of FLT3 ITD MRD testing [10], it is not yet clear how this testing will be employed and what the clinically important cut-offs will be, thus it would be premature to begin standardizing testing.
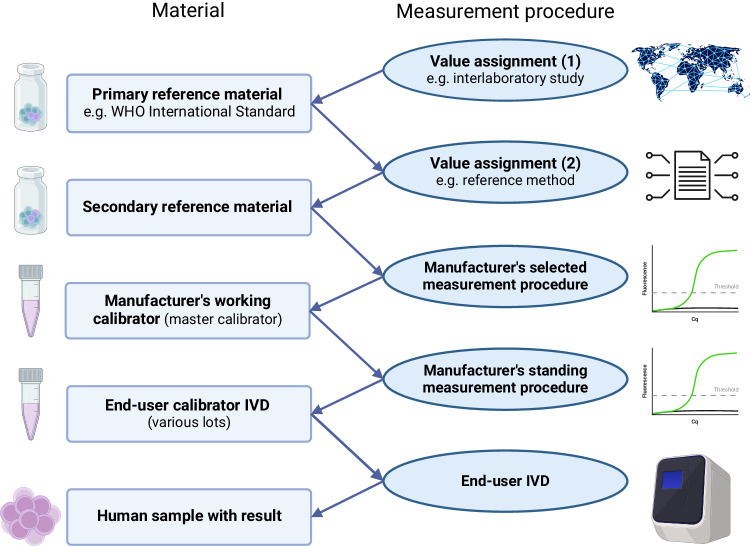
The development of higher order, primary reference materials (RMs) was the priority of the board (Supplementary Table 4). Primary RMs are at the top of the calibration hierarchy from which other quality control (QC) materials can be calibrated (Fig. 2).
Internationally recognized certification would be important to any reference materials produced, as is compliance with the European Union In Vitro Diagnostics Regulation (EU-IVDR). Certification of reference materials demonstrates that the material has been produced to a high standard, assuring laboratories of its quality, driving uptake. The EU-IVDR regulation, Chapter 1, Section 1, Article 1, point 3 specifically exempts certified reference materials (CRMs); however, they do require any IVD in-kit controls or calibrators to demonstrate traceability to reference materials of a higher metrological order; therefore, any reference materials produced should be of a sufficient order to satisfy this requirement and be available to IVD manufacturers for this purpose.
The pre-board surveys suggested ISO 17034:2016 [11] or ISO 13485:2016 [12] would be the most appropriate form of certification for any reference materials produced (Supplementary Table 5); however, further discussion in the meeting suggested that manufacturing reference materials according to World Health Organization (WHO) guidelines [13] and approval by the WHO Expert Committee on Biological Standardization (ECBS) would be the most appropriate approach given the current context of AML MRD testing where a reference method is not currently available.
Higher order CRMs for RT-PCR based assays should be cell based as this allows for full process control, capturing the uncertainty generated in the RNA extraction and cDNA synthesis processes known to impact on the final normalized ratio result (Supplementary Table 6).
Values for RT-PCR based MRD assays should only be assigned to any reference materials produced using ABL1 as a reference gene to encourage standardization. Most laboratories performing molecular AML MRD testing use the ABL1 reference gene to normalize testing results; however, a small subset uses alternative reference genes, such as GUSB, B2M and HMBS. The use of non-ABL1 reference genes by around 15% of laboratories has proven problematic to the standardization of BCR::ABL1 in CML, with conversion factors relating to GUSB being shown to be potentially more unstable when compared with their ABL1 counterparts despite extensive standardization [14].
The development of research use only (RUO) or QC materials calibrated to the primary CRM would also be beneficial to the field.
Fig. 2. Structure of a calibration hierarchy whereby patient results are traceable to higher order reference materials such as World Health Organisation (WHO) International Standard materials, through a series of linked calibrations.
An example of this is in the standardization of BCR::ABL1/reference gene ratio to the International Scale. Modified from ISO 17511:2020 Figure 4 model calibration hierarchy for “international conventional calibrator” that defines the quantity intended to be measured (“measurand”) [15]. IVD, in vitro diagnostic medical device. Created with BioRender.com.
These recommendations should act as a starting point for commercial and academic consortia to work together to develop the resources outlined above to ensure that clinical trials results, and ultimately the management of patients with AML, are both based on the highest quality data possible.
Supplementary information
Author contributions
SS produced the advisory board pre-read and pre-board surveys, chaired the advisory board, analyzed the survey data, and wrote the letter. AD reviewed the pre-read, survey, contributed to the advisory board, designed Fig. 2 and reviewed the letter. RD assisted with the pre-read, contributed to the advisory board, and reviewed the survey and letter. CT, NC, HW, LLC, KM, NP, and AD reviewed the pre read, surveys, contributed to the advisory board, and reviewed the letter. CH, JR, AC, VL, GH, DD, TM, KG, AC, and LW reviewed the pre-read, surveys, contributed to the advisory board, and reviewed the letter.
Competing interests
Whilst the diverse composition of the panel brought a breadth of expertise and perspectives, it also introduced potential conflicts of interest, particularly regarding the commercial providers. These entities may have a vested interest in the outcomes of the advisory panel due to their affiliations and the nature of their businesses. Each member has disclosed their respective affiliations with commercial bodies. The discussion was led by individuals from non-commercial entities and all recommendations and findings have been derived through consensus and with the commitment to advancing clinical and laboratory practice. These disclosures have been made explicit to uphold transparency and integrity in the reporting of the panel’s findings.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41375-024-02275-x.
References
- 1.Bhansali RS, Pratz KW, Lai C. Recent advances in targeted therapies in acute myeloid leukemia. J Hematol Oncol. 2023;16:1–27. doi: 10.1186/s13045-023-01424-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Food and Drug Administration. Hematologic Malignancies: Regulatory Considerations for Use of Minimal Residual Disease in Development of Drug and Biological Products for Treatment Guidance for Industry. 2020 Jan. United States Food and Drug Administration. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/hematologic-malignancies-regulatory-considerations-use-minimal-residual-disease-development-drug-and (accessed 4 Aug 2023).
- 3.Schuurhuis GJ, Heuser M, Freeman S, Béné M-C, Buccisano F, Cloos J, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131:1275–91. doi: 10.1182/blood-2017-09-801498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UK NEQAS LI Home Page - Measurable Residual Disease for AML by Molecular Methods (Pilot - Not Accredited). https://www.ukneqasli.co.uk/eqa-pt-programmes/molecular-haemato-oncology-programmes/measurable-residual-disease-for-aml-by-molecular-methods-pilot-not-accredited/ (accessed 4 Aug 2023).
- 5.Scott S, Cartwright A, Francis S, Whitby L, Sanzone AP, Mulder A, et al. Assessment of droplet digital polymerase chain reaction for measuring BCR-ABL1 in chronic myeloid leukaemia in an international interlaboratory study. Br J Haematol. 2021;194:53–60. doi: 10.1111/bjh.17521. [DOI] [PubMed] [Google Scholar]
- 6.Scott S, Dillon R, Thiede C, Sadiq S, Cartwright A, Clouston HJ, et al. Assessment of acute myeloid leukemia molecular measurable residual disease testing in an interlaboratory study. Blood Adv. 2023;7:3686–94. doi: 10.1182/bloodadvances.2022009379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White HE, Matejtschuk P, Rigsby P, Gabert J, Lin F, Lynn Wang Y, et al. Establishment of the first World Health Organization International Genetic Reference Panel for quantitation of BCR-ABL mRNA. Blood. 2010;116:111–17. doi: 10.1182/blood-2010-06-291641. [DOI] [PubMed] [Google Scholar]
- 8.Leaders and aspiring leaders in healthcare science - National Physical Laboratory. https://www.npl.co.uk/leaders-in-healthcare-science (accessed 10 Oct 2023).
- 9.Alpermann T, Schnittger S, Eder C, Dicker F, Meggendorfer M, Kern W, et al. Molecular subtypes of NPM1 mutations have different clinical profiles, specific patterns of accompanying molecular mutations and varying outcomes in intermediate risk acute myeloid leukemia. Haematologica. 2016;101:e55–8. doi: 10.3324/haematol.2015.133819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dillon LW, Gui G, Page KM, Ravindra N, Wong ZC, Andrew G, et al. DNA sequencing to detect residual disease in adults with acute myeloid leukemia prior to hematopoietic cell transplant. JAMA. 2023;329:745–55. doi: 10.1001/jama.2023.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Organization for Standardization. ISO 17034:2016—General requirements for the competence of reference material producers. Available from: https://www.iso.org/standard/29357.html (accessed 9 Aug 2023).
- 12.International Organization for Standardization. ISO 13485:2016—Medical devices—Quality management systems—Requirements for regulatory purposes. Available from: https://www.iso.org/standard/59752.html (accessed 9 Aug 2023).
- 13.WHO. Expert Committee on Biological Standardization WHO Expert Committee on Biological Standardization: Fifty-ninth report. WHO Technical Report Series 2012;1-240. Available from: https://www.who.int/groups/expert-committee-on-biological-standardization.
- 14.White HE, Salmon M, Albano F, Andersen CSA, Balabanov S, Balatzenko G, et al. Standardization of molecular monitoring of CML: results and recommendations from the European treatment and outcome study. Leukemia. 2022;36:1834–42. doi: 10.1038/s41375-022-01607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Organization for Standardization. ISO 17511:2020 In vitro diagnostic medical devices — Requirements for establishing metrological traceability of values assigned to calibrators, trueness control materials and human samples. 2020. Available from: https://www.iso.org/standard/69984.html (accessed 9 Aug 2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.