Abstract
This study aimed to examine the effect of Phosphodiesterase 4 (PDE4) inhibition on Aquaporin-5 (AQP5) and its potential cell signaling pathway in the ovarian ischemia reperfusion (OIR) model. Thirty adult female rats were divided into five groups: Group 1; Control: Sham operation, Group 2; OIR that 3 hour ischemia followed by 3 hour reperfusion, Group 3; OIR + Rolipram 1 mg/kg, Group 4; OIR + Rolipram 3 mg/kg, Group 5; OIR + Rolipram 5 mg/kg. Rolipram was administered intraperitoneally to the rats in groups 3-4 and 5 at determined doses 30 minutes before reperfusion. From ovary tissue; Tumor necrosis factor-a (TNF-α), Cyclic adenosine monophosphate (cAMP), Nuclear factor kappa (NF-κB), Interleukin-6 (IL-6), Phosphodiesterase 4D (PDE4D), Mitogen-activated protein kinase (MAPK) and AQP5 levels were measured by ELISA. We also measured the level of AQP5 in ovary tissue by real-time reverse-transcription polymerase chain reaction (RT-PCR). In the OIR groups; TNF-α, NF-κB, IL-6, MAPK inflammatory levels increased, and cAMP and AQP5 levels decreased, which improved with the administration of rolipram doses. Also histopathological results showed damaged ovarian tissue after OIR, while rolipram administration decrased tissue damage in a dose dependent manner. We propose that the protective effect of PDE4 inhibition in OIR may be regulated by AQP5 and its potential cell signaling pathway and may be a new target in OIR therapy. However, clinical studies are needed to appraise these data in humans.
Keywords: Ischemia, Ovary, PDE4, Reperfusion, Rolipram
Introduction
Ovarian torsion is a gynecological emergency that can affect women of all ages, particularly women of reproductive age, and leads to ovarian ischemia reperfusion (OIR) injury through detorsion [1]. However, reperfusion that occurs as a result of detorsion of the ovaries has some systemic and local effects. Thus, with the ischemic damage caused by torsion, reperfusion damage consist of in the ovaries and this causes an ischemia/reperfusion (I/R) issue [2]. This is accompanied by the release of large amounts of free radicals, with oxidative tissue damage having a significant impact on the damage caused by OIR [3] In cases of ovarian torsion, an I/R injury may develop due to the release of free radicals and reactive oxygen species (ROS) during the detorsion process [4]. Excessive production of ROS, which are hydroxyl radicals, hydrogen peroxide and superoxide radicals, is one of the complications of I/R that causes serious damage to reproductive tissues [5]. Ovarian torsion occurs with a frequency of 2.7-7.4%, depending on the series, and most frequently during the reproductive years, and patients are usually in their mid-20s [6] and occur with a frequency of about 15% among infants and children [7].Therefore, early diagnosis and treatment is important in this disease and increases the chance of preserving ovarian function and fertility [8]. Phosphodiesterases (PDEs) are enzymes consisting of 11 subtypes PDE1-PDE11 and hydrolyzing two secondary messengers, cyclic guanosine mono-phosphate (cGMP) and cyclic adenosine monophosphate (cAMP) [9]. PDEs are involved in the regulation of many physiological mechanisms including gene expression, cell proliferation, differentiation, apoptosis, metabolism, visual transduction, inflammation [10]. PDE4 is one of the PDE families responsible for the hydrolysis of cAMP. This is accompanied by the release of large quantity of free radicals, with oxidative tissue damage having a significant impact on the damage caused by OIR and separated into four subtypes PDE4A/4B/4C/4D [11]. PDE4 is commonly found in immune and inflammatory cells such as neutrophils, macrophages and monocytes [12]. Selective inhibition of PDE4 contributes to the suppression of many aspects of the inflammatory response by increasing the cAMP content in many immunomodulatory and inflammatory cells [13]. Few studies have reported signals of activation of inflammatory cells due to an increase in cAMP [14]. However, the importance of activation of cAMP signaling on the overall response of inflammatory cells is mostly unknown [15]. Rolipram is known as a selective PDE4 inhibitor has been shown in previous experimental studies to suppress local increases in vascular permeability and neutrophil recruitment following I/R injury in a dose-dependent manner (1 to 10 mg/kg) and increases intracellular cAMP levels in many tissues and cell types [16].
Aquaporins (AQPs) not only regulate transepithelial fluid transport across membranes, but are also involved in the regulation of key events crucial for the inflammatory response. Several studies have shown that AQPs including AQP5, have important roles in inflammatory diseases. This clearly indicates that AQPs may be potential targets in inflammatory diseases including I/R [17, 18]. As there is an urgent need for further preventive treatment to preserve ovary tissue during OIR injuries, we considered examining the different pathways that cause such damage. Hence, this study aimed to investigate the relationship between PDE4, which is widely present in inflammatory cells, and AQP5 in the development of inflammation in OIR injury. Therefore, we tried to appraise for the first time the role of PDE4 inhibitor rolipram in an OIR-induced injury model and its relationship in distinct pathways mediating this injury to IL-6/ NF-κB/TNF-α /MAPK and cAMP/AQP5.
Materials and Methods
Chemicals
Rolipram (R0110) was purchased from TCI (Zwijndrecht, Belgium). TNF-α (Catalog #201-11-0765), IL-6 (Catalog #201-11-0136), cAMP (Catalog #201-11-029), AQP5 (Catalog #201-11-0570), MAPK (Catalog#201-11-1064), NF-κB (Catalog#201-11-0288), cAMP Spesific (PDE4D) (Catalog #201-12-4539) ELISA kits were obtained from SunRed (Shanghai, China) and AQP5 250 rxn (Rn00562837_m1) was purchased from Thermo Fisher Scientific (Budapest, Hungary).
Animals
Wistar albino rats, approximately 12 weeks old and weighing 220-240 grams, were kept in steel cages under standard conditions (7am-8pm light period, 55% relative humidity, and 21±2 °C) throughout the experiments, and were given tap water and standard pellet feed ad libitum. All animal protocols and care were confirmed by Experimental Animal Ethics Committee of Ataturk University (13.01.2023/13).
Preparation and Treatment
Rats were separated into five groups (n=6), fasted for 24 hours, and administered the following chemicals:
Group 1: Control: Sham operation performed.
Group 2: OIR
Group 3: OIR + Rolipram (1 mg/kg) single i.p. dose 30 min before reperfusion
Group 4: OIR + Rolipram (3mg/kg) single i.p. dose 30 min before reperfusion
Group 5: OIR + Rolipram (5mg/kg) single i.p. dose 30 min before reperfusion
Surgical Protocol
All procedures were performed under sterile conditions and general anesthesia. For general anesthesia, each rat was injected intraperitoneally with 5 mg/kg xylazine hydrochloride and 45 mg/kg ketamine hydrochloride. The abdominal skin was shaved and cleaned with 10% povidone iodine. The lower abdomen was opened with a 2 cm midline incision and bilateral ovaries were exposed. After the ovaries were identified, ischemia was created with atraumatic vascular clamps (Bulldog clamps) just below the left ovary and the incisions were closed with 4-0 silk sutures. After a 3 hours period of ischaemia, the atraumatic vascular clamps were removed and reperfusion was achieved for 3 hours. In the control group, only laparotomy was performed. 1, 3 and 5 mg/kg rolipram [16, 19, 20] was administered intraperitoneally to the rats in groups 3, 4 and 5 respectively, 30 minutes before reperfusion. To protect the rats from hypothermia, the operating table was heated using a lamp from above and a heater from below. During the waiting period, the incision line in the abdominal area was closed with 3-0 silk suture. In the final stage of the experiment, all rats were sacrificed, their ovaries were removed and stored in %10 formaldehyde for histopathological and molecular examinations [21].
Molecular Measurements
Measurement of Ovary Tissue
After the surgical procedures, about 100 mg of ovary tissue was homogenized in 2 ml of phosphate-buffered saline (PBS) in eppendorf tubes using a homogenizer (TissueLyser II by QIAGEN), and then centrifuged. AQP5, NF-κB, MAPK, PDE4D, cAMP, IL-6, and TNF-α levels from the obtained supernatants were measured by ELISA method in Epoch Spectrophotometer System and Take3 Plate device. An equation was obtained from the absorbance of the standards by plotting a standard curve. Linear AQP5, NF-κB, MAPK PDE4, cAMP, IL-6, and TNF-α concentrations were calculated according to this equation as previously described [22]. All ELISA kits were used according to manufacturer’s directives and measured using a BioTek Epoch Microplate Spectrophotometer.
Evaluation Of AQP5 With real-time reverse-transcription polymerase chain reaction (RT-PCR)
Ribonucleic Acid(RNA) Extraction from Ovary Tissue
Rat ovary tissue was individually weighed, homogenized in the Tissue Lyser II device (350 µl of RLT buffer was added to 20-30 mg of tissue) and RNA was extracted in the QIAcube RNA isolation tool. Total RNA was isolated in the Qiaqube RNA isolation tool using the RNeasy Mini Kit. Total mRNA quantity was measured by nano drop spectrophotometry at 260/280 nm. The resulting RNA was stored -80 °C under the necessary conditions.
Reverse Transcriptase Reaction and Synthesis of Complementary DNA (cDNA)
Using the High Capacity cDNA Reverse Transcription Kit enzyme, cDNA synthesis was performed from total RNA. Each reaction was actualized with 10 μl of RNA and cDNA synthesis was achieved with Veriti 96 Well Thermal Cycler according to the specified temperature values. The quantity of cDNA was determined by nano drop spectrophotometry and stored at -20˚C. For cDNA synthesis, 10 μl total RNA; 10 X RT 30 Buffer 2 μl; MultiScribe 10 X RT Random Primers 2 μl; Reverse Transcriptase 1 μl; 25 X dNTPs mix 0.8 μl; DEPC-H2O 4.2 μl) were used [23].
Quantitative Detection of AQP5 mRNA Expressions by RT-PCR
The relative expression analyses of AQP5 were performed with the StepOnePlus Real Time PCR System (Applied Biosystems) using cDNA synthesized from rat ovary RNA. The real-time quantitative reverse transcriptase PCR was run using Primer Perfect Probe mix, TaqMan Probe-based technology (Primer Design Ltd., Southampton, UK), and the results were expressed as the relative-fold change in expression as compared with that in the control animals. Specific primer were used for rat gene transcripts Rn00562837_m1. The gene expression levels were normalized using β-actin (Rn00667869_m1) as a housekeeping gene. For each tissue, triplicate determinations were performed in a 96-well optical plate for all parameters using 9 μL of cDNA (100 ng), 1 μL of Primer Perfect Probe mix, and 10 μL of QuantiTect Probe PCR Master mix (Qiagen) in each 20 ml reaction. The plates were heated for 2 min at 50 °C and 10 min at 95 °C, followed by 40 cycles of 15 s at 94 °C and 60 s at 60 °C. All data are expressed as the fold-change in expression as compared with the expression in other animal groups, using the 2−ΔΔCt method [24, 25].
Histopathological Analyzes
Ovary tissue samples were fixed in 10% formalin solution for 72 hours. Subsequently, routine tissue processing was performed. Tissues were first washed in running water for 20 minutes and formalin was removed. Then, water was removed from the tissues by passing one hour through increasing alcohol series (50, 60, 70, 80, 96 and 99). Then, the tissue samples were made transparent by passing through the xylene series 3 times for 15 minutes. Finally, the tissues were thrown into molten paraffin, which was kept in an oven at 60°C, and 2 changes were kept for 1 hour each, and the paraffin was absorbed into the tissue. Tissue samples were then blocked for sectioning. Paraffin blocks were cut with a microtome at a thickness of 5 microns. Sections were stained with hematoxylin and eosin stains. The sections were examined and photographed with an Olimpus CX 21 camera-attached microscope. Finally, the images were brought together with Photoshop CS5.
Statistical Analyses
All the results were expressed as mean standard deviation (SD) of the mean. The numerical data regarding molecular and biochemical analyses were subjected to one-way analysis of variance (ANOVA) using the IBM SPSS Statistics 20.0 software program (SPSS Inc., Chicago, IL). Differences among the groups were determined using the Tukey test and were considered to be significant when the p values was less than 0.05 in a 95% confidence interval.
Results
Molecular Findings
Inflammatory Cytokine ELISA Results
TNF-α levels were measured in ovary tissue. TNF-α levels were significantly increased in the ovary tissue of OIR induced rats compared to the control group (p<0.001). With the application of PDE4 inhibitor rolipram, TNF-α levels were significantly decreased in the OIR+ROL3, OIR+ROL5 group compared to the OIR group (p<0.01) (Fig. 1).
Fig. 1.
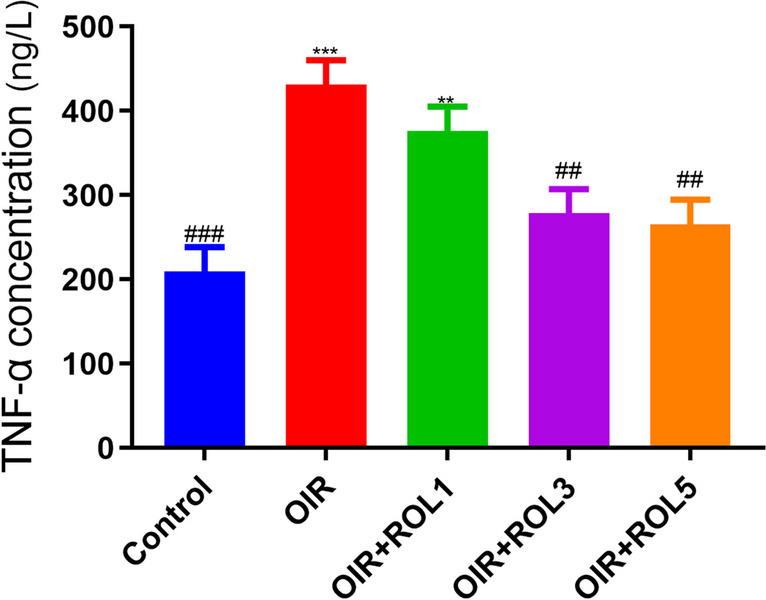
Effects of Rolipram (ROL) on TNF-α levels in ovarian ischemia-reperfusion injury. *p<0.05 **p<0.01 ***p<0.001 indicate comparison with control group. #p<0.05, ##p<0.01 and ###p<0.001 indicate the comparison by OIR group according to Tukey test. Results are given as mean ± standard deviation. ROL 1: Rolipram 1 mg/kg, ROL 3: Rolipram 3 mg/kg, ROL 5: Rolipram 5 mg/kg
IL-6 levels were measured in ovary tissue. IL-6 levels in the ovary tissue of OIR-induced rats were significantly increased compared to the control group (p<0.01). With the administration of PDE4 inhibitor rolipram, IL-6 levels decreased in a dose-dependent manner compared to the OIR group. Namely, while it decreased significantly in the OIR+ROL1 group (p<0.05); It was evaluated that OIR+ROL3 (p<0.01), OIR+ROL5 (p<0.01) decreased significantly (Fig. 2).
Fig. 2.
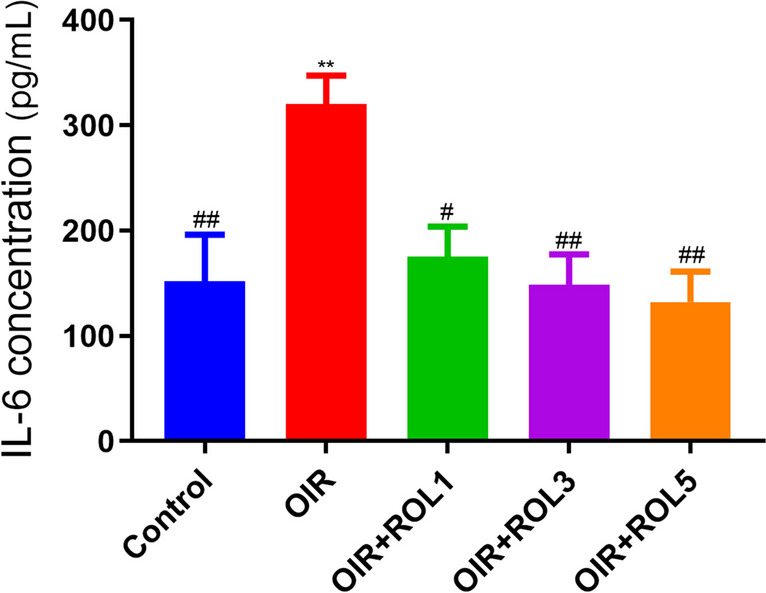
Effects of Rolipram (ROL) on IL-6 levels in ovarian ischemia-reperfusion injury. *p<0.05 **p<0.01 ***p<0.001 indicate comparison with control group. #p<0.05, ##p<0.01 and ###p<0.001 indicate the comparison by OIR group according to Tukey test. Results are given as mean ± standard deviation. ROL 1: Rolipram 1 mg/kg, ROL 3: Rolipram 3 mg/kg, ROL 5: Rolipram 5 mg/kg
AQP5 ELISA Result
In our study, AQP5 levels were measured in ovary tissue. AQP5 levels were significantly decreased in the ovary tissue of OIR induced animals compared to the control group (p<0.001). No significant increase was observed in the OIR+ROL1 group compared to the OIR group. In the OIR+ROL3 group, AQP5 levels were increase compared to the OIR group (p<0.001). In the OIR+ROL5 group in which the highest dose of rolipram was applied, AQP5 levels showed the highest increase compared to the OIR group (p<0.001) (Fig. 3).
Fig. 3.
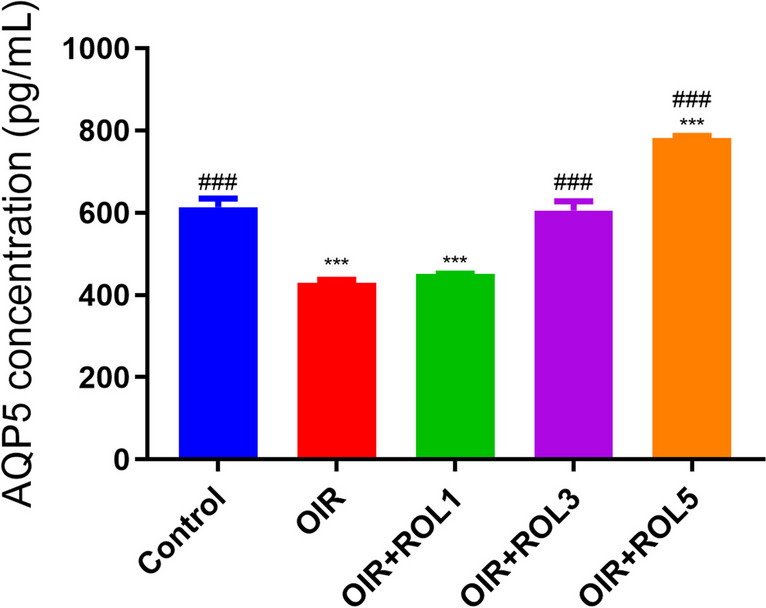
Effects of Rolipram (ROL) on AQP5 levels in ovarian ischemia-reperfusion injury. *p<0.05 **p<0.01 ***p<0.001 indicate comparison with control group. #p<0.05, ##p<0.01 and ###p<0.001 indicate the comparison by OIR group according to Tukey test. Results are given as mean ± standard deviation. ROL 1: Rolipram 1 mg/kg, ROL 3: Rolipram 3 mg/kg, ROL 5: Rolipram 5 mg/kg
NF-κB ELISA Result
NF-κB signal was measured in ovary tissue in our study. NF-κB levels were significantly increased in the ovary tissue of OIR induced animals compared to the control group (p<0.01). With the application of PDE4 inhibitor rolipram, a significant decrease was observed in NF-κB levels in the OIR+ROL5 group compared to the OIR group (p<0.05) (Fig. 4).
Fig. 4.
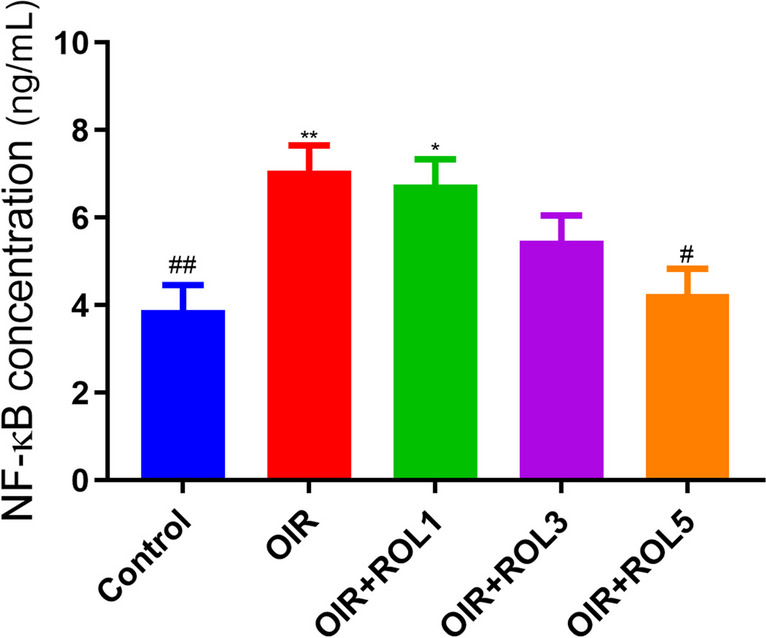
Effects of Rolipram (ROL) on NF-κB levels in ovarian ischemia-reperfusion injury. *p<0.05 **p<0.01 ***p<0.001 indicate comparison with control group. #p<0.05, ##p<0.01 and ###p<0.001 indicate the comparison by OIR group according to Tukey test. Results are given as mean ± standard deviation. ROL 1: Rolipram 1 mg/kg, ROL 3: Rolipram 3 mg/kg, ROL 5: Rolipram 5 mg/kg
MAPK ELISA Result
MAPK levels were measured in ovary tissue. A significant increase was observed in MAPK levels in the ovary tissue of OIR-induced animals compared to the control group (p<0.001). With the application of PDE4 inhibitor rolipram, a dose-dependent decrease in MAPK levels was observed in the OIR+ROL3, OIR+ROL5 groups compared to the OIR group (p<0.001) (Fig. 5).
Fig. 5.
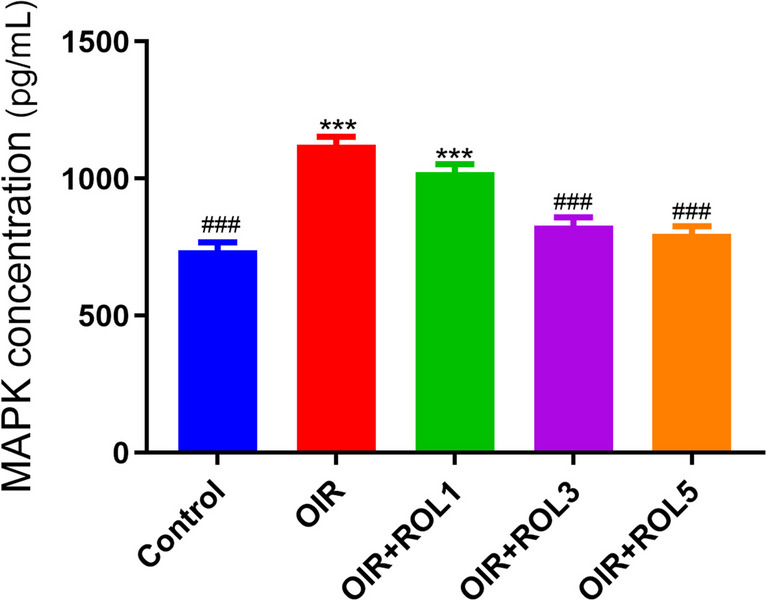
Effects of Rolipram (ROL) on MAPK levels in ovarian ischemia-reperfusion injury. *p<0.05 **p<0.01 ***p<0.001 indicate comparison with control group. #p<0.05, ##p<0.01 and ###p<0.001 indicate the comparison by OIR group according to Tukey test. Results are given as mean ± standard deviation. ROL 1: Rolipram 1 mg/kg, ROL 3: Rolipram 3 mg/kg, ROL 5: Rolipram 5 mg/kg
PDE4D ELISA Result
PDE4D levels were measured in ovary tissue. PDE4D levels in the ovary tissue of OIR induced animals were significantly increased compared to the control group (p<0.01). Administration of PDE4 inhibitor rolipram to OIR treated animals caused a dose-dependent decrease in PDE4D level. Namely; A significant decrease was observed in the OIR+ROL3 (p<0.01) and OIR+ROL5 (p<0.01) groups, while a decrease was observed in the OIR+ROL1 (p<0.05) group compared to the OIR group (Fig. 6).
Fig. 6.
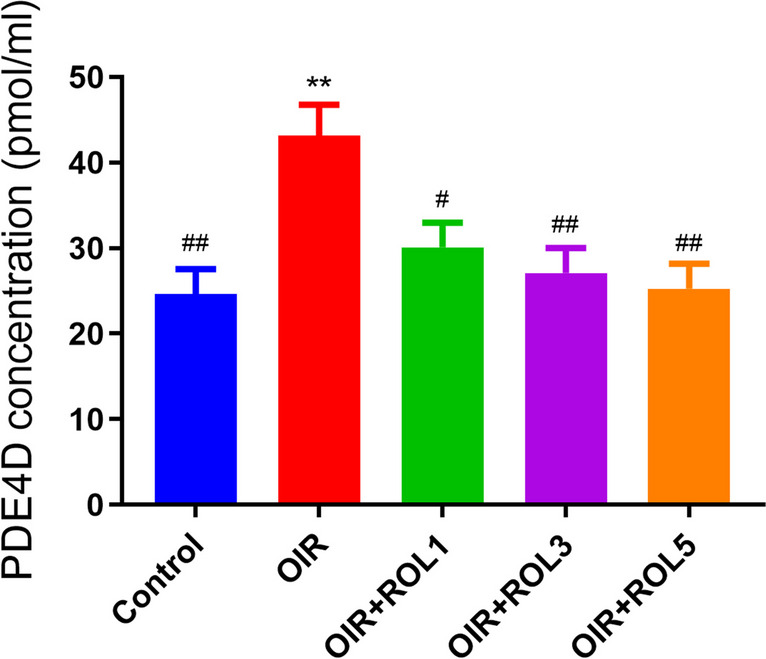
Effects of Rolipram (ROL) on PDE4D levels in ovarian ischemia-reperfusion injury. *p<0.05 **p<0.01 ***p<0.001 indicate comparison with control group. #p<0.05, ##p<0.01 and ###p<0.001 indicate the comparison by OIR group according to Tukey test. Results are given as mean ± standard deviation. ROL 1: Rolipram 1 mg/kg, ROL 3: Rolipram 3 mg/kg, ROL 5: Rolipram 5 mg/kg
cAMP ELISA Result
cAMP levels were measured in ovary tissue. In the OIR group, cAMP levels were significantly decreased compared to the control group (p<0.05). With the administration of the PDE4 inhibitor rolipram, cAMP levels increased significantly in the OIR+ROL1 (p<0.01), OIR+ROL3 (p<0.001), OIR+ROL5 (p<0.001) groups compared to the OIR group (Fig. 7).
Fig. 7.
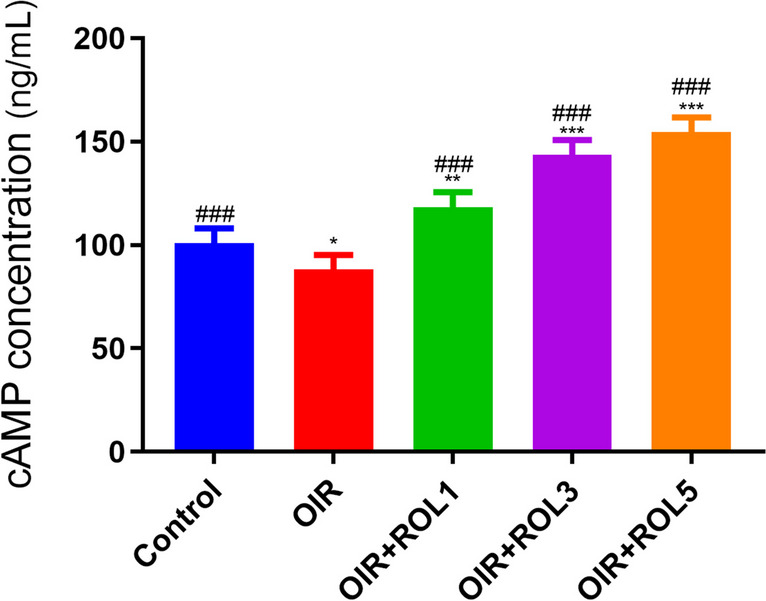
Effects of Rolipram (ROL) on cAMP levels in ovarian ischemia-reperfusion injury. *p<0.05 **p<0.01 ***p<0.001 indicate comparison with control group. #p<0.05, ##p<0.01 and ###p<0.001 indicate the comparison by OIR group according to Tukey test. Results are given as mean ± standard deviation. ROL 1: Rolipram 1 mg/kg, ROL 3: Rolipram 3 mg/kg, ROL 5: Rolipram 5 mg/kg
Expression of AQP5 in Over Tissues
When AQP5 mRNA Expression levels in the ovary tissue were examined in the OIR model, it was observed that there was a significant decrease in the OIR group compared to the control group (p<0.05). With the application of PDE4 inhibitor rolipram, AQP5 mRNA expression levels were not observed to increase significantly in the OIR+ROL1 group compared to the OIR group, whereas in the OIR+ROL3 (p<0.05) and OIR+ROL5 (p<0.001) groups, AQP5 mRNA expression levels were observed to increase compared to the OIR group (Fig. 8).
Fig. 8.
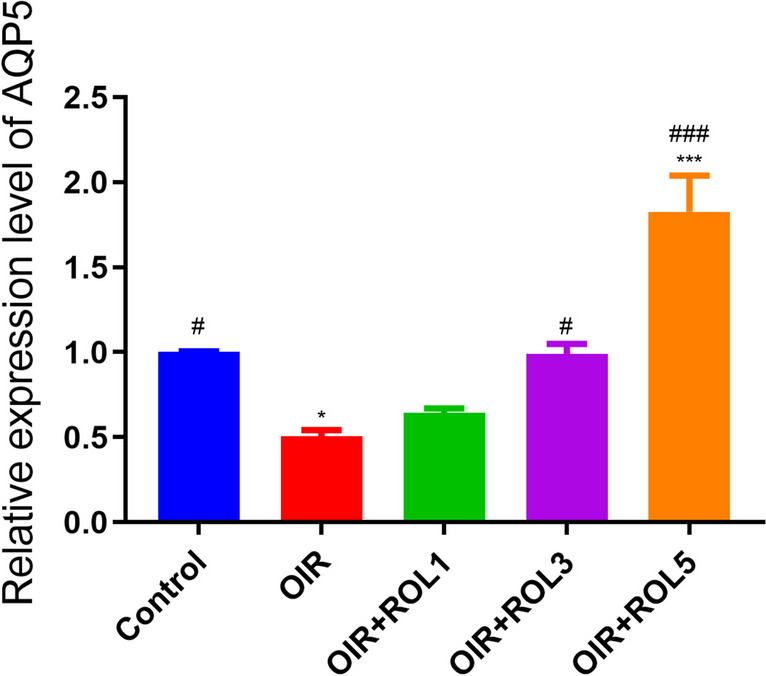
Effects of Rolipram (ROL) on AQP5 mRNA expression levels in ovarian ischemia-reperfusion injury. *p<0.05 **p<0.01 ***p<0.001 indicate comparison with control group. #p<0.05, ##p<0.01 and ###p<0.001 indicate the comparison by OIR group according to Tukey test. Results are given as mean ± standard deviation. ROL 1: Rolipram 1 mg/kg, ROL 3: Rolipram 3 mg/kg, ROL 5: Rolipram 5 mg/kg
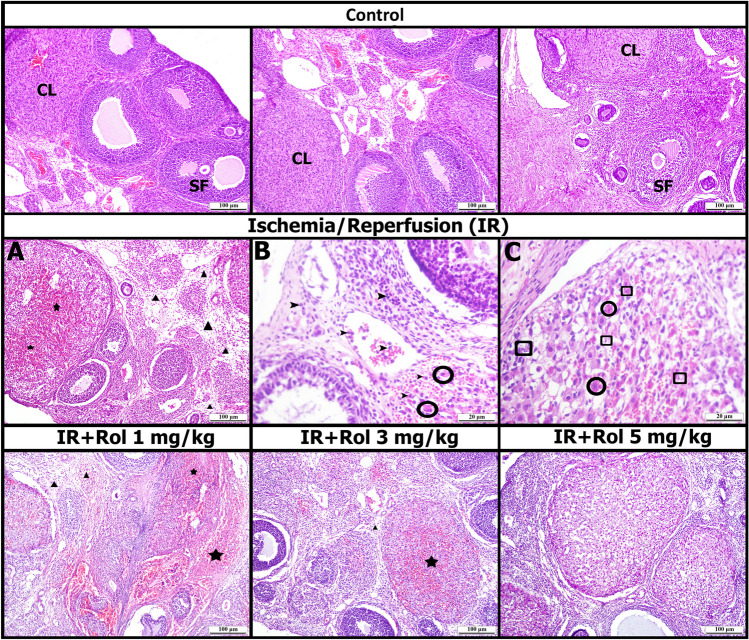
Histopathological Results
The medulla and cortex of the ovary were examined in the control group. Control-looking primary, secondary follicles and copus luteum were observed in the cortex. Normal-looking blood vessels were observed in the medulla (Fig. 9, Table 1).
Fig. 9.
Histopathological findings (Ischemia and Reperfusion: IR, Rol: Rolipram, Star: Hemorrhage, Triangle: Edema, Round Ring: necrotic cell, Square: Apoptotic cell, Arrowhead: Inflammatory cell, CL: Corpus luteum, SF: Secondary follicle)
Table 1.
Histopathological findings scoring
| GROUPS | Hemorrhage | Edema | Inflammatory Cell | Necrotic Cell |
|---|---|---|---|---|
| Control | - | - | - | - |
| OIR | ++ | ++ | ++ | ++ |
| OIR+ROL 1 | ++ | ++ | ++ | + |
| OIR+ROL 3 | + | + | + | - |
| OIR+ROL 5 | -/+ | - | - | - |
In the OIR group, moderate hemorrhage was observed in the cortex and corpus luteum, while edema findings were observed in the medulla. While leukocyte infiltration from blood vessels into parenchymal tissue was observed at high magnifications, apoptotic and necrotic cells were observed in parenchyma (Fig. 9, Table 1).
Moderate hemorrhage and edema were observed in the OIR+ROL 1 mg/kg group, as in the ischemia-reperfusion group, and apoptotic and necrotic cells were observed in the parenchyma. Mild hemorrhage and edema were observed in the OIR+ROL 3 mg/kg group, and rarely apoptotic and necrotic cells in the parenchyma. While hemorrhage and edema areas were not observed in the OIR+ROL 5 mg/kg group, apoptotic and necrotic cells were not observed in the parenchyma. The general appearance of the OIR+ROL 5 mg/kg group was found to be similar to that of control (Fig. 9, Table 1).
Discussion
OIR is a complex pathological disease that begins with the cessation of oxygen supply in response to overproduction of free oxygen radicals, then continues with an inflammatory response, and ends with apoptosis and cell death [26]. In this study, The PDE4 inhibitor rolipram was first tested in a rat model of ovarian ischemia reperfusion and its inflammatory levels were determined in each rat group to investigate whether it has a therapeutic effect on OIR. Although rolipram is not currently in active clinical use, its additional anti-inflammatory effect, originally described for its antidepressant properties, is under clinical and experimental investigation in indications for autoimmune disorders [27]. Therefore, the demonstration of the efficacy of PDE4 inhibition in our study suggests that rolipram or similar newly developed PDE4 inhibitors may be used in OIR patients and/or other ischemia reperfusion conditions in the acute intervention phase in addition to other indications. Many studies have shown the beneficial effects of PDE inhibition, in models of I/R injury [28]. In one study, it was shown that PDE inhibitors inhibit the production of proinflammatory genes such as NF-κB and TNF-α in macrophages and microglia by increasing cAMP [29]. Rolipram is known as a selective PDE4 inhibitor and increases intracellular cAMP levels in many tissues and cell types [30]. cAMP is a tightly arranged second messenger involved in different intracellular processes and plays an important role in these processes [31]. Inhibition of PDE4 reduces inflammatory effects by increasing cAMP in these cells [30]. During OIR-induced damage, it causes dysregulation and ovarian damage in the processes of apoptosis and oxidative stress, inflammation by disturbances in the cAMP pathway. Several studies have shown that damage to I/R is reduced by phosphodiesterase inhibitors such as pentoxifylline, aminophylline and rolipram, all of which are known to increase intracellular cAMP levels [32]. In our study, rolipram significantly reduced tissue damage and inflammatory cell count in the OIR group and we also observed an increase in cAMP levels due to increasing doses of rolipram in our results. This can be a result of PDE inhibition by rolipram, for PDE enzymes metabolizes cAMP and when we blocked PDE4 activity cAMP accumulation might be occurred in ROL administered groups. In this context we suggest that there may be a relationship between the cAMP pathway and the anti-inflammatory effects of rolipram.
We also examined the relationship of rolipram with AQP5 to evaluate its protective effects and advantages in OIR. AQPs, are a family of small membrane proteins that transport water across biological membranes. It is important for water transport, many metabolic processes and the survival of living cells [33]. Almost all AQPs except AQP0 and AQP10 were found in the female reproductive system [34]. It has been suggested that AQPs may contrubute to the modulation of ovarian response to exogenous gonadotrophin and positively associated with fertilization rate [35].
AQP1-AQP12 (except AQP0 and AQP10) has been shown to be found in the ovaries and ovaries of rodents [36]. AQP5 in mammals; It was detected in the lung, salivary gland, reproductive system, kidney, eye, gastrointestinal system. Also, AQP5 expression has been detected in the oviduct and uterus of many mammalian species, including rats [37–42]. In addition, in a study, it was shown that AQP5 is localized at the protein level on the ovarian epithelial side of the ovarian bursa. Such interesting upregulations of AQP5 in discrete compartments on the ovarian epithelial side of the ovarian bursa indicate their important role in intra-bursa fluid homeostasis [43]. Additionally, a study by Starowicz et al found that while immunofluorescence staining of AQP5 was seen in most oocytes, it was also present in the apical membrane of the epithelial cells of the oviduct ampoule, and after ovulation in rats, AQP5 was shown to play a role in the intracellular movement of water in oocytes and the oviduct ampoule [44]. Previous studies have shown that AQP5 expression was decreased at both mRNA and protein levels in the I/R group compared to the control group [45–47]. Furthermore in an I/R rat study, it was determined that the cAMP-PKA signaling pathway may play a role in the expression of AQP5 protein, and mRNA increases the expression of AQP5 when the cAMP-PKA signaling pathway is activated [48]. Our findings revealed that AQP5 expression decreased in OIR and could prevent the decrease of AQP5 expression after rolipram treatment. Based on these results, we hypothesize that rolipram may affect AQP5 expression by activating the cAMP pathway. However, AQP5 in the ovarian bursa still needs future explanation [43]. In another study, AQP5 immunoreactivity was not detected in mouse ovaries [34]. Therefore, information regarding the role and expression of AQPs in rat female reproductive tissues is still very limited. Therefore, future studies for both rats and humans should be focused on.
During ovarian ischemia, tissues are exposed to destructive pro-inflammatory cytokines and ROS released by inflammatory cells, leading to inflammatory damage [49]. Moreover, unexpectedly, the reperfusion of ischemic tissue following ischemia leads to an elevation in ROS levels due to tissue oxygenation [50]. Therefore; future studies focusing on other possible pathways involving in OIR damage such as oxidative status should be planned. In reperfusion injury, with the influx of oxygen into tissues, ROS and activated neutrophils release pro-inflammatory molecules such as TNF-α and IL-6, which directly cause tissue damage and are potent activators of other neutrophils [51]. Studies have also shown that levels of inflammatory cytokines such as TNF-a and IL-6 are increased in ischemic and/or reperfused tissue [52]. Therefore, I/R is characterized by increased proinflammatory cytokines, including TNF-α and IL-6 [53]. We observed that the IL-6 and TNF-α level in the OIR group was elevated compared to the control. These findings suggest that triggering the inflammatory response may play an important role in the pathogenesis and progression of OIR injury. We found that rolipram reduced protein levels of pro-inflammatory factors, including TNF-α and IL-6, which are used to assess the inflammatory response. Therefore, our findings showed that rolipram could inhibit pro-inflammatory cytokines; This suggested that the anti-inflammatory roles of rolipram in OIR may be related to this mechanism.
Studies have shown that the MAPK signaling pathway and NF-κB signaling pathway, are involved in the regulation of inflammation [54]. Activation of the NF-κB signaling pathway, is functionally associated with its upregulation leading to generation of proinflammatory cytokines. In addition, high levels of other interleukins and TNF-α directly trigger NF-κB signaling, amplification of the initial inflammatory effect [55]. Therefore, many OIR studies have shown that NF-κB levels are increased, suggesting that the NF-κB signaling pathway may increase inflammation and apoptosis and may be the primary oxidative stress-response pathway [56–58]. Similar to our results, we observed that NF-κB levels increased in the I/R group compared to the control. We think that NF-κB and signaling pathways are activated in OIR and rolipram may limit this activation. Therefore, this situation; it suggests that NF-κB may be part of the mechanism by which rolipram exerts its effects.
To appraise the advantages and protective effects of rolipram treatment on OIR, we also examined on the MAPK signaling pathway. Recent studies have clearly demonstrated that MAPKs are associated with increased I/R damage [59]. Studies have shown that the MAPK signaling pathway is involved in a variety of cellular activities, inflammation, innate immunity, proliferation, differentiation, survival and apoptosis [60]. Therefore, stress-activated protein kinases, including c-jun N-terminal kinase (JNK) and p38 MAPK, have been suggested to contribute to the promotion of cell apoptosis and causes overproduction of proinflammatory factors [61]. Recent medical and experimental studies have clearly indicated that MAPKs are linked to an elevated risk of I/R injury [62]. Numerous studies have demonstrated that the protein expression level of MAPK was significantly increased in I/R-associated diseases compared to the control group [63–66]. In our study, we observed that the MAPK level in the OIR group was increased compared to the control and that treatment with increasing doses of rolipram reduces MAPK levels. Based on these results, we think that rolipram may play a protective role in OIR via the MAPK pathway. Thus, regulation of the inflammation-related pathways, the MAPK pathway and the NF-κB pathway may be helpful for OIR.
Conclusion
The PDE4 inhibitor rolipram can preserve ovary tissue from the harmful effects of OIR damage. The underlying mechanisms may be upregulation of cAMP and downregulation of IL-6/TNF-α/NF-κB/MAPK by rolipram, which may reduce OIR-induced ovarian damage. Rolipram can reduce OIR injury by inhibiting NF-κB, MAPK occurence, reducing TNF-α and IL-6 inflammation levels, and upregulating AQP5 expression. The PDE4 inhibitor rolipram and the different pathways such as IL-6/NF-κB/TNFα/MAPK and cAMP/AQP5 mediating its effects against ovarian torsion damage may be a promising target for ovarian damage or infertility. Therefore, PDE4 inhibition may be a new therapeutic strategy in the treatment of OIR. However, future experimental and clinical studies are needed to detect these results in humans.
Acknowledgements
We thank the anonymous reviewers for examining this manuscript.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). Health Institutes of Türkiye (TUSEB) Project Number: 16490.
Data availability
The data that support the findings of this study are available from the corresponding author, EC, upon reasonable request.
Declarations
Consent for Publication
All the participants provided written informed consent for publication.
Conflict of İnterest
There is no conflict of interest between our authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barghi B, Shokoohi M, Khaki AA, Khaki A, Moghimian M, Soltani M. Eugenol Improves Tissue Damage and Oxidative Stress in Adult Female Rats after Ovarian Torsion/Detorsion. J Obstetrics Gynaecology : J Institute Obstetrics Gynaecol. 2021;41:933–938. doi: 10.1080/01443615.2020.1816938. [DOI] [PubMed] [Google Scholar]
- 2.Shokoohi M, Shoorei H, Soltani M, Abtahi-Eivari S-H, Salimnejad R, Moghimian M. Protective Effects of the Hydroalcoholic Extract of Fumaria Parviflora on Testicular Injury Induced by Torsion/Detorsion in Adult Rats. Andrologia. 2018;50:e13047. doi: 10.1111/and.13047. [DOI] [PubMed] [Google Scholar]
- 3.Refaie MMM, El-Hussieny M. Protective Effect of Pioglitazone on Ovarian Ischemia Reperfusion Injury of Female Rats via Modulation of Peroxisome Proliferator Activated Receptor Gamma and Heme-Oxygenase 1. Int Immunopharmacol. 2018;62:7–14. doi: 10.1016/j.intimp.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz EPT, Un H, Gundogdu B, Polat E, Askin S, Topdagi YE, Halici Z. Protective Effect of Lycopene against Reperfusion Injury in Rats with Ovarian Torsion: A Biochemical and Histopathological Evaluation. J Lab Phys. 2020;12:32–37. doi: 10.1055/s-0040-1715553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shokoohi M, Soltani M, Abtahi-Eivary S-H, Niazi V, Poor MR, Ravaei H, Salimnejad R, Moghimian M, Shoorei H. Effect of Hydro-Alcoholic Extract of Olea Europaea on Apoptosis-Related Genes and Oxidative Stress in a Rat Model of Torsion/Detorsion–Induced Ovarian Damage. Asian Pacific J Reprod. 2019;8:148. doi: 10.4103/2305-0500.262831. [DOI] [Google Scholar]
- 6.Sasaki KJ, Miller CE. Adnexal Torsion: Review of the Literature. J Minimal Invasive Gynecol. 2014;21:196–202. doi: 10.1016/j.jmig.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Cass DL. Ovarian Torsion. Sem Ped Surg. 2005;14:86–92. doi: 10.1053/j.sempedsurg.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Geimanaite L, Trainavicius K. Ovarian Torsion in Children: Management and Outcomes. J Pedia Surg. 2013;48:1946–1953. doi: 10.1016/j.jpedsurg.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Bender AT, Beavo JA. Cyclic Nucleotide Phosphodiesterases: Molecular Regulation to Clinical Use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 10.Baillie GS, Tejeda GS, Kelly MP. Therapeutic Targeting of 3′,5′-Cyclic Nucleotide Phosphodiesterases: Inhibition and Beyond. Nature Rev Drug Disc. 2019;18:770–796. doi: 10.1038/s41573-019-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgin AB, Magnusson OT, Singh J, Witte P, Staker BL, Bjornsson JM, Thorsteinsdottir M, Hrafnsdottir S, Hagen T, Kiselyov AS, Stewart LJ, Gurney ME. Design of Phosphodiesterase 4D (PDE4D) Allosteric Modulators for Enhancing Cognition with Improved Safety. Nature Biotechnol. 2010;28:63–70. doi: 10.1038/nbt.1598. [DOI] [PubMed] [Google Scholar]
- 12.Spadaccini M, D’Alessio S, Peyrin-Biroulet L, Danese S. PDE4 Inhibition and Inflammatory Bowel Disease: A Novel Therapeutic Avenue. Int J Mole Sci. 2017;18:1276. doi: 10.3390/ijms18061276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakkas LI, Mavropoulos A, Bogdanos DP. Phosphodiesterase 4 inhibitors in immune-mediated diseases: mode of action, clinical applications, current and future perspectives. Current Medicinal Chemistry. 2017;24. 10.2174/0929867324666170530093902 [DOI] [PubMed]
- 14.VanUffelen B, de Koster B, Elferink J. Interaction of Cyclic GMP and Cyclic AMP during Neutrophil Migration: Involvement of Phosphodiesterase Type III. Biochem Pharmacol. 1998;56:1061–1063. doi: 10.1016/S0006-2952(98)00147-6. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Yee C, Beavo JA. CD3- and CD28-Dependent Induction of PDE7 Required for T Cell Activation. Science. 1999;283:848–851. doi: 10.1126/science.283.5403.848. [DOI] [PubMed] [Google Scholar]
- 16.Kim HK, Kwon JY, Yoo C, Abdi S. The Analgesic Effect of Rolipram, a Phosphodiesterase 4 Inhibitor, on Chemotherapy-Induced Neuropathic Pain in Rats. Anesthesia Analgesia. 2015;121:822–828. doi: 10.1213/ANE.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 17.Mariajoseph-Antony LF, Kannan A, Panneerselvam A, Loganathan C, Shankar EM, Anbarasu K, Prahalathan C. Role of Aquaporins in Inflammation—a Scientific Curation. Inflammation. 2020;43:1599–1610. doi: 10.1007/s10753-020-01247-4. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Mao J, Wang T, Fu X. Downregulation of Aquaporin-4 Protects Brain Against Hypoxia Ischemia via Anti-Inflammatory Mechanism. Molecular Neurobiol. 2017;54:6426–6435. doi: 10.1007/s12035-016-0185-8. [DOI] [PubMed] [Google Scholar]
- 19.Dastgheib M, Shetab-Boushehri SV, Baeeri M, Gholami M, Karimi MY, Hosseini A. Rolipram and Pentoxifylline Combination Ameliorates Experimental Diabetic Neuropathy through Inhibition of Oxidative Stress and Inflammatory Pathways in the Dorsal Root Ganglion Neurons. Metabolic Brain Dis. 2022;37:2615–2627. doi: 10.1007/s11011-022-01060-y. [DOI] [PubMed] [Google Scholar]
- 20.Han K-H, Kim S-H, Jeong IC, Lee Y-H, Chang S-J, Park B-N-R, Kim SW. Electrophysiological and Behavioral Changes by Phosphodiesterase 4 Inhibitor in a Rat Model of Alcoholic Neuropathy. J Korean Neurosurg Soc. 2012;52:32. doi: 10.3340/jkns.2012.52.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halici Z, Karaca M, Keles ON, Borekci B, Odabasoglu F, Suleyman H, Cadirci E, Bayir Y, Unal B. Protective Effects of Amlodipine on Ischemia-Reperfusion Injury of Rat Ovary: Biochemical and Histopathologic Evaluation. Fertility Steril. 2008;90:2408–2415. doi: 10.1016/j.fertnstert.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Bayir Y, Cadirci E, Polat B, Kilic Baygutalp N, Albayrak A, Karakus E, Un H, Keles MS, Kocak Ozgeris FB, Toktay E, Karaca M, Halici Z. Aliskiren – a Promising Strategy for Ovarian Ischemia/Reperfusion Injury Protection in Rats via RAAS. Gynecol Endocrinol. 2016;32:675–683. doi: 10.3109/09513590.2016.1153055. [DOI] [PubMed] [Google Scholar]
- 23.Aydin P, Magden ZBA, Uzuncakmak SK, Halici H, Akgun N, Mendil AS, Mokhtare B, Cadirci E. Avanafil as a Novel Therapeutic Agent Against LPS-Induced Acute Lung Injury via Increasing CGMP to Downregulate the TLR4-NF-ΚB-NLRP3 Inflammasome Signaling Pathway. Lung. 2022;200:561–572. doi: 10.1007/s00408-022-00564-9. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Cinar I, Sirin B, Aydin P, Toktay E, Cadirci E, Halici I, Halici Z. Ameliorative Effect of Gossypin against Acute Lung Injury in Experimental Sepsis Model of Rats. Life Sci. 2019;221:327–334. doi: 10.1016/j.lfs.2019.02.039. [DOI] [PubMed] [Google Scholar]
- 26.Nayki UA, Nayki C, Cetin N, Cimen FK, Coban A, Mammadov R, Tas IH, Malkoc I. Effect of Kineret® on Ovarian Ischemia Reperfusion Injury in a Rat Model. J Obstetrics Gynaecol Res. 2016;42:1525–1533. doi: 10.1111/jog.13095. [DOI] [PubMed] [Google Scholar]
- 27.Castro A, Jerez MJ, Gil C, Martinez A. Cyclic Nucleotide Phosphodiesterases and Their Role in Immunomodulatory Responses: Advances in the Development of Specific Phosphodiesterase Inhibitors. Medicinal Res Rev. 2005;25:229–244. doi: 10.1002/med.20020. [DOI] [PubMed] [Google Scholar]
- 28.Souza DG, Cassali GD, Poole S, Teixeira MM. Effects of Inhibition of PDE4 and TNF-α on Local and Remote Injuries Following Ischaemia and Reperfusion Injury. British J Pharmacol. 2001;134:985–994. doi: 10.1038/sj.bjp.0704336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh M, Aguirre V, Wai K, Felfly H, Dietrich WD, Pearse DD. The Interplay between Cyclic AMP, MAPK, and NF- κ B Pathways in Response to Proinflammatory Signals in Microglia. BioMed Res Int. 2015;2015:1–18. doi: 10.1155/2015/308461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montana JG, Dyke HJ. Update on the Therapeutic Potential of PDE4 Inhibitors. Exp Opinion Invest Drugs. 2002;11:1–13. doi: 10.1517/13543784.11.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Astakhova LA, Kapitskiĭ SV, Govardovskiĭ VI, Firsov ML. [CAMP as a regulator of the phototransduction cascade]. Rossiiskii fiziologicheskii zhurnal imeni I.M. Sechenova. 2012;98:1273–85. [PubMed]
- 32.Hoffmann SC, Bleiweis MS, Jones DR, Paik HC, Ciriaco P, Egan TM. Maintenance of CAMP in Non-Heart-Beating Donor Lungs Reduces Ischemia-Reperfusion Injury. Ame J Resp Critical Care Med. 2001;163:1642–1647. doi: 10.1164/ajrccm.163.7.9911060. [DOI] [PubMed] [Google Scholar]
- 33.Verkman AS, Mitra AK. Structure and Function of Aquaporin Water Channels. Ame J Physiol-Renal Physio. 2000;278:F13–F28. doi: 10.1152/ajprenal.2000.278.1.F13. [DOI] [PubMed] [Google Scholar]
- 34.Im JW, Lee CY, Kim D-H, Bae H-R. Differential expressions of aquaporin subtypes in female reproductive tract of mice. Develop Reprod. 2020;24:177–185. 10.12717/DR.2020.24.3.177. [DOI] [PMC free article] [PubMed]
- 35.Lee HJ, Jee BC, Kim SK, Kim H, Lee JR, Suh CS, Kim SH. Expressions of Aquaporin Family in Human Luteinized Granulosa Cells and Their Correlations with IVF Outcomes. Human Reproduc (Oxford, England) 2016;31:822–31. doi: 10.1093/humrep/dew006. [DOI] [PubMed] [Google Scholar]
- 36.Skowronski MT, Kwon T-H, Nielsen S. Immunolocalization of Aquaporin 1, 5, and 9 in the Female Pig Reproductive System. J Histochem Cytochem : official J Histochem Soc. 2009;57:61–7. doi: 10.1369/jhc.2008.952499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein C, Troedsson M, Rutllant J. Expression of Aquaporin Water Channels in Equine Endometrium Is Differentially Regulated During the Oestrous Cycle and Early Pregnancy. Reproduc Dom Animals. 2013;48:529–537. doi: 10.1111/rda.12116. [DOI] [PubMed] [Google Scholar]
- 38.Lindsay LA, Murphy CR. Redistribution of Aquaporins 1 and 5 in the Rat Uterus Is Dependent on Progesterone: A Study with Light and Electron Microscopy. Reprod (Cambridge, England) 2006;131:369–78. doi: 10.1530/rep.1.00914. [DOI] [PubMed] [Google Scholar]
- 39.Brañes MC, Morales B, Ríos M, Villalón MJ. Regulation of the Immunoexpression of Aquaporin 9 by Ovarian Hormones in the Rat Oviductal Epithelium. Ame J Physio-Cell Physio. 2005;288:C1048–C1057. doi: 10.1152/ajpcell.00420.2003. [DOI] [PubMed] [Google Scholar]
- 40.Huang H-F, He R-H, Sun C-C, Zhang Y, Meng Q-X, Ma Y-Y. Function of Aquaporins in Female and Male Reproductive Systems. Human Reprod Update. 2006;12:785–795. doi: 10.1093/humupd/dml035. [DOI] [PubMed] [Google Scholar]
- 41.Aralla M, Borromeo V, Groppetti D, Secchi C, Cremonesi F, Arrighi S. A Collaboration of Aquaporins Handles Water Transport in Relation to the Estrous Cycle in the Bitch Uterus. Theriogenol. 2009;72:310–321. doi: 10.1016/j.theriogenology.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 42.Skowronski MT. Distribution and Quantitative Changes in Amounts of Aquaporin 1, 5 and 9 in the Pig Uterus during the Estrous Cycle and Early Pregnancy. Reprod Bio Endocrinol. 2010;8:109. doi: 10.1186/1477-7827-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, Zhang Y, Zhao H, Zhang Y, Chen Q, Peng H, Lei L, Qiao J, Shi J, Cao Z, Duan E, Jin Y. Hormonal regulation of ovarian bursa fluid in mice and involvement of aquaporins. PLoS ONE. 2013;8:e63823. 10.1371/journal.pone.0063823. [DOI] [PMC free article] [PubMed]
- 44.Starowicz A, Grzesiak M, Mobasheri A, Szoltys M. Immunolocalization of Aquaporin 5 during Rat Ovarian Follicle Development and Expansion of the Preovulatory Cumulus Oophorus. Acta Histochemica. 2014;116:457–465. doi: 10.1016/j.acthis.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Rabb H, Wang Z, Nemoto T, Hotchkiss J, Yokota N, Soleimani M. Acute Renal Failure Leads to Dysregulation of Lung Salt and Water Channels. Kidney Int. 2003;63:600–606. doi: 10.1046/j.1523-1755.2003.00753.x. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto N, Yoneda K, Asai K, Sobue K, Tada T, Fujita Y, Katsuya H, Fujita M, Aihara N, Mase M, Yamada K, Miura Y, Kato T. Alterations in the Expression of the AQP Family in Cultured Rat Astrocytes during Hypoxia and Reoxygenation. Mole Brain Res. 2001;90:26–38. doi: 10.1016/S0169-328X(01)00064-X. [DOI] [PubMed] [Google Scholar]
- 47.Qi QYC, Chen W, Li XL, Wang YW, Xie XH. H2S Protecting against Lung Injury Following Limb Ischemia-Reperfusion by Alleviating Inflammation and Water Transport Abnormality in Rats. Biomed Environ Sci : BES. 2014;27:410–8. doi: 10.3967/bes2014.070. [DOI] [PubMed] [Google Scholar]
- 48.Wu ZY, Yao Y, Hu R, Dai FF, Zhang H, Mao ZF. Cyclic adenosine monophosphate-protein kinase A signal pathway may be involved in pulmonary aquaporin-5 expression in ischemia/reperfusion rats following deep hypothermia cardiac arrest. Genetics and Molecular Research. 2016;15. 10.4238/gmr.15017377. [DOI] [PubMed]
- 49.Cadirci E, Oral A, Odabasoglu F, Kilic C, Coskun K, Halici Z, Suleyman H, Nuri Keles O, Unal B. Atorvastatin Reduces Tissue Damage in Rat Ovaries Subjected to Torsion and Detorsion: Biochemical and Histopathologic Evaluation. Naunyn-Schmiedeberg’s Arch Pharmacol. 2010;381:455–466. doi: 10.1007/s00210-010-0504-y. [DOI] [PubMed] [Google Scholar]
- 50.Karaman E, Onder GO, Goktepe O, Karakas E, Mat OC, Bolat D, Koseoglu E, Tur K, Baran M, Ermis M, Balcioglu E, Yay A. Protective Effects of Boric Acid Taken in Different Ways on Experimental Ovarian İschemia and Reperfusion. Biol Trace Element Res. 2023 doi: 10.1007/s12011-023-03871-1. [DOI] [PubMed] [Google Scholar]
- 51.Polat B, Albayrak A, Halici Z, Karakus E, Bayir Y, Demirci E, Cadirci E, Odaci E, Yayla M, Atamanalp SS. The Effect of Levosimendan in Rat Mesenteric Ischemia/Reperfusion Injury. J Invest Surg : official J Acad Surg Res. 2013;26:325–33. doi: 10.3109/08941939.2013.806615. [DOI] [PubMed] [Google Scholar]
- 52.Polat B, Albayrak A, Halici Z, Karakus E, Bayir Y, Demirci E, Cadirci E, Odaci E, Yayla M, Atamanalp SS. The Effect of Levosimendan in Rat Mesenteric Ischemia/Reperfusion Injury. J Invest Surg. 2013;26:325–333. doi: 10.3109/08941939.2013.806615. [DOI] [PubMed] [Google Scholar]
- 53.Çolak S, Koc K, Yıldırım S, Geyikoğlu F. Effects of Boric Acid on Ovarian Tissue Damage Caused by Experimental Ischemia/Reperfusion. Biotech Histochem. 2022;97:415–422. doi: 10.1080/10520295.2021.2012823. [DOI] [PubMed] [Google Scholar]
- 54.Xu D, Kong T, Shao Z, Liu M, Zhang R, Zhang S, Kong Q, Chen J, Cheng B, Wang C. Orexin-A alleviates astrocytic apoptosis and inflammation via inhibiting OX1R-mediated NF-ΚB and MAPK signaling pathways in cerebral ischemia/reperfusion injury. Biochimica et Biophysica Acta (BBA) - Mole Basis Dis. 2021;1867:166230. 10.1016/j.bbadis.2021.166230. [DOI] [PubMed]
- 55.Sun H, Zhou Z, Xuan H, Yan Z. Anti-Inflammatory and Protective Effects of Combined Treatment with Sitagliptin and Melatonin in Cardiac Ischemia Reperfusion Injury in Obese Rats: Involvement of TLR-4/NF-ΚB Pathway. Eur J Inflam. 2021;19:205873922110662. doi: 10.1177/20587392211066201. [DOI] [Google Scholar]
- 56.Refaie MMM, El-Hussieny M, Shehata S. TLR4/NF-ΚB/TNFα and CAMP/SIRT1 Signaling Cascade Involved in Mediating the Dose-Dependent Effect of Cilostazol in Ovarian Ischemia Reperfusion-Induced Injury. Immunopharmacol Immunotox. 2022;44:338–346. doi: 10.1080/08923973.2022.2043901. [DOI] [PubMed] [Google Scholar]
- 57.Kirmizi DA, Baser E, Okan A, Kara M, Yalvac ES, Doganyigit Z. The Effect of a Natural Molecule in Ovary Ischemia Reperfusion Damage: Does Lycopene Protect Ovary? Exp Animals. 2021;70:37–44. doi: 10.1538/expanim.20-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bayram P, Karamese SA, Erol HS, Ozdemir B, Toktay E, Salum C. Protective Effects of a Natural Product, Paeoniflorin, on Ischemia Reperfusion Injury on Rat Ovary Tissue: Histopathological, Immunohistochemical, and Biochemical Study. J Histotechnol. 2023;46:170–183. doi: 10.1080/01478885.2023.2227409. [DOI] [PubMed] [Google Scholar]
- 59.Park KM, Kramers C, Vayssier-Taussat M, Chen A, Bonventre JV. Prevention of Kidney Ischemia/Reperfusion-Induced Functional Injury, MAPK and MAPK Kinase Activation, and Inflammation by Remote Transient Ureteral Obstruction. J Bio Chem. 2002;277:2040–2049. doi: 10.1074/jbc.M107525200. [DOI] [PubMed] [Google Scholar]
- 60.Kim EK, Choi E-J. Compromised MAPK Signaling in Human Diseases: An Update. Archiv Toxicol. 2015;89:867–882. doi: 10.1007/s00204-015-1472-2. [DOI] [PubMed] [Google Scholar]
- 61.Himaya SWA, Ryu B, Qian Z-J, Li Y, Kim S-K. 1-(5-Bromo-2-Hydroxy-4-Methoxyphenyl)Ethanone [SE1] Suppresses pro-Inflammatory Responses by Blocking NF-ΚB and MAPK Signaling Pathways in Activated Microglia. Eur J Pharmacol. 2011;670:608–616. doi: 10.1016/j.ejphar.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 62.Sapmaz-Metin M, Topcu-Tarladacalisir Y, Uz YH, Inan M, Omurlu IK, Cerkezkayabekir A, Kizilay G, Akpolat M. Vitamin E Modulates Apoptosis and C-Jun N-Terminal Kinase Activation in Ovarian Torsion-Detorsion Injury. Exp Mole Pathol. 2013;95:213–9. doi: 10.1016/j.yexmp.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 63.Li L, Shu F, Wang X-Q, Wang F, Cai L, Zhao X, Lv H-G. Propofol alleviates intestinal ischemia/reperfusion injury in rats through p38 MAPK/NF-ΚB signaling pathway. Eur Rev Med Pharmacol Sci. 2021;25:1574–1581. 10.26355/eurrev_202102_24867. [DOI] [PubMed]
- 64.Jia L, Cui W, Chen J, Yang J, Xue X, Cai J, Zhao W, Gao W. Erythropoietin Alleviates Acute Lung Injury Induced by Ischemia-Reperfusion through Blocking P38 MAPK Signaling. Human Exp Toxic. 2021;40:S593–S602. doi: 10.1177/09603271211043480. [DOI] [PubMed] [Google Scholar]
- 65.Yang Z-H, Lu Y-J, Gu K-P, Xiang Z-Y, Huang H-M. Effect of ulinastatin on myocardial ischemia-reperfusion injury through JNK and P38 MAPK signaling pathways. Eur Rev Med Pharmacol Sci. 2019;23:8658–8664. 10.26355/eurrev_201910_19183. [DOI] [PubMed]
- 66.Ming Y-C, Chao H-C, Chu S-M, Luo C-C. Heparin-Binding Epidermal Growth Factor-like Growth Factor (HB-EGF) Protected Intestinal Ischemia-Reperfusion Injury through JNK and P38/MAPK-Dependent Pathway for Anti-Apoptosis. Ped Neonatol. 2019;60:332–336. doi: 10.1016/j.pedneo.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Aksak Karamese S, Toktay E, Unal D, Selli J, Karamese M, Malkoc I. The Protective Effects of Beta-Carotene against Ischemia/Reperfusion Injury in Rat Ovarian Tissue. Acta Histochemica. 2015;117:790–797. doi: 10.1016/j.acthis.2015.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, EC, upon reasonable request.