Preimplantation genetic diagnosis (PGD) involves genetic analysis of artificially fertilised embryos to select an embryo with a desired genotype before it is implanted.1,2 Since the 1980s, over 2500 cycles of PGD have been performed worldwide.3 The technique has been used to test for disorders caused by a single gene (cystic fibrosis, thalassaemia, sickle cell disease, muscular dystrophy) and chromosomal abnormalities (Down's syndrome, trisomy 18).4 The procedure is regulated in the United Kingdom by the Human Fertilisation and Embryo Authority, which says it should be used only for detecting “very serious, life threatening conditions”5 and not for minor genetic abnormalities.4,6
The technique has been used to detect genes for adult onset disorders such as Huntington's disease and for familial predisposition to cancer, such Li-Fraumeni syndrome (which involves mutations in p53 cancer suppressor genes).3 It has been used in Australia by fertile couples without a history of sex linked disorders to select the sex of their child.7
Summary points
A common objection to using preimplantation genetic diagnosis (PGD) to choose an embryo that may produce a child who would provide stem cells for an existing person is that children conceived for the benefit of their siblings are not valued in their own right
The uptake of this procedure will have few social consequences and is likely to be a reasonable use of limited health resources
Using PGD to choose a stem cell donor is unlikely to cause harm to anyone and is likely be beneficial to some
In countries where PGD is already permitted, using PGD solely for choosing a HLA compatible embryo to provide stem cells for treating an existing person should also be permitted
PGD for the benefit of a relative
Children have been conceived to provide stem cells for their siblings. In the most publicised case, the Ayala case,8 Marissa Ayala was conceived in 1989 to provide stem cells for her sister Anissa. A later report noted, “Marissa is now a healthy four year old, and, by all accounts, as loved and cherished as her parents said she would be. The bone marrow transplant was a success and Anissa is now a married, leukemia-free bank clerk.”9 Assisted reproduction has been used to conceive children to provide stem cells for siblings.10
At the end of 1999, a couple in the United States underwent in vitro fertilisation and for the first time used PGD to screen their embryos for those whose tissue type matched that of their daughter, who had Fanconi's anaemia. Over four treatment cycles, five suitable embryos were implanted; one survived and at birth blood from the umbilical cord was harvested. This blood was used in a successful stem cell transplant for the daughter.11 In Britain the parents of a 2 year old boy with β thalassaemia applied in October 2001 to the Human Fertilisation and Embryo Authority for permission to select an embryo, using PGD, that can provide him with a matched stem cell transplant, again through umbilical cord blood taken at birth.12 These are the first publicly recorded cases of requests for PGD for the benefit of a relative; they are likely to herald further requests.
In both these cases, the technique fulfils two functions. Firstly, it is used to select embryos that do not have the genetic mutation that affects the family (Fanconi's anaemia or thalassaemia). This is a standard indication for PGD. Secondly, it is used to select an HLA compatible stem cell donor from these embryos.
Even more controversially, in both the United States and Australia there have been requests to use PGD to select a HLA compatible embryo to serve as a stem cell donor in the absence of any family history of genetic disease. Recently a British couple went to the United States to have in vitro fertilisation and PGD to select a stem cell donor for their child with relapsed leukaemia. The woman is currently pregnant.13
Practice guidelines, public attitudes, and the law
Currently, there are no clear professional guidelines specifically related to using PGD for the benefit of an existing person. The legal position is developing, and public opinion is not well known.
Professional organisations
The UK Human Genetics Commission recommends PGD be used only to detect “specific and serious conditions” in the embryo (see box).14 The World Health Organization has stated that prenatal diagnosis should be used only to gain information about the health of the fetus, but it does not give specific guidance on the indications for PGD.15
In a meeting last year, the American Society of Reproductive Medicine and the American Medical Association, considering the specific issue of PGD for the benefit of an existing person, agreed that the procedure was justified.16
Recommendations of Human Genetics Commission14
PGD should be limited to the detection of specific and serious conditions—“serious” is difficult to define in this context
PGD should not be used for trait selection or such that it could give rise to eugenic outcomes
Consistency is needed between conditions considered as appropriate for PGD and for prenatal diagnosis by amniocentesis or chorionic villus sampling
PGD to detect carrier status for an autosomal recessive condition should where possible be avoided
Guidance regarding PGD to select and implant embryos that are affected by a genetic condition has not yet been formulated
Legal considerations
Use of the technique is regulated in Britain by the Human Fertilisation and Embryology Authority, whose role is to interpret the Human Fertilisation and Embryology Act.6 In 1993, the authority made it clear that PGD for sex selection for social reasons would not be licensed. It has stated that testing for conditions which are “not associated with disability or a serious medical condition” would not be acceptable.7 Currently a joint Working Party of the Human Fertilisation and Embryo Authority and the Human Genetics Commission is considering a response to their recent public consultation exercise on PGD. It is likely that they will give guidance on the appropriate indications for PGD later this year.
Public attitudes
A recent UK survey by the Human Genetics Commission found support for using genetic information to detect disabling conditions before birth but considerable opposition to sex selection or to the selection of mental and physical characteristics of children.17 The public's views on selecting an embryo to attempt to save an existing person's life are not known.
Views of people with genetic disorders
Many people with particular genetic conditions support prenatal testing for that condition, but some oppose such testing on the basis that it discriminates against people living with the same condition.18,19 In contrast, the Genetic Interest Group, which represents support groups for families in Britain affected by genetic conditions, opposes “any and all attempts to restrict the range of medical conditions for which pre-implantation diagnosis can be performed.”20 The Human Genetics Commission has set up a consultative panel to canvass the views of people with genetic conditions on issues such as these, but currently their views regarding PGD for the benefit of an existing person are unclear.
Ethical issues
Commodification
Lord Winston described creating children to provide stem cells as “using an unborn child as a commodity.”5 The commonest objection to this procedure is that it is wrong to bring children into existence “conditionally.” This objection finds its philosophical foundation in Immanuel Kant's famous dictum, “Never use people as a means but always treat them as an end.”
Though common, this objection is difficult to sustain.21 Though we might aspire to a world where parents always dote on their children as unconditional ends, in reality many children are born for a purpose: to care for their parents, as a companion to a sibling, or to run the family business. Actually Kant's dictum was “Never use people solely as a means.” Provided that parents love their child, there is little problem with that child benefiting others. And, as the Ayala case illustrates, a child conceived for stem cell donation is likely to be valued as a person.
Best interests of the child
What if the stem cell transplant is unsuccessful? Would parents unconsciously blame the donor child? What will life be like for the child conceived to produce stem cells?
A principle of the Human Fertilisation and Embryology Act is that the best interests of the child produced by assisted reproduction must be paramount. Before the birth of Marissa Ayala, grim predictions were made about her prospects, but these proved to be false. Blanket predictions about how parents will treat their children, and defining a set of conditions under which it is appropriate to allow people to parent, are dangerous and liable to be mistaken. Moreover, it is important to remember that the alternative for the child who was conceived to provide stem cells is not another life in which he or she was conceived in another way, but non-existence. If Abe Ayala had not had his vasectomy reversed to conceive Marissa as a stem cell donor, she simply would not have existed. Thus psychological harm to the offspring is unpredictable, unlikely to occur, and, even if it did occur, unlikely to be so severe that it would be better for that particular child never to have existed.
Pareto optimality and rational choice
Economists describe a Pareto optimal state of affairs as one that is at least as good as all alternative states of affairs in all relevant respects and better in some respects. Many would argue that it is rational to bring about a Pareto optimal state of affairs, assuming that resources may not be better used elsewhere. In the case before the Human Fertilisation and Embryo Authority involving a boy with thalassaemia, the couple would be entitled to use PGD to select an embryo that does not have the thalassaemia genes. Why not let them select one that would also be a compatible stem cell donor? Assuming that the couple can produce sufficient numbers of embryos, using PGD in this circumstance would bring about a Pareto optimal state of affairs: it would produce a child without thalassaemia, and it may save the life of an existing child. All the alternatives are equally likely to produce a new child without thalassaemia but less likely to save the existing child.
If it is rational to allow fertile couples with a history of genetic disease to use PGD in this circumstance, then the same principle of Pareto optimality makes it rational for fertile couples without a history of genetic disease to use in vitro fertilisation and PGD to have a child who will provide stem cells for an existing child. All the alternatives are likely to produce a new child but less likely to save the existing child.
Reduced genetic diversity
Some people might argue that by selecting embryos we risk reducing the genetic diversity of our species and exposing the human race to unforeseen risks. Such arguments are speculative. Moreover, the number of requests for PGD is likely to remain limited given the emotional and financial costs of the procedure.
Destruction of embryos
Another objection to this use of in vitro fertilisation and PGD is that it results in the unnecessary destruction of embryos that are non-compatible tissue donors but likely to be healthy. However, UK legislation allows embryos to be destroyed until 14 days of age.6,22 To prohibit couples from rejecting healthy but unwanted embryos in a society that condones the destruction of hundreds of thousands of healthy but unwanted fetuses would be wildly inconsistent. Moreover, couples should be encouraged to donate their healthy but unwanted embryos to other couples who cannot conceive.
Harm to society—“eugenics”
Is it harmful to society if families choose their children on the basis of their genetic makeup? There is opposition to the practice of seeking “designer babies,” fuelled by concerns about eugenics at an individual family and societal level.23 Though a compulsory national screening programme to prevent the implantation of embryos with certain genotypes would be eugenic, discriminatory, and akin to the Nazi eugenic project, the best way to prevent state-sponsored eugenics is to ensure that couples—not the state, professionals or other organisations—retain control over reproduction and the decision of which children to have.24
Moreover, selection of children on a much grander scale is already commonplace. An estimated 18 000 amniocenteses take place annually in Britain, mainly to detect chromosomal anomalies such as Down's syndrome.4 Using PGD to select a stem cell donor will have little if any effect on the gene pool or on society more generally.
Moral disapproval by society
Some people find the use of PGD to select children distasteful and offensive. The Human Fertilisation and Embryology Act has arguably been formed to reflect a dominant Christian morality and to protect against offence to that morality. However, liberal societies have a presumption in favour of individual freedom of action unless there is a clear harm to others. Although the United Kingdom and other democratic countries may have laws that prevent gratuitous offence to others (such as creating earrings from embryos), we should be loath to restrict liberty in the absence of evidence of serious harm to others, especially in private behaviour and especially when the activity in question is potentially life-saving (and life-creating).9
The famous legal scholar H L A Hart in the 1960s argued effectively against Lord Devlin in relation to the Wolfenden report on sexual behaviour that there is a sphere of private conduct that should be immune to legislation regardless of popular opinion, and that popular opinion or even morals are not always sufficient grounds for legislation. Such arguments were important in repealing the laws that made homosexuality illegal. Similarly, unless our private reproductive decisions cause harm to others, they should remain immune to legislation even if some people morally disapprove of them.25,26
Rationing of resources
To justify providing these procedures within a public health service such as the NHS, it must be shown that allocation of the necessary resources is appropriate. The lifetime treatment costs for someone with β thalassaemia in Britain are estimated at close to £200 000 ($295 000).27 This is likely to be considerably higher than the cost of tissue typing using PGD and subsequent stem cell transplantation, although a precise costing for this procedure is not available.28,29 There is clearly no economic justification for restricting this procedure.
For couples with no family history of genetic disease (who would not be entitled under current arrangements to use in vitro fertilisation and PGD within the NHS), in vitro fertilisation could be funded as a part of the cost of the treatment of the sibling (as it provides a stem cell source) or funded by the couple privately.
Conclusions
Who is harmed by allowing PGD to be performed solely for the benefit of a relative? Not the couple who wish to produce an embryo. Nor the child who would not otherwise have existed. Nor the person who receives the stem cell transplant that might save his or her life. We must avoid the trap of interfering with individual liberty by preventing such procedures for no good reason, simply out of the “genophobia” that grips much of society today. Some people object to using PGD along with in vitro fertilisation for any indication. But if these procedures are acceptable, as they are in many countries, it is reasonable to use them to both bring a new person into the world and to help save an existing life.
Figure 1.
MARK ENGEBRETTSON/UNIVERSITY OF MINNESOTA
Molly Nash (left), who had Fanconi's anaemia, received stem cells from Adam, seen here with Dr John Wagner
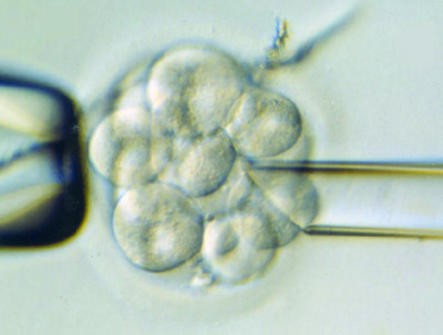
Figure 2.
Cell biopsy for preimplantatation genetic diagnosis
Footnotes
Competing interests: JS is employed by the Murdoch Children's Research Institute, which conducts research into children's health, including genetic research.
References
- 1.Handyside AH, Konotogianni EH, Hardy K, Winston RML. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature. 1990;244:768–770. doi: 10.1038/344768a0. [DOI] [PubMed] [Google Scholar]
- 2.Handyside AH, Lesko JG, Tarin JJ, Winston RML, Hughes MR. Birth of a normal girl after IVF and preimplantation diagnostic testing for cystic fibrosis. N Engl J Med. 1992;327:905–909. doi: 10.1056/NEJM199209243271301. [DOI] [PubMed] [Google Scholar]
- 3.Verlinsky Y, Rechitsky S, Verlinsky O, et al. Preimplantation diagnosis for p53 tumour suppressor gene mutations. Reproductive Biomedicine Online. 2000;2:102–105. doi: 10.1016/s1472-6483(10)62233-x. www.rbmonline.com/4DCGI/Article/Detail?38%091%09=%2055%09 (accessed 2 Nov 2001). [DOI] [PubMed] [Google Scholar]
- 4.Human Fertilisation and Embryology Authority and Advisory Committee on Genetic Testing 2000. Consultation document on preimplantation genetic diagnosis. www.hfea.gov.uk/pgd/pgdpaper.pdf (accessed 2 Nov 2001).
- 5.Parents seek test tube “lifesaver”. BBC News 1 Oct 2001. http://news.bbc.co.uk/hi/english/uk/england/newsid_1572000/1572106.stm (accessed 1 Oct 2001).
- 6.Human Fertilisation and Embryology Act 1990 (C.37). Norwich: HMSO; 1990. www.hmso.gov.uk/acts/acts1990/Ukpga_19900037_en_1.htm (accessed 2 Nov 2001). [Google Scholar]
- 7.Savulescu J. Sex selection—the case for. Med J Austr. 1999;171:373–375. doi: 10.5694/j.1326-5377.1999.tb123697.x. [DOI] [PubMed] [Google Scholar]
- 8.Rachels J. When philosophers shoot from the hip. Bioethics. 1991;5:66–71. doi: 10.1111/j.1467-8519.1991.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 9.Hastings Center Report. 1994. May/June:2. [Google Scholar]
- 10.Lamperd R. Race for life. Sun Herald 1998 Jul 2:1.
- 11.Verlinsky Y, Rechitsky S, Schoolcraft W, Strom C, Kuliev A. Preimplantation diagnosis for Fanconi anemia combined with HLA matching. JAMA. 2001;285:3130–3133. doi: 10.1001/jama.285.24.3130. [DOI] [PubMed] [Google Scholar]
- 12.Kmietowicz Z. Couple asks permission to select an embryo to save son's life. BMJ. 2001;323:767. [Google Scholar]
- 13.Meek J. The designer child from love and desperation. The Age 2001 Oct 22:B4.
- 14.Human Genetics Commission. Response to the Human Fertilisation and Embryology Authority on the consultation on preimplantation genetic diagnosis. March 2001. www.hgc.gov.uk/business_publications_statement_pgd.htm (accessed 2 Nov 2001).
- 15.Foubister V. Ethicists debate new use of genetic testing. American Medical News 2001 Jan 15. www.ama-assn.org/sci-pubs/amnews/pick_01/prse0115.htm (accessed 2 Nov 2001).
- 16.Proposed international guidelines on ethical issues in medical genetics and genetic services. Report of a WHO meeting on ethical issues in medical genetics. Geneva: World Health Organization; 1998. www.who.int/ncd/hgn/hgnethic.htm . (WHO/HGN/ GL/ETH/98.1) www.who.int/ncd/hgn/hgnethic.htm (accessed 2 Nov 2001). (accessed 2 Nov 2001). [PubMed] [Google Scholar]
- 17.Human Genetics Commission. Public attitudes to human genetic information: People's Panel quantitative study conducted for the Human Genetics Commission. www.servicefirst.gov.uk/2001/panel/hgc/index.htm (accessed 2 Nov 2001).
- 18.Conway SP, Allenby K, Pond MN. Patient and parental attitudes toward genetic screening and its implications at an adult cystic fibrosis centre. Clin Genet. 1994;45:308–312. doi: 10.1111/j.1399-0004.1994.tb04037.x. [DOI] [PubMed] [Google Scholar]
- 19.Shakespeare T. Eugenics? Slipping down the slope. Splice of Life 1998/9:5(2). www.geneticsforum.org.uk/eugenic.htm (accessed 2 Nov 2001).
- 20.Genetic Interest Group. Genetic testing, screening and “eugenics”. London: Genetic Interest Group; 1999. www.gig.org.uk/docs/gig_eugenics.pdf (accessed 2 Nov 2001). [Google Scholar]
- 21.Burley J, Harris J. Human cloning and child welfare. J Med Ethics. 1999;25:108–113. doi: 10.1136/jme.25.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Secretary of State for Health. Government response to the recommendations made in the chief medical officer's expert group report “Stem cell research: medical progress with responsibility”. London: Stationery Office; 2000. www.doh.gov.uk/cegc/govresp.htm . (Cm 4833.) www.doh.gov.uk/cegc/govresp.htm (accessed 2 Nov 2001). (accessed 2 Nov 2001). [DOI] [PubMed] [Google Scholar]
- 23.Appleyard B. Brave new worlds: genetics and the human experience. London: Harper Collins; 1999. [Google Scholar]
- 24.Glover J. Eugenics: Some lessons from the Nazi experience. In: Harris J, Holm S, editors. The future of human reproduction: ethics, choice and regulation. Oxford: Clarendon Press; 1998. pp. 55–65. [Google Scholar]
- 25.Devlin P. The enforcement of morals. London: Oxford University Press; 1959. [Google Scholar]
- 26.Hart HLA. Law, liberty and morality. Stanford, CA: Stanford University Press; 1963. [Google Scholar]
- 27.Karnon J, Zeuner D, Brown J, Ades AE, Wonke B, Modell B. Lifetime treatment costs of beta-thalassaemia major. Clin Lab Haematol. 1999;21:377–385. doi: 10.1046/j.1365-2257.1999.00262.x. [DOI] [PubMed] [Google Scholar]
- 28.Smith TJ, Hillner BE, Schmitz N, Linch DC, Dreger P, Goldstone AH, et al. Economic analysis of a randomised clinical trial to compare filgrastim-mobilized peripheral-blood progenitor-cell transplantation and autologous bone marrow transplantation in patients with Hodgkin's and non-Hodgkin's lymphoma. J Clin Oncol. 1997;15:5–10. doi: 10.1200/JCO.1997.15.1.5. [DOI] [PubMed] [Google Scholar]
- 29.Lavery SA, Aurell R, Turner C, Taylor DM, Winston RM. An analysis of the demand for and cost of preimplantation genetic diagnosis in the United Kingdom. Prenat Diagn. 1999;19:1205–1208. [PubMed] [Google Scholar]