Abstract
The trans-Golgi network (TGN) is putatively the site where varicella-zoster virus is enveloped. gE is targeted to the TGN by selective retrieval from the plasmalemma in response to signaling sequences in its endodomain. gI lacks these sequences but forms a complex with gE. We now find that gI is targeted to the TGN and plasma membrane when expressed in Cos-7 cells; nevertheless, surface labeling revealed that gI is not retrieved from the plasma membrane. TGN targeting of gI depended on the T338 of its endodomain and was lost when T338 was deleted or mutated to A, S, or D. The endodomain of gI was sufficient, if it contained T338, to target a fusion protein containing the ectodomain of the human interleukin-2 receptor to the TGN. A truncated protein consisting only of the gI ectodomain was secreted and taken up by nontransfected cells. This uptake of the secreted gI ectodomain was blocked by mannose 6-phosphate. Following cotransfection, both gI and gE were retrieved to the TGN from the plasma membrane in 26.7% of cells, neither gI nor gE was internalized in 18.3%, and gE was retrieved to the TGN while gI remained at the plasma membrane in 55%. We suggest that the T338 of its endodomain is necessary to retain gI in the TGN; moreover, because gI and gE interact, the signaling sequences of each glycoprotein reinforce one another in ensuring that both glycoproteins are concentrated in the TGN yet remain on the cell surface.
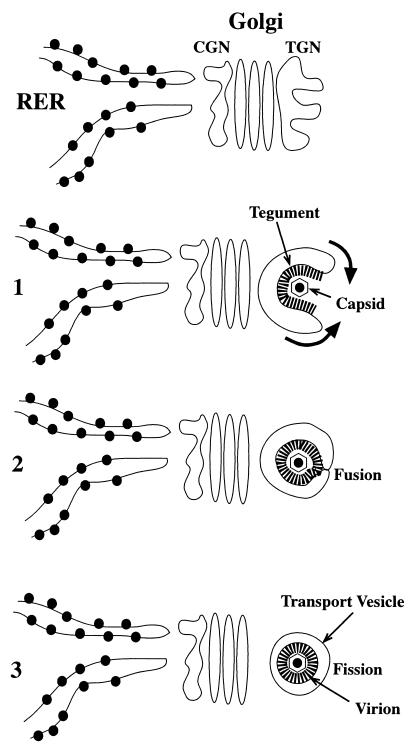
Envelopment of varicella-zoster virus (VZV) has been proposed to be a two-step process that is completed in the trans-Golgi networks (TGN) of infected cells (8). According to this hypothesis, nucleocapsids assemble in the nucleus and acquire a primary envelope by budding through the inner nuclear membrane. Budding delivers the temporarily enveloped virions to the perinuclear cisterna, which is continuous with the rough endoplasmic reticulum (RER). Fusion of the primary envelope with the membranes of the RER enables the nucleocapsids to gain access to the cytosol, though which they are translocated to the TGN. The VZV glycoproteins (gps) are synthesized on attached polyribosomes and are transported as integral or peripheral membrane proteins from the RER via the Golgi apparatus to the TGN, where they are concentrated for incorporation into the final viral envelope. Tegument proteins lack signal sequences (6) and must thus be synthesized on free polyribosomes in the cytosol; nevertheless, there is evidence that tegument proteins that are included in assembled virions also concentrate in the region of the TGN, where they are associated with the cytosolic domains (endodomains) of integral membrane gps (8). Envelopment within the TGN is thought to involve the restriction of gps to the concave surface of flattened sacs that wrap around nucleocapsids (Fig. 1). Tegument is trapped by the wrapping process and so becomes included in the final virion. A process of fusion of membranes with subsequent fission converts the enveloping sac into an inner viral envelope and an outer transport vesicle. In cells that contain a lysosomal pathway, the transport vesicle membrane is rich in the large cation-independent mannose 6-phosphate receptor (MPR). Perhaps for this reason, virions are directed in tissue culture cells to acidic post-Golgi vacuoles that have been identified as late endosomes (7, 8). Virions appear to be inactivated in these vacuoles, accounting for the strict cell association of VZV when propagated in tissue culture.
FIG. 1.
Heuristic model depicting the envelopment of VZV. Viral gps are synthesized in the RER and transported through the cis-Golgi network (CGN) and Golgi stack to the TGN, where they concentrate. Step 1: curvilinear vacuoles appear in the TGN. Tegument is bound to the concave face of these vacuoles, while their convex face is smooth. The gps are concentrated in the tegument-coated membrane of the concave face. Capsids adhere to the tegument. The lips of the curvilinear vacuole wrap around a capsid (arrows). Step 2: the extending lips of the curvilinear vacuole fuse, trapping a capsid and the tegument that surrounds it within a cavity delimited by the concave membrane. Step 3: subsequent fission of the fused lips of the vesicle creates two separate membrane-enclosed structures, an inner virion and an outer transport vesicle. The original, tegument-lined concave face becomes the viral envelope, while the smooth convex face becomes a transport vesicle that carries the enveloped virion to post-Golgi destinations.
The hypothesis that the final site of VZV envelopment is the TGN requires that all of the components of the final virion be targeted to this organelle. For the integral membrane gps, targeting could involve signal sequences or patches in the primary structure of the proteins. Alternatively, gps that lack such targeting information (passenger gps) could still be sorted to the TGN if they were to form complexes with gps that do contain targeting sequences (navigator gps). Previous data has indicated that gE, the most abundant gp of VZV (9), is targeted to the TGN as a result of the presence in its endodomain of a targeting sequence, AYRV, and a signal patch rich in acidic amino acids (2, 46). These signaling regions resemble those which have been found in the endodomains of the endogenous TGN proteins furin (12, 39) and TGN38 (3, 11, 25, 27, 38, 40). The targeting of gE to the TGN is an indirect process that includes its endocytosis from the plasma membrane, followed by selective retrieval to the TGN (2, 45). In keeping with this route of transport, there is an endocytosis signal in the endodomain of gE (21).
The mechanism by which gI is concentrated in the TGN is less clear than that for gE. Since the N-terminal domain of gI forms a complex with gE in the RER (14, 42), gI might be expected to be simply a passenger gp and follow the itinerary navigated by gE. If this were to be the case, then gI would not be targeted to the TGN in transfected cells that express gI by itself. Targeting of gI to the TGN would only occur in cells that express both gI and gE. There is evidence that gI is recycled from the plasma membrane by endocytosis when it is expressed by itself in HeLa cells (19); however, a contrary study reports that HeLa cells that express gI restrict it to the plasma membrane and do not reinternalize gI (1). This latter report also maintains that gI is not targeted to the TGN unless it is coexpressed in HeLa cells together with gE. In contrast, gI has been found to be targeted to the TGN when expressed by itself in transfected Cos-7 cells (36). Conceivably, foreign proteins could be transported differently in different types of cell. To be transported, a foreign protein would have to contain a signaling domain, but the cell would also have to express an endogenous receptor capable of responding to the signal sequence or patch in the protein. The successful targeting of gI to the TGN of Cos-7 cells indicates that TGN targeting information is present in the primary sequence of gI, which can be recognized by at least some types of cell. That phenomenon is significant, because there is no reason to believe that Cos-7 cells are unique. The cellular machinery that enables Cos-7 cells to respond to signaling information in the primary sequence of gI is thus likely also to be present in additional cell types.
The current experiments were undertaken to identify and characterize the signaling domain that is responsible for the ability of Cos-7 cells to target gI to the TGN. An additional goal was to compare the routes to the TGN followed by transported gI and gE. Because gI and gE form a complex (13, 41, 42), the sorting of these proteins was analyzed in cells in which they were expressed individually and also in those in which they were coexpressed. The data confirm that gI is targeted to the TGN when expressed by itself in Cos-7 cells, the responsible targeting signal resides in the endodomain of gI, and T338 is critical for targeting. In contrast to gE, the independent targeting of gI does not involve retrieval from the plasma membrane but retention of gI in the TGN during intracellular transport. Truncation of the C-terminal domain of gI, including the transmembrane region, causes the mutant protein to be secreted. Nontransfected cells take up the secreted protein by an endocytic mechanism that is inhibited by mannose 6-phosphate (Man 6-P).
MATERIALS AND METHODS
Cells and antibodies.
Cos-7 cells were grown in Dulbecco's modified Eagle's medium containing 10% heat-inactivated fetal bovine serum, 100 U of penicillin per ml, and 100 U of streptomycin per ml. Cultures were maintained at 37°C with 5% CO2. Monoclonal antibodies to gE and gI were purchased from Viro Research Inc. (Rockford, Ill.). Rabbit polyclonal antibodies to recombinant gE and gI, raised in our laboratory and characterized as previously described, were also used (36). Antibodies to detect the adaptin protein complex AP-1 (monoclonal anti-γ-adaptin clone 100/3) were obtained from Sigma Chemical Co. (St. Louis, Mo.) (2, 29). Antibodies to the human interleukin-2 receptor (tac) were purchased from Biodesign International (Kennebunk, Maine).
PCR cloning.
DNA encoding gI and gE was cloned from VZV (Ellen strain) genomic DNA (36, 46). For both gps, reaction mixtures were initially incubated for 3 min at 94°C and then subjected to 35 cycles of 1 min at 94°C, 1 min at 58°C, and 3 min at 72°C. For gI, Vent polymerase (New England Biolabs) was employed for amplification, and the resulting products were cloned into pZErO-2 (Invitrogen, Carlsbad, Calif.). The cloned DNA was digested with EcoRI and XbaI and gel purified. For gE, Taq polymerase was used for amplification (46). The PCR products were digested with Asp718 and XbaI and gel purified. The resulting DNA encoding gI or gE was cloned into the multiple cloning sites of the eucaryotic expression vector pSVK3 (Pharmacia, Piscataway, N.J.). The primers used to clone gI were 5′-CCCGAATTCTCATTTAATCGCGATGTT-3′ and 5′-CCCTCTAGATAATTAGTTCTATTTAAC-3′. The primers used to clone gE were 5′-CCCGGTACCGAGGGTCGCCTGTAATAT-3′ and 5′-CCCTCTAGATGCCCCGGTTCGGTGATCA-3′. The primers employed to produce truncated and mutated gI constructs are shown in Table 1. Chimeric cDNA constructs encoding chimeric proteins in which the transmembrane and endodomains of gI were fused to the extracellular domain of tac were prepared (10, 30). Essentially, the terminal primers amplified two overlapping DNA fragments and overlapping internal primers that encompassed the desired mutant. Gel-purified products of the first step of amplification were employed for a second round of PCR amplification in which only the terminal primers were used. The terminal 5′ tac primer was 5′-CCCGGTACCAAGGGTCAGGAAGATGGA-3′. This amplimer was paired with the upstream internal primer 5′-ATCATTAAGGGATGTTGTAAATATGGAGGT-3′, the downstream internal primer 5′-TTTACAACATCCCTTAATGATCCTCCA-3′, and an appropriate gI wild-type (gIwt) or mutant 3′ amplimer (Table 1). The authenticity of all constructs was verified by sequencing.
TABLE 1.
PCR amplimers used to prepare truncated and mutated gI constructs
| Construct | 3′ primer |
|---|---|
| gIwt | 5′-CCCTCTAGATAATTAGTTCTATTTAAC-3′ |
| gIδ1 | 5′-CCCTCTAGATACAACGGAATGTGGGGG-3′ |
| gIδ2 | 5′-CCCTCTAGATGGGGGGGATTCTTCGCG-3′ |
| gIδ3 | 5′-CCCTCTAGATTCTTCGCGAATCGTTGC-3′ |
| gIδ4 | 5′-CCCTCTAGATATTACAATAACAATAAC-3′ |
| gIδ5 | 5′-CCCTCTAGATTGTGCAATGGCGGCCTC-3′ |
| gIδ6 | 5′-CCCTCTAGATATGCCCCTTCTTGTTTT-3′ |
| gIδ7 | 5′-CCCTCTAGATATTACAATAACAATAAC-3′ |
| gIδ2KK | 5′-CCCTCTAGAGGGGGATTTTTTGCGAATCGT-3′ |
| gIδ3T→A | 5′-CCCTCTAGAGCGAATCGCTGCTAGTTGTGC-3′ |
| gIδ3T→S | 5′-CCCTCTAGAGCGAATCGATGCTAGTTGTGC-3′ |
| gIδ3T→D | 5′-CCCTCTAGAGCGAATGTCTGCTAGTTGTGC-3′ |
| gIδ3R→E | 5′-CCCTCTAGACTCAATCGTTGCTAGTTGTGC-3′ |
Transfection and culture of cells.
For transfection, cells were grown on glass coverslips in two- or eight-well chambers. Cells were transfected with cDNA constructs using Lipofectin (Life Technologies, GIBCO, Grand Island, N.Y.) according to the manufacturer's directions. Cells were incubated at 37°C in an atmosphere of 5% CO2. The medium was changed after 12 h, and incubation was continued for another 48 h. In most experiments the cells were then fixed with 2% formaldehyde (freshly prepared from paraformaldehyde) in phosphate-buffered saline (PBS) at pH 7.4 for 2 h at room temperature. In the remaining experiments, endocytosis of expressed proteins was studied. For this purpose, polyclonal antibodies to gI or gE (5 ng/ml) were added to cells that had been transfected with cDNA encoding gIwt or gEwt. Cells were incubated for 30 min in the presence of the added antibodies and were then fixed as described above.
Immunocytochemistry.
Fixed cells were washed with PBS and permeabilized with 0.1% Triton X-100 in PBS containing bovine serum albumin (BSA) (2.0 mg/ml). Primary antibodies were then applied for 2 h at room temperature. To visualize the sites of primary antibody binding, the cells were thoroughly washed, and appropriate affinity-purified secondary antibodies coupled to contrasting fluorophores were applied for 1 h at room temperature. In initial experiments, the secondary antibodies were goat anti-rabbit or anti-mouse immunoglobulin G labeled with fluorescein isothiocyanate (Kirkegaard & Perry, Gaithersburg, Md.) or cyanine-3 (Jackson Immunoresearch Laboratories, Inc., West Grove, Pa.). In subsequent studies, the secondary antibodies were coupled to Alexa fluor-488 or Alexa-594 (Molecular Probes Inc., Eugene, Oreg.), because of the brighter fluorescence of the newer fluorophores and the lack of overlap in their emission spectra. All secondary antibodies were applied in a dilution of 1:80 in PBS with BSA (2 mg/ml). Slides were mounted in glycerol-PBS (8:1) containing β-nitrophenol to minimize fading. When only proteins at the cell surface were examined by immunocytochemistry, cells were processed in the same way except that the permeabilization step was omitted. For the experiments on endocytosis of antibodies, fixed cells were permeabilized but probed only with an appropriate secondary antibody. The lipid N-(ɛ-7-nitrobenz-2-oxa-1,3-diazo-4-yl-aminocaproyl)-d-erythro-sphingosine (C6-NBD-ceramide) was used as a marker to locate the TGN (23). The TGN was also marked in transfected cells by its ability to recruit the adapter complex AP-1, which was visualized with antibodies to γ-adaptin (see above) (2, 29).
RESULTS
A signal in its endodomain is sufficient to target gI to the TGN.
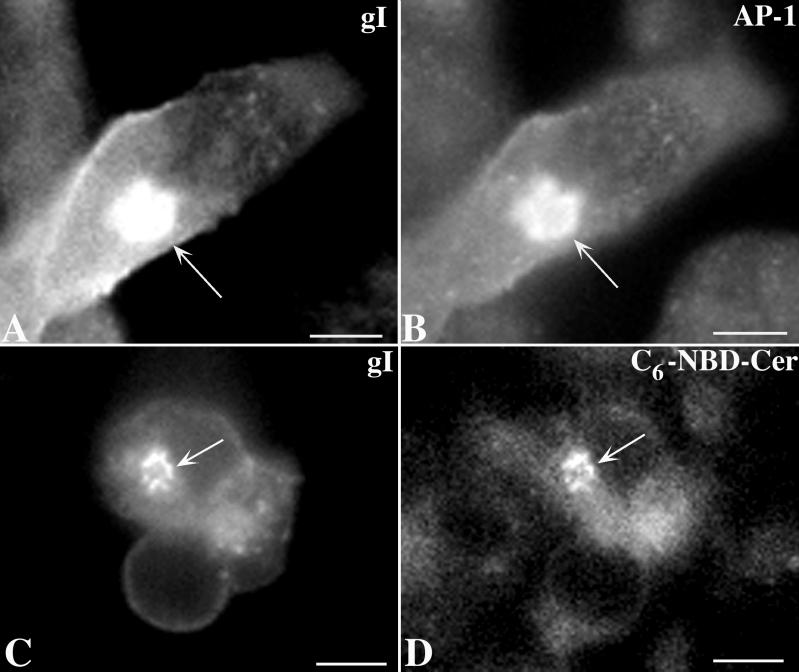
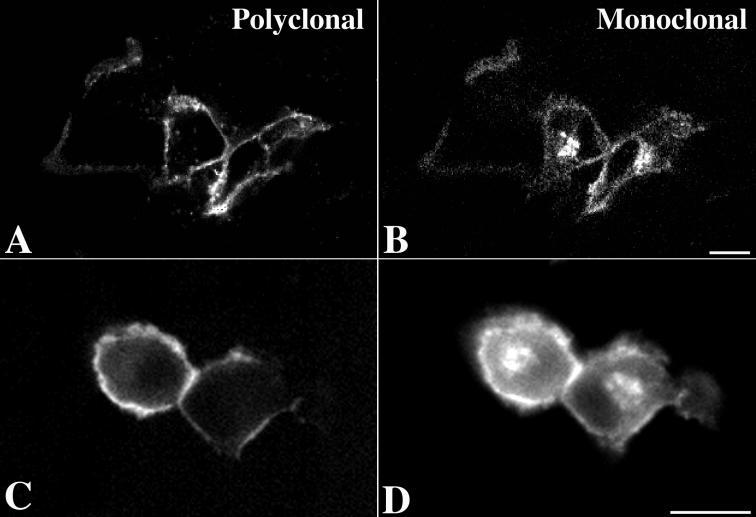
Constructs encoding gIwt or truncated mutant forms of gI lacking all or part of its endodomain (Fig. 2, Table 1) were transfected into Cos-7 cells. The expressed proteins were then localized immunocytochemically using polyclonal and/or monoclonal antibodies to gI. The TGN was identified by simultaneously localizing the immunoreactivity of γ-adaptin (AP-1) (2, 29) and intracellular sites of concentration of C6-NBD-ceramide (23). Expressed gI (Fig. 3A and C) was found to be transported to the plasma membrane but also to be concentrated in the TGN, where it colocalized with γ-adaptin immunoreactivity (Fig. 3B) and with C6-NBD-ceramide (Fig. 3D).
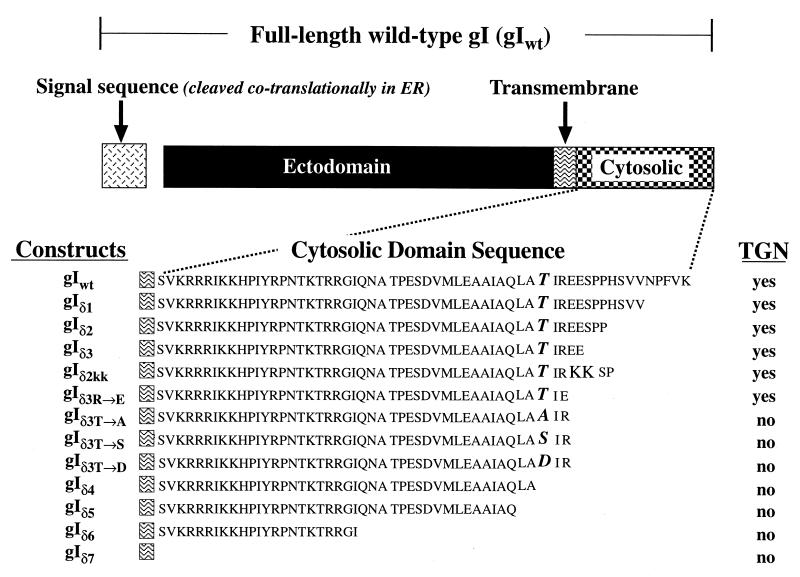
FIG. 2.
Domains of the full-length gIwt and mutations made at sites within the sequence of the putative endodomain of gI. The nomenclature used to refer to each construct is shown to the left of its sequence. The sequence of the putative endodomain is shown beginning at the deduced transmembrane region. Also shown (to the right of each construct), is whether or not the corresponding expressed protein was found to be concentrated in the TGN of transfected cells. In addition to the transport of these membrane-anchored forms of gI, a truncated soluble protein, consisting only of the gI ectodomain (not illustrated), was also expressed in transfected cells. This protein was found in the TGN due to secretion and reuptake into endosomes (see text).
FIG. 3.
Immunoreactivity of gI colocalizes with TGN markers in transfected cells. (A and B) The immunoreactivity of gI (A) is coincident with that of γ-adaptin, used as a marker for the AP-1 adapter complex. (C and D) The immunoreactivity of gI (C) is coincident with the fluorescence of C6-NBD-ceramide, a lipid fluorophore that concentrates in membranes of the TGN (8, 23). Coincident concentration of gI immunoreactivity with the TGN markers in the TGN region of the transfected cells is indicated by the arrows. Bars, 10 μm.
To test the hypothesis that gI is targeted to the TGN because of a signal in its endodomain, the targeting of expressed protein was analyzed in cells that had been transfected with constructs encoding truncated mutants of gI (Fig. 2, Table 1). Again, gIwt was found to be targeted to the TGN (Fig. 4A); however, when the endodomain of gI was entirely lacking, the expressed protein, gIδ7, did not reach the TGN (Fig. 4K). Instead, the pattern of immunoreactivity suggested that gIδ7 remained in the RER. All of the mutant constructs that, like gIwt, gave rise to expressed proteins that were targeted to the TGN, gIδ1 (Fig. 4B), gIδ2 (Fig. 4C), gIδ3 (Fig. 4D), gIδKK (Fig. 4E), and gIδ3R→E (Fig. 4F), had in common the presence of T338.
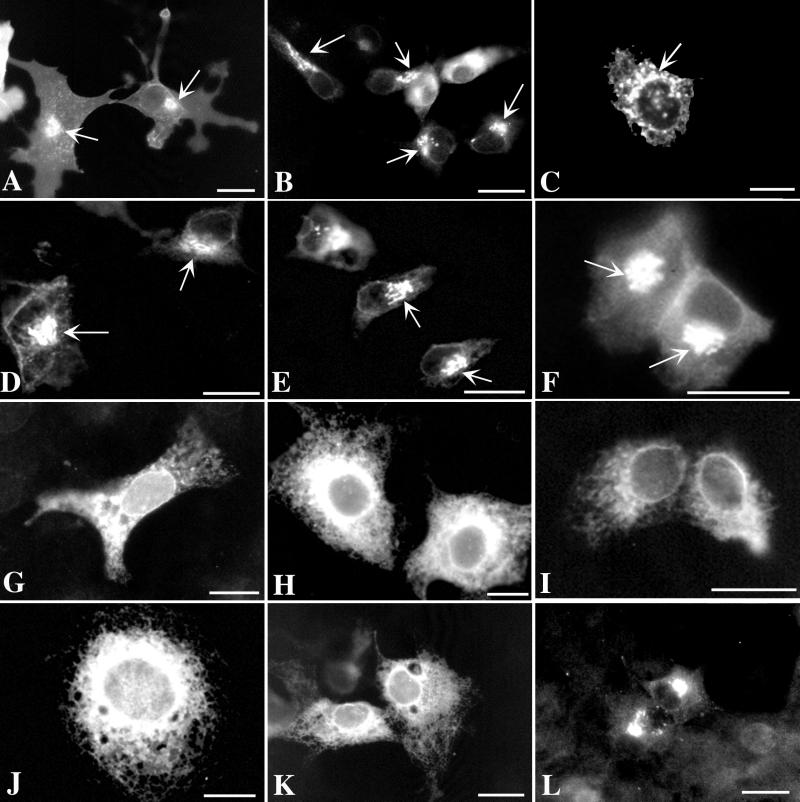
FIG. 4.
Signal in the endodomain of gI encompassing T338 is necessary for targeting expressed protein to the TGN. The nomenclature and corresponding sequences of the gI mutant constructs used to transfect Cos-7 cells are depicted in Fig. 2. The arrows indicate fluorescence in the TGN region of transfected cells. (A) Expressed gIwt is concentrated in the TGN and plasma membrane. (B) Expressed gIδ1 (lacking the five C-terminal amino acids of gIwt) is still targeted to the TGN and plasma membrane. (C and D) Expressed gIδ2 (lacking the nine C-terminal amino acids of gIwt) and gIδ3 (lacking the 12 C-terminal amino acids of gIwt) are still targeted to the TGN and plasma membrane. (E) Expressed gIδ2KK (E341 to K341 and E342 to K342) is targeted to the TGN and plasma membrane. This mutation destroys a consensus casein kinase II site. (F) Expressed gIδR→E (R340 to E340; also lacking the 12 C-terminal amino acids of gIwt) is targeted to the TGN and plasma membrane. R340 is thus not a component of a TGN targeting signal. (G) Expressed gIδ3t→A (T338 to A338; also lacking the 12 C-terminal amino acids of gIwt) is not targeted to the TGN or plasma membrane. The pattern of immunoreactivity suggests that the expressed gIδ3t→A may be retained in the RER. The same results are obtained when T338 is mutated to S338 or D338 (not illustrated). T338 is thus required for TGN targeting. (H to K) Expressed gIδ4, gIδ5, gIδ6, and gIδ7, respectively (which lack, respectively, the 17, 19, 36, and 59 [entire endodomain] C-terminal amino acids of gIwt and thus the critical T338), are not targeted to the TGN or plasma membrane. Note that gIδ5 contains all of the amino acids N-terminal to T338. (L) Expressed gIδcd+tm is concentrated in the TGN. Note that gIδcd+tm differs from gIδ7 (K), which lacks the endodomain of gIwt, in that the transmembrane domain of gIwt is also deleted in gIδcd+tm. Bars, 10 μm.
The fact that mutation of E341 and E342 to K341 (Fig. 4E) did not affect sorting to the TGN suggests that acidic amino acids at these locations are not part of a TGN targeting sequence. Similarly, the lack of effect on TGN targeting of mutating R340 to E340 (Fig. 4F) suggests that a basic amino acid is not required at this site. In contrast, the mutation of T338 to A338, S338, or D338 eliminated TGN targeting and resulted in apparent retention of the expressed protein in the RER (Fig. 4G). These observations imply that T338 is critical for the targeting of gI to the TGN. The E341 and E342 to K341 and K342 mutation eliminates a consensus site for phosphorylation by casein kinase II (X-S/TP-X-X-E-X). If TGN targeting of gI requires the phosphorylation of T338, casein kinase II is probably not the responsible enzyme. Mutations of T338 to S338 and D338 were generated to test the possibility that phosphorylation of T338 by any serine/threonine protein kinase contributes to TGN targeting. An asparagine residue often behaves as if it were a constitutively phosphorylated threonine (5, 33). The failure of S338 and D338 to substitute for T338 thus does not suggest that the phosphorylation of T338 by a serine/threonine protein kinase plays a role in the targeting of gI to the TGN.
A gI mutant lacking both cytosolic and membrane domains is concentrated in the TGN and endosomes and is secreted.
Surprisingly, when both the transmembrane and endodomains of gI (gIδcd+tm) were deleted, the expressed protein was again concentrated in the Golgi region of the cells or the TGN (Fig. 4L). This observation was unexpected, because deletion of the endodomain of gI abolished TGN targeting when the transmembrane domain was retained. In addition to the Golgi/TGN, expressed gIδcd+tm protein was also found in small cytosolic vesicles (Fig. 4L and 4A), which were not seen in cells transfected with constructs encoding either gIwt or any of the other mutants that contained a transmembrane domain but lacked elements of the endodomain.
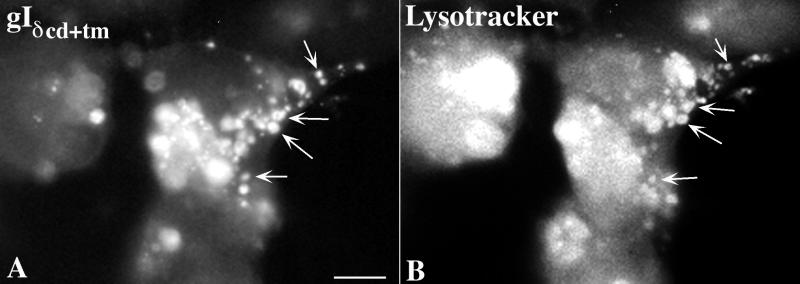
Since the small vesicles that contained gI immunoreactivity in cells transfected with cDNA encoding gIδcd+tm resembled endosomes, experiments were done to test the hypothesis that gIδcd+tm is partially sorted to endosomes. Cells that were transfected with constructs encoding gIδcd+tm were incubated for 30 min with an endosomal tracer, Lysotracker, prior to fixation. Lysotracker, which is fluorescent, is taken up by endocytosis and thus marks the endocytic compartment of cells that have taken it up. The 30-min time of incubation is not sufficient for Lysotracker to be transferred from endosomes to lysosomes. Following fixation of the cells, the immunoreactivity of gI was demonstrated simultaneously with the fluorescence of Lysotracker. A partially coincident localization of gI immunoreactivity (Fig. 5A) with Lysotracker (Fig. 5B) was observed, confirming that a subpopulation of endosomes contains gIδcd+tm. These observations indicate that gIδcd+tm is sorted to endosomes as well as to the TGN.
FIG. 5.
Subset of the cytoplasmic vesicles of transfected cells that contain gIδcd+tm are endosomes. Transfected cells were incubated with the fluorescent endosomal tracer Lysotracker in order to determine whether the expressed mutant construct enters the endosomal pathway. A coincident localization of gI immunoreactivity and Lysotracker fluorescence was found in many of the cytoplasmic vesicles of transfected cells expressing gIδcd+tm (arrows). These observations suggest that gIδcd+tm gains access to the endosomal pathway. Bars, 10 μm.
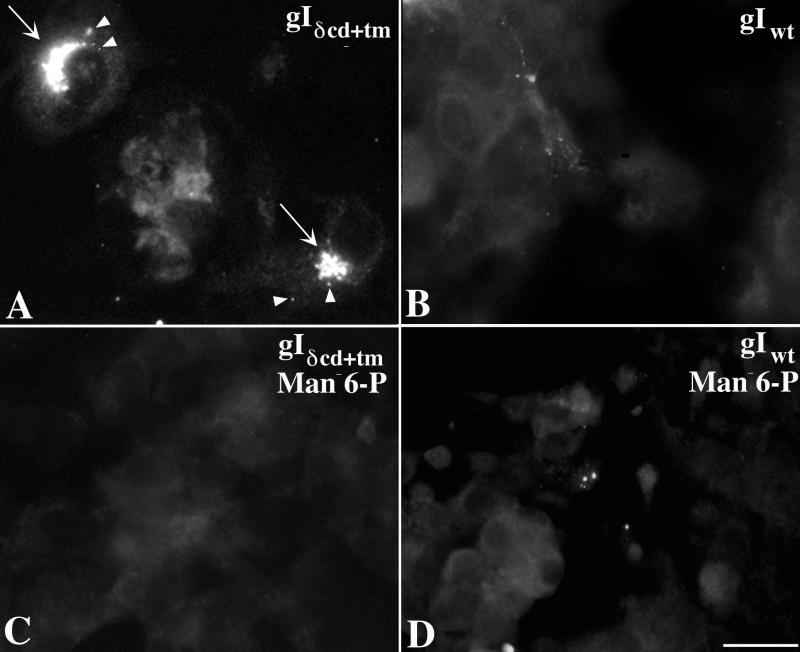
Since gIδcd+tm contains a signal sequence but no transmembrane domain, the protein would be expected to be translocated to the lumen of the RER. A similar truncated form of gE is secreted (45). The appearance of gIδcd+tm in endosomes could be explained if gIδcd+tm were similarly to be secreted and then taken up by endocytosis. The protein could then be transported to the TGN within endocytic vesicles. To determine whether this postulated process occurs, medium conditioned by the growth of Cos-7 cells expressing gIδcd+tm was collected and transferred to fresh cultures of nontransfected Cos-7 cells. As a control, conditioned medium was also collected from cultures of cells expressing gIwt and similarly transferred to cultures of nontransfected cells. The transferred medium was rendered cell-free prior to transfer by centrifugation at 1,000 × g and passage through a 0.2-μm filter. gI immunoreactivity was found in a Golgi pattern and small vesicles in nontransfected Cos-7 cells exposed to medium from cells transfected with gIδcd+tm (Fig. 6A), but no such immunoreactivity appeared in cells exposed to medium from cells transfected with gIwt (Fig. 6B). These observations suggest that transfected cells that express gIδcd+tm secrete the protein into the medium and that secreted gIδcd+tm can be taken up by nontransfected cells.
FIG. 6.
Expressed gIδcd+tm is secreted and taken up by a Man 6-P-dependent mechanism. Cells were transfected with either gIδcd+tm or gIwt (control). Medium was collected, rendered cell-free, and passed to recipient cultures of nontransfected Cos-7 cells. (A) The immunofluorescence of gI is concentrated in some cytoplasmic vesicles and in the TGN (arrows) of recipient cells exposed to medium conditioned by cells transfected with gIδcd+tm. (B) No gI immunofluorescence is found in the TGN of recipient cells exposed to medium conditioned by cells transfected with gIwt. (C) Addition of Man 6-P (20 μM) to the medium prevents the appearance of gI immunofluorescence in recipient cells exposed to medium conditioned by cells transfected with gIδcd+tm. (D) Addition of Man 6-P (20 μM) to the medium does not alter the appearance of recipient cells exposed to medium conditioned by cells transfected with gIwt. There is no recognizable gI immunofluorescence. Bars, 10 μm.
The ectodomains of gI and other VZV gps are known to contain Man 6-P groups (7). Lysosomal enzymes and other proteins that contain Man 6-P groups are known to be taken up by receptor-mediated endocytosis following their binding to plasmalemmal MPRs (37, 44). Since uptake mediated by an MPR would be inhibited by Man 6-P, the ability of Man 6-P to antagonize the uptake of gIδcd+tm was explored. Addition of exogenous Man 6-P (20 mM) completely prevented the uptake of gIδcd+tm from the transferred conditioned medium (Fig. 6C). Man 6-P (20 mM) did not affect the appearance of cells exposed to medium from cells expressing gIwt (Fig. 6D). These observations support the idea that cell surface MPRs mediate the uptake of secreted gIδcd+tm.
A fusion protein containing the transmembrane and endodomains of gI and the ectodomain of tac is targeted to the TGN.
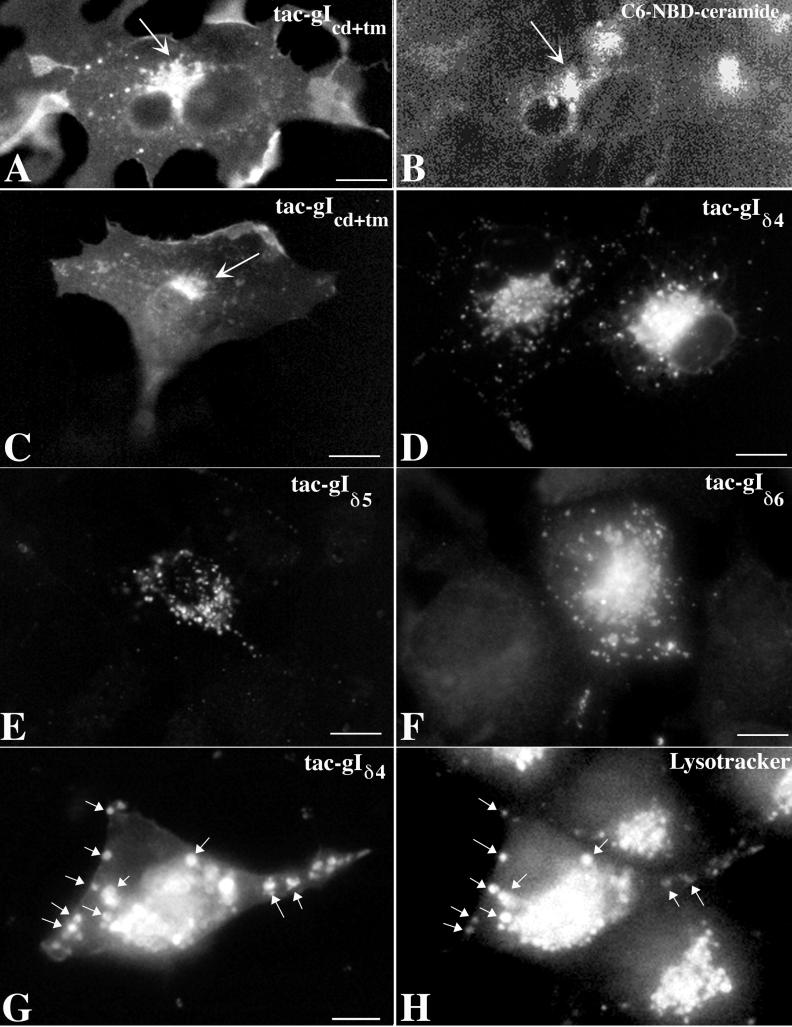
To confirm whether the endodomain of gI contains TGN targeting information, chimeric proteins were constructed in which the ectodomain of tac was fused to the transmembrane and endodomains of gI (tac-gIcd+tm). Cos-7 cells that are transfected with cDNA encoding the full-length tac target the expressed protein only to the plasma membrane (45, 46). Cos-7 cells transfected with the tac-gIcd+tm construct were found to target the expressed protein to both the plasma membrane and the TGN (Fig. 7A and C). The location of the TGN marker C6-NBD-ceramide (Fig. 7B) coincided with that of the protein expressed within the same cells transfected with the tac-gIcd+tm construct (Fig. 7A), confirming that the tac-gIcd+tm fusion protein is targeted to the TGN. The localization of tac-gIcd+tm is thus essentially the same as that seen in cells that express the full-length gIwt (compare Fig. 3A and C with Fig. 4A).
FIG. 7.
Signal in the endodomain of gI can target the tac ectodomain to the TGN. Constructs were prepared encoding proteins in which the ectodomain of tac was fused to the cytosolic and transmembrane domains of gI. In some of these constructs the endodomain of gI was mutated. These mutations were designed to correspond to those shown in Fig. 2. The location of the expressed fusion proteins in transfected cells, demonstrated by tac immunofluorescence, can be compared to the gI immunoreactivity of the corresponding gI mutants shown in Fig. 4. (A and B) The immunoreactivity of tac is coincident with the fluorescence of the TGN marker C6-NBD-ceramide in cells expressing tac-gIcd+tm. The arrows indicate coincident concentration of tac immunoreactivity with C6-NBD-ceramide in the transfected cells. (C) Expressed tac-gIcd+tm is concentrated in the TGN (arrow). (D to F) Expressed tac-gIδ4 (D), tac-gIδ5 (E), and tac-gIδ6 (F) (which lack, respectively, the 17, 19, and 36 C-terminal amino acids of the endodomain of gIwt and thus the critical T338) are concentrated in cytoplasmic vesicles but not in the TGN. Although these vesicles are often numerous in the Golgi region of transfected cells (D), the immunofluorescence is not distinctly localized to the TGN itself (compare D [vesicles] with C [TGN]). (G and H) Transfected cells were incubated with Lysotracker in order to determine whether the expressed mutant construct enters the endosomal pathway. A coincident localization of tac immunoreactivity and Lysotracker fluorescence was found in many of the cytoplasmic vesicles of transfected cells expressing tac-gIδ4 (arrows). Similar results were obtained with tac-gIδ5 and tac-gIδ6 (not illustrated). These observations suggest that chimeric proteins in which the tac ectodomain is fused to the transmembrane and elements of the endodomain of gI that lack T338 gain access to the endosomal pathway. Bars, 10 μm.
Mutations were made in the endodomains of the tac-gIcd+tm construct so that the resulting mutations corresponded to those in the endodomain of gI illustrated in Fig. 2 that were used to investigate gI sorting. Mutations that eliminated the threonine corresponding to T338 of gI all abolished the TGN targeting of the tac-gI proteins expressed by transfected cells (Fig. 7D to F). The expressed chimeric proteins, however, did not remain in the RER, as did the gI mutants. Instead, the chimeric proteins lacking T338 became concentrated in large cytoplasmic vacuoles. This localization is identical to that of fusion proteins expressed by cells transfected with constructs encoding the ectodomain of tac fused to the endodomain of gE from which the TGN targeting signal was deleted (46). Thus, constructs that contain the cytosolic domain of gI, which lack T338, are not targeted to the TGN even if they escape from the RER.
Lysotracker was used to determine whether the vacuoles containing the expressed tac-gI proteins lacking T338 were endosomes (Fig. 7G and H). Coincident localization was found in vesicles between the fluorescence of Lysotracker and that due to tac immunoreactivity. This observation suggests that gI-tac chimeric proteins lacking T338 are sorted to endosomes. The inclusion of T338 evidently prevents the chimeric proteins from leaving the TGN in endosomes.
Plasmalemmal gI does not recycle to the TGN.
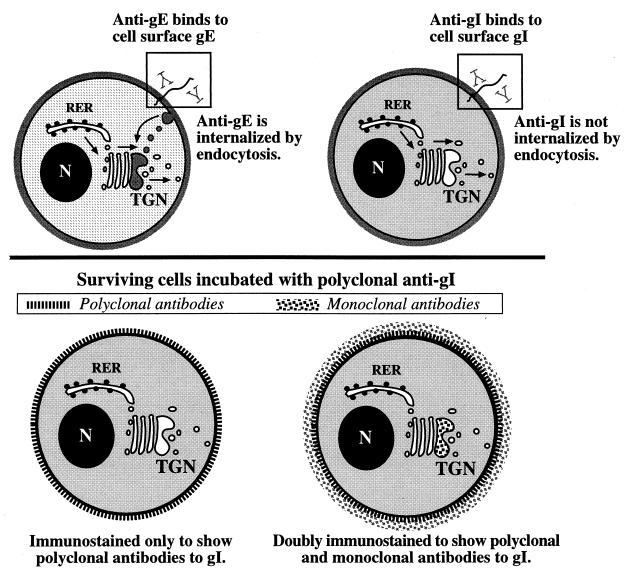
The targeting of gE to the TGN involves its endocytotic retrieval from the plasma membrane and subsequent transport in endosomes (2, 45) (Fig. 8). This transport depends on a tyrosine-containing sequence (AYRV) and a patch of acidic amino acids containing a casein kinase II phosphorylation site in the endodomain of gE. Identical determinants cannot be recognized in the endodomain of gI. Experiments were thus carried out to compare the transport of gI to the TGN with that of gE. Transfected cells expressing gI were incubated, while alive, with polyclonal antibodies to gI. These antibodies can bind to the exposed extracellular domains of plasmalemmal gI, but the antibodies cannot permeate the plasma membrane. Internalization of the polyclonal antibodies to gI thus requires uptake by endocytosis. The incubated cells were fixed, permeabilized, and immunostained with monoclonal antibodies to gI. In contrast to the polyclonal antibodies to gI, which had access only to gI at the cell surface, the monoclonal antibodies, which were applied to cells after fixation and permeabilization, had access to the entire universe of gI molecules within the cells. By determining the location of the polyclonal antibodies following incubation, the endocytic uptake of gI from the cell surface could be analyzed (Fig. 8). Double labeling with species-specific secondary antibodies enabled gI molecules labeled with polyclonal and monoclonal antibodies to be distinguished from one another. Examination of immunofluorescence by both standard and laser-scanning confocal microscopy ensured that internal and plasmalemmal immunoreactivity could be distinguished (Fig. 9). The design of the experiments thus made it possible to determine whether a proportion of the gI molecules at any given site of gI concentration within the cells were exposed at the cell surface during the period of incubation with polyclonal antibodies to gI.
FIG. 8.
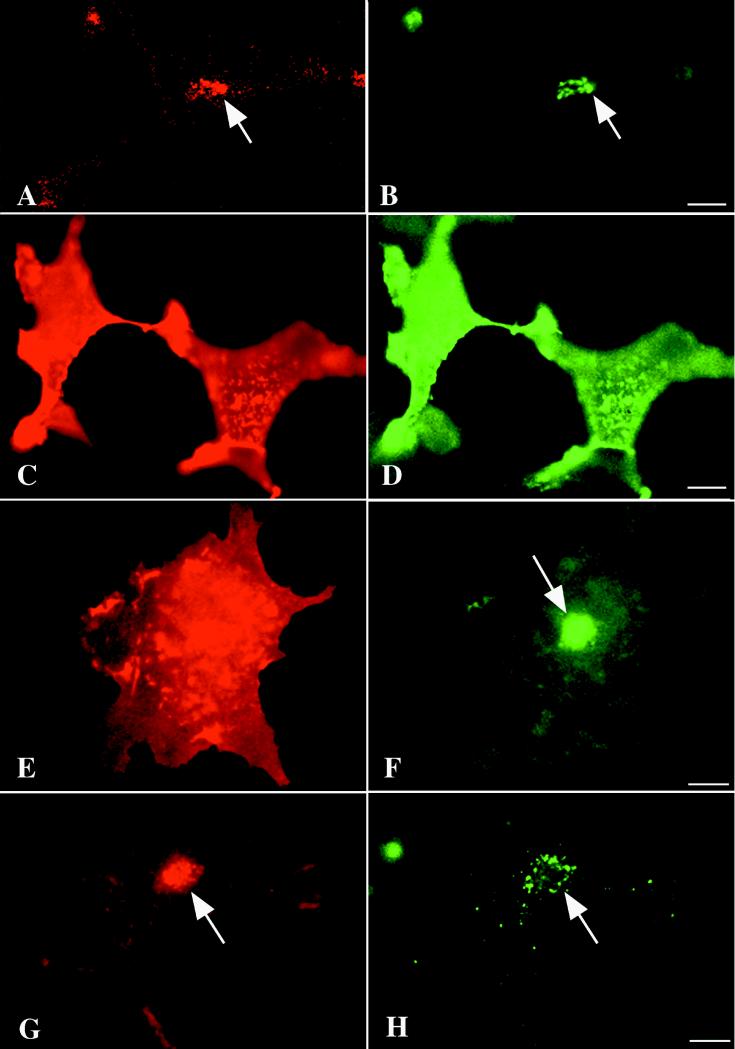
Diagram depicting the labeling of expressed gE and gI at the surface of living cells with antibodies in order to test the hypothesis that their concentration in the TGN depends on retrieval from the plasma membrane. (Top) Transfected cells expressing gE (left) or gI (right) are incubated while alive with antibodies that react with the ectodomains of the molecules. Antibodies to gE are internalized, while antibodies to gI are not. The internalized gE is routed to the TGN. In contrast, no antibodies to gI are found in the TGN of transfected cells. (Bottom) Surviving cells are incubated with polyclonal antibodies to gI to label the gp at the cell surface. Following fixation and permeabilization, however, the cells are doubly immunostained with monoclonal antibodies to gI. Species-specific secondary antibodies labeled with contrasting fluorophores thus make it possible to recognize sites reached by molecules of gI that were at the cell surface during the period of incubation with polyclonal antibodies from the total universe of intracellular gI. Polyclonal antibodies to gI label only molecules at the cell surface. The concentration of gI within the TGN is labeled only with monoclonal antibodies. These experiments reveal that expressed gE and gI both reach the cell surface but that gE is retrieved by endocytosis while gI is not. Despite the failure to be retrieved from the plasma membrane, molecules of gI still concentrate in the TGN. These molecules of gI do not recycle to the cell surface. Micrographs illustrating these observations are shown in Fig. 9.
FIG. 9.
Expressed gI is found at the cell surface and in the TGN but does not reach the TGN by retrieval from the plasma membrane. (A and C) When surviving cells that express gI are incubated with polyclonal antibodies that react with gI, the immunoreactivity is not internalized but remains at the cell surface. The cell surface restriction of immunoreactivity is apparent in both optical sections through the cells by laser-scanning confocal microscopy (A) and in images of whole cells examined by conventional vertical fluorescence microscopy (C). (B and D) Examination of fixed and permeabilized cells with monoclonal antibodies that react with gI reveals that gI is present in the TGN as well as at the cell surface. The gI in the TGN evidently does not cycle to and from the plasma membrane. The presence of gI immunoreactivity in the TGN is often made clear when cells are optically sectioned by laser-scanning confocal microscopy (B), which readily distinguishes internal fluorescence from signal originating at the cell surface. Still, unless cells are very flat, the fluorescence of the gI immunoreactivity in the TGN can be distinguished through that of the overlying cell surface (D). The same cells are imaged to show polyclonal immunoreactivity in A and B and monoclonal immunoreactivity in C and D. Bars, 10 μm.
Labeling of gI by polyclonal antibodies was seen only at the cell surface (Fig. 8A and C). The polyclonal antibodies to gI were thus not internalized to a significant degree within the 30-min period of incubation. In contrast, after fixation and permeabilization, monoclonal antibodies were found to label gI both at the cell surface and at internal sites (Fig. 8B and D). The cytoplasmic immunostaining was concentrated in the TGN, within which labeling by the polyclonal antibodies was never detected. These observations indicate that although gI is targeted to the TGN, it is not routed to this structure by retrieval from the plasma membrane. The targeting mechanism that directs gI to the TGN must therefore be different from that which directs gE (Fig. 8), which does reach the TGN following retrieval from the plasma membrane (2, 45).
Complex results were obtained when gE and gI were expressed simultaneously in the same cell following cotransfection of the respective cDNAs. Again, cells were preincubated with polyclonal antibodies to gI, but in this case, after fixation and permeabilization they were immunostained with monoclonal antibodies to gE. Three different outcomes were observed. In 27% (16 of 60) of cotransfected cells, there was a coincident localization in the TGN of polyclonal antibodies to gI with gE immunoreactivity (Fig. 10A and B). These cells evidently retrieved gI together with gE from the plasma membrane and targeted the gI-gE complex, like gE, to the TGN. In 18% (11 of 60) of cotransfected cells, neither gI nor gE immunoreactivity was found in the TGN, but both were intense at the plasma membrane (Fig. 10C and D). These data suggest that the gI-gE complex can be targeted in some cells more like gI than like gE. IN the third and most common, 55% (33 of 60), type of cell, gI immunoreactivity remained localized at the plasma membrane, but gE immunoreactivity was prominent in the TGN (Fig. 10E and F). In these cells, a gI-gE complex either did not form or was split, enabling the two gps to be targeted independently. As a control, cells were incubated for 30 min with rabbit polyclonal antibodies directed against gE instead of gI and subsequently immunostained after fixation and permeabilization with monoclonal antibodies to gI. When this was done, gI immunoreactivity was found in the TGN, where it colocalized with internalized anti-gE (Fig. 10G and H). Plasma membrane immunostaining of gI, which varies in intensity, is apparent in these cells; very little anti-gE can be detected on the plasma membrane.
FIG. 10.
Intracellular trafficking of gI and gE may be influenced by the co-expressed protein in co-transfected cells. In order to follow the trafficking of gps from the cell surface, cotransfected cells were incubated while alive with antibodies to gI. The cells were then fixed, permeabilized, and immunostained to demonstrate gE. This experiment thus locates the entire universe of gE but only the gI that was exposed at the cell surface during the time the surviving cells were incubated with antibodies to gI. Three outcomes were observed. (A and B) Surface-labeled gI (A) is colocalized with gE (B) in the TGN. The coexpression of gE has thus led to the internalization of gI and its retrieval to the TGN (compare with the trafficking of gI expressed by itself in Fig. 9). (C and D) Both surface-labeled gI (C) and gE immunoreactivities are confined to the cell surface. There is no apparent internalization of gE and no labeling of either gp in the TGN. The coexpressed gI evidently interfered with the internalization and trafficking of gE to the TGN. (E and F) Surface-labeled gI remains at the cell surface (E), but gE is concentrated in the TGN (F). Despite the known ability of gI and gE to form a complex, the two gps evidently can, in some cotransfected cells, traffic independently. Note the resemblance of the localization of surface-labeled gI in these cotransfected cells to that of surface-labeled gI in transfected cells that express only this gp (Fig. 9). Bars, 10 μm.
DISCUSSION
Observations made in the current study suggest that Cos-7 cells target gI to the TGN as well as to the cell surface. Thus, the intracellular location of expressed gIwt was found to be coincident with that of the TGN markers AP-1 and C6-NBD-ceramide and was also identical to that of expressed gE, which is known to be targeted to the TGN (2, 45, 46). The information that causes gI to be targeted to the TGN must therefore reside in a sequence within its primary structure, since the transfected cells express no other viral proteins. HeLa cells, which do not sort gI to the TGN (1), may lack the ability to either recognize or respond to the TGN signaling domain of gI. Three types of evidence suggested that the endodomain is the region that contains the targeting information that directs gI to the TGN when gI is expressed as an integral membrane protein. (i) When the gI endodomain was deleted, intact transmembrane and ectodomains were not able to direct expressed protein to the TGN. (ii) Chimeric proteins consisting of the tac ectodomain fused to the transmembrane and endodomains of gI were targeted to the TGN. (iii) Specific mutations of the endodomains of gI or tac-gI chimeric constructs prevented expressed proteins from being sorted to the TGN.
The mechanism by which Cos-7 cells target expressed gI to the TGN was found to be substantially different from that used by these cells to target expressed gE to the same organelle. The endodomain of gI contains neither the tyrosine-based sequence nor the patch rich in acidic amino acids in the endodomain that target gE to the TGN (2, 46). An analogous tyrosine-based targeting signal and an acidic cluster of amino acids are also critical in the trafficking to the TGN of the resident proteins TGN38 (3, 11, 25–27, 38, 40) and furin (12, 28, 32, 35). Analysis of mutants revealed that gI constructs that lacked T338 were not targeted to the TGN and the TGN targeting of gI constructs was lost when T338 was mutated to A338, S338, or D338. Instead of the tyrosine-based signal and cluster of acidic amino acids that target gE, TGN38, and furin to the TGN, the TGN targeting of gI thus depends on the T338 residue of its endodomain.
The observation that a threonine residue is critical in the targeting of gI to the TGN raises the possibility that the responsible signal involves the phosphorylation of T338 by a serine/threonine protein kinase. This possibility, however, is not supported by the observations that neither S nor D is able to substitute for T. S would be expected to be phosphorylated by a serine/threonine protein kinase, and D often mimics a constitutively phosphorylated T (5, 33). In addition, the substitution of K341 K342 for E341 E342, which eliminates a casein kinase II consensus site in the endodomain of gI that might cause T338 to be phosphorylated, fails to affect the targeting of gI constructs to the TGN. If the viral serine/threonine protein kinases (ORF47 and ORF66) phosphorylate serine as well as threonine, then the failure of S and D to substitute for T would also argue against the participation of viral serine/threonine protein kinases in the TGN targeting of gI. Since the basic R340 can also be mutated to an acidic E340, T338 is unlikely to be a component of a signal sequence that extends beyond this residue toward the C-terminal end of the molecule. The efficient trafficking of the resident protein TGN38 from endosomes to the TGN requires a free hydroxyl group in the amino acid at position 331 of its endodomain, which, in contrast to the critical T338 of gI, can be provided by either S or T (27). As is also true of the T338 of gI, the critical S/T331 of TGN38 cannot be replaced by D. The observation that an S/T residue is involved in its targeting has been taken to support the idea that TGN38 undergoes a signal-mediated step in its passage from endosomes to the TGN. TGN38 (3, 11, 25–27, 38, 40), furin (28, 32, 35), and gE (1, 22, 46) are all targeted to the TGN following their endocytic retrieval from the plasma membrane. T/S331 is important in the intracellular trafficking of TGN38 because it is necessary for the linkage of TGN38 because to the actin cytoskeleton via neurabin F-actin binding proteins (31). Neither previous studies in HeLa cells (1, 19) nor the current observations, however, suggest that the endocytic retrieval of gI, in the absence of coexpressed gE, directs membrane-anchored gI to the TGN. There is thus reason to suspect that mechanisms analogous to those that regulate the trafficking of TGN38, furin, or gE from endosomes to the TGN might not be operative in the TGN targeting of gI.
In contrast to gE, expressed gI, labeled in living cells at the cell surface with antibodies, did not appear in the TGN. In some cells, the coexpression of gE and gI was found to cause surface-labeled gI to be retrieved to the TGN. This phenomenon, which has also been observed in prior studies (1, 19), is likely to be due to the formation of a gE-gI complex. Because it is bound to gE, the surface-labeled gI is internalized with it and follows the internal itinerary of its gE partner. In other cells, however, both gE and the surface-labeled gI remained at the plasma membrane; neither appeared in the TGN. This phenomenon is also consistent with the formation of a gE-gI complex. The internalization of gE may be prevented by its binding to gI, which is retained in the plasma membrane. In a final group of cells gE was targeted normally to the TGN, while in the same cells, surface-labeled gI remained in the plasma membrane; the gE-gI complex thus either did not form or was not stable, enabling the two gps to traffic independently.
The TGN is a region of the cell where sorting of membrane and soluble proteins into different transport vesicles normally occurs (34). These proteins leave the TGN in a constitutive pathway of vesicles bound for the cell surface, in a regulated pathway of secretory vesicles, and in vesicles of the endosomal pathway. Proteins that contain Man 6-P residues bind to MPRs and become diverted to the endosomal vesicles, which are clathrin-coated as they form in the TGN (16). TGN38, furin, and gE therefore have to be sorted in the TGN to vesicles of the constitutive pathway for delivery and insertion into the plasma membrane before these proteins can be internalized by endocytosis and retrieved by the TGN from endosomes. An alternative mechanism by which gI might become concentrated in the TGN without retrieval from the plasma membrane would be to prevent a portion of the newly synthesized gI that enters the TGN from the Golgi stacks from exiting the TGN via the constitutive pathway (24). Such a TGN retention mechanism has indeed been found to occur in targeting a resident protein (vacuolar alkaline phosphatase) to the TGN of the yeast Saccharomyces cerevisiae (4). In this case, one signal slows the rate of exit from the TGN and a second targeting sequence directs retrieval from a post-Golgi compartment. Since gIwt is not retrieved from the plasma membrane when it is expressed alone, it seems likely that the TGN targeting of gIwt is due to its retention in the organelle, a process for which T338 is evidently essential. In view of the fact that a gE-gI complex forms in the RER of infected cells, the retention signal of gI and the retrieval signal of gE probably function synergistically. The presence of T338 in gI may help to retain the gE-gI complex in the TGN, while the tyrosine-based motif and acidic amino acids in gE help to retrieve the complex from the plasma membrane. The synergism would be expected to ensure that both gps are well concentrated in the TGN, enabling proper viral envelopment to take place. Similarly, the presence of gI in the gE-gI complex may slow the internalization of gE at the plasma membrane so that gE and gI can function in mediating cell-cell fusion (18) and the display of Fc receptors (17, 19–22, 43).
Conversion of gI from a membrane to a soluble protein by eliminating its transmembrane domain had a surprising effect on the intracellular localization of the expressed protein. An analogous truncated form of gE, which lacks transmembrane and endodomains, is also partially secreted, but mainly remains in the RER (45). Mutant forms of gI that lacked either the entire endodomain or its T338 residue also appeared to be retained in the RER. The further elimination of the transmembrane domain of gI, however, caused the mutant proteins to concentrate in the TGN region of transfected cells and to reach cytoplasmic vesicles, which were found (with the probe Lysotracker) to be endosomes. The ectodomain of gI thus appears better equipped than that of gE to be transported out of the lumen of the RER to reach the Golgi apparatus and TGN. Within the Golgi/TGN, the soluble ectodomain of gI could, in theory, be sorted either to secretory vesicles (of constitutive or regulated pathways) or to endosomes. Transport to endosomes might cause the expressed gI ectodomain to concentrate in the TGN region because of the cycling of endosomal vesicles to and from the TGN. Alternatively, the gI ectodomain could reach endosomes if it were secreted and subsequently taken up by endocytosis. Since the ectodomain of gI contains Man 6-P groups (7), its diversion within the TGN to the endosomal pathway (15, 16, 34) and its uptake by receptor-mediated endocytosis (37, 44) would both be anticipated results. The observations that gI immunoreactivity appeared in the TGN of nontransfected cells exposed to cell-free medium conditioned by cells expressing the gI ectodomain and that concentration was antagonized by adding Man 6-P to the medium support the idea that soluble gI is secreted and taken up by endocytosis. Exogenous Man 6-P would not be expected to enter cells and interfere with the sorting of molecules within the TGN. In any case, these observations indicate that T338 and the endodomain of gI are only necessary for the TGN targeting of gI when it is an integral membrane protein.
The efficacy of Man 6-P in preventing the uptake of the secreted ectodomain of gI is analogous to the similar efficacy of Man 6-P in preventing infection of target cells by cell-free VZV (7, 8). When a virion becomes attached to the plasma membrane of a target cell, the ectodomains of the gps in its envelope are apposed to receptors in the target cell's plasma membrane. As a result, Man 6-P groups, which are found in the ectodomains of VZV gps, including gI (7, 8), are positioned so that they can, potentially, interact with MPRs. It is therefore possible that Man 6-P is able to prevent both the uptake of the secreted gI ectodomain and the infection of target cells because it competes with the Man 6-P groups of the ectodomains of virions for access to MPRs of the plasma membrane. This hypothesis is supported by the current observations but remains to be confirmed.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI 12718.
We thank Saul Silverstein for helpful comments on the manuscript.
REFERENCES
- 1.Alconada A, Bauer U, Baudoux L, Piette J, Hoflack B. Intracellular transport of the glycoproteins gE and gI of the varicella-zoster virus. J Biol Chem. 1998;273:13430–13436. doi: 10.1074/jbc.273.22.13430. [DOI] [PubMed] [Google Scholar]
- 2.Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- 3.Bos K, Wraight C, Stanley K K. TGN38 is maintained in the trans-Golgi network by a tyrosine-containing motif in the cytoplasmic domain. EMBO J. 1993;12:2219–2228. doi: 10.1002/j.1460-2075.1993.tb05870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryant N J, Stevens T H. Two separate signals act independently to localize a yeast late Golgi membrane protein through a combination of retrieval and retention. J Cell Biol. 1997;136:287–297. doi: 10.1083/jcb.136.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casanova J E, Breitfeld P P, Ross S A, Mostov K E. Phosphorylation of the polymeric immunoglobulin receptor required for its efficient transcytosis. Science. 1990;248:742–745. doi: 10.1126/science.2110383. [DOI] [PubMed] [Google Scholar]
- 6.Davison A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 7.Gabel C, Dubey L, Steinberg S, Gershon M, Gershon A. Varicella-zoster virus glycoproteins are phosphorylated during posttranslational maturation. J Virol. 1989;63:4264–4276. doi: 10.1128/jvi.63.10.4264-4276.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gershon A, Zhu Z, Sherman D L, Gabel C A, Ambron R T, Gershon M D. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J Virol. 1994;68:6372–6390. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grose C. Glycoproteins encoded by varicella-zoster virus: biosynthesis, phosphorylation, and intracellular trafficking. Annu Rev Microbiol. 1990;44:59–80. doi: 10.1146/annurev.mi.44.100190.000423. [DOI] [PubMed] [Google Scholar]
- 10.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 11.Humphrey J S, Peters P J, Yuan L C, Bonifacino J S. Localization of TGN38 to the trans-Golgi network: involvement of a cytoplasmic tyrosine-containing sequence. J Cell Biol. 1993;120:1123–1135. doi: 10.1083/jcb.120.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones B G, Thomas L, Molloy S S, Thulin C D, Fry D, Walsh K A, Thomas G. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 1995;14:5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura H, Straus S E, Williams R K. Baculovirus expression, purification, and properties of varicella-zoster virus gE, gI, and the complex they form. J Infect Dis. 1998;178(Suppl. 1):S13–S15. doi: 10.1086/514256. [DOI] [PubMed] [Google Scholar]
- 14.Kimura H, Straus S E, Williams R K. Varicella zoster virus glycoproteins E and I expressed in insect cells form a heterodimer that requires the N-terminal domain of glycoprotein I. Virology. 1997;233:382–391. doi: 10.1006/viro.1997.8625. [DOI] [PubMed] [Google Scholar]
- 15.Kornfeld S. Trafficking of lysosomal enzymes. FASEB J. 1987;1:462–468. doi: 10.1096/fasebj.1.6.3315809. [DOI] [PubMed] [Google Scholar]
- 16.Le Borgne R, Hoflack B. Mannose 6-phosphate receptors regulate the formation of clathrin-coated vesicles in the TGN. J Cell Biol. 1997;137:335–345. doi: 10.1083/jcb.137.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litwin V, Grose C. Herpesviral Fc receptors and their relationship to the human Fc receptors. Immunol Res. 1992;11:226–238. doi: 10.1007/BF02919129. [DOI] [PubMed] [Google Scholar]
- 18.Mallory S, Sommer M, Arvin A M. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J Virol. 1997;71:8279–8288. doi: 10.1128/jvi.71.11.8279-8288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson J, Grose C. Complex formation facilitates endocytosis of the varicella-zoster virus gE:gI Fc receptor. J Virol. 1998;72:1542–1551. doi: 10.1128/jvi.72.2.1542-1551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson J K, Bishop G A, Grose C. Varicella-zoster virus Fc receptor gE glycoprotein: serine/threonine and tyrosine phosphorylation of monomeric and dimeric forms. J Virol. 1997;71:110–119. doi: 10.1128/jvi.71.1.110-119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson J K, Grose C. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J Virol. 1997;71:4042–4054. doi: 10.1128/jvi.71.5.4042-4054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson J K, Santos R A, Grose C. Varicella-zoster virus glycoprotein gE: endocytosis and trafficking of the Fc receptor. J Infect Dis. 1998;178(Suppl. 1):S2–S6. doi: 10.1086/514255. [DOI] [PubMed] [Google Scholar]
- 23.Pagano R E, Sepanski M A, Martin O C. Molecular trapping of a fluorescent ceramide analogue at the Golgi apparatus of fixed cells: interaction with endogenous lipids provides a trans-Golgi marker for both light and electron microscopy. J Cell Biol. 1989;109:2067–2079. doi: 10.1083/jcb.109.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelham H R, Munro S. Sorting of membrane proteins in the secretory pathway. Cell. 1993;75:603–605. doi: 10.1016/0092-8674(93)90479-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponnambalam S, Rabouille C, Luzio J P, Nilsson T, Warren G. The TGN38 glycoprotein contains two non-overlapping signals that mediate localization to the trans-Golgi network. J Cell Biol. 1994;125:253–268. doi: 10.1083/jcb.125.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reaves B, Horn M, Banting G. TGN38/41 recycles between the cell surface and the TGN: brefeldin A affects its rate of return to the TGN. Mol Biol Cell. 1993;4:93–105. doi: 10.1091/mbc.4.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roquemore E P, Banting G. Efficient trafficking of TGN38 from the endosome to the trans-Golgi network requires a free hydroxyl group at position 331 in the cytosolic domain. Mol Biol Cell. 1998;9:2125–2144. doi: 10.1091/mbc.9.8.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schäfer W, Stroh A, Berghöfer S, Seiler J, Vey M, Kruse M L, Kern H F, Klenk H D, Garten W. Two independent targeting signals in the cytoplasmic domain determine trans-Golgi network localization and endosomal trafficking of the proprotein convertase furin. EMBO J. 1995;14:2424–2435. doi: 10.1002/j.1460-2075.1995.tb07240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seaman M N J, Sowerby P J, Robinson M S. Cytosolic and membrane-associated proteins involved in the recruitment of AP-1 adaptors onto the trans-Golgi network. J Biol Chem. 1996;271:25446–25451. doi: 10.1074/jbc.271.41.25446. [DOI] [PubMed] [Google Scholar]
- 30.Sharon J, Kao C Y, Sompuram S R. Oligonucleotide-directed mutagenesis of antibody combining sites. Int Rev Immunol. 1993;10:113–127. doi: 10.3109/08830189309061689. [DOI] [PubMed] [Google Scholar]
- 31.Stephens D J, Banting G. Direct interaction of the trans-Golgi network membrane protein, TGN38, with the F-actin binding protein, neurabin. J Biol Chem. 1999;274:30080–30086. doi: 10.1074/jbc.274.42.30080. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi S, Nakagawa T, Banno T, Watanabe T, Murakami K, Nakayama K. Localization of furin to the trans-Golgi network and recycling from the cell surface involves Ser and Tyr residues within the cytoplasmic domain. J Biol Chem. 1995;270:28397–28401. doi: 10.1074/jbc.270.47.28397. [DOI] [PubMed] [Google Scholar]
- 33.Thorsness P E, Koshland D E., Jr Inactivation of isocitrate dehydrogenase by phosphorylation is mediated by the negative charge of the phosphate. J Biol Chem. 1987;262:10422–10425. [PubMed] [Google Scholar]
- 34.Traub L M, Kornfeld S. The trans-Golgi network: a late secretory sorting station. Curr Opin Cell Biol. 1997;9:527–533. doi: 10.1016/s0955-0674(97)80029-4. [DOI] [PubMed] [Google Scholar]
- 35.Voorhees P, Deignan E, van Donselaar E, Humphrey J, Marks M S, Peters P J, Bonifacino J S. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 1995;14:4961–4975. doi: 10.1002/j.1460-2075.1995.tb00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Gershon M D, Lungu O, Panagiotidis C A, Zhu Z, Hao Y, Gershon A A. Intracellular transport of varicella-zoster glycoproteins. J Infect Dis. 1998;178:S7–S12. doi: 10.1086/514268. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe H, Grubb J H, Sly W S. The overexpressed human 46-kDa mannose 6-phosphate receptor mediates endocytosis and sorting of B-glucouonidase. Proc Natl Acad Sci USA. 1990;87:8036–8040. doi: 10.1073/pnas.87.20.8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilde A, Dempsey C, Banting G. The tyrosine-containing internalization motif in the cytoplasmic domain of TGN38/41 lies within a nascent helix. J Biol Chem. 1994;269:7131–7136. [PubMed] [Google Scholar]
- 39.Wolins N, Bosshart H, Kuster H, Bonifacino J S. Aggregation as a determinant of protein fate in post-Golgi compartments: role of the luminal domain of furin in lysosomal targeting. J Cell Biol. 1997;139:1735–1745. doi: 10.1083/jcb.139.7.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong S H, Hong W. The SXYQRL sequence in the cytoplasmic domain of TGN38 plays a major role in trans-Golgi network localization. J Biol Chem. 1993;268:22853–22862. [PubMed] [Google Scholar]
- 41.Yao Z, Grose C. Unusual phosphorylation sequence in the gpIV (gI) component of the varicella-zoster virus gpI-gpIV glycoprotein complex (VZV gE-gI complex) J Virol. 1994;68:4204–4211. doi: 10.1128/jvi.68.7.4204-4211.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao Z, Jackson W, Forghani B, Grose C. Varicella-zoster virus glycoprotein gpI/gpIV receptor: expression, complex formation, and antigenicity within the vaccinia-T7 RNA polymerase transfection system. J Virol. 1993;67:305–314. doi: 10.1128/jvi.67.1.305-314.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye M, Duus K M, Peng J, Price D H, Grose C. Varicella-zoster virus Fc receptor component gI is phosphorylated on its endodomain by a cyclin-dependent kinase. J Virol. 1999;73:1320–1330. doi: 10.1128/jvi.73.2.1320-1330.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.York S J, Arneson L S, Gregory W T, Dahms N M, Kornfeld S. The rate of internalization of the mannose 6-phosphate/insulin-like growth factor II receptor is enhanced by multivalent ligand binding. J Biol Chem. 1999;274:1164–1171. doi: 10.1074/jbc.274.2.1164. [DOI] [PubMed] [Google Scholar]
- 45.Zhu Z, Gershon M D, Hao Y, Ambron R T, Gabel C A, Gershon A A. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J Virol. 1995;69:7951–7959. doi: 10.1128/jvi.69.12.7951-7959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Z, Hao Y, Gershon M D, Ambron R T, Gershon A A. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by a signal sequence (AYRV) and patch in the cytosolic domain of the molecule. J Virol. 1996;70:6563–6575. doi: 10.1128/jvi.70.10.6563-6575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]