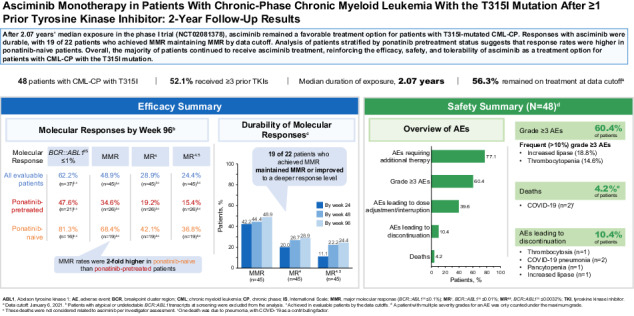
Abstract
Asciminib targets the BCR::ABL1 myristoyl pocket, maintaining activity against BCR::ABL1T315I, which is resistant to most approved adenosine triphosphate–competitive tyrosine kinase inhibitors. We report updated phase I results (NCT02081378) assessing safety/tolerability and antileukemic activity of asciminib monotherapy 200 mg twice daily in 48 heavily pretreated patients with T315I-mutated chronic-phase chronic myeloid leukemia (CML-CP; data cutoff: January 6, 2021). With 2 years’ median exposure, 56.3% of patients continued receiving asciminib. Overall, 62.2% of evaluable patients achieved BCR::ABL1 ≤1% on the International Scale (IS); 47.6% and 81.3% of ponatinib-pretreated and -naive patients, respectively, achieved BCR::ABL1IS ≤1%. Of 45 evaluable patients, 48.9% achieved a major molecular response (MMR, BCR::ABL1IS ≤0.1%), including 34.6% and 68.4% of ponatinib-pretreated and -naive patients, respectively. MMR was maintained until data cutoff in 19 of 22 patients who achieved it. The most common grade ≥3 adverse events (AEs) included increased lipase level (18.8%) and thrombocytopenia (14.6%). Five (10.4%) patients experienced AEs leading to discontinuation, including 2 who discontinued asciminib and died due to COVID-19; these were the only deaths reported. These results show asciminib’s effectiveness, including in almost 50% of ponatinib pretreated patients, and confirm its risk-benefit profile, supporting its use as a treatment option for T315I-mutated CML-CP.
Subject terms: Chronic myeloid leukaemia, Molecularly targeted therapy
Introduction
BCR::ABL1 tyrosine kinase inhibitors (TKIs) have improved survival for patients with chronic myeloid leukemia (CML) [1, 2]. However, many patients experience resistance or intolerance through successive adenosine triphosphate (ATP)–competitive TKI therapies, resulting in decreased probability of survival [2–6]. A common mechanism of resistance is emerging BCR::ABL1 mutations [2, 7]. The T315I mutation is among the most frequently identified BCR::ABL1 mutations, occurring in 2% to 16% of patients with imatinib- or second-generation TKI-resistant CML and increasing in frequency with subsequent lines of therapy [8, 9]. The T315I mutation confers resistance to all currently approved ATP-competitive TKIs except ponatinib and olverembatinib (approved in China for TKI-resistant CML in chronic phase [CP] or accelerated phase [AP] with the T315I mutation), limiting treatment options for affected patients [1, 2, 10–12].
Patients with the T315I mutation may have adverse outcomes, including decreased overall survival (OS) and progression-free survival [8, 9, 13, 14]. Ponatinib has demonstrated activity in patients, although it may be associated with safety concerns [1, 13, 15–18]. In the OPTIC trial, which assessed ponatinib starting doses of 45, 30, and 15 mg QD, 51.6%, 35.5%%, and 25.3%, respectively, of patients with T315I-mutated CML-CP achieved BCR::ABL1 on the International Scale (IS) ≤1% by 12 months (predictive of long-term survival) [1, 16, 19, 20]. Safety concerns associated with ponatinib include the risk of cardiovascular (CV) adverse events (AEs), including arterial occlusive events (AOEs), in 14% to 31% of patients with CML-CP, although rates can be reduced by approximately 60% using response-based dose-reduction strategies [1, 13, 18, 21].
Asciminib is the first approved BCR::ABL1 inhibitor that works by specifically targeting the ABL myristoyl pocket, inhibiting BCR::ABL1 kinase activity by locking it in an inactive conformation via allosteric binding [12, 22–25]. Asciminib has high specificity and selectivity for the ABL kinase family with limited off-target activity [12, 23]. By targeting the myristoyl-binding pocket, asciminib maintains activity against BCR::ABL1 kinase domain mutations, including T315I, that confer resistance to ATP-competitive TKIs [12, 22, 23, 26].
A previous analysis of the phase I X2101 trial (NCT02081378) in heavily pretreated patients with Ph+ CML-CP/AP, including T315I-mutated CML-CP/AP, first demonstrated the safety of asciminib monotherapy QD or twice daily (BID) at 10 to 200 mg [26]. After a median follow-up of approximately 14 months, asciminib demonstrated a favorable safety and tolerability profile, with 11% and 6% of patients with CML-CP with or without the T315I mutation, respectively, discontinuing therapy due to AEs [26]. The maximum tolerated dose of asciminib was not reached [26]. Preclinical observations suggested that a 4- to 13-fold higher asciminib concentration was required for adequate inhibition of BCR::ABL1T315I compared with non-mutated BCR::ABL1, and in X2101 the majority of patients with T315I-mutated CML-CP who achieved responses had received doses of ≥150 mg BID [12, 23, 26, 27]. Use of the highest tested dose of asciminib, 200 mg BID, was justified by overall major molecular response (MMR; BCR::ABL1IS ≤0.1%) rates predicted by pharmacokinetic/pharmacodynamic analysis in patients with T315I-mutated CML-CP and may improve the probability of response by maximizing the predicted proportion of patients with asciminib exposure above the preclinical 90% maximal effective concentration [28]. Results from this trial supported full approval of asciminib 200 mg BID for patients with T315I-mutated CML-CP in the US with subsequent approvals worldwide [29–31].
We report efficacy and safety data in 48 patients with T315I-mutated CML-CP receiving asciminib 200 mg BID monotherapy with a median duration of exposure of 2 years in X2101; separate analyses of patients with CML-CP receiving asciminib 150 or 160 mg and patients with CML-AP receiving asciminib 200 mg BID are also included.
Methods
Study oversight
The study was designed by the sponsor (Novartis Pharmaceuticals) in collaboration with study investigators. The sponsor collected and analyzed data in conjunction with the authors. All authors contributed to the development and writing of the manuscript and vouch for the accuracy and completeness of the data and the fidelity of the study to the protocol.
Study design
This phase I, first-in-human, dose-finding study was described previously [25, 26]. The current analysis focused on 48 patients with CML-CP with a confirmed T315I mutation who received a starting dose of asciminib monotherapy 200 mg BID in the dose-escalation or -expansion phases of this study arm. BCR::ABL1 mutational analyses were performed centrally by ICON (Portland, OR, USA) using Sanger sequencing. Patients were ≥18 years old with cytogenetically confirmed Ph+ CML-CP with the T315I mutation who were resistant to or intolerant of ≥1 prior TKI (per 2009 European LeukemiaNet recommendations) [32]. Efficacy and safety data for patients with T315I-mutated CML-CP who received asciminib 150 mg BID (n = 5) and 160 mg BID (n = 6) and efficacy data for patients with T315I-mutated CML-AP who received asciminib 200 mg BID (n = 4) are reported. The primary objective was to determine the maximum tolerated dose and/or recommended dose for expansion of asciminib monotherapy. Secondary objectives included assessing the safety and tolerability, pharmacokinetics, and preliminary antileukemic activity of asciminib.
Study assessments
Coding and grading of AEs, assessment of molecular response, and BCR::ABL1 mutational analysis were described previously [25].
Statistical analyses
Assessment of molecular response rates by time point was defined previously [25]. The analyses herein are based on data collected by the January 6, 2021, cutoff, when all patients had completed their week 60 follow-up visit or discontinued earlier and represent cumulative rates unless otherwise specified.
Results
Patients
This analysis included all 48 patients, enrolled from October 2016 to October 2019, with T315I-mutated CML-CP receiving asciminib monotherapy 200 mg BID (Supplemental Fig. S1). Two patients had additional mutations at baseline (E255K [n = 1], E355G [n = 1]). At data cutoff, more than half of patients (27 [56.3%]) remained on treatment (Table 1). With 2.07 years (range, 0.04–4.12) median follow-up and median duration of exposure, 21 (43.8%) patients discontinued treatment, primarily due to AEs (8.3%) and physician decision (22.9%), including loss of response (n = 3), loss/lack of response followed by stem cell transplant (n = 3), lack of efficacy (n = 2), to receive stem cell transplant (n = 2), and transfer to investigator-initiated trial (n = 1). Thirty-six (75.0%) and 27 (56.3%) patients received treatment for ≥48 and ≥96 weeks, respectively; most (77.8% and 74.1%, respectively) were receiving 200 mg BID at those cutoffs (Supplemental Table S1). Fifteen (31.3%) patients received treatment for ≥144 weeks. Two (4.2%) patients died on study; one died on treatment (within 30 days of last study drug dose) due to COVID-19 pneumonia, and the other died >30 days post study discontinuation due to pneumonia with COVID-19 as the contributing reason.
Table 1.
Patient disposition.
| Disposition reason, n (%) | Ponatinib pretreated n = 29 |
Ponatinib naive n = 19 |
All patients N = 48 |
|---|---|---|---|
| Patients treated | |||
| Treatment ongoinga | 15 (51.7) | 12 (63.2) | 27 (56.3) |
| End of treatment | 14 (48.3) | 7 (36.8) | 21 (43.8) |
| Primary reason for end of treatment | |||
| Physician decisionb | 8 (27.6) | 3 (15.8) | 11 (22.9) |
| Adverse event | 2 (6.9) | 2 (10.5) | 4 (8.3) |
| Patient/guardian decision | 0 | 0 | 0 |
| Progressive disease | 2 (6.9)c | 0 | 2 (4.2)c |
| Death | 0 | 2 (10.5) | 2 (4.2) |
| Technical problems | 1 (3.4)d | 0 | 1 (2.1)d |
| Protocol deviation | 1 (3.4)e | 0 | 1 (2.1)e |
aPatients ongoing at the time of the cutoff (January 6, 2021).
bReasons for physician decision included loss of response (n = 3), loss or lack of response with transfer to stem cell transplant (n = 3), lack of efficacy (n = 2), to receive stem cell transplant (n = 2), and transfer to investigator-initiated trial (n = 1).
cPatients with disease progression to accelerated phase.
dPatient travel to trial site restricted due to COVID-19. Moved to a managed access program.
ePatient required prohibited medication (hydroxyurea). Continued treatment under a managed access program.
Most patients were heavily pretreated, with 15 (31.3%), 17 (35.4%), and 8 (16.7%) having received 2, 3, and ≥4 prior TKIs, respectively (Table 2). No patients previously received olverembatinib. Twenty-nine (60.4%) patients previously received ponatinib (Supplementary Table S2); these patients received a median of 3 (range, 1–5) prior TKIs. Eleven of 26 patients with available prior ponatinib dosing data received an initial ponatinib dose of 45 mg. Twenty-six of 29 patients received ponatinib as the last TKI before study entry. Patients discontinued ponatinib due to intolerance (n = 9), resistance (n = 14), enrollment in X2101 (n = 4), or completion of prescribing regimen (n = 2). Baseline BCR::ABL1IS levels of 26 evaluable patients separated by reason for ponatinib discontinuation are shown in Supplementary Table S3.
Table 2.
Patient demographics and baseline clinical characteristics.
| Demographic variable | All patients N = 48 |
Evaluable patients N = 45 |
|---|---|---|
| Age, median (range), years | 56.5 (26–86) | 54.0 (26–86) |
| Age category, n (%) | ||
| 18 to <65 years | 32 (66.7) | 31 (68.9) |
| ≥65 years | 16 (33.3) | 14 (31.1) |
| ≥75 years | 4 (8.3) | 4 (8.9) |
| Sex, n (%) | ||
| Male | 37 (77.1) | 36 (80.0) |
| Female | 11 (22.9) | 9 (20.0) |
| Race, n (%) | ||
| White | 23 (47.9) | 21 (46.7) |
| Asian | 12 (25.0) | 12 (26.7) |
| Unknown | 6 (12.5) | 5 (11.1) |
| Other | 6 (12.5) | 6 (13.3) |
| Black or African American | 1 (2.1) | 1 (2.2) |
| Ethnicity, n (%) | ||
| East Asian | 10 (20.8) | 10 (22.2) |
| Other | 16 (33.3) | 14 (31.1) |
| Not reported | 14 (29.2) | 13 (28.9) |
| Unknown | 3 (6.3) | 3 (6.7) |
| Southeast Asian | 2 (4.2) | 2 (4.4) |
| Hispanic or Latino | 3 (6.3) | 3 (6.7) |
| ECOG performance status, n (%) | ||
| 0 | 36 (75.0) | 33 (73.3) |
| 1 | 12 (25.0) | 12 (26.7) |
| No. of prior TKIs | ||
| 1a | 8 (16.7) | 8 (17.8) |
| 2 | 15 (31.3) | 14 (31.1) |
| 3 | 17 (35.4) | 16 (35.6) |
| ≥4 | 8 (16.7) | 7 (15.6) |
| Individual prior TKIs | ||
| Bosutinib | 3 (6.3) | 2 (4.4) |
| Dasatinib | 33 (68.8) | 33 (73.3) |
| Imatinib | 27 (56.3) | 25 (55.6) |
| Nilotinib | 26 (54.2) | 23 (51.1) |
| Ponatinib | 29 (60.4) | 26 (57.8) |
| Radotinib | 4 (8.3) | 4 (8.9) |
| Reason for discontinuation of last dose of ponatinib | ||
| Intolerance | 9 (31.0) | 7 (26.9) |
| Resistance | 14 (48.3) | 13 (50.0) |
| Other | 6 (20.7) | 6 (23.1) |
| Mutations at screening, n (%) | ||
| T315I alone | 46 (95.8) | 43 (95.6) |
| T315I and E255K | 1 (2.1) | 1 (2.2) |
| T315I and E355G | 1 (2.1) | 1 (2.2) |
| BCR::ABL1IS at screening, n (%) | ||
| >0.01% to 0.1% | 0 | 0 |
| >0.1% to 1% | 8 (16.7) | 8 (17.8) |
| >1% to 10% | 11 (22.9) | 11 (24.4) |
| >10% | 26 (54.2) | 26 (57.8) |
| Atypical /e1a2/unknown transcriptsb | 3 (6.3) | 0 |
ECOG Eastern Cooperative Oncology Group, TKI tyrosine kinase inhibitor.
aPrior TKIs included dasatinib (n = 5), nilotinib (n = 2), and radotinib (n = 1).
be19a2 transcript, e13a3 (b2a3) transcript, no transcript detected (n = 1, each).
Efficacy
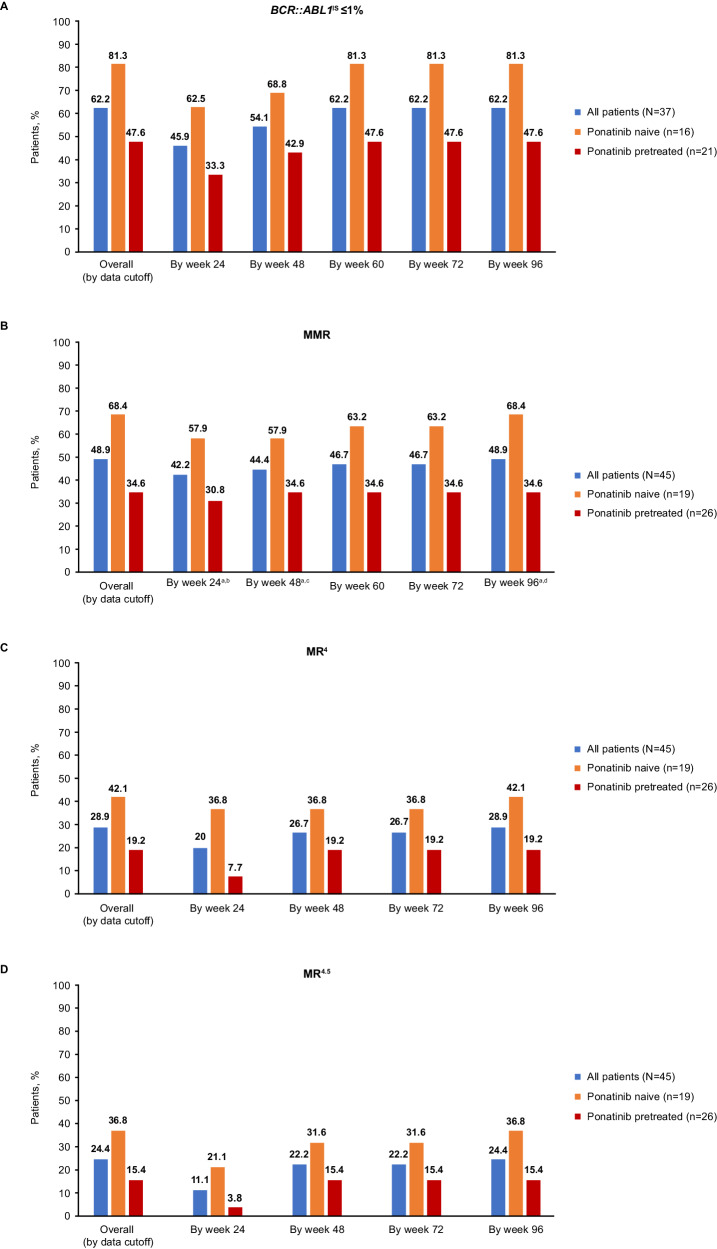
Of the 48 total patients, 3 were excluded from efficacy analyses due to atypical BCR::ABL1 transcripts at baseline (n = 2) or no transcripts detected (n = 1) (Table 2; Supplementary Fig. S1). Of the remaining 45 patients, 37 had BCR::ABL1IS > 1% at baseline and were evaluable for BCR::ABL1IS ≤1% achievement (Fig. 1). Twenty-three (62.2%) patients achieved BCR::ABL1IS ≤1% with 17 (45.9%) doing so by week 24 (Fig. 2). The response level of most (6 of 8) patients with BCR::ABL1IS ≤1% at baseline deepened by ≥2 logs compared with baseline with treatment; 2 of these 8 patients lost this response.
Fig. 1. Cumulative molecular response in ponatinib-pretreated and -naive patients without the indicated response at baseline.
Cumulative (A) BCR::ABL1IS ≤1%, (B) MMR, (C) MR4, and (D) MR4.5 in evaluable patients. IS International Scale, MMR major molecular response, MR4 BCR::ABL1IS ≤0.01%, MR4.5 BCR::ABL1IS ≤0.0032%. a CIs are based on the Clopper–Pearson method. 95% CIs were used for all patients, and 90% CIs were used for ponatinib-pretreated and -naive patients. b The rate of MMR by week 24 was 42.2% (95% CI, 27.7–57.8%) in all patients, 57.9% (90% CI, 36.8–77.0%) in ponatinib-naive patients, and 30.8% (90% CI, 16.3–48.7%) in ponatinib-pretreated patients. c The rate of MMR by week 48 was 44.4% (95% CI, 29.6–60.0%) in all patients, 57.9% (90% CI, 36.8–77.0%) in ponatinib-naive patients, and 34.6% (90% CI, 19.4–52.6%) in ponatinib-pretreated patients. d The rate of MMR by week 96 was 48.9% (95% CI, 33.7–64.2%) in all patients, 68.4% (90% CI, 47.0–85.3%) ponatinib-naive patients, and 34.6% (90% CI, 19.4–52.6%) in ponatinib-pretreated patients.
Fig. 2. Cumulative BCR::ABL1IS ≤1% by time point in patients with baseline BCR::ABL1IS > 1%a.
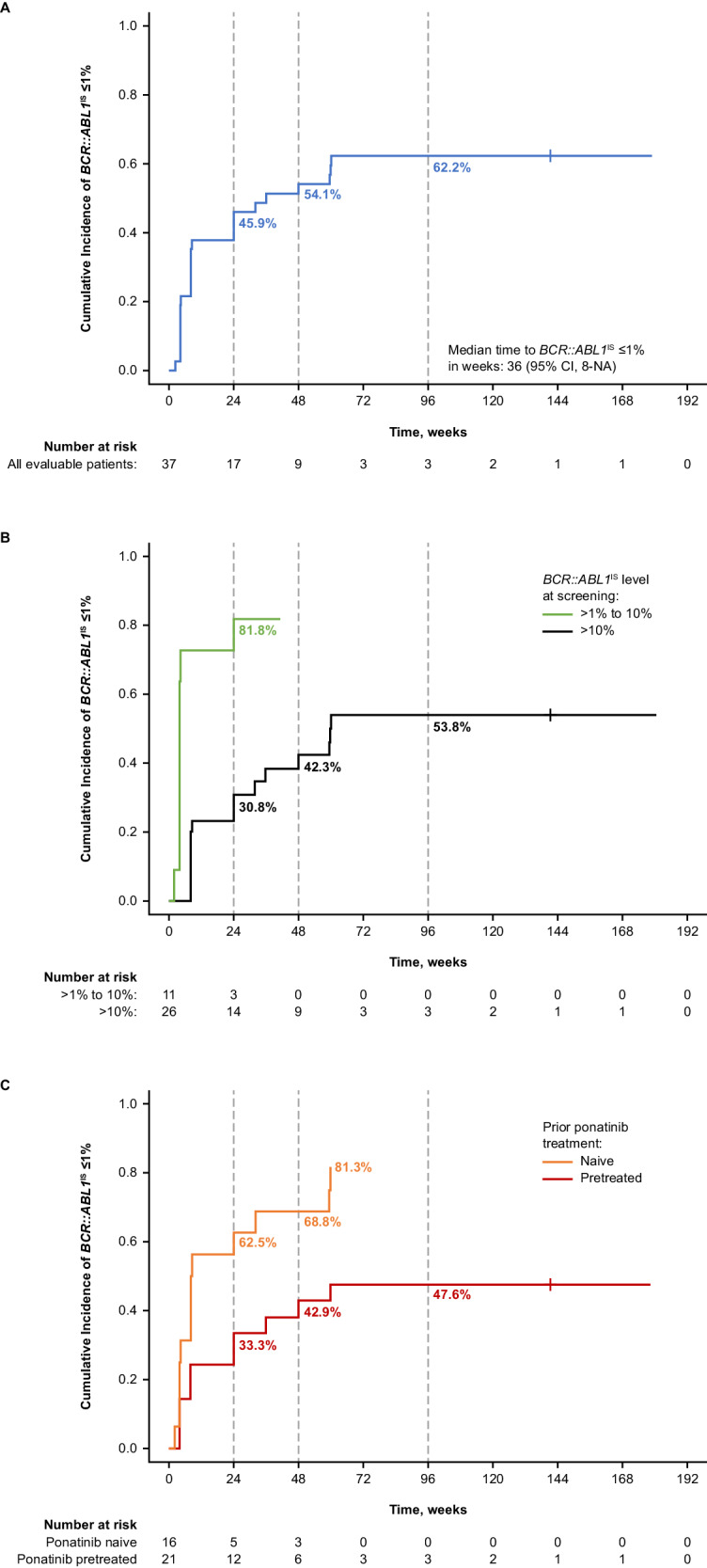
Cumulative BCR::ABL1IS ≤1% in all evaluable patients (A) overall, (B) by BCR::ABL1IS at baseline, and (C) by prior treatment with ponatinib. IS International Scale. a Treatment discontinuations for any reason were treated as competing events.
All 45 evaluable patients had BCR::ABL1IS > 0.1% at baseline and were evaluable for MMR. Twenty-two (48.9%) patients achieved MMR, with 19 (42.2%) doing so by week 24 (Fig. 3). Two (9.1%) of these 22 patients achieved MMR after dose reduction to 160 mg BID to manage AEs. MMR was achieved in 6 (75%), 9 (81.8%), and 7 (26.9%) patients with BCR::ABL1IS >0.1% to ≤1%, >1% to ≤10%, and >10% at baseline (Supplementary Table S4).
Fig. 3. Cumulative MMR by time point in patients not in MMR at baseline.
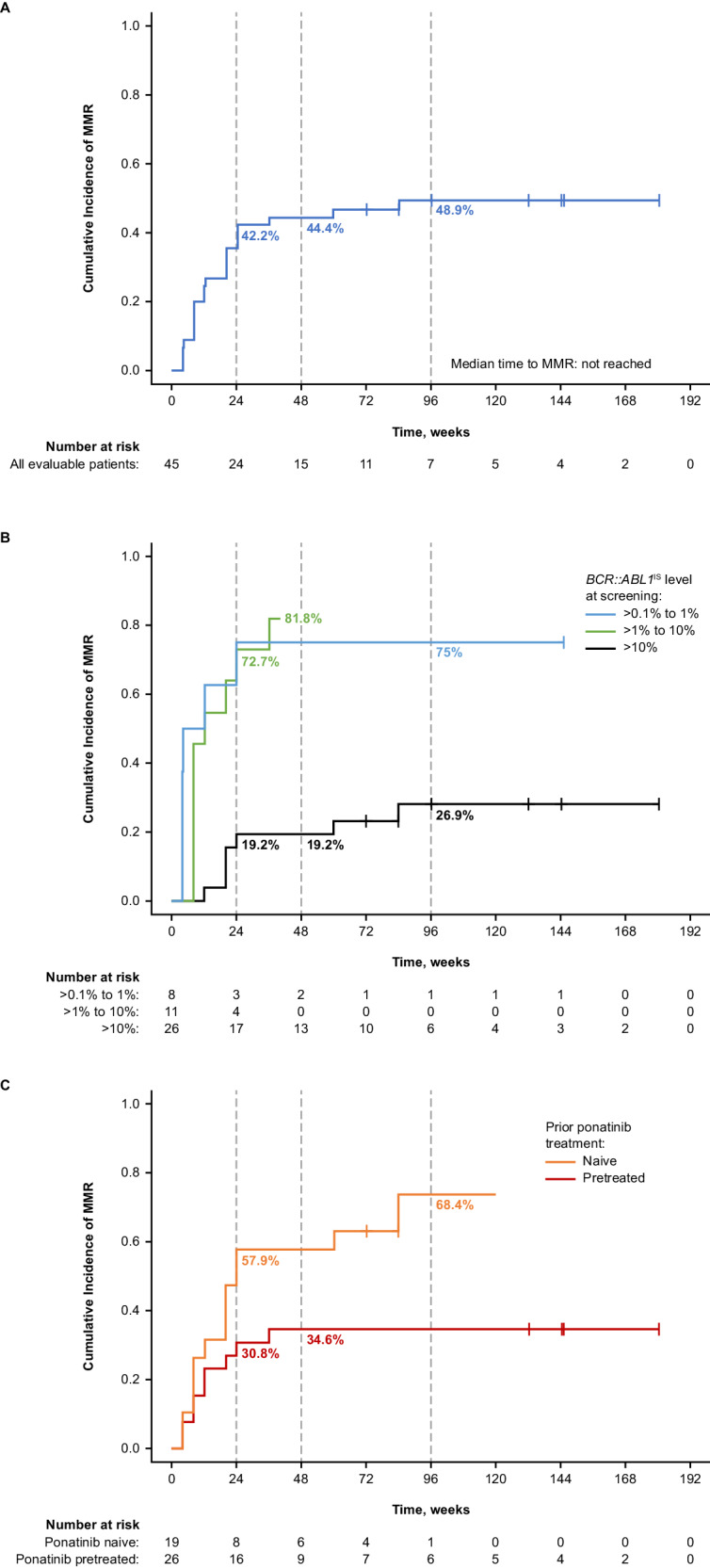
a Cumulative MMR in all evaluable patients (A) overall, (B) by BCR::ABL1IS at baseline, and (C) by prior treatment with ponatinib. IS International Scale, MMR major molecular response. a Treatment discontinuations for any reason were treated as competing events.
Nineteen of 22 patients who achieved MMR maintained MMR by data cutoff. All 3 patients who lost MMR discontinued study treatment (1 due to technical reasons [could not travel due to COVID-19 and was moved to the managed access program] 1 withdrew consent, and 1 to receive transplant). The Kaplan–Meier estimated rate of durable MMR at week 96 was 84% (95% CI, 68.1–100.0%). Deep molecular responses were also observed: 9 (20.0%), 12 (26.7%), and 13 (28.9%) patients achieved at least MR4 including 5 (11.1%), 10 (22.2%), and 11 (24.4%) who achieved MR4.5 by week 24, 48, and 96, respectively (Fig. 1). Of the 13 patients who achieved at least MR4 by week 96, 6 patients had BCR::ABL1IS ≤1% at baseline and the other 7 patients had BCR::ABL1IS >1% at baseline.
An exploratory analysis of evaluable patients stratified by ponatinib pretreatment status suggested that response rates were higher in ponatinib-naive than -pretreated patients. Thirteen of 16 (81.3%) ponatinib-naive and 10 of 21 (47.6%) ponatinib-pretreated patients achieved BCR::ABL1IS ≤1%. MMR was achieved by 13 of 19 (68.4%) ponatinib-naive and 9 of 26 (34.6%) ponatinib-pretreated patients (Fig. 1), including patients who discontinued ponatinib due to intolerance (4/7 [57.1%]), resistance (2/13 [15.4%]), or other reasons (3/6 [50%]) (Supplementary Table S5). Two of 3 patients who lost MMR by data cutoff were ponatinib pretreated. The Kaplan–Meier estimated rate of durable MMR at week 96 was 91% (95% CI, 73.9–100.0%) in ponatinib-naive and 78% (95% CI, 50.6–100.0%) in ponatinib-pretreated patients. Of the 9 ponatinib-pretreated patients achieving MMR by cutoff, 7 had BCR::ABL1IS ≤10% at baseline and 2 had BCR::ABL1IS > 10% at baseline (Supplementary Table S6).
In all 48 patients, the Kaplan–Meier estimated rate of event-free survival at week 96 was 87% (95% CI, 77–97%) (Supplementary Fig. S2A). The median time to event was not reached. Two patients with additional mutations at baseline did not achieve MMR (Supplementary Table S7); one remained on therapy and achieved BCR::ABL1IS ≤1% by data cutoff; the other discontinued the study due to physician decision. Six patients acquired additional mutations during the study (M244V, M351T, F359I, A337T, and F359V). The patient who acquired F359V did so after they had achieved MMR and lost it at week 96 (no mutations detectable at that time); at the next assessment, F359V was detected. BCR::ABL1IS levels continued to increase until the patient discontinued treatment to receive a transplant. The 5 remaining patients did not achieve MMR; 4 discontinued treatment (3 due to lack of efficacy, 1 due to disease progression), and 1 (with M244V) remained on study drug and achieved BCR::ABL1IS ≤1% by data cutoff.
In patients who received asciminib 150 mg BID (n = 5) or 160 mg BID (n = 6), BCR::ABL1IS ≤1% and MMR rates did not increase after week 24 (Supplementary Tables S8 and S9). Among evaluable patients, 1 patient with 150 mg BID and 2 with 160 mg BID were in MMR by week 96.
Of 4 patients in AP (Supplementary Table S10), 2 remained on treatment by data cutoff for ≥72 and ≥168 weeks. These 2 patients had BCR::ABL1IS >1% to ≤10% at baseline. One achieved MMR by week 48, the other achieved first MR4.5 by week 24, and both maintained responses at cutoff. Two patients had BCR::ABL1IS >10% at baseline and discontinued treatment, one after treatment for ≥24 weeks without achieving BCR::ABL1IS ≤1% and the other after treatment for 23 days without postbaseline assessment.
Safety
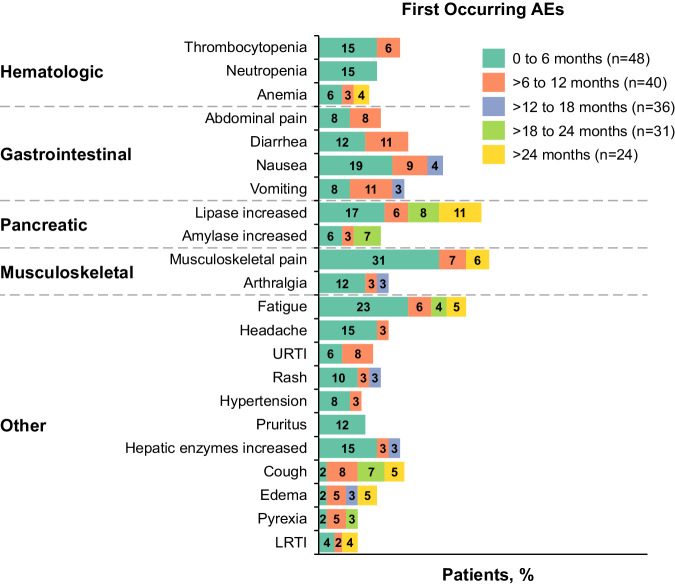
No safety signals were observed with asciminib 150 mg BID or 160 mg BID (Supplementary Table S11), supporting the evaluation of asciminib 200 mg BID. For patients who received asciminib 200 mg BID, all-grade AEs occurring in ≥20% of patients included increased lipase level (29.2%), fatigue (29.2%), nausea (27.1%), and diarrhea (20.8%) (Table 3). Grade ≥3 events occurred in 29 (60.4%) patients; those occurring in ≥10% of patients were increased lipase level (18.8%) and thrombocytopenia (14.6%). Most AEs occurred in the first 6 months of treatment, and incidence rates generally decreased over time (Fig. 4 and Supplementary Fig. S3). AEs led to study discontinuation in 5 (10.4%) patients, including the 2 who died of COVID-19 (Supplementary Table S12); the 3 remaining patients discontinued due to pancytopenia, thrombocytosis, and increased lipase level (n = 1 each). AEs led to dose adjustment or interruption in 19 (39.6%) patients and required additional therapy in 37 (77.1%). No obvious differences in overall safety categories were observed between ponatinib-naive and -pretreated patients; rates of AEs leading to dose adjustment were higher in ponatinib-pretreated (44.8%) than ponatinib-naive (31.6%) patients.
Table 3.
AEs reported irrespective of study treatment relationship by preferred term reported in ≥10% of patients.
| Preferred term, n (%) | All patients N = 48 |
|
|---|---|---|
| All grades | Grade ≥ 3 | |
| No. of patients with ≥1 event | 48 (100) | 29 (60.4) |
| Lipase increased | 14 (29.2) | 9 (18.8) |
| Fatigue | 14 (29.2) | 1 (2.1) |
| Nausea | 13 (27.1) | 0 |
| Diarrhea | 10 (20.8) | 1 (2.1) |
| Vomiting | 9 (18.8) | 3 (6.3) |
| Musculoskeletal paina | 9 (18.8) | 0 |
| Thrombocytopenia | 8 (16.7) | 7 (14.6) |
| Headache | 8 (16.7) | 1 (2.1) |
| Arthralgia | 8 (16.7) | 0 |
| Alanine aminotransferase increased | 7 (14.6) | 3 (6.3) |
| Abdominal pain | 7 (14.6) | 3 (6.3) |
| Cough | 7 (14.6) | 0 |
| Amylase increased | 6 (12.5) | 2 (4.2) |
| Back pain | 6 (12.5) | 1 (2.1) |
| Pruritus | 6 (12.5) | 0 |
| Aspartate aminotransferase increased | 6 (12.5) | 1 (2.1) |
| Hypertension | 5 (10.4) | 3 (6.3) |
| Anemia | 5 (10.4) | 3 (6.3) |
| Edema peripheral | 5 (10.4) | 2 (4.2) |
AE adverse event.
aMusculoskeletal pain includes preferred terms for musculoskeletal pain and pain in extremity.
Fig. 4. Adverse reactions (≥10% at first occurrence) over time.
a,bADR adverse drug reaction, AE adverse event, LRTI lower respiratory tract infection, URTI upper respiratory tract infection. a Includes reported AEs and adverse drug reactions. b Proportions are calculated based on the number of patients at risk of an event (ie, patients ongoing treatment and event-free at the start of the interval). A patient with multiple occurrences of an event in the same time interval is counted only once in that time interval. The safety topics correspond to either single preferred terms or groups of preferred terms according to the adverse drug reaction definitions.
Clinically important safety information grouped by special interest categories is reported in Table 4. The most frequently reported categories included gastrointestinal toxicity (47.9%), pancreatic toxicity (31.3%: mainly enzyme elevations; 1 clinical event [grade 2 pancreatitis]), hypersensitivity (27.1%, mainly mild dermatologic events), hepatotoxicity (27.1%, mainly enzyme elevations), and myelosuppression (25%). The most common (≥20%) gastrointestinal toxicities were nausea (27.1%) and diarrhea (20.8%). Grade ≥3 gastrointestinal events occurred in 5 (10.4%) patients. Pancreatic enzyme elevations included increased lipase (31.3%) and amylase (12.5%). Grade ≥3 events occurred in 22.9% of patients (Supplementary Table S13). Events mostly resolved without dose adjustment or with temporary interruption. The grade 2 pancreatitis occurred at day 393 in a patient with prior imatinib, nilotinib, and ponatinib treatment who initiated asciminib 200 mg BID and was receiving asciminib 80 mg BID at onset. Hepatotoxicity included mainly enzyme elevations; grade 3 events occurred in 10.4% of patients. No grade ≥3 hypersensitivity events occurred. Myelosuppression-related any-grade events occurring in ≥10% of patients included thrombocytopenia (18.8%), neutropenia (14.6%), and anemia (10.4%); grade ≥3 events included thrombocytopenia (16.7%), neutropenia (12.5%), anemia (6.3%), and pancytopenia (2.1%).
Table 4.
Adverse events of special interest.
| Safety topic,a n (%) | All patients N = 48 |
|
|---|---|---|
| All grades | Grade ≥ 3 | |
| Gastrointestinal toxicity | 23 (47.9) | 5 (10.4) |
| Hypersensitivityb | 13 (27.1) | 0 |
| Hepatotoxicity (including laboratory terms)c | 13 (27.1) | 5 (10.4) |
| Myelosuppressiond | 12 (25.0) | 10 (20.8) |
| Thrombocytopenia | 9 (18.8) | 8 (16.7) |
| Leukopenia | 7 (14.6) | 6 (12.5) |
| Erythropenia (anemia) | 5 (10.4) | 3 (6.3) |
| Cytopenias affecting >1 lineage | 1 (2.1) | 1 (2.1) |
| Pancreatic toxicity (including laboratory terms) | 15 (31.3) | 11 (22.9) |
| Pancreatic toxicity (clinical events) | 1 (2.1) | 0 |
| Edema and fluid retention | 8 (16.7) | 3 (6.3) |
| Hemorrhage | 9 (18.8) | 1 (2.1) |
| Ischemic heart and CNS conditions | 5 (10.4) | 2 (4.2) |
| Ischemic CNS vascular conditions | 3 (6.3) | 1 (2.1) |
| Ischemic heart disease | 3 (6.3) | 1 (2.1) |
| Arterial occlusive event | 4 (8.3) | 2 (4.2) |
| Phototoxicity | 1 (2.1) | 0 |
| QTc prolongation | 1 (2.1) | 1 (2.1) |
CNS central nervous system.
aNumbers (n) represent counts of patients. A patient with multiple severity grades for an adverse event is only counted under the maximum grade. MedDRA version 23.1, CTCAE version 4.03, Case Retrieval Strategy version released February 25, 2021.
bMainly mild dermatologic events, including rash, rash maculopapular, dermatitis acneiform, eczema, and urticaria. One event of allergic rhinitis was also observed.
cMainly enzyme elevations.
dMyelosuppression includes anemia, leukopenia, thrombocytopenia, and cytopenias affecting >1 lineage.
Four (8.3%) patients had AOEs (Supplementary Table S14); 2 (4.2%) were grade 1 and 2 (4.3%) were grade 3. When adjusted for patient-year exposure, the incidence of all-grade and grade 3 AOEs was 4.3% and 2.2%, respectively (Supplementary Table S15). One patient experienced cerebrovascular accident (grade 3) 13 days after hospitalization due to COVID-19 and died 23 days post-hospitalization due to pneumonia with COVID-19 as a contributing factor. Another patient experienced 3 grade 3 AOEs (peripheral arterial occlusive disease of the right and left side and coronary artery disease), all of which occurred separately over >1300 days of treatment. Percutaneous transluminal angioplasty, electrocardiography, and heart catheterization were performed, and a stent was implanted. Peripheral arterial occlusive disease of both sides later improved to grade 2. Grade 1 AOEs included left carotid artery disease (n = 1) and cerebrovascular accident (n = 1; reported 2 days after the patient discontinued asciminib due to disease progression). Of the 4 patients with AOEs, all had prior exposure to ≥3 TKIs (imatinib [n = 4], nilotinib [n = 3], bosutinib [n = 1], dasatinib [n = 3], and ponatinib [n = 3]). A lower exposure-adjusted incidence of AOEs was observed in ponatinib-naive (2.4%) than in ponatinib-pretreated (5.9%) patients. All patients with AOEs had ≥1 past and/or active CV risk factor at baseline, including arterial hypertension (n = 4), coronary artery disease (n = 1), and cerebrovascular accident (n = 1). No AOEs led to asciminib dose adjustment, interruption, or discontinuation.
Cardiac failure occurred in 1 patient (Supplementary Table S16) who had exposure to 4 prior antileukemic therapies and multiple baseline CV risk factors. On study day 22, pre-existing degenerative aortic valve disease worsened to grade 2, and aortic valve disease (aortic valve vitium) and tricuspid valve incompetence (both grade 1) developed. The patient continued receiving asciminib until hospitalization due to COVID-19 (day 888), a cause of death.
Discussion
This updated analysis of a phase I trial continues to demonstrate the clinical efficacy and safety of asciminib monotherapy 200 mg BID in patients with T315I-mutated CML-CP—a population that typically has poor prognosis. Molecular responses were observed in few patients who received asciminib 150 mg BID and 160 mg BID doses; the observed efficacy and consistent safety profile in this analysis supports 200 mg BID as the optimal dose for patients with the T315I mutation. After 2 years’ median exposure, most (56.3%) patients continued receiving asciminib. Remarkably, only 4 (8.3%) patients discontinued asciminib due to AEs.
A high proportion (62.2%) of patients achieved BCR::ABL1IS ≤1%, most by month 11 (week 48). The achievement of BCR::ABL1IS ≤1% or complete cytogenetic response (CcyR) within 12 months predicts long-term survival [1, 19, 20]; achievement at 12 months is associated with higher 6-year OS rate (with CcyR, 93%; without CcyR, 79%) [33] and a lower 5-year rate of disease progression (with CcyR 3%; without major cytogenetic response, 19%) [34]. BCR::ABL1IS ≤1% therefore is an important response milestone, particularly for patients in whom ≥2 prior TKIs have not provided MMR [1, 19, 20]. For context, in PACE, 66% and 70% of patients with T315I-mutated CML-CP receiving ponatinib 45 mg QD, the recommended starting dose, achieved CCyR by 12 and 57 months, respectively [13, 18, 35]. In OPTIC, response to treatment was dose dependent, with 51.6%, 35.5%, and 25.3% of patients with T315I-mutated CML-CP at ponatinib starting doses of 45, 30, and 15 mg, respectively, achieving this response by 12 months and 60.0%, 25.0%, and 10.5%, respectively, by 3 years [14, 16].
The achievement of MMR is an important treatment milestone that predicts improved OS, and progression-free survival [1, 33, 36, 37], and is associated with improved durations of CCyR [38]. Almost half of patients (22 [48.9%]), including most patients who achieved BCR::ABL1IS ≤1% (16 of 23), achieved MMR. MMR was achieved by patients with all observed baseline BCR::ABL1IS levels and in patients who discontinued prior ponatinib for both intolerance and resistance. Responses were sustained, with most patients (19 of 22) who achieved MMR maintaining MMR by data cutoff. In PACE, 56% and 58% of patients with T315I-mutated CML-CP receiving ponatinib 45 mg QD achieved MMR by 12 and 57 months, respectively; 60% of patients who achieved a response by 57 months were estimated to maintain MMR at 5 years [13, 35]. In OPTIC, 34.4%, 24.7%, and 23.1% of patients with CML-CP with or without the T315I mutation at ponatinib starting doses of 45, 30, and 15 mg, respectively, had MMR at cutoff (32 months’ median follow-up) [16].
Deep molecular response (MR4 and MR4.5) is a strong predictor of OS [39] and can minimize risk of loss of CCyR or MMR [40]. MR4 is required for treatment-free remission eligibility [1, 41], and MR4.5 has been associated with better event-free survival and failure-free survival than CCyR [39, 42]. In this analysis, >50% of patients who achieved MMR improved to a deeper response level, with 13 (28.9%) and 11 (24.4%) patients achieving MR4 and MR4.5, respectively.
After 2 years of median exposure, safety results were consistent with the known safety profile of asciminib; no new or worsening safety signals arose in this heavily pretreated patient population. Few patients (5 [10.4%]) experienced AEs leading to discontinuation. Most AEs occurred early (within the first 6 months of treatment); this pattern was also observed in pretreated patients with CML-CP without the T315I mutation from this trial and the phase III ASCEMBL trial [25, 43]. New AEs occurring in ≥10% of patients predominantly after 6 months of treatment included increased lipase level, upper respiratory tract infection, cough, edema, pyrexia, dizziness, increased amylase level, vomiting, anemia, and lower respiratory tract infection; most were grade 1/2.
In the treatment of CML, TKIs have been associated with CV toxicity [18, 29, 44–50], and CV risk profile is a clinical concern in selecting, starting, and monitoring TKI treatment [46]. Results from preclinical models suggest that asciminib may have less potential for cardiotoxicity than ponatinib [51, 52]. In the current analysis, few patients (4 [8.3%]) experienced AOEs. The role of asciminib in these events remains uncertain due to multiple confounding factors, including treatment with multiple prior TKIs and baseline CV risk factors; data from ongoing studies of asciminib in treatment-naive patients will further clarify the CV risk profile of asciminib. In a cohort of 115 patients with CML-CP without the T315I mutation from this trial treated with asciminib doses ranging from 10 to 200 mg QD or BID, with 4.2 years’ median exposure, 10 (8.7%) patients experienced AOEs; none led to treatment discontinuation [25]. In the ASCEND clinical trial of asciminib 40 mg BID starting dose in 101 newly diagnosed patients with median follow-up of 23 months, 1 patient had 2 vascular events [53]. In ASCEMBL, AOEs occurred in 3.2% of patients who received asciminib 40 mg BID with a median follow-up of 1.2 years [24]. In an updated analysis of ASCEMBL with a median follow-up of 2.3 years, the frequency of AOEs rose to 5.1%, but the exposure-adjusted AOE rate (3.0 per 100 patient-years) decreased from that of the primary analysis (3.3) [43, 54]. Patients in these analyses who experienced AOEs while receiving asciminib received several prior TKIs and had multiple baseline CV risk factors [25, 43, 55]. Of 270 patients with CML-CP who received ponatinib 45 mg QD in PACE, 84 (31%) experienced ≥1 AOEs (including CV [16%], cerebrovascular [13%], and peripheral vascular [14%] events) after a median exposure of 32.1 months (range, 0.1–73.0) [13]. OPTIC used ponatinib dose-reduction strategies to assess treatment efficacy while limiting the occurrence of AOEs; after 32 months median follow-up, AOEs were reported in 9.6%, 5.3%, and 3.2% of patients receiving ponatinib 45, 30, and 15 mg, respectively [16]. Ponatinib response-based dose-reduction strategies reduced the risk of AOEs by approximately 60% [21]. In this analysis of X2101, only 1 patient experienced cardiac failure, which occurred contemporaneously with COVID-19 pneumonia from which the patient later died. The role of asciminib remains uncertain due to pretreatment with multiple prior TKIs, baseline CV risk factors, and lack of pretreatment assessment of ejection fraction. While the reported CV events do not constitute a safety signal, risk factors and comorbidities should be closely monitored and managed during asciminib therapy in accordance with clinical practice recommendations.
Pancreatic toxicity poses a safety concern for patients with CML receiving TKI therapy and warrants clinical awareness as enzyme elevations may occur in the absence of evidence of pancreatitis, although the risk factors and mechanism of TKI-associated pancreatic toxicity are unknown [27, 56, 57]. In this analysis with asciminib, pancreatic toxicity events (31.3%) were mainly asymptomatic enzyme elevations. Only 1 patient experienced pancreatitis, which was grade 2 and did not require treatment changes. In a previous analysis of this trial, which included patients with CML-CP/AP with and without the T315I mutation receiving asciminib monotherapy 10 to 200 mg QD or BID (14 months’ median follow-up), clinical pancreatitis occurred in 3% of patients and resolved in all patients within 10 days of discontinuing asciminib [26]. In a cohort of 115 patients with CML-CP without the T315I mutation from this trial, pancreatic enzyme elevations and clinical pancreatitis were reported in 46 (40.0%) and 8 (7.0%) patients, respectively [25]. In contrast, no cases of pancreatitis were reported in ASCEMBL [24, 43]. The asciminib prescribing information recommends monitoring patients monthly or as clinically indicated for pancreatic toxicity, with dose modifications as needed [29].
In the current study, across all observed molecular endpoints, more patients achieved milestones receiving asciminib without prior ponatinib treatment; overall rates were 1.7-, 2-, 2.2-, and 2.4-fold higher in ponatinib-naive than -pretreated patients for BCR::ABL1IS ≤1%, MMR, MR4, and MR4.5, respectively. A similar trend was observed in a real-world analysis of patients with CML treated with asciminib, including 4% with the T315I mutation; in ponatinib-pretreated and -naive patients, respectively, without the indicated response at baseline, 27.3% and 53.3% achieved CCyR, 20% and 52.2% achieved MMR, and 10.5% and 19.4% achieved MR4.5 after a median follow-up of 30 months [58]. In the current study, rates of BCR::ABL1IS ≤1% and MMR were 2.3- and 3.7-fold higher, respectively, in ponatinib-pretreated patients who discontinued ponatinib due to intolerance versus resistance. Responses were sustained regardless of prior treatment with ponatinib. The safety profile of asciminib was independent of prior ponatinib treatment, with no differences observed between populations in rates of grade ≥3 AEs or AEs requiring additional therapy, reinforcing the safety of asciminib across lines of treatment. AOEs were more frequent in ponatinib-pretreated than -naive patients. The tolerability of asciminib and higher efficacy in ponatinib-naive patients may provide rationale for using asciminib before ponatinib in patients with T315I-mutated CML-CP, although asciminib also retains activity after ponatinib.
In conclusion, asciminib exhibited clinical efficacy, a sustained safety profile, and tolerability in patients with T315I-mutated CML-CP who have limited treatment options. Sustained responses were observed in both ponatinib-naïve and -pretreated patients, and >50% of patients remained on therapy at 2 years’ median exposure. This analysis reinforces the utility of asciminib as a treatment option for patients with T315I-mutated CML-CP, regardless of ponatinib pretreatment status.
Supplementary information
Acknowledgements
This study was conducted by Novartis Pharmaceuticals Corporation. We thank the patients who participated in the trial and their families, and the staff at each site who assisted with the study. We also thank Chris Hofmann, PhD (Nucleus Global) for medical editorial assistance with this manuscript, which was funded by Novartis Pharmaceutical Corporation.
Author contributions
All authors (JEC, KS, D-WK, TPH, GE, MJM, AH, FL, MCH, MB, MD, YTG, JJWMJ, MT, VGGdS, PlC, DJD, AD, SC, FP, NA, DR) contributed to acquiring and interpreting data, writing anśd reviewing the manuscript, and reviewing and approving the final manuscript.
Data availability
Novartis is committed to sharing with qualified external researchers, access to patient-level data, and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described at www.clinicalstudydatarequest.com. The sequencing data described in this publication will be made available for any qualified request. To submit a request, please contact: novartis.datasharing@novartis.com.
Competing interests
JEC Novartis, Pfizer, Takeda, Sun Pharma, Terns Pharma, Incyte, and Bristol Myers Squibb: consulting fees; Novartis, Pfizer, Takeda, Sun Pharma, Ascentage: institutional research support. KS Novartis: research funding, honoraria. D-WK Novartis, Bristol Myers Squibb, Pfizer, IL-YANG, and Takeda: grants. TPH Novartis, Bristol Myers Squibb, and Enliven: consultancy, research funding. GE BMS, Incyte Biosciences, Novartis, and Pfizer: speaker. MJM Bristol Myers Squibb, Takeda, and Pfizer: personal fees. AH Bristol Myers Squibb, Pfizer: institutional research support; Novartis and Incyte: institutional research support, personal fees. FL Bristol Myers Squibb, Incyte, and Celgene: consultancy, honoraria; Novartis: consultancy, honoraria, and research funding. MCH Novartis, Deciphera, Theseus, and Blueprint Medicines: consultancy; Deciphera: speaker’s bureau; Jonathan David Foundation, VA Merit Review Grant (I01BX005358), and NCI R21 grant (R21CA263400): partial salary support. Prior to 2019, MCH held an equity interest in MolecularMD. MCH holds multiple patents on the diagnosis and/or treatment of gastrointestinal stromal tumors; one patent on treatment has been licensed by Oregon Health & Science University to Novartis. MB Bristol Myers Squibb, Celgene, Pfizer, Incyte, and Novartis: consultancy and honoraria; AbbVie: consultancy. MD Fusion Pharma, Blueprint, Pfizer, Novartis, Medscape, and Sangamo: consultancy; Pfizer: research funding; Takeda, Sangamo, and Blueprint: membership on board of directors or advisory committees. YTG Pfizer, Johnson & Johnson, Amgen, MSD Pharma, Novartis, EUSA Pharma, Roche, Bristol Myers Squibb, and AbbVie: honoraria. JJWMJ Novartis and Bristol Myers Squibb: research funding; Incyte: speaker’s fee; AbbVie, Novartis, Pfizer, and Incyte: honoraria; AbbVie, Alexion, Amgen, Astellas, Bristol Myers Squibb, Daiichi Sankyo, Janssen-Cilag, Olympus, Incyte, Sanofi Genzyme, Servier, Jazz, and Takeda: support for Apps for Care and Science nonprofit foundation of which J.J.W.M.J. is president. MT IMAGO: consultancy; Novartis and Takeda: research funding; Constellation Pharmaceuticals and Bristol Myers Squibb: membership on board of directors or advisory committees. VGGS Novartis, Pfizer, Bristol Myers Squibb, and Incyte: grants, nonfinancial support, and honoraria. PC Pfizer, Novartis, and Incyte: honoraria. DJD AbbVie, Novartis, Blueprint, and GlycoMimetics: grants; AbbVie, Novartis, Blueprint, and GlycoMimetics: research funding; AbbVie, Amgen, Autolus, Blueprint, Forty-Seven, GlycoMimetics, Incyte, Jazz, Kite, Novartis, Pfizer, Servier, and Takeda; consulting. AbbVie, Amgen, Autolus, Blueprint, Forty-Seven, GlycoMimetics, Incyte, Jazz, Kite, Novartis, Pfizer, Servier, and Takeda: personal fees. AD, SC, FP, and NA are employees of Novartis. DR Novartis, Pfizer, and Incyte: personal fees.
Ethics approval
The protocol was approved by the sites’ institutional review boards and/or ethics committees. The study was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Conference on Harmonization. All patients provided written informed consent.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41375-024-02278-8.
References
- 1.Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34:966–84. doi: 10.1038/s41375-020-0776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel AB, O’Hare T, Deininger MW. Mechanisms of resistance to ABL kinase inhibition in chronic myeloid leukemia and the development of next generation ABL kinase inhibitors. Hematol Oncol Clin N Am. 2017;31:589–612. doi: 10.1016/j.hoc.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortes J, Lang F. Third-line therapy for chronic myeloid leukemia: current status and future directions. J Hematol Oncol. 2021;14:44. doi: 10.1186/s13045-021-01055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akard LP, Albitar M, Hill CE, Pinilla-Ibarz J. The “hit hard and hit early” approach to the treatment of chronic myeloid leukemia: implications of the updated National Comprehensive Cancer Network clinical practice guidelines for routine practice. Clin Adv Hematol Oncol. 2013;11:421–32. [Google Scholar]
- 5.Jabbour E, Kantarjian H, Cortes J. Use of second- and third-generation tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia: an evolving treatment paradigm. Clin Lymphoma Myeloma Leuk. 2015;15:323–34. doi: 10.1016/j.clml.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosi GR, Fogliatto LM, Costa TEV, Grokoski KC, Pereira MP, Bugs N, et al. What happens to intolerant, relapsed or refractory chronic myeloid leukemia patients without access to clinical trials? Hematol Transfus Cell Ther. 2019;41:222–8. doi: 10.1016/j.htct.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jabbour E, Parikh SA, Kantarjian H, Cortes J. Chronic myeloid leukemia: mechanisms of resistance and treatment. Hematol Oncol Clin N Am. 2011;25:981–95. doi: 10.1016/j.hoc.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicolini FE, Ibrahim AR, Soverini S, Martinelli G, Muller MC, Hochhaus A, et al. The BCR-ABLT315I mutation compromises survival in chronic phase chronic myelogenous leukemia patients resistant to tyrosine kinase inhibitors, in a matched pair analysis. Haematologica. 2013;98:1510–6. doi: 10.3324/haematol.2012.080234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soverini S, Branford S, Nicolini FE, Talpaz M, Deininger MW, Martinelli G, et al. Implications of BCR-ABL1 kinase domain-mediated resistance in chronic myeloid leukemia. Leuk Res. 2014;38:10–20. doi: 10.1016/j.leukres.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Dhillon S. Olverembatinib: first approval. Drugs. 2022;82:469–75. doi: 10.1007/s40265-022-01680-9. [DOI] [PubMed] [Google Scholar]
- 11.Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–80. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 12.Manley PW, Barys L, Cowan-Jacob SW. The specificity of asciminib, a potential treatment for chronic myeloid leukemia, as a myristate-pocket binding ABL inhibitor and analysis of its interactions with mutant forms of BCR-ABL1 kinase. Leuk Res. 2020;98:106458. doi: 10.1016/j.leukres.2020.106458. [DOI] [PubMed] [Google Scholar]
- 13.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393–404. doi: 10.1182/blood-2016-09-739086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deininger MW, Apperley JF, Arthur CK, Chuah C, Hochhaus A, De Lavallade H, et al. Post hoc analysis of responses to ponatinib in patients with chronic-phase chronic myeloid leukemia (CP-CML) By baseline BCR-ABL1 level and baseline mutation status in the OPTIC Trial. Blood. 2021;138:307. [Google Scholar]
- 15.Boddu P, Shah AR, Borthakur G, Verstovsek S, Garcia-Manero G, Daver N, et al. Life after ponatinib failure: outcomes of chronic and accelerated phase CML patients who discontinued ponatinib in the salvage setting. Leuk Lymphoma. 2018;59:1312–22. doi: 10.1080/10428194.2017.1379076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortes J, Apperley J, Lomaia E, Moiraghi B, Undurraga Sutton M, Pavlovsky C, et al. Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: a randomized, open-label phase 2 clinical trial. Blood. 2021;138:2042–50. doi: 10.1182/blood.2021012082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molica M, Scalzulli E, Colafigli G, Foa R, Breccia M. Insights into the optimal use of ponatinib in patients with chronic phase chronic myeloid leukaemia. Ther Adv Hematol. 2019;10:2040620719826444. doi: 10.1177/2040620719826444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iclusig (Ponatinib) [package insert]. Cambridge MTPCL.
- 19.Jabbour E, Kantarjian H, O’Brien S, Shan J, Quintas-Cardama A, Faderl S, et al. The achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood. 2011;118:4541–6. doi: 10.1182/blood-2011-04-348110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hehlmann R, Lauseker M, Jung-Munkwitz S, Leitner A, Muller MC, Pletsch N, et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-alpha in newly diagnosed chronic myeloid leukemia. J Clin Oncol. 2011;29:1634–42. doi: 10.1200/JCO.2010.32.0598. [DOI] [PubMed] [Google Scholar]
- 21.Jabbour E, Apperley J, Cortes J, Rea D, Deininger M, Abruzzese E, et al. Dose modification dynamics of ponatinib in patients with chronic-phase chronic myeloid leukemia (CP-CML) from the PACE and OPTIC trials. Leukemia. 2024;38:475–81. doi: 10.1038/s41375-024-02159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoepfer J, Jahnke W, Berellini G, Buonamici S, Cotesta S, Cowan-Jacob SW, et al. Discovery of asciminib (ABL001), an allosteric inhibitor of the tyrosine kinase activity of BCR-ABL1. J Med Chem. 2018;61:8120–35. doi: 10.1021/acs.jmedchem.8b01040. [DOI] [PubMed] [Google Scholar]
- 23.Wylie AA, Schoepfer J, Jahnke W, Cowan-Jacob SW, Loo A, Furet P, et al. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature. 2017;543:733–7. doi: 10.1038/nature21702. [DOI] [PubMed] [Google Scholar]
- 24.Rea D, Mauro MJ, Boquimpani C, Minami Y, Lomaia E, Voloshin S, et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood. 2021;138:2031–41. doi: 10.1182/blood.2020009984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mauro MJ, Hughes TP, Kim D-W, Réa D, Cortes JE, Hochhaus A, et al. Asciminib monotherapy in patients with CML-CP without BCR::ABL1 T315I mutations treated with at least two prior TKIs: 4-Year phase 1 safety and efficacy results. Leukemia. 2023;37:1048–59. [DOI] [PMC free article] [PubMed]
- 26.Hughes TP, Mauro MJ, Cortes JE, Minami H, Rea D, DeAngelo DJ, et al. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. N Engl J Med. 2019;381:2315–26. doi: 10.1056/NEJMoa1902328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novartis Pharmaceuticals Corporation. NDA/BLA Multi-disciplinary Review and Evaluation {NDA 215358} SCEMBLIX (asciminib). 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/215358Orig1s000,Orig2s000MultidisciplineR.pdf. Accessed 29 Apr 2024.
- 28.Combes FP, Li YF, Hoch M, Lorenzo S, Ho YY, Sy SKB. Exposure-efficacy analysis of asciminib in Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase. Clin Pharmacol Ther. 2022;112:1040–50. [DOI] [PubMed]
- 29.Scemblix (asciminib) [package insert]. East Hanover, NJ; Novartis Pharmaceutical Corporation. 2023.
- 30.Hughes TP, Cortes JE, Réa D, Mauro MJ, Hochhaus A, Kim D-W, et al. Asciminib provides durable molecular responses in patients with chronic myeloid leukemia in chronic phase (CML-CP) with the T315I mutation: updated efficacy and safety data from a phase I trial. Presented at: EHA2022 Congress; June 9-17, 2022; Vienna, Austria, and Online. Poster P704.
- 31.Scemblix (asciminib) [prescribing information]. Macquarie Park N, Australia; Novartis Pharmaceuticals Australia Pty Limited; 2022.
- 32.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–51. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castagnetti F, Gugliotta G, Breccia M, Stagno F, Iurlo A, Albano F, et al. Long-term outcome of chronic myeloid leukemia patients treated frontline with imatinib. Leukemia. 2015;29:1823–31. doi: 10.1038/leu.2015.152. [DOI] [PubMed] [Google Scholar]
- 34.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 35.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783–96. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hehlmann R, Lauseker M, Saussele S, Pfirrmann M, Krause S, Kolb HJ, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31:2398–406. doi: 10.1038/leu.2017.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jabbour E, Kantarjian HM, Saglio G, Steegmann JL, Shah NP, Boque C, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2014;123:494–500. doi: 10.1182/blood-2013-06-511592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cortes J, Quintas-Cardama A, Kantarjian HM. Monitoring molecular response in chronic myeloid leukemia. Cancer. 2011;117:1113–22. doi: 10.1002/cncr.25527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hehlmann R, Muller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014;32:415–23. doi: 10.1200/JCO.2013.49.9020. [DOI] [PubMed] [Google Scholar]
- 40.Falchi L, Kantarjian HM, Wang X, Verma D, Quintas-Cardama A, O’Brien S, et al. Significance of deeper molecular responses in patients with chronic myeloid leukemia in early chronic phase treated with tyrosine kinase inhibitors. Am J Hematol. 2013;88:1024–9. doi: 10.1002/ajh.23560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Horton A, Lipton JH. Treatment outcomes in chronic myeloid leukemia: does one size fit all? J Natl Compr Canc Netw. 2020;18:1421–8. doi: 10.6004/jnccn.2020.7627. [DOI] [PubMed] [Google Scholar]
- 42.Etienne G, Dulucq S, Nicolini FE, Morrisset S, Fort MP, Schmitt A, et al. Achieving deeper molecular response is associated with a better clinical outcome in chronic myeloid leukemia patients on imatinib front-line therapy. Haematologica. 2014;99:458–64. doi: 10.3324/haematol.2013.095158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hochhaus A, Rea D, Boquimpani C, Minami Y, Cortes JE, Hughes TP, et al. Asciminib vs bosutinib in chronic-phase chronic myeloid leukemia previously treated with at least two tyrosine kinase inhibitors: longer-term follow-up of ASCEMBL. Leukemia. 2023;37:617–26. [DOI] [PMC free article] [PubMed]
- 44.Santoro M, Mancuso S, Accurso V, Di Lisi D, Novo G, Siragusa S. Cardiovascular issues in tyrosine kinase inhibitors treatments for chronic myeloid leukemia: a review. Front Physiol. 2021;12:675811. doi: 10.3389/fphys.2021.675811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cirmi S, El Abd A, Letinier L, Navarra M, Salvo F. Cardiovascular toxicity of tyrosine kinase inhibitors used in chronic myeloid leukemia: an analysis of the FDA adverse event reporting system database (FAERS) Cancers. 2020;12:826. doi: 10.3390/cancers12040826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aghel N, Delgado DH, Lipton JH. Cardiovascular toxicities of BCR-ABL tyrosine kinase inhibitors in chronic myeloid leukemia: preventive strategies and cardiovascular surveillance. Vasc Health Risk Manag. 2017;13:293–303. doi: 10.2147/VHRM.S108874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gleevec (imatinib) [package insert]. East Hanover, NJ; Novartis Pharmaceuticals Corporation. 2024.
- 48.Tasigna (nilotinib) [package insert]. East Hanover, NJ; Novartis Pharmaceuticals Corporation. 2024.
- 49.Sprycel (dasatinib) [package insert]. Princeton, NJ; Bristol-Myers Squibb Company. 2023.
- 50.Bosulif (bosutinib) [package insert]. New York, NY; Pfizer Labs. 2023.
- 51.Singh AP, Glennon MS, Umbarkar P, Gupte M, Galindo CL, Zhang Q, et al. Ponatinib-induced cardiotoxicity: delineating the signalling mechanisms and potential rescue strategies. Cardiovasc Res. 2019;115:966–77. doi: 10.1093/cvr/cvz006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng S, Jin P, Li H, Pei D, Shu X. Evaluation of CML TKI induced cardiovascular toxicity and development of potential rescue strategies in a zebrafish model. Front Pharmacol. 2021;12:740529. doi: 10.3389/fphar.2021.740529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeung DT, Shanmuganathan N, Reynolds J, Branford S, Walia M, Yong ASM, et al. Excellent early and major molecular responses observed with asciminib treatment for CP-CML: results from the ALLG CML13 Ascend-CML study. Presentation at: 65th ASH Annual Meeting and Exposition; December 9-12, 2023; San Diego, CA, & Online. Abstract P704.
- 54.Réa D, Hochhaus A, Mauro MJ, Minami Y, Lomaia E, Voloshin S, et al. Efficacy and safety results from ASCEMBL, a phase 3 study of asciminib vs bosutinib in patients with chronic myeloid leukemia in chronic phase after =/>2 prior tyrosine kinase inhibitors: wk 96 update. Presented at: EHA2022 Congress; June 9-17, 2022; Vienna, Austria, and Online. Abstract S155.
- 55.Rea D, Mauro MJ, Boquimpani C, Minami Y, Lomaia E, Voloshin S, et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after >/=2 prior TKIs. Blood. 2021;138:2031–41. [DOI] [PMC free article] [PubMed]
- 56.Steegmann JL, Baccarani M, Breccia M, Casado LF, Garcia-Gutierrez V, Hochhaus A, et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30:1648–71. doi: 10.1038/leu.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rea D. Management of adverse events associated with tyrosine kinase inhibitors in chronic myeloid leukemia. Ann Hematol. 2015;94:S149–58. doi: 10.1007/s00277-015-2318-y. [DOI] [PubMed] [Google Scholar]
- 58.Luna A, Perez-Lamas L, Boque C, Giraldo P, Xicoy B, Ruiz Nuno C, et al. Real-life analysis on safety and efficacy of asciminib for ponatinib pretreated patients with chronic myeloid leukemia. Ann Hematol. 2022;101:2263–70. doi: 10.1007/s00277-022-04932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Novartis is committed to sharing with qualified external researchers, access to patient-level data, and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described at www.clinicalstudydatarequest.com. The sequencing data described in this publication will be made available for any qualified request. To submit a request, please contact: novartis.datasharing@novartis.com.